Abstract
Background
Alopecia areata (AA) is an autoimmune disease characterized by chronic inflammation, the pathogenesis of which is unknown. Stress is believed to play a role; however, evidence remains insufficient. A recent study showed that substance P (SP) damaged hair follicles by causing neurogenic inflammation, activating perifollicular mast cells, and inducing keratinocyte apoptosis.
Objective
We aimed at studying the role of SP in AA pathogenesis. We investigated the SP levels in the lesional scalp tissues and serum. We also studied the effect of SP on the inflammatory response and hair growth in the outer root sheath (ORS) cells.
Methods
We compared the serum levels of SP in 58 AA patients and 28 healthy subjects. Then, we checked the expression of SP and SP receptor, neurokinin-1 receptor (NK-1R) in the scalps of AA patients and healthy controls using immunohistochemical staining. Finally, we analyzed the mRNA expression of inflammatory cytokines and hair growth-related factors in ORS cells.
Results
SP and NK-1R expression were markedly higher in the hair follicles and interfollicular epidermis of the scalp lesions of AA patients. However, there was no statistically significant difference in serum SP levels between controls and patients, regardless of the type of alopecia. SP significantly increased the mRNA expression of inflammatory cytokines and decreased hair growth-related growth factors in ORS cells, but the results were not dramatic.
Conclusion
SP triggered a localized micro-inflammation in lesional hair follicles, provoked an inflammatory response, and inhibited hair growth, thereby confirming the pathogenic role of SP in AA.
Keywords: Alopecia areata, Pathogenesis, Substance P
INTRODUCTION
Alopecia areata (AA) is an autoimmune disorder characterized by acute non-scarring hair loss and involves peri- and intra-hair follicular infiltration of CD4+ and CD8+ T lymphocytes, as well as Langerhans cells and histiocytes1. Although the pathogenesis of AA is not fully understood, stress is believed to play a role in the development of AA by releasing an immunomodulatory neuropeptide, substance P (SP), which causes neurogenic inflammation and activates perifollicular mast cells2; SP has also been demonstrated to prevent hair growth and induce keratinocyte apoptosis3. When SP was injected into AA-affected C3H/HeJ mice, mast cell degranulation and catagen development in hair follicles were identified. In addition, moderate infiltration of CD8+ T cells and a notable increase in granzyme B expression by these cells were observed, which destroyed the hair follicles4. CD8+ T cells infiltrating the follicular epithelium express the neurokinin-1 molecule, which can bind to the receptor of SP2. In the lesional scalp area of patients with AA, expression of numerous inflammatory cytokines, such as interferon-γ, interleukins (ILs), and tumor necrosis factor-α, were identified, which are well-known for their roles in the pathogenesis of the disease5.
For hair growth, various growth factors, such as vascular endothelial growth factor (VEGF) and keratinocyte growth factor (KGF), are required. VEGF works directly on hair follicle dermal papilla cells as an autocrine growth factor and increases angiogenesis, leading to extension of the anagen phase and hair regrowth6. This study examined the differences in SP levels in the lesional scalp tissues and sera of AA patients and compared them to those in the control group. We also attempted to confirm the effect of SP on inflammatory response and hair growth to identify the putative functional role of SP in AA.
MATERIALS AND METHODS
Immunohistochemistry
Tissue samples were fixed using 10% formaldehyde, embedded in paraffin, and cut into 4 µm-thick sections. The sections were deparaffinized in xylene and rehydrated using an alcohol series. Then, the sections were incubated overnight with a primary antibody diluted 1:100 (SP, neurokinin-1 receptor [NK-1R]; Abcam, Cambridge, MA, USA) at 4℃. Subsequently, incubation was carried out with a secondary antibody at room temperature for 1 hour, with diaminobenzidine tetrachloride solution for 1 minute, followed by counterstaining with Mayer’s hematoxylin. The intensity of immunohistochemical staining was analyzed using ImageJ (http://imagej.nih.gov/ij/docs/index.html). Written informed consent was obtained from the donors of the skin samples, and the study was approved by the ethical committee of the Institutional Review Board of Chungnam National University Hospital (IRB No. 2019-05-038).
Sample preparation
Venous blood samples were obtained under sterile conditions from all subjects and then centrifuged for 15 min at approximately 1,000 g (3,000 rpm) to separate the serum. Serum samples were stored at –20℃ or –80℃ before the assay.
Enzyme-linked immunosorbent assay
Serum SP levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s instructions. Culture medium was collected and secreted IL-1β, IL-6, and IL-8 were determined using commercial ELISA kits purchased from R&D Systems.
Cell culture and SV40 transformation
Human scalp tissues were obtained under the written informed consent of donors, following the Institutional Review Board of Chungnam National University Hospital (IRB No. 2016-07-009). We obtained six lesional scalp tissues from patients with active AA and six normal scalp tissues. In addition, outer root sheath (ORS) cells were cultured from tissues of the occipital scalp skin that was the remnant of scalp surgeries performed on four male individuals. The study was conducted in accordance with the principles of the Declaration of Helsinki. Hair follicles were isolated from the scalp specimens, according to a previously reported method7. Hair follicles were incubated with 0.25% trypsin and 0.02% ethylenediaminetetraacetic acid in phosphate-buffered saline for 10 minutes. Hair follicles were then vigorously pipetted to obtain single-cell populations. The dissociated cells were rinsed in Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), and centrifuged for 5 minutes at 200 g. ORS cells were then resuspended in keratinocyte-serum free medium supplemented with epidermal growth factor and bovine pituitary extract (Gibco) and seeded onto culture dishes. The cultures were maintained at 37℃ in a humidified atmosphere containing 5% CO2. For SV40 transformation, the retroviral vector pLXIN-SV40T was stably transfected into PT67 cells (Clontech Laboratories, Mountain View, CA, USA), a recombinant retrovirus packaging cell line. The retrovirus-containing medium was collected, filtered through a 0.22-µm low protein-binding filter (Millipore, Billerica, MA, USA), and transferred to primary culture of ORS cells. After overnight infection, the retrovirus-containing medium was replaced with fresh medium, and the cells were incubated for two additional days. SV40-transformed cells were detected by growing them for four weeks in a selective medium containing G418 (200 µg/ml), as reported previously.
Western blot analysis
The cells were lysed in Proprep solution (Intron, Daejeon, Korea). Total protein was measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Samples were run on sodium dodecyl sulfate–polyacrylamide gels, transferred onto nitrocellulose membranes, and incubated with appropriate primary antibodies. Blots were then incubated with peroxidase-conjugated secondary antibodies and visualized using enhanced chemiluminescence (Translab, Daejeon, Korea).
Quantitative real-time polymerase chain reaction
Total RNA was isolated using an Easy-Blue RNA extraction kit (Intron) and reverse-transcribed using Moloney-murine leukemia virus reverse transcriptase (Elpis Biotech, Daejeon, Korea). Aliquots of RT mixture were amplified using SYBR Green real-time PCR master mix (Applied Biosystems, Waltham, MA, USA). The following primer sequences were used: IL-1β, 5’-TTAAAGCCCGCCTGACAGA-3’ and 5’-GCGAATGACAGAGGGTTTCTTAG-3’; IL-6, 5’-CTGCGCAGCTTTAAGGAGTTC-3’ and 5’-CCATGCTACATTTGCCGAAGA-3’; IL-8, 5’-CCTTTCCACCCCAAATTTATCA-3’ and 5’-TTTCTGTGTTGGCGCAGTGT-3’; VEGF, 5’-CGAGGGCCTGGAGTGTGT-3’ and 5’-CGCATAATCTGCATGGTGATG-3’; KGF, 5’-CTTCTGTCGAACACAGTGGTACCT-3’ and 5’-TCATCTCTTGGGTCCCTTTTACTT-3’.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 8.0.1 for Windows (GraphPad Software, San Diego, CA, USA). Mann–Whitney test, Kruskal–Wallis test, and t-test were used to compare the serum SP levels and examine the relationship between AA and control subjects. A p-value of <0.05 was considered to be statistically significant.
RESULTS
Increased expression of SP and NK-1R in scalp tissue of patients with AA
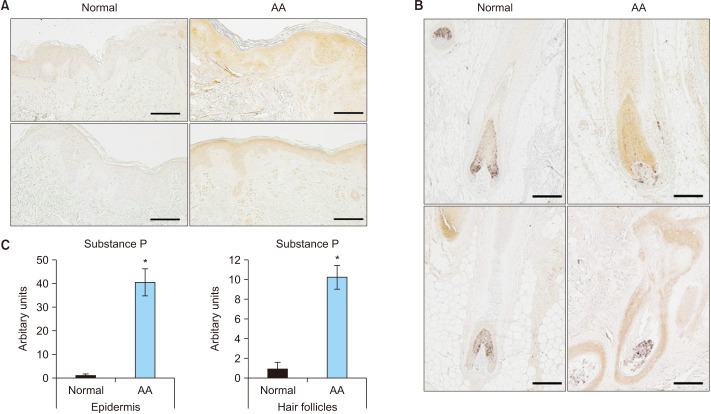
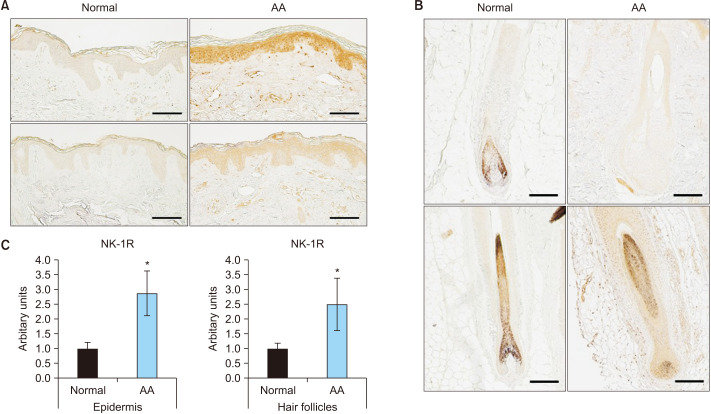
The expression of SP in the skin lesions of patients with AA was compared to that in normal scalp skin. First, immunohistochemical staining showed that SP was mainly expressed in AA’s interfollicular epidermis and hair follicle cells (Fig. 1, Supplementary Fig. 1). Next, the expression of the NK-1R, a receptor for SP, was determined; it showed a significant increase in the interfollicular epidermis, hair follicle cells, and infiltrated inflammatory cells around the hair follicles in AA (Fig. 2). These results indicated that the expression of SP and NK-1R was elevated in the skin lesions of patients with AA.
Fig. 1. Expression of substance P in normal and alopecia areata (AA) scalp (n=9; scale bar: A, 200 µm; B, 100 µm). (A) Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-substance P. Examination of the expression of substance P in the interfollicular epidermis. (B) Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-substance P. Examination of the expression of substance P in the hair follicles. (C) Quantification of the relative staining area. *Statistically significant (p<0.05).
Fig. 2. Expression of neurokinin-1 receptor (NK-1R) in normal and alopecia areata (AA) scalp (n=3; scale bar: A, 200 µm; B 100 µm). (A) Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-NK-1R. Examination of NK-1R expression in the interfollicular epidermis. (B) Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-NK-1R. Examination of NK-1R expression in the hair follicles. (C) Quantification of the relative staining area. *Statistically significant (p<0.05).
Serum SP levels in patients with AA
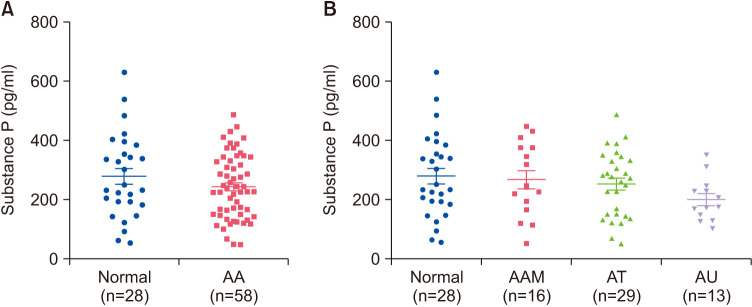
We assessed the serum SP levels in AA patients and healthy controls using ELISA kits. There were no statistically significant differences in serum SP levels between the normal subjects and patients with AA (p=0.360) (Fig. 3A). Additionally, the differences in serum SP levels were analyzed according to the type of AA (alopecia areata multiplex, alopecia totalis, and alopecia universalis), which showed no statistical differences between the groups (p=0.310) (Fig. 3B).
Fig. 3. Serum substance P levels in healthy controls and patients with AA. (A) Serum substance P levels were measured using ELISA and there was no statistical difference between patients with AA and healthy controls (p=0.360). (B) No correlation according to AA type (p=0.310). AA: alopecia areata, AAM: alopecia areata multiplex, AT: alopecia totalis, AU: alopecia universalis.
Effect of SP treatment on ORS cells
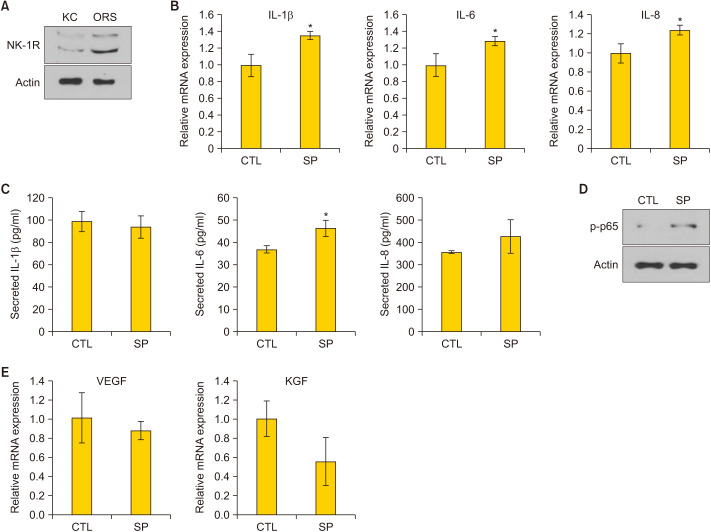
The effect of SP on ORS cells was evaluated in vitro. First, the expression of NK-1R was detected in keratinocytes and ORS cells using western blotting (Fig. 4A). Treatment of ORS cells with SP recombinant protein resulted in a significant increase in mRNA expression of inflammatory cytokines, such as IL-1β, IL-6, and IL-8 (p=0.031, 0.016, and 0.034, respectively) (Fig. 4B). We also examined that SP treatment increased the secretion of IL-6 and did not affect the level of IL-1b and IL-8 (Fig. 4C). On the treatment of ORS cells with SP, nuclear factor kappa B (NF-κB) p65 phosphorylation increased, indicating the activation of the NF-κB pathway by SP (Fig. 4D). Further, the mRNA expression of hair growth-related factors, including VEGF and KGF, slightly decreased with SP treatment, but the difference was not statistically significant (p=0.599 and 0.189, respectively) (Fig. 4E). These results demonstrated that SP increased inflammation and slightly decreased hair growth-related factors in ORS cells.
Fig. 4. Effect of SP in production of inflammatory cytokines and hair growth-related factors in outer root sheath cells. (A) Detection of neurokinin-1 receptor (NK-1R) in epidermal keratinocytes (KC) and outer root sheath (ORS) cells, using western blot analysis. (B) Treatment of ORS cells with recombinant SP (1 µM) for 2 hours. Assessment of the mRNA expression of inflammatory cytokines by quantitative PCR (q-PCR) (n=6). (C) Treatment of ORS cells with recombinant SP (1 µM) for 24 hours. Assessment of the secretion of inflammatory cytokines by ELISA (n=3). (D) Treatment of ORS cells with recombinant SP (1 µM) for 30 minutes. Determination of protein level of phospho-p65 by western blot analysis. (E) Treatment of ORS cells with recombinant SP (1 µM) for 24 hours. Assessment of the mRNA expression of vascular endothelial growth factor (VEGF) and keratinocyte growth factor (KGF) by q-PCR (n=6). IL: interleukin, CTL: control, SP: substance P. *Statistically significant (p<0.05).
DISCUSSION
AA, a chronic and relapsing hair loss disorder, is considered a T cell-mediated autoimmune disease that results in non-scarring hair loss. However, the pathogenesis of AA is not entirely understood. According to previous studies, AA is caused by the destruction of immune privilege in healthy hair follicles8. One of the mechanisms behind relative immune privilege is the downregulation of surface molecules, such as major histocompatibility complex (MHC) class Ia and beta (2)-microglobulin and downregulation of MHC class II immunoreactivity required for auto antigen presentation to CD8+ T lymphocytes9. However, in AA, pro-inflammatory signals are present in people with a genetic predisposition, and SP is also believed to play a significant role in the expression of this inflammatory signals4. SP is a stress-associated neuropeptide that plays a crucial role in intracutaneous neurogenic inflammation10. Several roles of SP in human hair follicles have been revealed. An earlier study using organ-cultured human anagen hair follicles showed that SP inhibited hair shaft elongation in a dose-dependent manner and promoted catagen-like hair bulb morphology. In the anagen hair bulb, MHC class I and ectopic MHC class I molecules was upregulated by SP, indicating disruption of the immune privilege of hair follicles. In addition, SP downregulated the receptor of SP (NK-1R) in the hair bulb and upregulated neutral endopeptidase (NEP), a known SP-degrading enzyme.
Furthermore, deleterious effects of neurogenic inflammation and perifollicular mast cell degranulation induced by SP played a role in developing stress-associated AA3. SP and NK-1R are associated with stress-reactive circuits in animals, particularly the hypothalamic-pituitary-adrenal axis11. In addition, evidence of interaction between SP/NK-1R and catecholamine neurotransmitters, including dopamine and norepinephrine, has been reported in the literature. Stressors induce the release of catecholamines into the brain’s cortical regions and SP/NK-1R and the release of dopamine and norepinephrine in the frontal cortex12. According to studies in mice, stress caused incomplete intrafollicular keratinocyte apoptosis and catagen development in hair follicles. It also induced neurogenic inflammation through mast cell degranulation and inhibited the proliferation of hair follicle keratinocytes13,14,15.
The role of mast cells in AA was published in several studies. Mast cells are found in connective tissue sheath of murine and human HF and can drive antigen-specific cytotoxic CD8+ T-cell responses. So they can play a significant role as stimulators of AA16. In addition, by the mast cell activation, SP also induces selective tumor necrosis factor-a secretion, a cytokine identified for its keratinocyte-apoptotic and hair growth-inhibitory effects3. With our results, we could hypothesize that interaction between perifollicular mast cells and SP activation could induce deleterious neurogenic inflammation, resulting in immune privilege collapse and aggravating the inflammatory status of AA. Moreover, SP level in the skin can affect the hair cycle itself17,18. The mechanism of change of SP level during the hair cycle has not yet been fully understood. Still, on the early and mid anagen cycle, the level of SP increased, preceded by nerve growth factor, which stimulates sprouting of nerve fibers and modulates the synthesis and expression of neuropeptides17. The reason for increasing SP level on telogen phase also has not been clarified. Still, Zhou et al.17 speculate that some peptidases (NEP, angiotensin-converting enzyme) are decreased in telogen skin, resulting in an apparent increase in SP level.
In this study, we confirmed that SP and NK-1R expression increased in the lesional scalp tissues of AA patients compared to those in the control group. However, serum SP levels in AA patients were not significantly different from those in the control group. Based on the results, the effect of SP in AA patients can be considered to have a localized function rather than a systemic function. Clinically, these results could be evidence that neurogenic inflammation plays a role in the pathogenesis and progression of AA by regulating the micro-milieu of inflammation of hair follicles. In addition, our results showed that SP increased the inflammatory cytokines, such as IL-1β, IL-6, and IL-8, in vitro and activated NF-κB signaling. IL-1 is a potent pro-inflammatory cytokine that promotes the migration of T lymphocytes, neutrophils, and macrophages to inflamed tissues19. In addition, IL-1 is considered to be a potent inducer of alopecia and an inhibitor of hair growth in vitro. IL-1α and IL-1β markedly increased in the murine hair growth cycle, especially at the beginning of the catagen phase20. In the human lesional scalp of patients with AA, IL-1β expression was markedly increased at the early stage of the disease5. In addition, IL-1β is a potent inhibitor of human hair growth in vitro and is upregulated during the catagen period in murine skin21,22. Inflammatory responses in human ORS cells through the NOD-like receptor Family Pyrin Domain Containing 3 inflammasome and the NF-κB pathway involving cytokines, such as IL-1β, are the inducible factors of AA23. In our study, SP also slightly decreased the mRNA expression of hair growth-related factors, including VEGF and KGF; however, the difference was not statistically significant. Explanations regarding the ability of SP to inhibit hair growth have already been reported through evidence-based biology. Several mechanisms have been revealed; they include reducing pro-proliferative SP-signaling in the keratinocytes of the hair follicle, deleterious neurogenic inflammation by activation of perifollicular mast cells, and intense immune attack on the immune-privileged epithelium of hair follicles3.
This study examined the increased expression of SP and its receptor in hair follicles and the interfollicular epidermis in patients with AA. Moreover, treatment with SP increased the mRNA expression of inflammatory cytokines and decreased the expression of growth factors in ORS cells. In conclusion, our data provided evidence that SP triggered localized micro-inflammation in lesional hair follicles, provoked an inflammatory response, and inhibited follicular growth, thereby supporting the pathogenic role of SP in AA.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2019R1A2C1004114, 2021R1I1A1A01061447).
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad.21.161-s001.pdf
Expression of substance P in epidermis (A) and hair follicles (B) of healthy subjects and alopecia areata patients. Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-substance P (n=9; scale bar: A, 200 µm; B, 100 µm).
References
- 1.Shin JM, Ko JW, Kwon IS, Choi JW, Hong DK, Lee JH, et al. Clinical relevance for serum cold-inducible RNA-binding protein level in alopecia areata. Ann Dermatol. 2019;31:387–392. doi: 10.5021/ad.2019.31.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalish RS. Clues from alopecia areata on the role of neuropeptides in the initiation of autoimmunity. J Invest Dermatol. 2007;127:1289–1291. doi: 10.1038/sj.jid.5700655. [DOI] [PubMed] [Google Scholar]
- 3.Peters EM, Liotiri S, Bodó E, Hagen E, Bíró T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–1886. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–1497. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflamm. 2010;2010:928030. doi: 10.1155/2010/928030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachgar S, Moukadiri H, Jonca F, Charveron M, Bouhaddioui N, Gall Y, et al. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 7.Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, a beta-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009;54:6–11. doi: 10.1016/j.jdermsci.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson B, Burell G, Kristiansson P, Björkegren K, Nyberg F, Svärdsudd K. Decline of substance P levels after stress management with cognitive behaviour therapy in women with the fibromyalgia syndrome. Scand J Pain. 2019;19:473–482. doi: 10.1515/sjpain-2018-0324. [DOI] [PubMed] [Google Scholar]
- 12.Schank JR, Heilig M. Substance P and the neurokinin-1 receptor: the new CRF. Int Rev Neurobiol. 2017;136:151–175. doi: 10.1016/bs.irn.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–2538. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- 14.Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005;83:386–396. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- 15.Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. 2020;29:703–725. doi: 10.1111/exd.14155. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Kawana S, Aoki E, Katayama M, Nagano M, Suzuki H. Dynamic changes in nerve growth factor and substance P in the murine hair cycle induced by depilation. J Dermatol. 2006;33:833–841. doi: 10.1111/j.1346-8138.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Katayama M, Aoki E, Suzuki H, Kawana S. Foot shock stress prolongs the telogen stage of the spontaneous hair cycle in a non-depilated mouse model. Exp Dermatol. 2007;16:553–560. doi: 10.1111/j.1600-0625.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 20.Hoffmann R, Wenzel E, Huth A, van der Steen P, Schäufele M, Henninger HP, et al. Cytokine mRNA levels in Alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530–533. doi: 10.1111/1523-1747.ep12395722. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235–238. doi: 10.1038/sj.jidsp.5640218. [DOI] [PubMed] [Google Scholar]
- 23.Shin JM, Choi DK, Sohn KC, Kim SY, Min Ha J, Ho Lee Y, et al. Double-stranded RNA induces inflammation via the NF-κB pathway and inflammasome activation in the outer root sheath cells of hair follicles. Sci Rep. 2017;7:44127. doi: 10.1038/srep44127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of substance P in epidermis (A) and hair follicles (B) of healthy subjects and alopecia areata patients. Paraffin-embedded normal scalp skin and AA lesions are immunohistochemically stained with anti-substance P (n=9; scale bar: A, 200 µm; B, 100 µm).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.