Key Features.
The National Diet and Nutrition Survey Rolling Programme (NDNS RP) is a cross-sectional, annual survey designed to collect detailed, quantitative information on food consumption, nutrient intake and nutritional status of the general United Kingdom (UK) population aged ≥1.5 years.
NDNS RP uses a stratified sampling design to generate a random sample of private UK households each year and a nationally representative core sample of around 1000 participants (500 adults, 500 children) each year. Design provides for additional recruitment at country level.
Data/samples include: sociodemographic data; dietary assessment; anthropometry; physical activity; energy expenditure; blood and urine samples for nutritional biomarker analysis; National Health Service (NHS) Central Registry and Cancer Registry linkage; contact for further research.
The NDNS RP dataset (2008–19) comprises: dietary data (7999 adults and 7656 children); blood biomarkers (4181 adults and 2014 children); spot urine (3246 adults and 2318 children); and total energy expenditure (using doubly labelled water) (419 adults and 352 children). Results are published and disseminated via UK Government; survey data accessible via the UK Data Service.
Stored biological samples are accessible for further health-related research in the public interest through the NDNS Bioresource.
NDNS RP data underpin monitoring and development of nutrition policy. Data are used for chemical exposure risk assessment and modelling for consumer safety.
Data resource basics
The purpose of the UK National Diet and Nutrition Survey (NDNS) is to provide detailed quantitative data on food consumption, nutrient intakes and the sources of nutrients for the UK population, and an assessment of population nutritional status through objective biomarker analysis. The UK NDNS reports official statistics for ongoing monitoring of diet, to provide the evidence base for developing and evaluating effectiveness of policy and interventions, and for exposure assessment.
The UK NDNS was set up in 1992 following the first Dietary and Nutritional Survey of British Adults1 and was initially conducted as a series of discrete, stand-alone, cross-sectional surveys of different age groups: pre-school children,2,3 older adults,4,5 young people6,7 and adults.8–10 From 2008, to increase the ability to track changes over time and enable more rapid response to changing policy needs, the primary data collection moved to a continuous rolling programme (RP) format, sampling across all ages from 1.5 years. This Data Resource Profile focuses on the UK National Diet and Nutrition Survey Rolling Programme (NDNS RP) Years 1–11, 2008–19,11 for which the results dataset is publicly available; however, NDNS RP data collection is ongoing and is presently funded to Year 15, 2023. The wider NDNS surveillance programme also includes assessment and monitoring of population salt intakes through adjunct Urinary Sodium Surveys performed periodically for individual UK countries (England, Scotland, Wales and Northern Ireland). For completeness, information about the NDNS Urinary Sodium Surveys (2000–19), past NDNS and other UK nutrition surveys are presented in Supplementary Tables S1 and S2, available as Supplementary data at IJE online.
The NDNS RP seeks to collect data from a representative sample of the UK population of around 1000 participants (500 adults, 500 children) each year, with provision for additional representative sampling for country-level analyses in Scotland, Wales and Northern Ireland (‘boost’ sample). The primary aim is the assessment of dietary intake, collected alongside anthropometric, sociodemographic and behaviour variables, following which participants provide blood and urine samples for nutritional status analyses. The NDNS RP has also included doubly labelled water (DLW) sub-studies for measurement of Total Energy Expenditure for assessment of misreporting.
The NDNS RP has employed a dietary assessment method designed to provide full description, quantification and detail of all foods and drinks consumed during the dietary recording period and capable of capturing habitual consumption when conducted over a number of days. Issues of seasonality have been managed through the continuous fieldwork model. Prior to the RP, the age-based surveys used a weighed food diary method (either 4 or 7 days). At the start of the NDNS RP (2008), in order to reduce burden for participants given concerns about falling response rates, an estimated (un-weighed) food diary (collected over 4 consecutive days) was introduced following a dietary methods review and comparison study.12,13 This method was used for 11 years of the NDNS RP (2008–19). From Year 12 (2019), the NDNS RP moved to collect four 24-h recalls (over non-consecutive days) using an automated web-based dietary data collection tool, Intake24 [https://intake24.org/].
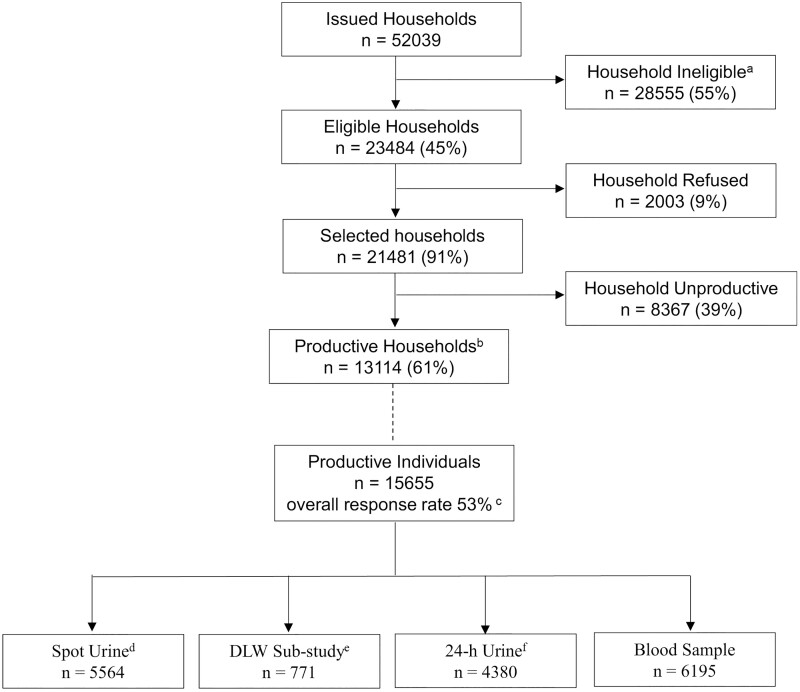
The overall response rate is based on completion of the dietary assessment element of the survey and ranged from 45.7% to 57.5% for Years 1–11 (Table 1). The published dataset for NDNS RP Years 1–11 includes a total of 7999 adults and 7656 children with dietary information, 4181 adults and 2014 children with blood biomarker data, 4380 participants with 24-h urine collection data (Years 1–5) and 5564 participants with spot urine collection data (Years 6–11) (Figure 1). The survey aimed for ∼10% of the sample to complete the periodic DLW sub-study (conducted every 4–5 years); 771 DLW participants are included in the published dataset.
Table 1.
Sample size and response in the UK National Diet and Nutrition Survey Rolling Programe Years 1–11 (2008–19)
| Core and boost sample combined, unweighted numbers | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample type | Response (I) | Response (II) | Response (III) | Survey year |
||||||||||
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | Year 11 | ||||
| 2008/9 | 2009/10 | 2010/11 | 2011/12 | 2012/23 | 2103/14 | 2104/15 | 2015/16 | 2016/17 | 2017/18 | 2018/19 | ||||
| n | n | n | n | n | n | n | n | n | n | n | ||||
| Core + country boost | Household responsea | Issued | 5049 | 5265 | 5265 | 5994 | 3726 | 4424 | 4424 | 4648 | 4424 | 4480 | 4340 | |
| Eligible | 2367 | 2345 | 2319 | 2826 | 1688 | 1937 | 1948 | 2154 | 1980 | 2061 | 1859 | |||
| Selected | 2128 | 2132 | 2133 | 2583 | 1490 | 1748 | 1721 | 1860 | 1905 | 1987 | 1794 | |||
| Productive | 1375 | 1409 | 1313 | 1643 | 982 | 1124 | 1136 | 1143 | 1056 | 1009 | 924 | |||
| Response rate %b | 56.0 | 57.5 | 54.9 | 55.5 | 55.7 | 55.2 | 55.4 | 50.2 | 50.6 | 45.7 | 46.7 | |||
| Individual response | Adults | Dietc | 801 | 812 | 782 | 1055 | 625 | 663 | 703 | 714 | 647 | 631 | 566 | |
| 24-h urined | 473 | 513 | 438 | 650 | 431 | – | – | – | – | – | – | |||
| Spot urined | – | – | – | – | – | 561 | 588 | 571 | 517 | 523 | 486 | |||
| DLW sub-studyd | 108 | – | 90 | – | – | 216 | 5 | – | – | – | – | |||
| Blood | 370 | 450 | 384 | 565 | 372 | 358 | 349 | 355 | 301 | 342 | 335 | |||
| Children | Dietc | 845 | 857 | 783 | 893 | 572 | 686 | 650 | 656 | 606 | 580 | 528 | ||
| 24-hour urined | 398 | 410 | 372 | 422 | 273 | – | – | – | – | – | – | |||
| Spot urined | – | – | – | – | – | 461 | 402 | 402 | 375 | 347 | 331 | |||
| DLW sub-studyd | 93 | – | 81 | – | – | 172 | 6 | – | – | – | – | |||
| Blood | 206 | 231 | 213 | 252 | 158 | 194 | 156 | 173 | 138 | 144 | 149 | |||
| Country boost | Individual response | Scotland | Dietc | 306 | 306 | 260 | 501 | – | – | – | – | – | – | – |
| Wales | Dietc | – | 167 | 148 | 162 | 158 | 159 | 139 | 199 | 151 | – | – | ||
| Northern Ireland | Dietc | 209 | 201 | 210 | 202 | – | 169 | 161 | 160 | 157 | 149 | 128 | ||
DLW, Doubly Labelled Water. Dash = that element of the study was not included that survey year or no country boost sample for that year. 24-h urine samples were collected in Years 1 to 5, spot urine samples in Years 6 to 11 and the doubly labelled water sub-study was included in Years 1, 3, 6 and 7.
The classification of eligible/selected/productive households changed over the National Diet and Nutrition Survey Rolling Programme years and may differ slightly to published reports.
Response is calculation of: the proportion of eligible addresses that are productive multiplied by the proportion of total individuals that are productive. Productive individuals are those that completed 3 or 4 diary days.
Completed 3 or 4 diary days.
Ages 4 years and above only.
Figure 1.
Flow diagram of recruitment numbers and response rates for the UK National Diet and Nutrition Survey Rolling Programme Years 1–11, 2008–2019. DLW, doubly labelled water. aThe majority were addresses selected for the child boost sample that were screened out because they did not contain any children in the eligible age range (1.5 to 18 years). The remainder included vacant or derelict properties and institutions. bThose in which one or more participant(s) completed 3 or 4 diary days. cResponse is calculation of: the proportion of eligible addresses that are productive multiplied by the proportion of total individuals that are productive. Productive individuals are those that completed 3 or 4 diary days. dIncluded in Years 6 to 11 for participants aged 4 years and above only. eIncluded in Years 1, 3, 6 and 7 for participants aged 4 years and above only. fIncluded in Years 1 to 5 for participants aged 4 years and above only
Survey components are carried out in stages and are voluntary (participants could decline or withdraw at any stage); hence, different numbers of participants have completed individual survey elements. Table 2 provides an age/sex breakdown of all participants for key survey components.
Table 2.
Profile of participants in the UK National Diet and Nutrition Survey Rolling Programe Years 1–11 (2008–19): all years combineda
| Core and boost sample combined, unweighted numbers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Individual response | Ageb |
|||||||
| 1.5–3 years | 4–10 years | 11–18 years | Total | 19–64 years | 65 years | Total | All | |
| children | and over | adults | participants | |||||
| n | n | n | n | n | n | n | n | |
| Diet | ||||||||
| Male | 717 | 1571 | 1619 | 3907 | 2519 | 781 | 3300 | 7207 |
| Female | 658 | 1440 | 1651 | 3749 | 3617 | 1082 | 4699 | 8448 |
| Total | 1375 | 3011 | 3270 | 7656 | 6136 | 1863 | 7999 | 15 655 |
| 24-h urinec,d | ||||||||
| Male | – | 445 | 509 | 954 | 823 | 237 | 1060 | 2014 |
| Female | – | 430 | 491 | 921 | 1135 | 310 | 1445 | 2366 |
| Total | – | 875 | 1000 | 1875 | 1958 | 547 | 2505 | 4380 |
| Spot urined,e | ||||||||
| Male | – | 642 | 616 | 1258 | 1023 | 329 | 1352 | 2610 |
| Female | – | 474 | 586 | 1060 | 1468 | 426 | 1894 | 2954 |
| Total | – | 1116 | 1202 | 2318 | 2491 | 755 | 3246 | 5564 |
| DLWd,f | ||||||||
| Male | – | 74 | 104 | 178 | 145 | 59 | 204 | 382 |
| Female | – | 73 | 101 | 174 | 151 | 64 | 215 | 389 |
| Total | – | 147 | 205 | 352 | 296 | 123 | 419 | 771 |
| Blood sample | ||||||||
| Male | 76 | 366 | 631 | 1073 | 1341 | 393 | 1734 | 2807 |
| Female | 82 | 299 | 560 | 941 | 1904 | 543 | 2447 | 3388 |
| Total | 158 | 665 | 1191 | 2014 | 3245 | 936 | 4181 | 6195 |
At time of writing, data only published/available for Years 1–11 (2008–18/19).
The age groups shown are those used for the National Diet and Nutrition Survey Rolling Programme reports.
24-h urine samples were included in Years 1 to 5 for participants aged 4 years and above only.
Some numbers differ from published reports due to the different ways of classifiying ‘obtained samples’.
Spot urine samples were included in Years 6 to 11 for participants aged 4 years and above only.
The doubly labelled water sub-study was included in Years 1, 3, 6 and 7 for participants aged 4 years and above only.
NDNS RP conforms to the Declaration of Helsinki and operates under UK National Health Service (NHS) Health Research Authority Research Ethics Committee (REC) Approval (Years 1–5, Oxfordshire REC A, REF 07/H0604/113; Years 6–10 and 11–15, East of England-Cambridgeshire South REC, REF 13/EE/0016).
Data collected
The NDNS RP is funded by UK Government, currently delivered through the Office for Health Improvements and Disparities, Department of Health and Social Care in England (OHID, DHSC), and the UK Food Standards Agency (FSA). Devolved government departments in Scotland, Wales and Northern Ireland have funded additional NDNS RP recruitment and stand-alone NDNS urinary sodium surveys for their respective countries. The programme is commissioned on a cyclical basis and is currently carried out by a consortium comprising NatCen Social Research (NatCen) and the Medical Research Council (MRC) Epidemiology Unit, University of Cambridge. Interviewer fieldwork in Northern Ireland has been carried out by the Northern Ireland Statistics and Research Agency (NISRA). Survey methods, progress and results publications are overseen by the NDNS Project Board.
Sampling design
The NDNS RP sampling plan follows a multistage clustered stratified design to generate a new random sample of UK private households each year. The sample has been drawn annually from the ‘small users’ sub-file of the Postcode Address File prepared by the national Post Office. For cost effectiveness, addresses were clustered into small geographical areas, Primary Sampling Units (PSUs), based on postcode sectors randomly selected from across the UK. Sorted first by region, PSUs were then grouped within each region into five equal bands based on Index of Multiple Deprivation score; subsequently, PSUs were sorted by population density. This ensured the sample of addresses was representative of the UK with respect to these three measures. Addresses were then randomly selected from each PSU for the core sample. Additional addresses were selected in Scotland (Years 1–4), Wales (Years 2–9) and Northern Ireland (NI) (Years 1–4 and Years 6–14) to boost respective country sample size for comparisons to be made with the UK as a whole. At each address, the interviewer selected one household at random (if two or more). In order to achieve (as far as possible) equal numbers of adults and children in the sample, at some addresses only children were selected to take part. The interviewer randomly selected up to one adult (aged ≥19 years) and one child (aged 1.5–18 years) from each selected household.
Prior to analysis, weighting factors are applied to all data to account for unequal selection probabilities and non-response so that results are generalisable to the UK population as a whole. The applied weights put the four UK countries into their correct population proportions. In years where recruitment was boosted, the sample design included an adjustment for selecting more addresses in respective individual countries.
Survey schedule
NDNS RP fieldwork has been organized practically in two stages—interviewer and nurse stages—both undertaken in households of participants (Table 3). The two stages have generally been carried out between 2 to 4 months apart. The interviewer stage comprised three visits/contacts with the household for participant selection and recruitment, administration of the food diary and other protocols (with additional visits for DLW sub-study participants). Participants who completed 3 or 4 diary days were invited to take part in the nurse stage to obtain further physical measurements and a blood sample.
Table 3.
Survey components used in the UK National Diet and Nutrition Survey Rolling Programme Years 1 to 11 (2008–19)
| Survey stage | Component and method | Completed by (age if not completed by all participants) | Survey Yearsa |
|---|---|---|---|
| Interviewer stage | Computerised Assisted Personal Interview (CAPI): | ||
| Household composition, socio | Household representative and | All Years | |
| economic status, shopping and | main food provider | ||
| cooking practices | |||
| Usual eating habits, general health, oral health, dietary supplement use | All participants | All Years | |
| Sun exposure | All participants | Years 1–10 | |
| Physical activity | Ages 4 to 15 years | Years 6–11 | |
| Consent for NHS Register and Cancer Register data linkage | Age ≥16 years | All Years | |
| Consent to be contacted for follow-up/further research | All participants (parent/carer on behalf of children) | All Years | |
| Self-completion questionnaires: | |||
| Smoking and drinking | Age ≥8 years | All Years | |
| Physical activity | Age ≥16 years | All Years | |
| Four-day food diary | All participants | All Years | |
| Physical measures: | |||
| Height and weight | All participants | All Years | |
| Actigraph (physical activity monitor) | Ages 4 to 15 yearsb | Years 1 to 5 | |
| Spot urine sample | Age ≥4 years | Year 6 onwards | |
| Doubly labelled water sub study: | |||
| Total energy expenditure | Age ≥4 years | Years 1, 3, 6 and 7 | |
| Nurse stage | Computerised Assisted Personal Interview (CAPI): | ||
| Current medication | All participants | All Years | |
| Physical measures: | |||
| Waist and hip measurements | Age ≥11 years | All Years | |
| Mid upper arm circumferences | Ages 2 to 15 years | Years 1 to 5 | |
| Infant length | Ages 18 months to 2 years | All Years | |
| Blood pressure | Age ≥4 years | Years 1 to 10 | |
| Blood samples | All participants | All Years | |
| 24-h urine sample and PABAc | Age ≥4 years | Years 1 to 5 |
Year 1 2008–09; Year 2 2009–10; Year 3 2010–11; Year 4 2011–12; Year 5 2012–13; Year 6 2013–14; Year 7 2014 15; Year 8 2015–16; Year 9 2016–17; Year 10 2017–18; Year 11 2018–19.
In Year 1, ActiGraph was only collected for children aged 4 to 10 years.
Para-aminobenzoic acid.
Italicized text denotes a major component.
Survey components
Questionnaire information was collected with verbal agreement, and physical measures and biological samples under written consent from participants (aged ≥16 years; parent/carer for younger ages). With consent, a sub-set of biological samples are retained for long-term sample storage. Consent has also been obtained for data linkage to the NHS Central Registry and Cancer Registry and re-contact for further research.
Content and protocols for individual survey components vary with age. Details of questionnaire variables and measurement protocols are regularly reviewed between survey years, and over time some have been modified for improvement, priority, efficiency and/or burden.
Days of food recording are randomly assigned with the aim to evenly represent all days of the week in the NDNS dataset each year. Completed paper food diaries were manually coded using a bespoke diet coding and analysis programme (Diet In, Nutrients Out, DINO).14 Coded foods and dietary supplements are linked to food composition data in the NDNS nutrient databank15 which draws on information from the Composition of Foods Integrated Dataset (CoFID),16 FSA Food Recipes Database17 and manufacturers’ data gathered through food labels and web information. Dietary data variables are listed in Supplementary Table S3, available as Supplementary data at IJE online.
Height and weight have been measured using portable stadiometer and weighing scales (demispan for ≥65 years, or those for whom there is no reliable height measure) to calculate body mass index (BMI). During the nurse stage, additional physical measures have included seated blood pressure, infant length, waist and hip circumferences and mid upper arm circumferences (Table 3).
For participants aged ≥16 years, following use of a bespoke physical activity (PA) questionnaire during Year 1, the Recent Physical Activity Questionnaire was introduced from Year 2.18 For Years 1–10, the PA questionnaires also included sunlight exposure questions.
PA data were initially collected from children aged 4 to 15 years using ActiGraph, a physical device worn at the hip over 7 consecutive days, and subsequently by PA questionnaire based on questions asked in the Health Survey for England [https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england].
Overnight fasted blood samples (non-fasted for ages 1.5–3 years and those unable/unwilling to fast) were collected by venepuncture by a qualified nurse/paediatric phlebotomist and taken by the nurse to a locally recruited laboratory for immediate processing or posted directly to the central NDNS laboratory according to pre-analytical protocol. Following processing and sub-aliquoting, blood samples were stored at −80°C prior to analysis. Urine samples, currently a single spot sample (from Year 6) and previously a 24-h urine sample (Years 1–5), have also been collected for nutritional biomarker analysis from participants aged ≥4 years. All data and biological samples are labelled (link-anonymized, latterly using barcodes) for tracking and traceability.
At intervals, the DLW sub-study was implemented in a sub-sample of participants for measurement of Total Energy Expenditure for assessment of misreporting. Participants were recruited on an age/sex quota basis and provided a pre-dose baseline urine sample and then 10 daily spot urine samples after drinking a body-weight-specific dose of DLW (2O).
Details of biochemical analyses are provided in Tables 4 and 5. Full details of data and sample collection protocols and analytical procedures are available with published reports and publicly available datasets.
Table 4.
Blood biomarkers in the UK National Diet and Nutrition Survey Rolling Programe Years 1–11 (2008–19)a
| Measurement | Sample type | Age (years) | Unit | Assay and comments |
|---|---|---|---|---|
| Full blood count | Whole blood | ≥1.5 | Multiple | Beckman Coulter LH700 (Years 1–8); Siemens Advia 2120 (Years 8–11). Performed at Addenbrooke's Hospital |
| HbA1cb | Whole blood | ≥1.5 | mmol/mol | HPLC (Tosoh Automated Glycohemoglobin Analyser). Years 1–10. |
| Performed at Addenbrooke's Hospital | ||||
| C-reactive protein (CRP) | Serum | ≥1.5 | mg/L | Siemens Dimension RXL (Years 1–5), Xpand (Years 6–10), |
| EXL200 (Year 11) clinical chemistry analysers (manufacturer’s method (extended range)). From Year 11 performed at CBAL | ||||
| Ferritin | Plasma (Years 1–10), serum Year 11 | ≥1.5 | μg/L | Dade Behring immunonephelometry on Siemens BN ProSpec (Years 1–5), Siemens immunoturbidimetric method on Dimension |
| Xpand (Years 6–10), EXL200 (Year 11) clinical chemistry analysers. From Year 11 performed at CBAL | ||||
| Soluble transferrin receptor (sTfR) | Plasma | ≥7 | mg/ml | Enzyme immunoassay. Years 1–4 |
| Creatinine | Plasma (Years 1–9), serum Year 10 | ≥1.5 | μmol/L | Siemens Dimension Xpand clinical chemistry analyser (manufacturer’s method). Changed from compensated kinetic Jaffe to enzymatic method during Year 9 |
| Glucose (only if fasted)b | Plasma | ≥7 | mmol/L | Siemens Dimension Xpand clinical chemistry analyser (manufacturer’s method). Years 1–5 |
| Total and HDL cholesterol, triglycerides | Serum | ≥1.5 | mmol/L | Siemens Dimension RXL (Years 1–5), Xpand (Years 6–10), |
| EXL200 (Year 11) clinical chemistry analysers (manufacturer’s methods). From Year 11 performed at CBAL | ||||
| LDL cholesterol | Serum | ≥1.5 | mmol/L | Calculated (Friedewald equation) |
| Thiamin (vitamin B1) | Red blood cell haemolysates | ≥1.5 | n/a | ETKAC |
| Riboflavin (vitamin B2) | Red blood cell haemolysates | ≥1.5 | n/a | EGRAC |
| Vitamin B6 (pyridoxal-5-phosphate and pyridoxic acid) | Plasma | ≥1.5 | nmol/L | HPLC with fluorimetric detection |
| Vitamin B12 | Serum | ≥1.5 | ng/L | Siemens Advia Centaur (manufacturer’s competitive protein binding method). Performed at Addenbrooke's Hospital |
| Holo-transcobalamin | Serum | ≥1.5 | pmol/L | ELISA (Axis Shield Diagnostics, Dundee). Years 6–11 |
| Homocysteine | Plasma | ≥7 | μmol/L | Immunoturbidimetric assay on Siemens BN ProSpec (manufacturer’s method). Years 1–5 |
| Folate | Serum | ≥1.5 | nmol/L | LC-MS/MS (During Years 1–6 performed at CDC) |
| Whole blood folate | Whole blood | ≥1.5 | nmol/L | Microbiological method, performed at CDC. Red cell folate is calculated from whole blood folate, serum folate and haematocrit. |
| Vitamin C | Plasma | ≥1.5 | μmol/L | Fluorescent enzymatic assay |
| Retinol (vitamin A), retinyl palmitate, α-tocopherol, γ-tocopherol (vitamin E), α- and β-cryptoxanthin, lycopene, lutein and zeaxanthin (combined), α- and β –carotene | Plasma | ≥1.5 | μmol/L | HPLC with photodiode array detection. Retinyl palmitate measured in Years 1–4 only |
| Vitamin D (25-hydroxyvitamin D) | Serum | ≥1.5 | nmol/L | CLIA (DiaSorin Liaison (Years 1–6)). LC-MS/MS (Years 7–11) |
| Selenium and zinc | Plasma (Years 1–10), serum Year 11 | ≥7 | μmol/L | Inductively coupled plasma mass spectrometry (ICP-MS). From |
| Year 11, performed at University Hospital Southampton. Measured in all ages from Year 11 | ||||
| Repository samples | Plasma/serum | ≥1.5 | n/a |
CBAL, Core Biochemical Assay Laboratory, Addenbrooke's Hospital; CDC, Centers for Disease Control; CLIA, Chemiluminescence immunoassay; EGRAC, erythrocyte gluthathione reductase activation coefficient; ELISA, enzyme-linked immunosorbent assay; ETKAC, erythrocyte transketolase activation coefficient; HbA1c, glycated haemoglobin; HDL, high density lipoprotein; HPLC, high performance liquid chromatography; LC-MS/MS, liquid chromatography tandem mass spectrometry; LDL, low density lipoprotein [Friedewald equation: LDL = Total cholesterol – HDL cholesterol – (triglycerides/2.2)].
Analyses were peformed at Medical Research Council Elsie Widdowson Laboratory (Years 1–10) or Nutritional Biomarker Laboratory, MRC Epidemiology Unit (Year 11 onwards) unless otherwise stated.
Analysis of these analytes was funded separately by Diabetes UK.
Table 5.
Urine biomarkers in the UK National Diet and Nutrition Survey Rolling Programe Years 1–11 (2008–19)a
| Measurement | Age (years) | Unit | Assay |
|---|---|---|---|
| 24-h urine: | Years 1–5 only | ||
| Sodium, potassium | ≥4 | mmol/L | Ion-specific electrode (Integrated Multisensor Technology) on |
| Siemens Dimension Xpand (manufacturer's method) | |||
| Creatinine, urea | ≥4 | mmol/L | Siemens Dimension Xpand clinical chemistry analyser (manufacturer’s method). Changed from compensated kinetic Jaffe to enzymatic method during Year 9 |
| Para-amino benzoic acid | ≥4 | mg/L | High-performance liquid chromatography with UV detection |
| Nitrogen | ≥4 | g/L | FP-428 LECO Nitrogen Determinator, performed at Institute of Grassland and Environmental Research (IGER), now the Institute for Biological, Environmental and Rural Sciences (IBERS), |
| University of Aberystwyth, UK. | |||
| Repository samples | ≥4 | ||
| Spot urine: | Years 6–11 only | ||
| Iodine | ≥4 | μmol/L | Inductively coupled plasma mass spectrometry, performed at |
| University Hospital Southampton | |||
| Repository samples | ≥4 | ||
| Doubly labelled water:b | Years 1, 3, 6 and 7 only | ||
| TEE | ≥4 | kcal/day | Isotope ratio mass spectrometry: Platinum equilibration and CO2 equilibration |
TEE, total energy expenditure; UV, ultra-violet.
Analyses were peformed at Medical Research Council Elsie Widdowson Laboratory (Years 1–10) or Nutritional Biomarker Laboratory, MRC Epidemiology Unit (Year 11 onwards) unless otherwise stated.
Participants received monetary gift vouchers following completion of individual survey components and were offered written feedback on height, weight and BMI (≥16 years), dietary intake, blood pressure and, with written consent for themselves and/or their General Practitioner, results of clinically relevant biomarkers.
Survey personnel (including fieldwork, data analysts, biochemical analysts) are recruited with specialist expertise and receive training including demonstration and practice sessions, quality control and competency assessments (e.g. anthropometry, dietary assessment, sample collection, bioanalysis). Fieldworker training has been undertaken at the beginning of each new fieldwork year, with refresher and additional briefing training at intervals throughout the year. Data checks for reconciliation, quality control and assurance are carried out according to standard operating procedures. Laboratory assays include gold-standard methodologies, use of internal quality controls to monitor performance over time and, where available, participation in external quality assessment schemes to monitor performance and/or accuracy (dependent on the scheme).
Data collection from Year 12
NDNS data collection is ongoing. From Year 12 (October 2019), in response to methodological and scientific advances and challenges including the drive for cost effectiveness,22,23 dietary assessment changed from paper-based food diary to online 24-h recalls using Intake24. In parallel, PA questionnaires also moved online and, for efficiency, participant selection within a household increased. An evaluation of methodological changes is in progress.24
In response to the global COVID-19 pandemic, fieldwork was initially suspended in March 2020 and resumed in October 2020 with an adapted remote fieldwork model enabling continued data collection for questionnaire-based variables and self-reported physical measures and a DLW sub-study; collection of blood and spot urine samples resumed in October 2021. The next NDNS RP data release is expected in 2023.
Data resource use
NDNS RP results are regularly published via Government in paired or combined data years for reasons of sample size. Publications include summary statistics for the UK as a whole25–31 and the devolved countries, Scotland,32 Northern Ireland33,34 and Wales,35,36 separately. The next NDNS RP results publication is expected in 2023 to follow completion of Years 12–14 (2019–22).
NDNS RP reports are published as Official Statistics and are regularly used by government, researchers, health professionals and industry to provide evidence on diet, nutrient intake and nutritional status in UK population age/sex groups.37 They underpin policy development and monitoring of diet and nutrition objectives, such as UK Government’s long-term initiatives to reduce childhood obesity,38,39 the calorie reduction programme,40 introduction of the 2018 UK soft drinks industry levy41 and the recent decision to introduce mandatory folic acid fortification of flour [https://www.gov.uk/government/consultations/adding-folic-acid-to-flour/outcome/proposal-to-add-folic-acid-to-flour-consultation-response]. Over the past 20 years, NDNS data have informed reports and position statements of the UK Scientific Advisory Committee on Nutrition,42–46 feeding into such policy development. Data are used by the FSA for risk assessments and consideration of food safety implications, including dietary contaminants and toxicology exposure modelling and assessing implications of novel foods entering the UK food chain. NDNS data contribute to global health through international datasets (e.g. the European Food Safety Authority, World Health Organization, Food and Agriculture Organization of the United Nations) and initiatives such as the Vitamin D Standardisation Programme and identification of high prevalence of vitamin D deficiency throughout the European population.47 NDNS RP data have also informed development of guidelines for interpretation of micronutrient biomarkers and risk factors for anaemia.48,49 The NDNS RP DLW data provide a unique nationally representative reference dataset for the UK population and have recently been used to describe and estimate components of energy expenditure.50
Strengths and weaknesses
The NDNS RP provides a unique source of nationally representative, highly detailed, quantitative information on food consumption, providing reliable estimates of usual dietary intakes and distributions, with blood and urine samples providing objective evidence of nutritional status for the UK population. The continuous nature of the Rolling Programme provides a powerful opportunity for time trend analyses of nutritional health and wellbeing across the age spectrum.28 The collection of detailed anthropometric, sociodemographic, economic and other contextual and behavioural information enables analysis by population sub-groups when data years are combined. NDNS RP reports include detailed analyses by age and sex, and previous reports have included comparison of intakes and status by income and other indicators of deprivation.25,28,32–36 The availability of this comprehensive dataset and access to biological samples through the NDNS Bioresource offers potential for further research in the public interest and scope for data linkage at the individual level.
As with all dietary surveys and studies, assessment of usual diet relies on self-report instruments that can be prone to measurement error and bias. The use of a comprehensive and detailed dietary assessment instrument conducted over 3 to 4 days accounts for day-to-day variation and provides potential to adjust for dietary data for usual intakes. Along with regular DLW assessments carried out within the survey sample for periodic quantification of misreporting of energy, this enables comprehensive measurement of diet and understanding of data limitations. The rigour of NDNS survey methods and quality of resulting data are underpinned by gold-standard nutritional biomarker methods and investment in international initiatives for data harmonization. Furthermore, the complex sample design, intensive fieldwork model and weighting strategy all help to ensure that data are representative of the UK population.
Similar to the picture seen in other national surveys,51 NDNS RP response rates have declined in recent years. An internal review in 2018 found no substantive evidence linking the decrease in response rates to a possible increase in bias of NDNS RP estimates.
Long-running surveillance programmes depend on standardized measurement with continuity over time to provide comparability of data between assessments. However, it is also vital that survey methods remain fit for purpose to ensure relevance and efficiency. Where methods are subject to change within the NDNS RP, implications are assessed to identify aspects of data discontinuity with previous years of the NDNS RP, including through evaluation and cross-validation studies where feasible to facilitate data harmonization.
Data resource access
NDNS RP reports are openly available and published regularly via [gov.uk]. Anonymized individual-level data are available to registered users through the UK Data Service (UKDS). NDNS RP biological samples are accessible via the NDNS Bioresource. For enquiries contact Polly Page [polly.page@mrc-epid.cam.ac.uk].
Ethics approval
The NDNS RP conforms to the Declaration of Helsinki and operates under UK National Health Service (NHS) Health Research Authority Research Ethics Committee (REC) Approval (Years 1–5, Oxfordshire REC A, REF 07/H0604/113; Years 6–10 and 11–15, East of England-Cambridgeshire South REC, REF 13/EE/0016).
Supplementary Material
Acknowledgements
We wish to thank: all NDNS participants, NatCen fieldworkers, past and present colleagues working on NDNS RP and members of the NDNS Project Board. We also acknowledge the contributions and support from: MRC Elsie Widdowson Laboratory, Cambridge [previously MRC Human Nutrition Research (MC_U105960384)] for scientific leadership and delivery (Years 1–10); University College London for their contribution (Years 1–5); network of UK field laboratories; Laboratory Research Services and Core Biochemical Assay Laboratory, Cambridge University Hospitals NHS Foundation Trust, Cambridge (Year 11); Centers for Disease Control and Prevention (CDC), USA (Years 1–11); Southampton University Hospital Trace Element Laboratory, University Hospital Southampton NHS Foundation Trust (Year 11) and Aberystwyth University.
Conflict of interest
None declared.
Contributor Information
Michelle C Venables, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Caireen Roberts, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Sonja Nicholson, Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Beverley Bates, NatCen Social Research, London, UK.
Kerry S Jones, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK.
Robert Ashford, NatCen Social Research, London, UK.
Suzanne Hill, NatCen Social Research, London, UK.
Anila Farooq, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Albert Koulman, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Nicholas J Wareham, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK.
Polly Page, Medical Research Council Epidemiology Unit, School of Clinical Medicine, University of Cambridge, Cambridge, UK; Medical Research Council Elsie Widdowson Laboratory, Cambridge, UK.
Data Availability
See Data Resource Access, above.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
M.C.V., C.R. and P.P. wrote the manuscript. S.N., R.A., B.B., K.J., A.K. and N.J.W. provided input to drafting. S.H. and A.F. populated the tables. All authors read and approved the final manuscript.
Funding
The NDNS RP Years 1–11, 2008–19, was commissioned and funded by Public Health England (PHE, an executive agency of the Department of Health) and the UK Food Standards Agency (FSA). Responsibility for NDNS transferred from PHE to OHID in the Department of Health and Social Care in England in October 2021 and the survey is now funded by OHID and the FSA. This work was also supported by the National Institute for Health Research Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme [IS-BRC-1215-20014] through the Nutrition Measurement Platform (Dietary Assessment, Anthropometry, Physical Activity, and the Nutritional Biomarker Laboratory) at the MRC Epidemiology Unit, University of Cambridge. Diabetes UK funded HbA1C and glucose in NDNS RP Years 1–5.
References
- 1. Gregory F, Fister K, Tyler H, Wiseman M.. The Dietary and Nutritional Survey of British Adults. London: HMSO, 1990. [Google Scholar]
- 2. Gregory J, Collins D, Davies PS, Hughes J, Clarke P.. National Diet and Nutrition Survey: Children aged 1.5 to 4.5 Years. London: HMSO, 1995. [Google Scholar]
- 3. Hinds K, Gregory J.. National Diet and Nutrition Survey: Children Aged 1.5 to 4.5 Years. London: HMSO, 1995. [Google Scholar]
- 4. Finch S, Doyle W, Lowe C. et al. The National Diet and Nutrition Survey: People Aged 65 Years and Over. London: The Stationery Office, 1998. [Google Scholar]
- 5. Steele J, Sheiham A, Marcens W, Wallis AWG.. National Diet and Nutrition Survey: People aged 65 Years and Over. London: The Stationery Office, 1998. [Google Scholar]
- 6. Gregory J, Lowe S, Bates CJ. et al. National Diet and Nutrition Survey (NDNS): Young People Aged 4-18 Years. London: The Stationery Office, 2000. [Google Scholar]
- 7. Walker A, Gregory J, Bradnock GJ, Nunn J, White D.. National Diet and Nutrition Survey: Young People Aged 4 to 18 Years. London: The Stationery Office, 2000. [Google Scholar]
- 8. Henderson L, Gregory J, Irving K, Swan G.. The National Diet and Nutrition Survey (NDNS): Adults Aged 19-64 Years. Volume 2: Energy, protein, carbohydrate, fat and alcohol intake. London: The Stationery Office, 2002. [Google Scholar]
- 9. Henderson L, Gregory J, Swan G.. The National Diet and Nutrition Survey (NDNS): Adults Aged 19-64 Years. Volume 1: Types and quantities of food consumed. London: The Stationery Office; 2002. [Google Scholar]
- 10. Henderson L, Irving K, Gregory J. et al. The National Diet and Nutrition Survey (NDNS): Adults Aged 19 to 64 Years . London: The Stationery Office; 2003. [Google Scholar]
- 11.NatCen Social Research, MRC Elsie Widdowson Laboratory. National Diet and Nutrition Survey Years 1-11, 2008/09-2018/19. [Data Collection]. 16th edn. UK Data Service. SN: 6533, 2021. 10.5255/UKDA-SN-6533-16 (22 March 2022, date last accessed). [DOI]
- 12. Ashwell M, Barlow S, Gibson S, Harris C.. National diet and nutrition surveys: the British experience. Public Health Nutr 2006;9:523–30. [DOI] [PubMed] [Google Scholar]
- 13. Stephen A, Teucher B, Bluck L. et al. A Comparison of Results by Dietary Assessment Method: Repeat 24-Hour Recall and Four-Day Estimated Diet Diary. 2007. https://www.mrc-epid.cam.ac.uk/research/studies/ndns/reports-and-publications/ (22 March 2022, date last accessed).
- 14. Fitt E, Cole D, Ziauddeen N. et al. DINO (Diet In Nutrients Out)—an integrated dietary assessment system. Public Health Nutr 2015;18:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health England. NDNS from Years 1 to 4: Appendix A. 2019. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (22 March 2022, date last accessed).
- 16.McCance and Widdowson's Composition of Foods Integrated Dataset.2019. https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (22 March 2022, date last accessed).
- 17.MRC Human Nutrition Research. Food Standards Agency Standard Recipes Database, 1992-2012. [Data Collection]. UK Data Service. SN: 8159, 2017. 10.5255/UKDA-SN-8159-1 (22 March 2022, date last accessed). [DOI]
- 18.Public Health England. NDNS from Years 1 to 4: Appendix V. 2019. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (22 March 2022, date last accessed).
- 19. Schoeller DA, , RavussinE, , SchutzY, , AchesonKJ, , BaertschiP, , Jequier E.. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. AmJ Physiol Regul Integr Comp Physiol 1986;250:R823–30. [DOI] [PubMed] [Google Scholar]
- 20. Jequier E, , AchesonK, , Schutz Y.. Assessment of Energy Expenditure and Fuel Utilization in Man. Annu Rev Nutr 1987;7:187–208. [DOI] [PubMed] [Google Scholar]
- 21. Elia M, , Livesey G.. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr 1988;47:591–607. [DOI] [PubMed] [Google Scholar]
- 22. Timmins KA, Vowden K, Husein F, Burley V.. Making the Best Use of New Technologies in the National Diet and Nutrition Survey: A Review. 2014. https://www.researchgate.net/publication/305033366_Making_the_best_use_of_new_technologies_in_the_National_Diet_and_Nutrition_Survey_a_review (22 March 2022, date last accessed).
- 23. Amoutzopoulos B, Steer T, Roberts C. et al. Traditional methods v. new technologies—dilemmas for dietary assessment in large-scale nutrition surveys and studies: a report following an international panel discussion at the 9th International Conference on Diet and Activity Methods (ICDAM9), Brisbane, 3 September 2015. J Nutr Sci 2018;7:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts C, Collins D, Amoutzopoulos B. et al. Evaluation of Changes in the Dietary Methodology in the National Diet and Nutrition Survey Rolling Programme from Year 12 (2019 to 2020) Stage 1. 2021. https://www.gov.uk/government/publications/evaluation-of-change-in-dietary-methodology-in-ndns-rolling-programme-stage-1 (22 March 2022, date last accessed).
- 25. Bates B, Lennox A, Prentice A. et al. National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012). 2014. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (22 March 2022, date last accessed).
- 26. Roberts C, Steer T, Maplethorpe N. et al. National Diet and Nutrition Survey Results from Years 7 and 8 (Combined) of the Rolling Programme (2014/2015–2015/2016). 2018. https://www.gov.uk/government/statistics/ndns-results-from-years-7-and-8-combined (22 March 2022, date last accessed).
- 27. Bates B, Bates CJ, Prentice A, Swan G.. National Diet and Nutrition Survey: Headline Results from Years 1 and 2 (Combined) of the Rolling Programme 2008-9–2009-10. 2011. https://www.gov.uk/government/publications/national-diet-and-nutrition-survey-headline-results-from-years-1-and-2-combined-of-the-rolling-programme-2008-9-2009-10 (22 March 2022, date last accessed).
- 28. Bates B, Collins D, Cox L. et al. National Diet and Nutrition Survey Years 1 to 9 of the Rolling Programme (2008/2009–2016/2017): Time Trend and Income Analyses. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/772434/NDNS_UK_Y1-9_report.pdf (22 March 2022, date last accessed).
- 29. Bates B, Collins D, Jones K. et al. National Diet and Nutrition Survey Rolling programme Years 9 to 11 (2016/2017 to 2018/2019). 2020. https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019 (22 March 2022, date last accessed).
- 30. Bates B, Cox L, Nicholson S. et al. National Diet and Nutrition Survey Results from Years 5 and 6 (Combined) of the Rolling Programme (2012/2013–2013/2014). 2016. https://www.gov.uk/government/statistics/ndns-results-from-years-5-and-6-combined (22 March 2022, date last accessed).
- 31. Bates B, Lennox L, Prentice A, Bates CJ, Swan G.. National Diet and Nutrition Survey Headline results from Years 1, 2 and 3 (Combined) of the Rolling Programme 2008/09–2010/11. 2012. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-headline-results-from-years-1-2-and-3-combined-of-the-rolling-programme-200809-201011 (22 March 2022, date last accessed).
- 32. Bates B, Lennox A, Prentice A. et al. National Diet and Nutrition Survey Rolling Programme (NDNS RP): Results from Years 1-4 (Combined) for Scotland (2008/09-2011/12): A Survey Carried Out on Behalf of the Food Standards Agency in Scotland and Public Health England. London: The Stationery Office, 2017. https://www.foodstandards.gov.scot/publications-and-research/publications/national-diet-and-nutrition-survey-rolling-programme-results-from-years-1-4 (May 2022, date last accessed).
- 33. Bates B, Lennox A, Prentice A, et al. National Diet and Nutrition Survey (NDNS RP): Results from Years 1-4 (combined) for Northern Ireland (2008/09-2011/12): A Survey Carried Out on Behalf of the Food Standards Agency in Northern Ireland and Public Health England. London: The Stationery Office, 2017.
- 34. Bates B, Clifford R, Collins D. et al. National Diet and Nutrition Survey (NDNS RP): Results for Years 5 to 9 (combined) of the Rolling Programme for Northern Ireland (2012/13–2016/17) and Time Trend and Income Analysis (Years 1 to 9; 2008/09–2016/17): A Survey Carried Out on Behalf of the Food Standards Agency in Northern Ireland and Public Health England. London: The Stationery Office, 2019.
- 35. Bates B, Collins D, Cox L. et al. National Diet and Nutrition Survey: results for Years 5 to 9 of the Rolling Programme for Wales (2012/2013–2016/2017) and time trend and income analysis (Years 1 to 9; 2008/09 –2016/17). London: The Stationery Office, 2019. [Google Scholar]
- 36. Bates B, Lennox A, Prentice A. et al. National Diet and Nutrition Survey Rolling Programme (NDNS RP) Results from Years 2-5 (combined) for Wales (2009/10-2012/13): A Survey Carried Out on Behalf of the Food Standards Agency in Wales, Welsh Government and Public Health England. London: The Stationery Office, 2017. https://gov.wales/national-diet-and-nutrition-survey-rolling-programme-ndns-results-years-2-5-combined (May 2022, date last accessed).
- 37.Public Health England. Government Dietary Recommendations: Government Recommendations for Energy and Nutrients for Males and Females Aged 1–18 Years and 19+ Years. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/618167/government_dietary_recommendations.pdf (22 March 2022, date last accessed).
- 38.HM Government. Childhood Obesity: A Plan for Action. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/546588/Childhood_obesity_2016__2__acc.pdf (March 2022, date last accessed).
- 39.HM Government. Childhood Obesity: A Plan for Action.2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/718903/childhood-obesity-a-plan-for-action-chapter-2.pdf (March 2022, date last accessed).
- 40. Tedstone A, Targett V, Mackinlay B. et al. Public Health England Calorie Reduction: The Scope and Ambition for Action. 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/800675/Calories_Evidence_Document.pdf (March 2022, date last accessed).
- 41.HM Revenue and Customs. The Soft Drinks Industry Levy Regulations. 2018. http://www.legislation.gov.uk/uksi/2018/41/pdfs/uksi_20180041_en.pdf (March 2022, date last accessed).
- 42.The Scientific Advisory Committee on Nutrition. Dietary Reference Values for Energy. London: The Stationery Office, 2011.
- 43.The Scientific Advisory Committee on Nutrition. Carbohydrates and Health. London: The Stationery Office, 2015.
- 44.The Scientific Advisory Committee on Nutrition. Iron and Health Report. London: The Stationery Office, 2010.
- 45.The Scientific Advisory Committee on Nutrition. Vitamin D and Health. London: The Stationery Office, 2016.
- 46.The Scientific Advisory Committee on Nutrition. Update on Folic Acid. London: The Stationery Office, 2017.
- 47. Cashman KD, Dowling KG, Skrabakova Z. et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 2016;103:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS; BRINDA Working Group. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106:333S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suchdev PS, Namaste SM, Aaron GJ. et al. ; BRINDA Working Group. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv Nutr 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brage S, Lindsay T, Venables M. et al. Descriptive epidemiology of energy expenditure in the UK: findings from the National Diet and Nutrition Survey 2008-15. Int J Epidemiol 2020;49:1007–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gazan R, Vieux F, Mora S, Havard S, Dubuisson C.. Potential of existing online 24-h dietary recall tools for national dietary surveys. Public Health Nutr 2021;24:5361–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See Data Resource Access, above.