Abstract
Soon after asymmetric septation in sporulating Bacillus subtilis cells, ςF is liberated in the prespore from inhibition by SpoIIAB. To initiate transcription from its cognate promoters, ςF must compete with ςA, the housekeeping sigma factor in the predivisional cell, for binding to core RNA polymerase (E). To estimate the relative affinity of E for ςA and ςF, we made separate mixtures of E with each of the two sigma factors, allowed reconstitution of the holoenzyme, and measured the concentration of free E remaining in each mixture. The affinity of E for ςF was found to be about 25-fold lower than that for ςA. We used quantitative Western blotting to estimate the concentrations of E, ςA, and ςF in sporulating cells. The cellular concentrations of E and ςA were both about 7.5 μM, and neither changed significantly during the first 3 h of sporulation. The concentration of ςF was extremely low at the beginning of sporulation, but it rose rapidly to a peak after about 2 h. At its peak, the concentration of ςF was some twofold higher than that of ςA. This difference in concentration cannot adequately account for the replacement of ςA holoenzyme by ςF holoenzyme in the prespore, and it seems that some further mechanism—perhaps the synthesis or activation of an anti-ςA factor—must be responsible for this replacement.
Soon after the asymmetric septation that occurs early in the sporulation of Bacillus subtilis, ςF is activated in the prespore (7, 29, 37). However, other sigma factors besides ςF are present in the cell early in sporulation, for example, the vegetative “housekeeping” ςA factor and the postexponential-phase-specific ςH factor (16), and these, like ςF, are capable of binding to core RNA polymerase (here called core RNAP or E). Although ςA activity disappears after asymmetric septation (26), ςA protein can be detected during sporulation, albeit not in a form that copurifies with core RNAP in the way that ςA from vegetative cells does (37). In this study, we sought to discover how, notwithstanding the presence of ςA, ςF is incorporated into the holoenzyme in the prespore. We did not consider how ςF is released from inhibition by SpoIIAB (see references 9, 29, and 37).
One can readily envisage three possible (nonexclusive) means by which one sigma factor might replace another. The usurper might have a higher affinity for E, it might achieve a higher concentration, or it might supplant the resident sigma factor through the latter’s becoming inactivated. Although there was much interest some years ago in the exchange of sigma factors in Bacillus (reviewed in reference 28), relatively few experiments on this topic have been reported recently. To investigate the mechanism by which E-ςF replaces E-ςA in the prespore, we have determined the relative affinities of ςA and ςF for E and have measured the intracellular concentrations of E, ςA and ςF. We concluded that the replacement of E-ςA by E-ςF cannot be explained in terms of either a higher concentration of ςF or a greater affinity for E, and we suggest that an anti-ςA factor may be synthesized or activated in sporulating cells at about the time of asymmetric septation.
MATERIALS AND METHODS
Overproduction and purification of proteins.
Plasmid pLC2 (21) was used for the overproduction of ςA protein. It was transformed into the overexpression host Escherichia coli BL21(DE3) (38). Freshly transformed cells were grown at 37°C in 2YT containing 100-μg/ml ampicillin and 0.4% (wt/vol) glucose. At an A595 of 0.6 to 0.7, the culture was induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG), transferred to 30°C, and incubated for 3 h with shaking. Growth at 30°C after induction minimizes the formation of inclusion bodies and ensures that about 60% of the ςA protein is soluble. Cells were harvested and stored at −20°C. Cell pellets were resuspended in 20 ml of lysis buffer (100 mM Tris-HCl [pH 8.5], 10 mM EDTA, 5 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF]), sonicated briefly, and then passed through a French press. Lysates were clarified by centrifugation at 16,000 rpm for 90 min. The supernatant was loaded on a DEAE-Sepharose column, and a linear 0- to 1-M gradient of NaCl in buffer A (20 mM Tris-HCl [pH 8.5], 0.5 mM DTT, 0.1 mM EDTA) was applied to the column. The fractions containing ςA were pooled and loaded onto a Superdex-75 preparative gel filtration column equilibrated with buffer B (20 mM Tris-HCl [pH 8.0], 2 mM EDTA, 2 mM DTT, 20 mM MgCl2, 100 mM NaCl) and eluted in the same buffer. Fractions enriched in ςA were applied to a Mono-Q (HR5/5) column attached to a Pharmacia fast protein liquid chromatograph and eluted with a 0.25- to 0.4-M gradient of NaCl in a buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 0.1 mM DTT) containing 10% glycerol. Eluted fractions were concentrated with Centricon-10 concentrators (Amicon) and dialyzed against storage buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 1 mM DTT, 10 mM MgCl2, 50 mM NaCl, 50% glycerol). Alternatively, ςA protein was purified from inclusion bodies by previously published protocols (3, 21).
For ςF purification, E. coli BL21(DE3) transformants harboring pEAC (30) were grown, induced, and broken as previously described (5). Cell extracts were purified by DEAE-Sepharose chromatography and Superdex-75 chromatography as described for ςA. A third purification step involved chromatography on a Mono-Q (HR5/5) column with a shallow gradient of 0.2 to 0.5 M NaCl in 80 ml of TGED (10 mM Tris-HCl [pH 8.5], 0.1 mM EDTA, 0.1 mM DTT, 10% glycerol). ςF fractions were concentrated by Centricon-10 concentrators and dialyzed versus storage buffer.
Core RNAP was purified from B. subtilis SG38 by a modification of previously published procedures (2, 15, 17). Cell pellets from a 4-liter culture were resuspended in 80 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 2 mM EDTA, 0.1 mM DTT, 1 mM β-mercaptoethanol, 233 mM NaCl, 10% glycerol, 1 mM PMSF) and passed through a French press. After Polymin P fractionation and ammonium sulfate precipitation (17), the precipitate was resuspended in 8 ml of TGED containing 0.5 M NaCl and loaded onto a Sephacryl S-300 (Pharmacia) column. RNA polymerase-containing fractions were pooled, dialyzed overnight into TGED containing 50 mM NaCl, and subjected to DNA-cellulose affinity chromatography. Elution was accomplished with 0.7 M NaCl. Fractions were dialyzed versus TGED (pH 7.0) containing 50 mM NaCl and applied to BioRex 70. The core enzyme was eluted with a 40-ml linear gradient of 0.5 to 1.0 M NaCl in TGED (pH 7.0) and dialyzed into storage buffer.
Immobilization of ςA on the sensor chip surface.
ςA (0.2 mg/ml) was dialyzed into phosphate-buffered saline (pH 7.4) containing 1 mM DTT at 4°C. ςA was immobilized on the dextran surface of one flow cell of sensor chip CM5 by the amine coupling method, and HBS buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 0.0005% surfactant P20 [Pharmacia]) was the running buffer throughout. All injections carried out in the immobilization procedure used a flow rate of 10 μl/min. Dialyzed ςA was diluted 10-fold into coupling buffer (10 mM sodium acetate, pH 3.8). The sensor chip was activated by a 40-μl injection of a 1:1 mixture of N-hydroxysuccinimide and N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide hydrochloride (Pharmacia). Immediately after activation, the diluted ςA was injected in a volume of 40 to 70 μl. This injection was followed by a 40-μl injection of 1 M ethanolamine-HCl (pH 8.5) that acted to block any excess activated dextran. Typical immobilizations resulted in the attachment of 500 to 1,000 resonance units (RU) of ligand. Binding experiments were always carried out within 12 h of immobilization.
Measurement of free E after preincubation with ςA or ςF.
ςA was immobilized to the sensor chip as described above. This ligand was used to determine the concentration of free E after preincubation with ςA or ςF. The running buffer contained 10 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 50 mM NaCl, 1 mM EDTA, 1 mM DTT, and 10% glycerol; E, ςA, and ςF were dialyzed into this buffer. Samples of 100 nM E were incubated with ςA at a range of concentrations (0, 12.5, 25, 50, 100, 250, 500, and 750 nM) for 40 min at room temperature to allow reconstitution of the holoenzyme prior to sample injection (flow rate, 10 μl/min; injection volume, 40 μl). A 50-μl injection of running buffer containing 0.5 M NaCl was used to regenerate the sensor chip surface after each injection. The concentration of free E was calculated by comparing the maximum number of resonance units of the E-ςA sensorgram (after subtraction of the blank flow cell signal) obtained in the absence of ςA in the preincubation (100% free E) with the maximum number of resonance units obtained at increasing concentrations of ςA. The concentration of ςA required to reduce free E by 50% in the preincubation (KςA) was calculated from a plot of the log10 ςA concentration (x axis) versus the percentage of free E (y axis). KςF was obtained by conducting similar experiments, with the concentration range of ςF running from 0 to 6,000 nM.
Induction of sporulation.
B. subtilis SG38 was induced to sporulate as described previously (11). Times (hours) after resuspension in starvation medium are called t0, t1, etc. Efficiency of sporulation was confirmed through monitoring of alkaline phosphatase levels in sporulating cell extracts as described previously (8).
Intracellular protein concentrations.
B. subtilis cell extracts were prepared by incubating 1-ml cell pellets in 250 μl of 50 mM Tris-HCl (pH 7.5), containing 5-mg/ml lysozyme, 10% glycerol, and 1 mM PMSF for 10 min at 37°C. A 250-μl volume of sodium dodecyl sulfate (SDS) sample loading buffer (33) was added to each incubation mixture, and the samples were boiled for 5 min. Assays of ςA, ςF, and core RNAP were made by immunoblotting with purified antibodies after the proteins from cell extracts had been separated by SDS–10% (ςA and core RNAP) or −15% (ςF) polyacrylamide gel electrophoresis (PAGE). Immunoblotting was carried out as described previously (29) with the following modification: an alkaline phosphatase-conjugated secondary antibody (Bio-Rad) was used, and blots were developed by exposure to alkaline phosphatase reagent (Amersham) for 5 min (1 ml/blot). Each blot included known volumes of standard solutions of purified proteins, and the quantities of protein in the unknown samples were determined by use of a Fluoroimager (Molecular Dynamics). The ImageQuant package was employed to calculate the quantities of the unknowns and a set of known samples that covered the range. For core RNAP, both the α subunit band and the combined densities of the β and β′ subunit bands were used in the quantitation. Average intracellular protein concentration values (micromolar) were derived by assuming the number of cells per microliter of a sporulating culture to be 3.2 × 108 and the volume of each cell to be 1.8 × 10−15 liter; any inaccuracy in this estimation will, of course, affect all protein concentrations equally. For each protein, average values were derived from analysis of five or six different blots.
RESULTS AND DISCUSSION
Comparison of the relative affinities of ςA and ςF for E.
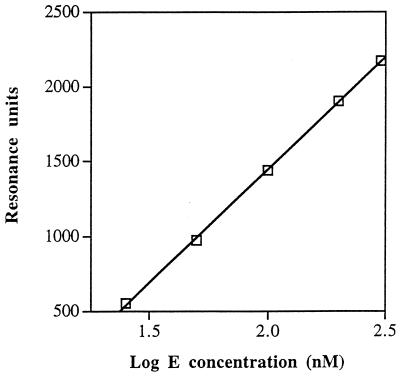
One possible means by which ςF could replace ςA would be that the former has a higher affinity for E than the latter. “Direct displacement” of this kind was suggested by Losick and Pero (28) as a means by which ςE (the first mother cell-specific sigma factor) could replace ςA early in sporulation. (At the time of that publication [28], ςF had not been identified.) To compare the affinities of ςA and ςF for E, we used a back-titration method, in which we measured the free E remaining in solution after separate mixtures of E and one of the sigma factors had been allowed to come to equilibrium with the holoenzyme formed in the mixture. In each such mixture, we determined the concentration of free E by surface plasmon resonance, using ςA as the ligand immobilized to the sensor chip that responds to E. We first constructed a standard curve, relating the height of the sensorgram in resonance units to the concentration of E, by passing increasing concentrations of a pure solution of E over the chip (Fig. 1). We then preincubated standard concentrations of E with increasing concentrations of ςA in solution to allow holoenzyme reconstitution and applied each mixture to the immobilized ςA. Back titration of the E samples with increasing amounts of ςA resulted in a steady decrease in the concentration of free E reported by the sensor chip. Such experiments allowed us to determine the concentration of ςA required in the preincubation to cause a 50% reduction in the concentration of free E. This concentration is termed KςA.
FIG. 1.
Standard curve for determination of the concentration of free core RNAP (E). With ςA immobilized on the sensor chip, the response in resonance units given by different concentrations of E is plotted against the log10 E concentration.
Back titration was first performed with an E concentration of 50 nM. The concentration of ςA in the preincubation required to reduce the height of the 50 nM E sensorgram to the height characteristic of a 25 nM E sensorgram was found to be 50 nM (mean of three titrations with a range of 44 to 54 nM). These results are shown in Fig. 2a (filled circles). When the concentration of E was 100 nM, the concentration of ςA required in the preincubation to reduce the height of the sensorgram for free E to a height corresponding to that of 50 nM was 70 nM (range of three values, 61 to 83 nM; filled circles in Fig. 2b). From these data, we calculated that the concentration of ςA in solution when E was half saturated with ςA was 20 to 25 nM (mean, 22.5 nM).
FIG. 2.
Sequestration of free E by ςA and ςF. Percentage of maximal sensorgram height was plotted against the log10 sigma factor concentration. Panels: a, binding to 50 nM E; b, binding to 100 nM E. Symbols: ●, ςA; ○, ςF.
The concentration of ςF needed to back titrate free E was much greater than the concentration of ςA needed. The average concentration of ςF required to reduce the concentration of free E from 50 to 25 nM was 540 nM (range of three values, 524 to 560 nM), and the average concentration of ςF required to reduce the concentration of free E from 100 to 50 nM was 657 nM (range of three values, 561 to 754 nM; open circles in Fig. 2a and b). The concentration of free ςF in solution when E is half saturated with ςF is therefore 515 to 607 nM (mean, 561 nM). Hence, the affinity of E for ςA is 25-fold higher than that for ςF (561 divided by 22.5).
The validity of this comparison of E-sigma affinities relies on the assumption that the relatively low affinity of ςF for E is not due to the sigma’s being partially inactivated. SDS-PAGE analysis of the ςF samples revealed that no detectable degradation of ςF had occurred during the course of the sporulation experiments. In addition, the ςF used was tested for the ability to bind to SpoIIAB. Surface plasmon resonance experiments demonstrated that, in the presence of ATP, ςF had a Kd for immobilized SpoIIAB of 16 nM, a value almost identical to the 14 nM found previously for a different ςF sample (29). Moreover, native-PAGE analysis indicated that all of the ςF sample formed a complex with SpoIIAB in the presence of ATP (results not shown). Furthermore, in vitro transcription assays showed that on incubation with core RNAP and substrate DNA, the preparation of ςF used in this study yielded amounts of transcript similar to those produced by other ςF preparations (results not shown). Taken together, these results suggest that the ςF sample used in the experiments with E was fully active.
The ςA preparation was also examined by SDS-PAGE and appeared to be undegraded. Moreover, it was capable of inhibiting transcription directed by ςF when E was limiting (results not shown). Direct measurement by surface plasmon resonance of the dissociation constant for the interaction between E and ςA yielded a Kd of 3 to 4 nM, very similar to the value reported for the interaction between E and ς70 of E. coli (13). The possibility cannot be absolutely excluded that a fraction of the preparation was inactive in binding to core RNAP, but any such inactivation would lead to an underestimate of the difference in the affinity of E for the two sigma factors.
One possible reason for the high affinity of ςA may be that this factor has an extra N-terminal sequence akin to that of E. coli ς70. Although this N-terminal extension may not itself be directly involved in the binding of E, its presence in ςA may help in stabilization of the E-ςA complex; for example, the N-terminal extension may aid in propagating conformational changes between E and ςA in the E-ςA interaction similar to those apparent between E. coli E and ς70 (14, 42). (Such an effect would be additional to the well-known role of the extension in preventing binding of the sigma factor to the promoter in the absence of E [6].) We should point out that the comparison of ςF with ςA relies on the assumption that the two proteins utilize the same binding site on E, so that when ςF has bound E, the resulting holoenzyme is unable to interact with ςA. This assumption seems likely, since alternative sigma factors have been shown to compete for E both in vivo and in vitro (4, 10, 18). E binding has been shown to involve the same region, region 2.1, of several sigma factors—E. coli ς70 and ς32 (23, 24, 35), B. subtilis ςE (36), and bacteriophage T4 Gp55 (22). Since all bacterial sigma factors are closely similar at region 2.1 (27), it seems likely that ςA and ςF use the same site to bind to E.
Intracellular concentrations of ςA, ςF, and core RNAP.
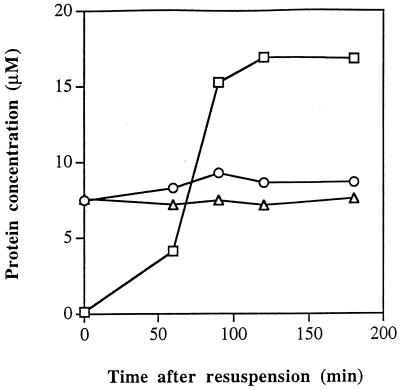
We cannot rigorously exclude the possibility that the affinities of the relevant proteins in the cell are very different from those in vitro. However, there is no evidence to support such a suggestion, and we found that E, ςA, and ςF were highly active in transcription assays in buffers similar to those used for the surface plasmon resonance experiments (results not shown). If we therefore assume that the results described in the previous section give a reasonable approximation to the situation in the cell, they seem to rule out the possibility that the replacement of E-ςA in the prespore by E-ςF is due to ςF’s having a higher affinity for the core RNAP. An alternative possibility was that, after asymmetric septation, the concentration of ςF in the prespore was much higher than that of ςA. Accordingly, we used quantitative immunoblotting to measure the intracellular concentrations of ςA, E, and ςF during the first 3 h of sporulation. We found that the ςA and core RNAP concentrations were constant, with ςA being slightly more concentrated than core RNAP (Fig. 3). A similar conclusion was reached for ςA many years ago (39). (For the above measurements, the core RNAP concentration was based on scanning of the α subunit band. The core RNAP concentration based on scanning of the β and β′ bands together was 10 to 15% lower [results not shown].) As previously reported (29), ςF was undetectable in vegetative cells (t0 samples), but its concentration rose rapidly at the time of asymmetric septation, reaching a peak value at t2 which was some twofold higher than that of ςA.
FIG. 3.
Intracellular concentrations of ςA (○), ςF (□), and core RNAP based on estimation of the α subunit (▵) during the first 3 h of sporulation. Samples were collected and assayed as described in Materials and Methods.
After asymmetric septation, the total concentration of sigma factors (ςA plus ςF) considerably exceeded the concentration of E. Given that a large fraction of the RNAP is engaged in elongating RNA and is thus unavailable for binding to sigma, these values imply that ςA and ςF must be in competition for a limited number of core RNAP molecules. Similarly, a recent study has suggested that in E. coli, two sigma factors, ς70 and ςS, compete in stationary phase for a limited supply of E (10). We note that the concentration of core RNAP estimated here corresponds to about 8,000 molecules/cell, a value similar to that (4,800 to 8,000 molecules/cell) reported by Bremer and Dennis (1) for E. coli grown at a comparable doubling time and a little higher than that reported for E. coli by Ishihama et al. (19).
To what extent is the interpretation of these findings modified by a possible asymmetry of distribution of sigma factors between the prespore and the mother cell? Immunofluorescence experiments with sporulating cells indicate that ςF shows no obvious asymmetry in its location in the sporangium; fluorescence was observed throughout cells that had already formed their asymmetric septum (25). Recently, similar immunofluorescence experiments have been used to monitor the location of ςA during sporulation. As with ςF, ςA was located throughout the sporangium and apparently at roughly equal concentrations in the prespore and the mother cell (10a). We therefore conclude that the concentrations of ςA and ςF in the prespore are unlikely to be very different from those measured here in the whole cell. In summary, we can say that, at its peak soon after asymmetric septation, the ςF concentration in the prespore may be, at most, a few fold higher than that of ςA but is very unlikely to be high enough to overcome the unfavorable ratio of the affinities of the two sigma factors for core RNAP.
Mechanism of replacement of EςA by EςF.
Given the above results, how can we account for the replacement of EςA by EςF in the prespore? One possibility is that ςA is inactivated by a modification that leaves unaffected both its ability to interact with antibody and its mobility on polyacrylamide gels. A more likely possibility is that an anti-ςA factor is synthesized or activated during sporulation. A cell constituent that interferes with the function of ςA and which is metabolically unstable was described many years ago (34, 40). An anti-ςA factor (e.g., a protein inhibitor) could allow ςF and ςG in the prespore and ςE and ςK in the mother cell to replace ςA as a component of the holoenzyme during sporulation. At the time of germination, ςA could be freed by specific loss of its anti-sigma factor, and its concentration could be supplemented by fresh synthesis of ςA as described by Qi et al. (32). Since the completion of the experimental work described here, a specific anti-ς70 factor, Rsd, has been reported to be important in sigma factor exchange in E. coli (20).
At first sight, the results of Fujita and Sadaie (12), also published after the completion of the work described here, appear to conflict with ours. Those workers reported that the RNAP holoenzyme contained ςA which could be detected by immunoblotting no matter from which stage of sporulation the enzyme had been isolated. However, given that we have shown that the concentration of ςA is unchanged throughout the first 3 h of sporulation (Fig. 3), and given the very low dissociation constant for the interaction between core RNAP and ςA (see above), it may be that the method of preparing the holoenzyme used by Fujita and Sadaie (12) leads to an association of the core with ςA that does not occur in the cell. The presence of an anti-sigma factor attached to ςA would not necessarily prevent such an association, since we have evidence that SpoIIAB, the anti-sigma factor of ςF, can associate with the ςF holoenzyme (27a; see also reference 31).
Our results have some features in common with those of Williams et al. (41). Those workers found that the sigma factor encoded by gene 55 of phage T4 had a lower affinity for E than ς70 but nonetheless displaced the latter during phage infection. Subsequent experiments with this system showed that the ability of the gene 55 product to displace ς70 was due, in all probability, to the synthesis of an anti-ς70 factor by another gene, asiA, of T4 (31).
ACKNOWLEDGMENTS
We thank J. D. Helmann for providing plasmid pLC2; D. A. Harris for much valuable advice and for reading the manuscript; and J. Errington, R. Losick, and I. Lucet for reading the manuscript.
We thank the Biotechnology and Biological Sciences Research Council and the Medical Research Council for financial support and the Wellcome Trust for providing the BIACore facility.
REFERENCES
- 1.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1527–1542. [Google Scholar]
- 2.Burgess R R, Jendrisak J J. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- 3.Chang B-Y, Doi R H. Overproduction, purification, and characterization of Bacillus subtilis RNA polymerase ςA factor. J Bacteriol. 1990;172:3257–3263. doi: 10.1128/jb.172.6.3257-3263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelm B K, Duffy J J, Geiduschek E P. Interaction of Bacillus subtilis RNA polymerase core with two specificity-determining subunits. J Biol Chem. 1982;257:6501–6508. [PubMed] [Google Scholar]
- 5.Diederich B, Wilkinson J F, Magnin T, Najafi S M A, Errington J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 6.Dombroski A J, Walter W A, Gross C A. Amino-terminal amino acids modulate ς-factor DNA-binding activity. Genes Dev. 1993;7:2446–2553. doi: 10.1101/gad.7.12a.2446. [DOI] [PubMed] [Google Scholar]
- 7.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Errington J, Mandelstam J. Variety of sporulation phenotypes resulting from mutations in a single regulatory locus, spoIIA, in Bacillus subtilis. J Gen Microbiol. 1983;129:2091–2101. doi: 10.1099/00221287-129-7-2091. [DOI] [PubMed] [Google Scholar]
- 9.Errington J, Feucht A, Lewis P J, Lord M, Magnin T, Najafi S M A, Wilkinson J F, Yudkin M D. Control of the cell-specificity of ςF activity in Bacillus subtilis. Philos Trans R Soc Lond B Biol Sci. 1996;351:537–542. doi: 10.1098/rstb.1996.0052. [DOI] [PubMed] [Google Scholar]
- 10.Farewell A, Kvint K, Nystrom T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 10a.Feucht, A., and J. Errington. Personal communication.
- 11.Feucht A, Magnin T, Yudkin M D, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Sadaie Y. Rapid isolation of RNA polymerase from sporulating cells of Bacillus subtilis. Gene. 1998;221:185–190. doi: 10.1016/s0378-1119(98)00452-1. [DOI] [PubMed] [Google Scholar]
- 13.Gill S C, Weitzel S E, von Hippel P H. Escherichia coli ς70 and NusA proteins. I. Binding interactions with core RNA polymerase in solution and within the transcription complex. J Mol Biol. 1991;220:307–324. doi: 10.1016/0022-2836(91)90015-x. [DOI] [PubMed] [Google Scholar]
- 14.Greiner D P, Hughes K A, Gunasekera A H, Meares C P. Binding of the ς70 protein to the core subunits of Escherichia coli RNA polymerase, studied by iron-EDTA protein footprinting. Proc Natl Acad Sci USA. 1996;93:71–75. doi: 10.1073/pnas.93.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hager D A, Jin D J, Burgess R R. Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry. 1990;29:7890–7894. doi: 10.1021/bi00486a016. [DOI] [PubMed] [Google Scholar]
- 16.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmann J D, Masiarz F R, Chamberlin M J. Isolation and characterization of the Bacillus subtilis ς28 factor. J Bacteriol. 1988;170:1560–1567. doi: 10.1128/jb.170.4.1560-1567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks K A, Grossman A D. Altering the level and regulation of the major sigma subunit of RNA polymerase affects gene expression and development in Bacillus subtilis. Mol Microbiol. 1996;20:201–212. doi: 10.1111/j.1365-2958.1996.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishihama A, Taketo M, Saitoh T, Fukuda R. Control of formation of RNA polymerase in Escherichia coli. In: Losick R, Chamberlin M, editors. RNA polymerase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1976. pp. 485–502. [Google Scholar]
- 20.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major ς subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juang Y-L, Helmann J D. The δ subunit of Bacillus subtilis RNA polymerase. An allosteric effector of the initiation and core-recycling phases of transcription. J Mol Biol. 1994;239:1–14. doi: 10.1006/jmbi.1994.1346. [DOI] [PubMed] [Google Scholar]
- 22.Léonetti J-P, Wong K, Geiduschek E P. Core-sigma interaction: probing the interaction of the bacteriophage T4 gene 55 promoter recognition protein with E. coli RNA polymerase core. EMBO J. 1998;17:1467–1475. doi: 10.1093/emboj/17.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesley S A, Burgess R R. Characterization of the Escherichia coli transcription factor ς70: localization of a region involved in the interaction with core RNA polymerase. Biochemistry. 1989;28:7728–7734. doi: 10.1021/bi00445a031. [DOI] [PubMed] [Google Scholar]
- 24.Lesley S A, Brow M A D, Burgess R R. Use of in vitro protein synthesis from polymerase chain reaction-generated templates to study interactions of Escherichia coli transcription factors with core RNA polymerase and for epitope mapping of monoclonal antibodies. J Biol Chem. 1991;266:2632–2638. [PubMed] [Google Scholar]
- 25.Lewis P J, Magnin T, Errington J. Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor ςF of Bacillus subtilis. Genes Cells. 1996;1:881–894. doi: 10.1046/j.1365-2443.1996.750275.x. [DOI] [PubMed] [Google Scholar]
- 26.Linn T G, Greenleaf A L, Shorenstein R G, Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci USA. 1973;70:1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Lord, M., and M. D. Yudkin. Unpublished data.
- 28.Losick R, Pero J. Cascades of sigma factors. Cell. 1981;25:582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- 29.Magnin T, Lord M, Yudkin M D. Contribution of partner switching and SpoIIAA cycling to regulation of ςF activity in Bacillus subtilis. J Bacteriol. 1997;179:3922–3927. doi: 10.1128/jb.179.12.3922-3927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of Bacillus subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 31.Orsini G, Ouhammouch M, le Caer J-P, Brody E N. The asiA gene of bacteriophage T4 codes for the anti-ς70 protein. J Bacteriol. 1993;175:85–93. doi: 10.1128/jb.175.1.85-93.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi F-X, He X-S, Doi R H. Localization of a new promoter, P5, in the sigA operon of Bacillus subtilis and its regulation in some spo mutant strains. J Bacteriol. 1991;173:7050–7054. doi: 10.1128/jb.173.21.7050-7054.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Segall J, Tjian R, Pero J, Losick R. Chloramphenicol restores sigma factor activity to sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 1974;71:4860–4863. doi: 10.1073/pnas.71.12.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Severinova E, Severinov K, Fenyö D, Marr M, Brody E N, Roberts J W, Chait B T, Darst S A. Domain organization of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1996;263:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 36.Shuler M F, Tatti K M, Wade K H, Moran C P., Jr A single amino acid substitution in ςE affects its ability to bind core RNA polymerase. J Bacteriol. 1995;177:3687–3694. doi: 10.1128/jb.177.13.3687-3694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 38.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 39.Tjian R, Losick R. An immunological assay for the sigma subunit of RNA polymerase in extracts of vegetative and sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 1974;71:2872–2876. doi: 10.1073/pnas.71.7.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tjian R, Stinchcomb D, Losick R. Antibody directed against Bacillus subtilis ς factor purified by sodium dodecyl sulfate slab gel electrophoresis. J Biol Chem. 1974;250:8824–8828. [PubMed] [Google Scholar]
- 41.Williams K P, Kassavetis G A, Geiduschek E P. Interactions of the bacteriophage T4 gene 55 product with Escherichia coli RNA polymerase. J Biol Chem. 1987;262:12365–12371. [PubMed] [Google Scholar]
- 42.Wu F Y-H, Yarbrough L R, Wu C-W. Conformational transition of Escherichia coli RNA polymerase induced by the interaction of ς subunit with core enzyme. Biochemistry. 1976;15:3254–3258. doi: 10.1021/bi00660a014. [DOI] [PubMed] [Google Scholar]