Abstract
Purpose
The objective of this review is to define live birth rate (LBR) and clinical pregnancy rate (CPR) for women ≥ 40 undergoing ovulation induction (OI)/intrauterine insemination (IUI).
Methods
A systematic review was performed in accordance with PRISMA guidelines using PubMed and Google Scholar. The primary and secondary outcomes of interest were LBR and CPR, respectively.
Results
There were 636 studies screened of which 42 were included. In 8 studies which provided LBR for partner sperm, LBR/cycle ranged from 0 to 8.5% with majority being ≤ 4%. Cumulative LBR was 3.6 to 7.1% over 6 cycles with the majority of pregnancies in the first 4. In the four studies providing LBR for donor sperm cycles, LBR/cycle ranged from 3 to 7% with cumulative LBR of 12 to 24% over 6 cycles. The majority of pregnancies occurred in the first 6 cycles. There were three studies with LBR or CPR/cycle ≥ 1% for women ≥ 43. No studies provided data above this range for women ≥ 45. In 4 studies which compared OI/IUI and IVF, the LBR from IVF was 9.2 to 22% per cycle. In 7 studies which compared outcomes by stimulation protocol, no significant differences were seen.
Conclusion
For women ≥ 40 using homologous sperm, the highest probability of live birth is via IVF. However, if IVF is not an option, OI/IUI may be considered for up to 4 cycles in those using partner sperm or 6 cycles with donor sperm. For women > 45, OI/IUI is likely futile but a limited trial may be considered for psychological benefit while encouraging consideration of donor oocyte IVF or adoption. Use of gonadotropins does not appear to be more effective than oral agents in this age group.
Keywords: Intrauterine insemination, Advanced reproductive age, Advanced maternal age, Ovulation induction, In vitro fertilization
Introduction
Ovulation induction (OI) and intrauterine insemination (IUI) is often recommended as a first-line treatment for couples with infertility with at least one patent fallopian tube and either normal or mildly abnormal semen parameters given it is less costly and less invasive than in vitro fertilization (IVF) [1–5]. In addition, in many parts of the United States (US), couples have limited access to centers capable of performing IVF while IUI may be more readily available [6–8]. However, just as natural fecundity decreases with advancing female age, so do success rates with all forms of fertility treatment including OI/IUI [9–12]. As such, couples with female partners of advanced reproductive age are often encouraged to start treatment with more aggressive measures including IVF with or without the use of donor oocytes [13–18]. For some patients, treatment with IVF may be unacceptable for any number of reasons including high cost of IVF, lack of insurance coverage, limited access to or long wait times for IVF at their local or regional center, religious objection, concern for maternal and fetal risks associated with IVF, and, in the case of donor oocytes, strong desire for genetic contribution to offspring [19, 20]. For this reason, many infertility clinics continue to offer IUI to women well past the age of 40. Limited resources are currently available for clinicians to provide guidance as to what female age OI/IUI treatments become futile, or do not improve chances of pregnancy beyond continued attempts to conceive spontaneously [21]. In this systematic review, we sought to critically examine the available literature for information on outcomes of women undergoing OI/IUI over the age 40 to enhance decision-making and inform age-related cutoffs for infertility clinics.
Materials and methods
A search was conducted on September 4th, 2021, using PubMed and Google Scholar databases (1992 to 2020) in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [22]. In PubMed, the search terms “advanced female age and IUI,” “advanced female age and intrauterine insemination,” “advanced reproductive age and IUI,” “advanced reproductive age and intrauterine insemination,” “advanced maternal age and IUI,” “advanced maternal age and intrauterine insemination,” “female age over 40 and IUI,” “female age over 40 and intrauterine insemination,” “female age > 40 and IUI,” and “female age > 40 and intrauterine insemination” were used. A title search in Google Scholar was performed using search terms as above plus “over 40 IUI,” “over 40 intrauterine insemination,” “ > 40 IUI,” “ > 40 intrauterine insemination,” “over forty IUI,” and “over forty intrauterine insemination.” Only full text publications in English were included. Articles were excluded which did not relate specifically to OI/IUI in women greater than or equal to 40 years, including articles which included unmedicated cycles only. Due to the small number of studies providing live birth data, studies including some unstimulated IUI cycles, particularly with use of donor sperm, were included. Studies which included women ≥ 40 years but did not provide specific outcomes in this group, i.e., reported on women ≥ 35 and included all women over 40 in this cohort, were not included. Studies that reported on age groups over but not including women ≥ 40 (i.e., women ≥ 42) were included. Case reports, case series, and systematic reviews were excluded. Titles and abstracts were screened and study quality/potential bias was assessed by the junior author with subsequent review by the two senior authors. The primary outcomes of interest were cumulative live birth rate (LBR) and LBR per cycle. Secondary outcomes of interest were cumulative clinical pregnancy rate (CPR) and CPR per cycle.
Results
Figure 1 provides details of study screening and inclusion. There were 634 studies screened with 338 articles remaining after removal of duplicates. After removal of non-full text articles, articles not relevant to the topic, articles not specifically providing data in women ≥ 40, and articles not in English, 42 studies were eligible for inclusion. The included studies were heterogeneous in terms of whether or not unstimulated IUI cycles were included, which type of stimulation was used, specific cycle protocols, whether partner or donor sperm was used, and outcomes reported. While inclusion and exclusion criteria were heterogenous, almost uniformly all studies excluded women with bilateral tubal disease, intrauterine pathology, and severe male factor infertility unless donor sperm was used. Specific details of the individual included studies which provided cumulative LBR data and LBR/cycle data can be found in Table 1 and Table 2, respectively. Details of studies which provided cumulative CPR and CPR/cycle data can be found in Table 3 and Table 4, respectively.
Fig. 1.
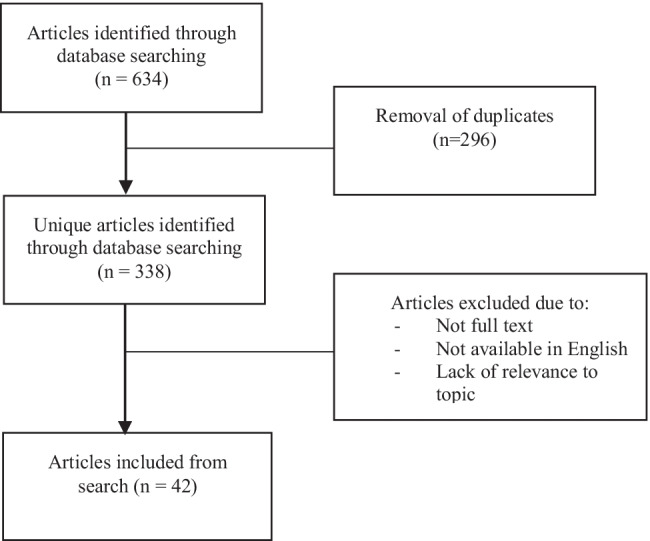
Article identification and screening
Table 1.
Studies including data on cumulative live birth rate following OI/IUI in women ≥ 40
| Authors | Year | Country | Study type | Patients/cycles | # of cycles | Age range | Intervention | Sperm source | Results | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Auyeung et al | 2001 | United States | Retrospective | 401 cycles in 152 women ≥ 40 (58 IVF or GIFT cycles in 45 women and 343 IUI cycles in 119 women). Excluded those with tubal disease, uterine cavity anomalies, unilateral ovary or tube, advanced endometriosis, and uncorrected male factor. Infertility diagnoses were ovulatory dysfunction (48%), male factor (36.1%), endometriosis (7.6%), cervical factor (6.7%), unexplained (4.2%), and other (5%) | 1 to 8 | 40–48 years | Natural cycle/OPK/IUI (n = 38), CC/OPK/IUI with USF/hCG only if no + OPK (n = 194), or GN/USF//hCG/IUI (n = 111) | Not specified | 3.2% Cumulative LBR | Significantly higher LBR in IVF/GIFT group (15.5%) compared with IUI. No difference in outcome by IUI protocol |
| Buyalos et al | 1997 | United States | Prospective | 380 cycles in 119 women. 227 cycles in 70 women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal or uterine factors, cervical factor, and unexplained. Couples with male factor were excluded unless using donor sperm | Not specified | 38–47 | CC (40 cycles) or CC + GN (171 cycles), or GN/USF/hCG/IUI (106 cycles), or natural cycle/OPK/donor sperm IUI (6 cycles) | Partner or donor |
For age 40 (84 cycles, 21pts), cumulative LBR 23.8% For age 41 (62 cycles, 18 pts), cumulative LBR 16.7% For age 42 (50 cycles, 17 pts), cumulative LBR 23.5% |
No pregnancies occurred in women ≥ 43 |
| De Brucker et al | 2013 | Belgium | Retrospective | 764 cycles in 150 women ≥ 40, 46 primary ICSI cycles in 23 women > 40, and 129 ICSI cycles in 63 women after failed IUI (3 groups, switching after 1–3 cycles IUI, 4–6 cycles IUI, or 7–12 cycles IUI) | 1 to > 12 | 40–45 | natural cycle/USF/hCG/IUI, CC/USF/hCG/IUI, or GN/USF/hCG/IUI | Donor | Cumulative LBR 24% (6 cycles) | For primary ICSI, LBR/cycle (#1) 22%, cumulative LBR 29% after 6 cycles. Cumulative LBR not statistically significantly different than for IUI. For switchers, cumulative LBR 29% (3 cycles). No significant difference between primary ICSI and switchers in any group. Time to pregnancy with IUI was 194 days versus 44 days for ICSI |
| Linara-Demakakou et al | 2020 | United Kingdom | Retrospective | 8922 cycles in 3333 women. 1604 cycles in women age 40–42, 675 cycles in women ≥ 43. Patients had a normal fertility work-up, including ovarian reserve markers and pelvic ultrasound. Tubal patency was confirmed | Not specified | Not specified | natural cycle/OPK/IUI (48% in women 40–42, 44% in women ≥ 43), or CC vs GN/USF/hCG/IUI (52% in women 40–42, 56% in women ≥ 43) | Donor | For age 40–42, cumulative LBR 12% (6 cycles) | Plateau after 6 cycles in all groups. Stimulated cycles were significantly more successful in those 40–42y. No difference for women ≥ 43 with stimulation or no stimulation. The oldest patient achieving live birth was 44 |
| Frederick et al | 1994 | United States | Retrospective | 210 completed cycles in 77 women ≥ 40. IUI was chosen as therapy when post-coital tests were poor (6%), semen analyses were abnormal (18%), the patient was oligo-ovulatory (22%), or no obvious diagnosis was made (54%). Tubal patency was documented in the 6 months prior to treatment | Not specified | 40–46 | CC/USF/OPK vs hCG/IUI (31%), or CC + GN/USF/OPK vs hCG/IUI (39%), or GN/USF/OPK vs hCG/IUI | Partner | Cumulative LBR 3.9% | No difference in outcomes based on stimulation protocol |
| Hull et al | 1992 | United Kingdom | Retrospective | 186 cycles, 28 cycles from women ≥ 40. Main indications were tubal damage, unexplained infertility, endometriosis, and male factor. Women with ovarian failure or serious uterine abnormality were excluded | Not specified | 22–46 | CC + GN/USF/hCG/IUI | Partner | There was 1 live birth (4%) | |
| Malchau et al | 2017 | Denmark | Retrospective | 19,884 couples, 833 with female partner ≥ 40 undergoing IUI | Not specified | < 45 years | Natural cycle, CC, or GN (majority CC). No data regarding OPK vs US monitoring or whether trigger was used | Partner | Cumulative LBR 12.4% within 2 years from first cycle, 13.1% within 3 years from first cycle, and 13.5% within 5 years | For women who started treatment with ART, zero had a live birth as a result of IUI |
| Vichinsartvichai et al | 2015 | Thailand | Retrospective | 466 cycles from 221 couples, 31 women > 40. Patients were excluded with tubal factor or severe male factor infertility | 1 to 4 | 21–49 | CC/USF/hCG/IUI, CC + GN/USF/hCG/IUI, or GN/USF/hCG/IUI | Partner | Cumulative LBR 3.6% | Plateau after 4 cycles |
Table 2.
Studies including data on live birth rate per cycle following OI/IUI in women ≥ 40
| Authors | Year | Country | Study type | Patients/cycles | # of cycles | Age range | Intervention | Sperm source | Results | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Bou Nemer et al | 2017 | United States | Retrospective | 239 cycles in women ≥ 40. Causes of infertility included unexplained (50.6%), diminished ovarian reserve (25.9%), male factor (5.4%), ovulatory dysfunction (2%), uterine factor (0.4%), and other (13%) | Not specified | Not specified | CC + GN/USF/hCG/IUI | Not specified | LBR/cycle 1.28% | LBR/cycle was 9.2% for IVF |
| Brzechffa et al | 1997 | United States | Retrospective | 363 cycles in 184 women who had failed at least 3 ovulatory cycles of CC/IUI. 111 cycles in women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal factor, uterine or cervical factor, unexplained, or male factor | Not specified | 25–46 | GN/USF/hCG/IUI | Partner | LBR/cycle 2.7% | No pregnancies occurred with > 1500 IU of hMG was used |
| Buyalos et al | 1997 | United States | Prospective | 380 cycles in 119 women. 227 cycles in 70 women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal or uterine factors, cervical factor, and unexplained. Couples with male factor were excluded unless using donor sperm | Not specified | 38–47 | CC (40 cycles) or CC + GN (171 cycles), or GN/USF/hCG/IUI (106 cycles), or natural cycle/OPK/donor sperm IUI (6 cycles) | Partner or donor |
For age 40 (84 cycles, 21pts), LBR/cycle 5.9% For age 41 (62 cycles, 18 pts), LBR/cycle 4.8% For age 42 (50 cycles, 17 pts), LBR/cycle 8% |
No pregnancies occurred in women ≥ 43 |
| Corsan et al | 1996 | United States | Retrospective | 469 cycles in 168 women ≥ 40 with infertility for > 1 year in setting of tubal patency and normal uterine cavity. Infertility diagnoses included unexplained (n = 35), endometriosis (n = 59), male factor (n = 63), cervical factor (n = 8), ovulatory dysfunction (n = 33). Eighteen percent of patients had multifactorial infertility | Not specified | 40–47 | CC + GN/USF/hCG/IUI (45.8%), or GN/USF/hCG/IUI (54.2%) | Not specified |
For age 40 (135 cycles), LBR/cycle 9.6% For age 41 (114 cycles), LBR/cycle 5.2% For age 42 (84 cycles), LBR/cycle 2.4% For age ≥ 43 (136 cycles), there were no live births |
|
| De Brucker et al | 2013 | Belgium | Retrospective | 764 cycles in 150 women ≥ 40, 46 primary ICSI cycles in 23 women > 40, and 129 ICSI cycles in 63 women after failed IUI (3 groups, switching after 1–3 cycles IUI, 4–6 cycles IUI, or 7–12 cycles IUI) | 1 to > 12 | 40–45 | Natural cycle/USF/hCG/IUI, CC/USF/hCG/IUI, or GN/USF/hCG/IUI | Donor | LBR/cycle 7% |
For primary ICSI, LBR/cycle 22% Time to pregnancy with IUI was 194 days versus 44 days for ICSI |
| Linara-Demakakou et al | 2020 | United Kingdom | Retrospective | 8922 cycles in 3333 women, 1604 cycles in women age 40–42, 675 cycles in women ≥ 43. Patients had a normal fertility work-up, including ovarian reserve markers and pelvic ultrasound. Tubal patency was confirmed | Not specified | Not specified | Natural cycle/OPK/IUI (48% in women 40–42, 44% in women ≥ 43), or CC vs GN/USF/hCG/IUI (52% in women 40–42, 56% in women ≥ 43) | Donor |
For age 40–42, LBR/cycle 5.4% For women ≥ 43, LBR/cycle 0.4% |
Plateau after 6 cycles in all groups. Stimulated cycles were significantly more successful in those 40–42 y. No difference for women ≥ 43 with stimulation or no stimulation. The oldest patient achieving live birth was 44 |
| Ferrera et al | 2002 | United Kingdom | Retrospective | 1056 cycles in 261 patients, 339 cycles in 61 patients > 40. 212 single women, 49 women with same-sex partnerships. Tubal patency was confirmed. Patients (n = 10) with ovulatory dysfunction were treated with CC or GN | 1 to 8 | Not specified | Natural cycle/OPK/IUI (122 cycles), CC/USF/hCG/IUI (101 cycles), or GN/USF/hCG/IUI (116 cycles) | Donor | LBR/cycle 3% | No differences in outcome by cycle protocol |
| Frederick et al | 1994 | United States | Retrospective | 210 completed cycles in 77 women ≥ 40. IUI was chosen as therapy when post-coital tests were poor (6%), semen analyses were abnormal (18%), the patient was oligo-ovulatory (22%), or no obvious diagnosis was made (54%). Tubal patency was documented in the 6 months prior to treatment | Not specified | 40–46 | CC/USF/OPK vs hCG/IUI (31%), or CC + GN/USF/OPK vs hCG/IUI (39%), or GN/USF/OPK vs hCG/IUI | Partner | LBR/cycle 1.4% | No difference in outcomes based on stimulation protocol |
| Haebe et al | 2002 | Canada | Retrospective | 1117 cycles, 106 in women ≥ 40. 24 cycles were performed in women ≥ 43. Couples had patent tubes and absence of severe male factor | Not specified | up to 47 | Natural cycle, CC, CC + GN, GN, or GnRH-agonist + GN/USF/ + / − hCG/IUI | Partner |
For all ≥ 40, LBR/cycle 8.5% For age 40–42, LBR/cycle 9.8% For age ≥ 43, LBR/cycle 4.2% For natural cycle, LBR/cycle 4.8% For CC, LBR/cycle 8% For CC + GN, LBR/cycle 0% For GN, LBR/cycle 10% For GNRHa/GN, LBR/cycle 12.5% |
There were no statistically significant differences in outcomes by cycle type. Oldest woman to have a live birth was 44 y. No difference in terms of outcomes by FSH level > 10 or < 10 |
| Harris et al | 2012 | United States | Retrospective | 262 cycles from 130 women ≥ 38 including 73 women age ≥ 40 who had been trying to conceive for > 1 year. The minimum evaluation included a semen analysis, ovulation assessment, tubal patency, and uterine cavity assessment. Infertility diagnoses included male factor (n = 21), ovulatory dysfunction (n = 8), endometriosis (n = 18), tubal factor (n = 6); diminished ovarian reserve (n = 55), uterine abnormalities (n = 10), and unexplained infertility (n = 30) | 1 to 3 | Not specified | GN/USF/hCG/IUI | Partner | LBR/cycle 2.0% | All live births occurred in the 1st cycle |
| Kang et al | 1996 | United States | Retrospective | 408 cycles from 79 women, 89 cycles in women ≥ 40. Women with abnormal HSG, endometriosis, intrauterine synchiae, or myomas were excluded. Indications for treatment were single women (n = 16) or severe male factor (n = 45) | 1 to 12 | Not specified | Natural cycle/OPK/IUI, CC/USF/OPK vs hCG/IUI, CC + GN/USF/OPK vs hCG/IUI, or GN/USF/OPK vs hCG/IUI | Donor | LBR/cycle 4.5% | No pregnancies beyond cycle #7. No pregnancies in women ≥ 45. No differences in outcome based on stimulation protocol when stratified by age including OPK vs hCG |
| Nuojua-Huttunen et al | 1999 | Finland | Retrospective | 811 cycles, 98 from women ≥ 40. Infertility for > 1 year. Women with PCOS and/or only unilateral tubal patency were excluded | Not specified | Not specified | CC + GN/USF/hCG/IUI | Partner | LBR/cycle 3.1% | No pregnancies in women > 42 |
| Osaikhuwuomwan et al | 2018 | Nigeria | Retrospective | 217 couples, 26 with female partner ≥ 40. Patients had > 1 year of infertility and bilateral tubal patency. Treatment was offered to couples with mild male factor, ovulatory dysfunction, or unexplained infertility | 1 cycle only | Not specified | CC + GN/USF/hCG/IUI | Partner | LBR/cycle 0% | |
| Soares et al | 2019 | Spain | Retrospective | 7228 cycles from 3807 patients, 868 cycles in women ≥ 40. Comparing outcomes to single women (n = 652), same-sex female couples (n = 50), and heterosexual couples using donor sperm (n = 75) to heterosexual couples using partner sperm (n = 91) of equal quality. Included ovulatory and anovulatory women. Included only women who had their first treatment during the study period | Not specified | Not specified | GN/USF/hCG/IUI | Partner and donor | In all groups LBR < 7% | No differences in outcome based on group |
| Wiser et al | 2012 | Canada | Retrospective | 247 women ≥ 40, 85 undergoing IUI results compared to women undergoing IVF (n = 124) and IVM (n = 38) from the same age group. Inclusion criteria were diminished ovarian reserve or unexplained infertility | 1 cycle only | Not specified | CC/USF/hCG/IUI (n = 46) or GN/USF/hCG/IUI (n = 39) | Not specified | For GN/IUI, LBR 2.6%. No pregnancies in CC group | No statistically significant differences in outcomes between treatment groups. Compared to women undergoing IVF over 40, LBR was 13.7% |
Table 3.
Studies including data on cumulative clinical pregnancy rate following OI/IUI in women ≥ 40
| Authors | Year | Country | Study type | Patients/cycles | # of cycles | Age range | Intervention | Sperm source | Results | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al | 1996 | United States | Retrospective | 664 cycles, 130 cycles in women ≥ 41 attempting to conceive for at least 6 months. All patients had at least one patent tube. Both ovulatory (n = 200) and anovulatory (n = 90) patients were included. Patients with severe male factor were excluded | 1 to 6 | 22–48 years | CC/USF/IUI + / − hCG trigger | Partner or donor | 24% cumulative CPR (4 cycles) | No significant difference in outcomes between ovulatory and anovulatory women. The majority of pregnancies occurred in the first 4 cycles regardless of age (> 85%) |
| Bedaiwy et al | 2009 | Canada | Retrospective | 258 cycles in 142 women ≥ 40. Infertility diagnoses were ovarian factor (46.9%), male factor (25.2%), unexplained (22.9%), endometriosis (2.7%), other (2.3%) | Not specified | Not specified | LTZ + GN/USF/hCG/IUI (134 cycles in 90 women) or FSH/USF/hCG/IUI (124 cycles in 69 women) | Not specified |
For LTZ + GN, 13.3% cumulative CPR For GN only, 13.0% cumulative CPR |
No difference with + / − LTZ but fewer cancelled cycles and lower doses of GN used |
| Buyalos et al | 1997 | United States | Prospective | 380 cycles in 119 women. 227 cycles in 70 women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal or uterine factors, cervical factor, and unexplained. Couples with male factor were excluded unless using donor sperm | Not specified | 38–47 | CC (40 cycles) or CC + GN (171 cycles), or GN/USF/hCG/IUI (106 cycles), or natural cycle/OPK/donor sperm IUI (6 cycles) | Partner or donor |
For age 40 (84 cycles, 21pts), cumulative CPR 23.8% For age 41 (62 cycles, 18 pts), cumulative CPR 38.9% For age 42 (50 cycles, 17 pts), cumulative CPR 29.4% |
No pregnancies occurred in women ≥ 43 |
| Campana et al | 1996 | Switzerland | Retrospective | 1115 cycles in 332 couples, 202 cycles in 47 women ages 40–44, 36 cycles in 10 women > 44 | 1 to 9 | Not specified | Natural cycle/USF/OPK/IUI in women with ovulatory cycles, CC/USF/OPK/IUI or GN/USF/hCG/IUI in anovulatory women | Partner | For 40–44, cumulative CPR 10.6% | No pregnancies in women > 44 |
| Dickey et al | 2002 | United States | Prospective, observational | 3381 IUI cycles, 53 cycles in women ≥ 43. Patients were categorized as having ovulatory dysfunction, tubal factor, endometriosis, or other (cervical factor, male factor, or unexplained infertility) | 1 to 14 | Not specified | CC/OPK/IUI or CC/USF/hCG/IUI | Donor or partner | Cumulative CPR 9% (3 cycles) | No pregnancies beyond 3rd cycle |
| Dovey et al | 2008 | United States | Retrospective | 4199 cycles in 1738 patients, 166 cycles in 81 patients ages 41–42 and 120 cycles from 55 patients age > 42. Patients had a structurally normal uterine cavity with at least one open tube without radiographic evidence of peritubal adhesions, were ovulatory or oligo ovulatory, and had at least 5 million total motile sperm postprocessing | 1 to 6 for 41–42. 1 to 8 for > 42 | 20–48 | CC/OPK/IUI | Not specified |
For age 41–42, cumulative CPR 7.4% For women > 42, cumulative CPR 1.8% |
For age 41–42, 66.7% of pregnancies occurred in first 3 cycles, 83.3% of pregnancies occurred in first 4. For > 42, one pregnancy occurred in the 3rd cycle |
| Ferrera et al | 2002 | United Kingdom | Retrospective | 1056 cycles in 261 patients, 339 cycles in 61 patients > 40. 212 single women, 49 women with same-sex partnerships. Tubal patency was confirmed. Patients (n = 10) with ovulatory dysfunction were treated with CC or GN | 1 to 8 | Not specified | Natural cycle/OPK/IUI (122 cycles), CC/USF/hCG/IUI (101 cycles), or GN/USF/hCG/IUI (116 cycles) | Donor | Cumulative CPR 32% | No differences in outcome by cycle protocol |
| Frederick et al | 1994 | United States | Retrospective | 210 completed cycles in 77 women ≥ 40. IUI was chosen as therapy when post-coital tests were poor (6%), semen analyses were abnormal (18%), the patient was oligo-ovulatory (22%), or no obvious diagnosis was made (54%). Tubal patency was documented in the 6 months prior to treatment | Not specified | 40–46 | CC/USF/OPK vs hCG/IUI (31%), or CC + GN/USF/OPK vs hCG/IUI (39%), or GN/USF/OPK vs hCG/IUI | Partner | Cumulative CPR 14.3% | No difference in outcomes based on stimulation protocol. Miscarriage rate 72.7% |
| Harris et al | 2012 | United States | Retrospective | 262 cycles from 130 women ≥ 38 including 73 women age ≥ 40 who had been trying to conceive for > 1 year. The minimum evaluation included a semen analysis, ovulation assessment, tubal patency, and uterine cavity assessment. Infertility diagnoses included male factor (n = 21), ovulatory dysfunction (n = 8), endometriosis (n = 18), tubal factor (n = 6); diminished ovarian reserve (n = 55), uterine abnormalities (n = 10), and unexplained infertility (n = 30) | 1 to 3 | Not specified | GN/USF/hCG/IUI | Partner | Cumulative CPR 12.3% | |
| Hull et al | 1992 | United Kingdom | Retrospective | 186 cycles, 28 cycles from women ≥ 40. Main indications were tubal damage, unexplained infertility, endometriosis, and male factor. Women with ovarian failure or serious abnormality of the uterus were excluded | Not specified | 22–46 | CC + GN/USF/hCG/IUI | Partner | For women ≥ 40, there was 1 pregnancy (4%) | |
| Kang et al | 1996 | United States | Retrospective | 408 cycles from 79 women, 89 cycles in women ≥ 40. Women with abnormal HSG, endometriosis, intrauterine synchiae, or myomas were excluded. Indications for treatment were single women (n = 16) or severe male factor (n = 45) | 1 to 12 | Not specified | Natural cycle/OPK/IUI, CC/USF/OPK vs hCG/IUI, CC + GN/USF/OPK vs hCG/IUI, or GN/USF/OPK vs hCG/IUI | Donor | Cumulative CPR 42% (7 cycles) | No pregnancies beyond cycle #7. No pregnancies in women ≥ 45. No differences in outcome based on stimulation protocol when stratified by age including OPK vs hCG |
| Merviel et al | 2010 | France | Retrospective | 1038 cycles in 353 couples, 16 couples with female partner ≥ 40. Inclusion criteria were at least one patent fallopian tube, an FSH level below 12 IU/L, and more than 500,000 motile, normal spermatozoa | 1 to 9 | Not specified | GN/USF/hCG/IUI | Partner |
Cumulative CPR 25% Cumulative ongoing pregnancy rate (> 12 w GA) 12.5% |
|
| Sahakyan et al | 1999 | United States | Retrospective | 613 cycles from 274 patients, 47 patients ≥ 40. All couples had > 1 year of infertility. Infertility diagnoses included mild male factor (n = 32), anovulation (n = 73), endometriosis (n = 55), tubal factor (n = 17), and unexplained (n = 97) | 1 to 6 | 24–47 | GN/USF/hCG/IUI | Partner | Cumulative CPR 39% (6 cycles) | |
| Schorsch et al | 2013 | Germany | Retrospective | 4246 cycles from 1612 couples, 315 cycles from 133 women ≥ 40. Included ovulatory and anovulatory patients. All patients with mild male factor. All patients with demonstrated tubal patency | 1 to 14 | 19–45 | GN/USF/hCG/IUI | Partner |
For age 40–41 (222 cycles, 94 pts), cumulative CPR 21.28% For age 42–43 (27 women, 64 cycles), cumulative CPR 14.8% For women > 43 (12 women, 29 cycles), cumulative CPR 8.33% |
Few pregnancies after the third cycle |
| Vichinsartvichai et al | 2015 | Thailand | Retrospective | 466 cycles from 221 couples, 31 women > 40. Patients were excluded with tubal factor or severe male factor infertility | 1 to 4 | 21–49 | CC/USF/hCG/IUI, CC + GN/USF/hCG/IUI, or GN/USF/hCG/IUI | Partner | Cumulative CPR 3.6% | Plateau after 4 cycles |
Table 4.
Studies including data on clinical pregnancy rate per cycle following OI/IUI in women ≥ 40
| Authors | Year | Country | Study type | Patients/cycles | # of cycles | Age range | Intervention | Sperm source | Results | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al | 1996 | United States | Retrospective | 664 cycles, 130 cycles in women ≥ 41 attempting to conceive for at least 6 months. All patients had at least one patent tube. Both ovulatory (n = 200) and anovulatory (n = 90) patients were included. Patients with severe male factor were excluded | 1 to 6 | 22–48 years | CC/USF/IUI + / − hCG trigger | Partner or donor | 5.4% CPR per cycle | No significant difference in outcomes between ovulatory and anovulatory women in any age group |
| Andersen et al | 2006 | Multiple European countries | Retrospective | 78,505 cycles using partner sperm with 2,759 cycles from women ≥ 40. 14,779 cycles using donor sperm with 1,335 cycles from women ≥ 40 | Not specified | Not specified | Not specified | Partner or donor |
6.9% CPR/cycle (partner sperm) 6.7% CPR/cycle (donor sperm) |
|
| Ashrafi et al | 2013 | Iran | Retrospective | 1348 cycles from 632 women, 248 cycles in women ≥ 40. Of women > 40, 47.6% unexplained, 23.8% ovulatory dysfunction, 9.5% male factor, 9.5% tuboperitoneal factor, 9.5% multifactorial | 1 to 6 | Not specified | CC or CC/GN or GN + USF/hCG/IUI | Partner | 8.47% CPR/cycle | |
| Auyeung et al | 2001 | United States | Retrospective | 401 cycles in 152 women ≥ 40 (58 IVF or GIFT cycles in 45 women and 343 IUI cycles in 119 women). Excluded those with tubal disease, uterine cavity anomalies, unilateral ovary or tube, advanced endometriosis, and uncorrected male factor. Infertility diagnoses were ovulatory dysfunction (48%), male factor (36.1%), endometriosis (7.6%), cervical factor (6.7%), unexplained (4.2%), and other (5%) | 1 to 8 | 40–48 years | Natural cycle/OPK/IUI (n = 38), CC/OPK/IUI with USF/hCG only if no + OPK (n = 194), or GN/USF//hCG/IUI (n = 111) | Not specified | 5.5% CPR per cycle | No difference in outcome by IUI protocol |
| Bedaiwy et al | 2009 | Canada | Retrospective | 258 cycles in 142 women ≥ 40. Infertility diagnoses were ovarian factor (46.9%), male factor (25.2%), unexplained (22.9%), endometriosis (2.7%), other (2.3%) | Not specified | Not specified | LTZ + GN/USF/hCG/IUI (134 cycles in 90 women) or FSH/USF/hCG/IUI (124 cycles in 69 women) | Not specified |
For LTZ + GN, 9% CPR/cycle For GN only, CPR/cycle 7.3% |
No statistically significant difference in CPR per cycle between women age 40–42 and women > 42. No difference with + / − LTZ but fewer cancelled cycles and lower doses of GN used |
| Belloc et al | 2008 | France | Retrospective | 9,793 cycles, 947 in women ≥ 42. Infertility diagnoses were 35.6% combined male and female factor (35.6%), male factor (23.4%), unexplained (20.3%), “cervical hostility” (12.8%), and multifactorial female causes (7.9%) | Not specified | Not specified | CC/USF/hCG/IUI (60%) or GN/USF/hCG//IUI (40%) | Not specified | CPR/cycle 8.9% | Miscarriage per pregnancy 46.4% |
| Bonow et al | 2019 | Brazil | Retrospective | 381 cycles from 261 patients. 46 cycles from women ≥ 40. Causes of infertility were unexplained (35.9%), ovulatory dysfunction (26.2%), and endometriosis (15.2%) | Not specified | Not specified | GN/USF/hCG/IUI (n = 328), GN + CC/USF/hCG/IUI (n = 42), CC/USF/hCG/IUI (n = 9), natural cycle (n = 2) | Partner | CPR/cycle 4.35% | |
| Bou Nemer et al | 2017 | United States | Retrospective | 239 cycles in women ≥ 40. Causes of infertility included unexplained (50.6%), diminished ovarian reserve (25.9%), male factor (5.4%), ovulatory dysfunction (2%), uterine factor (0.4%), and other (13%) | Not specified | Not specified | CC + GN/USF/hCG/IUI | Not specified | CPR/cycle 5.44% (3% for women age 40 and 7% for those ≥ 41) | CPR/cycle for IVF was 12% compared to 5.4% with IUI. For women age 40, CPR was 15% for IVF vs 3% for IUI. For women ≥ 41, CPR was 7% with IVF and 7% with IUI miscarriage rate was 46.2% |
| Brzechffa et al | 1997 | United States | Retrospective | 363 cycles in 184 women who had failed at least 3 ovulatory cycles of CC/IUI. 111 cycles in women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal factor, uterine or cervical factor, unexplained, or male factor | Not specified | 25–46 | GN/USF/hCG/IUI | Partner | CPR/cycle 3.6% | No pregnancies occurred with > 1500 IU of hMG was used |
| Buyalos et al | 1997 | United States | Prospective | 380 cycles in 119 women. 227 cycles in 70 women ≥ 40. Causes of infertility included ovulatory dysfunction, endometriosis, tubal or uterine factors, cervical factor, and unexplained. Couples with male factor were excluded unless using donor sperm | Not specified | 38–47 | CC (40 cycles) or CC + GN (171 cycles), or GN/USF/hCG/IUI (106 cycles), or natural cycle/OPK/donor sperm IUI (6 cycles) | Partner or donor |
For age 40 (84 cycles, 21 pts), CPR/cycle 7.1% For age 41 (62 cycles, 18 pts), CPR/cycle 11.2% For age 42 (50 cycles, 17 pts), CPR/cycle 10% |
No pregnancies occurred in women ≥ 43 |
| Campana et al | 1996 | Switzerland | Retrospective | 1115 cycles in 332 couples, 202 cycles in 47 women ages 40–44, 36 cycles in 10 women > 44 | 1 to 9 | Not specified | Natural cycle/USF/OPK/IUI in women with ovulatory cycles, CC/USF/OPK/IUI or GN/USF/hCG/IUI in anovulatory women | Partner | For 40–44 CPR/cycle 2.5%, cumulative CPR 10.6% | No pregnancies in women > 44 |
| Corsan et al | 1996 | United States | Retrospective | 469 cycles in 168 women ≥ 40 with infertility for > 1 year in setting of tubal patency and normal uterine cavity. Infertility diagnoses included unexplained (n = 35), endometriosis (n = 59), male factor (n = 63), cervical factor (n = 8), ovulatory dysfunction (n = 33). Eighteen percent of patients had multifactorial infertility | Not specified | 40–47 | CC + GN/USF/hCG/IUI (45.8%), or GN/USF/hCG/IUI (54.2%) | Not specified |
For age 40 (135 cycles), CPR 13.3% For age 41 (114 cycles), CPR 7.9% For age 42 (84 cycles), CPR 4.8% For age ≥ 43 (136 cycles), there was a single clinical pregnancy |
|
| Dovey et al | 2008 | United States | Retrospective | 4199 cycles in 1738 patients, 166 cycles in 81 patients ages 41–42 and 120 cycles from 55 patients age > 42. Patients had a structurally normal uterine cavity with at least one open tube without radiographic evidence of peritubal adhesions, were ovulatory or oligo ovulatory, and had at least 5 million total motile sperm postprocessing | 1 to 6 for 41–42. 1 to 8 for > 42 | 20–48 | CC/OPK/IUI | Not specified |
For age 41–42, CPR/cycle 4.3% For women > 42, CPR/cycle 1% |
|
| Ferrera et al | 2002 | United Kingdom | Retrospective | 1056 cycles in 261 patients, 339 cycles in 61 patients > 40. 212 single women, 49 women with same-sex partnerships. Tubal patency was confirmed. Patients (n = 10) with ovulatory dysfunction were treated with CC or GN | 1 to 8 | Not specified | Natural cycle/OPK/IUI (122 cycles), CC/USF/hCG/IUI (101 cycles), or GN/USF/hCG/IUI (116 cycles) | Donor |
CPR/cycle 5.4% For natural cycle, CPR/cycle 3.3% For CC, CPR/cycle 0.9% For GN, CPR/cycle |
No differences in outcome by cycle protocol |
| Frederick et al | 1994 | United States | Retrospective | 210 completed cycles in 77 women ≥ 40. IUI was chosen as therapy when post-coital tests were poor (6%), semen analyses were abnormal (18%), the patient was oligo-ovulatory (22%), or no obvious diagnosis was made (54%). Tubal patency was documented in the 6 months prior to treatment | Not specified | 40–46 | CC/USF/OPK vs hCG/IUI (31%), or CC + GN/USF/OPK vs hCG/IUI (39%), or GN/USF/OPK vs hCG/IUI | Partner | CPR/cycle 5.2% | No difference in outcomes based on stimulation protocol. Miscarriage rate 72.7% |
| Gomez et al | 2014 | Germany | Retrospective | 5346 cycles in 2180 patients, 328 cycles in women 40–41 and 203 cycles in women > 41. Indication for treatment in all patients was mild male factor | Not specified | 19–45 | Natural cycle/OPK/IUI (n = 433), CC/USF/hCG/IUI (n = 596), or GN/USF/hCG/IUI (n = 4317) | Partner |
For age 40–41, CPR/cycle 8.84% For age > 41, CPR/cycle 3.43% For women 40–41, CPR/cycle for natural cycle 2.8% (n = 36) for GN 10% (n = 231), and for CC 8.2% (n = 61) For women > 41, CPR/cycle for natural cycle 4% (n = 50) for GN 4.5% (n = 111) and for CC 0% (n = 43) |
|
| Haebe et al | 2002 | Canada | Retrospective | 1117 cycles, 106 in women ≥ 40. 24 cycles were performed in women ≥ 43. Couples had patent tubes and absence of severe male factor | Not specified | up to 47 | Natural cycle, CC, CC + GN, GN, or GnRH-agonist + GN/USF/ + / − hCG/IUI | Partner |
For all ≥ 40, CPR/cycle = 17.3% For natural cycle, CPR/cycle 23.8% For CC, CPR/cycle 20% For CC + GN, CPR 10% For GN, CPR/cycle 33% For GNRHa/GN, CPR/cycle 12.5% |
There were no statistically significant differences in outcomes by cycle type. Miscarriage rate 52.6%. Oldest woman to become pregnant 46 years. No difference in terms of outcomes by FSH level > 10 or < 10 |
| Harris et al | 2012 | United States | Retrospective | 262 cycles from 130 women ≥ 38 including 73 women age ≥ 40 who had been trying to conceive for > 1 year. The minimum evaluation included a semen analysis, ovulation assessment, tubal patency, and uterine cavity assessment. Infertility diagnoses included male factor (n = 21), ovulatory dysfunction (n = 8), endometriosis (n = 18), tubal factor (n = 6); diminished ovarian reserve (n = 55), uterine abnormalities (n = 10), and unexplained infertility (n = 30) | 1 to 3 | Not specified | GN/USF/hCG/IUI | Partner | CPR/cycle 4.1% | |
| Houmard et al | 2002 | United States | Retrospective | 658 cycles from 248 patients, 208 cycles from women ≥ 40. 93% of cycles included IUI. 8.7% of patients had ovulatory dysfunction | Not specified | 24–47 | CC + GN/USF/hCG/IUI | Not specified | CPR/cycle 2.4% | |
| Iberico et al | 2004 | Spain | Cross sectional | 1010 cycles from 470 patients, 49 cycles in women ≥ 40. Criteria for inclusion were > 1 year of infertility, normal ovulation history or ovulate response to medication, bilateral patent fallopian tubes, and male partner with at least two semen analyses and at least one trial sperm washing with a quantity of > 5 million motile sperm | Not specified | 18–43 | GN/USF/hCG/IUI | Partner | CPR/cycle 12.2% | |
| Kang et al | 1996 | United States | Retrospective | 408 cycles from 79 women, 89 cycles in women ≥ 40. Women with abnormal HSG, endometriosis, intrauterine synchiae, or myomas were excluded. Indications for treatment were single women (n = 16) or severe male factor (n = 45) | 1 to 12 | Not specified | Natural cycle/OPK/IUI, CC/USF/OPK vs hCG/IUI, CC + GN/USF/OPK vs hCG/IUI, or GN/USF/OPK vs hCG/IUI | Donor | CPR/cycle 5.6% | No pregnancies in women ≥ 45. No differences in outcome based on stimulation protocol when stratified by age including OPK vs hCG |
| Khalil et al | 2001 | Denmark | Retrospective | 2473 cycles from 893 patients, 39 cycles in women ≥ 40. Criteria included infertility for > 2 years, at least one patent tube, and at least 1 million motile sperm on semen analysis × 2. Ovulatory and anovulatory patients were included | 1 to 9 | Not specified | CC/USF/hCG/IUI or CC + GN/USF/hCG/IUI or GN/USF/hCG/IUI | Partner | CPR/cycle 10.3% | |
| Michau et al | 2019 | France | Retrospective | 4146 cycles from 1312 couples. Patient had at least one patent fallopian tube | Not specified | 18–42 | GN/USF/hCG/IUI | Partner | CPR/cycle 8.3% | |
| Nuojua-Huttunen et al | 1999 | Finland | Retrospective | 811 cycles, 98 from women ≥ 40. Infertility for > 1 year. Women with PCOS and/or only unilateral tubal patency were excluded | Not specified | Not specified | CC + GN/USF/hCG/IUI | Partner | CPR/cycle 4.1% | No pregnancies in women > 42 |
| Osaikhuwuomwan et al | 2018 | Nigeria | Retrospective | 217 couples, 26 with female partner ≥ 40. Patients had > 1 year of infertility and bilateral tubal patency. Treatment was offered to couples with mild male factor, ovulatory dysfunction, or unexplained infertility | 1 cycle only | Not specified | CC + GN/USF/hCG/IUI | Partner | CPR/cycle 7.7% | |
| Plosker et al | 1994 | Canada | Retrospective | 381 cycles from 215 couples, 25 cycles from women ≥ 40. All patients had > 1 year infertility. Categorized as having non-severe male factor (i.e., > 1 mil sperm/mL), endometriosis, non-endometriosis tubal factor (at least one patent tube), idiopathic, ovarian dysfunction, or multifactorial infertility | 1 to 6 | Not specified | natural cycle CC, CC + GN, or GN, or GN + GNRHa/USF/OPK vs lupron or HCG trigger/IUI | Partner | CPR/cycle 4% | |
| Sahakyan et al | 1999 | United States | Retrospective | 613 cycles from 274 patients, 47 patients ≥ 40. All couples had > 1 year of infertility. Infertility diagnoses included mild male factor (n = 32), anovulation (n = 73), endometriosis (n = 55), tubal factor (n = 17), and unexplained (n = 97) | 1 to 6 | 24–47 | GN/USF/hCG/IUI | Partner | CPR/cycle 7% | |
| Schorsch et al | 2013 | Germany | Retrospective | 4246 cycles from 1612 couples, 315 cycles from 133 women ≥ 40. Included ovulatory and anovulatory patients. All patients with mild male factor. All patients with demonstrated tubal patency | 1 to 14 | 19–45 | GN/USF/hCG/IUI | Partner |
For age 40–41 (222 cycles, 94 pts), CPR/cycle 9.01% For age 42–43 (27 women, 64 cycles), CPR/cycle 6.25% For women > 43 (12 women, 29 cycles), CPR/cycle 3.45% |
Few pregnancies after the third cycle |
| Steiner et al | 2021 | Canada | Retrospective | 1596 stimulated IUI cycles, 846 from women ≥ 40. All patients had at least one patent fallopian tube. Patients with stage 3 or greater endometriosis or submucosal fibroids were excluded | 1 to 3 | 38–43 | CC or LTZ/USF/hCG/IUI (n = 161) or GN/USF/hCG/IUI (n = 685) | Partner and donor (approximately 8–10% in each group) | CPR/cycle for CC or LTZ 6.8% CPR/cycle for GN 4.8% | Women in the GN group were significantly older than the PO medication group, had lower basal FSH levels, had more follicles with stimulation, and thicker endometrial stripes but no differences in clinical pregnancy rates between PO meds and GN when controlling for these factors |
| Stone et al | 1999 | United States | Retrospective | 9963 cycles (2,825 patients ≥ 40) | Not specified | Up to 50 | No stim (n = 1367), CC (n = 149), GN (n = 128) + / − CC (n = 37), or GNRHa (n = 6)/OPK vs USF/hCG/IUI | Partner and donor |
For age 40–45, total CPR/cycle 4.7% For age > 45, CPR/cycle 0.5% |
Age 40 — 6% ongoing pregnancy/pt Age 41 — 4.7% ongoing pregnancy/pt Age 42 — 3.3% ongoing pregnancy/pt Age 43 — 2.2% ongoing pregnancy/pt Age 44 — 3.3% ongoing pregnancy/pt Age 45 — 1.2% ongoing pregnancy/pt. No other ongoing pregnancies except a single pregnancy in a 48yo pt, and two miscarriages, one in a 46yo, and one in a 47yo |
| Tay et al | 2007 | Malaysia | Retrospective | 507 cycles from 317 patients, 33 cycles in women > 40. All couples had > 1 year of infertility. Tubal patency investigated either up front or after 1–2 failed cycles of IUI. Couples were grouped by primary and secondary infertility and by diagnosis; mild male factor (n = 69), anovulation (n = 100), severe endometriosis (n = 48), tubal factor (n = 92), and unexplained (n = 198) | Not specified | Not specified | CC + GN/USF/hCG/IUI | Partner | CPR/cycle 6.1% | |
| Van der Westerlaken et al | 1998 | Netherlands | Retrospective | 1763 cycles from 466 couples, 21 women were ≥ 40. Categories included one-sided tubal pathology, mild male factor, ovulatory dysfunction, or unexplained infertility | 1 to 8 | Not specified | CC/USF/OPK vs hCG/IUI | Partner | No pregnancies occurred in women ≥ 40 | |
| Wiser et al | 2012 | Canada | Retrospective | 247 women ≥ 40, 85 undergoing IUI results compared to women undergoing IVF (n = 124) and IVM (n = 38) from the same age group. Inclusion criteria were diminished ovarian reserve or unexplained infertility | 1 cycle only | Not specified | CC/USF/hCG/IUI (n = 46) or GN/USF/hCG/IUI (n = 39) | Not specified | For GN/IUI, CPR/cycle 2.6%. No pregnancies in CC group | No statistically significant differences in outcomes between treatment groups. Compared to women undergoing IVF over 40, CPR was 16.9% |
Oral ovulation induction agents
Nine of the included studies provided specific outcomes from use of oral ovulation induction agents (i.e., clomiphene citrate (CC) or letrozole (LTZ)) in women ≥ 40 [23–31]. None of the included studies provided data on cumulative LBR (Table 1). Only one study provided LBR/cycle data specifically for oral OI agents (CC only) [23]. This was a retrospective study in which patient age ranged from 40 to 47 but vast majority of cycles were within women age 40–42. Only cycles using partner sperm were included. Cycles which were monitored via ovulation predictor kit (OPK) and those monitored by ultrasound with or without administration of an hCG trigger were included. LBR/cycle in this study was 8% (n = 25 cycles) (Table 2). The remaining studies provided CPR data. The details of these studies can be found in Table 3 and Table 4. Cumulative CPR ranged from 7.4% over 6 cycles (n = 6/81 patients) up to 24% over 4 cycles (n = 130 cycles) [24–26] (Table 3). CPR/cycle ranged from 0 to 8.2% (n = 5/61) [24–30] (Table 4). None of the studies which provided data on CPR in oral OI/IUI cycles included specific data on miscarriage rates in women ≥ 40 except for one at 52.6% (n = 10/19 pregnancies) [23].
None of these studies included data on LBR by cycle number. In studies which examined CPR by cycle number, the vast majority of pregnancies occurred within the first 3–4 cycles [24, 25, 31]. The outcomes for four of these studies are from age groups ranging from 40 to 43 [23, 25, 27, 28]. The cumulative CPR for women ≥ 42 was 1.8% over 8 cycles (n = 1/55) [25]. The CPR/cycle for women ≥ 42 ranged from 0% (n = 0/43) to 0.8% (n = 1/120) [25, 27]. The cumulative CPR for women ≥ 43 was 9% over 3 cycles (n = 53 cycles) [31]. All but one study had a variety of indications for oral OI/IUI and did not stratify results based on infertility diagnosis specifically in women ≥ 40 [23–26, 28–31]. These studies were also heterogenous in terms of use of donor or partner sperm. In terms of factors predictive for success specifically for use of oral OI, one study provided information on this and reported that there were no differences in outcome based on whether the patient was ovulatory or anovulatory [24].
Injectable ovulation induction agents
Thirteen of the included studies provided data specifically on pregnancy outcomes in gonadotropin (GN)/IUI cycles in women ≥ 40 [23, 26–28, 32–40]. One study provided cumulative LBR data at 7.8% over 1–3 cycles [23] (Table 1). The details of this study were previously discussed above. In studies which provided LBR/cycle data, LBR/cycle ranged from 2.0% (n = 73 women) to 12.5% (n = 5/40 cycles) [23, 32–34] (Table 2). Other than the study by Haebe et al., the LBR/cycle when using partner sperm ranged from 2.0 to 6.6% (n = 91cycles) [32–34]. In one of these studies, all included patients had previously failed to conceive with three CC/IUI cycles [32]. In this study, the age ranged from 40 to 46 [32]. The other two studies did not specify age range [33, 34]. All patients in these studies had ultrasound monitoring and hCG trigger. In one study with LBR data for donor sperm cycles, LBR/cycle was 6.0% (n = 777 cycles). Indication for donor sperm included single parent and same-sex female partnership procreation as well as severe male factor infertility [34]. The remaining studies provided CPR data. Details of these studies can be seen in Tables 3 and 4. Cumulative CPR ranged from 21.3% over 14 cycles (n = 94 patients) to 39% over 6 cycles (n = 47 patients) [35–37]. One study reported cumulative ongoing pregnancy rate (> 12 weeks’ gestation) of 12.5% (n = 16 women) [35]. None of the other included studies providing CPR data provided data on miscarriage rates specifically for women ≥ 40 (Table 3). CPR/cycle ranged from 4.5% (n = 5/111) to 12.2% (n = 6/49) [26–28, 35, 36, 38–40] (Table 4).
In studies which provided data on pregnancy outcome per cycle, all pregnancies occurred in the first 1–4 cycles [33, 37]. In studies which stratified by age, cumulative pregnancy rates over a range of 1 to 14 cycles were 21.3% for women age 40–41, 14.8% for women age 42–43, and 8.3% for women > 43 [37]. CPR/cycle was 9% (n = 94 cycles) to 10% (n = 328 cycles) for women age 40–41, 4.5% (n = 203 cycles) to 6.3% (n = 64 cycles) for women ≥ 42, and 3.5% (n = 29 cycles) for women ≥ 43 [27, 37]. These studies were heterogenous in terms of indication for GN/IUI and whether partner or donor sperm was used. Very few of the above studies investigated predictive factors for success specifically in women ≥ 40. One study reported that no pregnancies occurred in women ≥ 40 who required ≥ 1500 U human menopausal gonadotropin (hMG) during their cycle (n = 111 cycles) [32]. The only other predictive factor, or lack thereof, was in the study by Soares et al. in which there were no differences in LBR/cycle seen for women ≥ 40 years regarding whether they used donor sperm due to being unpartnered, in a same-sex female couple, or in a heterosexual couple with severe male factor or were a heterosexual couple using autologous sperm (n = 868 cycles) [34].
Oral ovulation induction agents combined with injectables
There were seven studies which provided data uniquely regarding cycles which combined oral and injectable agents [23, 38, 41–45]. One study provided cumulative LBR data for CC + GN/IUI cycles of 4% LBR (n = 28 cycles). The number of cycles per patient was not specified. Patient age range was 40–46. Only cycles using partner sperm were included (Table 1) [42]. In studies that reported on LBR/cycle, the range was from 0% (n = 26 women for Osaikhuwuomwan et al. and n = 10 women for Haebe et al.) to 3.1% (n = 98 women) [23, 43, 44] (Table 2). All three of these studies used CC as the oral OI agent and all three used exclusively partner sperm. The remainder of the details of these studies can be found in Table 2. The remainder of the studies provided CPR data. The details of these studies can be found in Tables 3 and 4. There was only one study which reported a cumulative pregnancy rate at 13% (n = 90 women, number of cycles not specified) [38] (Table 3). CPR/cycle ranged from 2.4% (n = 5/208) to 10% (n = 1/10) [23, 38, 41, 45]. In studies that provided information on rate of miscarriage in women ≥ 40, range was 46.2% (n = 6/13 pregnancies) to 52.6% (n = 10/19 pregnancies) [23, 41].
None of the above studies provided data on outcome by cycle number. In terms of stratification by age, CPR/cycle was 3% in those age 40 (n = 3/291) and 7% in those ≥ 41 (n = 10/479) [41]. Age stratification of outcomes was not performed for LBR data. One study commented that no pregnancies occurred in women ≥ 42 in their study (n = 98 cycles) [43]. The above studies utilized CC in addition to GN with the exception of the Bedaiwy et al. study which used letrozole [38]. Studies were heterogenous in terms of use of partner or donor sperm and some did not specify sperm source. None of these studies investigated for factors predictive of success in combination with oral and injectable OI/IUI cycles.
Other included studies
The remainder of studies used multiple different IUI protocols including cycles without stimulation and did not stratify data based on type of stimulation used [46–60]. In cycles using partner sperm, the cumulative LBR rate ranged from 3.6% (n = 31 women) to 12.4% (n = 833 women) [46–48]. Number of cycles was only reported for one of these studies [48]. In the Malchau et al. study, patient age ranged from 40 to 45. The majority of cycles were using CC but some natural cycles and GN cycles were included too with results not stratified by stimulation type. Time interval was in the 2 years following initiation of treatment [46]. The age range in the Frederick et al. study was 40 to 46 and for the Vichinsartvichai et al. study was 40 to 49 [47, 48]. Both of these studies included CC + GN or GN alone cycles [47, 48]. The remainder of details of these studies can be found in Table 1. LBR/cycle ranged from 1.4% (n = 3/210) to 8.5% (n = 9/106) [23, 47] (Table 2). The details of these two studies have previously been discussed. In cycles using donor sperm, cumulative LBR ranged from 12% (n = 2,279 cycles) to 24% (n = 764 cycles) over 6 cycles [49, 50] (Table 1). Both studies included both unmedicated and medicated cycles (including both CC and GN cycles) [49, 50]. For the De Brucker et al. study, age range was 40 to 45 [50]. Age range was not specified in the Linara-Demakakou et al. study [49]. LBR/cycle ranged from 3% (n = 339 cycles) to 7% (n = 150 cycles) [26, 49–51] (Table 2). Two of these studies were previously discussed above [49, 50]. The remaining two studies included unmedicated cycles as well as cycles with oral agents and injectable agents without stratification of results [26, 51]. One study did clarify that medicated cycles were only performed in women with ovulatory dysfunction [26]. Both of these studies included single women [26, 51]. One included couples with severe male factor [51]. The other included same-sex female couples but not heterosexual couples with male factor [26]. The remainder of the details of these studies can be found in Table 2. Studies which exclusively evaluated donor sperm cycles generally reported a plateau in outcomes between 5 and 7 cycles though one reported not reaching plateau until after 9 [26, 49–51]. Only one of the studies utilizing donor sperm provided data stratified by age. In this study, the LBR/cycle was 5.4% in women 40–42 years (n = 1604 cycles) and 0.4% in women ≥ 43 (n = 675 cycles) [49]. One study commented that no live births occurred in women ≥ 45 [51].
The remaining studies either did not specify sperm source or used a combination of partner and donor sperm [30, 52–54]. Cumulative LBR ranged from 3.2% (n = 119 women) to 17% (n = 12/70) (number of cycles not specified) [52, 53] (Table 1). The LBR/cycle range was 2.6% (n = 1/39) to 5.3% (n = 12/227) [30, 53, 54] (Table 2). In studies which stratified by age, cumulative LBR was 23.8% for women age 40, 16.7% for women age 41, and 23.5% for women age 42 [53] (Table 1). LBR/cycle ranged from 5.9% (n = 84 cycles) to 9.6% (n = 135 cycles) for women age 40, from 4.8% (n = 62 cycles) to n = 5.2% (n = 114 cycles) for women age 41, and 2.4% (n = 84 cycles) to 8% (n = 50 cycles) for women age 42 [53, 54]. In one study, no live births occurred in women ≥ 43 (n = 136 cycles) [54]. There were an additional nine studies which provided CPR data not previously discussed, the details of which are outlined in Tables 3 and 4 [55–63]. Notable points from these studies are that in one study, no pregnancies occurred in women ≥ 44 (n = 36 cycles from 10 women) [58]. One study provided data for women ≥ 45 with CPR/cycle of 0.5% (n = 1135 women) [60].
In terms of cycle factors predictive of success, the studies that compared cycle types found no significant difference between stimulation protocols [23, 27, 30, 47, 49, 52, 53]. The one exception is that Bedaiwy et al. found that adding letrozole to GN/IUI cycles decreased the number of cancelled cycles and doses of gonadotropin needed [38]. For donor sperm cycles, in the study by Linara-Demakakou et al., stimulated cycles were more successful in those 40–42 years (LBR/cycle 4.1% vs 6.7%) but there was no difference in outcome between stimulated and unstimulated cycles for women ≥ 43 (0.3% vs 0.5% LBR/cycle) [49]. However, no difference was seen in outcomes in other studies using donor sperm [26, 51]. In terms of patient factors, no difference was seen in outcomes for women ≥ 40 with FSH greater than or less than 10 mIU/mL [23].
Cycle outcomes compared to IVF
Some of the included studies provided data comparing pregnancy outcomes between women over 40 years undergoing IUI versus IVF (Fig. 2) or ICSI (Fig. 3). Two of these studies used conventional fertilization and did not specify sperm source [41, 52]. Two of the studies fertilized oocytes via intracytoplasmic sperm injection (ICSI); one of these studies specified that only donor sperm was used while the other did not specify sperm source [30, 50]. In a study by Auyeung et al. in which IVF and GIFT yielded a LBR of 15.5% per cycle (n = 58 cycles) which was nearly 5 times the LBR/cycle for IUI (3.2%, n = 343 cycles) [52]. Similar results were seen by Bou Nemer et al. with LBR/cycle of 1.28% (n = 3/239) for IUI and 9.2% (n = 49/531) for IVF (Fig. 2) [41]. Wiser et al. reported LBR/cycle of 1.2% (n = 1/85) for all OI/IUI cycles compared to 13.7% (n = 17/124) for ICSI [30]. Interestingly, De Brucker et al. performed a study comparing IUI to ICSI in women ≥ 40 years using donor sperm and found that while the LBR/cycle was only 7% (n = 10/150) for first IUI cycles and 22% (n = 5/23) for IVF/ICSI cycles but there was no statistically significant difference in cumulative LBR after 6 treatment cycles (24% for IUI versus 26% for ICSI) (Fig. 3). Furthermore, they compared outcomes of patients who underwent ICSI as primary treatment versus those who switched from IUI to ICSI after failed treatment of varying lengths and found no difference in overall outcomes despite there being a delay in beginning treatment with ICSI even in patients having done up to 12 cycles of IUI. It should be noted, though, that the mean time to clinical pregnancy resulting in live birth was significantly longer in the group starting with IUI (194 days) compared to that of the group starting with ICSI group (44 days) [50].
Fig. 2.
Live birth rates per cycle for IVF versus IUI in women ≥ 40
Fig. 3.
Live birth rates per cycle for ICSI versus IUI in women ≥ 40
Summary of results
In summary, 42 unique articles with data pertaining to pregnancy outcomes in women ≥ 40 were included. Seventeen of these studies provided LBR data. For all cycles using exclusively partner sperm, cumulative LBR ranged from 3.6 to 12.1% [33, 46–48]. In studies where number of cycles were available, this ranged from 3.6 to 7.1% over 3–4 cycles [33, 48] (Table 1). LBR/cycle ranged from 0 to 8.5% [23, 32, 33, 42, 43, 47]. It should be noted that after the study with the highest LBR/cycle, the next highest was only 4% [23, 42]. The majority of pregnancies occurred within the first 1–4 cycles [24, 25, 31, 33, 37]. For cycles using donor sperm only, cumulative LBR when reported was 12 to 24% over 6 cycles [49, 50]. LBR/cycle ranged from 3 to 7% [47, 49–51] (Table 2). Studies which exclusively evaluated donor sperm cycles generally reported a plateau in outcomes on average at 6 cycles [47, 49–51].
Conclusions
The aim of this systematic review is to synthesize the data available regarding IUI outcomes in women over 40 and to provide recommendations for how this treatment may be implemented into clinical practice.
Should OI/IUI or IVF be the treatment of choice for women over 40?
Per the American Society of Reproductive Medicine (ASRM), futility is defined as a treatment cycle that has a 0–1% chance of achieving live birth. This is in contrast to the definition of very poor prognosis, i.e., < 5% chance of live birth per cycle [21]. All studies but one relatively small study using partner sperm were within this range as were half of studies using donor sperm [26, 32, 33, 42, 43, 47, 51]. The LBR/cycle from IVF in the included studies comparing outcomes between the two treatments are much more consistently above this range for women over 40 [30, 41, 50, 52]. While not included in this review given the inclusion of women 38–42 years without stratification for women > 40 years, the FORT-T trial, a randomized controlled trial comparing live birth rate of CC/IUI, GN/IUI, and IVF, demonstrated consistent results with LBR over 2 cycles of 15.7% for CC/IUI and 13.5% for GN/IUI, and 31.4% for IVF [64]. When counseling women ≥ 40 using homologous sperm about the treatment which provides the best chance of achieving pregnancy using their own oocytes, IVF should be recommended as first line. This is especially true for women with a very poor prognosis, i.e., significant diminished ovarian reserve, significant endometriosis or tubal disease despite tubal patency, or severe male factor. In women using donor sperm, it seems reasonable to offer the patient the option of either a trial of up-front OI/IUI or IVF especially given the patient may not have been exposed to sperm at all previously or sperm without significant abnormalities and, therefore, may not have female infertility.
Is there a role for OI/IUI in women over forty?
Despite counseling regarding higher probability of achieving live birth with IVF, for some women, this may not be an option due to financial constraints, moral, ethical, or religious objections to IVF, logistical obstacles to presenting for frequent ultrasound and lab draws needed for monitoring, or concern about risks. Some patients may also not be candidates for IVF based on center-specific criteria for proceeding to oocyte retrieval. For example, a patient may have undergone multiple attempts at IVF in the past and may not have developed more than 1–2 mature follicles despite maximum dose gonadotropins and protocol optimization over multiple stimulation attempts. Other patients may have a strongly suspected poor outcome if IVF is pursued based on evidence of severe diminished ovarian reserve on serum and ultrasound evaluation. Finally, some women may desire to proceed with up-front IVF but the center where they access care may have a waitlist and they may be interested in pursuing IUI while awaiting IVF. In these patients, a limited trial of OI/IUI may be considered as an alternative option.
Is OI/IUI or IVF cost effective for women over forty?
There is very limited data regarding cost effectiveness of treatment with IUI versus IVF in women over 40 [10, 60, 64]. For younger women, the FASTT trial demonstrated improved cost effectiveness with a streamlined approach of three IUI cycles with subsequent transition to IVF versus six cycles of IUI (3 each of CC and GN) prior to proceeding with IVF [65]. From the studies that do exist for women ≥ 40 years, it appears that, despite the lower probability of pregnancy per cycle, the cost per successful outcome is significantly lower with IUI [60, 66]. However, patients should be counseled that even the cost for IUI treatments can be expected to be significantly higher than for younger women, due to lower chance of success per cycle and use of gonadotropins in some cases [10].
If OI/IUI is offered to women over forty, which stimulation protocol should be used?
Based on the data in the included articles, there does not appear to be convincing evidence that choosing gonadotropins over oral agents significantly improves outcomes [23, 27, 30, 47, 49, 52, 53]. We would suggest oral agents in women ≥ 40 and undergoing IUI due to lower cost and risk of multiples unless adequate ovarian stimulation is not able to be achieved with maximum dose oral agents. In these women, a combination of both agents may decrease the number of cancelled cycles and dose of gonadotropins needed, therefore decreasing costs [38]. However, the data to support this comes from a single small retrospective study.
Is there an age cutoff at which OI/IUI should not be offered as an option to women over forty?
In some of the included studies, LBR outcomes appear to be relatively consistent through age 42 or 43 [23, 49, 53, 54]. There are a very small number of studies which provide pregnancy and live birth rates outside the range of futility for women ages 43–45, primarily those with CPR and not LBR data [23, 31, 37]. The remainder reported no pregnancies or live births in this group or outcomes within the range of futility [25, 27, 43, 47, 54]. No studies which provided data for women ≥ 45 had values outside of the range of futility [51, 60]. This is true even in studies utilizing donor sperm [49, 51]. Therefore, we would not recommend offering IUI to women over 45 unless it is in the setting of providing treatment for psychologic closure before terminating fertility treatments or moving on to donor oocyte or donor embryo ART cycles regardless of whether homologous or donor sperm is used [21].
If OI/IUI is offered to women over forty, how many cycles should be offered?
In included studies which commented on pregnancy outcomes by cycle number using homologous sperm, the majority described a decline in cycle fecundity after 1–4 cycles [24, 25, 31, 33, 37]. This is consistent with other large studies in younger women undergoing infertility treatment with IUI [65, 67, 68]. Conversely, in the majority of studies investigating IUI using donor sperm, pregnancy rates were not seen to plateau until after 6 cycles on average [47, 49–51]. Based on this data, we recommend no more than four OI/IUI cycles in women over 40 using homologous sperm and no more than 6 cycles using donor sperm. However, the high cost of purchasing donor sperm must be considered in these patients. The exception to this would be women awaiting IVF who are willing to incur the cost of additional IUI cycles with the understanding that the available data suggests her probability of becoming pregnant with IUI is likely lower even than in her previous IUI cycles.
Which women over forty are most likely to be successful with OI/IUI?
There is limited data in this specific population regarding which patients are most likely to be successful from OI/IUI. Based on the studies included, it appears there is no difference between women who are ovulatory and anovulatory [24]. There do not appear to be differences in outcomes by FSH greater than or less than 10. However, the authors expressed concern that this may be due to small sample and different results may be seen if replicated in a larger group [23]. Women who require very high doses of gonadotropins may also have a lower probability of achieving pregnancy [38]. It should be noted that each of these statements is based on data from single, relatively small studies. Further studies are needed to determine which specific patient groups over 40 are the most likely to benefit from treatment with IUI.
In conclusion, women ≥ 40 using partner sperm should be counseled that the highest probability of live birth is achieved with IVF; however, if IVF is not an immediate option, OI/IUI may be considered as an alternative for up to 4 cycles. For those using donor sperm, consideration can be given to extending treatment to a total of 6 cycles. Live birth is uncommon after the age of 45 and we would suggest not offering OI/IUI cycles to women of this age except perhaps on a limited basis as a means of providing the opportunity for conception if the patient is not otherwise exposed to sperm or supportive care of the couple to obtain closure while encouraging consideration of other treatment options to include IVF with donor oocytes, donor embryos, or adoption [21]. We would suggest that gonadotropin/IUI in women ≥ 40 offers does not appear to be superior in ovulatory women and oral agents should be preferentially used due to lower cost and decreased complexity of treatment.
Strengths of this review include the relatively large number of individual data-generating studies which are available in a diverse range of populations including a wide variety of stimulation protocols and sperm sources. Limitations include the relatively small number of studies providing data on LBR and limited information for age tiers above the age of 40. Other weaknesses include the heterogeneity of populations used in regard to infertility diagnosis, age range, stimulation protocols, and sperm sources with few studies stratifying data by any of these factors. The majority of studies included are also small. Future directions which would be helpful include meta-analyses of the above data with sub-analyses by infertility diagnosis, age, stimulation protocol, and sperm source.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allahbadia G. Intrauterine insemination: fundamentals revisited. J Obstet Gynaecol India. 2017 doi: 10.1007/s13224-017-1060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung L. Patient selection for assisted reproductive technology treatments. Hong Kong Med J. 2000;6:177–183. [PubMed] [Google Scholar]
- 3.Dodson WC, Haney AF. Controlled ovarian hyperstimulation and intrauterine insemination for treatment of infertility. Fertil Steril. 1991 doi: 10.1016/S0015-0282(16)54168-5. [DOI] [PubMed] [Google Scholar]
- 4.American Society for Reproductive Medicine (ASRM) Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril. 2020 doi: 10.1016/j.fertnstert.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. J Urol. 2021 doi: 10.1097/JU.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists (ACOG). ACOG committee opinion no. 586: Health disparities in rural women. Obstet Gynecol. 2014; 10.1097/01.AOG.0000443278.06393.d6. [DOI] [PubMed]
- 7.American Society for Reproductive Medicine (ASRM) Disparities in access to effective treatment for infertility in the United States: an Ethics Committee Opinion. Fertil Steril. 2021 doi: 10.1016/j.fertnstert.2015.07.1139. [DOI] [PubMed] [Google Scholar]
- 8.Harris JA, Menke MN, Haefner JK, Moniz MH, Perumalswami CR. Geographic access to assisted reproductive technology health care in the United States: a population-based cross-sectional study. Fertil Steril. 2017 doi: 10.1016/j.fertnstert.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists (ACOG) and American Society for Reproductive Medicine (ASRM). Committee Opinion No. 589: age related fertility decline. Fertil Steril. 2014; 10.1016/j.fertnstert.2013.12.032
- 10.Broekmans FJ, Klinkert ER. Female age in ART: when to stop? Gynecol Obstet Invest. 2004 doi: 10.1159/000080794. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Am J Human Biol. 1998 doi: 10.1016/s0378-5122(98)00068-1. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. N Engl J Med. 1982 doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong S, Akande V. What is the best treatment option for infertile women aged 40 and over? J Assist Reprod Genet. 2013 doi: 10.1007/s10815-013-9980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabry R, Merviel P, Hazout A, Belloc S, Dalleac A, Copin H, et al. Management of infertility in women over 40. Maturitas. 2014 doi: 10.1016/j.maturitas.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Case A. Advanced reproductive age and fertility. J Obstet Gynaecol Can. 2011 doi: 10.1016/S1701-2163(16)35087-3. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig AK, Diedrich K, Ludwig M. The process of decision-making in reproductive medicine. Semin Reprod Med. 2005 doi: 10.1055/s-2005-923392. [DOI] [PubMed] [Google Scholar]
- 17.Marinakis G, Nikolaou D. National survey of the current management of infertility in women aged 40 and over in the UK. Obstet Gynaecol. 2012 doi: 10.3109/01443615.2012.663424. [DOI] [PubMed] [Google Scholar]
- 18.Tsafrir A, Simon A, Margalioth EJ, Laufer N. What should be the first-line treatment for unexplained infertility in women over 40 years of age-ovulation induction and IUI or IVF? Reprod Biomed Online. 2009;19:4334. doi: 10.1016/S1472-6483(10)61069-3. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell E, Mathews M, Mulay S. The impact of access barriers on fertility treatment decision making: a qualitative study from the perspectives of patients and service providers. Obstet Gynaecol Can. 2018 doi: 10.1016/j.jogc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Weigel G, Ranji U, Long M, Salganicoff A. Coverage and use of fertility services in the U.S. Kaiser family foundation website. Accessed Sep 4, 2021. https://www.kff.org/womens-health-policy/issue-brief/coverage-and-use-of-fertility-services-in-the-u-s/.
- 21.American Society for Reproductive Medicine (ASRM) Fertility treatment when the prognosis is very poor or futile: an Ethics Committee opinion. Fertil Steril. 2019 doi: 10.1016/j.fertnstert.2019.01.033. [DOI] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haebe J, Martin J, Tekepety F, Tummon I, Shepherd K. Success of intrauterine insemination in women aged 40–42 years. Fertil Steril. 2002 doi: 10.1016/S0015-0282(02)03168-0. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal SK, Buyalos RP. Clomiphene citrate with intrauterine insemination: is it effective therapy in women above the age of 35 years? Fertil Steril. 1996 doi: 10.1016/s0015-0282(16)58210-7. [DOI] [PubMed] [Google Scholar]
- 25.Dovey S, Sneeringer RM, Penzias AS. Clomiphene citrate and intrauterine insemination: analysis of more than 4100 cycles. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara I, Balet R, Grudzinskas JG. Intrauterine insemination with frozen donor sperm. Pregnancy outcome in relation to age and ovarian stimulation regime. Hum Reprod. 2002 doi: 10.1093/humrep/17.9.2320. [DOI] [PubMed] [Google Scholar]
- 27.Gomez R, Schorsch M, Steetskamp J, Hahn T, Heidner K, Seufert R, et al. The effect of ovarian stimulation on the outcome of intrauterine insemination. Arch Gynecol Obstet. 2014 doi: 10.1007/s00404-013-2952-3. [DOI] [PubMed] [Google Scholar]
- 28.Steiner N, Ruiter-Ligeti J, Frank R, Al Shatti M, Badeghiesh A, Rotshenker-Olshinka K, et al. Do oral ovulation induction agents offer benefits in women 38 to 43 years of age undergoing insemination cycles? Eur J Obstet Gynecol Reprod Biol. 2021 doi: 10.1016/j.ejogrb.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Westerlaken LA, Naaktgeboren N, Helmerhorst FM. Evaluation of pregnancy rates after intrauterine insemination according to indication, age, and sperm parameters. J Assist Reprod Genet. 1998 doi: 10.1023/A:1022576831691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiser A, Shalom-Paz E, Reinblatt SL, Son WY, Das M, Tulandi T, et al. Ovarian stimulation and intrauterine insemination in women aged 40 years or more. Reprod Biomed Online. 2012 doi: 10.1016/j.rbmo.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril. 2002 doi: 10.1016/s0015-0282(02)04212-7. [DOI] [PubMed] [Google Scholar]
- 32.Brzechffa PR, Buyalos RP. Female and male partner age and menotrophin requirements influence pregnancy rates with human menopausal gonadotrophin therapy in combination with intrauterine insemination. Hum Reprod. 1997 doi: 10.1093/humrep/12.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Harris ID, Missmer SA, Hornstein MD. Poor success of gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination for older women. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 34.Soares SR, Cruz M, Vergara V, Requena A, García-Velasco JA. Donor IUI is equally effective for heterosexual couples, single women and lesbians, but autologous IUI does worse. Hum Reprod. 2019 doi: 10.1093/humrep/dez179. [DOI] [PubMed] [Google Scholar]
- 35.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Sahakyan M, Harlow BL, Hornstein MD. Influence of age, diagnosis, and cycle number on pregnancy rates with gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 1999 doi: 10.1016/s0015-0282(99)00300-3. [DOI] [PubMed] [Google Scholar]
- 37.Schorsch M, Gomez R, Hahn T, Hoelscher-Obermaier J, Seufert R, Skala C. Success rate of inseminations dependent on maternal age? An analysis of 4246 insemination cycles. Geburtshilfe Frauenheilkd. 2013 doi: 10.1055/s-0033-1350615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedaiwy MA, Shokry M, Mousa N, Claessens A, Esfandiari N, Gotleib L, et al. Letrozole co-treatment in infertile women 40 years old and older receiving controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Ibérico G, Vioque J, Ariza N, Lozano JM, Roca M, Llácer J, et al. Analysis of factors influencing pregnancy rates in homologous intrauterine insemination. Fertil Steril. 2004 doi: 10.1016/j.fertnstert.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 40.Michau A, El Hachem H, Galey J, Le Parco S, Perdigao S, Guthauser B, et al. Predictive factors for pregnancy after controlled ovarian stimulation and intrauterine insemination: a retrospective analysis of 4146 cycles. J Gynecol Obstet Hum Reprod. 2019 doi: 10.1016/j.jogoh.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Bou Nemer L, Weitzman VN, Arheart KL, Barrionuevo MJ, Christie DR, Mouhayar Y, et al. In vitro fertilization versus mild stimulation intrauterine insemination in women aged 40 and older. Reprod Sci. 2017 doi: 10.1177/1933719116667215. [DOI] [PubMed] [Google Scholar]
- 42.Hull MGR, Eddowes HA, Fahy U, Abuzeid MI, Mills MS, Cahill DJ, et al. Expectations of assisted conception for infertility. Br Med J. 1996 doi: 10.1136/bmj.304.6840.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod. 1999 doi: 10.1093/humrep/14.3.698. [DOI] [PubMed] [Google Scholar]
- 44.Osaikhuwuomwan J, Osemwenkha A, Iribhogbe O, Aziken M, Orhue A. The effect of female age on the outcome of intrauterine insemination treatment in a public hospital-assisted reproduction technology unit. Niger J Clin Pract. 2018 doi: 10.4103/njcp.njcp_248_16. [DOI] [PubMed] [Google Scholar]
- 45.Houmard BS, Juang MP, Soules MR, Fujimoto VY. Factors influencing pregnancy rates with a combined clomiphene citrate/gonadotropin protocol for non-assisted reproductive technology fertility treatment. Fertil Steril. 2002 doi: 10.1016/s0015-0282(01)02990-9. [DOI] [PubMed] [Google Scholar]
- 46.Malchau SS, Henningsen AA, Loft A, Rasmussen S, Forman J, Nyboe Anderson A, Pinborg A. The long-term prognosis for live birth in couples initiating fertility treatments. Hum Reprod. 2017 doi: 10.1093/humrep/dex096. [DOI] [PubMed] [Google Scholar]
- 47.Frederick JL, Denker A, Rojas A, Horta I, Stone SC, Asch RH, et al. Is there a role for ovarian stimulation and intra-uterine insemination after age 40? Hum Reprod. 1994 doi: 10.1093/oxfordjournals.humrep.a138438. [DOI] [PubMed] [Google Scholar]
- 48.Vichinsartvichai P, Siriphadung S, Traipak K, Promrungrueng P, Manolertthewan C, Ratchanon S. The influence of women age and successfulness of intrauterine insemination (IUI) cycles. J Med Assoc Thai. 2015;98:833–838. [PubMed] [Google Scholar]
- 49.Linara-Demakakou E, Bodri D, Wang J, Arian-Schad M, Macklon N, Ahuja K. Cumulative live birth rates following insemination with donor spermatozoa in single women, same-sex couples and heterosexual patients. Reprod Biomed Online. 2020 doi: 10.1016/j.rbmo.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 50.De Brucker M, Camus M, Haentjens P, Verheyen G, Collins J, Tournaye H. Assisted reproduction using donor spermatozoa in women aged 40 and above: the high road or the low road? Reprod Biomed Online. 2013 doi: 10.1016/j.rbmo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Kang BM, Wu TC. Effect of age on intrauterine insemination with frozen donor sperm. Obstet Gynecol. 1996 doi: 10.1016/0029-7844(96)00074-9. [DOI] [PubMed] [Google Scholar]
- 52.Auyeung A, Klein ME, Ratts VS, Odem RR, Williams DB. Fertility treatment in the forty and older woman. J Assist Reprod Genet. 2001 doi: 10.1023/a:1013159116180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buyalos RP, Daneshmand S, Peter MD, Brzechffa R. Basal estradiol and follicle-stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction therapy. Fertil Steril. 1997 doi: 10.1016/s0015-0282(97)81514-2. [DOI] [PubMed] [Google Scholar]
- 54.Corsan G, Trias A, Trout S, Kemmann E. Ovulation induction combined with intrauterine insemination in women 40 years of age and older: is it worthwhile? Hum Reprod. 1996 doi: 10.1093/oxfordjournals.humrep.a019306. [DOI] [PubMed] [Google Scholar]
- 55.Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nyren KG. Assisted reproductive technology in Europe, 2002. Results generated from European registers by ESHRE. Hum Reprod. 2006;10.1093/humrep/del075. [DOI] [PubMed]
- 56.Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Barcie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008 doi: 10.1016/s1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 57.Bonow MP, Donne RDD, da Rosa VB, Lucca JA, Hillesheim CM, Schuffner A. Intrauterine insemination as a primary viable option to infertile couples: evaluation of patients in a private center. J Bras Reprod Assist. 2019 doi: 10.5935/1518-0557.20190014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campana A, Sakkas D, Stalberg A, Bianchi PG, Comte I, Pache T, et al. Intrauterine insemination: evaluation of the results according to the woman’s age, sperm quality, total sperm count per insemination and life table analysis. Hum Reprod. 1996 doi: 10.1093/oxfordjournals.humrep.a019244. [DOI] [PubMed] [Google Scholar]
- 59.Plosker SM, Jacobson W, Amato P. Predicting and optimizing success in an intra-uterine insemination programme. Hum Reprod. 1994 doi: 10.1093/oxfordjournals.humrep.a138385. [DOI] [PubMed] [Google Scholar]
- 60.Stone BA, Vargyas JM, Ringler GE, Stein AL, Marrs RP. Determinants of the outcome of intrauterine insemination: analysis of outcomes of 9963 consecutive cycles. Am J Obstet Gynecol. 1999 doi: 10.1016/s0002-9378(99)70048-7. [DOI] [PubMed] [Google Scholar]
- 61.Tay PYS, Mohan Raj VR, Kulenthran A, Sitizawiah O. Prognostic factors influencing pregnancy rate after stimulated intrauterine insemination. Med J Malaysia. 2007;62:286–289. [PubMed] [Google Scholar]
- 62.Ashrafi M, Rashidi M, Ghasemi A, Arabipoor A, Daghighi S, Pourasghari P, et al. The role of infertility etiology in success rate of intrauterine insemination cycles: an evaluation of predictive factors for pregnancy rate. Int J Fertil Steril. 2013;7:100–107. [PMC free article] [PubMed] [Google Scholar]
- 63.Khalil RM, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. Homologous intrauterine insemination. An evaluation of prognostic factors based on a review of 2473 cycles. Acta Obs Gynecol Scand. 2001 doi: 10.1034/j.1600-0412.2001.800115.x. [DOI] [PubMed] [Google Scholar]
- 64.Goldman MB, Thornton KL, Ryley D, Alper MM, Fung JL, Hornstein MD, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T) Fertil Steril. 2014 doi: 10.1016/j.fertnstert.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reindollar RH, Regan MM, Neumann PJ, Levine B, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 66.Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in-vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost-effectiveness analysis. Lancet. 2000 doi: 10.1016/S0140-6736(99)04002-7. [DOI] [PubMed] [Google Scholar]
- 67.Morshedi M, Duran HE, Taylor S, Oehninger S. Efficacy and pregnancy outcome of two methods of semen preparation for intrauterine insemination: a prospective randomized study. Fertil Steril. 2003 doi: 10.1016/s0015-0282(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 68.Soria M, Pradillo G, Garcia J, Ramón P, Castillo A, Jordana C, Paricio P. Pregnancy predictors after intrauterine insemination: analysis of 3012 cycles in 1201 couples. J Reprod Infertil. 2012;13:158–166. [PMC free article] [PubMed] [Google Scholar]




