Abstract
Modern agri-food systems generate large amounts of crop-based biomass that are unfit for direct human consumption but potentially suitable for livestock feeding in production of meats, milk, and eggs. This study aims to develop novel feeds for cattle from some of those biomass materials through the natural microbial-driven processes of ensiling. Fruit and vegetables resembling supermarket discards were ensiled alone or co-ensiled with corn crop residues, mushroom wastes, etc. via laboratory experiments. Longitudinal sample analyses showed that (co-)ensiling was successful, with pH and fermentation acids changing rapidly into desirable ranges (pH < 4.5, the acids 5–13% DM with lactic acid dominating). The (co-)ensiled products had key nutritional parameters comparable to those of good quality forages commonly used on dairy farms. Additionally, in vitro incubation experiments indicated that the ensiled products could substitute certain conventional feeds while maintaining diet digestibility. Findings from this pilot study provide a proof of principle that quality novel feeds for cattle can be generated by co-ensiling food discards and low-value crop residues. Future research and animal feeding trials to demonstrate the utility of this approach can help societies more effectively utilize untapped biomass resources, strengthening the regenerative capacity of agri-food systems towards a more sustainable food future.
Subject terms: Environmental sciences, Environmental impact
Introduction
The global food system has and will continue to weather unprecedented challenges in multiple dimensions. At the core are fundamental issues of meeting ever-growing demand in terms of food availability and equity1–3 along with the pressing needs to mitigate food’s footprints on climate change, environmental degradation, and unsustainable resource extraction4,5. In this context, strategies that promote circularity and expand the regenerative capacity of the agri-food systems are of paramount importance6,7. Toward this end, recovery and re-use of biomass materials that are already produced in primary production but ‘lost’ from the linear food supply chain present a unique opportunity. These biomass materials exit the food supply chain mainly as indigestible, unpalatable, or undesirable biomass (IUUB) typically unfit for direct human consumption. Oftentimes, these materials are still rich in carbohydrates, proteins, and other macro- or micro-nutrients8,9. These nutrients can be upcycled via livestock feeding, because of farm animals’ innate capability to digest a wide variety of biomass. Therefore, optimizing the use of IUUB materials through livestock presents a viable solution for producing more food with less resource-, environment-, and climate-burdens.
There are three broad categories of IUUB materials10: (i) crop residues upon removal of human-edible parts, typically inedible/indigestible; (ii) processing byproducts (or co-products) consisting of the remaining residues from food/beverage processing industries, generally unpalatable; (iii) food waste/discards from various stages of the food supply chain, usually undesirable to humans. Tremendous amounts of IUUB materials are routinely used as feedstuffs, totaling 1140 million metric tons (MT) of crop residues and 600 MT processing byproducts annually worldwide11. Still, very large amounts remain untapped resources for livestock-based upcycling. This is particularly true for food waste/discards, which is estimated to be 1300–1600 MT globally12,13 and projected to increase in coming decades14.
Feeding food waste to animals has had a long history, but the practice has become less common in modern production systems9,15. Among the reasons are the economics of precision feeding16, the inherent variability in nutritional attributes of food wastes from diverse sources17, concerns over disease transmission 18, and undervaluation of impacts of food waste on society and sustainability19. With the pressing challenges of sustainable food security amid climate change, there have been renewed interests in transforming food waste into feeds for livestock9,20,21. It has been demonstrated that proper thermal treatment can render post-consumer food waste (from restaurants and homes) safe for monogastric species; risk of transmission of pathogens and parasites is minimal18. Japan and South Korea reportedly convert and utilize 36% and 45% of such food waste for animal feeding22–24. Notably, food waste is not created equal20. Pre-consumer plant-based food discards would be more suitable for feeding ruminants. For example, feeding studies have demonstrated that marketplace fruit and vegetable discards could replace up to 50% of concentrate feeds in the control diet to support satisfactory steer growth25, or substitute 6–18% of concentrate feeds to support milk production (25 kg day−1) of Holstein cows26. Interestingly, there remains a huge opportunity as wasted fruit and vegetables constitute the single largest food waste stream globally and nationally in the United States27–29. To exploit the opportunity requires scientific and management intervention because fresh fruit and vegetable discards are prone to spoilage, which can reduce palatability and nutritional value and increase risks of microbial contamination30. Therefore, finding ways to extend the storage life and conserve the nutrients for safe feeding is essential.
Here, we report the efficacy of preserving fruit and vegetable discards via ensiling. Ensiling is a microbial-driven process commonly used on dairy farms for the very purpose of preserving freshly harvested feed crops (around 35% DM) for prolonged storage and feeding. But data are scarce on the utility and robustness of ensiling fruit and vegetable discards, which are much wetter (moisture around 85%, compared to 65% for feed crops typically ensiled). We hypothesized that fresh fruit and vegetables can be co-ensiled with crop residue biomass to produce high quality feeds for cattle. To test the hypothesis, we conducted a series of laboratory experiments in which fresh fruit and vegetables were ensiled alone or in combination (i.e. co-ensiling) with plant biomass such as corn crop residues or spent mushroom compost (see Methods for details). The overarching goal of our research is to develop viable solutions for optimal utilization of IUUB materials, contributing to sustainable livestock production toward enhanced regenerative agri-food systems. Specific objectives included: (i) assess the feasibility of ensiling fresh fruit and vegetables (FFV) alone, or co-ensiling with various biomass substrates via longitudinal studies, (ii) determine key nutritional characteristics of ensiled products, and (iii) evaluate the digestibility of ensiled products via in vitro incubation experiments.
Results and discussion
Ensiling efficacy
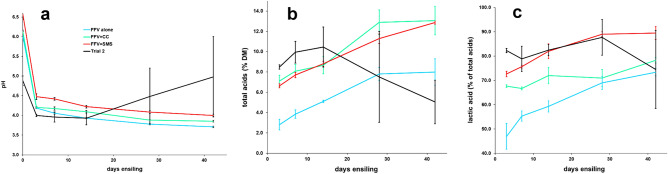
Three ensiling trials were conducted (see Methods and Supplementary Tables 1 and 2 for details). In Trial 1, ensiling FFV alone (a mixture of 10 types of fruit and vegetables, see Methods) was effective, where pH dropped to 4.2 by day 3 and maintained in a narrow range of 3.7–4.1 thereafter (Fig. 1a). Volatile fatty acids (VFAs) from fermentation increased steadily from 7.1% DM (day 3) to 13.1% (day 28) and maintained near the highest value on day 42 (Fig. 1b). Both pH and VFAs were within the desirable ranges (3.5–4.5 pH, 10–14% DM) for effective preservation of fresh-cut crops such as corn silage31,32. Not surprisingly, a considerable amount of liquid accumulated at the bottom of the ensiling vessel; about 110–150 mL per vessel (or 160–210 mL per kg of ensiling substrate) were collected on day 42 (i.e. end of ensiling trial) by gravity drainage. The liquid samples contained 7% DM, with soluble carbohydrates comprising 39.5% of DM. The samples were also enriched in P and Na concentrations but had lower pH, compared to the liquid sample that seeped from the bulk materials of diced FFV during ensiling preparation (i.e. day 0; see Methods). Clearly, proper effluent management is required if FFV is to be ensiled alone on farms.
Figure 1.
Ensiling parameters pH (a), volatile fatty acids (b), and lactic acid as a percentage of the volatile fatty acids (c) in longitudinal samples collected on days 3, 7, 14, 28, and 42. Abbreviations: FFV, fresh fruits and vegetables; CC, corn cobs; SMC, spent mushroom compost. Error bars are ± 1 standard deviation about the mean. Trial 2 was co-ensiling of FFV with corn stalks, mushroom stumps, spent mushroom compost and wet brewers’ grains.
Co-ensiling FFV with corn cobs (CC) or spent mushroom compost (SMC), as part of Trial 1, had key parameters falling within the desirable ranges as well (Fig. 1). A sharp drop in pH (from 6.0–6.6 to 4.2–4.5) by day 3 was followed by gradual decrease then steady maintenance around 3.9 and 4.0. VFAs increased from 7 to 13% DM (day 3 to day 42) for FFV + SMC, which closely mirrored that of FFV alone (Fig. 1). For FFV + CC, VFAs concentrations were considerably less (3% and 8% DM on day 3 and day 42), but still adequate to attain and maintain pH in a desirable range. It is not clear what might have hindered acids from attaining greater amounts in FFV + CC. Interestingly, FFV + SMC had pH consistently higher (by 0.2–0.3 unit) than that of FFV alone or FFV + CC throughout the ensiling trial, despite greater amounts of the acids (Fig. 1). This is probably due to the SMC substrate having a higher buffering capacity.
In Trial 2, FFV was co-ensiled with corn stalks (CS), mushroom stems (MS), wet brewers’ grains (WBG), and SMC, all originated from local sources (Methods; Supplementary Tables 1 and 2). Ensiling progressed normally in the first two weeks (days 3, 7, 14), with pH and VFAs patterns similar to treatments in Trial 1 (Fig. 1). However, samples obtained on day 28 and day 42 had mean pH higher (4.4 and 5.0) and VFAs lower (8% and 5%) than the earlier-day samples (Fig. 1). We noticed that one of the three replicates on day 28 and day 42 actually had pH and the acids within or close to normal range (3.7 and 4.3 for pH and 12% and 7% for total acids); we thus suspect some mishaps with the possibility of breached anaerobic conditions involving the other ensiling vessels.
Lactic acid, as the most desirable acid among the various fermentation acids generated from the ensiling process, was produced in the greatest quantities throughout the experiment in all cases (Fig. 1c). The ensiled products (i.e. day 42 samples) had lactic acid concentrations averaging 2–5% of DM and 73–90% of total fermentation acids. Acetic acid was 11–26% of VFAs with concentrations of 0.7–2.9% DM, which is within the acceptable range of 1–4% DM for corn silage31–33. Propionic acid was present at low concentrations (0.2–0.4% of DM) in some samples, mostly the co-ensiling in Trial 2. Butyric acid or isobutyric acid were not detected in any of the samples.
Trial 3 was conducted to generate ensiled products for use in subsequent in vitro digestibility experiment (Methods; Supplementary Table 1). The ensiled products FFV + CS and FFV + CS + WBG had pH in the desirable range (mean pH 3.9 and 4.0). However, FFV + CS + WBG had acetic acid (instead of lactic acid) dominating (71% of VFAs). We do not know the mechanisms that led to the high level of acetic acid formation. In conventional (corn, legume, grass) silage making, low to moderate amounts of acetic acid can be beneficial because it inhibits yeasts, resulting in improved stability when silage is exposed to air during the feeding phase34. However, high concentrations of acetic acid produced during ensiling generally indicates suboptimal fermentation conditions35. Nevertheless, when consumed by ruminants, acetic acid in silage can be absorbed from the rumen and utilized for energy by cows34.
The fundamental purpose of ensiling is to preserve the biomass that is otherwise susceptible to spoilage, whether it is fresh-cut forage crops or fruit and vegetable discards such as in our case. Listed in Table 1 are established goals and characteristics of good quality corn, legume, and grass silages. Comparatively, the ensiled products in our study had all fermentation parameters (pH, VFAs, and NH3-N) falling within the ranges of desirable values, except for FFV + CS + WBG with acetic and propionic acids exceeding the references.
Table 1.
Fermentation parameters of ensiled products in the present study, as compared to those ‘established goals’ as well as typical values for conventional corn silage, legume silage, or grass silagea.
| Analyte | Corn silage | Legume / Grass silage | The present study | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Goal | Typical range | Goal | Typical range | Trial 1 | Trial 2 | Trial 3 | |||||
| (28–32% DM) | Legume silage (28–32% DM) | Grass silage (32–36% DM) | FFV | FFV + CC | FFV + SMC | FFV + CS + SMC + WBG + MS | FFV + CS | FFV + CS + WBG | |||
| pH | 3.9 | 3.88 | < 4.5 | 4.91 | 4.57 | 3.7 ± 0.02 | 3.9 ± 0.02 | 4.0 ± 0.03 | 4.0 ± 0.5 | 3.9 ± 0.01 | 4.4 ± 0.09 |
| Lactic acid (%DM) | 4–7% | 5.16% | 4–7% | 4.87% | 4.72% | 10.2 ± 1.0 | 5.9 ± 0.9 | 12.0 ± 0.8 | 4.0 ± 1.6 | 5.9 ± 0.4 | 1.2 ± 0.6 |
| Acetic acid (%DM) | < 2% | 3.49% | < 3% | 3.80% | 2.05% | 2.9 ± 0.4 | 2.1 ± 0.4 | 1.4 ± 0.4 | 0.7 ± 0.4 | 4.2 ± 0.3 | 6.2 ± 0.8 |
| Propionic acid (%DM) | < 0.5% | 0.35% | < 0.5% | 0.33% | 0.13% | n.d.b | n.d | n.d | 0.2 ± 0.1 | n.d | 0.9 ± 0.07 |
| Butyric acid(%DM) | < 0.01% | 0.03% | < 0.1% | 0.91% | 0.34% | n.d | n.d | n.d | n.d | n.d | n.d |
| Total acids(%DM) | 9.05% | 9.9% | 7.2% | 13.1 ± 1.4 | 8.0 ± 1.3 | 12.9 ± 0.2 | 5.1 ± 2.1 | 10.1 ± 0.6 | 8.4 ± 0.8 | ||
| Lactic acid (% total acids) | 65–70% | 57.25% | 65–70% | 49.1% | 65.2% | 78.2 ± 1.1 | 73.4 ± 1.6 | 89.5 ± 2.7 | 74.5 ± 16.0 | 58.4 ± 0.8 | 16.5 ± 7.0 |
| NH3-N (% total N) | < 7% | 8.58% | < 10% | 16.4% | 9.12% | 2.2 ± 0.4 | 2.5 ± 0.7 | 1.1 ± 0.1 | 1.4 ± 0.8 | 1.7 ± 0.04 | 1.6 ± 0.04 |
| 1,2 Propanediol (when present) | 1.30% | n.d | n.d | n.d | n.d | n.d | n.d | ||||
aData for the goals and conventional silages were from Ward and de Ondarza35.
bAbbreviations: FFV for fresh fruit and vegetables; CC for corn cobs; CS for corn stalks; SMC for spent mushroom compost; WBG for wet brewers’ grains; MS for mushroom stems, and n.d. for analyte not detected. Deviations from reference values are in italics.
Conventional silage-making requires substrate dry matter to be > 30–32% to minimize the risks of Clostridia growth. Clostridia bacteria are one of the most common undesirable bacteria that may persist in unstable silage, leading to higher dry matter loss and poor silage palatability34,35. In our study, the wet substrates (< 25% DM in all co-ensiling treatments; Supplementary Table 1) did not succumb to Clostridia growth because no butyric acid was detected (Clostridia bacteria convert lactic acid to butyric acid). The rapid decrease in pH within the first few days of fermentation might have inhibited Clostridia growth, which requires pH 4.5 or above36. It is also possible that the substrates (FFV and the other biomass) were not laden with Clostridia bacteria, unlike forage crops harvested directly from agricultural fields that may be contaminated with soil.
Nutritional attributes of ensiled products
Crude protein (CP) concentrations in ensiled products ranged from 6.9% to 18.1% DM (Table 2). Compared to FFV alone, the addition of corn crop residues diluted CP content whereas wet brewers’ grains and mushroom stumps elevated CP in the co-ensiled products. Soluble protein concentration was highest in ensiled FFV alone (63.1% CP) but considerably less in most of the co-ensiled products (27.2–50.4% CP). Protein bound to ADF and NDF ranged from 2.7 to 22.5% (Supplementary Table 3). The ADF-bound protein is indigestible in ruminants and passes into manure. The difference between NDF- and ADF-bound protein provides a measure of bypass protein for intestinal degradation and absorption. Compared with conventional forages typically used in feeding dairy cows, the ensiled products of FFV alone or in combination with corn residues (CC, CS) had CP levels similar to that of corn silage (around 8.5% DM), whereas co-ensiling FFV with WBG, MS, and SMC led to ensiled products with CP levels close to that of grass or alfalfa hay (13–20% DM, Table 2).
Table 2.
Summary of key nutritional parameters of ensiled products, as compared to those of corn silage, legume silage, and grass silage.
| Parameter | FFVa | FFV + CCa | FFV + SMSa | Trial 2 mixture | FFV + CSa | FFV + CS + WBGa | Corn silageb | Alfalfa hayb | Grass hayb |
|---|---|---|---|---|---|---|---|---|---|
| CPc (% DM) | 10.5 | 7.4 | 13.8 | 18.1 | 7.6 | 16.0 | 8.0 | 19.7 | 13.6 |
| SPd (% CP) | 65.3 | 50.4 | 30.2 | 27.2 | 41.2 | 32.6 | 61.7 | 40.1 | 29.5 |
| ADF (% DM) | 13.6 | 36.5 | 20.4 | 39.2 | 40.4 | 37.7 | 26.1 | 32.8 | 34.9 |
| NDF (% DM) | 15.9 | 57.2 | 32.4 | 48.0 | 57.8 | 55.4 | 39.7 | 41.6 | 54.4 |
| Lignin (% DM) | 2.3 | 4.8 | 18.7 | 10.1 | 6.4 | 6.4 | 2.8 | 7.6 | 5.2 |
| Sugar (% DM) | 38.6 | − e | − e | 1.3 | 1.0 | 0.5 | 1.1 | 9.7 | 8.2 |
| Starch (% DM) | − e | − e | − e | 5.1 | 6.9 | 8.4 | 35.5 | 1.6 | 2.6 |
| TDN | − e | − e | − e | 53.0 | 54.9 | 59.0 | 70 | 52 | 52 |
| Crude fat (% DM) | 8.8 | − e | − e | − e | 1.6 | 4.2 | 3.2 | 2.9 | 3.1 |
| Ash (% DM) | 8.0 | 3.8 | 21.5 | 3.9 | 8.3 | 8.0 | 4.3 | 10.0 | 6.0 |
aAbbreviations: same as Table 1 footnotes. bValues for the conventional dairy feed silages are from the feed dictionary of UPenn Dairy Ration Analyzer. cCrude protein. dSoluble protein as fraction of crude protein. eData not available.
Fibrous feedstuffs are indispensable in ruminant diets for maintaining normal rumen fermentation and rumination, lowering the risk for rumen acidosis and post-calving disorders 37,38. In our study, ensiled FFV alone had lower ADF (13.6%) and NDF (15.9% DM) concentrations relative to conventional forages. Co-ensiled products had ADF concentrations in the range of 20.4–40.4% DM and NDF concentrations 32.4–57.8%, which are comparable to those found in grass or alfalfa hay (Table 2). The ensiled FFV + SMC had high lignin content (18.7% DM), originating from the raw SMC substrate (32.6% lignin; Supplementary Table 2). Higher lignin content of forages is generally associated with lower amounts of nutrients for the animal. Restricting FFV + SMC to a relatively low ratio in a total mixed ration/diet can help limit the dietary lignin level to within an acceptable range. Future research is needed to evaluate the use of mechanical processing to differentiate various fiber fractions in raw SMC materials. Additionally, the high ash content in FFV + SMC (21.5% DM) could also be lowered via dilution in total mixed rations and/or SMC substrate processing. Ash content in other ensiled products evaluated in this study were mostly < 10% DM (Table 2). Conventional silages generally have ash concentrations of about 4.4% (corn silage) and 10.9% (legume silage;35).
The parameter of total digestible nutrients (TDN) is the sum of digestible fiber, protein, lipid, and carbohydrate components of a feedstuff. It is the simplest form of energy evaluation of a feed, directly related to digestible energy of the feed39. High quality forages such as alfalfa have TDN typically ranging from 50 to 60% DM while low quality forages range from 40 to 50% 40. Accordingly, ensiled products evaluated in our study had TDN concentrations ranging from 50 to 60% which are equivalent to TDN content commonly observed in medium–high quality forages (Table 2).
Feed hygiene of ensiled products
Ensiled products tended to be ‘cleaner’ (lower molds and yeast counts) than day 0 samples (Table 3). Mold counts were within the range considered safe (< 5.7 log10 CFU g−1) or relatively safe (< 6.0 log10 CFU g−1) for conventional feeds (https://www.foragelab.com/Services/Forage-and-Feed/Mold-and-Yeast-Evaluation/), except for FFV + CC. In the latter case, a crack in the lid for one of the three ensiling vessels was observed at the end of the experiment, indicating a probable breach of anaerobic conditions and thus resulting in greater mold growth (mean value 6.48 log10 CFU g−1). Higher than acceptable mold counts may lead to depressed digestibility, feed intake, and animal performance. Fusarium and Mucor species were the predominant mold species identified in the samples. Fusarium is a large genus of filamentous fungi widely distributed in soil and plants; some have been reported to produce mycotoxins41. Mucor does not produce mycotoxins42. Yeast concentrations in our samples were considered acceptable for well-preserved conventional silage (< 4 log10 yeast CFU g−1;43), except for the ensiled product of Trial 2 (Table 3) presumably due to experimental mishap as mentioned earlier.
Table 3.
Mean and standard deviation of mold and yeast analyses on selected samples from ensiling experimentsa, values are log10 CFU g substratea.
| Mold counts | Yeast counts | |||||||
|---|---|---|---|---|---|---|---|---|
| FFV | FFV + CC | FFV + SMC | Trial 2 | FFV | FFV + CC | FFV + SMC | Trial 2 | |
| Day 0 | 2.82 ± 2.46 | 4.47 ± 4.27 | 4.22 ± 4.11 | – | 3.22 ± 3.31 | 6.91 ± 6.68 | 6.10 ± 5.82 | – |
| Day 42 | 2.70 ± 0.0 | 6.48 ± 6.64b | 3.78 ± 3.85 | 5.76 ± 0.42 | 2.82 ± 2.46 | 3.00 ± 2.94 | 2.70 ± 0.0 | 7.13 ± 0.99 |
| Mold counts concerning animal feedingc | ||||||||
| Mold count (log10) | Guidance | |||||||
| < 5.7 | Safe | |||||||
| 5.7–6.0 | Relatively safe | |||||||
| 6.0–6.3 | Discount energy (0.95), feed with caution | |||||||
| 6.3–6.5 | Discount energy (0.95), closely observe animals and performance | |||||||
| 6.5–6.7 | Discount energy (0.95), closely observe animals and performance, dilute with other feeds | |||||||
| > 6.7 | Discontinue feeding | |||||||
aAbbreviations: same as Table 1 footnotes. bHigh mold count in one ensiling vessel due to crack in lid and air entry. cSource: CVAS (https://www.foragelab.com/).
In vitro incubation outcomes
In vitro incubation is a commonly employed method that uses rumen fluid to digest feed samples in incubation vials; fermentation parameters are determined to assess the digestibility of treatment diets when exposed to rumen microbes, ultimately helping to predict potential impact on animal performance. After 24 h incubation in our study, all treatments had fermentation parameters characterized by decreases in pH (by 1.8–1.9 unit), increases in ammonia-N concentration (by up to 2.8 mg dL−1), and changes in VFAs makeup, compared to 0 h (Supplementary Tables 4 and 5). Additionally, gas production was in the range of 86–106 mL per vial. Such gas is generally a mixture of methane and carbon dioxide plus trace amounts of other compounds. Future investigation to determine the amount and ratio of methane in the gaseous emissions can provide further insights regarding carbon footprint mitigation related to novel feeds in diet. Taken together, our results suggested normal fermentation activities taking place in the incubation vials, as treatment diets did not differ from the control diet in terms of gas production, ammonia-N concentration, and VFAs in most cases, with pH slightly higher (by < 0.07 unit on average; Table 4). In essence, there were little differences in digestibility between the control and treatment diets containing novel feeds. This implies that the novel feeds could potentially substitute conventional feeds (5% or 10%) to support animal requirement, although actual cow performance remains to be determined experimentally via feeding studies.
Table 4.
In vitro fermentation parameters after 24 h incubation. Values are means of three replicates ± one standard deviation; the same letters following a parameter value in a row are not significantly different using Fisher’s protected least significant difference test (Pr > F) at a probability level of 0.05.
| Analyte | Unit | Dieta | ||||||
|---|---|---|---|---|---|---|---|---|
| TMR | TMR + 5%NF1 | TMR + 10%NF1 | TMR + 5%NF2 | TMR + 10%NF2 | TMR + 10%NF1 + C + P | TMR + 10%NF2 + C + P | ||
| pH | 5.16 ± 0.01c | 5.21 ± 0.02b | 5.25 ± 0.02a | 5.23 ± 0.01ab | 5.25 ± 0.04a | 5.20 ± 0.02b | 5.22 ± 0.02ab | |
| Gas production | mL | 106 ± 3a | 103 ± 4a | 104 ± 5a | 99 ± 5a | 86 ± 23a | 77 ± 29a | 88 ± 25a |
| NH3-N | mg dL-1 | 11.65 ± 0.74a | 10.70 ± 0.65ab | 9.95 ± 0.22bc | 11.95 ± 0.84a | 11.33 ± 1.91ab | 8.49 ± 0.33c | 11.91 ± 0.98a |
| Acetic acid | % mmol | 48.37 ± 1.25a | 50.21 ± 2.14a | 50.22 ± 1.04a | 50.29 ± 1.83a | 50.78 ± 1.01a | 48.52 ± 1.71a | 50.37 ± 1.79a |
| Propionic acid | % mmol | 30.25 ± 0.43ab | 29.45 ± 0.75bc | 29.26 ± 0.50c | 29.19 ± 0.61c | 28.98 ± 0.46c | 30.44 ± 0.49a | 29.50 ± 0.52abc |
| Butyric acid | % mmol | 16.49 ± 0.73a | 15.52 ± 0.85a | 15.52 ± 0.52a | 15.62 ± 0.84a | 15.12 ± 0.59a | 16.31 ± 0.85a | 15.42 ± 0.91a |
| Isobytyric acid | % mmol | 0.74 ± 0.08a | 0.73 ± 0.18a | 0.73 ± 0.08a | 0.69 ± 0.08a | 0.82 ± 0.14a | 0.87 ± 0.09a | 0.73 ± 0.15a |
| Isovaleric acid | % mmol | 1.78 ± 0.06a | 1.80 ± 0.20a | 1.91 ± 0.07a | 1.87 ± 0.20a | 1.95 ± 0.03a | 1.79 ± 0.21a | 1.77 ± 0.16a |
| Valeric acid | % mmol | 2.36 ± 0.12a | 2.29 ± 0.22a | 2.37 ± 0.11a | 2.33 ± 0.19a | 2.35 ± 0.08a | 2.27 ± 0.17a | 2.21 ± 0.16a |
aAbbreviation: TMR, total mixed ration; NF1, novel feed 1 (ensiled product of fresh fruit and vegetables with corn stalks); NF2, novel feed 2 (ensiled product of fresh fruit and vegetables with corn stalks and wet brewers’ grains); C + P, ground corn plus protein mix.
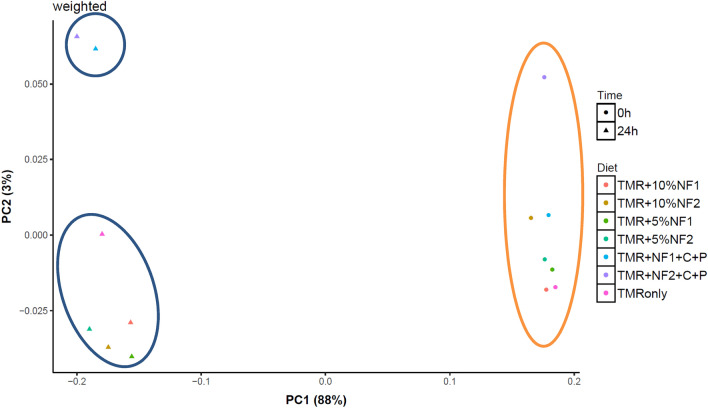
Microbial compositions at the community level at 24 h differed dramatically from that of 0 h (Fig. 2), a result of rumen microbes responding to the diets during in vitro incubation. Inclusion of novel feeds alone (5% or 10%) did not change microbial community makeup at 24 h as compared to the control diet, but the addition of C + P apparently triggered some microbial differences as shown in Fig. 2 (PC2 on Y axis). The addition of C + P was to balance dietary nutrients against cow requirements in the ration formulation model (Methods, Supplementary Table 4). At the phylum level, Firmicutes and Bacteroidetes were the most abundant phyla in all treatments, together accounting for 90% of bacterial abundance, whereas Proteobacteria, Spirochaetes, and Tenericutes were less abundant (Fig. 3). The community-level difference between C + P and the other treatment diets were reflected at the phylum level, with the dominating Firmicutes even more abundant (by a few percentage) while Proteobacteria, Spirochaetes, and Tenericutes further less (Fig. 3). For the dominating Firmicutes at the genus level, Butyrivibrio, unclassified Clostridiales, and Clostridium were in higher abundance with the C + P treatments compared to all other treatments (Supplementary Table 6). Taken together, our findings from the in vitro incubation study suggest that the novel feeds at 5% or 10% inclusion rates could maintain diet digestibility, thus supporting milk production. The more nuanced changes in microbial profiles with the addition of ground corn and protein mixes and its potential implications regarding animal productivity as well as carbon flow pathways deserve further investigation. Future studies through actual feeding trials are warranted.
Figure 2.
Comparison of bacterial communities in 0 vs. 24 h in vitro incubation samples. TMR for total mixed ration, TMR only served as control; NF1 for ensiled novel feed FFV + CS; NF2 for ensiled novel feed FFV + CS + WBG; C + P for ground corn and protein mixes. See Table 1 footnotes for additional abbreviations.
Figure 3.
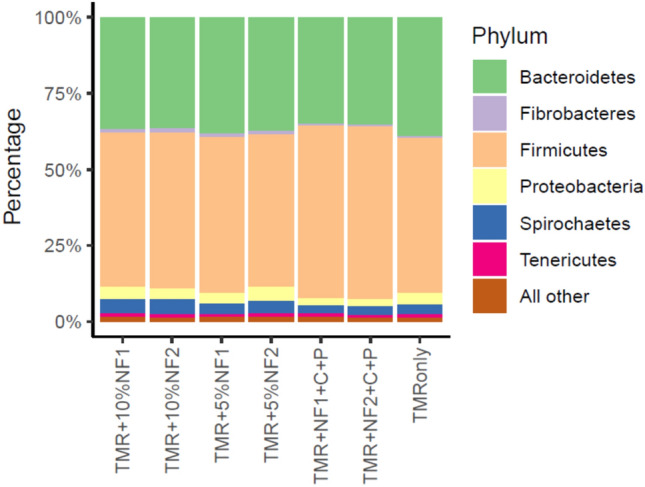
Comparison of individual bacterial phyla after 24 h in vitro incubation. Treatments and abbreviations are the same as in Fig. 2 caption.
Perspectives
Previous studies have tested the ensiling of various fruit or vegetable residues, such as cabbage and lettuce leaves, carrot residues, yacon tuber, pineapple residues, or mixed vegetables discarded from marketplaces44–49. These studies focused on characterization of ensiled products at the end point. Results from our study with samples collected longitudinally over time allowed better understanding of the ensiling processes of FFV and crop biomass as they progressed, in addition to nutritional attributes of the end products. We found that desirable conditions for preserving the substrates were attained rapidly. More importantly and for the first time to our knowledge, we show that co-ensiling FFV with drier crop residue materials has the synergistic benefits of minimizing silage effluent and retaining soluble nutrients meanwhile making it possible to create value-added quality feeds from crop residues that are otherwise underutilized or under-valued.
Future investigations need to test the utility of making quality novel feeds via scale-up studies to pave the way for eventual on-farm adoption. Animal feeding trials will be essential to document animal responses to diets containing novel feeds regarding key performance parameters such as dry matter intake, milk yield and milk components (protein and fat fractions), as well as health indices. Studies with in-depth evaluation of rumen microbial responses can help shed light on whether novel feeds would modify methanogen profiles and potential implications regarding enteric methane emissions. Further, IUUB materials generated in agri-food systems are diverse and versatile. Studies expanding the scope to test various crop biomass as co-ensiling substrates with fresh produce materials could bring about new upcycling opportunities. For fresh produce discards, particularly unsellable fruit and vegetables from retail markets, understanding the variability (amounts, composition, seasonal flow, etc.) and how it may affect animal performance is very important. Additionally, logistics such as source distribution and transport distance will need to be considered for lifecycle-based assessment of carbon footprints. Comprehensive analyses to address multi-sustainability objectives, e.g. socioeconomic impact, climate change, land-, water-, and nutrient-footprints from novel feeds substituting conventional feed ingredients will be critical to demonstrate broader impacts and to inform resource- and climate-smart policymaking.
At the farm level, feed security with adequate and uninterrupted supply of quality feeds is central to sustain a given livestock operation. A farm’s feed security, or feed insecurity risks, are subject to various factors. Some risks are external and beyond the farmer’s control. Examples include feed supply and price volatility, competition from non-agriculture sectors, or increasing adverse weather events aggravated by climate change leading to crop failure or harvest loss. Developing low-impact non-competing novel feeds from wasted food as well as inexpensive, reliable, locally available crop biomass can help alleviate some of the uncertainties and mitigate relevant risks. This would help enhance farming resilience and benefit societies with nutrient-rich food produced more sustainably.
Global demands for meats, milk, and eggs are expanding, stemming increasingly from developing economies. The livestock sector must strive to meet the growing demands meanwhile addressing sustainability challenges and lowering unintended consequences. Innovative strategies and practices that mitigate feed vs. food competition and leverage livestock to upcycle human-unfit biomass are essential. Findings in our pilot study can help advance the endeavor in developing viable solutions to support sustainable livestock production while strengthening the regenerative capacity of the agri-food system toward a more livable future.
Materials and methods
Description of ensiling substrates
Three ensiling trials were conducted for a period spanning over a year. For consistency as well as cross comparison, we created a formula to make fresh fruit and vegetable mixtures with items obtained from a discount produce market to be used in each trial. The formula included ten types of fruit and vegetables that topped the list of unsellable fresh produce in US supermarkets, as reported by Buzby et al.27,28. Together, they accounted for 55.3% of the total (by weight) of the national supermarket fruit and vegetable waste data27. Our formula consisted of: (i) watermelon, romaine lettuce, apple, potato, and tomato, each making up 14% by weight in the mix, plus (ii) orange, cantaloupe melon, onion, bell pepper, and banana, each 6%.
Substrates for co-ensiling trials included corn cobs (CC), corn stalks (CS), mushroom stumps (MS), spent mushroom compost (SMC), and wet brewers’ grains (WBG), in addition to FFV. The raw materials for CS were obtained from one southeast Pennsylvania dairy farm and CC from another. Corn cobs were sieved to pass 7 mm. Corn stalks were processed first through a silage chopper then ground in a cutting mill to pass 1 mm. Mushroom stumps are the lower part of the stem that is removed and discarded upon harvest and prior to processing and packaging. Spent mushroom compost is the growth media cleared out of the mushroom house after the growing cycles, which consists of remains of the original components such as wheat straw, corn stalks, peat moss, etc.50. Both MS and SMC substrates were obtained from a local large-scale mushroom facility. For ensiling preparation, the MS specimen was brush-cleaned of clinging compost materials and the SMC specimen was air-dried and clumps broken down by hand to smaller pieces. The WBG originated from a local brewery and was kept under refrigeration until beginning the ensiling experiment. Analyses of physical, chemical, and nutritional parameters of the raw substrates are in Supplementary Table 2.
Ensiling trials
Three ensiling trials were conducted. Trial 1 tested the ensiling of FFV alone, co-ensiling of FFV with CC, and co-ensiling of FFV with SMC. Trial 2 tested co-ensiling of FFV with CS, MS, SMC, and WBG. Trial 3 was conducted with the co-ensiling of FFV + CS and FFV + CS + WBG, respectively; the ensiled products were used in a subsequent in vitro incubation experiment. Ratios of substrates in the co-ensiling treatments are listed in Supplementary Table 1; samples obtained at the beginning of the experiments (day 0) had 24–25% DM for all co-ensiling treatments. FFV alone had 12.3% DM.
For each ensiling trial, a fresh batch of FFV was made according to the formula described earlier. Raw items were cut into 14 mm cubes using a commercial food processor (Robot Coupe model CL 50, Robot Coupe USA, Inc., Ridgeland, MS). To avoid clogging the processing unit, hard stems from peppers and bananas as well as rinds from melons were manually cut to approximately 14 mm pieces then added to the mixture. Processed FFV was bulked in a plastic tub, the content was thoroughly mixed by hand upon ensiling preparation.
Ensiling was conducted using 0.95 L polyethylene containers with snap-on lids. The containers and lids were wiped with 70% ethanol immediately prior to filling. Each co-ensiling treatment was prepared by weighing out the substrates into a tub and homogenizing manually. FFV alone or with co-ensiling materials was packed into the ensiling containers, tamped down to eliminate air pockets and filled to the top to limit air-filled head space. Lids were snapped on, with circumferences coated with waterproof silicone sealant to prevent air exchange. To permit gas release while maintaining anaerobic conditions in the vessel, a water-filled fermentation lock was inserted through a rubber grommet on the lid and sealed with silicone sealant. Vessels were placed on a laboratory bench under ambient light and temperature (approximately 20 °C). Preliminary trials indicated that the temperature inside the vessels fluctuated in a narrow range of 18–20 °C during ensiling; temperature was not monitored in subsequent trials.
The longitudinal experiments were conducted for 42 days in Trials 1 and 2; sample collection took place on days 0, 3, 7, 14, 28 and 42. Trial 3 was conducted for 28 days, and samples were collected on day 28 to be used for the in vitro experiment. At each sampling time, three replicates of the vessels per treatment were removed and the ensiling process terminated; the content was emptied into a plastic tub and mixed thoroughly with a sterile plastic scoop for sampling and analysis.
In vitro experiment
In vitro incubation was conducted to evaluate digestibility when the ensiled products were added (as novel feeds) to total mixed ration (TMR) made for lactating cows at the Marshak Dairy. The latter is a 180-cow research and teaching facility at the University of Pennsylvania, School of Veterinary Medicine. The TMR consisted of grass hay, corn silage, triticale, ground corn, proteins, byproducts, minerals and vitamins. Each of the ensiled products (FFV + CS, labeled as novel feed 1, NF1 in short; FFV + CS + WBG, novel feed 2, NF2 in short) was a composite made from equal aliquots of three replicates. All feed samples were oven-dried and ground to pass 2 mm in a high-speed spice grinder. The in vitro incubation experiment consisted of seven treatments, in triplicate, with inclusion rates of 5% and 10% per novel feed, plus ground corn and protein mixes (C + P) added to diets of 10% novel feeds for the purpose of balancing nutrients against cow requirements (Supplementary Table 4).
Rumen fluid was obtained via stomach tubing51 from three cows at the Marshak Dairy following IACUC protocols approved by the Office of Animal Welfare at the University of Pennsylvania. Rumen fluid was checked for pH, poured into purged 250 mL bottles, and kept in a warm container until being transferred to the laboratory. At the laboratory, rumen fluid from all three cows was poured into a purged 1 L bottle which was maintained in a water bath at 37 °C under constant flow of CO2, to make a pooled inoculum. The inoculum was added to 21 glass vials (seven treatments in triplicate), each containing 0.75 g feed sample and 12 mL of MacDougall’s buffer. To add inoculum, each vial was purged with CO2 for 30 s, then 6 mL inoculum was pipetted in, and the vial was purged again for 30 s. Vials were sealed with rubber septa and metal lids and crimped. Once all 21 vials had been filled, 60 mL syringes were inserted into the top for collecting and recording gas production, and the vials were placed into the water bath with gentle agitation at 37 °C for 24 h. Upon completing the incubation, all vials were removed from the water bath, gas volumes were recorded, subsamples (~ 2–3 mL each) were taken to check pH, and the remaining contents in the vial were strained through 4 layers of cheesecloth to separate the solid and liquid fractions. Approximately 500 mg of the solid fraction and 0.75 mL of the liquid fraction from each vial were placed into 2-mL Eppendorf tubes (in duplicate) and stored at − 80 °C until extraction for DNA. Additionally, to prepare samples for VFAs/ammonia analysis, 5 mL of the liquid fraction was spun at 10,000 × g for 10 min. Four mL of the supernatant was transferred to a new tube and 800 µL of 36% metaphosphoric acid was added, and the tube was spun at 15,000 × g for 20 min. The remaining supernatant was removed and stored in a -20 °C freezer until sending to a certified service laboratory (Cumberland Valley Analytical Services, Waynesboro, PA) for analysis.
The same steps were repeated for 21 control vials with 0 h incubation. After inoculum was added, vials were gently agitated and then immediately processed for sampling following the same procedure described above.
Sample analysis
For samples collected during the ensiling experiment, a subsample of approximately 75 g was used for gravimetric DM determination using a forced-draft oven (80 ˚C 24 h). Another subsample, 50 g, was used for pH determination (1:1 ratio in deionized water). A third subsample, roughly 400 g, was sent to the same certified laboratory (above) for analyses. The remaining materials were archived in a − 20 °C freezer.
Analyses of ensiling process parameters included concentrations of lactic, acetic, propionic, butyric, and iso-butyric acids plus 1, 2 propanediol, in addition to pH and DM. These analyses were conducted for longitudinal samples collected during the course of the ensiling experiments. Additionally, selected samples were analyzed for a suite of nutritional indices (the “CPM Plus” analytical package by wet chemistry, https://www.foragelab.com/Services/Forage-and-Feed/Chemistry). The nutritional indices included all macro- and micro-nutrients as well as fiber profiles. The selected samples included those ensiled products i.e. at the end of ensiling trial (day 42 or day 28), and in some cases samples obtained at the beginning of experiments (day 0 or day 3). Furthermore, selected samples were analyzed for yeast and mold counts with mold identification. Additionally, liquid effluent from the ensiling of FFV alone was obtained by gravity drainage and analyzed for dry matter, water-soluble carbohydrates and minerals.
For samples obtained from the in vitro incubation, a portion of the liquid fraction from each vial was analyzed at the certified service laboratory (above) for VFAs (acetic, propionic, butyric, isobutyric, isovaleric, and valeric) in addition to ammonia. Genomic DNA was extracted from 250 µL of the liquid fraction and 250 mg of the solid fraction of each incubation vial using the repeated bead beating and column (RBB + C) method followed by extraction with a commercial kit (QIAmp Fast DNA Stool Mini Kit; Qiagen Sciences, Germantown, MD) as described in Yu and Morrison52. Extracted DNA was pooled by fraction and treatment and the V1-V2 region of the bacterial 16S rRNA gene was PCR-amplified in triplicate using the bacterial-specific primers F27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and R338 (5′-TGCTGCCTCCCGTAGGAGT-3′) barcoded with a unique 12-base error-correcting Golay code for multiplexing as described in Song et al.53. Polymerase chain reaction was performed using the Accuprime Taq DNA Polymerase System (Invitrogen; Carlsbad, CA). Thermal cycling conditions involved an initial denaturing step at 95 °C for 5 min followed by 20 cycles (denaturing at 95 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C for 90 s) and a final extension step at 72 °C for 8 min. Amplicons from each sample were combined and each library was added to a pool in equimolar concentration. The final pool was bead-purified using Agencourt AMPure XP Beads (Beckman Coulter, Brea, CA). Sequencing was performed at the PennCHOP Microbiome Core using the Illumina MiSeq platform.
Bioinformatics and data analysis
Ensiled sample results for nutritional, ensiling process, and mold/yeast evaluation parameters reported by the certified laboratories were entered in a Microsoft Excel spreadsheet, means and standard deviations calculated. Graphical presentation of results was developed in Excel. In vitro fermentation parameters analysis of variance was conducted using SAS General Linear Models54 with mean separation by Fisher’s protected least significant difference test at a probability level of 0.05. Pairwise comparisons of in-vitro fermentation parameters at initial conditions vs. 24 h incubation were by one-sided t-test in SAS.
The raw 16S-rRNA amplicon sequencing data was processed through the QIIME2 (2020.6) pipeline55. Briefly, paired end sequence data was de-multiplexed and amplicon sequence variants (ASV) were assigned using the DADA2 plugin56. A phylogenetic tree was constructed using FastTree 257. Taxonomy was assigned based on a pre-trained Naive Bayes classifier trained on the Greengenes database (v13.8) for the 16S rRNA gene spanning the V1-V2 region58. The between sample diversity (weighted and unweighted UniFrac distances) were computed using the ‘qiime diversity’ plugin.
A nonparametric permutational multivariate ANOVA (PERMANOVA) test59 implemented in the vegan package for R was used for beta diversity matrices. Pairwise Wilcoxon Rank Sum Test was used to determine differences in bacterial genera between treatment groups. The P values were adjusted using the Bonferroni correction method. A P value of 0.05 was used to define significance.
Supplementary Information
Acknowledgements
We thank Dr. Jianxin Liu of Zhejiang University and Dr. Chuncheng Xu of China Agricultural University for helpful discussion regarding ensiling experimental setup and conditions. This research is supported in part by a Penn Global Engagement grant and a Pennsylvania Department of Agriculture research grant. Additional support is provided by Agriculture and Food Research Initiative Competitive Grant no. 2022-68014-36664 from the USDA National Institute of Food and Agriculture.
Abbreviations
- CC
Corn cobs
- CS
Corn stalks
- C + P
Ground corn and protein mix
- FFV
Fresh fruit and vegetables
- IUUB
Indigestible, unpalatable, or undesirable biomass
- MS
Mushroom stumps
- VFAs
Volatile fatty acids
- SMC
Spent mushroom compost
- TMR
Total mixed ration
- WBG
Wet brewers’ grains
Author contributions
Z.D. designed and directed the research; L.B. and J.B. identified/acquired source materials used in the study and advised on ensiling and nutritional matters; J.T. performed ensiling trials and data management; J.D., R.S., T.C. and Y.L. assisted in preliminary trials and ensiling preparation; M.H., B.V. conducted in vitro incubation and sample handling; N.I. performed bioinformatics analysis; D.P. coordinated in vitro experimentation with microbial data interpretation; B.P., J.D., B.H. and G.S. participated in brainstorming and project discussion. Z.D. wrote the manuscript with contributions from several co-authors. All authors reviewed the manuscript.
Data availability
All data associated with this work are available in the supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17812-w.
References
- 1.FAO, IFAD, UNICEF, WFP and WHO. The state of food security and nutrition in the world 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all.10.4060/cb4474en (2021).
- 2.Food and Agriculture Organization of the United Nations (FAO). Land use statistics and indicators. Global, regional and country trends 1990–2019. (Food and Agriculture Statistics, 2022).
- 3.Khokhar, T. Chart: Globally, 70% of freshwater is used for agriculture. (The World Bank, 2017).
- 4.Food and Agriculture Organization of the United Nations (FAO). Statistical yearbook−World food and agriculture 2021. (FAO Statistics, 2021).
- 5.The World Bank. Water in agriculture. https://documents1.worldbank.org/curated/en/875921614166983369/pdf/Water-in-Agriculture-Towards-Sustainable-Agriculture.pdf (2021).
- 6.de Boer, I. J. M. & van Ittersum, R. K. Circularity in agricultural production. Mansholt Lecture 2018. https://www.wur.nl/upload_mm/7/5/5/14119893-7258-45e6-b4d0-e514a8b6316a_Circularity-in-agricultural-production-20122018.pdf. (Wageningen Univ. and Research, 2022).
- 7.Van Zanten H, et al. Defining a land boundary for sustainable livestock consumption. Global Change Biol. 2018;24:4185–4194. doi: 10.1111/gcb.14321. [DOI] [PubMed] [Google Scholar]
- 8.Dou ZX, Toth JD. Global primary data on consumer food waste: rate and characteristics—a review. Resour. Conserv. Recy. 2020;168:105332. doi: 10.1016/j.resconrec.2020.105332. [DOI] [Google Scholar]
- 9.Shurson GC. "What a waste"-can we improve sustainability of food animal production systems by recycling food waste streams into animal feed in an era of health, climate, and economic crises? Sustainability. 2020;12(17):7071. doi: 10.3390/su12177071. [DOI] [Google Scholar]
- 10.Dou Z. Leveraging livestock to promote a circular food system. Front. Agric. Sci. Eng. 2021;8(1):188–192. doi: 10.15302/J-FASE-2020370. [DOI] [Google Scholar]
- 11.Mottet A, et al. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017;14:1–8. doi: 10.1016/j.gfs.2017.01.001. [DOI] [Google Scholar]
- 12.Gustavsson, J., Cederberg, C., Sonesson, U., van Otterdijk, R. & Meybeck, A. Global food losses and food waste: Extent, causes and prevention. (Food and Agriculture Organization of the United Nations, 2011).
- 13.Porter SD, Reay DS, Higgins P, Bomberg E. A half-century of production-phase greenhouse gas emissions from food loss & waste in the global food supply chain. Sci. Tot. Environ. 2016;571:721–729. doi: 10.1016/j.scitotenv.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Lopez Barrera E, Hertel T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy. 2021;98:101874. doi: 10.1016/j.foodpol.2020.101874. [DOI] [Google Scholar]
- 15.Dou Z, Toth JD, Westendorf M. Food waste for livestock feeding: Feasibility, safety, and sustainability implications. Glob. Food Secur. 2018;17:154–161. doi: 10.1016/j.gfs.2017.12.003. [DOI] [Google Scholar]
- 16.Luciano A, et al. Potentials and challenges of former food products (food leftover) as alternative feed ingredients. Animals. 2020;10:125. doi: 10.3390/ani10010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajeh C, Saoud IP, Kharroubi S, Naalbandian S, Abiad MG. Food loss and food waste recovery as animal feed: A systematic review. J. Mater. Cycles Waste Manage. 2021;23:1–17. doi: 10.1007/s10163-020-01102-6. [DOI] [Google Scholar]
- 18.Shurson GC, Urriola PE, van de Ligt JLG. Can we effectively manage parasites, prions, and pathogens in the global feed industry to achieve one health? Transboundary Emerg. Dis. 2022;69:4–30. doi: 10.1111/tbed.14205. [DOI] [PubMed] [Google Scholar]
- 19.Orias NE, et al. Food loss and waste: Not all food waste is created equal. Front. Nutr. 2021;8:615550. doi: 10.3389/fnut.2021.615550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dou, Z., Galligan, D., Shurson, G. & Thomson, A. Food supply chain and waste in climate mitigation. The Role of Agricultural Science and Technology in Climate 21 Project Implementation. (eds. Baltensperger, D. et al.) 14–17 https://www.cast-science.org/publication/the-role-of-agricultural-science-and-technology-in-climate-21-project-implementation/. (Council for Agricultural Science and Technology, 2021).
- 21.Zu Ermgassen EK, Phalan B, Green RE, Balmford A. Reducing the land use of EU pork production: Where there’s swill, there’s a way. Food Policy. 2016;58:35–48. doi: 10.1016/j.foodpol.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M-H, Kim J-W. Comparison through a LCA evaluation analysis of food waste disposal options from the perspective of global warming and resource recovery. Sci. Tot. Environ. 2010;408:3998–4006. doi: 10.1016/j.scitotenv.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 23.Padeyanda Y, Jang Y-C, Ko Y, Yi S. Evaluation of environmental impacts of food waste management by material flow analysis (MFA) and life cycle assessment (LCA) J. Mater. Cycl. Waste Manage. 2016;18(3):493–508. doi: 10.1007/s10163-016-0510-3. [DOI] [Google Scholar]
- 24.Salemdeeb R, Zu Ermgassen EK, Kim MH, Balmford A, Al-Tabbaa A. Environmental and health impacts of using food waste as animal feed: A comparative analysis of food waste management options. J. Cleaner Prod. 2017;140:871–880. doi: 10.1016/j.jclepro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das NG, Huque KS, Amanullah SM, Dharmapuri S, Makkar HPS. Study of chemical composition and nutritional values of vegetable wastes in Bangladesh. Vet. Anim. Sci. 2018;5:31–37. doi: 10.1016/j.vas.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angulo J, et al. Nutritional evaluation of fruit and vegetable waste as feedstuff for diets of lactating Holstein cow. J. Environ. Manage. 2012;95:S210–S214. doi: 10.1016/j.jenvman.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Buzby JC, Bentley JT, Padera B, Ammon C, Campuzano J. Estimated fresh produce shrink and food loss in U.S. supermarkets. Agric. Basel. 2015;5:626–648. doi: 10.3390/agriculture5030626. [DOI] [Google Scholar]
- 28.Buzby, J. C., Bentley, J. T., Padera, B., Campuzano, J. & Ammon, C. Updated supermarket shrink estimates for fresh foods and their implications for ERS loss-adjusted food availability data. Economic Information Bulletin Number 155. (USDA-ERS, 2016).
- 29.Xue L, et al. China’s food loss and waste embodies increasing environmental impacts. Nat. Food. 2021;2:519–528. doi: 10.1038/s43016-021-00317-6. [DOI] [PubMed] [Google Scholar]
- 30.Davis, C., Wiggins, L. & Hersom, M. Utilization of cull vegetables as feedstuffs for cattle. Publ. AN280. (University of Florida Extension, 2018).
- 31.Ishler, V. A., Jones, C. M., Heinrichs, J. & Roth, G. W. From harvest to feed: Understanding silage management. (Penn State Extension, 2017).
- 32.Van Saun, R. J. & Heinrichs, J. Troubleshooting silage problems. (Penn State Extension, 2016).
- 33.Kung, L. & Shaver, R. Interpretation and use of silage fermentation analysis results. Focus on Forage13, 3. (University of Wisconsin Madison Division of Extension, 2001).
- 34.Kung L, Jr, Shaver RD, Grant RJ, Schmidt RJ. Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018;101(5):4020–4033. doi: 10.3168/jds.2017-13909. [DOI] [PubMed] [Google Scholar]
- 35.Ward, R. & de Ondarza, M. B. Fermentation analysis of silage: use and interpretation. (Cumberland Valley Analytical Services, 2008).
- 36.Driehuis F, Wilkinson JM, Jiang Y, Ogunade I, Adesogan AT. Silage review: animal and human health risks from silage. J. Dairy Sci. 2018;101:4093–4110. doi: 10.3168/jds.2017-13836. [DOI] [PubMed] [Google Scholar]
- 37.Erickson PS, Kalscheur KF. Nutrition and feeding of dairy cattle. In: Bazer FW, Lamb GC, Wu G, editors. Animal Agriculture: Sustainability Challenges and Innovations. Elsevier: Academic Press; 2020. pp. 157–180. [Google Scholar]
- 38.Grant RJ, Ferraretto LF. Silage feeding management: silage characteristics and dairy cow feeding behavior. J. Dairy Sci. 2018;101(5):4111–4121. doi: 10.3168/jds.2017-13729. [DOI] [PubMed] [Google Scholar]
- 39.Rasby, R. & Martin, J. Understanding feed analysis. (Institute of Agriculture and Natural Resources, University of Nebraska−Lincoln, 2022).
- 40.Van Emon, M., Glunk, E. & Buck, C. Forage analysis interpretation. Publ. MT 201609AG (Montana State University Extension, 2016).
- 41.D’Mello JPF, Placinta CM, Macdonald AMC. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999;80(3–4):183–205. doi: 10.1016/S0377-8401(99)00059-0. [DOI] [Google Scholar]
- 42.Botha, A. & Botes, A. Mucor. Encyclopedia of Food Microbiology, 2nd edn, (eds Batt, C. & Patel, P.) 834–840 (Academic Press, 2014).
- 43.Glewen M, Kung L, Shaver RD, Hoffman PC. Dealing with high yeast levels in high moisture corn and corn silage. University of Wisconsin Madison; 2010. [Google Scholar]
- 44.Cao Y, et al. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J. Dairy Sci. 2011;94:3902–3912. doi: 10.3168/jds.2010-3623. [DOI] [PubMed] [Google Scholar]
- 45.Froetschel MA, et al. Nutritional value of ensiled grocery food waste for cattle. J. Anim. Sci. 2014;92:5124–5133. doi: 10.2527/jas2014-8126. [DOI] [PubMed] [Google Scholar]
- 46.Gowda NKS, et al. Study on evaluation of silage from pineapple (Ananas comosus) fruit residue as livestock feed. Trop. Anim. Health Prod. 2015;47:557–561. doi: 10.1007/s11250-015-0762-2. [DOI] [PubMed] [Google Scholar]
- 47.Özkul H, Kılıç A, Polat M. Evaluation of mixtures of certain market wastes as silage. Asian Austral. J. Anim. Sci. 2011;24(9):1243–1248. doi: 10.5713/ajas.2011.10460. [DOI] [Google Scholar]
- 48.Rinne M, et al. Carrot by-product fermentation quality and aerobic spoilage could be modified with silage additives. Agric. Food Sci. 2019;28:59–69. doi: 10.23986/afsci.79829. [DOI] [Google Scholar]
- 49.Wang L, Guan L, Fang J, Cai Y, Cao Y. Fermentation characteristic and in vitro ruminal digestion of yacon residue silage with lactic acid bacteria inoculant or beet pulp. Rev. Bras. Zootecn. 2019;4:e20180152. doi: 10.1590/rbz4820180152. [DOI] [Google Scholar]
- 50.Beyer, D. M. Spent mushroom substrate. (Penn State Extension, 2011).
- 51.Duffield T, et al. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J. Dairy Sci. 2004;87:59–66. doi: 10.3168/jds.S0022-0302(04)73142-2. [DOI] [PubMed] [Google Scholar]
- 52.Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36:808–813. doi: 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- 53.Song SJ, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SAS Institute. SAS ver. 9.4. (SAS Institute, Inc., 2020).
- 55.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price MN, Dehal PS, Arkin AP. FastTree 2−approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson MJ. A new method for non parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this work are available in the supplementary materials.