Abstract
The risk factors for non-alcoholic fatty liver disease (NAFLD) progression are not completely known. Porphyromonas gingivalis infection is a risk factor for systemic diseases. We investigated the association of P. gingivalis infection with the risk of non-alcoholic steatohepatitis progression. Here, hematological tests, periodontal examination, and saliva collection were performed for 164 patients with NAFLD. P. gingivalis was identified in saliva using polymerase chain reaction. Hepatic steatosis and stiffness were evaluated using vibration-controlled transient elastography (VCTE) and magnetic resonance imaging. In patients with NAFLD, P. gingivalis positivity (P. gingivalis ratio ≥ 0.01%) in saliva correlated with liver stiffness determined using magnetic resonance elastography (MRE; p < 0.0001). A P. gingivalis ratio of 0.01% corresponds to 100,000 cells/mL and indicates the proportion of P. gingivalis in the total number of bacteria in the oral cavity. Patients with NAFLD and advanced fibrosis on MRE showed significantly elevated endotoxin activity; those who had > 10 periodontal pockets with depths ≥ 4 mm had significantly increased hepatic stiffness on both VCTE and MRE.
Subject terms: Dental diseases, Gastroenterology, Saliva
Introduction
Non-alcoholic fatty liver disease (NAFLD) is now recognized as one of the most common chronic liver diseases1. It is common knowledge that people with high alcohol consumption rates develop fatty livers; however, recent studies have found that fatty liver also occurs in individuals who consume little or no alcohol2. NAFLD is an umbrella term for simple fatty liver (in which fat is deposited in the liver with little or no inflammation) and non-alcoholic steatohepatitis (NASH), which is a risk factor for fibrosis and liver cancer. It is believed that insulin resistance, oxidative stress, inflammatory cytokines, and other factors contribute to the progression of simple fatty liver to NASH, which finally leads to cirrhosis and hepatocellular carcinoma. Thus, NAFLD is an important disease, affecting approximately 30% of the population in Japan3,4. It has been reported that NAFLD is closely associated with the constituents of metabolic syndrome, such as obesity5, type 2 diabetes mellitus6, hypertension7, and hyperlipidemia8. In addition, this disease has been noted to co-occur with other diseases such as sleep apnea syndrome9 and hypothyroidism10. It is clear that diet modification is effective in patients with NAFLD11–15; however, no gold standard has been established for drug therapy16–21.
Periodontal disease refers to chronic inflammation of the supporting tissues of teeth. It is associated with many metabolic diseases such as diabetes mellitus22, cardiovascular disease23, and NAFLD24. In animal studies, periodontal disease has been found to induce increased blood concentrations of proinflammatory molecules and increase the oxidative stress that occurs because of these mediators25–34. These studies support the hypothesis that periodontal disease may be a risk factor for NAFLD. Animal studies have demonstrated a relationship between Porphyromonas gingivalis and fatty liver disease35, and human studies have shown the involvement of P. gingivalis in NAFLD progression36; however, the relationship between NAFLD and periodontal disease in humans remains unclear.
We hypothesized that deep periodontal pockets and P. gingivalis bacterial content would be associated with hepatic fibrogenesis. Therefore, this study aimed to investigate the relationship between the pathogenesis of NAFLD and intraoral findings.
Results
According to the sample size calculation, 164 patients who met the eligibility criteria were identified and included between August 2015 and April 2019.
Of the 164 participants, P. gingivalis represented ≥ 0.01% and < 0.01% of all bacteria in the saliva of 45 and 119 patients, respectively.
A P. gingivalis ratio of 0.01% corresponds to approximately 100,000 cells/mL and indicates the ratio of P. gingivalis to the total number of bacteria in the oral cavity quantified by performing polymerase chain reaction (PCR) of the DNA obtained from saliva samples37,38. The baseline demographics and disease characteristics of the patient groups with a P. gingivalis ratio ≥ 0.01% and a P. gingivalis ratio < 0.01% in saliva were almost similar (Table 1). Liver stiffness measured by magnetic resonance elastography (MRE) was significantly higher in the group with a P. gingivalis ratio ≥ 0.01% than in the group with a P. gingivalis ratio < 0.01% in saliva (3.0 ± 1.3 vs. 3.7 ± 1.0, p = 0.0064, Table 1). In addition, there was a significant difference between these groups in terms of the level of the inflammatory marker, ferritin (215 ± 198 vs. 282 ± 142, p = 0.04, Table 1).
Table 1.
Baseline characteristics of the trial population.
| Variables | All (n = 164) | P. gingivalis < 0.01% (n = 119) | P. gingivalis ≥ 0.01% (n = 45) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 57 (15) | 55 (15) | 60 (15) | 0.043 |
| Male (%) | 92 (56) | 70 (59) | 22 (49) | 0.255 |
| Comorbidities | ||||
| Type 2 diabetes (%) | 56 (34) | 41 (34) | 15 (33) | 0.893 |
| Hyperlipidemia (%) | 59 (36) | 46 (39) | 13 (29) | 0.248 |
| Hypertension (%) | 57 (35) | 43 (36) | 14 (31) | 0.55 |
| Hyperuricemia (%) | 15 (9) | 9 (8) | 6 (13) | 0.255 |
| Cardiovascular disease (%) | 6 (4) | 6 (5) | 0 (0) | 0.126 |
| Thyroid disease (hypothyroidism) (%) | 3 (2) | 2 (2) | 1 (2) | 0.819 |
| Concomitant drug use | ||||
| Anti-diabetic (%) | 57 (35) | 41 (34) | 16 (35) | 0.896 |
| DPP4-inhibitor (%) | 39 (24) | 26 (22) | 13 (29) | 0.348 |
| Metformin (%) | 31 (19) | 21 (18) | 10 (22) | 0.507 |
| Sulfonylurea (%) | 17 (10) | 11 (9) | 6 (13) | 0.446 |
| Anti-lipidemic (%) | 56 (34) | 43 (36) | 13 (29) | 0.386 |
| Anti-hypertensive (%) | 56 (34) | 42 (35) | 14 (31) | 0,617 |
| Anti-platelet (%) | 0 (0) | 0 (0) | 0 (0) | |
| Laxatives, regular use (%) | 0 (0) | 0 (0) | 0 (0) | |
| Liver imaging | ||||
| CAP on VCTE (dB/m) | 305 (60) | 310 (56) | 295 (69) | 0.168 |
| LSM on VCTE (kPa) | 7.7 (4.3) | 7.3 (4.4) | 8.6 (3.8) | 0.114 |
| Liver fat content on MRI-PDFF (%) | 12.4 (6.6) | 13.0 (6.6) | 10.8 (6.4) | 0.091 |
| Liver stiffness on MRE (kPa) | 3.2 (1.2) | 3.0 (1.3) | 3.7 (1.0) | 0.0064 |
| Endotoxin | ||||
| EAA (× 102) | 0.1 (0.09) | 0.1 (0.08) | 0.2 (0.08) | 0.001 |
| Metabolic factors | ||||
| Weight (kg) | 72.6 (15.6) | 73 (16) | 71 (15) | 0.473 |
| BMI (kg/m2) | 27 (5.3) | 27.3 (5.0) | 26.1 (6.0) | 0.177 |
| Glucose (mg/dL) | 109 (35) | 112 (38) | 101 (26) | 0.093 |
| Insulin (μU/mL) | 20 (28) | 21 (31) | 15 (18) | 0.183 |
| HOMA-R | 6 (10) | 7 (11) | 4 (8) | 0.2 |
| Liver function | ||||
| Platelet count | 22 (8) | 22 (9) | 20 (4) | 0.182 |
| AST (U/L) | 37 (21) | 35 (21) | 42 (22) | 0.056 |
| ALT (U/L) | 50 (38) | 48 (40) | 54 (30) | 0.434 |
| GGT (U/L) | 77 (97) | 73 (69) | 86 (147) | 0.447 |
| ALP (U/L) | 241 (91) | 238 (88) | 250 (99) | 0.476 |
| T.Bil (mg/dL) | 1.2 (4.8) | 1.3 (5.7) | 0.7 (0.3) | 0.428 |
| Lipids | ||||
| Tcho (mg/dL) | 198 (40) | 199 (38) | 196 (44) | 0.591 |
| LDL-C (mg/dL) | 119 (85) | 121 (98) | 112 (35) | 0.56 |
| HDL-C (mg/dL) | 43 (82) | 40 (95) | 50 (15) | 0.459 |
| TG (mg/dL) | 182 (130) | 189 (143) | 162 (86) | 0.244 |
| Inflammatory markers | ||||
| hsCRP (mg/L) | 0.2 (0.3) | 0.2 (0.4) | 0.1 (0.2) | 0.512 |
| Ferritin (ng/mL) | 233 (187) | 215 (198) | 282 (142) | 0.042 |
| Fibrosis marker | ||||
| Type IV collagen 7s (ng/mL) | 4.2 (1.0) | 4.2 (1.0) | 4.3 (1.0) | 0.483 |
| Periodontal assessment | ||||
| PPD (mm) | 2.5 (0.5) | 2.4 (0.5) | 2.7 (0.5) | 0.0091 |
| CAL (mm) | 2.5 (0.5) | 2.4 (0.5) | 2.7 (0.5) | 0.0091 |
| BOP (site) | 23 (30) | 19 (28) | 32 (31) | 0.0118 |
| Stability of teeth | 0 | 0 | 0 | |
| PPD ≥ 4 mm (site) | 14 (23) | 12 (22) | 22 (23) | 0.01 |
| Oral bacteria, P. gingivalis (× 106 cells/mL) | 0.4 (1.0) | 0.2 (0.5) | 1.2 (1.6) | < 0.0001 |
| Antibody titer for P. gingivalis FDC381 | 0.6 (1.8) | 0.5 (1.9) | 0.8 (1.4) | 0.28 |
| Antibody titer for P. gingivalis SU63 | 0.9 (2.0) | 0.5 (1.5) | 2.0 (2.6) | < 0.0001 |
| IMT mean (R) | 0.9 (0.3) | 0.9 (0.3) | 1.0 (0.3) | 0.0402 |
| IMT mean (L) | 0.9 (0.3) | 0.9 (0.2) | 1.0 (0.3) | 0.008 |
| IMT max (R) | 1.1 (0.5) | 1.1 (0.5) | 1.2 (0.4) | 0.239 |
| IMT max (L) | 1.1 (0.5) | 1.1 (0.6) | 1.2 (0.5) | 0.346 |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BOP bleeding on probing, CAL clinical attachment level, CAP controlled attenuation parameter, DPP4 dipeptidyl peptidase-4, EAA endotoxin activity assay, GGT gamma-glutamyl transferase, HDL-C high-density lipoprotein cholesterol, HOMA-R homeostasis model assessment-estimated insulin resistance, hsCRP high-sensitivity C-reactive protein, IMT (R: right, L: left) intima-media thickness (R, L), LDL-C low-density lipoprotein cholesterol, LSM liver stiffness measurement, MRE magnetic resonance elastography, MRI magnetic resonance imaging, PDFF proton density fat fraction, PPD periodontal pocket depth, T.Bil total bilirubin, Tcho total cholesterol, TG triglycerides, VCTE vibration-controlled transient elastography.
aData are reported as means (SDs) or numbers (percentages). The p-values were determined using the t-test.
The baseline periodontal pocket depth (PPD) [P. gingivalis in saliva ≥ 0.01% group, mean (standard deviation, SD): 2.7 (0.5) mm; P. gingivalis in saliva < 0.01% group, 2.4 (0.5) mm], PPD ≥ 4 mm [%; P. gingivalis in saliva ≥ 0.01% group, 43 (96%) patients; P. gingivalis in saliva < 0.01% group, 119 (73%) patients], and antibody titer for P. gingivalis SU63 [P. gingivalis in saliva ≥ 0.01% group mean (SD): 2.0 (2.6); P. gingivalis in saliva < 0.01% group: 0.5 (1.5)] indicated that this study population had a stable periodontal disease condition (Table 1, Fig. 1).
Figure 1.
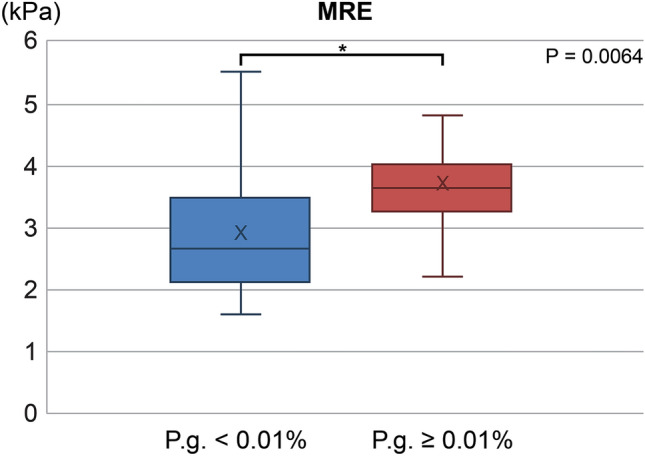
Liver stiffness in groups of patients with < 0.01% and ≥ 0.01% P. gingivalis in saliva. MRE, magnetic resonance elastography.
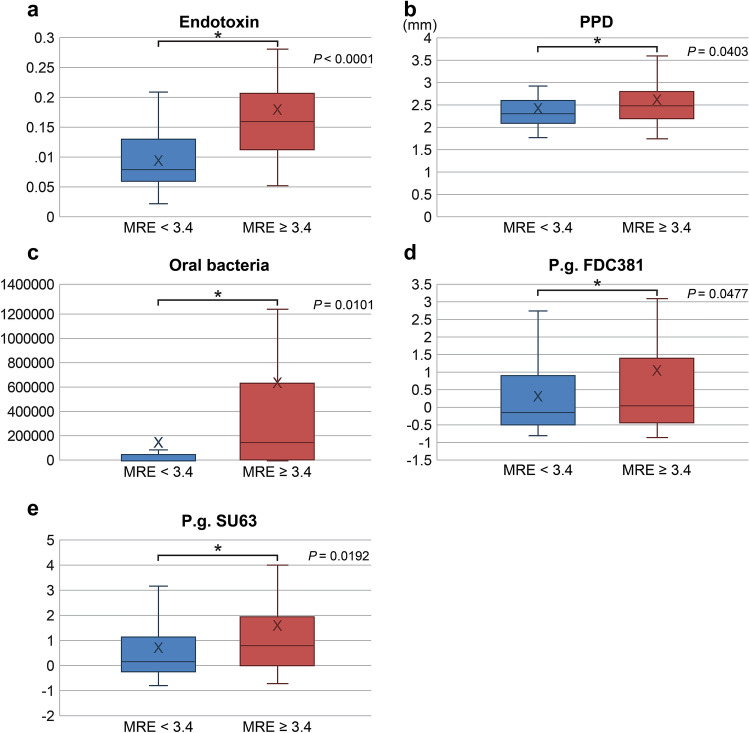
The high elasticity group (MRE ≥ 3.4 kPa) included 64 participants, and the low elasticity group (MRE < 3.4 kPa) included 100 participants. We found significant differences between these two groups in terms of the presence of oral P. gingivalis (6.0 ± 1.3 vs. 0.2 ± 0.5 × 106 cells/mL, respectively; p < 0.01), results of endotoxin activity assay (0.2 ± 0.12 vs. 0.1 ± 0.05, respectively; p < 0.0001), and antibody titers for P. gingivalis FDC381 (1.0 ± 2.4 vs. 0.3 ± 1.1, respectively; p < 0.0477) and SU63 (1.6 ± 2.6 vs. 0.7 ± 1.7, respectively; p < 0.019). These data suggest that an increased liver stiffness (determined by MRE) is associated with a high number of P. gingivalis bacteria in the oral cavity, increased endotoxin levels, and high antibody titers for P. gingivalis FDC381 and SU63 (Table 2, Fig. 2a–e).
Table 2.
Baseline characteristics of the trial population.
| Variables | All (n = 164) | MRE < 3.4 kPa (n = 100) | MRE ≥ 3.4 kPa (n = 64) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 57 (15) | 56 (14) | 60 (14) | |
| Male (%) | 92 (56) | 57 (57) | 25 (39) | |
| Comorbidities | ||||
| Type 2 diabetes (%) | 56 (34) | 33 (33) | 23 (36) | |
| Hyperlipidemia (%) | 59 (36) | 38 (38) | 21 (31) | |
| Hypertension (%) | 57 (35) | 35 (35) | 22 (34) | |
| Hyperuricemia (%) | 15 (9) | 9 (9) | 6 (9) | |
| Cardiovascular disease (%) | 6 (4) | 3 (3) | 3 (0) | |
| Thyroid disease (hypothyroidism) (%) | 3 (2) | 1 (1) | 2 (0) | |
| Concomitant drug use | ||||
| Anti-diabetic (%) | 57 (35) | 32 (32) | 25 (35) | |
| DPP4-inhibitor (%) | 39 (24) | 22 (22) | 17 (27) | |
| Metformin (%) | 31 (19) | 21 (21) | 10 (16) | |
| Sulfonylurea (%) | 17 (10) | 10 (10) | 7 (11) | |
| Anti-lipidemic (%) | 56 (34) | 37 (37) | 19 (30) | |
| Anti-hypertensive (%) | 56 (34) | 34 (34) | 22 (34) | |
| Anti-platelet (%) | 0 (0) | 0 (0) | 0 (0) | |
| Laxatives, regular use (%) | 0 (0) | 0 (0) | 0 (0) | |
| Liver imaging | ||||
| CAP on VCTE (dB/m) | 305 (60) | 309 (57) | 297 (67) | |
| LSM on VCTE (kPa) | 7.7 (4.3) | 6.3 (3.2) | 10.9 (4.8) | < 0.0001 |
| Liver fat content on MRI-PDFF (%) | 12.4 (6.6) | 12.5 (7.2) | 12.2 (5.5) | |
| Liver stiffness on MRE (kPa) | 3.2 (1.2) | 2.4 (0.5) | 4.3 (1.3) | < 0.0001 |
| Endotoxin | ||||
| EAA (× 102) | 0.1 (0.09) | 0.1 (0.05) | 0.2 (0.12) | < 0.0001 |
| Metabolic factors | ||||
| Weight (kg) | 72.6 (15.6) | 75 (15) | 69 (15) | 0.022 |
| BMI (kg/m2) | 27 (5.3) | 27.9 (4.9) | 26.6 (4.5) | |
| Glucose (mg/dL) | 109 (35) | 113 (33) | 111 (44) | |
| Insulin (μU/mL) | 20 (28) | 20 (26) | 18 (18) | |
| HOMA-R | 6 (10) | 6 (8) | 6 (11) | |
| Liver function | ||||
| Platelet count | 22 (8) | 23 (9) | 19 (6) | 0.0239 |
| AST (U/L) | 37 (21) | 36 (17) | 46 (24) | 0.0065 |
| ALT (U/L) | 50 (38) | 54 (44) | 55 (27) | |
| GGT (U/L) | 77 (97) | 71 (70) | 100 (145) | |
| ALP (U/L) | 241 (91) | 236 (58) | 268 (135) | |
| T.Bil (mg/dL) | 1.2 (4.8) | 1.5 (7.0) | 0.8 (0.6) | |
| Lipids | ||||
| Tcho (mg/dL) | 198 (40) | 199 (39) | 202 (40) | |
| LDL-C (mg/dL) | 119 (85) | 127 (119) | 115 (29) | |
| HDL-C (mg/dL) | 43 (82) | 34 (117) | 51 (20) | |
| TG (mg/dL) | 182 (130) | 192 (140) | 175 (99) | |
| Inflammatory markers | ||||
| hsCRP (mg/L) | 0.2 (0.3) | 0.2 (0.4) | 0.2 (0.3) | |
| Ferritin (ng/mL) | 233 (187) | 223 (207) | 271 (147) | |
| Fibrosis marker | ||||
| Type IV collagen 7s (ng/mL) | 4.2 (1.0) | 4.0 (0.7) | 4.9 (1.2) | < 0.0001 |
| Periodontal assessment | ||||
| PPD (mm) | 2.5 (0.5) | 2.4 (0.5) | 2.6 (0.6) | 0.0403 |
| CAL (mm) | 2.5 (0.5) | 2.4 (0.5) | 2.6 (0.6) | 0.0403 |
| BOP (site) | 23 (30) | 13 (18) | 38 (40) | < 0.0001 |
| Stability of teeth | 0 | 0 | 0 | |
| PPD ≥ 4 mm (site) | 10 (14) | 6 (12) | 16 (17) | 0.0004 |
| Oral bacteria, P. gingivalis (× 106 cells/mL) | 0.4 (1.0) | 0.2 (0.5) | 6.0 (1.3) | 0.0101 |
| Antibody titer for P. gingivalis FDC381 | 0.6 (1.8) | 0.3 (1.1) | 1.0 (2.4) | 0.0477 |
| Antibody titer for P. gingivalis SU63 | 0.9 (2.0) | 0.7 (1.7) | 1.6 (2.6) | 0.0192 |
| IMT mean (R) | 0.9 (0.3) | 0.8 (0.2) | 1.1 (0.3) | < 0.0001 |
| IMT mean (L) | 0.9 (0.3) | 0.8 (0.2) | 1.1 (0.3) | < 0.0001 |
| IMT max (R) | 1.1 (0.5) | 1.0 (0.4) | 1.4 (0.5) | < 0.0001 |
| IMT max (L) | 1.1 (0.5) | 1.0 (0.5) | 1.5 (0.6) | < 0.0001 |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BOP bleeding on probing, CAP controlled attenuation parameter, CAL clinical attachment level, DPP4 dipeptidyl peptidase-4, EAA endotoxin activity assay, GGT gamma-glutamyl transferase, HDL-C high-density lipoprotein cholesterol, HOMA-R homeostasis model assessment-estimated insulin resistance, hsCRP high-sensitivity C-reactive protein, IMT (R: right, L: left) intima-media thickness (R, L), LDL-C low-density lipoprotein cholesterol, LSM liver stiffness measurement, MRE magnetic resonance elastography, MRI magnetic resonance imaging, PDFF proton density fat fraction, PPD periodontal pocket depth, T.Bil total bilirubin, Tcho total cholesterol, TG triglycerides, VCTE vibration-controlled transient elastography.
aData are reported as means (SDs) or numbers (percentages). P-values were determined using the t-test.
Figure 2.
Endotoxin activity (a), PPD (b), periodontal disease-causing bacteria, P. gingivalis (c), antibody titer for P. gingivalis FDC381 (d), and antibody titer for P. gingivalis SU63 (e) in the groups with MRE < 3.4 kPa and MRE ≥ 3.4 kPa. MRE magnetic resonance elastography, PPD periodontal pocket depth.
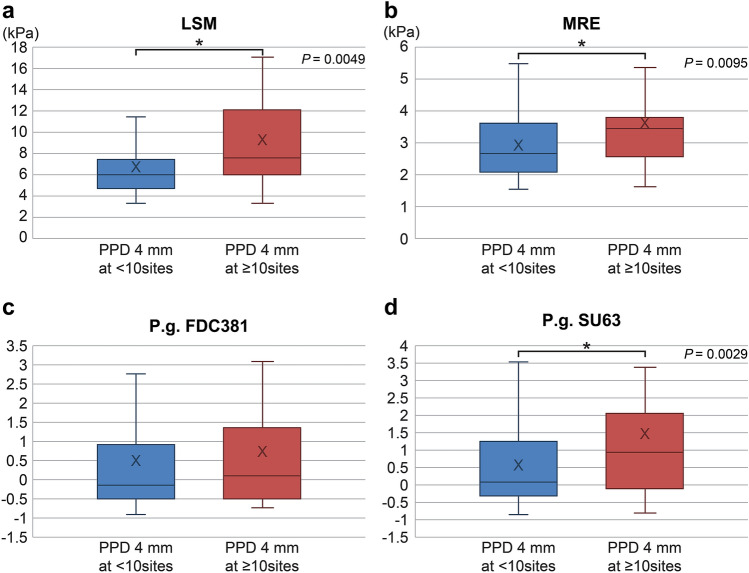
Among the 164 study participants, 52 and 112 had less than 10 (3 ± 3 sites) and more than 10 (26 ± 17 sites) periodontal pockets with depths ≥ 4 mm, respectively. The liver stiffness values were significantly higher in the group with > 10 PPD sites than in the corresponding group with < 10 PPD sites (3.6 ± 1.5 vs. 3.0 ± 1.0 kPa, respectively; p < 0.0095; Table 3, Fig. 3a–d). The mean of PPD ≥ 4 mm was 4.0 mm (± 0.8). Moreover, there were significant differences between these groups regarding the periodontal conditions. These data indicate that liver stiffness increases in case of a greater number of deep periodontal pockets.
Table 3.
Baseline characteristics of the study population.
| Variables | All (n = 164) | PPD ≥ 4 mm at < 10 sites (n = 112) | PPD ≥ 4 mm at ≥ 10 sites (n = 52) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 57 (15) | 57 (15) | 56 (13) | |
| Male (%) | 92 (56) | 66 (59) | 26 (50) | |
| Comorbidities | ||||
| Type 2 diabetes (%) | 56 (34) | 36 (32) | 20 (38) | |
| Hyperlipidemia (%) | 59 (36) | 42 (38) | 17 (33) | |
| Hypertension (%) | 57 (35) | 40 (36) | 17 (33) | |
| Hyperuricemia (%) | 15 (9) | 10 (9) | 5 (10) | |
| Cardiovascular disease (%) | 6 (4) | 5 (4) | 1 (2) | |
| Thyroid disease (hypothyroidism) (%) | 3 (2) | 1 (1) | 2 (4) | |
| Concomitant drug use | ||||
| Anti-diabetic (%) | 57 (35) | 37 (33) | 20 (38) | |
| DPP4-inhibitor (%) | 39 (24) | 24 (22) | 15 (29) | |
| Metformin (%) | 31 (19) | 20 (18) | 11 (21) | |
| Sulfonylurea (%) | 17 (10) | 10 (9) | 7 (13) | |
| Anti-lipidemic (%) | 56 (34) | 40 (36) | 16 (31) | |
| Anti-hypertensive (%) | 56 (34) | 40 (36) | 16 (31) | |
| Anti-platelet (%) | 0 (0) | 0 (0) | 0 (0) | |
| Laxatives, regular use (%) | 0 (0) | 0 (0) | 0 (0) | |
| Liver imaging | ||||
| CAP on VCTE (dB/m) | 305 (60) | 305 (56) | 304 (68) | |
| LSM on VCTE (kPa) | 7.7 (4.3) | 7.0 (3.9) | 9.2 (4.8) | 0.0049 |
| Liver fat content on MRI-PDFF (%) | 12.4 (6.6) | 12.4 (6.8) | 12.1 (6.3) | |
| Liver stiffness on MRE (kPa) | 3.2 (1.2) | 3.0 (1.0) | 3.6 (1.5) | 0.0095 |
| Endotoxin | ||||
| EAA (× 102) | 0.1 (0.09) | 0.1 (0.05) | 0.2 (0.13) | < 0.0001 |
| Metabolic factors | ||||
| Weight (kg) | 72.6 (15.6) | 72 (16) | 72 (16) | |
| BMI (kg/m2) | 27 (5.3) | 27.0 (4.9) | 26.7 (6.1) | |
| Glucose (mg/dL) | 109 (35) | 109 (32) | 106 (40) | |
| Insulin (μU/mL) | 20 (28) | 21 (32) | 17 (19) | |
| HOMA-R | 6 (10) | 6 (11) | 6 (11) | |
| Liver function | ||||
| Platelet count | 22 (8) | 22 (9) | 21 (6) | |
| AST (U/L) | 37 (21) | 38 (22) | 35 (19) | |
| ALT (U/L) | 50 (38) | 51 (40) | 45 (28) | |
| GGT (U/L) | 77 (97) | 80 (109) | 65 (54) | |
| ALP (U/L) | 241 (91) | 256 (102) | 231 (32) | |
| T.Bil (mg/dL) | 1.2 (4.8) | 1.3 (5.8) | 0.8 (0.6) | |
| Lipids | ||||
| Tcho (mg/dL) | 198 (40) | 201 (39) | 193 (43) | |
| LDL-C (mg/dL) | 119 (85) | 124 (101) | 108 (31) | |
| HDL-C (mg/dL) | 43 (82) | 40 (98) | 49 (19) | |
| TG (mg/dL) | 182 (130) | 185 (135) | 178 (126) | |
| Inflammatory markers | ||||
| hsCRP (mg/L) | 0.2 (0.3) | 0.2 (0.4) | 0.2 (0.3) | |
| Ferritin (ng/mL) | 233 (187) | 228 (200) | 241 (151) | |
| Fibrosis marker | ||||
| Type IV collagen 7s (ng/mL) | 4.2 (1.0) | 4.2 (0.9) | 4.4 (1.2) | |
| Periodontal assessment | ||||
| PPD (mm) | 2.5 (0.5) | 2.3 (0.3) | 3.0 (0.5) | < 0.0001 |
| CAL (mm) | 2.5 (0.5) | 2.3 (0.3) | 3.0 (0.5) | < 0.0001 |
| BOP (site) | 23 (30) | 12 (15) | 47 (40) | < 0.0001 |
| Stability of teeth | 0 | 0 | 0 | |
| PPD ≥ 4 mm (site) | 10 (14) | 3 (3) | 26 (17) | < 0.0001 |
| Oral bacteria, P. gingivalis (× 106 cells/mL) | 0.4 (1.0) | 0.1 (0.3) | 0.9 (1.6) | < 0.0001 |
| Antibody titer for P. gingivalis FDC381 | 0.6 (1.8) | 0.5 (1.5) | 0.8 (2.4) | |
| Antibody titer for P. gingivalis SU63 | 0.9 (2.0) | 0.6 (1.3) | 1.5 (2.5) | 0.0029 |
| IMT mean (R) | 0.9 (0.3) | 0.8 (0.2) | 1.2 (0.2) | < 0.0001 |
| IMT mean (L) | 0.9 (0.3) | 0.8 (0.2) | 1.1 (0.3) | < 0.0001 |
| IMT max (R) | 1.1 (0.5) | 1.0 (0.4) | 1.5 (0.4) | < 0.0001 |
| IMT max (L) | 1.1 (0.5) | 1.0 (0.5) | 1.5 (0.5) | < 0.0001 |
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BOP bleeding on probing, CAP controlled attenuation parameter, CAL clinical attachment level, DPP4 dipeptidyl peptidase-4, EAA endotoxin activity assay, GGT gamma-glutamyl transferase, HDL-C high-density lipoprotein cholesterol, HOMA-R homeostasis model assessment-estimated insulin resistance, hsCRP high-sensitivity C-reactive protein, IMT (R: right, L: left) intima-media thickness (R, L), LDL-C low-density lipoprotein cholesterol, LSM liver stiffness measurement, MRE magnetic resonance elastography, MRI magnetic resonance imaging, PDFF proton density fat fraction, PPD periodontal pocket depth, T.Bil total bilirubin, Tcho total cholesterol, TG triglycerides, VCTE vibration-controlled transient elastography.
aData are reported as means (SDs) or numbers (percentages). The p-values were determined using the t-test.
Figure 3.
LSM (a), MRE (b), antibody titer for P. gingivalis FDC381 (c), and antibody titer for P. gingivalis SU63 (d) in patients with periodontal pockets ≥ 4 mm at a depth of < 10 mm or ≥ 10 sites. LSM liver stiffness measurement, MRE magnetic resonance elastography, PPD periodontal pocket depth.
The results of logistic regression analysis using MRE ≥ 3.4 kPa as the dependent variable for all participants are shown in Table 4. The prevalence of NAFLD in advanced fibrosis was associated with weight [odds ratio (OR) = 0.948; p = 0.011], bleeding on probing (BOP) (OR = 1.045; p = 0.001), P. gingivalis ≥ 0.01 (OR = 4.05; p = 0.04), intima-media thickness (IMT) mean (OR = 1.21; p = 0.03), and PPD (OR = 2.045; p = 0.026).
Table 4.
Factors associated with NAFLD in advanced fibrosis.
| Covariate | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Endotoxin | 1.050 | 0.946–1.166 | 0.337 |
| Weight (kg) | 0.948 | 0.907–0.991 | 0.011 |
| Age | 0.998 | 0.951–1.048 | 0.954 |
| Platelet count (× 104/μL) | 0.977 | 0.900–1.060 | 0.523 |
| AST (U/L) | 1.013 | 0.984–1.044 | 0.341 |
| Type VII collagen 7s (ng/mL) | 1.705 | 0.889–3.271 | 0.098 |
| BOP | 1.045 | 1.013–1.079 | 0.001 |
| P. gingivalis ≥ 0.01% | 4.05 | 1.056–15.52 | 0.04 |
| IMT mean | 1.21 | 1.056–1.387 | 0.003 |
| PPD (mm) | 2.045 | 0.875–1.393 | 0.026 |
AST aspartate aminotransferase, BOP bleeding on probing, IMT intima-media thickness, NAFLD non-alcoholic fatty liver disease, PPD periodontal pocket depth.
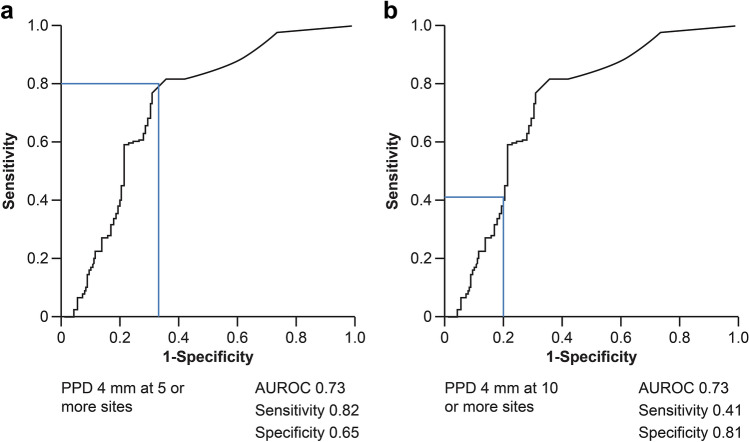
We also constructed receiver operating characteristic curves for patients using at least 0.01% P. gingivalis in saliva and the number of periodontal pockets with depths ≥ 4 mm as independent variables. When periodontal pockets with depths ≥ 4 mm were observed in at least five sites, the area under the receiver operating characteristic curve, sensitivity, and specificity were 0.73, 0.82, and 0.65, respectively. Similarly, area under the curve of 0.73, sensitivity of 0.41, and specificity of 0.81 were obtained in case the number of periodontal pockets with depths ≥ 4 mm were greater than or equal to 10 (Fig. 4a,b).
Figure 4.
AUROC curves for patients with PPDs ≥ 4 mm at ≥ 5(a) and ≥ 10 (b) sites. AUROC area under the receiver operating characteristic, PPD periodontal pocket depth.
Patients with NAFLD who had > 10 periodontal pockets with depths ≥ 4 mm on periodontal examination had significantly increased liver stiffness on both vibration-controlled transient elastography (VCTE) and MRE. Furthermore, in patients with liver stiffness ≥ 3.4 kPa, there were significant differences in salivary and serum P. gingivalis antibody levels, which may have been related to P. gingivalis’ endotoxin.
Discussion
This is a study to demonstrate that the association between NAFLD pathology (especially liver stiffness), P. gingivalis bacteria contents, and oral findings (PPD). This observational study examining the relationship between periodontal disease and NAFLD pathogenesis produced three major findings. First, it demonstrated that the group with ≥ 0.01% P. gingivalis in saliva had a higher MRE-determined liver stiffness than the group with < 0.01% P. gingivalis in saliva. P. gingivalis migrates relatively easily from the oral cavity into the blood circulation39,40; therefore, its endotoxin (present in the oral cavity) can reach the liver41.
Second, it found that the group with MRE-determined liver stiffness values of ≥ 3.4 kPa had higher serum P. gingivalis FDC381 and SU63 antibody levels. P. gingivalis is an obligate anaerobic gram-negative bacillus with strong adhesion; therefore, it forms a biofilm with other gram-negative bacteria associated with periodontal disease. In addition to producing a proteolytic enzyme called gingipains, P. gingivalis expresses lipopolysaccharides (which are endotoxins of the outer bacterial membrane) and is an anaerobic bacterium associated with periodontal disease progression. Inflammation caused by the cytokines and proteins produced during P. gingivalis infection is associated with liver stiffness42,43. Furthermore, the histological lesions in NASH are unevenly distributed throughout the liver parenchyma; therefore, liver biopsy errors can result in substantial stratification and staging inaccuracies44. We consider it important to perform non-invasive, safe, low-cost, and short-term clinical trials as proof-of-concept studies. Many studies have reported that the steatosis grade could be evaluated using the controlled attenuation parameter45–50 (which is based on the properties of ultrasonographic signals acquired with VCTE) or magnetic resonance imaging (MRI) proton density fat fraction51,52 (which is an MRI-based method for quantitatively assessing hepatic steatosis and is available for several types of MRI scanners). Moreover, VCTE and MRE have excellent diagnostic abilities for steatosis and fibrosis in patients with NAFLD53,54. We consider that non-invasive evaluation of the pathogenesis of NAFLD using VCTE and MRI may replace invasive methods, such as liver biopsy. For this reason, we included non-invasive methods for assessing NASH/NAFLD progression as secondary endpoints and compared the results with those derived from liver biopsies.
Third, the group with ≥ 10 periodontal pockets with depths ≥ 4 mm had increased liver stiffness compared to the group with < 10 periodontal pockets with depths ≥ 4 mm. The presence of more than 10 periodontal pockets with depths > 4 mm suggests high morbidity in patients with periodontal disease55. The factor involved in PPD and liver stiffness in this study may have been age, and not sex. With increasing age, the prevalence of periodontal disease rises, and periodontal pockets become deeper56. However, the average age of the patients was 53.0 years, and age was not associated with the other examined factors in this study.
Inflammation caused by the cytokines and proteins produced in response to P. gingivalis infection is associated with liver stiffness42,43. Therefore, the amount of bacteria in the oral cavity and serum antibody titers were examined in this cross-sectional study, and the relationship between periodontal disease and liver stiffness in patients with NAFLD was evaluated.
NAFLD is common in patients with metabolic abnormalities, such as type 2 diabetes and obesity; its prevalence is rapidly increasing globally with the growing obese population5. The main risk associated with this disease is that it progresses from liver cirrhosis to liver cancer over a long time without manifestation of accompanying subjective symptoms, and mortality due to cerebral or myocardial infarction is also high. It is thought that the pathology in patients with NASH progresses as periodontopathic bacteria enter the blood, leading to inflammation in the fatty liver. Therefore, it is necessary to actively interfere with the link between periodontal disease and NAFLD.
The strength of this study lies in the use of non-invasive MRI (in place of liver biopsy) to assess changes in liver fat content. Importantly, this non-invasive method revealed a seemingly mutual relationship between P. gingivalis and liver stiffness.
The study has a certain limitation. It would be important to differentiate NASH from NAFLD in the patients enrolled in this study; however, currently, biopsy is not used to diagnose and differentiate liver tissue.
In summary, patients with NAFLD who had P. gingivalis infection and deeper periodontal pockets had higher liver stiffness values than those who did not have periodontal disease.
Furthermore, the serum antibody titers for P. gingivalis FDC381 and SU63 were high in the patient group with increased liver stiffness, and logistic regression analysis suggested that periodontal disease is associated with liver stiffness. These results indicate that periodontal disease may be associated with NAFLD. As this was a cross-sectional study, a causal relationship could not be ascertained. Additional randomized controlled trials are needed to investigate the relationship between periodontal disease and NAFLD, and further investigation of patients with NAFLD who do not have periodontal disease is needed. As this study showed that liver fibrosis and periodontal disease are likely to be related, future interventional studies with treatment for fibrosis as an endpoint will be necessary.
Methods
The recruitment process for this study was carried out at Kanagawa Dental University Yokohama Clinic, Kanagawa Dental University, Iwasaki Internal Medicine Clinic, and Yokohama City University Hospital Cohort. Patient recruitment process was conducted over a period of 8 h per day, 5 days a week. Eligible patients were screened by the attending physicians and deputy researchers (gastroenterologists and periodontists). The design of our research required patient involvement; specifically, the development of the research question was based on experiences of the patients. The Kanagawa Dental University Committee for Research Screening approved this study (approval number: 323; approval date: August 21, 2015). All procedures were performed in accordance with the tenets of the Declaration of Helsinki.
Each participant was examined to assess the amount of periodontal disease-causing bacteria (e.g., P. gingivalis, T. fosythensis, and T. denticola) present in oral cavity, the degree of infection, and periodontal disease severity. All examinations were performed by dentists affiliated with Kanagawa Dental University Hospital and Kanagawa Dental University Yokohama Clinic. The amount of P. gingivalis in saliva was measured using quantitative PCR, and the infection level was evaluated using serum immunoglobin G (IgG) antibody titer tests for P. gingivalis FDC381 and SU63 based on enzyme-linked immunosorbent assays. The severity of periodontal disease was determined by assessing the PPD, clinical attachment level, level of gingival BOP at six sites per tooth using a calibrated periodontal probe (HU-FRIEDY PQ-OW Thin Williams, Hu-Friedy , Chicago, IL, USA), and the stability of the teeth. The patients with NAFLD recruited in this study met the inclusion and exclusion criteria listed in Supplementary Tables S1 and S2, respectively. Fatty liver, steatosis grade, and fibrosis stage were evaluated using non-invasive methods such as ultrasonography (VCTE) and MRI.
Registration
For patients judged to be eligible, the principal investigator or collaborator filled out a patient registration form with the necessary information and sent these data to the Patient Enrollment Center (Yokohama City University). The Patient Enrollment Center confirmed the patient's eligibility based on the registration form, registered the patient, and informed the main researcher or collaborator of the patient’s identification and assignment numbers. All participants provided informed consent to participate in the study prior to the commencement of the study.
Sample size calculation
A retrospective analysis of patients with NAFLD and periodontitis (P. gingivalis ratio < 0.01% vs. P. gingivalis ratio ≥ 0.01%) in Yokohama City University Hospital showed a mean liver stiffness of 2.8 and 3.6 in the P. gingivalis < 0.01% and P. gingivalis ≥ 0.01% groups, respectively, on MRE. We decided to calculate the number of patients required for a proper analysis of the variance t-test based on these data. Considering the liver stiffness (determined with MRE) in the P. gingivalis < 0.01% and P. gingivalis ≥ 0.01% groups to be 2.8 and 3.6, respectively, with a common SD of 1.8, a total of 159 patients were needed to reach an 80% statistical power with a two-sided significance level of 5%. To compensate for dropouts, we proposed an increase in the number of patients to 164.
Statistical analyses
In this study, statistical analyses were performed mainly for the following items. As primary analysis, stratification analysis was performed using the P. gingivalis bacterial content, MRE, and periodontal pockets as indexes. At the secondary endpoint, the P. gingivalis IgG antibody titer in the blood was required for each participant, and the paired Wilcoxon signed-rank test was performed. Data are presented as means ± SDs or numbers (percentages). The significance level was set at p < 0.05. We used statistical software for all analyses (SAS version 9.4, SAS Institute, Cary, NC, USA).
Trial steering and data monitoring committees
The pilot committee consisted of three people appointed by independent clinical and basic researchers (general and palliative care specialists and statisticians at Yokohama City University School of Medicine). They provided overall supervision and ensured all registered examinations. These investigators were anonymous and randomly selected. The Independent Data Monitoring Committee comprised two members of staff from the Department of Biostatistics, Yokohama City University School of Medicine. The management team monitored the monthly progress of patients and data with phone, email, and/or web conferencing. If the oversight committee determined that field monitoring was necessary, a member of the Independent Data Monitoring Committee visited the site for face-to-face monitoring.
Ethics declarations
This study involves human participants and was approved by the Kanagawa Dental University Committee for Research Screening (Approval No: 323). All procedures were performed in accordance with the Declaration of Helsinki.
Consent to participate/Consent to publish
All participants provided informed consent to participate in the study prior to initiation of the study. Participants also signed a form indicating their agreement to the presentation of their data at conferences and in papers in a manner that does not disclose their personal information.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Supplementary Information
Author contributions
Conceptualization: Y.K. Data curation: T.K. and K.H. Formal analysis: Y.K. and M.T. Funding acquisition: M.M. Investigation: T.S., H.U., and K.W. Methodology: S.S. and Y.K. Resources: T.K. Software: S.S. Supervision: S.T., N.H., T.K., and A.N. Validation: T.K. Visualization: T.M. Writing—original draft: S.S. Writing—review & editing: Y.K., T.H., and M.Y. All authors reviewed the manuscript.
Funding
This investigator-initiated cross-sectional study was conducted by the Kanagawa Dental University—as the sponsor—and funded by the Ministry of Health, Labour, and Welfare, Japan (Grant number: 16K11872). The funders of this trial played no role in the design of this study; collection, analysis, and interpretation of data; or writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Satsuki Sato, Yohei Kamata, Takaomi Kessoku and Tomoko Shimizu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17917-2.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Perumpail BJ, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimba S, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet. Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi Y, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 6.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: A population-based study. Gut. 2010;59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 7.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Sonmez A, et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J. Clin. Lipidol. 2015;9:576–582. doi: 10.1016/j.jacl.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Asfari MM, Niyazi F, Lopez R, Dasarathy S, McCullough AJ. The association of nonalcoholic steatohepatitis and obstructive sleep apnea. Eur. J. Gastroenterol. Hepatol. 2017;29:1380–1384. doi: 10.1097/MEG.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugari S, Mantovani A, Nascimbeni F, Lonardo A. Hypothyroidism and nonalcoholic fatty liver disease—A chance association? Horm. Mol. Biol. Clin. Invest. 2018;41:20180047. doi: 10.1515/hmbci-2018-0047. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda M, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang MA, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: A pilot study. Am. J. Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 13.Toshimitsu K, et al. Changes of anthropometric and biological parameters in patients with nonalcoholic steatohepetitis for dietary modification. J. Metab. Clin. Nutr. 2009;12:337–346. [Google Scholar]
- 14.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang RT, Koretz RL, Yee HF. Is weight reduction an effective therapy for nonalcoholic fatty liver? Am. J. Med. 2003;115:554–559. doi: 10.1016/S0002-9343(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal AJ, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis—A systematic review and meta analysis. J. Hepatol. 2011;55:1383–1390. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: A pilot study. Aliment. Pharmacol. Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 20.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 21.Zein CO, et al. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz M, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018;45:138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 23.Holmlund A, Lampa E, Lind L. Poor response to periodontal treatment may predict future cardiovascular disease. J. Dent. Res. 2017;96:768–773. doi: 10.1177/0022034517701901. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki K, et al. Oral pathobiont-induced changes in gut microbiota aggravate the pathology of nonalcoholic fatty liver disease in mice. Front. Immunol. 2021;12:766170. doi: 10.3389/fimmu.2021.766170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ao M, et al. Dental infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS ONE. 2015;10:e0137249. doi: 10.1371/journal.pone.0137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi C, Gudino CV, Gibson FC, Genco CA. Review: Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol. Oral Microbiol. 2010;25:305–316. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riewe SD, et al. Human trophoblast responses to Porphyromonas gingivalis infection. Mol. Oral Microbiol. 2010;25:252–259. doi: 10.1111/j.2041-1014.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth GA, et al. Porphyromonas gingivalis infection and cell death in human aortic endothelial cells. FEMS Microbiol. Lett. 2007;272:106–113. doi: 10.1111/j.1574-6968.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa-Nakamura K, et al. The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J. Periodontal Res. 2011;46:497–504. doi: 10.1111/j.1600-0765.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 30.Tateishi F, et al. Detection of Fusobacterium nucleatum in chorionic tissues of high-risk pregnant women. J. Clin. Periodontol. 2012;39:417–424. doi: 10.1111/j.1600-051X.2012.01855.x. [DOI] [PubMed] [Google Scholar]
- 31.Noack B, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 32.Ebersole JL, et al. Periodontitis in humans and non-human primates: Oral-systemic linkage inducing acute phase proteins. Ann. Periodontol. 2002;7:102–111. doi: 10.1902/annals.2002.7.1.102. [DOI] [PubMed] [Google Scholar]
- 33.Tamaki N, et al. Short-term effects of non-surgical periodontal treatment on plasma level of reactive oxygen metabolites in patients with chronic periodontitis. J. Periodontol. 2009;80:901–906. doi: 10.1902/jop.2009.080640. [DOI] [PubMed] [Google Scholar]
- 34.Kato T, et al. Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere. 2018;3:e00460-18. doi: 10.1128/mSphere.00460-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomofuji T, et al. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J. Periodontol. 2007;78:1999–2006. doi: 10.1902/jop.2007.070056. [DOI] [PubMed] [Google Scholar]
- 36.Kuraji R, et al. Porphyromonas gingivalis induced periodontitis exacerbates progression of non-alcoholic steatohepatitis in rats. Clin. Exp. Dent. Res. 2016;2:216–225. doi: 10.1002/cre2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 38.Araujo MWB, et al. Reproducibility of probing depth measurements using a constant-force electronic probe: Analysis of inter- and intraexaminer variability. J. Periodontol. 2003;74:1736–1740. doi: 10.1902/jop.2003.74.12.1736. [DOI] [PubMed] [Google Scholar]
- 39.Taylor GW, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J. Periodontol. 1996;67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- 40.Ebersole JL, et al. Aging, inflammation, immunity and periodontal disease. Periodontol. 2016;2000(72):54–75. doi: 10.1111/prd.12135. [DOI] [PubMed] [Google Scholar]
- 41.Furusho H, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J. Gastroenterol. 2013;48:1259–1270. doi: 10.1007/s00535-012-0738-1. [DOI] [PubMed] [Google Scholar]
- 42.Tsukasaki M, et al. Hosts. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018;9:701. doi: 10.1038/s41467-018-03147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasaki A, et al. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci. Rep. 2020;10:4134. doi: 10.1038/s41598-020-60904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratziu V, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 45.Sasso M, et al. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Obara N, et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J. Gastroenterol. 2008;43:720–728. doi: 10.1007/s00535-008-2225-2. [DOI] [PubMed] [Google Scholar]
- 47.Alisi A, Pinzani M, Nobili V. Diagnostic power of fibroscan in predicting liver fibrosis in nonalcoholic fatty liver disease. Hepatology. 2009;50:2048–2049. doi: 10.1002/hep.23345. [DOI] [PubMed] [Google Scholar]
- 48.Chang PE, et al. Prospective evaluation of transient elastography for the diagnosis of hepatic fibrosis in Asians: Comparison with liver biopsy and aspartate transaminase platelet ratio index. Aliment. Pharmacol. Ther. 2008;28:51–61. doi: 10.1111/j.1365-2036.2008.03711.x. [DOI] [PubMed] [Google Scholar]
- 49.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2014;29:1470–1476. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 50.de Lédinghen V, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Reeder SB, et al. Quantification of hepatic steatosis with MRI: The effects of accurate fat spectral modeling. J. Magn. Reson. Imaging. 2009;29:1332–1339. doi: 10.1002/jmri.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Permutt Z, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment. Pharmacol. Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imajo K, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637.e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Yoneda M, et al. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD) Gut. 2007;56:1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergström J, Eliasson S. Prevalence of chronic periodontal disease using probing depth as a diagnostic test. J. Clin. Periodontol. 1989;16:588–592. doi: 10.1111/j.1600-051X.1989.tb02142.x. [DOI] [PubMed] [Google Scholar]
- 56.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.