Abstract
The phenotypes of atopic dermatitis (AD) are diverse, and ethnic differences have been suggested. To date, few studies have explored large-scale national data on the treatment patterns of AD in Asians. Therefore, we aimed to examine real-world treatment patterns for AD, including the probability of discontinuation of AD treatment and restart after discontinuation. A retrospective observational study was conducted using the nationwide healthcare database in South Korea between January 1, 2016 to July 31, 2020. We identified 944,559 pediatric patients and 1,066,453 adults with AD. Topical corticosteroids and antihistamines were the most commonly prescribed medications in all age groups. The frequency of topical corticosteroid prescription decreased as the age increased. Although immunosuppressive drugs were not widely used in both children and adults, cyclosporine was the most frequently prescribed immunosuppressant, particularly among those aged 12 years or more (1–2%). Pediatric patients were more likely to discontinue treatment than adult patients. Treatment restart for moderate-to-severe AD was earlier than that for overall AD. In conclusion, significant differences were observed in the treatment patterns of AD between pediatric and adult patients. These findings will improve our understanding of the latest treatment patterns for AD, which may contribute to decision-making in clinical practice.
Subject terms: Atopic dermatitis, Epidemiology
Introduction
Atopic dermatitis (AD), also known as atopic eczema, is a common chronic inflammatory skin disorder that affects 11–20% of children and 5–8% of adults. While the prevalence of AD has decreased or plateaued in some countries, its global prevalence is generally increasing1,2. The onset of AD is most common during the first five years of life; however, it can begin at any age and may persist for a long period, as the disease has a relapsing–remitting course with repeated acute flareups3. Indeed, the pathogenesis of AD is complex, involving genetic predisposition, immune dysfunction, and environmental factors, which poses therapeutic challenges to clinicians4.
To date, there is no definite cure for AD; thus, the treatment aims to manage the symptoms and reduce inflammation to improve the quality of life of patients. Current treatment guidelines for AD largely depend on its severity and the age of patients5. Topical corticosteroids (CSs) are recommended as the first-line treatment for flares and for controlling mild AD in both pediatric and adult patients. Systemic therapies, including cyclosporine and dupilumab, are approved for use in recalcitrant chronic AD. Meanwhile, off-label treatments have been frequently used for refractory AD, which is not well controlled by conventional treatments6. Accordingly, understanding the current prescription patterns for AD is important for addressing the gap between clinical practice and relevant guidelines. At present, there are limited data on real-world treatment patterns for AD, particularly in Asian populations7. The phenotypes of AD are diverse, and differences according to ethnicity have also been suggested8.
Herein, we aimed to describe real-world treatment patterns for AD, stratified by age group, using South Korea’s nationwide healthcare database. In addition, we evaluated the time to discontinuation of AD treatment and time to treatment restart after discontinuation.
Materials and methods
Study design and data source
This retrospective observational study was conducted using data collected from the Health Insurance Review and Assessment Service (HIRA) database in South Korea from January 1, 2016 to July 31, 2020. This database covers approximately 98% of the South Korean population and contains comprehensive information on healthcare services. It includes an individual’s anonymized identifier with demographic characteristics (i.e., age, sex, and health insurance type), inpatient and outpatient diagnoses based on the International Classification of Diseases 10th Revision (ICD-10), and information on drug prescriptions (i.e., prescription date, dosage, duration, and route of administration). This study was approved by the institutional review board of Sungkyunkwan University, South Korea (No. 2020-09-009). Because this study used anonymized administrative data, the requirement for informed consent was waived by the institutional review board of Sungkyunkwan University. All research was conducted in accordance with guidelines and regulations of the institutional and national research committee and with the 1964 Helsinki declaration.
Study population
We identified all patients who were newly diagnosed with AD and subsequently received at least one prescription for AD treatment between January 1, 2017 and July 31, 2020. The date of the first prescription for AD treatment served as the index date. We excluded patients diagnosed with AD in 2016 to identify only incident patients diagnosed with AD within our study period. In addition, we excluded patients with diagnoses of immune-mediated inflammatory diseases during the 1-year period preceding the index date to avoid the possibility of including patients who were treated for immune-mediated diseases other than AD (Table S1). Lastly, patients who could not be followed up for at least 1 year from the index date were excluded.
We then constructed two cohorts based on age at the index date. Patients aged < 18 years on the index date were classified as the pediatric cohort and those aged ≥ 18 years at the index date were classified as the adult cohort.
Treatment patterns
We defined the following medications to observe the treatment patterns of AD: topical CSs, topical calcineurin inhibitors (CIs), antihistamines, systemic CSs, immunosuppressants (cyclosporine, azathioprine, methotrexate, mycophenolate mofetil, and interferon-γ), intravenous immunoglobulin (IVIG), alitretinoin, montelukast, dupilumab, and phototherapy. Based on the World Health Organization classification of topical CSs, we categorized the medications into three levels: low (class 6–7), medium (class 3–5), and high (class 1–2)9. In South Korea, dupilumab was eligible for reimbursement for the indication of AD, as of January 1, 2020.
The prevalence of medication use at the index date (treatment initiation) and during the entire observation were examined. To exclude the possibility of potential use for related conditions other than AD, we restricted medications to prescriptions for primary or secondary AD diagnosis. In addition, as an exploratory analysis, we identified the factors associated with oral CSs use and treatment discontinuation.
Discontinuation and restart of AD treatment
Furthermore, we assessed the time to treatment discontinuation and the time to restart after discontinuation in the pediatric and adult cohorts, respectively. Discontinuation of AD treatment was defined as the absence of a prescription for AD treatment for ≥ 6 months. Follow-up began on the index date and ended on the date of discontinuation or at the end of the study period. Restart of AD treatment was defined as the prescription of AD medication after the discontinuation of AD treatment (≥ 6 months gap).
Additionally, to observe treatment patterns focused on systemic treatments for moderate-to-severe AD, the discontinuation and restart of moderate-to-severe AD treatment were evaluated separately. Moderate-to-severe AD treatment was defined as treatment with at least one immunosuppressant, IVIG, alitretinoin, dupilumab, or phototherapy. Patients were followed up from their date of first moderate-to-severe AD prescription until the date of discontinuation or the end of the study period.
Patient characteristics
Demographic characteristics, such as age, sex, insurance type, and region of residence, were assessed on the index date. Different comorbid conditions were measured within a 1-year period before the index date for pediatric and adult cohorts, as common comorbidities may differ between children and adults. Proxies of overall health status, including the duration of hospitalization, number of hospital visits, and Charlson comorbidity index score, were also evaluated for the period during the year before the index date10.
Statistical analysis
Baseline characteristics are presented as frequency (proportion) for categorical variables and as mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables. To estimate the prevalence of AD medication at treatment initiation and during the entire observation period, the results were stratified by age group: 0–1, 2–5, 6–11 and 12–17 for the pediatric cohort and 18–39, 40–59 and ≥ 60 years for the adult cohort. The prevalence was calculated as the number of patients that were prescribed treatment for each medication class, where the denominator represented the total number of patients in each age group. The prevalence of AD medication use between the pediatrics and adults were compared using χ2 test or Fisher’s exact test for categorical variables and Student t-test for continuous variables. p-values of < 0.05 were defined as statistically significant.
To identify the factors associated with oral CSs use and treatment discontinuation, multivariable logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs).
Kaplan–Meier survival curves were used to quantify the time to discontinuation and restart after the discontinuation of AD treatment. We compared the survival curves of the pediatric and adult cohorts using the log-rank test. All the analyses were performed using SAS, version 9.4 (SAS Institute).
Results
Patient characteristics
Among 4,872,859 patients diagnosed with AD between January 1, 2017 and July 31, 2020, 2,011,012 patients met our inclusion criteria (pediatric patients [n = 944,559] and adult patients [n = 1,066,453]) (Fig. S1). The mean (SD) ages of the pediatric and adult cohorts were 5.7 (4.8) and 45.4 (18.2), respectively (Table 1). Allergic rhinitis and conjunctivitis were the most common comorbidities in both the pediatric and adult patients. During the observation period, 1.5% of pediatric patients and 4.2% of adult patients received at least one moderate-to-severe AD medication. The median (IQR) duration of observation period was 29.7 (15.6) months and 27.9 (16.0) months for pediatrics and adults, respectively.
Table 1.
Demographics and clinical characteristics of pediatric (< 18 years) and adult patients (≥ 18 years) with atopic dermatitis.
| Characteristics | Pediatrics (n = 944,559) | Adults (n = 1,066,453) |
|---|---|---|
| Age (years), mean (SD); median | 5.7 (4.8); 4.0 | 45.4 (18.2); 44.0 |
| Sex, male, n (%) | 483,637 (51.2) | 452,175 (42.4) |
| Medical aid recipients, n (%) | 17,101 (1.8) | 38,081 (3.6) |
| Region of residence, n (%) | ||
| Metropolitan | 173,655 (18.4) | 264,668 (24.8) |
| Urban | 294,921 (31.2) | 296,705 (27.8) |
| Rural | 475,983 (50.4) | 505,080 (47.4) |
| Comorbidities, n (%) | ||
| Allergic conditions | ||
| Allergic urticaria | 162,360 (17.2) | 160,852 (15.1) |
| Allergic rhinitis | 624,517 (66.1) | 464,593 (43.6) |
| Asthma | 174,446 (18.5) | 66,539 (6.2) |
| Chronic sinusitis | 99,133 (10.5) | 66,343 (6.2) |
| Conjunctivitis | 260,647 (27.6) | 202,112 (19.0) |
| Skin infections | ||
| Bacterial infections | 184,046 (19.5) | 181,240 (16.7) |
| Fungal infections | 32,805 (3.5) | 125,705 (11.2) |
| Viral infections | 181,952 (19.3) | 76,459 (7.2) |
| Impetigo | 78,899 (8.4) | 16,836 (1.6) |
| Eczema Herpeticum | 32,487 (3.4) | 2308 (0.2) |
| Skin cancer | – | 7979 (0.1) |
| Neuropsychiatric disorders | ||
| Anxiety | 4109 (0.4) | 53,588 (5.0) |
| Depression | 2555 (0.3) | 49,317 (4.6) |
| Sleep disorder | 1775 (0.2) | 54,687 (5.1) |
| ADHD | 5053 (0.2) | 998 (0.1) |
| Cardiovascular comorbidities | ||
| Hypertension | – | 189,500 (17.8) |
| Dyslipidemia | – | 162,068 (15.2) |
| Diabetes | – | 110,533 (9.4) |
| Myocardial infarction | – | 3175 (0.3) |
| Stroke | – | 17,441 (1.6) |
| CCI, mean (SD) | – | 0.48 (1.0) |
| Duration of hospitalization (days), mean (SD) | 1.2 (5.8) | 1.9 (10.2) |
| No. of outpatient visits, mean (SD) | 18.8 (18.0) | 21.7 (26.8) |
| Moderate-to-severe AD*, n (%) | 14,268 (1.5) | 44,298 (4.2) |
| Duration of observation period† (months), mean (SD) | 29.2 (9.2) | 28.0 (9.2) |
| Duration of observation period† (months), median (IQR) | 29.7 (15.6) | 27.9 (16.0) |
AD atopic dermatitis, ADHD attention deficit hyperactivity disorder, CCI Charlson comorbidity index, IQR interquartile range, SD standard deviation.
*Moderate-to-severe AD was defined as receiving at least one immunosuppressant, alitretinoin, intravenous immunoglobulin, dupilumab, or phototherapy, throughout the observational period.
†Duration from the first prescription of any AD medication to the end of the study period (Jul 31, 2020).
Patterns of treatment initiation
Overall, the most common medications prescribed at the initiation of treatment were topical CSs in both pediatric and adult patients, with a prescription rate ranging from 58–85%. (Table 2). The prevalence of individual AD treatments were generally higher in adults than in pediatric patients at treatment initiation. In particular, prescription of systemic CSs was more prevalent in adults than in pediatrics (p < 0.001). Conversely, the frequency of topical CSs was higher among the pediatrics compared with adults (p < 0.001). Immunosuppressants were rarely prescribed at the initiation of treatment.
Table 2.
Treatment initiation pattern for atopic dermatitis.
| Treatment category | Pediatrics | Adults | p-value‡ | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1 year (n = 286,178) | 2–5 years (n = 249,070) | 6–11 years (n = 263,880) | 12–17 years (n = 145,431) | 18–39 years (n = 451,924) | 40–59 years (n = 357,229) | ≥ 60 years (n = 257,300) | ||
| Medications at treatment initiation, mean (SD) | 1.30 (0.53) | 1.68 (0.70) | 1.81 (0.78) | 2.05 (0.85) | 2.02 (0.90) | 1.88 (0.84) | 1.77 (0.81) | < 0.001 |
| Prescription with ≥ 2 distinct medications | 76,835 (26.8) | 136,383 (54.8) | 155,244 (58.8) | 99,072 (68.1) | 294,143 (65.1) | 212,333 (59.4) | 138,912 (54.0) | < 0.001 |
| Type of medication at treatment initiation | ||||||||
| Topical treatment | ||||||||
| Corticosteroids* | 244,439 (85.4) | 184,337 (74.0) | 191,972 (72.7) | 104,294 (71.7) | 289,791 (64.1) | 207,966 (58.2) | 151,614 (58.9) | < 0.001 |
| Low | 216,612 (75.7) | 133,292 (53.5) | 114,994 (43.6) | 42,334 (29.1) | 97,552 (21.6) | 60,366 (16.9) | 40,499 (15.7) | < 0.001 |
| Medium | 25,996 (9.1) | 41,946 (16.8) | 53,685 (20.3) | 30,811 (21.2) | 76,151 (16.9) | 55,475 (15.5) | 38,919 (15.1) | 0.005 |
| High | 5,664 (2.0) | 14,782 (5.9) | 33,512 (12.7) | 42,766 (29.4) | 144,545 (32.0) | 102,435 (28.7) | 78,506 (30.5) | < 0.001 |
| Calcineurin inhibitors | 335 (0.1) | 9551 (3.8) | 16,009 (6.1) | 11,491 (7.9) | 47,167 (10.4) | 33,958 (9.5) | 21,037 (8.2) | < 0.001 |
| Systemic treatment | ||||||||
| Antihistamines | 109,426 (38.2) | 172,424 (69.2) | 186,313 (70.6) | 110,679 (76.1) | 327,085 (72.4) | 245,826 (68.8) | 168,199 (65.4) | < 0.001 |
| Corticosteroids | 13,605 (4.8) | 39,947 (16.0) | 67,498 (25.6) | 65,849 (45.3) | 228,114 (50.5) | 169,640 (47.5) | 105,844 (41.1) | < 0.001 |
| Oral | 12,953 (4.5) | 38,946 (15.6) | 65,717 (24.9) | 62,635 (43.1) | 209,488 (46.4) | 148,793 (41.7) | 86,826 (33.7) | < 0.001 |
| Median daily dose (IQR)† (mg) | 5.0 (5.3) | 6.0 (5.0) | 7.5 (5.0) | 10.0 (5.0) | 10.0 (7.5) | 10.0 (7.5) | 10.0 (7.5) | |
| Parenteral | 1032 (0.4) | 2111 (0.9) | 5178 (2.0) | 11,958 (8.2) | 64,414 (14.3) | 65,299 (18.3) | 47,059 (18.3) | < 0.001 |
| Immunosuppressants | ||||||||
| Cyclosporine | 6 (0.0) | 18 (0.0) | 118 (0.0) | 428 (0.3) | 3843 (0.9) | 3632 (1.0) | 2396 (0.9) | < 0.001 |
| Median daily dose (IQR)† (mg) | 50.0 (10.0) | 50.0 (50.0) | 100.0 (50.0) | 100.0 (150.0) | 100.0 (125.0) | 100.0 (150.0) | 100.0 (150.0) | |
| Methotrexate | 0 | 5 (0.0) | 6 (0.0) | 5 (0.0) | 33 (0.0) | 49 (0.0) | 38 (0.0) | < 0.001 |
| Oral | 0 | 4 (0.0) | 5 (0.0) | 4 (0.0) | 30 (0.0) | 44 (0.0) | 36 (0.0) | < 0.001 |
| Median weekly dose (IQR)† (mg) | – | 10.0 (2.5) | 5.0 (7.5) | 11.3 (13.8) | 10.0 (5.0) | 7.5 (5.0) | 5.0 (6.9) | |
| Parenteral | 0 | 1 (0.0) | 1 (0.0) | 1 (0.0) | 3 (0.0) | 5 (0.0) | 2 (0.0) | 0.084 |
| Azathioprine | 0 | 0 | 1 (0.0) | 56 (0.0) | 131 (0.0) | 94 (0.0) | 81 (0.0) | < 0.001 |
| Mycophenolate mofetil | 0 | 1 (0.0) | 0 | 4 (0.0) | 6 (0.0) | 13 (0.0) | 12 (0.0) | < 0.01 |
| Interferon-γ | 0 | 0 | 1 (0.0) | 1 (0.0) | 1 (0.0) | 2 (0.0) | 0 | 0.329 |
| Intravenous immunoglobulin | 4 (0.0) | 6 (0.0) | 3 (0.0) | 4 (0.0) | 9 (0.0) | 6 (0.0) | 2 (0.0) | 0.723 |
| Dupilumab | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n/a |
| Montelukast | 4598 (1.6) | 12,335 (5.0) | 12,126 (4.6) | 2588 (1.8) | 5222 (1.2) | 5177 (1.5) | 2665 (1.0) | < 0.001 |
| Alitretinoin | 0 | 1 (0.0) | 5 (0.0) | 27 (0.0) | 671 (0.2) | 874 (0.2) | 479 (0.2) | < 0.001 |
| Phototherapy | 245 (0.1) | 741 (0.3) | 2327 (0.9) | 2663 (1.8) | 10,274 (2.3) | 5018 (1.4) | 2452 (1.0) | < 0.001 |
Values are numbers (percentages) unless stated otherwise.
SD standard deviation.
*Topical corticosteroids were categorized into three levels based on the WHO potency-based classification of topical corticosteroids: low (class 6–7), medium (class 3–5) and high (class 1–2).
†Median daily dose during the observational period. For methotrexate, the median weekly doses were calculated.
‡The p-values denote comparison between pediatrics and adults.
Treatment patterns during the entire observational period
The most frequent medications prescribed during the observation period were topical CSs, followed by antihistamines, systemic CSs, and topical CIs (Table 3). The use of antihistamines use was particularly higher among those with allergic comorbidities (Fig. S2). When categorized by potency, those under 11 years of age were more likely to be prescribed low-potency topical CSs, whereas those aged > 12 years and the adults were more likely to be prescribed high-potency topical CSs. Montelukast was more frequently prescribed to pediatrics than adults (p < 0.001). Cyclosporine was the most commonly prescribed immunosuppressant, although overall immunosuppressants were rarely prescribed (< 2%). The median daily dose of cyclosporine ranged from 50.0 mg, for children, to 100.0 mg, for adults.
Table 3.
Treatment pattern for atopic dermatitis during the entire observation period.
| Treatment category | Pediatrics | Adults | p-value‡ | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1 years (n = 286,178) | 2–5 years (n = 249,070) | 6–11 years (n = 263,880) | 12–17 years (n = 145,431) | 18–39 years (n = 451,924) | 40–59 years (n = 357,229) | ≥ 60 years (n = 257,300) | ||
| Prescriptions for AD treatment per year, mean (SD) | 1.27 (1.75) | 1.01 (1.32) | 1.02 (1.43) | 1.43 (2.39) | 1.24 (1.99) | 1.12 (1.93) | 1.34 (2.67) | < 0.0001 |
| Prescriptions with ≥ 2 distinct medications | 152,465 (53.3) | 169,708 (68.1) | 181,334 (68.7) | 110,744 (76.1) | 324,822 (71.9) | 234,390 (65.6) | 158,881 (61.7) | < 0.0001 |
| Type of medication | ||||||||
| Topical treatment | ||||||||
| Corticosteroids* | 259,788 (90.8) | 201,234 (80.8) | 207,980 (78.8) | 114,420 (78.7) | 318,101 (70.4) | 226,692 (63.5) | 167,042 (64.9) | < 0.0001 |
| Low | 238,375 (83.3) | 155,542 (62.5) | 135,657 (51.4) | 56,103 (38.6) | 127,202 (28.2) | 72,377 (20.3) | 49,735 (19.3) | < 0.0001 |
| Medium | 49,942 (17.5) | 58,837 (23.6) | 70,735 (26.8) | 43,018 (29.6) | 101,190 (22.4) | 66,467 (18.6) | 47,612 (18.5) | < 0.0001 |
| High | 13,695 (4.8) | 24,106 (9.7) | 48,218 (18.3) | 58,122 (40.0) | 178,744 (39.6) | 120,261 (33.7) | 93,504 (36.3) | < 0.0001 |
| Calcineurin inhibitors | 6094 (2.1) | 17,936 (7.2) | 26,565 (10.1) | 21,757 (15.0) | 70,162 (15.5) | 40,657 (11.4) | 24,317 (9.5) | < 0.0001 |
| Systemic treatment | ||||||||
| Antihistamines | 169,471 (59.2) | 192,421 (77.2) | 202,088 (76.6) | 118,250 (81.3) | 347,241 (76.8) | 259,277 (72.6) | 180,138 (70.0) | < 0.0001 |
| Corticosteroids | 36,509 (12.8) | 58,827 (23.6) | 87,152 (33.0) | 79,244 (54.5) | 258,709 (57.2) | 186,284 (52.1) | 119,100 (46.3) | < 0.0001 |
| Oral | 35,104 (12.3) | 57,444 (23.1) | 84,883 (32.2) | 75,744 (52.1) | 239,681 (53.0) | 164,635 (46.1) | 98,660 (38.3) | < 0.0001 |
| Median daily dose (IQR)† (mg) | 5.0 (5.3) | 5.0 (5.1) | 7.5 (5.0) | 10.0 (5.0) | 10.0 (5.0) | 10.0 (10.0) | 10.0 (10.0) | |
| Parenteral | 3114 (1.1) | 3926 (1.6) | 9163 (3.5) | 20,165 (13.9) | 88,377 (19.6) | 78,477 (22.0) | 58,092 (22.6) | < 0.0001 |
| Immunosuppressants | ||||||||
| Cyclosporine | 38 (0.0) | 87 (0.0) | 509 (0.2) | 1739 (1.2) | 8419 (1.9) | 5542 (1.6) | 3449 (1.3) | < 0.0001 |
| Median daily dose (IQR)† (mg) | 50.0 (24.0) | 50.0 (40.0) | 75.0 (50.0) | 100.0 (150.0) | 100.0 (125.0) | 100.0 (150.0) | 100.0 (150.0) | |
| Methotrexate | 1 (0.0) | 11 (0.0) | 21 (0.0) | 57 (0.0) | 384 (0.1) | 182 (0.1) | 119 (0.0) | < 0.0001 |
| Oral | 1 (0.0) | 10 (0.0) | 20 (0.0) | 56 (0.0) | 373 (0.1) | 176 (0.1) | 113 (0.0) | < 0.0001 |
| Median weekly dose (IQR)† (mg) | 7.5 (-) | 10.0 (5.0) | 7.5 (5.0) | 10.0 (5.0) | 10.0 (5.0) | 10.0 (5.0) | 7.5 (5.0) | |
| Parenteral | 0 (0.0) | 5 (0.0) | 2 (0.0) | 1 (0.0) | 11 (0.0) | 7 (0.0) | 8 (0.0) | 0.0062 |
| Azathioprine | 0 | 0 | 4 (0.0) | 83 (0.1) | 232 (0.1) | 141 (0.0) | 116 (0.0) | < 0.0001 |
| Mycophenolate mofetil | 0 | 2 (0.0) | 2 (0.0) | 5 (0.0) | 16 (0.0) | 29 (0.0) | 25 (0.0) | < 0.0001 |
| Interferon-γ | 0 | 3 (0.0) | 3 (0.0) | 8 (0.0) | 11 (0.0) | 2 (0.0) | 0 | 0.6112 |
| Intravenous immunoglobulin | 18 (0.0) | 10 (0.0) | 5 (0.0) | 6 (0.0) | 10 (0.0) | 7 (0.0) | 3 (0.0) | 0.0032 |
| Dupilumab | 0 | 0 | 1 (0.0) | 24 (0.0) | 147 (0.0) | 31 (0.0) | 4 (0.0) | < 0.0001 |
| Montelukast | 18,747 (6.6) | 23,165 (9.3) | 19,243 (7.3) | 4359 (3.0) | 8144 (1.8) | 7709 (2.2) | 4113 (1.6) | < 0.0001 |
| Alitretinoin | 0 | 1 (0.0) | 13 (0.0) | 84 (0.1) | 1149 (0.3) | 1286 (0.4) | 658 (0.3) | < 0.0001 |
| Phototherapy | 954 (0.3) | 1761 (0.7) | 4331 (1.6) | 5109 (3.5) | 15,925 (3.5) | 6529 (1.8) | 3410 (1.3) | < 0.0001 |
Values are numbers (percentages) unless stated otherwise.
AD atopic dermatitis, SD standard deviation, IQR interquartile range.
*Topical corticosteroids were categorized into three levels based on the WHO potency-based classification of topical corticosteroids: low (class 6–7), medium (class 3–5) and high (class 1–2).
†Median daily dose during the observational period. For methotrexate, the median weekly doses were calculated.
‡The p-values denote comparison between pediatrics and adults.
For characteristics associated with oral CSs use, adults had higher odds compared with pediatrics (adjusted OR 2.84, 95% CI 2.82–2.86) and patients with allergic rhinitis were more likely to be prescribed oral CSs (1.18, 1.17–1.19) (Table S2).
Discontinuation of AD treatment
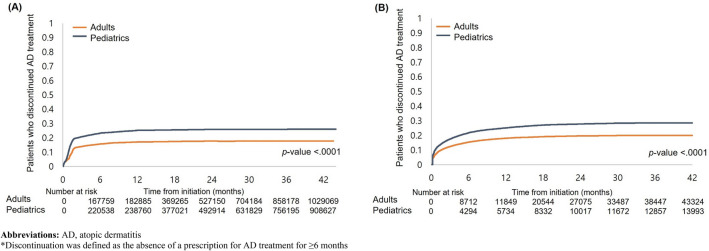
Figure 1A,B shows the Kaplan–Meier curves for time to discontinuation in the pediatric and adult cohorts. The overall probability of AD treatment discontinuation was significantly higher in pediatric patients than in adults (p < 0.001) (Fig. 1A). Specifically, the estimated rate of treatment discontinuation was 23.3% during the first 12 months for pediatric patients and 15.8% for adults. Furthermore, the probability of moderate-to-severe AD treatment discontinuation was higher in the pediatric group than in the adult group (p < 0.001) (Fig. 1B). The estimated rate of discontinuing moderate-to-severe AD treatment within the first 12 months was 21.8% in pediatric patients and 15.4% in adults.
Figure 1.
Kaplan–Meier curve for time to discontinue* (A) AD treatment among pediatrics and adult cohort and (B) moderate-to-severe AD treatment among those who were prescribed moderate-to-severe AD treatment. AD atopic dermatitis. *Discontinuation was defined as the absence of a prescription for AD treatment for ≥ 6 months.
For characteristics associated with treatment discontinuation, adults were less likely to discontinue AD treatment compared with pediatrics (adjusted OR 0.57, 95% CI 0.56–0.58) and patients with allergic rhinitis had lower odds of treatment discontinuation (0.85, 0.84–0.86) (Table S2).
Restart after discontinuation
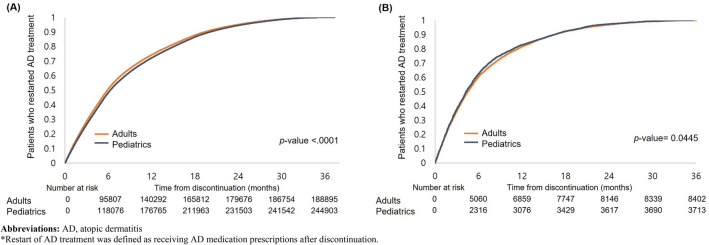
Figure 2A,B presents the Kaplan–Meier curves for the probability of restarting AD treatment among the discontinuers in the pediatric and adult cohorts. There was a significant difference in the probability of restarting any AD treatment among the discontinuers in pediatrics and adults, with a median time to restart of 368 and 356 days, respectively (Fig. 2A). Additionally, restarting tended to be earlier in patients taking medications for moderate-to-severe AD (Fig. 2B). The median time to restart of treatment for moderate-to-severe AD among those who discontinued moderate-to-severe AD treatment was 308 and 316 days for pediatric and adult patients, respectively.
Figure 2.
Kaplan–Meier curve for time to restart* after discontinuation of (A) AD treatment among pediatrics and adult cohort and (B) moderate-to-severe AD treatment among those who were prescribed moderate-to-severe AD treatment. AD atopic dermatitis. *Restart of AD treatment was defined as receiving AD medication prescriptions after discontinuation.
Discussion
This study aimed to assess real-world treatment patterns of patients with AD using a nationwide claims database in South Korea. The results showed significant differences in the treatment pattern between the pediatrics and adults patients with AD. Overall, topical CSs were the most commonly prescribed medications, both at the initiation of treatment and during the entire observation period in all age groups. This is in line with the results of previous studies that analyzed treatment patterns among children and adults with AD in the United States11. Not surprisingly, relevant guidelines also suggest topical CSs as the first-line treatment and maintenance therapy12,13. Of note, infants (0–1 years) and young children (2–5 years) had a higher prescription for topical CSs than other age groups despite the widespread steroid phobia among the parents of pediatric patients with AD14. Furthermore, we assessed the prevalence of topical CSs based on potency. We found that patients aged < 11 years were more likely to be prescribed low-potency topical CSs, whereas those aged > 12 years and the adults were more likely to be prescribed high-potency topical CSs. This may be attributed to the fact that physicians consider age before prescription; additionally, it can be assumed that the AD lesions occurring in infants and children are not as severe as those in adults.
Antihistamines were the second most commonly prescribed medications in all age groups, with a prescription rate ranging from 59 to 81%. In a study of the US medical claims database, antihistamines were prescribed to 40–50% of pediatric patients with AD7. Another study conducted in a tertiary hospital in India reported that 43% of patients with AD were prescribed antihistamines15. Although little to no evidence exists regarding the role of antihistamines in AD treatment, they are widely prescribed by physicians, perhaps, to reduce the itchiness that AD causes. However, it should be noted that the antipruritic effect of antihistamines cannot adequately alleviate AD, and additional or other treatments should be considered16. In addition, the current guidelines only recommend that antihistamines should be used for the purpose of sedation if a patient has sleep disturbance or other atopic comorbidities5. Another possible explanation for the high use of antihistamines would be the high proportion of patients with allergic comorbidities among those who were prescribed antihistamines, and this suggests that physicians may have prescribed them to concomitantly treat the pruritus of AD and other allergic comorbidities. Similarly, regular use of montelukast, specifically among the pediatric patients may be explained by the high prevalence of allergic rhinitis in patients with AD, along with the absence of major adverse effects of montelukast in children17.
Systemic CSs are also frequently prescribed to patients with AD, even at treatment initiation, although most guidelines discourage their use as a first-line therapy18. This finding is similar to the results of previous studies that reported a high prevalence of systemic CS use19,20. Taken together, systemic CSs are still widely prescribed for the treatment of AD despite unfavorable risk–benefit profiles, which indicates the need for safer and more effective systemic treatments21.
In this study, immunosuppressants were not commonly prescribed to either pediatric or adult patients. Among the medications, cyclosporine was the most frequently prescribed immunosuppressant, particularly in adults. This is in line with the current guidelines that recommend cyclosporine as the first-line therapy to treat severe refractory AD and the survey study that found cyclosporine as the preferred first-line therapy among the European dermatologists22,23. However, a previous study conducted in the UK reported methotrexate and azathioprine as the most frequently prescribed immunosuppressants, although their use is off-label24. Another study based on US pediatric and German populations also showed that methotrexate is prescribed more frequently than cyclosporine25. Discrepancies in the findings of the previous studies and those of the present study may be explained by the heterogeneity in the study populations and the medical environment. The prevalence of liver disease is relatively high among South Koreans, and hence, physicians might have been reluctant to prescribe methotrexate, which has a hepatotoxic risk profile26. In addition, previous studies have shown that Asian patients with AD present a unique phenotype involving increased hyperplasia and the increased activation of Th17 and Th2/Th228,27. Differences in phenotypes and serum biomarkers between Asian and European American patients with AD may affect the treatment response rate, which, in turn, influences the decision of physicians in prescribing medications. Moreover, in countries with different access to healthcare systems, cyclosporine, which requires regular monitoring of blood pressure and renal function, may not be commonly prescribed28.
The most notable change in guidelines for the treatment of AD in South Korea is the recommendation of dupilumab as an important treatment option for patients with moderate-to-severe AD29,30. Likewise, a recent study based on German AD registry reported dupilumab to be the leading treatment among the patients with moderate-to-severe AD31. However, the use of dupilumab was minimal in the current study, perhaps due to the reimbursement date of dupilumab in South Korea. Thus, future studies based on the latest data are in need to evaluate the prescription pattern of dupilumab.
In the present study, approximately 25% of pediatric patients and 15% of adults discontinued AD treatment during the observation period. In both the groups, the majority of patients who discontinued AD treatment did so within the first 12 months of treatment. Information on the reasons for discontinuation were not included in the database; however, one possible reason is that due to the chronic and episodic nature of AD, patients may discontinue their treatment during the remission period and restart at subsequent flare-ups32. The median time to restart of AD treatment since discontinuation was approximately 1 year for both adults and pediatrics, although adults tended to restart slightly earlier than pediatrics. Restarting the treatment for moderate-to-severe AD appeared to be earlier than that for overall AD, which suggests that moderate-to-severe AD patients have a shorter time to recurrence.
This study had several limitations. First, owing to the nature of the claims data, we identified patients with AD using the diagnostic code; it was not possible to use the Hanifin and Rajka criteria, which are the diagnostic standard for AD. The HIRA database does not include data on symptoms or clinical manifestations; hence, disease severity was defined based on the prescribed medications, not the Eczema Area and Severity Index. Second, investigating the reasons for discontinuation or restart was beyond the scope of our study because of the lack of information about the reasons in our data. Further studies are necessary to determine why patients discontinue (e.g., adverse events and ineffectiveness) or restart AD treatment. Finally, we cannot rule out the possibility that the medications were prescribed to treat conditions other than AD. To address this concern, we included only prescriptions with a primary diagnosis code for AD in our analysis.
Despite these limitations, this study is significant in that it analyzed the real-world treatment patterns for AD in over 2 million patients using the nationwide data that covers the entire South Korean population. Additionally, we used all AD prescription records in Korea, which allowed us to estimate prevalence according to the severity of AD based on medication use. This study will improve the understanding of the latest treatment patterns for AD in clinical practice, particularly among the Asian population wherein research is sparse.
Supplementary Information
Acknowledgements
This study used HIRA research data (M20200911753) made by Health Insurance Review & Assessment Service(HIRA). The views expressed are those of the author(s) and not necessarily those of the HIRA and the MOHW. We thank the Health Insurance Review and Assessment (HIRA) service of South Korea for providing data in the HIRA database.
Author contributions
J.L., A.C., J.-Y.S., and S.W.S. conceptualized and designed the study. J.L. and A.C. drafted the manuscript. A.C. and I.-S.O. performed the statistical analysis. All authors interpreted the results and contributed to the critical revision of the manuscript for important intellectual content. All authors contributed to revising the draft. J.-Y.S. and S.W.S. supervised the study.
Funding
This work was supported by Pfizer Pharmaceuticals, Korea Ltd.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to Korean Health Insurance Review and Assessment Service policy but are available from the corresponding author on reasonable request.
Competing interests
Ja-Young Jeon and Hyun-Jeong Yoo are employees and shareholders of Pfizer Inc. No potential conflicts of interest relevant to this article are reported. Ju-Young Shin received grants from the Ministry of Food and Drug Safety, the Ministry of Health and Welfare, the National Research Foundation of Korea, and pharmaceutical companies, including Daiichi Sankyo, GSK, and Pfizer, outside the submitted work. The other authors declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jihyun Lee and Ahhyung Choi.
Contributor Information
Ju-Young Shin, Email: shin.jy@skku.edu.
Sang Wook Son, Email: skin4u@korea.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17222-y.
References
- 1.Williams H, et al. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 2008;121(4):947–954. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 3.Avena-Woods C. Overview of atopic dermatitis. Am. J. Manag. Care. 2017;23(8 Suppl):S115–S123. [PubMed] [Google Scholar]
- 4.Thyssen JP, Rinnov MR, Vestergaard C. Disease mechanisms in atopic dermatitis: A review of aetiological factors. Acta Derm. Venereol. 2020;100:340–348. doi: 10.2340/00015555-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J. Am. Acad. Dermatol. 2014;71(2):327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morais-Almeida M, Cabral A. Off-label prescribing for allergic diseases in pre-school children. Allergol. Immunopathol. 2014;42(4):342–347. doi: 10.1016/j.aller.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Paller AS, Siegfried EC, Vekeman F, Gadkari A, Kaur M, Mallya UG, et al. Treatment patterns of pediatric patients with atopic dermatitis: A claims data analysis. J. Am. Acad. Dermatol. 2020;82(3):651–660. doi: 10.1016/j.jaad.2019.07.105. [DOI] [PubMed] [Google Scholar]
- 8.Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman SC, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Cli. Immunol. 2015;136(5):1254–1264. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 9.WHO model prescribing information: Drugs used in skin diseases: Annex: Classification of topical corticosteroids. apps.who.int/medicinedocs/en/d/Jh2918e/32.html#Jh2918e.32.1 [Internet].
- 10.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Silverberg J. Real-world outpatient prescription patterns for atopic dermatitis in the United States. Dermatitis. 2019;30(5):294–299. doi: 10.1097/DER.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 12.Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis. J. Am. Acad. Dermatol. 2004;50(3):391–404. doi: 10.1016/j.jaad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Mohan GC, Lio PA. Comparison of dermatology and allergy guidelines for atopic dermatitis management. JAMA Dermatol. 2015;151(9):1009–1013. doi: 10.1001/jamadermatol.2015.0250. [DOI] [PubMed] [Google Scholar]
- 14.Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: A systematic review. JAMA Dermatol. 2017;153(10):1036–1042. doi: 10.1001/jamadermatol.2017.2437. [DOI] [PubMed] [Google Scholar]
- 15.Giri VP, Giri OP, Kanodia S, Yadav L, Gupta SK. Prescription pattern in atopic dermatitis in skin outpatients in a tertiary care teaching hospital at Darbhanga, Bihar, India. Int. J. Sci. Study. 2014;2(18):10–13. [Google Scholar]
- 16.Wollenberg A, Szepietowski J, Taieb A, Ring J. Corrigendum: Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2019;33(7):1436. doi: 10.1111/jdv.15719. [DOI] [PubMed] [Google Scholar]
- 17.Lee A-Y. Is montelukast benefical in children with atopic dermatitis? Allergy, Asthma Immunol. Res. 2016;8(4):279–281. doi: 10.4168/aair.2016.8.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin. Immunol. 2018;14(2):52. doi: 10.1186/s13223-018-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horii KA, Simon SD, Liu DY, Sharma V. Atopic dermatitis in children in the United States, 1997–2004: Visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120(3):e527–e534. doi: 10.1542/peds.2007-0289. [DOI] [PubMed] [Google Scholar]
- 20.Heratizadeh A, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J. Eur. Acad. Dermatol. Venereol. 2020;34(6):1263–1272. doi: 10.1111/jdv.16078. [DOI] [PubMed] [Google Scholar]
- 21.Drucker A, Eyerich K, de Bruin-Weller M, Thyssen J, Spuls P, Irvine A, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br. J. Dermatol. 2018;178(3):768–775. doi: 10.1111/bjd.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J. Eur. Acad. Dermatol. Venereol. 2018;32(6):850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen FM, et al. The European TREatment of ATopic eczema (TREAT) Registry Taskforce survey: Prescribing practices in Europe for phototherapy and systemic therapy in adult patients with moderate-to-severe atopic eczema. Br. J. Dermatol. 2020;183(6):1073–1082. doi: 10.1111/bjd.18959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert L, Amand C, Gadkari A, Rout R, Hudson R, Ardern-Jones M. Treatment patterns in UK adult patients with atopic dermatitis treated with systemic immunosuppressants: Data from The Health Improvement Network (THIN) J. Dermatol. Treat. 2020;31(8):815–820. doi: 10.1080/09546634.2019.1639604. [DOI] [PubMed] [Google Scholar]
- 25.Hagenström K, Sauer K, Mohr N, Dettmann M, Glaeske G, Petersen J, et al. Prevalence and medications of atopic dermatitis in Germany: Claims data analysis. Clin. Epidemiol. 2021;13:593. doi: 10.2147/CLEP.S315888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin. Mol. Hepatol. 2020;26(2):209. doi: 10.3350/cmh.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen H-C, Czarnowicki T, Noda S, Malik K, Pavel AB, Nakajima S, et al. Serum from Asian patients with atopic dermatitis is characterized by TH2/TH22 activation, which is highly correlated with nonlesional skin measures. J. Allergy Clin. Immunol. 2018;142(1):324–328. doi: 10.1016/j.jaci.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Chidambaram P. Possible Adaptations to the United States from South Korea's Healthcare System. 2015.
- 29.Kim JE, et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (part II): Systemic treatment. Ann. Dermatol. 2015;27(5):578–592. doi: 10.5021/ad.2015.27.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, et al. Consensus update for systemic treatment of atopic dermatitis. Ann. Dermatol. 2021;33(6):497. doi: 10.5021/ad.2021.33.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traidl S, et al. Online survey to identify current challenges in atopic dermatitis management and guideline implementation in German-speaking countries. Eur. J. Dermatol. 2021;31(6):806–812. doi: 10.1684/ejd.2021.4167. [DOI] [PubMed] [Google Scholar]
- 32.Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol. Clin. 2017;35(3):291–297. doi: 10.1016/j.det.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to Korean Health Insurance Review and Assessment Service policy but are available from the corresponding author on reasonable request.