Abstract
Anemia is a blood disorder which is caused due to inadequate red blood cells and hemoglobin concentration. It occurs in all phases of life cycle but is more dominant in pregnant women and infants. According to the survey conducted by the World Health Organization (WHO) (McLean et al., Public Health Nutr 12(4):444–454, 2009), anemia affects 1.62 billion people constituting 24.8% of the population and is considered the world’s second leading cause of illness. The Peripheral Blood Smear (PBS) examination plays an important role in evaluating hematological disorders. Anemia is diagnosed using PBS. Being the most powerful analytical tool, manual analysis approach is still in use even though it is tedious, prone to errors, time-consuming and requires qualified laboratorians. It is evident that there is a need for an inexpensive, automatic and robust technique to detect RBC disorders from PBS. Automation of PBS analysis is very active field of research that motivated many research groups to develop methods using image processing. In this paper, we present a review of the methods used to analyze the characteristics of RBC from PBS images using image processing techniques. We have categorized these methods into three groups based on approaches such as RBC segmentation, RBC classification and detection of anemia, and classification of anemia. The outcome of this review has been presented as a list of observations.
Graphical abstract
Keywords: Peripheral blood smear, Red blood cells, Image processing, Computer-aided system, Anemia diagnosis
Introduction
Anemia is a condition described by insufficient red blood cells or based on hemoglobin content in the blood below a specific range estimated for specific sex and age of a person. Anemia is diagnosed using PBS where microscopic examination of blood smear provides useful information about alteration of RBC shape and size or presence of any inclusion bodies. RBC morphology is a key tool for hematologists to recommend appropriate clinical and laboratory follow-up and to select the best tests for definitive diagnosis. Anemia analysis can be done based on RBC morphology and clinical parameters. Morphological analysis using blood smear is performed by spreading a drop of blood thinly onto a glass slide and stained with coloring agents such as Giemsa, Leishman, and Wright-Giemsa and examined under a microscope by a qualified lab technician [93]. The blood smear contains different types of cells, namely White Blood Cells (WBCs), RBCs and platelets. An image of PBS indicating different blood cells is shown in Fig. 1.
Fig. 1.
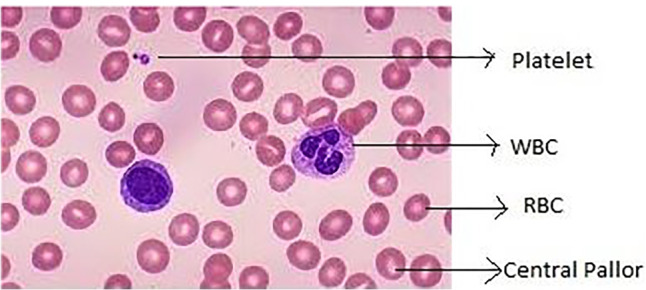
Microscopic view of blood smear image [95]
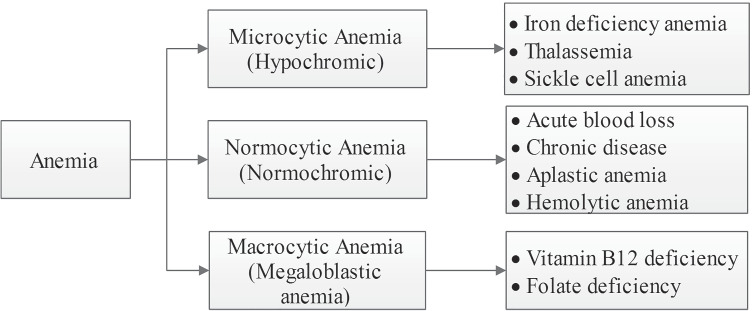
It can be observed that RBCs are more in number in comparison with WBCs and platelets. During this examination of the smear, the pathologists assess the size, shape, and color of the RBCs and WBCs. Also, they estimate the number of platelets present. The quality of RBC is characterized by red cell indices and any deviation in size, volume, or shape of red cells represents an abnormal red blood cell [78]. Anemia is classified based on the morphology of red cells, red cell indices and hemoglobin content as in Fig. 2.
Fig. 2.
Classification of anemia
Anemia is classified into hypochromic microcytic, normochromic normocytic and macrocytic anemia. Further, it is categorized into Iron Deficiency Anemia (IDA), Sickle Cell Anemia (SCA), thalassemia, Hereditary Spherocytosis (HS), Hereditary Elliptocytosis (HE), aplastic anemia and Hemolytic Anemia (HA) based on the RBC morphology. Based on the morphology, types of normal and abnormal red blood cells are shown in Fig. 3.
Fig. 3.
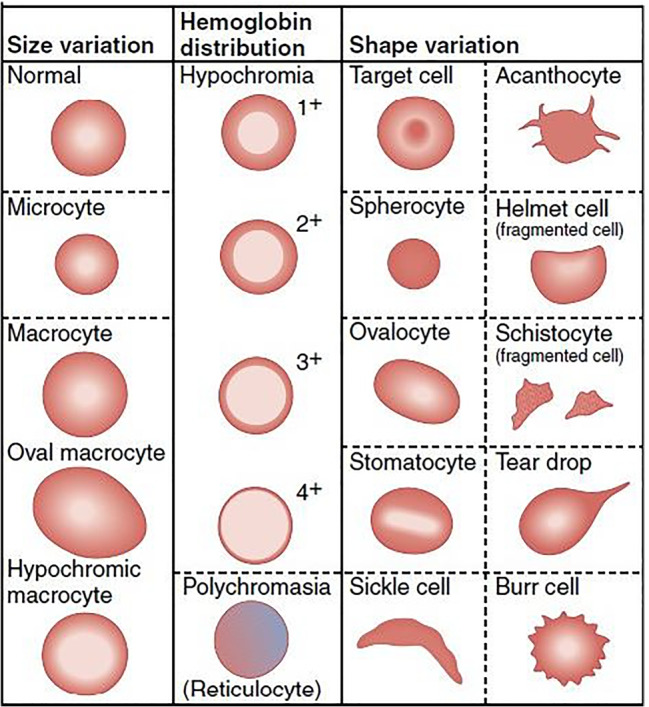
Normal and abnormal RBCs [95]
Anemia classification can also be performed based on the clinical parameters such as RBC count, RBC indices, namely Mean Corpuscular or Cell Volume (MCV in femtoliter), Mean Cellular Hemoglobin Concentration (MCHC), Mean Cell Hemoglobin Content (MCH in picograms), hematocrit (HCT) or Packed Cell Volume (PCV) and Red Cell Distribution Width (RDW). These parameters play an important role in the detection and classification of anemia. Hematologists usually examine PBS if RBC indices are abnormal [58]. The morphological classification of anemia based on the clinical diagnosis is as shown in Table 1.
Table 1.
Classification of anemia based on the clinical parameters
| Anemia Type | MCV fL | MCH pg | MCHc% |
|---|---|---|---|
| Macrocytic anemia | > 100 | > 32 | 32–35 |
| Normocytic anemia | 80–100 | 27–32 | 32–35 |
| Microcytic anemia | < 80 | < 27 | < 32 |
This paper presents a comprehensive review of automation of PBS images for detection of anemia. Many research groups have attempted this automation based on clinical or morphological analysis.
Approaches
This survey summarizes the various research works involved in the automation of analysis of the PBS images. The approaches are categorized as RBC segmentation and counting, RBC classification and detection of anemia, and anemia classification.
RBC segmentation and counting
In this section, we provide information about RBC segmentation and counting using image processing methods based on color and size variations. The segmentation techniques are categorized into different sub-sections based on the approaches.
Thresholding and transform-based segmentation methods
Thresholding is the simplest way of segmenting an image into foreground and background based on the different intensities or colors. Transform methods are used to identify the features in the other domains. Prasad et al. [124] developed a decision support system to detect malaria parasites in thin PBS images using color image analysis. Morphological operations were used to detect RBCs and color image processing techniques to extract the region of interest. This method could detect around 96% of the parasites for 200 Giemsa stained images of 100X magnification under uniform stain and illumination conditions [58] [90, 125]. Bhavnani et al. [40] proposed a method to segment and count RBCs and WBCs using Otsu thresholding and morphological operations. WBC counting was performed by counting number of connected components and obtained an average accuracy of 94.25%. RBC counting was performed using Watershed segmentation and Circular Hough Transform (CHT) and accuracies of 92.67% and 91.07% were obtained respectively. The principal objective of watershed segmentation [87] is to find the watershed lines which forms continuous path giving rise to continuous boundaries between the regions. It extracts nearly uniform objects from the background. CHT is an image transform [53] that extracts circular objects from an image. The transform can measure radius and the centroid of each circular object in an image by searching a 3D Hough space. Maji et al. [107] presented RBC counting method using Otsu thresholding and mathematical morphology and classified into circular, non-circular, overlapped cells or artifacts. The average accuracy obtained was 96.9% for circular and 97.1% for non-circular cells from 146 images. Ruberto et al. [65] proposed a method for malarial parasite-infected blood cells using HSV component based on color similarity and Watershed algorithm for 12 Giemsa stained images acquired at different magnifications with some variations in stain and lighting conditions. Ruberto et al. [66] proposed a method based on region proposal using edge boxes for detecting and quantifying RBCs and obtained accuracy in the range of 96–98% for 180 ALL-IDB images. The research groups [67] presented the same method for another malarial parasite MP-IDB database with 100 images and achieved accuracy in the range of 89–99%. Sharif et al. [144] presented a preliminary study on RBC segmentation method using masking and watershed algorithm for 20 images with 40X magnification. However, this method needs improvement in segmentation for large overlap. Biswas et al. [43] proposed blood cell segmentation method using Watershed Transform (WT) [118, 144] and Sobel filter in the spatial frequency domain [60, 126] and obtained 93% accuracy for 30 images measured using a structure similarity index matrix. Habibzadeh et al. [86] proposed a method for WBC and RBC segmentation using YIQ color space and WT and 90% accuracy was achieved for RBC segmentation using 10 images. However, they reported addressing large variations of blood cells and low quality images in their future work. Cruz et al. [57] proposed RBC counting method using blob analysis based on HSV component and WT and obtained an average accuracy of 95.6% for 10 blood samples taken with 40X and 100X magnifications. Segmentation of RBCs from PBS images using Hough Transform (HT) was reported by many research groups [14, 72, 83, 110, 113, 149, 155]. They reported the accuracy in the range of 94–96%. Mahamood et al. [104, 105] proposed color based blood cell segmentation in CIELAB color space and used CHT for cell extraction. The experiment was performed on ALL-IDB dataset with 108 Wright stain images of magnification ranging from 300X to 500X and obtained the average accuracy of 81% for WBCs and 64% for RBCs. Sarrafzadeh et al. [139] presented a circlet transform-based method to count RBCs and obtained a low error rate for 100 images with 100X magnification. However, the authors suggested to improve initial RBC mask for accurate segmentation. Yeldhos et al. [161] implemented FPGA based embedded system for counting RBC using CHT. YCbCr color conversion and WT segmentation method were used. An accuracy of 90.98% was achieved for 108 blood smear images of ALL-IDB dataset. Frejlichowski [80] proposed a method to detect RBCs based on pixel relationship and obtained 83% accuracy for 700 RBCs from May-Grunwald-Giemsa (MGG) stained images. Alomari et al. [28] proposed an iterative structured circle method to detect WBCs and RBCs and obtained average accuracy of 95.3% for RBCs and 98.4% for WBCs from 100 images of different magnifications ranging from 300 to 500X.
Edge based segmentation methods
Das et al. [60] proposed a method to identify RBCs and different types of WBCs using edge detection algorithms, namely Canny, Laplacian of Gaussian (LOG), Sobel and obtained 85% accuracy for 20 images. Poomcokrak et al. [123] proposed Canny edge algorithm based RBC counting method. The method obtained 74% accuracy for 59 RBCs and 59 non-RBCs using Multilayer Perceptron (MLP). MLP is a simple feed forward neural network that uses back propagation algorithm to train neurons [89, 92]. It consists of an input layer, an arbitrary number of hidden layers and an output layer. The hidden layer processes the input information and transmits to the output layer. MLPs are often applied to supervised learning problems. Backpropagation is used to adjust weights and biases to minimize the error. Hafiz et al. [19] proposed RBC segmentation algorithm using boundary-based thresholding and Canny edge detection methods and obtained average accuracy of 87.9% for five images from the Broad Bioimage Benchmark Collection (BBBC) dataset.
Clustering based segmentation methods
Abbas et al. [11] presented a method to segment blood cells using the YCbCr color space and K-means clustering method [45] for 90 Giemsa stained blood smear images. Blood cells were easily identified by this method using a unique color of every component. Wei et al. [157] proposed a method to detect and count overlapped RBCs in microscopic blood smear images. The H and S components were used to differentiate between WBCs and segmented RBCs. H and S components are closely related to the way humans feel color. H is the color sensed due to the wavelength. S indicates the purity of the color [126]. Watershed and K-means clustering algorithms were applied for segmentation. An accuracy of 92.9% was obtained for 100 Wright-Giemsa stained images. However, the authors of this paper suggested to fine tune the segmentation method for robustness. Acharya et al. [15] presented a method to separate RBCs from other components of blood using K-medoids and obtained 98% accuracy for 1000 Wright stained images. Savkare et al. [140] proposed a method to segment blood cells using K-means clustering algorithm and WT and obtained 95.5% accuracy for 78 Giemsa stained microscopic images. However, they reported that if cells are not well-stained and have low contrast, this method does not work well. Ruberto et al. [68] presented a fuzzy set based optimal threshold selection approach for blood cell segmentation. The local threshold was set using a histogram and average accuracy of 98% was obtained with a computation time less than a second.
Contour and matching based segmentation methods
Bronkorsta et al. [46] proposed a parametric deformable template-based online detection method to detect RBC shapes of 900 cells in 100X magnification and obtained accuracy of 95.7% for 10 images. This technique is based on the prior knowledge about the shape and appearance of the object. A template prototype and according energy function is defined for template description. However, a good initial guess for the shape, size, and location of the object is needed to find global minimum in this method. Bergen et al. [39] proposed a method for WBC and RBC segmentation using template matching and level set algorithm [141]. A Dice coefficient score of 0.96 was obtained for WBC segmentation from 155 images. Ritter et al. [133] proposed a blood cell segmentation method using a graph algorithm and obtained a success rate of 90% for 98 images. However, due to the diffuse area, this method failed to detect all platelets. Cai et al. [47] presented an RBC segmentation method based on an active appearance model incorporating shape and texture information of the cell.
Machine learning-based segmentation methods
Sadafi et al. [135] presented a fully convolutional neural network-based RBC segmentation method and obtained 90% accuracy for 5772 raw images of different stains. In this work, the authors suggested to use post-processing methods for touching cell split up to improve the accuracy. Kimbahune et al. [98] and Jun et al. [108] proposed blood cell image segmentation and counting method using Pulse-Coupled Neural Network and found that the method is time efficient. A machine learning approach based on the YOLO algorithm was presented by Alam et al. [20] to identify and count blood cells and obtained an accuracy of 96.09% for RBCs and 86.89% for WBCs from 364 100x magnified annotated images of Blood Cell Count Dataset (BCCD). Adagale et al. [16] proposed an overlapped RBC counting algorithm using Pulse Coupled Neural Network with a template matching technique and obtained 90% average accuracy for 40 images. Chari et al. [51] presented a pilot study on the analysis of MGG stained normal images using ShonitTM artificial intelligence system. The extracted cells were classified using three different deep neural network models based on images annotated by three experts. The precision of 93.9% was achieved for all WBC classes from 6000 WBCs. RBCs and platelets were identified based on the estimation of indices for 100 images from every 100 samples and obtained estimation within 10% reported value of Sysmex XN 3000TM hematology analyzer. Loddo et al. [101] proposed a blood cell counting method using nearest neighbor and SVM techniques. This method used ALL-IDB dataset with 368 images and obtained an average accuracy of 99.2% for WBCs and 98% for RBCs. Tran et al. [150, 151] presented deep learning semantic segmentation method for RBC and WBC segmentation and counting. It is pixel level segmentation of the image. An experiment was conducted on 42 ALL-IDB database images with 380 training images post augmentation. SegNet architecture was utilized to segment blood cells by labeling each pixel. It is a deep architecture for the segmentation of multi-class based on assigning each pixel of an image into a corresponding class [38]. The segmentation accuracy for WBCs, RBCs and the background reached 94.93%, 91.11% and 87.32%, respectively. For cell counting, Euclidean distance transform and binary dilation were used and accuracy of 93.3% for RBCs and 97.29% for WBCs was obtained. Shahzad et al. [143] designed semantic segmentation using a convolutional encoder-decoder framework along with VGG16 network and the model was trained and tested on the ALL-IDB dataset with 108 images. The proposed system achieved accuracies of 97.45%, 93.34%, and 85.11% for RBCs, WBCs, and platelets respectively. Amin et al. [33] presented a comparison of different classification algorithms using WEKA tool for hematological data. The experiment was conducted on two datasets from a total of 900 samples with CBC parameters. Three data mining classifiers were tried, namely J48 decision tree, MLP, and Naive Bayes, using which accuracies of 97.2%, 86.6% and 70% were achieved respectively.
Miscellaneous category
Gupta et al. [84] identified RBCs using blob detection method and obtained 75% accuracy for 88 RBCs. However, a few RBCs were left undetected due to the lighting conditions. Hidalgo et al. [81] proposed a novel method to count the number of circular and elongated RBCs using circumference and ellipse adjustment algorithms for 66 Giemsa stained images from erythrocytesIDB database. They used k-curvature for separating clustered RBCs and obtained 98% accuracy without pre-processing steps. Hegde et al. [90] presented a review on WBC, RBC, platelet analysis techniques and highlighted the importance of illumination and color shade variation correction to develop a robust system for PBS analysis.
A lot of work has been carried out to segment blood cells from PBS images. The distribution of segmentation techniques used in the literature is depicted in Fig. 4.
Fig. 4.
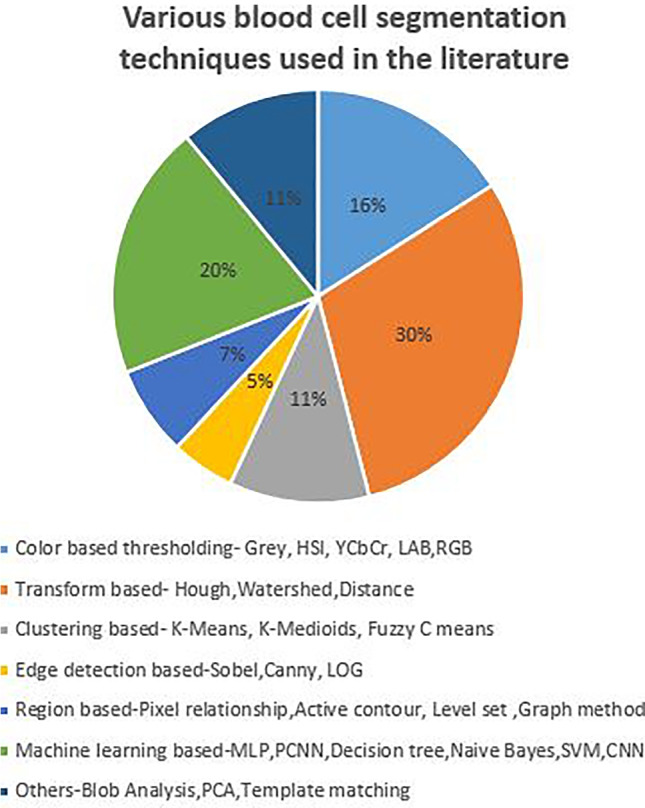
Distribution of blood cell segmentation methods
It is observed from the distribution that most of the literature used transform method, color thresholding and machine learning techniques for the segmentation. The summary of the literature described in Section 2.1 highlighting results of each method which is listed in Table 2.
Table 2.
RBC segmentation and counting methods
| Methods | No. of images(Stain) | Accuracy(%) | Remarks | Ref. |
|---|---|---|---|---|
| K-means clustering, WT | 60 (Giemsa )100 (Wright–Giemsa) | 93–98.9 | Robustness is not explained | [11, 140, 157] |
| Iterative structured circle detection, circlet transform | 100 | 95.3 | Incorrect hole filling leads to errors To improve initial RBCs mask for accurate segmentation | [28, 143] |
| Graph algorithm | 98 | 99 | Considered only non-overlapped cells | [133] |
| Parametric template matching, PCNN | 900 cells | 90–95.7 | Require prior knowledge about the appearance of the cell | [16, 46, 98] |
| YOLO algorithm | 364 | 96.1 | Satisfactory performance | [20] |
| HSV conversion, morphological operations | 200 (Giemsa) | 96 | Used uniform staining and illumination | [125] |
| Pixel relationship | 10 (MGG) | 83 | Occluded objects are rejected before the later stages | [80] |
| Canny, LOG, Sobel | 20–30 | 85–93 | Normal RBCs Less samples | [43, 60] |
| K-curvature, circumference and ellipse adjustments | 66 | 98 | Images are not preprocessed to reduce execution time | [81] |
| Blob analysis, WT | 10 blood samples | 90–96 | Need optimization to get accurate results | [57, 86] |
| CNN AlexNet | 5772 | 90 | Average execution time was 227 ms | [135] |
| Canny edge, MLP | 59 RBCs and 59 non RBCs | 74–88 | Increase training images | [123] [19] |
| K-medoids, distance transform | 1000 (Wright) | 98 | Processing of central pallor of RBCs consume more time | [15] |
| HT | 500 subjects | 91–94.9 | Many tunable parameters | [72, 110, 148, 155] |
| Deep neural network models | 100 (MGG) | Indices lie within the 10% of Sysmex reported value | Considered only normal blood smear images | [51] |
| CHT, NN, SVM | 368 | 98 | Achieved low false negative rate | [101] |
| LAB, YCbCr color space, CHT | 108(Wright) | 81–91 | Computational time is more | [105] [161] |
| Region proposal | 180 (Wright) | 96-98 | Tested on ALL-IDB and MP-IDB datasets | [65] |
| Semantic segmentation | 108 (Wright) | 91–97 | More labeled images are required | [143, 150, 151] |
RBC classification and detection of anemia
In this section, we provide the details of image processing techniques used to classify RBCs based on shape, size and texture variations in order to detect anemia.
Classification of RBCs into normal and abnormal was presented using image processing techniques [18, 23, 24, 54, 69, 99, 112, 121, 127, 147, 148, 154]. Various methods such as Otsu thresholding, CHT, statistical and moment invariants, and geometric texture features were used. The accuracies in the range of 83–94% were obtained using ANN, SVM and BPNN classifiers.
Shape feature and region based RBC classification methods
Wheeless et al. [158] presented a method to classify RBCs into normal, sickle or other abnormal cell using recursive partitioning and form factor. The recursive partitioning technique is based on the concept of finding the cutpoints for the features that best isolate different disease cases. The data are then divided according to these cutpoints [44]. Form factor provides a measure of circularity as given in equation. If more is the departure from the perfect circle value 1, lower is the form factor value.
| 1 |
An accuracy of 85% for normal RBCs, 83% for abnormal cells, and 81% for sickle cells from 3878 cell images was obtained. Safca et al. [136] proposed a method to classify RBCs into sickle cells, echinocytes and elliptocytes using morphological operations and shape features such as diameter, area and perimeter [13]. Morphological imaging operations done on a binary image to remove small objects, fill the cell holes and clearing the border to avoid edge touching cells [147]. An average accuracy of 96% was achieved for 34 images. Deb et al. [64] proposed an algorithm to classify RBCs using aspect ratio and Fourier descriptors and obtained average accuracy of 92% for 33 images. The authors also presented a method to count NRBCs and WBCs based on the roundness factor. Rezatofighi et al. [132] proposed RBC detection method using polar transformation and run-length matrix and a true positive rate of 97.73% was obtained for 22 blood smears. However, objects with large size variations failed to be detected by this method. Soltanzadeh et al. [146] presented a method to classify three types of RBCs using morphological methods. The method obtained 98.63% for elliptocyte, 96.7% for discocyte and 95.36% accuracy for echinocyte recognition for 200 images based on Euclidean distance. Frejlichowski [79] proposed RBC classification method using template matching and Fourier transform. A recognition rate of 93% was obtained for 55 MGG stained images. Arnau et al. [82] presented a method for RBC classification using an active contour segmentation. This method classified RBCs into normal, sickle cells and other cell deformations and obtained 95% accuracy for 45 images. Aruna et al. [34] proposed a method to detect sickle cells using Canny Edge, LOG, Prewitt, Robert and Sobel operators and found that the Canny edge detection method was preferable. Rakshit et al. [128] presented sickle cell detection method using Sobel edge detector and region properties and obtained an overall accuracy of 95.8%. Ahmadzadeh et al. [17] presented a method to group RBCs into three clusters (biconcave, stomatocyte, and sphero-echinocyte) using K-medoids, and K-means clustering and obtained 95% accuracy for Digital Holographic Microscopic (DHM) images. Chandrasiri et al. [48, 49] presented an algorithm to identify four common types of anemia, namely elliptocytes, microcytes, macrocytes and spherocytes, using HT and morphological operations. The researchers could obtain an accuracy in the range of 91–97% based on cell features for 40 images.
Machine learning-based RBC classification methods
Bhowmick et al. [41] presented classification of RBCs in scanning electron microscopic images using a Marker-controlled watershed segmentation method. It is combinational approach of edge-based segmentation and morphological operation methods that uses markers on some set of norms. A marker is a connected component that can easily segment boundaries from an image. With this approach, the regional minimal values occur only at marked locations [117]. The authors projected both structural and textural feature classification in this work and obtained an accuracy of 88.99% for 132 anemic blood samples using Bayesian classifier. Bayesian approach classifies the new instance by assigning the most possible target value, given the attribute values that represent the instance on the principle of Bayes’ Theorem [122]. Das et al. [61] proposed a method for RBC characterization in anemia using Marker-controlled watershed segmentation and morphological features. The algorithm classified five different types of RBCs such as elliptocyte, echinocyte, acanthocyte, sickle cell and teardrop cell in anemia and obtained an accuracy of 86.87% for 715 abnormal and 290 normal RBCs using logistic regression classifier. A method to recognize abnormal RBC shapes such as teardrop, echinocyte and elliptocyte using Hu’s moments for 300 anemia and 100 Leishman stained images of thalassemia cases was proposed by [62]. Elsalamony [74, 75] proposed a geometrical shape signature method to detect sickle and elliptocytosis using CHT and watershed segmentation and 100% accuracy was achieved for 30 images. Elsalamony [76] proposed benign and distorted cell detection methods using HT and WT and obtained 96.9% accuracy using NN and 92.9% using Classification and Regression (C&R) tree for 180 cells from 45 images and reported that NN was preferred over C&R tree to detect sickle cells. In another paper, Elsalamony [77] used Self-Organising Map (SOM) along with the above mentioned methods and reported that SOM does not require any target variables but gets slower in training the neurons. A neural network-based algorithm was proposed by Kim et al. [97] to distinguish abnormalities in RBCs and WBCs using Principal Component Analysis (PCA) and obtained 91% average recognition rate for RBCs in classifying 12 classes from 680 RBCs. Lee et al. [100] proposed RBC classification method using a hybrid neural network and identified sickle, horn and elliptocyte cells. An accuracy of 91% was achieved by this method for a dataset consisting of 200 normal and 200 abnormal single cells. Rodrigues et al. [134] proposed a method to classify RBCs into normal, sickle cells, and erythrocytes with other deformations using morphological properties and obtained 94.6% accuracy using SVM classifier for 626 images from the erythrocytesIDB dataset. They used ANOVA for feature selection and suggested to study unsupervised methods to identify the patterns in cells. Hirimutugoda et al. [91] presented a method to detect malarial parasites and thalassemia using 3-layered ANN for 200 Giemsa images of each case and obtained 86.54% correct recognition rate by defining ROIs. Aliyu et al. [26] presented RBC classification method using SVM and obtained 100% accuracy for normal, acanthocyte, teardrop cells and 73% for elliptocyte and 90% for sickle cells using SVM and 33% using deep learning for 250 images. They reported that the SVM classifier outperformed DL due to limited datasets. A research group [24] also proposed a method to detect sickle cell using Otsu thresholding and shape features and obtained 88% accuracy for 30 Giemsa stained images. Dalvi et al. [59] proposed a method to classify RBCs into four abnormal types, namely elliptocyte, echinocyte, teardrop and macrocyte, using thirteen geometric features and achieved better accuracy using ANN than the decision tree. The accuracy obtained was 96.04% for RBC counting and 90.54% for RBC classification. Razzak et al. [130] presented contour aware segmentation method based on CNN and extreme learning. The experiment was conducted on 64,000 blood cells from ALL-IDB database. RBCs and WBCs were segregated based on the color intensity features and were cropped to extract features using CNN and given for ELM for subtypes classification. The segmentation accuracy of 98.12% and 98.16% and classification accuracy of 94.71% and 98.68% was achieved for RBCs and WBCs respectively. Mundhra et al. [116] proposed deep learning-based ShonitTM system to localize and classify blood cells. U-net deep learning architecture was used to localize WBCs and platelets from 300 MGG and Leishman stained training images. Otsu thresholding in the green channel was used to identify RBCs and clumped cells were rejected. CNN architecture was used to classify WBCs and RBCs based on size and shape. The sensitivity for WBC extraction was 99.5%. The sensitivity and specificity of identification for the common cell types were above 91% and 98% respectively. Alom et al. [27] presented deep learning-based inception recurrent residual convolutional neural network for WBC and RBC classification. The recognition accuracies of 100% for 352 WBC images and 99.94% for 3737 RBC images were achieved. It is mentioned that the model requires a large number of network parameters. Durant et al. [71] proposed a method for RBC classification based on morphology using CNN for 10 classes. Around 3737, 100X magnified labeled cells were used and correct classification frequency of 90.60% was achieved. The researchers reported that the distribution of labels for cell types was not homogeneous.
Clinical parameters based RBC classification methods
Zahir et al. [162] presented an ANN-based method to detect RBC disorders anemia and polycythemia using Hb value, MCH and RBC count and obtained significant results for more than 90% of the 1000 blood samples with training time less than 15 minutes. Bacus et al. [36, 37] presented RBC classification method by extracting the features and obtained correlation coefficient of 0.965 for 100 cells from 4 specimens. Red cell indices along with the red cell differential counts were considered in this work. Maity et al. [106] presented a method to generate an anemia diagnosis report based on the CBC report and RBC morphology using red cell indices and shape features. A precision of 98.2% was achieved in classifying microcytic, macrocytic, sickle, teardrop, elliptocyte, and normal cells from 1500 Leishman blood smear images.
Figure 5 shows the distribution of RBC classification methods used in the related works to detect anemia. We observe from the distribution that, majority of the researchers used shape features and machine learning to classify RBCs. A brief overview of the RBC classification and anemia detection methods are listed in Table 3.
Fig. 5.
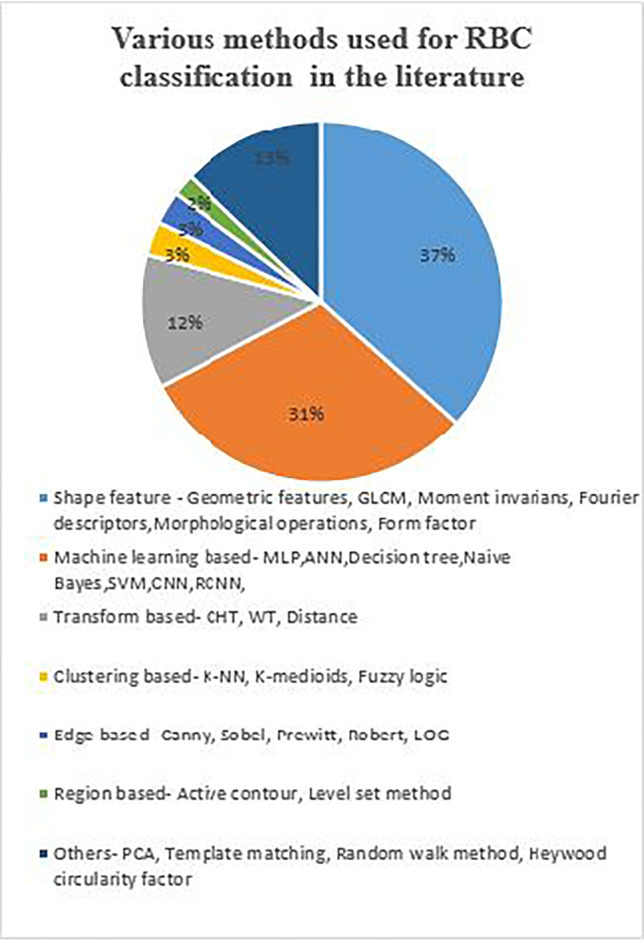
Distribution of RBC classification methods
Table 3.
RBC classification and anemia detection methods
| Methods | No. of images (Stain) | Performance metric | Remarks | Ref. |
|---|---|---|---|---|
| CHT, Heywood circularity factor, ANN, moment invariants, inclusion-tree structure, BPNN, PCA, SVM | 150–1000 samples | 80–99% accuracy for normal & abnormal RBCs | Lacks robustness | [18, 69, 147, 149, 154] |
| Morphological properties, Naive Bayes, K-NN, SVM, Sobel edge | 626 | 94.6–96% accuracy for normal and sickle cells | Consider unsupervised classifiers for more RBC patterns | [128, 134] |
| CHT, WT, NN, decision tree, SOM, SVM | 30–45 (Giemsa) | 97–100% accuracy for sickle and elliptocytosis | Geometrical shape signature is used for detection process | [74–76] |
| Recursive partitioning, form factor | 3878 cells | 85% for discocytes, 83% for abnormal cells and 81% for sickle cells | Form factor invariant to cell size and provides useful information on cell shape | [121, 158] |
| Hybrid neural network | 200 normal and 200 abnormal cells | 91% accuracy for sickle, horn and elliptocytes | Considered only convexity index feature | [100] |
| DL, SVM | 105 normal and 250 abnormal | Normal—100%, achantocyte—100%, sickle cell—90%, teardrop—100% and elliptocyte—73% accuracy using SVM | SVM classifier outperformed DL | [23, 26] |
| Rolling ball background, shape features, Naive Bayes, Bayesian classifier | 1500 (Leishman) | 98.2% precision for microcytic, macrocytic, sickle, teardrop, elliptocyte | Decision from CBC test measures is semi-automatic operation | [106] |
| ANN | 1000 blood samples | Less computational time | Used RBG values—from Hb, MCH and RBC count | [162] |
| CNN , ELM | 64,000 blood cells | 94.71% accuracy | Images from multiple sources are used | [130] |
| U-Net | 300 (MGG) and (Leishman) | 91% sensitivity and 98% specificity | Results are shown for a variety of smear and stain | [116] |
| Inception recurrent residual CNN | 352 WBCs and 3737 RBCs | 100% for WBC and 99.94% accuracy for RBC | Model require larger number of network parameters | [27] |
| CNN | 3737 labeled Cells | 90.6% accuracy for 10 RBC classes | Label distribution was not homogeneous | [71] |
Classification of anemia
This section provides details of image processing methods used to classify anemia based on the morphology and hematological parameters of RBCs.
Morphology based anemia classification methods
Chen et al. [52] presented a method to classify hemolytic anemia based on differential value and variation of chain codes in eight directions and irregularity of erythrocytes. Accuracy in the range of 95–97% was achieved for 24 microscopic images using Bayes classifier, logistic model trees and rule based classifiers. Nithyaa et al. [119] proposed a method to detect various blood disorders such as malaria, elephantiasis, trypanosomiasis, SCA and polycythemia using statistical features and Euclidean distance for 40 images. Azam et al. [35] presented a method to detect seven RBC types of anemic diseases using shape descriptors and obtained 92% accuracy for 100 instances using MLP and random forest classifier. Tyas et al. [153] presented a semi-automated algorithm to classify four types of abnormal RBCs such as teardrop, acanthocytes, sickle cell and target cell using GLCM features in the minor thalassemia cases. The ROI was selected manually in this method and accuracies of 93.22% and 92.55% were obtained using BPNN and CNN respectively for 256 images. Various methods [109, 129] have been used to detect thalassemia using K-means clustering, active contour, neural network, decision tree and an average accuracy in the range 82–95% was reported. Sharma et al. [145] proposed a method to detect SCA and thalassemia using Marker-controlled watershed segmentation and geometric features and accuracy of 80.6% was obtained for 100 images with KNN classifier. Fadhel et al. [13] proposed an algorithm to count normal and abnormal RBCs in the SCA slide using CHT and WT for 233 cells and proved that CHT is better than WT. Elsalamony [73] proposed a method to diagnose SCA using HT and obtained segmentation accuracy of 99.98%. This method also achieved a classification accuracy of 96.9% and 92.9% using NN and C&R tree respectively. Lotfi et al. [102] presented a technique for the automatic detection of IDA by identifying three types of abnormal red cells using region and Fourier descriptors. This method obtained accuracies of 99%, 97% and 100% for dacrocytes, elliptocytes and schistocyte cells respectively for 100 cells of each case using NN, SVM and KNN classifier. Tyagi et al. [152] presented a method to detect poikilocyte cells in IDA using GLCM features and moment invariants and obtained accuracy in the range of 75–81% for 100 images using ANN. Several methods have been proposed to detect the sickle-shaped RBC in SCD patients by [21, 22, 29, 55, 56, 88, 114, 115, 120, 131, 159, 160] using methods such as random walk, Sobel edge, geometric features, Fuzzy C means clustering, LOG, WT, HT and morphological filters. The average accuracy reported was in the range of 85–95%. Alzubaidi et al. [30] developed deep learning models for SCA diagnosis and achieved an accuracy of 99.54%. The researchers proposed three CNN models with different layers and filters and used data from erythrocytesIDB, ALL-IDB and other internet sources. The extracted features were used for training multi-class SVM and accuracy around 98–99% was obtained. Zhang et al. [163] proposed a method to segment subtypes of RBCs in sickle cell disease using deformable U-net on 266 raw microscopy images and obtained 99.12% accuracy. This method could segment blurred, clustered and heterogeneous shaped RBCs and performed better than baseline U-net. Aliyu et al. [25] proposed the Alexnet deep learning model for the classification of RBCs in SCA. Around 750 single RBCs from Giemsa stained blood smears were acquired for the experiment and classification accuracy of 95.92% was obtained. They reported low specificity due to less normal cells. Haan et al. [85] presented a deep learning framework based screening of sickle cells using a smartphone microscope. U-net architecture for image normalization and enhancement network and semantic segmentation for sickle cells were used and approximately 98% accuracy was achieved from 96 unique patient samples. Das et al. [63] presented an overview of enhancement, segmentation and classification techniques used for SCA detection. The review also highlights clinical uses, hardware implementation and future scope for the analysis of SCD.
Hematological parameters based anemia classification
Birndorf et al. [42] presented ANN-based hybrid system to evaluate microcytic anemia such as IDA, hemoglobinopathy and anemia of chronic disease using HCT, MCV, RDW and obtained 96.5% accuracy for 473 cases of microcytic anemia and anemia of chronic disease. Dogan et al. [70] proposed IDA detection method based on hematology parameters, namely Serum iron and total iron-binding capacity, using decision trees for 96 patients and the results got perfectly matched with the physician’s decisions. Lund et al. [103] presented an algorithm to classify microcytic and macrocytic anemia using image analysis techniques based on MCV and RBC size and obtained 95% accuracy for 4000 cells. Sanap et al. [137] proposed an anemia classification method based on CBC reports using C4.5 decision tree algorithm and SVM using WEKA tool with 514 instances and obtained accuracy of 99.42% and 88.13% respectively. Abdullah et al. [12] presented anemia types prediction method based on CBC reports using data mining techniques using WEKA tool for 41 patients. The experiment showed that the J48 decision tree performed better with 97% precision among Naive Bayes, MLP and SVM algorithms. Jaiswal et al. [94] presented anemia prediction method based on CBC reports using supervised machine learning algorithms. This method used eighteen attributes from 200 samples and obtained maximum accuracy of 96.09%. In this work, it was reported that Naive Bayes outperformed C4.5 and random forest. Khalaf et al. [96] presented machine learning approaches for the classification of SCD dosage levels using 13 attributes from 1168 sample points. They concluded that the random forest classifier performed overall better than RNN and feedforward neural networks. Amendolia et al. [31, 32] presented ANN-based method to detect α and β thalassemia using hemochromic parameters. A specialized ANN was used in the method and accuracy of 94% was achieved for 304 cases. Setsirichok et al. [142] proposed a method for classifying thalassemia using Hb and MCV parameters and obtained an average accuracy in the range of 93–99% for 8054 clinical trial samples using C4.5 decision tree, Naive Bayes classifier and MLP. However, they mentioned that Hb parameter is redundant for the study.
Table 4 summarizes the anemia classification methods used in literature.
Table 4.
Anemia classification methods
| Methods | No. of images | Accuracy (%) | Remarks | Ref. |
|---|---|---|---|---|
| GLCM, CNN | 256 | BPNN—93.2, CNN—92.6 for minor thalassemia case | Sample size is less | [153] |
| SVM, KNN, MLP | 304 records | MLP—92 , SVM—83 sensitivity for thalassemia | Using RBC, Hb, HCT, MCV parameters | [31, 32] |
| ANN | 473 cases | 96.5 for IDA, HA, ACD | Using HCT, MCV, RDW | [42] |
| Active contour, NN, DT | 15 groups | 82–93 for thalassaemia | False-positive and false negative errors are less than 1% and 2% | [?, 109] |
| C4.5 DT, Naive Bayes classifier and MLP | 8054 samples | 99.4 for 18 classes of thalassaemia | Using six Hb attributes and MCV | [142] |
| Marker-controlled Watershed segmentation, KNN | 100 | 80.6 for SCA and thalassaemia | Developed combined method | [145] |
| Fuzzy C means clustering, geometrical and statistical features | 80 | KNN—73.3, SVM—83.3, ELM—87.7 for SCD | Fuzzy C means overcomes the disadvantages of threshold segmentation | [55, 56] |
| HSI color space, K-means clustering | 60 | 94.6 for thalassemia | Detected α, β thalassemia, β-thalassemia trait | [129] |
| ANN, GLCM features | 100 | 75–81 for IDA | Classified 4 types of poikilocytes | [152] |
| CHT, marker-controlled WT, LOG, Fuzzy thresholding | 8–20 | 91.1 for SCD | CHT performed better, need improvement in de-noising method | [13] [21, 22, 88, 114, 115] |
| CLAHE, MLP and random forest | 100 instances | 92 for IDA and HA | Persistent results for any luminosity conditions | [35] |
| Deformable U-Net | 266 raw | 99.12 for SCD RBC | Method could segment blurred, clustered, heterogeneous shaped RBCs | [163] |
| Chain codes, Bayes classifier, logistic model trees and rules classifier | 24 | 96.6 for HA | HA is classified based on differential value of chain codes | [52] |
| Naive Bayes, C4.5 and random forest classifier | 200 samples | 96.1 for anemia detection | Used 18 attributes from CBC reports | [94] |
| DL, multi-class SVM | 100–250 | 99.5 for SCD | Proposed three CNN models with different layers and filters | [30] |
| DL-Alexnet | 750 single RBCs | 95.9 for SCA | Specificity was low due to less normal cells | [25] |
| U-net architecture, semantic segmentation | 96 unique samples | 98 for SCD | Developed smartphone microscope | [85] |
It is evident from Fig. 6 that, most of the research groups used the traditional machine learning approach for anemia classification. It can also be observed that, there is an increasing tendency towards the usage of deep learning classifier models.
Fig. 6.
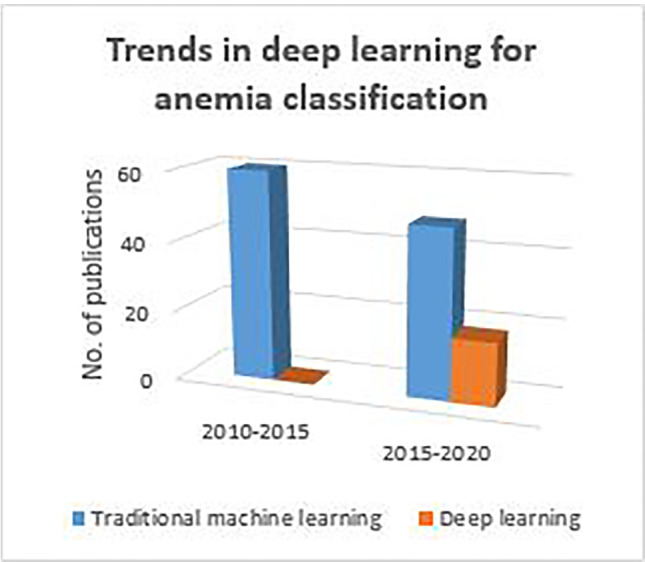
Application of traditional machine learning and deep learning for anemia classification
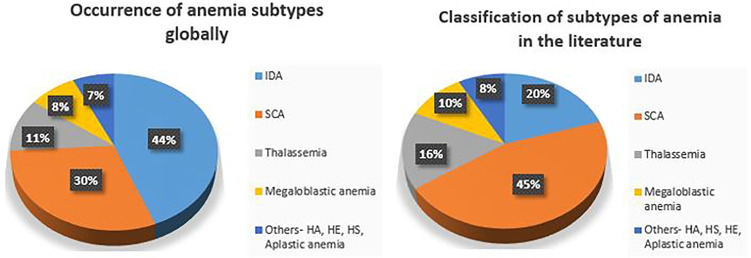
Occurrence of anemia worldwide according to WHO [50, 111] and papers on classification of subtypes of anemia are shown in Fig. 7.
Fig. 7.
Occurrence and classification of anemia subtypes
Out of many anemia subtypes, the frequency of SCA and IDA detection was high by the majority of the researchers which is depicted in Fig. 7. It can be observed from figure that occurrence of IDA globally is much higher than SCA. However, number of studies seen in the literature is not in the same proportion. There is scope for more study in IDA detection. Also, there is a need for other anemia subtypes detection and diagnosis in peripheral blood smear analysis.
Database
Many researchers have used proprietary datasets with blood smear images of different stains. A few publicly available databases are used for the performance analysis of the developed algorithm. An overview of these databases are given in Table 5.
Table 5.
Outline of the publicly available databases
| Database | No. of images | Annotations | Studies |
|---|---|---|---|
| BCCD [4] | 364 smear images | Available for RBCs, WBCs and platelets | [20] |
| erythrocytesIDB [6] | 196 smear images and 629 Giemsa stained single RBCs | Available for sickle cells of 80 smear images | [29, 81, 134] |
| ASH image bank [3] | 2100 hematologic Leishman stained images | Not available | - |
| Isfahan MISP [9] | 148 | Not available | [139] |
| PHIL [8] | 100 | Not available | [14] |
| BBBC [5] | 18 biological image sets | Available for RBC’s only | [19] |
| Telepathology 2012 [10] | Malarial parasite images | Tool available | [156] |
| LISC [7] | 400 Wright-Giemsa stained images | Available for WBC’s from 250 images only | [138] |
| ALL-IDB [1] | 108 smear images and 260 cropped normal and blast single cell images | Available for WBC’s only | [29, 65, 101, 104, 105, 130, 143, 150, 151, 161] |
BCCD is a small-scale publicly available dataset [4] that has 364 annotated images for blood cell detection taken originally from cosmicad and akshaylamba open sources. The erythrocytesIDB [6] contains 196 full field and 629 individual Giemsa stained peripheral blood smear images taken from SCD patients. ASH image bank [2] is a web-based image library that has a collection of hematologic images consists of normal and abnormal blood cells. However, studies have not explored available images for anemia detection. The atlas of hematology [3] provides normal and abnormal Leishman stained blood smear images for the morphological study of cells. Medical Image and Signal Processing (MISP) Research Center and Department of Pathology at Isfahan University of Medical Sciences [9] contributed for the dataset consists of 148 microscopic blood smear images. Public Health Image Library (PHIL) [8] contains a few blood smear images created by the Centers for Disease Control and Prevention (CDC) for reference. The Broad Bioimage Benchmark Collection (BBBC) [5] consists of publicly available image sets such as annotated biological images for the analysis of algorithms. Telepathology 2012 [10] consists of webmicroscope to acquire malarial parasite data along with annotation tool. Leukocyte Images for Segmentation and Classification (LISC) [7] for identification of different WBCs with ground truth for only 250 images. Acute Lymphoblastic Leukemia Image Database (ALL-IDB) [1] is a free, publicly available dataset for the evaluation of segmentation and classification methods. There are two datasets specifically for lymphoblasts detection. ALL-IDB1 consists of 108 blood smear images with labeled lymphocytes taken with 300 to 500 microscope magnifications. ALL-IDB2 is a collection of 260 cropped normal and blast cells that belongs to ALL-IDB1 dataset.
Even though a lot of work has been carried out on PBS images, annotations are not available for the publicly available datasets. A comparison of work would not be fair because ground truth depends on annotation done by individuals.
Discussion and future scope
Diagnosis of anemia is challenging, particularly in inadequate resource settings. Various state-of-the-art methods used in the literature for PBS analysis are mentioned in Section 2 and some of them are listed in Tables 2, 3 and 4. In this paper, we have provided a detailed report of the use of image processing methodologies to automate peripheral blood smear analysis for diagnosing morphology-based RBC disorders. Also, we can notice from Fig. 6 that detection and classification of anemia using deep learning is increasing over traditional machine learning approaches. From Table 5, it is evident that most of the research groups used a proprietary dataset for the analysis of the algorithms. As researchers used a publicly available datasets with different images and annotations, a comparison of the algorithms is not possible to accept the results. It is also observed that the number of images in dataset is in the range from 100 to 1000. As deep learning is becoming popular and many of classification algorithms are supervised learning approaches, it is desirable to have large PBS datasets with annotations. It can be observed from Fig. 7 that the occurrence of IDA is much higher than SCA and studies related to IDA is lesser. Hence, there is a need for more study of IDA and other subtypes of anemia. Figure 8 depicts the ways of classification of anemia. We can observe that around 88% of researchers used morphological parameters and only a few research groups used hematology parameters for the anemia diagnosis.
Fig. 8.
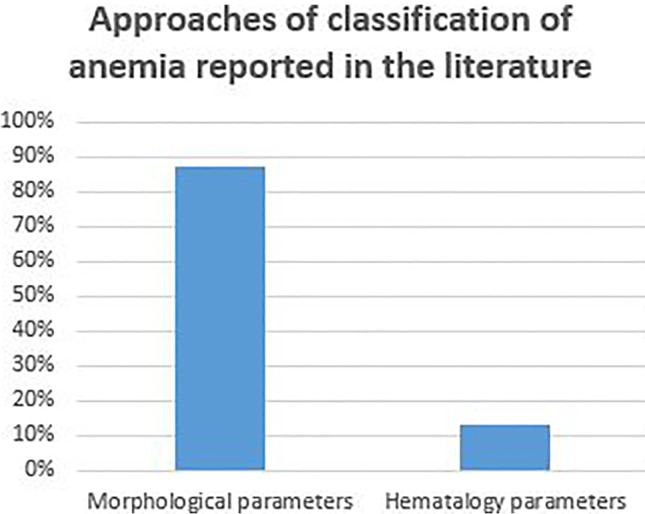
Ways of anemia classification
To detect anemia cases, mostly the shape and size of the abnormal RBCs are considered. The methods used by the research groups did not focus on other abnormalities of blood cells.
It can be noticed that multiple methods have been implemented to detect a few anemia cases like SCA, IDA and thalassemia that mainly depend on the input image taken from different lab setups and conditions. The summary table refers to the papers which are categorized into three divisions depending on the purpose and approaches mentioned in Section 2. All these research groups focused on developing computer-aided automated systems to reduce the task of hematologists in analyzing the peripheral blood smears. A major impediment of automated microscopic evaluation is that they are affected due to imaging and staining variations. An accurate identification of normal and abnormal cases is essential to assist pathologists for further diagnosis. Images are acquired from blood smear stained using a specific stain with different microscopic settings and lab arrangements. However, this poses many challenges for the automation of blood smear images. For example, as mentioned in Section 2 and Table 2, the methods presented by various researchers for RBC segmentation and counting were implemented on specific stained blood smear images under a controlled environment. However, it is observed from the past studies described in the listed papers that results vary due to lack of robustness in the methods. Although an automated decision support system is developed to reduce the burden on hematologists by eliminating manual inspection of blood smears, there is no integrated approach that has been developed to handle both standard and inconsistent microscopic blood smear images acquired from both the manual and automated workflow.
Conclusion
This review provides a summary of the current developments in computerized PBS analysis using microscopic blood smear images. The paper comprises of introduction to PBS analysis and anemia, an RBC disorder. Different sections have been summarized in this paper on existing automated image processing methods for identification, segmentation, feature extraction and classification of RBCs for further diagnosis of anemia. The literature on approaches of the classification of anemia also has been included in this review. Although, manual microscopic evaluation is a gold standard for PBS analysis, for quick and accurate diagnosis, an automated decision system is essential to overcome the limitations of microscopic analysis. Hence, the observations made during the process of the review are listed below.
To analyze RBC disorders rigorously, a large publicly available dataset with annotations of various types of blood cells is needed.
A robust system that can handle staining and imaging variations is desirable in PBS analysis for anemia detection.
A hybrid method which considers both morphological and clinical features would play an important role to improve the efficiency of classification of anemia subtypes.
An automated system plays a very important role in the PBS analysis. However, it is observed from the review that, there is a need for a large PBS publicly available database with appropriate annotations and robust system to assist clinicians for further diagnosis of disease.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Navya K.T., Email: navya.kt@manipal.edu.
Keerthana Prasad, Email: keerthana.prasad@manipal.edu.
Brij Mohan Kumar Singh, Email: brij.singh@manipal.edu.
References
- 1.ALL-IDB dataset. https://homes.di.unimi.it/scotti/all/
- 2.ASH image bank. http://imagebank.hematology.org/
- 3.Atlas of hematology. http://hematologyatlas.com/principalpage.htm
- 4.Blood cell count and detection. https://github.com/Shenggan/BCCDDataset
- 5.Broad bioimage benchmark collection. http://www.broadinstitute.org/bbbc/
- 6.ErythrocytesIDB dataset. http://erythrocytesidb.uib.es/
- 7.LISC database. http://users.cecs.anu.edu.au/hrezatofighi/Data/Leukocyte20Data.htm
- 8.Public health image library (PHIL). http://phil.cdc.gov/phil/home.asp
- 9.Red blood cells. https://hrabbani.site123.me/available-datasets/red-blood-cells
- 10.Telepathology 2012-webmicroscope. http://fimm.webmicroscope.net/Research/Momic/tp2012
- 11.Abbas N, Mohamad D, et al. Microscopic RGB color images enhancement for blood cells segmentation in YCbCr color space for k-means clustering. J Theor Appl Inf Technol. 2013;55(1):117–125. [Google Scholar]
- 12.Abdullah M, Al-Asmari S (2017) Anemia types prediction based on data mining classification algorithms, communication, management and information technology–Sampaio de Alencar (ed.)
- 13.AbdulraheemFadhel M, Humaidi AJ, RazzaqOleiwi S (2017) Image processing-based diagnosis of sickle cell anemia in erythrocytes. In: 2017 Annual conference on new trends in information & communications technology applications (NTICT), pp 203–207. IEEE
- 14.Acharjee S, Chakrabartty S, Alam MI, Dey N, Santhi V, Ashour AS (2016) A semiautomated approach using GUI for the detection of red blood cells. In: 2016 International conference on electrical, electronics, and optimization techniques (ICEEOT), pp 525–529. IEEE
- 15.Acharya V, Kumar P. Identification and red blood cell automated counting from blood smear images using computer-aided system. Med Biol Eng Comput. 2018;56(3):483–489. doi: 10.1007/s11517-017-1708-9. [DOI] [PubMed] [Google Scholar]
- 16.Adagale S, Pawar S (2013) Image segmentation using PCNN and template matching for blood cell counting. In: 2013 IEEE International conference on computational intelligence and computing research, pp 1–5. IEEE
- 17.Ahmadzadeh E, Jaferzadeh K, Lee J, Moon I. Automated three-dimensional morphology-based clustering of human erythrocytes with regular shapes: stomatocytes, discocytes, and echinocytes. J Biomed Opt. 2017;22(7):076015. doi: 10.1117/1.JBO.22.7.076015. [DOI] [PubMed] [Google Scholar]
- 18.Akrimi JA, Suliman A, George LE, Ahmad AR (2014) Classification red blood cells using support vector machine. In: Proceedings of the 6th international conference on information technology and multimedia, pp 265–269. IEEE
- 19.Al-Hafiz F, Al-Megren S, Kurdi H. Red blood cell segmentation by thresholding and Canny detector. Procedia Comput Sci. 2018;141:327–334. doi: 10.1016/j.procs.2018.10.193. [DOI] [Google Scholar]
- 20.Alam MM, Islam MT. Machine learning approach of automatic identification and counting of blood cells. Healthcare Technol Lett. 2019;6(4):103–108. doi: 10.1049/htl.2018.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albayrak B, Darici MB, Kiraci F, Ougrenci AS, Ozmen A, Ertez K (2018) Sickle cell anemia detection. IEEE Institute of Electrical and Electronics Engineers Inc
- 22.Algailani H, Hamad MES (2018) Detection of sickle cell disease based on an improved Watershed segmentation. In: 2018 International conference on computer, control, electrical, and electronics engineering (ICCCEEE), pp 1–4. IEEE
- 23.Aliyu HA, Razak MAA, Sudirman R. Normal and abnormal red blood cell recognition using image processing. Indonesian J Electr Eng Comput Sci. 2019;14(1):100–104. [Google Scholar]
- 24.Aliyu HA, Razak MAA, Sudirman R (2019) Segmentation and detection of sickle cell red blood image. In: AIP Conference proceedings, vol 2173, p 020004. AIP Publishing LLC
- 25.Aliyu HA, Razak MAA, Sudirman R, Ramli N. A deep learning AlexNet model for classification of red blood cells in sickle cell anemia. Int J Artif Intell. 2020;9(2):221–228. [Google Scholar]
- 26.Aliyu HA, Sudirman R, Razak MAA, Abd Wahab MA (2018) Red blood cell classification: deep learning architecture versus support vector machine. In: 2018 2nd International conference on biosignal analysis, processing and systems (ICBAPS), pp 142–147. IEEE
- 27.Alom MZ, Yakopcic C, Taha TM, Asari VK (2018) Microscopic blood cell classification using inception recurrent residual convolutional neural networks. In: NAECON 2018-IEEE national aerospace and electronics conference, pp 222–227. IEEE
- 28.Alomari YM, Abdullah S, Huda SN, Zaharatul Azma R, Omar K (2014) Automatic detection and quantification of WBCs and RBCs using iterative structured circle detection algorithm. Comput Math Methods Med, 2014 [DOI] [PMC free article] [PubMed]
- 29.Alzubaidi L, Fadhel MA, Al-Shamma O, Zhang J (2018) Robust and efficient approach to diagnose sickle cell anemia in blood. In: International conference on intelligent systems design and applications, pp 560–570. Springer
- 30.Alzubaidi L, Fadhel MA, Al-Shamma O, Zhang J, Duan Y. Deep learning models for classification of red blood cells in microscopy images to aid in sickle cell anemia diagnosis. Electronics. 2020;9(3):427. doi: 10.3390/electronics9030427. [DOI] [Google Scholar]
- 31.Amendolia SR, Brunetti A, Carta P, Cossu G, Ganadu M, Golosio B, Mura GM, Pirastru MG. A real-time classification system of thalassemic pathologies based on artificial neural networks. Med Decis Making. 2002;22(1):18–26. doi: 10.1177/0272989X0202200102. [DOI] [PubMed] [Google Scholar]
- 32.Amendolia SR, Cossu G, Ganadu M, Golosio B, Masala GL, Mura GM. A comparative study of k-nearest neighbour, support vector machine and multi-layer perceptron for thalassemia screening. Chemom Intell Lab Syst. 2003;69(1-2):13–20. doi: 10.1016/S0169-7439(03)00094-7. [DOI] [Google Scholar]
- 33.Amin MN, Habib MA. Comparison of different classification techniques using WEKA for hematological data. Amer J Eng Res. 2015;4(3):55–61. [Google Scholar]
- 34.Aruna N, Hariharan S. Edge detection of sickle cells in red blood cells. Int J Comput Sci Inform Technol. 2014;5(3):4140–4144. [Google Scholar]
- 35.Azam B, Rahman S, Ullah S, Hanan F (2017) Detection of the top anemic diseases in blood smear images using image quantization followed by ensemble of classifiers. In: Proceedings of the 2017 4th international conference on biomedical and bioinformatics engineering, pp 115–120
- 36.Bacus J, Belanger M, Aggarwal R, Trobaugh F., Jr Image processing for automated erythrocyte classification. J Histochem Cytochem. 1976;24(1):195–201. doi: 10.1177/24.1.1254916. [DOI] [PubMed] [Google Scholar]
- 37.Bacus J, Weens J. An automated method of differential red blood cell classification with application to the diagnosis of anemia. J Histochem Cytochem. 1977;25(7):614–632. doi: 10.1177/25.7.330716. [DOI] [PubMed] [Google Scholar]
- 38.Badrinarayanan V, Kendall A, Cipolla R. Segnet: a deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans Pattern Anal Mach Intell. 2017;39(12):2481–2495. doi: 10.1109/TPAMI.2016.2644615. [DOI] [PubMed] [Google Scholar]
- 39.Bergen T, Steckhan D, Wittenberg T, Zerfass T (2008) Segmentation of leukocytes and erythrocytes in blood smear images. In: 2008 30th Annual international conference of the IEEE engineering in medicine and biology society, pp 3075–3078. IEEE [DOI] [PubMed]
- 40.Bhavnani LA, Jaliya UK, Joshi MJ. Segmentation and counting of WBCs and RBCs from microscopic blood sample images. Int J Image Graph Signal Process. 2016;8(11):2016. [Google Scholar]
- 41.Bhowmick S, Das D, Maiti A, Chakraborty C. Structural and textural classification of erythrocytes in anaemic cases: a scanning electron microscopic study. Micron. 2013;44:384–394. doi: 10.1016/j.micron.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Birndorf NI, Pentecost JO, Coakley JR, Spackman KA. An expert system to diagnose anemia and report results directly on hematology forms. Comput Biomed Res. 1996;29(1):16–26. doi: 10.1006/cbmr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 43.Biswas S, Ghoshal D. Blood cell detection using thresholding estimation based watershed transformation with sobel filter in frequency domain. Proced Comput Sci. 2016;89:651–657. doi: 10.1016/j.procs.2016.06.029. [DOI] [Google Scholar]
- 44.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Belmont, CA: Wadsworth. Int Group. 1984;432:151–166. [Google Scholar]
- 45.Brewka G. Artificial intelligence—a modern approach by Stuart Russell and Peter Norvig, Prentice Hall. Series in artificial intelligence, Englewood Cliffs, NJ. Knowl Eng Rev. 1996;11(1):78–79. doi: 10.1017/S0269888900007724. [DOI] [Google Scholar]
- 46.Bronkorsta P, Reinders MJ, Hendriks EA, Grimbergen J, Heethaar RM, Brakenhoff G. On-line detection of red blood cell shape using deformable templates. Pattern Recogn Lett. 2000;21(5):413–424. doi: 10.1016/S0167-8655(00)00012-X. [DOI] [Google Scholar]
- 47.Cai R, Wu Q, Zhang R, Fan L, Ruan C (2012) Red blood cell segmentation using active appearance model. In: 2012 IEEE 11th International conference on signal processing, vol 3, pp 1641–1644. IEEE
- 48.Chandrasiri S, Samarasinghe P (2014) Automatic anemia identification through morphological image processing. In: 7th International conference on information and automation for sustainability, pp 1–5. IEEE
- 49.Chandrasiri S, Samarasinghe P (2014) Morphology based automatic disease analysis through evaluation of red blood cells. In: 2014 5th International conference on intelligent systems, modelling and simulation, pp 318–323. IEEE
- 50.Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann N Y Acad Sci. 2019;1450(1):15. doi: 10.1111/nyas.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chari PS, Prasad S. Pilot study on the performance of a new system for image based analysis of peripheral blood smears on normal samples. Indian J Hemat Blood Transfus. 2018;34(1):125–131. doi: 10.1007/s12288-017-0835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen HM, Tsao YT, Tsai SN. Automatic image segmentation and classification based on direction texton technique for hemolytic anemia in thin blood smears. Mach Vis Appl. 2014;25(2):501–510. doi: 10.1007/s00138-013-0585-y. [DOI] [Google Scholar]
- 53.Chen X, Lu L, Gao Y (2012) A new concentric circle detection method based on Hough transform. In: 2012 7th International conference on computer science & education (ICCSE), pp 753–758. IEEE
- 54.Chintawar I, Aishvarya M, Kuhikar C. Detection of sickle cells using image processing. Int J Sci Technol Eng. 2016;2(9):335–339. [Google Scholar]
- 55.Chy TS, Rahaman MA (2018) Automatic sickle cell anemia detection using image processing technique. In: 2018 International conference on advancement in electrical and electronic engineering (ICAEEE), pp 1–4. IEEE
- 56.Chy TS, Rahaman MA (2019) A comparative analysis by KNN, SVM & ELM classification to detect sickle cell anemia. In: 2019 International conference on robotics, electrical and signal processing techniques (ICREST), pp 455–459. IEEE
- 57.Cruz D, Jennifer C, Castor LC, Mendoza CMT, Jay BA, Jane LSC, Brian PTB et al (2017) Determination of blood components (WBCs, RBCs, and platelets) count in microscopic images using image processing and analysis. In: 2017 IEEE 9th International conference on humanoid, nanotechnology, information technology, communication and control, environment and management (HNICEM), pp 1–7. IEEE
- 58.Dacie JV (2006) Dacie and Lewis practical haematology. Elsevier Health Sciences
- 59.Dalvi PT, Vernekar N (2016) Computer aided detection of abnormal red blood cells. In: 2016 IEEE International conference on recent trends in electronics, information & communication technology (RTEICT), pp 1741–1746. IEEE
- 60.Das BK, Jha KK, Dutta HS (2014) A new approach for segmentation and identification of disease affected blood cells. In: 2014 International conference on intelligent computing applications, pp 208–212. IEEE
- 61.Das D, Chakraborty C, Mitra B, Maiti A, Ray A. Quantitative microscopy approach for shape-based erythrocytes characterization in anaemia. J Microsc. 2013;249(2):136–149. doi: 10.1111/jmi.12002. [DOI] [PubMed] [Google Scholar]
- 62.Das D, Ghosh M, Chakraborty C, Pal M, Maity AK (2010) Invariant moment based feature analysis for abnormal erythrocyte recognition. In: 2010 International conference on systems in medicine and biology, pp 242–247. IEEE
- 63.Das PK, Meher S, Panda R, Abraham A. A review of automated methods for the detection of sickle cell disease. IEEE Rev Biomed Eng. 2019;13:309–324. doi: 10.1109/RBME.2019.2917780. [DOI] [PubMed] [Google Scholar]
- 64.Deb N, Chakraborty S (2014) A noble technique for detecting anemia through classification of red blood cells in blood smear. In: International conference on recent advances and innovations in engineering (ICRAIE-2014), pp 1–9. IEEE
- 65.Di Ruberto C, Dempster A, Khan S, Jarra B. Analysis of infected blood cell images using morphological operators. Image Vis Comput. 2002;20(2):133–146. doi: 10.1016/S0262-8856(01)00092-0. [DOI] [Google Scholar]
- 66.Di Ruberto C, Loddo A, Putzu L (2019) A region proposal approach for cells detection and counting from microscopic blood images. In: International conference on image analysis and processing, pp 47–58. Springer
- 67.Di Ruberto C, Loddo A, Putzu L. Detection of red and white blood cells from microscopic blood images using a region proposal approach. Comput Biol Med. 2020;116:103530. doi: 10.1016/j.compbiomed.2019.103530. [DOI] [PubMed] [Google Scholar]
- 68.Di Ruberto C, Putzu L (2014) Accurate blood cells segmentation through intuitionistic fuzzy set threshold. In: 2014 Tenth international conference on signal-image technology and internet-based systems, pp 57–64. IEEE
- 69.Diaz G, Gonzalez FA, Romero E. A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J Biomed Inform. 2009;42(2):296–307. doi: 10.1016/j.jbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Dogan S, Turkoglu I. Iron-deficiency anemia detection from hematology parameters by using decision trees. Int J Sci Technol. 2008;3(1):85–92. [Google Scholar]
- 71.Durant TJ, Olson EM, Schulz WL, Torres R. Very deep convolutional neural networks for morphologic classification of erythrocytes. Clin Chem. 2017;63(12):1847–1855. doi: 10.1373/clinchem.2017.276345. [DOI] [PubMed] [Google Scholar]
- 72.Ejaz Z, Hassan A, Aslam H. Automatic red blood cell detection and counting system using Hough transform. Indo Amer J Pharmaceut Sci. 2018;5(7):7104–7110. [Google Scholar]
- 73.Elsalamony HA (2014) Sickle anemia and distorted blood cells detection using hough transform based on neural network and decision tree. In: Proceedings of the international conference on image processing, computer vision, and pattern recognition (IPCV). The Steering Committee of The World Congress in Computer Science, Computer ..., p 1
- 74.Elsalamony HA (2015) Detecting distorted and benign blood cells using the Hough transform based on neural networks and decision trees. In: Emerging trends in image processing, computer vision and pattern recognition, pp 457–473. Elsevier
- 75.Elsalamony HA. Healthy and unhealthy red blood cell detection in human blood smears using neural networks. Micron. 2016;83:32–41. doi: 10.1016/j.micron.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Elsalamony HA. Anaemia cells detection based on shape signature using neural networks. Measurement. 2017;104:50–59. doi: 10.1016/j.measurement.2017.03.012. [DOI] [Google Scholar]
- 77.Elsalamony HA. Detection of anaemia disease in human red blood cells using cell signature, neural networks and SVM. Multimed Tools Appl. 2018;77(12):15047–15074. doi: 10.1007/s11042-017-5088-9. [DOI] [Google Scholar]
- 78.Ford J. Red blood cell morphology. Int J Lab Hematol. 2013;35(3):351–357. doi: 10.1111/ijlh.12082. [DOI] [PubMed] [Google Scholar]
- 79.Frejlichowski D (2010) Pre-processing, extraction and recognition of binary erythrocyte shapes for computer-assisted diagnosis based on MGG images. In: International conference on computer vision and graphics, pp 368–375. Springer
- 80.Frejlichowski D. Detection of erythrocyte cells in microscopy images. Przeglad Elektrotechniczny. 2012;88(10b):264–267. [Google Scholar]
- 81.Gonzalez-Hidalgo M, Guerrero-Pena F, Herold-Garcia S, Jaume-i Capó A, Marrero-Fernández PD. Red blood cell cluster separation from digital images for use in sickle cell disease. IEEE J Biomed Health Inform. 2014;19(4):1514–1525. doi: 10.1109/JBHI.2014.2356402. [DOI] [PubMed] [Google Scholar]
- 82.Gual-Arnau X, Herold-Garcia S, Simo A. Erythrocyte shape classification using integral-geometry-based methods. Med Biol Eng Comput. 2015;53(7):623–633. doi: 10.1007/s11517-015-1267-x. [DOI] [PubMed] [Google Scholar]
- 83.Guan PP, Yan H (2011) Blood cell image segmentation based on the Hough transform and fuzzy curve tracing. In: 2011 International conference on machine learning and cybernetics, vol 4, pp 1696–1701. IEEE
- 84.Gupta M. Cell identification by blob detection. UACEE Int J Adv Electon Eng. 2012;2:56–59. [Google Scholar]
- 85.de Haan K, Koydemir HC, Rivenson Y, Tseng D, Van Dyne E, Bakic L, Karinca D, Liang K, Ilango M, Gumustekin E, et al. Automated screening of sickle cells using a smartphone-based microscope and deep learning. NPJ Digit Med. 2020;3(1):1–9. doi: 10.1038/s41746-019-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Habibzadeh M, Krzyzak A, Fevens T (2011) Application of pattern recognition techniques for the analysis of thin blood smear images. Journal of Medical Informatics and Technologies 18 (2011)
- 87.Hari J, Prasad AS, Rao SK (2014) Separation and counting of blood cells using geometrical features and distance transformed watershed. In: 2014 2nd International conference on devices, circuits and systems (ICDCS), pp 1–5. IEEE
- 88.Hariharan S, Parvathy H, Aruna S. An overview of sickle cell anemia–a special emphasis on image processing on SEM images. Int J Appl Eng Res. 2016;11(1):201–8. [Google Scholar]
- 89.Haykin S, Network N. A comprehensive foundation. Neur Netw. 2004;2(2004):41. [Google Scholar]
- 90.Hegde RB, Prasad K, Hebbar H, Sandhya I. Peripheral blood smear analysis using image processing approach for diagnostic purposes: a review. Biocybern Biomed Eng. 2018;38(3):467–480. doi: 10.1016/j.bbe.2018.03.002. [DOI] [Google Scholar]
- 91.Hirimutugoda Y, Wijayarathna G. Image analysis system for detection of red cell disorders using artificial neural networks. Sri Lanka J Bio-Med Inform. 2010;1(1):2010. doi: 10.4038/sljbmi.v1i1.1484. [DOI] [Google Scholar]
- 92.Hornik K, Stinchcombe M, White H. Multilayer feedforward networks are universal approximators. Neur Netw. 1989;2(5):359–366. doi: 10.1016/0893-6080(89)90020-8. [DOI] [Google Scholar]
- 93.Houwen B. Blood film preparation and staining procedures. Clin Lab Med. 2002;22(1):1–14. doi: 10.1016/S0272-2712(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 94.Jaiswal M, Srivastava A, Siddiqui TJ (2019) Machine learning algorithms for anemia disease prediction. In: Recent trends in communication, computing, and electronics, pp 463–469. Springer
- 95.Jones KW (2009) Evaluation of cell morphology and introduction to platelet and white blood cell morphology. Clinical Hematology and Fundamentals of Hemostasis, 93–116
- 96.Khalaf M, Hussain AJ, Keight R, Al-Jumeily D, Fergus P, Keenan R, Tso P. Machine learning approaches to the application of disease modifying therapy for sickle cell using classification models. Neurocomputing. 2017;228:154–164. doi: 10.1016/j.neucom.2016.10.043. [DOI] [Google Scholar]
- 97.Kim K, Kim P, Song J, Park Y (2000) Analyzing blood cell image to distinguish its abnormalities. In: Proceedings of the eighth ACM international conference on multimedia, pp 395–397
- 98.Kimbahune VV, Uke N. Blood cell image segmentation and counting. Int J Eng Sci Technol (IJEST) 2011;3(3):2448. [Google Scholar]
- 99.Kulasekaran S, Sheeba F, Mammen JJ, Saivigneshu B, Mohankumar S (2015) Morphology based detection of abnormal red blood cells in peripheral blood smear images. In: 7th WACBE world congress on bioengineering 2015, pp 57–60. Springer
- 100.Lee H, Chen YPP. Cell morphology based classification for red cells in blood smear images. Pattern Recogn Lett. 2014;49:155–161. doi: 10.1016/j.patrec.2014.06.010. [DOI] [Google Scholar]
- 101.Loddo A, Putzu L, Di Ruberto C, Fenu G (2016) A computer-aided system for differential count from peripheral blood cell images. In: 2016 12th International conference on signal-image technology & internet-based systems (SITIS), pp 112–118. IEEE
- 102.Lotfi M, Nazari B, Sadri S, Sichani NK (2015) The detection of dacrocyte, schistocyte and elliptocyte cells in iron deficiency anemia. In: 2015 2nd International conference on pattern recognition and image analysis (IPRIA), pp 1–5. IEEE
- 103.Lund P, Barnes R. Automated classification of anaemia using image analysis. The Lancet. 1972;300(7775):463–464. doi: 10.1016/S0140-6736(72)91857-0. [DOI] [PubMed] [Google Scholar]
- 104.Mahmood NH, Lim PC, Mazalan SM, Razak MAA. Blood cells extraction using color based segmentation technique. Int J Life Sci Biotechnol Pharma Res. 2013;2(2):2250–3137. [Google Scholar]
- 105.Mahmood NH, Mansor MA. Red blood cells estimation using Hough transform technique. Signal Image Process. 2012;3(2):53. [Google Scholar]
- 106.Maity M, Sarkar P, Chakraborty C (2012) Computer-assisted approach to anemic erythrocyte classification using blood pathological information. In: 2012 Third international conference on emerging applications of information technology, pp 116–121. IEEE
- 107.Maji P, Mandal A, Ganguly M, Saha S (2015) An automated method for counting and characterizing red blood cells using mathematical morphology. In: 2015 Eighth international conference on advances in pattern recognition (ICAPR), pp 1–6. IEEE
- 108.Mao-Jun S, Zhao-bin W, Hong-Juan Z, Yi-de M (2008) A new method for blood cell image segmentation and counting based on pcnn and autowave. In: 2008 3rd International symposium on communications, control and signal processing, pp 6–9. IEEE
- 109.Marzuki NIBC, bin Mahmood NH, bin Abdul Razak MA. Identification of thalassemia disorder using active contour. Indonesian J Electr Eng Comput Sci. 2017;6(1):160–165. doi: 10.11591/ijeecs.v6.i1.pp160-165. [DOI] [Google Scholar]
- 110.Mazalan SM, Mahmood NH, Razak MAA (2013) Automated red blood cells counting in peripheral blood smear image using circular Hough transform. In: 2013 1st International conference on artificial intelligence, modelling and simulation, pp 320–324. IEEE
- 111.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 112.Md Tomari MR, Wan Zakaria WN, et al. (2015) An empirical framework for automatic red blood cell morphology identification and counting ARPN. Journal of Engineering and Applied Sciences 10(2015)
- 113.Mogra M, Bansel A, Srivastava V. Comparative analysis of extraction and detection of RBCs and WBCs using Hough transform and k-means clustering algorithm. Int J Eng Res Gen Sci. 2014;2(5):670–674. [Google Scholar]
- 114.Mohamad AS, Halim NSA, Nordin MN, Hamzah R, Sathar J (2018) Automated detection of human RBC in diagnosing sickle cell anemia with laplacian of gaussian filter. In: 2018 IEEE Conference on systems, process and control (ICSPC), pp 214–217. IEEE
- 115.Mohamad AS, Hamzah R, Mokhtar AS, Sathar J (2017) Sickle cell disease verification via Sobel edge algorithms for image processing. In: 2017 International conference on engineering technology and technopreneurship (ICE2T), pp 1–4. IEEE
- 116.Mundhra D, Cheluvaraju B, Rampure J, Dastidar TR (2017) Analyzing microscopic images of peripheral blood smear using deep learning. In: Deep learning in medical image analysis and multimodal learning for clinical decision support, pp 178–185. Springer
- 117.Navya K, Pradeep G (2018) Lung nodule segmentation using adaptive thresholding and watershed transform. In: 2018 3rd IEEE International conference on recent trends in electronics, information & communication technology (RTEICT), pp 630–633. IEEE
- 118.Nee LH, Mashor MY, Hassan R. White blood cell segmentation for acute leukemia bone marrow images. J Med Imag Health Inform. 2012;2(3):278–284. doi: 10.1166/jmihi.2012.1099. [DOI] [Google Scholar]
- 119.Nithyaa A, Premkumar R, Kanchana D, Krishnan NA (2013) Automated detection and classification of blood diseases. In: Recent advancements in system modelling applications, pp 393–404. Springer
- 120.Parvathy H, Hariharan S, Aruna S. A real time system for the analysis of sickle cell anemia blood smear images using image processing. Int J Innov Res Sci Eng Technol. 2016;5:6200–6207. [Google Scholar]
- 121.Patil DN, Khot UP. Image processing based abnormal blood cells detection. Int J Tech Res Applic. 2015;31:37–43. [Google Scholar]
- 122.Poole DL, Mackworth AK (2010) Artificial intelligence: foundations of computational agents. Cambridge University Press
- 123.Poomcokrak J, Neatpisarnvanit C (2008) Red blood cells extraction and counting. In: The 3rd international symposium on biomedical engineering, pp 199–203
- 124.Prasad K, Winter J, Bhat UM, Acharya RV, Prabhu GK. Image analysis approach for development of a decision support system for detection of malaria parasites in thin blood smear images. J Digit Imaging. 2012;25(4):542–549. doi: 10.1007/s10278-011-9442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prasad MN, Prasad K, Navya K (2018) Color transfer method for efficient enhancement of color images and its application to peripheral blood smear analysis. In: International conference on recent trends in image processing and pattern recognition, pp 134–142. Springer
- 126.Rafael C (1992) Gonzalez, and Richard E. Woods. Digital Image Processing, 793
- 127.Rahmat R, Wulandari F, Faza S, Muchtar M, Siregar I (2018) The morphological classification of normal and abnormal red blood cell using self organizing map. In: IOP Conf. Series: Mater. Sci. Eng, vol 308, p 012015
- 128.Rakshit P, Bhowmik K. Detection of abnormal findings in human rbc in diagnosing sickle cell anaemia using image processing. Proced Technol. 2013;10:28–36. doi: 10.1016/j.protcy.2013.12.333. [DOI] [Google Scholar]
- 129.Rashid NZN, Mashor MY, Hassan R (2015) Unsupervised color image segmentation of red blood cell for thalassemia disease. In: 2015 2nd International conference on biomedical engineering (ICoBE), pp 1–6. IEEE
- 130.Razzak MI, Naz S (2017) Microscopic blood smear segmentation and classification using deep contour aware cnn and extreme machine learning. In: 2017 IEEE Conference on computer vision and pattern recognition workshops (CVPRW), pp 801–807. IEEE
- 131.Revathi T, Jeevitha S. Efficient Watershed based red blood cell segmentation from digital images in sickle cell disease. Int J Sci Eng Appl Sci. 2016;2:300–317. [Google Scholar]
- 132.Rezatofighi S, Roodaki A, Zoroofi R, Sharifian R, Soltanian-Zadeh H (2008) Automatic detection of red blood cells in hematological images using polar transformation and run-length matrix. In: 2008 9th International conference on signal processing, pp 806–809. IEEE
- 133.Ritter N, Cooper J (2007) Segmentation and border identification of cells in images of peripheral blood smear slides. In: Proceedings of the thirtieth Australasian conference on computer science, vol 62, pp 161–169. Australian Computer Society, Inc
- 134.Rodrigues LF, Naldi MC, Mari JF (2016) Morphological analysis and classification of erythrocytes in microscopy images. In: Proceedings of the 2016 Workshop de Visao Computacional, Campo Grande, Brazil, pp 9–11
- 135.Sadafi A, Radolko M, Serafeimidis I, Hadlak S (2018) Red blood cells segmentation: a fully convolutional network approach. In: 2018 IEEE Intl Conf on Parallel & Distributed Processing with Applications, Ubiquitous Computing & Communications, Big Data & Cloud Computing, Social Computing & Networking, Sustainable Computing & Communications (ISPA/IUCC/BDCloud/SocialCom/SustainCom), pp 911–914. IEEE
- 136.Safca N, Popescu D, Ichim L, Elkhatib H, Chenaru O (2018) Image processing techniques to identify red blood cells. In: 2018 22nd International conference on system theory, control and computing (ICSTCC), pp 93–98. IEEE
- 137.Sanap SA, Nagori M, Kshirsagar V (2011) Classification of anemia using data mining techniques. In: International conference on swarm, evolutionary, and memetic computing, pp 113–121. Springer
- 138.Sapna S, Renuka A (2017) Techniques for segmentation and classification of leukocytes in blood smear images-a review. In: 2017 IEEE International conference on computational intelligence and computing research (ICCIC), pp 1–5. IEEE
- 139.Sarrafzadeh O, Dehnavi AM, Rabbani H, Ghane N, Talebi A (2015) Circlet based framework for red blood cells segmentation and counting. In: 2015 IEEE workshop on signal processing systems (SiPS), pp 1–6. IEEE
- 140.Savkare S, Narote S (2015) Blood cell segmentation from microscopic blood images. In: 2015 International conference on information processing (ICIP), pp 502–505. IEEE
- 141.Sethian JA. A fast marching level set method for monotonically advancing fronts. Proc Natl Acad Sci. 1996;93(4):1591–1595. doi: 10.1073/pnas.93.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Setsirichok D, Piroonratana T, Wongseree W, Usavanarong T, Paulkhaolarn N, Kanjanakorn C, Sirikong M, Limwongse C, Chaiyaratana N. Classification of complete blood count and haemoglobin typing data by a C4.5 decision tree, a naive Bayes classifier and a multilayer perceptron for thalassaemia screening. Biomed Signal Process Control. 2012;7(2):202–212. doi: 10.1016/j.bspc.2011.03.007. [DOI] [Google Scholar]
- 143.Shahzad M, Umar AI, Khan MA, Shirazi SH, Khan Z, Yousaf W (2020) Robust method for semantic segmentation of whole-slide blood cell microscopic images. Comput Math Methods Med, 2020 [DOI] [PMC free article] [PubMed]
- 144.Sharif JM, Miswan M, Ngadi M, Salam MSH, bin Abdul Jamil MM (2012) Red blood cell segmentation using masking and Watershed algorithm: a preliminary study. In: 2012 International conference on biomedical engineering (ICoBE), pp 258–262. IEEE
- 145.Sharma V, Rathore A, Vyas G (2016) Detection of sickle cell anaemia and thalassaemia causing abnormalities in thin smear of human blood sample using image processing. In: 2016 International conference on inventive computation technologies (ICICT), vol 3, pp 1–5. IEEE
- 146.Soltanzadeh R, Rabbani H (2010) Classification of three types of red blood cells in peripheral blood smear based on morphology. In: IEEE 10th International conference on signal processing proceedings, pp 707–710. IEEE
- 147.Taherisadr M, Nasirzonouzi M, Baradaran B, Mehdizade A, Shiraz I. New approach to red blood cell classification using morphological image processing. Shiraz E-Med J. 2013;14(1):44–53. [Google Scholar]
- 148.Tomari M, Lias J, Musa R, Wan Zakaria WN, et al. (2015) Development of red blood cell analysis system using NI vision builder AI ARPN. Journal of Engineering and Applied Sciences 10(2015)
- 149.Tomari R, Zakaria WNW, Ngadengon R, Wahab MHA (2015) Red blood cell counting analysis by considering an overlapping constraint Ⓒ 2006-2015 Asian Research Publishing Network (ARPN) 10(3)
- 150.Tran T, Binh Minh L, Lee SH, Kwon KR (2019) Blood cell count using deep learning semantic segmentation. 10.20944/preprints201909.0075.v1
- 151.Tran T, Kwon O, Kwon K, Lee S, Kang K (2018) Blood cell images segmentation using deep learning semantic segmentation. In: 2018 IEEE International conference on electronics and communication engineering (ICECE), pp 13–16. IEEE
- 152.Tyagi M, Saini LM, Dahyia N (2016) Detection of poikilocyte cells in iron deficiency anaemia using artificial neural network. In: 2016 International conference on computation of power, energy information and commuincation (ICCPEIC), pp 108–112. IEEE
- 153.Tyas DA, Ratnaningsih T, Harjoko A, Hartati S (2017) The classification of abnormal red blood cell on the minor thalassemia case using artificial neural network and convolutional neural network. In: Proceedings of the international conference on video and image processing, pp 228–233
- 154.Veluchamy M, Perumal K, Ponuchamy T. Feature extraction and classification of blood cells using artificial neural network. Am J Appl Sci. 2012;9(5):615. doi: 10.3844/ajassp.2012.615.619. [DOI] [Google Scholar]
- 155.Venkatalakshmi B, Thilagavathi K (2013) Automatic red blood cell counting using Hough transform. In: 2013 IEEE Conference on information & communication technologies, pp 267–271. IEEE
- 156.Walliander M, Turkki R, Linder N, Lundin M, Konsti J, Ojansivu V, Meri T, Holmberg V, Lundin J (2013) Automated segmentation of blood cells in giemsa stained digitized thin blood films. In: Diagnostic pathology, vol 8, p. S37. Springer
- 157.Wei X, Cao Y, Fu G, Wang Y. A counting method for complex overlapping erythrocytes-based microscopic imaging. J Innov Opt Health Sci. 2015;8(06):1550033. doi: 10.1142/S1793545815500339. [DOI] [Google Scholar]
- 158.Wheeless LL, Robinson RD, Lapets OP, Cox C, Rubio A, Weintraub M, Benjamin LJ. Classification of red blood cells as normal, sickle, or other abnormal, using a single image analysis feature. Cytometry: J Int Soc Anal Cytol. 1994;17(2):159–166. doi: 10.1002/cyto.990170208. [DOI] [PubMed] [Google Scholar]
- 159.Xu M, Papageorgiou DP, Abidi SZ, Dao M, Zhao H, Karniadakis GE. A deep convolutional neural network for classification of red blood cells in sickle cell anemia. PLoS Comput Biol. 2017;13(10):e1005746. doi: 10.1371/journal.pcbi.1005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu M, Yang J, Zhao H (2017) A multivariate shape quantification approach for sickle red blood cell in patient-specific microscopy image data. In: Ninth international conference on digital image processing (ICDIP 2017), vol 10420, p 104203W. International Society for Optics and Photonics
- 161.Yeldhos M (2018) Red blood cell counter using embedded image processing techniques. Research Reports 2(2018)
- 162.Zahir S, Chowdhury R, Payne GW (2006) Automated assessment of erythrocyte disorders using artificial neural network. In: 2006 IEEE International symposium on signal processing and information technology, pp 776–780. IEEE
- 163.Zhang M, Li X, Xu M, Li Q (2018) Rbc semantic segmentation for sickle cell disease based on deformable U-Net. In: International conference on medical image computing and computer-assisted intervention, pp 695–702. Springer