Abstract
Photoluminescent gold clusters are functionally variable chemical modules by ligand design. Chemical modification of protective ligands and introduction of different metals into the gold clusters lead to discover unique chemical and physical properties based on their significantly perturbed electronic structures. Here we report the synthesis of carbon-centered Au(I)-Ag(I) clusters with high phosphorescence quantum yields using N-heterocyclic carbene ligands. Specifically, a heterometallic cluster [(C)(AuI-L)6AgI2]4+, where L denotes benzimidazolylidene-based carbene ligands featuring N-pyridyl substituents, shows a significantly high phosphorescence quantum yield (Φ = 0.88). Theoretical calculations suggest that the carbene ligands accelerate the radiative decay by affecting the spin-orbit coupling, and the benzimidazolylidene ligands further suppress the non-radiative pathway. Furthermore, these clusters with carbene ligands are taken up into cells, emit phosphorescence and translocate to a particular organelle. Such well-defined, highly phosphorescent C-centered Au(I)-Ag(I) clusters will enable ligand-specific, organelle-selective phosphorescence imaging and dynamic analysis of molecular distribution and translocation pathways in cells.
Subject terms: Computational chemistry, Photobiology, Organometallic chemistry, Chemical bonding
Photoluminescent gold clusters have unique chemical and physical properties based on their perturbed electronic structures. Here, the authors report the synthesis of carbon-centered Au(I)-Ag(I) clusters with high phosphorescence quantum yields using N-heterocyclic carbene ligands.
Introduction
Sub-nanoscale gold clusters with atomic precision are promising miniature nanoscale materials. Gold clusters often exhibit photoluminescence properties such as phosphorescence, in addition to the unique molecular structures and aurophilicity1,2. To date, several excellent protocols have been developed to improve the photoluminescence performance of gold clusters. Examples include alloying by metal kernels, supramolecular networking by self-assembly of clusters, surface hardening by additives, chemical modification by capping ligands with electron-donating/withdrawing groups, and so on3–10. The structure, electronic state, and reactivity of the gold cluster moiety can be greatly affected by the protective ligands and different metals that additionally bind to the gold atoms.
The octahedral hexagold(I) cluster with a hyper-coordinated carbon center (CAuI6), first developed by Schmidbaur et al. using phosphine ligands, is one of the most classical models of AuI clusters11,12. These clusters emit bright yellowish-green light in the solid-state, but not in solution. Wang et al. reported a heteronuclear metal cluster capable of emitting light even in solution by the pyridyl-phosphine bidentate ligand13,14. This method has made it possible to construct a series of heterometallic AuI clusters that exhibit strong red phosphorescence in solution15,16. This luminescence is thought to be due to the formation of a solid sphere in which the surface of the cluster is completely protected17. It has also been reported that such cluster complexes are a promising group of compounds that emit light at specific locations in the cell18.
More recently, N-heterocyclic carbene (NHC) ligands have been developed as one of the most promising organic ligands for Au0 clusters with high designability19–22. Carbene ligands have strong electron-donating properties and are known to enhance the stability of metal surfaces, metal nanoparticles, and metal nanorods23–25. Therefore, NHC ligands have been used in the synthesis of Au0 clusters26–30, and their interfacial structure, stability, and catalytic activity have been elucidated. In this context, we successfully applied the NHC ligand to the CAuI6 cluster in 201831–33. We found that when an imidazolylidene carbene ligand was attached to each gold atom of the C-centered CAuI6 cluster, the phosphorescence emission was red-shifted in the solid-state compared to clusters protected by phosphine ligands31. On the other hand, when a benzimidazolylidene ligand with one benzene ring fused to the imidazole ring was used, the phosphorescence emission showed a large blue shift of about 60 nm32,33. These two examples indicate that such a simple chemical modification can significantly change the photochemical properties of the clusters. Phosphorescent metal cluster complexes have the potential to precisely control the structure and electronic state of the metal cluster part by ligand design, and are expected to contribute not only to the creation of structure-specific photochemical functions but also to live-cell imaging and elucidation of intracellular molecular behaviors.
In this study, we design and synthesize bidentate ligands consisting of an NHC ligand linked to a pyridyl ligand, and clarify the detailed structure and photochemical properties of heteronuclear clusters of AuI and AgI, which exhibit very high phosphorescence quantum yields even in solution. Furthermore, the strong phosphorescence emission of these clusters is used to elucidate the cellular uptake and organelle-selective translocation pathways of the cluster complexes (Fig. 1). Interestingly, the NHC ligand-protected heterometallic clusters are translocated to a specific intracellular organelle in a ligand-specific manner. This is in sharp contrast to phosphine-protected clusters with the same metal core structure, which are non-selectively dispersed in the cytosol. Thus, the control of intracellular translocation of C-centered AuI clusters is achieved by a slight modification of organic ligands. These results not only show that NHC ligands can improve the phosphorescence emission efficiency of C-centered AuI clusters, but also guide the development of functions for metal cluster-based functional molecules in chemical synthesis approaches.
Fig. 1. Schematic diagram of this study.
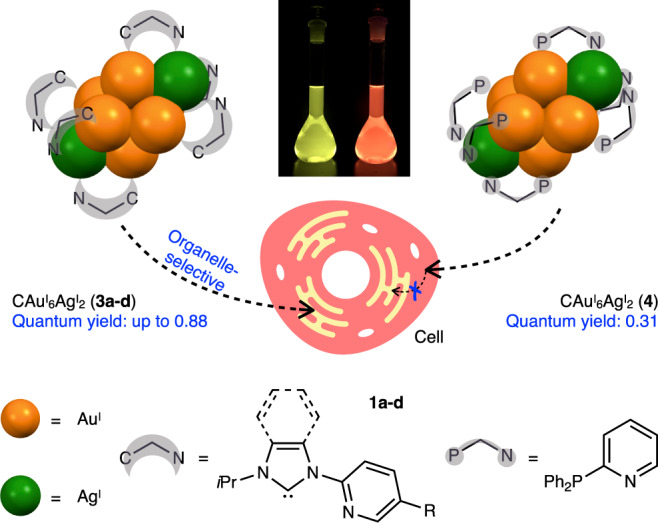
Carbon(C)-centered AuI-AgI clusters with N-heterocyclic carbene (NHC) ligands with intense phosphorescence and their ligand-specific, organelle-selective translocation in cells.
Results
Synthesis and characterization
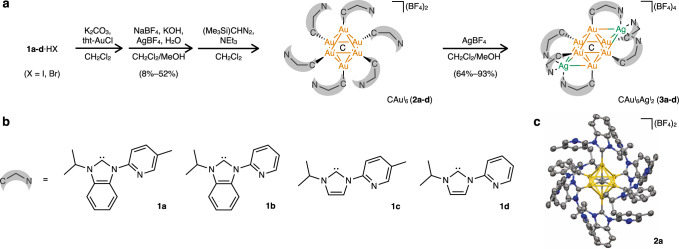
To further polynucleate the AuI cluster with different metals, a nitrogen donor was introduced into the wing-tip portion of each NHC ligand (N-isopropyl-N’−2-(5-methylpyridyl)benzimidazolylidene (1a), N-isopropyl-N’−2-pyridylbenzimidazolylidene (1b), N-isopropyl-N’−2-(5-methylpyridyl)imidazolylidene (1c), N-isopropyl-N’−2-pyridylimidazolylidene (1d); Supplementary Figs. 1–4). Specifically, CAuI6 complexes [(C)(AuI-L)6](BF4)2 (2a–d, L = 1a–d) in which only the carbene ligand is coordinated to AuI, were synthesized from unsymmetrical bidentate ligands 1a–d. The isolation yields of 2a–d were 8–52% based on the amount of AuI used according to previously reported literatures11–18, and their molecular structures in the solid-state were determined by single-crystal X-ray diffraction (ScXRD) (Fig. 2, Supplementary Figs. 5, 6, and Supplementary Table 8). It was found by NMR spectroscopy (Supplementary Figs. 8–27) and mass spectrometry (Supplementary Fig. 28) that the solid-state structure was maintained in solution.
Fig. 2. Heteronuclear CAuI6AgI2 clusters 3a–d were used in this study.
a Synthetic route for CAuI6AgI2 clusters 3a–d. b Chemical structures of ligands 1a–d. c Molecular structure of [(C)(AuI−1a)6](BF4)2 (2a, 25% probability) with the anions BF4- simplified.
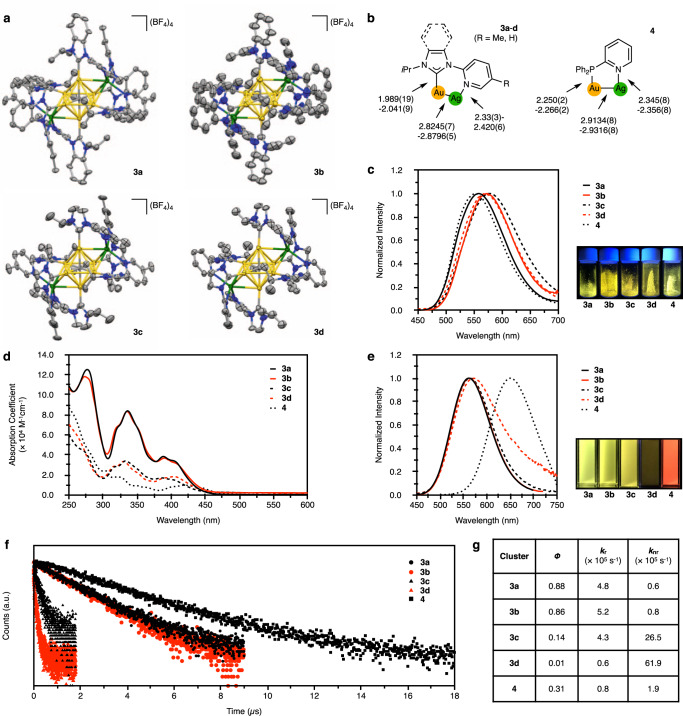
The heterometallic CAuI6AgI2 clusters, [(C)(AuI-L)6AgI2](BF4)4 (3a–d, L = 1a–d) were synthesized from CAuI6 clusters 2a–d with pyridyl pendants-introduced ligands 1a–d. The processes of complexation of CAuI6 clusters 2a–d with AgBF4 were investigated by UV-vis absorption, phosphorescence spectroscopy, mass spectrometry, and NMR spectroscopy (Supplementary Figs. 34–46). CAuI6AgI2 clusters, 3a–d, were isolated as single crystals by layering dry Et2O on a solution of each reaction mixture in CH2Cl2/CH3OH. As shown in Fig. 3a, the molecular structures of CAuI6AgI2 clusters, [(C)(AuI-L)6AgI2](BF4)4 (3a–d, L = 1a–d), were determined by ScXRD, and all clusters were found to have a bicapped octahedral core. The two AgI ions in each cluster are located on two opposite sides of the octahedron, each anchored by three pyridyl groups and interacting with three neighboring AuI atoms. These CAuI6AgI2 clusters 3a–d showed strong intramolecular C − H ∙ ∙ ∙ Au interactions34–36, with the shortest H-Au distances being 2.690, 2.818, 2.765, and 2.730 Å, respectively (Supplementary Fig. 47). The structures are similar to those of the previously reported [(C)(AuI-dppy)6AgI2](BF4)4 (4, dppy = 2-pyridyldiphenylphosphine)14, but differ in the following two points. First, because the C − AuI bond distances in 3a–d with NHC ligands are shorter than those of the P − AuI bonds in 4 with phosphine ligands, and also because the AuI-AgI distances are shorter in 3a–d, the overall structures of the CAuI6AgI2 parts of 3a–d are significantly smaller than that of 4 (Fig. 3b). The AuI-AgI distances in 3a–d are in a very similar range (Supplementary Table 13). Second, there are no intramolecular C − H ∙ ∙ ∙ Au interactions in 4.
Fig. 3. Molecular structures and photochemical properties of CAuI6AgI2 clusters 3a–d and 4.
a Molecular structures of 3a–d (50% probability for 3a, 3c, and 3d; 25% probability for 3b), with the anions BF4- simplified. b Comparison of the key structure parameters of 3a–d and 4. c Emission spectra of 3a–d and 4 in the solid-state, with λemmax being 559, 573, 578, 570, and 553 nm, respectively, and corresponding photos at room temperature. d UV-vis absorption spectra of 3a (ε336 = 8.4 × 104 M−1 cm−1), 3b (ε336 = 8.3 × 104 M−1 cm−1), 3c (ε333 = 3.4 × 104 M−1 cm−1), 3d (ε330 = 3.3 × 104 M−1 cm−1), and 4 (ε321 = 1.8 × 104 M−1 cm−1) in CH2Cl2 (293 K). e Emission spectra of 3a–d and 4 in CH2Cl2 at 293 K, with λemmax of 562, 562, 564, 571, and 650 nm, respectively, and the corresponding photos taken at room temperature. f Emission decay of 3a–d and 4 in CH2Cl2 at room temperature, with τ of 1.85, 1.66, 0.32, 0.16, and 3.74 µs, respectively. g Quantum yields, radiative rate constants (kr) and non-radiative rate constants (knr) of 3a–d and 4 in CH2Cl2 at room temperature. Note that a mixed solvent CH2Cl2/CH3OH (9:1, v:v) was used for 3d42.
Upon photoexcitation, 3a–d showed strong yellow luminescence in the solid-state with λemmax lying in the range from 559 to 578 nm, which significantly red-shifted as compared to 2a–d (from 482 to 490 nm), and is similar to 4 (553 nm, Fig. 3c and Supplementary Figs. 29 and 48). The two AgI atoms do not simply bind to the CAuI6 core but may change the electronic structure of the whole clusters13–17.
The CAuI6AgI2 clusters 3a–d were further characterized by solution-phase NMR spectroscopy (Supplementary Figs. 49–64). Two interesting features were observed in the 1H NMR spectra. First, in the 1H NMR spectra of 3a–d, the septet signals of the secondary hydrogen atoms of the isopropyl groups were shifted to the high field by about 0.5 ppm compared to 2a–d, which is a change similar to that observed when AgBF4 was added to 2a–d (Supplementary Figs. 42–45). This result confirmed the existence of C − H ∙ ∙ ∙ Au interactions in 3a–d even in solution36. The second feature is that split signals of methyl of isopropyl groups were observed in the both 1H and 13C NMR spectra of 3a–d. For example, the methyl groups of 2b showed only one set of signals in the 1H (1.49 ppm) and 13C NMR (21.9 ppm) spectra, whereas two sets of signals were observed in the 1H NMR (1.44 and 1.33 ppm) and 13C NMR (22.6 and 22.1 ppm) spectra for 3b. It was inferred from the molecular structures of 3a–d that this signal change was caused by the helical arrangement of the ligands in the bicapped octahedral CAuI6AgI2 and the two opposing CAuI3AgI moieties showing different helical directions14,15. Heterometallic species, [(C)(AuI-L)4AuIAgI](BF4)+ and [(C)(AuI-L)6AgI](BF4)2+ (L = 1a–d), were also observed in the MS spectra (Supplementary Fig. 65). These results strongly suggest that all of 3a–d maintain the bicapped octahedral structures even in solution.
Solutions of CAuI6AgI2 clusters 3a–d in CH2Cl2 or CH2Cl2/CH3OH showed multiple optical absorption bands in the range of 300–450 nm (Fig. 3d). These optical absorption modes were consistent with those observed when AgBF4 was added to the solutions of 2a–d. Their molar absorbance coefficients of 3a and 3b, protected with benzimidazolylidene ligands, were significantly higher than those of 3c and 3d, protected with imidazolylidene ligands.
Importantly, the use of the bidentate NHC ligands and the coordination of AgI ions have a synergistic effect, and the heterogeneous metal clusters emit very strong phosphorescence in solution (Fig. 3e and Supplementary Fig. 66). In comparison, the metal clusters 3a–d with NHC ligands emit yellow light in solution, whereas 4 with phosphine ligands emit red phosphorescence, with a difference in wavelength of about 90 nm. The phosphorescence quantum yields of 3a and 3b were determined to be 0.88 and 0.86 in CH2Cl2 (λemmax = 562 nm), respectively, the highest values among the reported AuI clusters (Fig. 3g). In contrast, single crystals of 3c and 3d with imidazolylidene ligands showed similar yellow luminescence, but the phosphorescence quantum yields in CH2Cl2 and CH2Cl2/CH3OH were remarkably low, 0.14 and 0.01, respectively. In addition, the phosphorescence lifetimes of 3a and 3b protected with benzimidazolylidene ligands were 1.85 and 1.66 μs, respectively, which were significantly longer than those of 3c and 3d protected with imidazolylidene ligands, 0.32 and 0.16 μs, respectively (Fig. 3f). The radiative (kr) and non-radiative rate constants (knr) shown in Fig. 3g indicate that clusters with NHC ligands have significantly higher radiative emission rates than clusters with phosphine ligands. Furthermore, the benzimidazolylidene ligand could dramatically improve the quantum yield and microsecond-level lifetime of the phosphorescence of the CAuI6AgI2 clusters, 3a and 3b, by significantly suppressing the non-radiative relaxation pathways.
Theoretical calculations of CAuI6AgI2 clusters
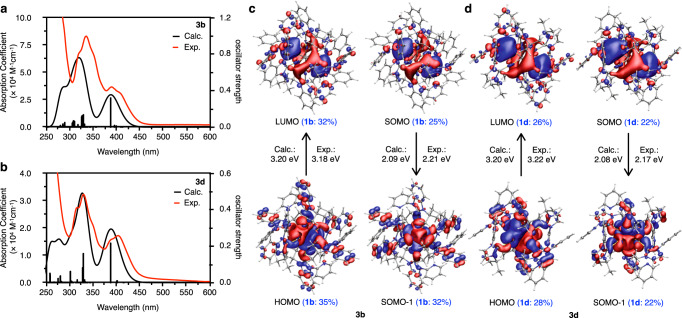
In order to mechanistically clarify how the photochemical properties change depending on the type of carbene ligands, the absorption and phosphorescence properties of 3b with benzimidazolylidene ligands and 3d with imidazolylidene ligands were theoretically calculated and comparatively analyzed (Fig. 4). As a result, the calculated absorption spectra agreed well with the experimental observations in the calculated energy range. The trend of the energy difference was reproduced; the first peaks for 3b and 3d were calculated to be 387 and 388 nm, respectively. The order of the molar absorbance coefficients (3b > 3d) was also well reproduced. The distributions of SOMO and SOMO − 1, which characterize phosphorescence, are similar to those of LUMO and HOMO, respectively (Fig. 4c, d). The imidazolylidene and benzimidazolylidene ligand moieties are involved in the SOMO − 1 orbitals. The calculated phosphorescence energies of 3b and 3d are 2.09 and 2.08 eV (592 and 596 nm, respectively), which are in close agreement with the experimental values of 2.21 and 2.17 eV, respectively, within error37 (Supplementary Table 21).
Fig. 4. TD-DFT calculations for CAuI6AgI2 clusters 3b and 3d.
a, b Calculated and experimental absorption spectra of 3b (calc. ε388 = 3.0 × 104 M−1 cm−1, exp. ε390 = 3.7 × 104 M−1 cm−1) and 3d (calc. ε389 = 2.0 × 104 M−1 cm−1, exp. ε385 = 1.6 × 104 M−1 cm−1). The excitation energies, oscillator strengths, and transition characters for the absorption spectra are summarized in Supplementary Tables 18, 19, and the associated molecular orbitals (MOs) are shown in Supplementary Figs. 68, 69. The lowest peaks observed around 400 nm for 3b and 3d were considered to be transitions between MOs consisting mainly of AuI, AgI and the central carbon. The strong absorption peaks around 330 nm for 3b and 3d are due to the MLCT transition from AuI to ligands37. The absorption in the high-energy region (<300 nm) is mainly due to the ππ* transition of the ligands. c, d Calculated MOs (lowest unoccupied molecular orbital, LUMO; highest occupied molecular orbital, HOMO; singly occupied molecular orbital, SOMO, in the triplet state), comparisons of calculated and experimental excitation energies, and analysis of orbital composition by Mulliken partition of ligands in 3b and 3d.
It is worth noting that the molecular structure of the clusters in the singlet state is very different from that in the triplet state8,9,38,39 (Supplementary Fig. 71 and Supplementary Table 23). For example, the AuI-AuI distances of 3b in the singlet state were found to be in the range of 3.090−3.203 Å, while in the triplet state they were found to be in the range of 2.931−3.443 Å. Therefore, we quantitatively analyzed the orbital compositions to evaluate the ligand effects. Mulliken partition analysis confirmed that even though the core of CAuI6AgI2 is mainly involved in the MOs, the ligands make significant contributions (Supplementary Tables 24–26). Unlike the phosphine ligand, the carbene ligand reduced the involvement of the ligand in the frontier orbitals and suppressed the involvement of the ligand in the charge transfer processes (see Supplementary Information for the details).
We then directly investigated the phosphorescence lifetimes and the radiative rate constants of 3b and 3d using the ZORA method with spin–orbit interaction in a perturbative way40 implemented in the ADF program package41. The results are compared to the experimental values, which approximate the calculated kr as kr = 1/τ (Supplementary Table 27). The phosphorescence is attributed to the three lowest, nearly degenerate spin–orbit states, which are about 0.1 eV lower than other states, with T1 being the dominant contribution. Although the order of the absolute values of τ differs from the experimental values, the trend of kr (3b > 3d) agrees with each other. Importantly, the kr values of imidazolylidene- and benzimidazolylidene-protected clusters were almost quantitatively reproduced in the calculations. Note that the low kr of 3d with imidazolylidene ligands is due to the solvent effect of CH3OH42. In addition, we analyzed the wavefunction and spin–orbit coupling of the low-lying spin–orbit states (Supplementary Tables 27 and 28). These states are described mainly by the T1, T2, and T3 components, with small contributions from the S1 and S3 components. It can be seen that the NHC ligand significantly changed the main component of each state and the coupling of the spin–orbit states. This is thought to be the origin of the different kr values of these compounds.
Finally, in order to evaluate knr, the minimum energy crossing point (MECP) between S0 and T1 states was calculated using the Harvey method43,44. The results showed that the core of CAuI6AgI2 is significantly deformed at the MECP. For example, the shape of CAuI6AgI2 of 3d is significantly changed compared to the shape of the T1 state (Supplementary Fig. 72). The energy barrier from the minimum of T1 state to MECP were calculated as 11.6 and 10.8 kcal/mol for 3b and 3d, respectively, which qualitatively agrees with the knr of these complexes (Supplementary Table 29). However, the energy difference of the MECP for 3b and 3d is small, so there may be another cause. Overall, although NHC ligands are less involved in the electronic structure of the clusters than phosphine ligands, they can accelerate radiative decay by enhancing the spin–orbit coupling of the low-lying spin–orbit states. On the other hand, when the benzimidazolylidene ligand was used, non-radiative decays did not occur preferentially, resulting in 3a and 3b having very high quantum yields.
Ligand-specific translocation in cells
Luminescent metal complexes have been used as promising bioimaging reagents for the past two decades45–47. Li and Wang et al. have reported on the nucleolus-selective labeling behavior of phosphine-protected CAuI6AgI2 cluster 418. Since carbene-protected CAuI6AgI2 clusters 3a and 3b emit phosphorescence sufficient for bioimaging in DMSO/PBS (1:1000, v:v) (Supplementary Figs. 73 and 74, Supplementary Table 30), we further examined their behavior in living cells to determine if there are differences in subcellular distribution and cell renewal pathway depending on the organic ligands.
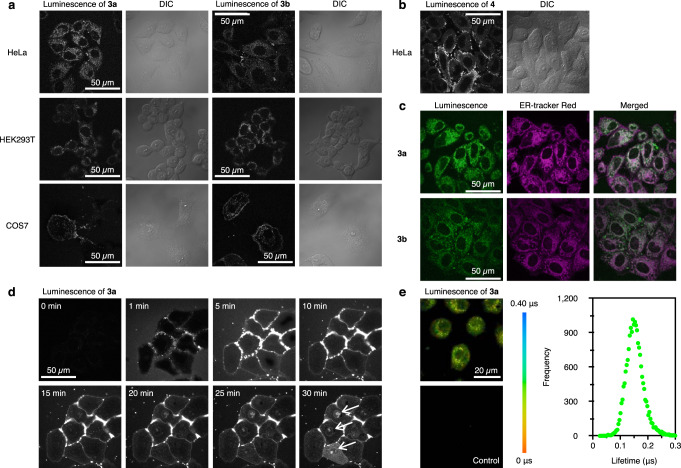
Figure 5 shows the optimized bioimaging results for 3a, 3b, and 4. At the concentration of 1.0 or 2.0 μM, where no cytotoxicity was observed by confocal microscopy analysis (Supplementary Figs. 75–82), 3a, 3b, and 4 entered HeLa cells within 10 min. Clusters 3a and 3b were suggested to accumulate in specific organelles, whereas cluster 4 was uniformly distributed in the cytosol. HEK293T and COS7 cells labeled with 3a or 3b showed a similar distribution pattern. To identify the accumulated structures, the cells labeled with 3a or 3b were further labeled with several organelle markers. Confocal microscope analysis showed that 3a and 3b were selectively located in the ER. The time-lapse imaging revealed that clusters 3a and 3b were transported into the cells within 5 min after accumulation on the surface. 3a or 3b had accumulated in the ER after 10 min, and the longer the incubation time, the more it accumulated in the nucleus region (Fig. 5d and Supplementary Figs. 78, 80). The accumulation of 3a in the ER, induced by a 10-min incubation, was maintained for 36 h (Supplementary Fig. 84). Thus, clusters with the NHC ligands can be selectively translocated to an intracellular organelle in a manner specific to the ligand structure. Furthermore, since these clusters have long emission lifetimes on the order of microseconds, phosphorescence lifetime imaging (PLIM) was conducted. The calculated lifetime of 3a was 0.15 μs, which was in good agreement with the in vitro result. Thus, 3a can be used as an ER-selective targeting reagent, and its phosphorescence can be well distinguishable from autofluorescence.
Fig. 5. Optimized bioimaging results of CAuI6AgI2 clusters 3a, 3b, and 4.
a Confocal luminescence and differential interference contrast (DIC) images of 3a and 3b in HeLa, HEK293T, and COS7 cells. Scale bar, 50 μm. b Confocal luminescence and DIC images of 4 in HeLa cells. c Co-localization images of 3a and 3b with ER-tracker Red in HeLa cells. d Time-lapse images of 3a in Hela cells. White arrows indicate nuclear accumulation. e PLIM images and lifetime plot of 3a in HeLa cells. Scale bar, 20 μm. Each experiment was independently repeated at least three times with similar results. As previously reported18, strong luminescence spots were observed in the nucleoli of the HeLa cells labeled with 4 at a concentration of 10 μM for 10 min. Time-lapse monitoring also confirmed that cellular uptake and nucleoli accumulation were fast under these conditions. At a concentration of 5.0 μM, similar localization was observed by extending the incubation time (30 and 60 min), but at the same time, the cells changed to a round shape and the nuclear envelope showed the characteristics of cells under stress45. Similar nucleoli spots and morphological changes were also observed in the cells labeled with 3a-c at a concentration of 5.0 or 10 μM. HEK293T cells and COS7 cells also showed a similar trend (Supplementary Figs. 75–81).
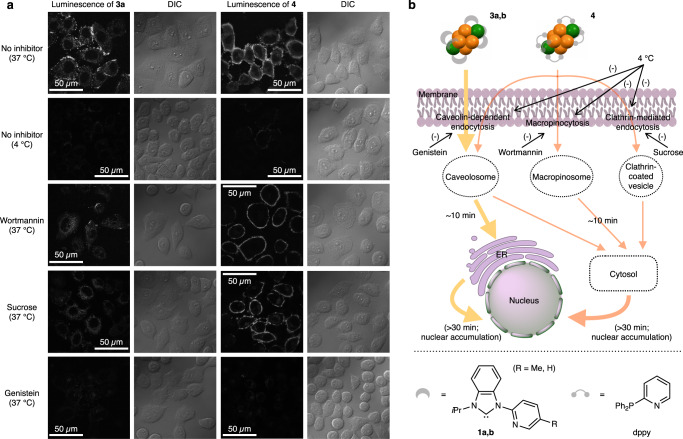
It is noteworthy that CAuI6AgI2 clusters protected by the NHC or phosphine ligands showed different distributions in the cells, even though they have almost the same core structure. To clarify the cellular uptake pathway of 3a and 4, cells were incubated with 3a and 4 at a lower temperature (4 °C), where endocytosis was expected to be non-specifically inhibited. As shown in Fig. 6a, the cells labeled under the condition did not show any luminescence signals. Then, route-specific inhibitors such as wortmannin, sucrose, and genistein were used at 37 °C to specifically inhibit macropinocytosis, clathrin- and caveolin-dependent endocytosis, respectively. For cluster 3a with benzimidazolylidene ligands, the genistein treatment effectively inhibited the ER accumulation. These results suggest that the uptake of cluster 3a is due to a caveolin-dependent endocytosis process. In contrast, the accumulation of cluster 4 with phosphine ligands in the cytosol was blocked by all inhibitors. Thus cluster 4 was taken up into the cells and dispersed in a nonspecific manner. Based on these results, we proposed a possible route of cellular uptake and intracellular translocation of NHC- and phosphine-protected CAuI6AgI2 clusters (Fig. 6b). These clusters first accumulate on the surface of the cell membrane and are then taken up into the cells by endocytosis. For phosphine-protected 4, the uptake is an energy-dependent, nonspecific process that is associated with macropinocytosis, clathrin-mediated, and/or caveolin-dependent endocytosis. In the case of NHC-protected 3a–d, caveolin-dependent endocytosis is the main pathway for cellular uptake. NHC-based clusters 3a and 3b were sequentially translocated to ER after intracellular uptake, but phosphine-based cluster 4 was released from intracellular compartments to cytosol within 10 min. Prolonged incubation allowed the nuclear accumulation of the clusters 3a, 3b, and 4, which in turn increased the permeability of their membranes. In addition, high concentration of clusters accelerated these processes.
Fig. 6. Mechanisms and proposed routes of cellular uptake of carbene- and phosphine-protected CAuI6AgI2 clusters.
a Confocal luminescence and DIC images of clusters 3a and 4 in HeLa cells at 37 °C without pretreatment of inhibitor (control), at 4 °C without pretreatment of inhibitor, or at 37 °C with pretreatment of endocytosis inhibitors wortmannin, sucrose or genistein. Scale bars, 50 μm. Each experiment was independently repeated at least three times with similar results. b Schematic representation of the proposed cellular uptake routes for carbene-protected 3a–d and phosphine-protected 4.
Discussion
In this study, we have established a rational design and synthesis method for a series of phosphorescent AuI-AgI clusters, and revealed that the organic ligands bound to the surface of the metal clusters are important for their photochemical property and subcellar distribution. The NHC ligands were found to remarkably shift the emission wavelength of the CAuI6AgI2 clusters, and significantly affecting the phosphorescence quantum yield and lifetime, compared to the same cluster protected by phosphine ligands. More importantly, the CAuI6AgI2 clusters were taken up into the living cells and showed structure-specific translocation pathways and distribution. This suggests that molecular design may be able to control molecular behaviors in the cell. Therefore, this study is expected to provide a strategy to create metal clusters with tunable functionalities, and also to lead to clear guidelines for designing active and applicable metallodrugs.
Methods
Synthesis of 1a·HI and 1b·HI
Under a nitrogen atmosphere, a Schlenk tube was charged with benzimidazole (5.00 g, 42.3 mmol), K2CO3 (3.90 g, 28.2 mmol), and the corresponding bromopyridine derivatives: 2-bromo-5-methylpyridine (2.43 g, 14.1 mmol) for 1a·HI and 2-bromopyridine (1.37 mL, 14.1 mmol) for 1b·HI. The reaction mixture was heated at 200 °C for 12 h and then allowed to cool to room temperature. After it was diluted with water (50 mL) and extracted with CH2Cl2 (50 mL × 3), the combined organic phase was washed with sat. Na2CO3 aqueous solution (50 mL × 3), and brine (50 mL), and then dried over anhydrous MgSO4 before filtration. Concentration under reduced pressure gave a light red oil. This intermediate was then transferred into a Schlenk flask and dissolved in CH3CN (50 mL) under a nitrogen atmosphere. 2-Iodopropane (1.51 mL, 15.2 mmol) was added to the solution, and the reaction mixture was heated at reflux for 12 h. After cooling to room temperature, the mixture was concentrated to ca. 5 mL under reduced pressure. When diethyl ether (50 mL) was added to this residue, a pale yellow precipitate was obtained as a crude product. Colorless crystals 1a·HI and 1b·HI were obtained by layering diethyl ether on CH2Cl2/CH3CN (9:1, v:v) solution containing the crude product. Yields: 2.30 g (43%, based on 2-bromo-5-methylpyridine) for 1a·HI; 1.90 g (37%, based on 2-bromopyridine) for 1b·HI.
For 1a·HI: 1H NMR (500 MHz, CDCl3, δ, ppm): 11.41 (s, 1H, benzimidazolyl), 8.77 (d, 1H, pyridyl), 8.61–8.59 (m, 1H, benzimidazolyl), 8.46 (d, 1H, pyridyl), 7.95 (dd, 1H, pyridyl), 7.84–7.82 (m, 1H, benzimidazolyl), 7.71 (td, 2H, benzimidazolyl), 5.39 (sept, 1H, isopropyl), 2.48 (s, 3H, methyl), 1.99 (d, 6H, isopropyl).
For 1b·HI: 1H NMR (500 MHz, CDCl3, δ, ppm): 11.46 (s, 1H, benzimidazolyl), 8.94 (d, 1H, pyridyl), 8.67 (m, 2H, benzimidazolyl + pyridyl), 8.16 (td, 1H, pyridyl), 7.86–7.84 (m, 1H, benzimidazolyl), 7.73 (t, 2H, benzimidazolyl), 7.54 (dd, 1H, pyridyl), 5.42 (sept, 1H, isopropyl), 2.01 (d, 6H, isopropyl).
Synthesis of 1c·HI
Under a nitrogen atmosphere, a Schlenk tube was charged with imidazole (2.04 g, 30.0 mmol), K2CO3 (2.76 g, 20.0 mmol), and 2-bromo-5-methylpyridine (1.72 g, 10.0 mmol). The reaction mixture was heated at 190 °C for 12 h and then allowed to cool to room temperature. After it was diluted with water (50 mL) and extracted with CHCl3 (50 mL × 3), the combined organic phases were washed with sat. Na2CO3 aqueous solution (50 mL × 3), and then dried over anhydrous MgSO4 before filtration. Concentration under reduced pressure gave a colorless oil, which was then transferred into a Schlenk flask and dissolved in CH3CN (50 mL) under a nitrogen atmosphere. 2-Iodopropane (1.70 g, 10.0 mmol) was added to the solution, and the reaction mixture was heated at reflux for 12 h. After cooling to room temperature, the mixture was concentrated to ca. 5 mL under reduced pressure. When diethyl ether (50 mL) was added to this residue, a pale yellow precipitate was obtained as a crude product. Colorless crystals of 1c·HI were obtained by layering diethyl ether on CH2Cl2/CH3CN (9:1, v:v) solution containing the crude product. Yield: 1.71 g (52%, based on 2-bromo-5-methylpyridine).
1H NMR (500 MHz, CDCl3, δ, ppm): 11.37 (s, 1H, imidazolyl), 8.54 (d, 1H, pyridyl), 8.32 (d, 1H, pyridyl), 8.26 (t, 1H, imidazolyl), 7.86 (dd, 1.8 Hz, 1H, pyridyl), 7.42 (t, 1H, imidazolyl), 5.27 (sept, 1H, isopropyl), 2.43 (s, 3H, methyl), 1.73 (d, 6H, isopropyl).
Synthesis of 2a–d
Imidazolium/benzimidazolium halide (1a·HI (114 mg, 0.30 mmol) for 2a; 1b·HI (110 mg, 0.30 mmol) for 2b; 1c·HI (98.7 mg, 0.30 mmol) for 2c; 1d·HBr (80.4 mg, 0.30 mmol) or 1d·HI (94.5 mg, 0.30 mmol) for 2d) was dissolved in dry CH2Cl2 (10 mL). Tht-AuCl (96.0 mg, 0.30 mmol) was added and then the solution was stirred for 5 min, which was followed by adding K2CO3 (828 mg, 6.00 mmol). After the mixture was stirred for 12 h in the dark, it was filtered through a thin layer of Celite. The solvent was then removed under reduced pressure using a rotary evaporator. After adding NaBF4 (165 mg, 1.50 mmol) and CH3OH (10 mL), the suspension was stirred for 5 min. CH2Cl2 (5 mL), a solution of KOH (28.0 mg, 0.50 mmol) in CH3OH (3 mL), a solution of AgBF4 (58.5 mg, 0.30 mmol) in CH3OH (1 mL), and H2O (50 μL) were sequentially dropwise added into the mixture under stirring, which leads to a brown suspension. After another 5 min stirring, the suspension was again filtered through Celite and evaporated to dryness. The solid was then transferred to a Schlenk flask with a nitrogen atmosphere, and dry CH2Cl2 (5 mL), Et3N (30.0 μL, 0.20 mmol), and a 2.0 M solution of Me3SiCHN2 in hexanes (48.0 μL, 0.10 mmol) were added. The resulting mixture was stirred for another 1 h. After filtration into a tube, a layer of dry Et2O was added to the CH2Cl2 solution, which gave the products colorless block-like crystals within 2 weeks. Yields: 59.5 mg (40%, based on tht-AuCl) for 2a; 74.3 mg (52%, based on tht-AuCl) for 2b; 31.3 mg (24%, based on tht-AuCl) for 2c; 11.6 mg (9%, based on tht-AuCl (1d·HBr)) for 2d; 10.0 mg (8%, based on tht-AuCl (1d·HI)) for 2d.
For 2a: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 8.40–8.15 (br, 6H, pyridyl), 7.91–7.38 (br, 30H, pyridyl + benzimidazolylidene), 7.22–6.72 (br, 6H, pyridyl), 5.75 (br, 6H, isopropyl), 1.45 (br, 54H, isopropyl + methyl). 13C NMR (126 MHz, CD2Cl2, δ, ppm): 187.9, 150.3, 148.5, 140.0, 135.6, 134.4, 132.4, 125.9, 125.7, 120.9, 113.8, 113.2, 54.3, 22.4, 18.3. ESI-MS (CH2Cl2): 1350.2 ([(C)(Au-1a)6]2+).
For 2b: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 8.43 (s, 6H, pyridyl), 7.86 (br, 6H, pyridyl), 7.75 (m, 12H, benzimidazolylidene), 7.53 (m, 12H, benzimidazolylidene), 7.30 (br, 6H, pyridyl), 6.45 (br, 6H, pyridyl), 5.71 (br, 6H, isopropyl), 1.49 (br, 36H, isopropyl). 13C NMR (126 MHz, CD2Cl2, δ, ppm): 186.6, 151.0, 149.4, 139.1, 134.1, 132.2, 125.7, 125.7, 123.7, 121.3, 115.0, 113.3, 53.9, 21.9. ESI-MS (CH2Cl2): 1308.3 ([(C)(Au-1b)6]2+).
For 2c: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 8.41 (d, 6H, pyridyl), 8.19 (s, 6H, pyridyl), 7.87 (s, 6H, imidazolylidene), 7.21 (s, 6H, imidazolylidene), 6.58 (d, 6H, pyridyl), 5.22 (sept, 6H, isopropyl), 2.08 (s, 18H, methyl), 1.15 (d, 36H, isopropyl). 13C NMR (126 MHz, CD2Cl2, δ, ppm): 179.8, 149.1, 148.7, 138.6, 133.9, 120.1, 117.2, 116.9, 53.6, 22.8, 18.1. ESI-MS (CH2Cl2): 1200.2 ([(C)(Au-1c)6]2+).
For 2d: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 8.58 (d, 6H, pyridyl), 8.38 (d, 6H, pyridyl), 7.91 (s, 6H, imidazolylidene), 7.21 (s, 6H, imidazolylidene), 7.06 (dd, 6H, pyridyl), 6.85 (t, 6H, pyridyl), 5.20 (sept, 6H, isopropyl), 1.15 (d, 36H, isopropyl). 13C NMR (126 MHz, CD2Cl2, δ, ppm): 179.9, 150.9, 149.2, 138.2, 123.8, 120.3, 117.5, 117.5, 53.8, 23.0. ESI-MS (CH2Cl2): 1158.2 ([(C)(Au-1d)6]2+).
Synthesis of 3a–d
CAuI6 cluster (2a (29.6 mg, 10.0 μmol) for 3a; 2b (28.8 mg, 10.0 μmol) for 3b; 2c (25.8 mg, 10.0 μmol) for 3c; 2d (24.9 mg, 10.0 μmol) for 3d) was dissolved in dry CH2Cl2 (3 mL). AgBF4 (6.0 mg, 30.0 μmol) in dry CH3OH (1 mL) was added to the solution under stirring. The mixture was then filtered and the filtrate was layered with dry Et2O. Yellow crystals can be obtained within 1 week. Yield: 30.3 mg (90%, based on 2a) for 3a; 30.2 mg (93%, based on 2b) for 3b; 26.3 mg (89%, based on 2c) for 3c; 18.3 mg (64%, based on 2d) for 3d.
For 3a: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 7.91 (d, 6H), 7.81 (d, 6H), 7.74 (s, 6H), 7.64 (dt, 12H), 7.59 (t, 6H), 7.44 (d, 6H), 5.34 (sept, 6H, isopropyl), 1.41 (d, 18H, isopropyl), 1.34 (s, 18H, isopropyl), 1.12 (s, 18H, methyl). 13C NMR (126 MHz, CD2Cl2, δ, ppm): 183.6, 151.8, 147.4, 143.1, 137.2, 134.5, 131.9, 127.6, 127.1, 123.5, 114.1, 113.4, 54.1, 22.3, 22.1, 16.4. ESI-MS (CH2Cl2): 2389.2 ([(C)(Au-1a)4AgAg2](BF4)2+).
For 3b: 1H NMR (500 MHz, CD2Cl2, δ, ppm): 8.14 (td, 6H), 7.84 (d, 6H), 7.77 (d, 6H), 7.71 (t, 6H), 7.64-7.61 (m, 12H), 7.37 (d, 6H), 6.30 (d, 6H), 5.28 (sept, 6H, isopropyl), 1.44 (d, 18H, isopropyl), 1.33 (d, 18H, isopropyl); 13C NMR (126 MHz, CD2Cl2, δ, ppm): 183.6, 151.6, 149.8, 143.2, 134.5, 131.9, 127.22, 127.16, 125.7, 124.7, 113.8, 112.9, 54.5, 22.6, 22.1. ESI-MS (CH2Cl2): 2897.5 ([(C)(Au−1b)6Ag](BF4)2+).
For 3c: 1H NMR (500 MHz, CD2Cl2/CD3OD = 9:1 (v:v), δ, ppm): 7.91 (d, 6H), 7.67 (s, 6H), 7.57 (s, 6H), 7.51 (d, 6H), 7.38 (s, 6H), 4.72 (sept, 6H, isopropyl), 2.14 (s, 18H, methyl), 1.19 (d, 18H, isopropyl), 0.94 (d, 18H, isopropyl; 13C NMR (126 MHz, CD2Cl2, δ, ppm): 178.6, 150.8, 149.6, 143.1, 136.6, 123.0, 121.8, 119.7, 54.7, 23.0, 22.4, 18.8. ESI-MS (CH2Cl2): 2681.5 ([(C)(Au−1c)6Ag](BF4)2+).
For 3d: 1H NMR (500 MHz, CD2Cl2/CD3OD = 3:1 (v:v), δ, ppm): 8.12 (t, 6H), 7.78 (d, 6H), 7.62 (d, 12H), 7.45 (d, 6H), 7.31 (t, 6H), 4.64 (sept, 6H, isopropyl), 1.16 (d, 18H, isopropyl), 0.96 (d, 18H, isopropyl). ESI-MS (CH2Cl2/CH3OH = 9:1 (v:v)): 1939.2 ([(C)(Au−1d)4AuAg](BF4)+).
X-ray crystallography
Intensity data of compounds 1a–c·HI, 2a–d, and 3a–d were collected on a Rigaku XtaLAB Synergy-DW system (CuKα) at 93 K. The structures were solved by direct methods, and non-hydrogen atoms except for the disordered BF4- and CH2Cl2 in 2b were refined anisotropically by the least squares on F2 using the SHELXTL program. The hydrogen atoms of organic ligands were generated geometrically; no attempt was made to locate the hydrogen atoms of disordered dichloromethane molecules in 2b and water molecules in 2d.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research was supported by JSPS KAKENHI Grants No. JP16H06509 (Coordination Asymmetry) and No. JP21H00453 to M.S., JSPS KAKENHI Grant No. JP19K05538 to M.E., and CREST (JPMJCR1752) from Japan Science and Technology (JST) to T.O. Part of the computation was performed using Research Center for Computational Science, Okazaki, Japan (Project: 20-IMS-C100).
Author contributions
Z.L. designed this work, performed synthetic experiments, analyzed data, and prepared the manuscript. M.Endo performed bioimaging experiments, analyzed data, and prepared the manuscript. H.U. designed this work and directed the research. T.S., P.Z., K.N., and M.Ehara performed theoretical calculations. X.-L.P. assisted in the synthetic experiments. T.E. performed PLIM experiments. T.K., and T.O. directed experiments. M.S. proposed and supervised this study and prepared the manuscript.
Peer review
Peer review information
Nature Communications thanks Hannu Häkkinen and other anonymous reviewer(s) for their contribution to the peer review of this work.
Data availability
The data that support the findings of this study are available within the manuscript and its supplementary information and from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2103969 (1a ∙ HI), CCDC 2103970 (1b ∙ HI), CCDC 2103971 (1c ∙ HI), CCDC 2103972 (2a), CCDC 2103973 (2b), CCDC 2103974 (2c), CCDC 2103975 (2d), CCDC 2103976 (3a), CCDC 2103977 (3b), CCDC 2103978 (3c), and CCDC 2103979 (3d). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhen Lei, Mizuki Endo.
Contributor Information
Masahiro Ehara, Email: ehara@ims.ac.jp.
Takeaki Ozawa, Email: ozawa@chem.s.u-tokyo.ac.jp.
Mitsuhiko Shionoya, Email: shionoya@chem.s.u-tokyo.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-31891-3.
References
- 1.Schmidbaur H, Schier A. A briefing on aurophilicity. Chem. Soc. Rev. 2008;37:1931–1951. doi: 10.1039/b708845k. [DOI] [PubMed] [Google Scholar]
- 2.Schmidbaur H, Schier A. Aurophilic interactions as a subject of current research: an up-date. Chem. Soc. Rev. 2012;41:370–412. doi: 10.1039/C1CS15182G. [DOI] [PubMed] [Google Scholar]
- 3.Kang X, Zhu M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019;48:2422–2457. doi: 10.1039/C8CS00800K. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Gonzalez B, et al. Permanent excimer superstructures by supramolecular networking of metal quantum clusters. Science. 2016;353:571–575. doi: 10.1126/science.aaf4924. [DOI] [PubMed] [Google Scholar]
- 5.Huang R-W, et al. Hypersensitive dual-function luminescence switching of a silver chalcogenolate cluster-based metal-organic framework. Nat. Chem. 2017;9:689–697. doi: 10.1038/nchem.2718. [DOI] [PubMed] [Google Scholar]
- 6.Lei Z, Pei X-L, Jiang Z-G, Wang Q-M. Cluster linker approach: preparation of a luminescent porous framework with NbO topology by linking silver ions with gold(I) clusters. Angew. Chem. Int. Ed. 2014;53:12771–12775. doi: 10.1002/anie.201406761. [DOI] [PubMed] [Google Scholar]
- 7.Ryo K, et al. Ultrabright luminescence from gold nanoclusters: rigidifying the Au(I)-thiolate shell. J. Am. Chem. Soc. 2015;137:8244–8250. doi: 10.1021/jacs.5b04210. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, et al. A mono-cuboctahedral series of gold nanocluster: photoluminescence origin, large enhancement, wide tunability, and structure−property correlation. J. Am. Chem. Soc. 2019;141:5314–5325. doi: 10.1021/jacs.8b13558. [DOI] [PubMed] [Google Scholar]
- 9.Weerawardene KLDM, et al. Luminescence and electron dynamics in atomically precise nanoclusters with eight superatomic electrons. J. Am. Chem. Soc. 2019;141:18715–18726. doi: 10.1021/jacs.9b07626. [DOI] [PubMed] [Google Scholar]
- 10.Yao C, et al. Giant emission enhancement of solid-state gold nanoclusters by surface engineering. Angew. Chem. Int. Ed. 2020;59:8270–8276. doi: 10.1002/anie.202001034. [DOI] [PubMed] [Google Scholar]
- 11.Scherbaum F, Grohmann A, Huber B, Krüger C, Schmidbaur H. “Aurophilicity” as a consequence of relativistic effects: the hexakis(triphenylphosphaneaurio)methane dication [(Ph3PAu)6C]2+ Angew. Chem. Int. Ed. Engl. 1988;27:1544–1546. doi: 10.1002/anie.198815441. [DOI] [Google Scholar]
- 12.Gabbaï FP, Schier A, Riede J, Schmidbaur H. Synthesis of the hexakis[(triphenylphosphane)gold(I)]methanium(2+) cation from trimethylsilyldiazomethane; crystal structure determination of the tetrafluoroborate salt. Chem. Ber. 1997;130:111–113. doi: 10.1002/cber.19971300118. [DOI] [Google Scholar]
- 13.Lei Z, Wang Q-M. Homo and heterometallic gold(I) clusters with hypercoordinated carbon. Coord. Chem. Rev. 2019;378:382–394. doi: 10.1016/j.ccr.2017.11.001. [DOI] [Google Scholar]
- 14.Jia J-H, Wang Q-M. Intensely luminescent gold(I)-silver(I) cluster with hypercoordinated carbon. J. Am. Chem. Soc. 2009;131:16634–16635. doi: 10.1021/ja906695h. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Pei X-L, Wang Q-M. Postclustering dynamic covalent modification for chirality control and chiral sensing. J. Am. Chem. Soc. 2013;135:16184–16191. doi: 10.1021/ja4075419. [DOI] [PubMed] [Google Scholar]
- 16.Jia J-H, Liang J-X, Lei Z, Cao Z-X, Wang Q-M. A luminescent gold(I)–copper(I) cluster with unprecedented carbon-centered trigonal prismatic hexagold. Chem. Commun. 2011;47:4739–4741. doi: 10.1039/c1cc10497g. [DOI] [PubMed] [Google Scholar]
- 17.Lei Z, Pei X-L, Guan Z-J, Wang Q-M. Full protection of intensely luminescent gold(I)-silver(I) cluster by phosphine ligands and inorganic anions. Angew. Chem., Int. Ed. 2017;56:7117–7120. doi: 10.1002/anie.201702522. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, et al. A phosphorescent silver(I)–gold(I) cluster complex that specifically lights up the nucleolus of living cells with FLIM imaging. Biomaterials. 2013;34:4284–4295. doi: 10.1016/j.biomaterials.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Hopkinson MN, Richter C, Schedler M, Glorius F. An overview of N-heterocyclic carbenes. Nature. 2014;510:485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 20.Smith CA, et al. N‐heterocyclic carbenes in materials chemistry. Chem. Rev. 2019;119:4986–5056. doi: 10.1021/acs.chemrev.8b00514. [DOI] [PubMed] [Google Scholar]
- 21.Zhukhovitskiy AV, MacLeod MJ, Johnson JA. Carbene ligands in surface chemistry: from stabilization of discrete elemental allotropes to modification of nanoscale and bulk substrates. Chem. Rev. 2015;115:11503–11532. doi: 10.1021/acs.chemrev.5b00220. [DOI] [PubMed] [Google Scholar]
- 22.Zhong R, Lindhorst AC, Groche FJ, Kühn FE. Immobilization of N‐heterocyclic carbene compounds: a synthetic perspective. Chem. Rev. 2017;117:1970–2058. doi: 10.1021/acs.chemrev.6b00631. [DOI] [PubMed] [Google Scholar]
- 23.Crudden CM, et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat. Chem. 2014;6:409–414. doi: 10.1038/nchem.1891. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, et al. Ballbot-type motion of N-heterocyclic carbenes on gold surfaces. Nat. Chem. 2017;9:152–156. doi: 10.1038/nchem.2622. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod MJ, et al. Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands. Nat. Chem. 2019;11:57–63. doi: 10.1038/s41557-018-0159-8. [DOI] [PubMed] [Google Scholar]
- 26.Polgar AM, Weigend F, Zhang A, Stillman MJ, Corrigan JF. A N‐heterocyclic carbene-stabilized coinage metal-chalcogenide framework with tunable optical properties. J. Am. Chem. Soc. 2017;139:14045–14048. doi: 10.1021/jacs.7b09025. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, et al. Trinuclear gold clusters supported by cyclic (alkyl)(amino)carbene ligands: mimics for gold heterogeneous catalysts. Angew. Chem., Int. Ed. 2014;53:9059–9063. doi: 10.1002/anie.201404665. [DOI] [PubMed] [Google Scholar]
- 28.Narouz MR, et al. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat. Chem. 2019;11:419–425. doi: 10.1038/s41557-019-0246-5. [DOI] [PubMed] [Google Scholar]
- 29.Narouz MR, et al. Robust, highly luminescent Au13 superatoms protected by N‐heterocyclic carbenes. J. Am. Chem. Soc. 2019;141:14997–15002. doi: 10.1021/jacs.9b07854. [DOI] [PubMed] [Google Scholar]
- 30.Shen H, et al. Highly robust but surface-active: an N-heterocyclic carbene-stabilized Au25 nanocluster. Angew. Chem., Int. Ed. 2019;58:17731–17735. doi: 10.1002/anie.201908983. [DOI] [PubMed] [Google Scholar]
- 31.Ube H, Zhang Q, Shionoya M. A carbon-centered hexagold(I) cluster supported by N‑heterocyclic carbene ligands. Organometallics. 2018;37:2007–2009. doi: 10.1021/acs.organomet.8b00291. [DOI] [Google Scholar]
- 32.Lei Z, Nagata K, Ube H, Shionoya M. Ligand effects on the photophysical properties of N,N’-diisopropylbenzimidazolylidene-protected C-centered hexagold(I) clusters. J. Organomet. Chem. 2020;917:121271. doi: 10.1016/j.jorganchem.2020.121271. [DOI] [Google Scholar]
- 33.Lei Z, Pei X-L, Ube H, Shionoya M. Reconstituting the C-centered hexagold(I) clusters with N-heterocyclic carbene ligands. Bull. Chem. Soc. Jpn. 2021;94:1324–1330. doi: 10.1246/bcsj.20210060. [DOI] [Google Scholar]
- 34.Schmidbaur H, Raubenheimer HG, Dobrzańska L. The gold–hydrogen bond, Au–H, and the hydrogen bond to gold, Au···H–X. Chem. Soc. Rev. 2014;43:345–380. doi: 10.1039/C3CS60251F. [DOI] [PubMed] [Google Scholar]
- 35.Rigoulet M, et al. Evidence for genuine hydrogen bonding in gold(I) complexes. Proc. Natl Acad. Sci. USA. 2019;116:46–51. doi: 10.1073/pnas.1817194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakar MA, Sugiuchi M, Iwasaki M, Shichibu Y, Konishi K. Hydrogen bonds to Au atoms in coordinated gold clusters. Nat. Commun. 2017;8:576. doi: 10.1038/s41467-017-00720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greisch J-F, et al. Gas-phase photoluminescence and photodissociation of silver-capped hexagold clusters. J. Phys. Chem. A. 2018;122:5799–5810. doi: 10.1021/acs.jpca.8b01864. [DOI] [PubMed] [Google Scholar]
- 38.Aikens CM. Electronic and geometric structure, optical properties, and excited state behavior in atomically precise thiolate-stabilized noble metal nanoclusters. Acc. Chem. Res. 2018;51:3065–3073. doi: 10.1021/acs.accounts.8b00364. [DOI] [PubMed] [Google Scholar]
- 39.Weerawardene KLDM, Aikens CM. Theoretical insights into the origin of photoluminescence of Au25(SR)18− nanoparticles. J. Am. Chem. Soc. 2016;138:11202–11210. doi: 10.1021/jacs.6b05293. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Ziegler T. A simplified relativistic time-dependent density-functional theory formalism for the calculations of excitation energies including spin-orbit coupling effect. J. Chem. Phys. 2005;123:154102. doi: 10.1063/1.2061187. [DOI] [PubMed] [Google Scholar]
- 41.Te Velde G, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 42.Zhou M, Lei Z, Guo Q, Wang Q-M, Xia A. Solvent dependent excited state behaviors of luminescent gold(I)−silver(I) cluster with hypercoordinated carbon. J. Phys. Chem. C. 2015;119:14980–14988. doi: 10.1021/acs.jpcc.5b03985. [DOI] [Google Scholar]
- 43.Harvey JN, Aschi M. Spin-forbidden dehydrogenation of methoxy cation: a statistical view. Phys. Chem. Chem. Phys. 1999;1:5555–5563. doi: 10.1039/a907723e. [DOI] [Google Scholar]
- 44.Harvey JN, Aschi M, Schwarz H, Koch W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor. Chem. Acc. 1998;99:95–99. doi: 10.1007/s002140050309. [DOI] [Google Scholar]
- 45.New, E. J., Parker, D., Smith, D. G. & Walton, J. W. Development of responsive lanthanide probes for cellular applications. Curr. Opin. Chem. Biol. 14, 238−246 (2010). [DOI] [PubMed]
- 46.Zhao Q, Huang C, Li F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011;40:2508–2524. doi: 10.1039/c0cs00114g. [DOI] [PubMed] [Google Scholar]
- 47.Lo KK-W. Molecular design of bioorthogonal probes and imaging reagents derived from photofunctional transition metal complexes. Acc. Chem. Res. 2020;53:32–44. doi: 10.1021/acs.accounts.9b00416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the manuscript and its supplementary information and from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2103969 (1a ∙ HI), CCDC 2103970 (1b ∙ HI), CCDC 2103971 (1c ∙ HI), CCDC 2103972 (2a), CCDC 2103973 (2b), CCDC 2103974 (2c), CCDC 2103975 (2d), CCDC 2103976 (3a), CCDC 2103977 (3b), CCDC 2103978 (3c), and CCDC 2103979 (3d). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.