Abstract
Purpose
Successful identification of transcriptomic biomarkers within human IVF embryos may enhance implantation prediction and provide insights not available through conventional embryo biopsy genomic analysis. We demonstrate proof-of-concept for a methodology to assess overall embryo gene expression using qPCR with blastocoel fluid-conditioned media by examining the comparative presence of apoptotic genes.
Methods
Blastocoel fluid-conditioned media were collected from 19 embryos (11 euploid) following trophectoderm biopsy of day-5 ICSI-IVF blastocysts. Media were assessed for apoptotic gene expression via qPCR. Statistical analysis of gene expression was conducted via Wilcoxon Signed-Ranks test (overall expression), multivariate ANOVA (functional gene groups), and chi-square test of independence (gene level).
Results
A significantly higher overall apoptotic gene expression within euploid versus aneuploid embryos (p = 0.001) was observed. There was significantly (p = 0.045) higher expression of pro-apoptotic genes between implanted and not implanted embryos. Pro- vs. anti-apoptotic gene expression from all euploid embryos approached significance (p = 0.053). The ploidy status-based claim is further substantiated at the gene level with significantly higher expression of BBC3 (p = 0.012) and BCL2L13 (p = 0.003) in euploid embryos compared to aneuploid embryos.
Conclusions
In this preliminary study, we demonstrate that (1) qualitative analysis of blastocoel fluid-conditioned media gene expression is possible, (2) global trends of expression are potentially related to clinical outcomes, and (3) gene-level expression trends exist and may be another viable metric for comparative expression between samples. The presence of statistical significance within analyses conducted with this sample size warrants a larger investigation of blastocoel fluid-conditioned media as an additional beneficial predictive tool for future IVF cases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02510-3.
Keywords: Blastocoel fluid, Apoptosis, Aneuploidy, qPCR, Embryo selection, Self-correction, Diagnostic, IVF
Introduction
Patients often seek assisted reproductive technology (ART) such as in vitro fertilization (IVF) after experiencing recurrent miscarriage most likely due to chromosomal aberrations [1, 2]. Selection of an embryo for transfer is often achieved by assessing the embryo morphology [3] or more recently with preimplantation genetic testing for aneuploidies (PGT-A) which involves biopsy of embryo trophectoderm cells at day 5 or 6, followed by the next-generation sequencing to assess ploidy status. Embryos with the correct number of chromosomes (euploid) are conventionally considered suitable for uterine transfer. However, the transfer of a euploid embryo does not guarantee successful implantation. This has been highlighted in a recent study that reported IVF-generated euploid implantation rates with single embryo transfers occurring in a range of 50–90% in their patient cohort [4]. The driving factor behind why some IVF-generated euploid embryos fail to implant could be due to an inherent biological factor within the embryo which may not be detectable by PGT-A. However, implantation failure can also be caused by non-embryo factors such as a non-receptive endometrium. To enhance uterine implantation rates of IVF-generated euploid embryos, the collection of more data regarding the status of the preimplantation embryo at the cellular and molecular level (reviewed by [5]) offer an opportunity to increase the rate of successful implantation.
A candidate biological source to obtain more information about implantation potential is blastocoel fluid. Blastocoel fluid resides in the blastocoel cavity of each developing preimplantation embryo and is known to contain various molecules, including cell-free DNA, proteins, mitochondrial DNA, miRNAs, and extracellular vesicles [6–13]. A likely hypothesis as to the origin of these molecules within the blastocoel fluid is as the remnants of apoptotic cells from the developing preimplantation embryo [14]. Evidence to support this rationale includes the detection of fragmented cfDNA [15] and mitochondrial DNA [10] in the blastocoel fluid which is consistent with what would be expected to be the remnants of cells that underwent apoptosis. Moreover, apoptosis is known to occur in the preimplantation embryo in both the inner cell mass and trophectoderm as an essential regulatory mechanism of embryonic development [16–18]. An early report from 1996 detected apoptosis within human preimplantation stage embryos via TUNEL analysis [19] and a later study demonstrated that apoptosis was shown to occur predominately at day 5 of blastocyst development [20]. A more recent study investigated the specific mechanism of apoptotic initiation and reported that activation of the spindle assembly checkpoint activated apoptosis at day 5 of embryo development [21]. Additionally, apoptotic gene expression and caspase activity were detected in human preimplantation embryos [22, 23] and apoptotic gene expression was shown to change at various stages in development [24, 25]. Collectively, these results provide substantial evidence of apoptosis during early embryo development, yet the specific reason why apoptosis occurs in this setting may be due to multiple interrelated reasons. One possibility is that apoptosis serves as a corrective mechanism for the embryo, sacrificing aneuploid or otherwise defective cells in order to maintain or improve overall embryo fitness. Apoptosis as a means of self-correction was recently demonstrated in a mouse model for mosaicism whereby aneuploidy was artificially induced in mouse embryos resulting in mosaic embryos. Apoptosis was selectively seen in the aneuploid cells of these mosaic embryos suggestive of a self-correction mechanism that purged the cells incompatible with a healthy embryo [26].
In recent years, analysis of the efficacy of blastocoel fluid has occurred in multiple laboratories. The analysis aimed at determining the concordance of blastocoel fluid predicted genotype with PGT-A has begun [27, 28]. However, there exist major differences between the goals of analyses conducted and the one we propose. Blastocoel fluid analysis is often proposed as a potential replacement of PGT-A, and hence, analyses are focused on developing chromosomal or other concordance between blastocoel fluid and conventional PGT-A-like analyses. However, fundamentally, it is likely that the blastocoel fluid of the embryo contains genetic material from cells eliminated during development. As such, one would not expect a concordance between the ploidy status extrapolated from within the blastocoel cavity and that of the embryo itself.
Nevertheless, conventionally assessed PGT-A remains a useful tool providing unique biological insight. While implantation outcome remains the primary metric by which IVF successes are assessed by this analysis, we continue to study relationships of blastocoel fluid expressions with ploidy status as a secondary metric of embryo viability. In this study, we assessed apoptotic gene expression levels in human blastocoel fluid-conditioned media from 19 embryos (11 euploid and 8 aneuploid). We hypothesized that pro-apoptotic gene expression would be higher in euploid embryos leading to successful implantation as this cellular process may regulate self-correction within the preimplantation embryo.
Materials and methods
Blastocoel fluid-conditioned media collection
Following standard procedures for PGT-A, extruded trophectoderm (TE) cells were biopsied following laser pulses between cellular junctions from ICSI-generated day-5 blastocyst stage embryos. The biopsied TE cells were removed by pipette for PGT-A analysis via next-generation sequencing (NGS) at a commercial sequencing company. Upon completion of the blastocyst biopsy procedure, the embryo self-collapses, resulting in blastocoel fluid being extruded out into the surrounding medium. The resulting blastocoel fluid-conditioned media (15–25-μL biopsy medium plus blastocoel fluid for each embryo), which is generally discarded, was collected, and saved post-biopsy from day 5 blastocyst stage human embryos obtained from patients undergoing IVF cycles at collaborating fertility clinics (San Antonio, TX, and Swansea, IL). The blastocoel fluid-conditioned media was snap-frozen prior to shipment to Greenville, SC, for further analysis. Biopsied embryos are cryopreserved pending the outcome of the NGS results. De-identified data including patient age, and ploidy status were provided by the collaborating fertility centers.
Apoptotic gene expression with TaqMan arrays
Blastocoel fluid-conditioned media from eleven euploid embryos (from eleven different patients) and eight aneuploid embryos (from eight different patients) were each subjected to DNase I (RNAase free, ThermoFisher, USA) treatment step for 30 min at 37 °C followed by inactivation at 65 °C for 10 min. Next cDNA synthesis (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, USA) was performed per the manufacturers’ instructions. cDNA concentration was subsequently assessed using an Eppendorf Bio Spectrometer. Twenty-nanograms of cDNA was required for each well of the 96-well TaqMan Fast Array-Human Apoptosis plate (Applied Biosystems, USA). cDNA obtained from each blastocoel fluid-conditioned media sample was diluted in 540 μL of nuclease-free water and combined with 540 μL of 2X TaqMan Master Mix (Applied Biosystems, USA). cDNA-Master Mix (10 μL) was then added to each well in the 96-well plate and prepared for thermal cycler as per the manufacturer’s instructions. Each plate was run using a 7500 Fast Real-Time PCR System (Applied Biosystems, USA) at 50 °C for 2 min (UNG activation), 95 °C for 20 s (polymerase activation), followed by 40 cycles of 95 °C for 3 s (denaturation) and 60 °C for 30 s (anneal/extend), all as per manufacturer’s instructions.
18S rRNA was included in each array plate as an intra-embryonic control, which involved a post hoc quality check of consistency of overall expression within samples. The lack of a control embryo group coupled with the fact that the fluorescence levels were auto-calculated prevented confident quantitative calculations via conventional means. Therefore, negative ΔCt (calculated with target Ct value normalized against 18S rRNA Ct) led to the determination of expression. Of note, many genes within samples resulted in “no expression” by failing to amplify within PCR despite multiple cycles and even at a low fluorescence threshold. This implies that there was no expression of the gene therefore the gene expression in those samples was categorized as having “no expression.” Quantitative analysis of the samples required the threshold consolidation method as mathematically described by [29].
Statistical analysis
Data were analyzed using IBM® SPSS® statistical software platform.
Whole apoptotic expression statistical analysis
A Wilcoxon signed-rank test was conducted to determine whether whole-gene expression was higher based on implantation group (positive implantation/negative implantation) and ploidy status (euploid/aneuploid).
Functional pathway statistical analysis
A multivariate factorial analysis of variance was conducted to determine whether the categorization of intrinsic/extrinsic and pro-apoptotic/anti-apoptotic affected the genetic expression of blastocoels. Post hoc analyses included analyses of variance and paired-sample t-tests.
Gene-level statistical analysis
A chi-square test of independence was used to assess whether genetic expression in the blastocysts was associated with implantation state (yes/no), reported ploidy status (euploid/aneuploid), and implanted blastocysts (chromosomally able to implant and result in live birth). Fisher exact tests were conducted when frequencies of genetic expression were fewer than 5 in each condition, where = Phi (effect size for chi-square), and = chi-square.
Ethics approval and consent to participate
Research approval was granted by the Institutional Review Board (IRB) of the University of South Carolina Office of Research Compliance. The study itself is conducted as Not Human Research (since the biopsy fluid samples are normally discarded and de-identified) set forth by the Code of Federal Regulations (45 CFR 46) and therefore exempt from further IRB review. The collection of blastocoel fluid-conditioned media was conducted under informed patient consent. The informed consent for treatment (American Society for Reproductive Medicine-Society for Assisted Reproductive Technology consent template; asrm.org) was modified to include that any unused biological material may be used for current or future research. Additionally, all patients signed a consent permitting PGT-A.
Facility: Vios Fertility Institute. All methods were performed in accordance with the relevant guidelines and regulations.
Facility: University of Texas Health Sciences Center San Antonio. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Detection of apoptotic gene expression in blastocoel fluid-conditioned media
Blastocoel fluid-conditioned media from euploid (n = 11; n = 5 implanted and n = 6 no implantation) and aneuploid (n = 8) blastocysts (ploidy status determined via conventional PGT-A) (Table 1) were assessed for apoptotic gene expression via real-time PCR. Implantation status was reported for all 11 of the fluid samples from the transferred euploid embryos assessed in this study. Apoptotic gene expression was observed in all samples with a comparative analysis of apoptotic expression (Fig. 1). The resulting picture of expression is a stochastic one, with significant deviations in overall gene expression between samples highlighting the unique developmental apoptotic trajectory experienced by each embryo.
Table 1.
Ploidy status (PGT-A), age, and implantation result (if applicable) associated with the embryos that harbored the blastocoel fluid-conditioned media used for this study. Sample number (first column) is an arbitrary identifier for samples
| Sample number | PGT-A | Age | Implantation outcome? |
|---|---|---|---|
| 1 | 46, XY | 37 | Yes |
| 2 | 46, XY | 26 | Yes |
| 3 | 46, XY | 30 | Yes |
| 4 | 46, XY | 32 | Yes |
| 5 | 46, XX | 29 | Yes |
| 6 | 46, XY | 29 | Yes |
| 7 | 46, XY | 31 | No |
| 8 | 46, XX | 35 | No |
| 9 | 46, XX | 33 | No |
| 10 | 46, XX | 36 | No |
| 11 | 46, XX | 41 | No |
| 12 | 47, XY + 21 | 41 | N/A |
| 13 | 47, XY + 21 | 33 | N/A |
| 14 | 47, XX + 21 | 39 | N/A |
| 15 | 47, XY + 21 | 41 | N/A |
| 16 | 47, XX + 21 | 30 | N/A |
| 17 | 45, XO | 32 | N/A |
| 18 | 45, XO | 32 | N/A |
| 19 | 45, XO | 30 | N/A |
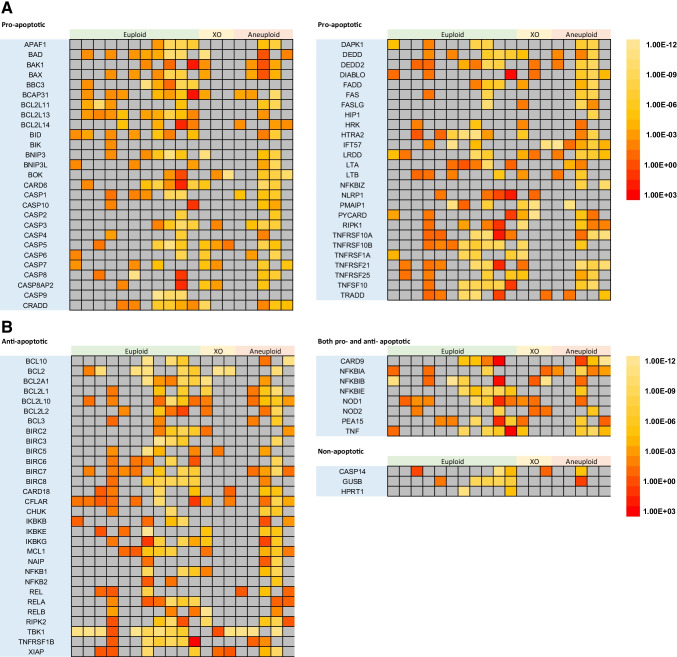
Fig. 1.
Relative apoptotic gene expression within the blastocoel fluid-conditioned media of all embryonic samples (in columns) with respect to many critical apoptotic genes demonstrates proof of capacity to assess quantitatively the relative concentration of gene expression within the cell-free fluid. A. Pro-apoptotic gene expression is shown. B. Anti-apoptotic and other classified gene expression is shown. Note that gray boxes represent gene expression for which qPCR analysis yielded no sustained amplification, likely implying the lack of any mRNA for the specific gene of interest within the specified blastocoel fluid-conditioned media. Sample expression is visualized upon a heat map that has color changes signifying exponential changes in expression. Hence, color differences represent the order of magnitude differences in expression. Comparative analysis (represented by colors) occurs between the comparison of gene expression for each gene (horizontal) (please refer to Supplemental Table 1 for citations used to classify genes as pro- or anti-apoptotic in this figure)
Whole apoptotic expression
The most immediate analysis we conducted was an exploration of whether apoptotic gene expression is higher within the two outcome groups (implantation outcome and ploidy status) via a Wilcoxon signed-rank test. While there existed no difference between implantation outcome and overall apoptotic gene expression, a significant difference existed between euploid (mean rank = 4.216) and aneuploid (mean rank = 0.161) gene expression, with p = 0.001. This difference is indicative of a global shift of expression of apoptotic genes to be higher within euploid embryos.
Analysis of apoptotic gene expression by functional gene classification
The relative apoptotic gene expression (quantitative differences) between blastocoel fluid-conditioned media samples with associated clinical outcomes (i.e., ploidy status, implantation success) provides an opportunity to determine if the implantation outcomes are associated with the specific cellular activity of interest. Specifically, classification of the genes into different groups, including canonical and pragmatically viable categorizations of “intrinsic” vs “extrinsic” apoptotic genes and “pro-apoptotic” and “anti-apoptotic” genes can offer a further specified understanding of the potential apoptotic activity of the system guided by the grouping of genes within mechanistically and functionally similar (or at least congruent pathway like) structures. Utilizing gene-specific literature, 90 genes of apoptotic interest assessed for gene expression within blastocoel fluid-conditioned media samples were classified by their association first as part of the (1) intrinsic, (2) extrinsic, and (3) both intrinsic and extrinsic pathways, as well as (1) pro-apoptotic, (2) anti-apoptotic, and (3) pro- and anti-apoptotic genes (Supplementary Table 1).
Statistical analyses offered insights into the potential associations that exist between embryonic outcomes and functional gene expression. A multivariate ANOVA revealed significantly (p = 0.045) higher pro-apoptotic expression in positive versus negative implantation and differences approaching significance (p = 0.053) for higher pro-apoptotic expression in euploid embryos compared to aneuploid embryos (Table 2). Additionally, ANOVA revealed ploidy status was not statistically different for intrinsic or extrinsic pathway genes (data not shown).
Table 2.
Results of a multivariate analysis of variance revealed associations between embryonic outcomes and the quantitative associated gene expression. Notably, there exists a significant relationship between pro- and anti-apoptotic expression with positive and negative implantation outcomes, wherein pro-apoptotic activity is significantly more expressed within embryos with positive implantation outcomes. Pro-apoptotic expression in euploid embryos versus aneuploid embryos also approaches significance
| Mean (Std. deviation) | Pro/anti-apoptotic expression | |
|---|---|---|
| Euploid and positive implantation |
Pro: 3.6002 (8.69067) Anti: 0.2362 (0.61232) |
F(1,72) = 4.160, p = .045, η2 = .055 |
| Euploid and negative implantation |
Pro: 4.7741 (20.66306) Anti: 0.4736 (1.48358) |
F(1,72) = 1.203, p = .276, η2 = .016 |
| Mean (Std. deviation) | Pro/anti apoptotic expression | |
| Euploid |
Pro: 4.1338 (10.16470) Anti: 0.3441 (0.75272) |
F(1,72) = 3.86, p = .053, η2 = .051 |
| Aneuploid |
Pro: 0.2016 (0.47979) Anti: 0.1186 (0.14383) |
F(1,72) = .791, p = .377, η2 = .011 |
Gene-level expression analysis
Having determined a potential relationship between functional apoptotic gene group expression and embryonic outcomes, we assessed whether the global associations can be further indicated towards specific genes which play important roles within the formation of the specific apoptotic functions.
Upon initial surveying of gene expression, we discovered four genes of interest whose expression in different samples was associated with different embryo outcomes.
BBC3 was expressed to a higher extent within euploid blastocoel fluid-conditioned media compared to aneuploid blastocoel fluid-conditioned media, with chi-square analysis yielding χ2 = 6.78, Φ = 0.579, and p = 0.012.
BCL2L13 was expressed to a higher extent within euploid blastocoel fluid-conditioned media compared to aneuploid blastocoel fluid-conditioned media, with chi-square analysis yielding χ2 = 8.93, Φ = 0.685, and p = 0.003.
IFT57 was expressed to a higher extent within aneuploid blastocoel fluid-conditioned media compared to euploid blastocoel fluid-conditioned media, with chi-square analysis yielding χ2 = 4.23, Φ = 0.472, and p = 0.04.
Finally, CASP9, an executioner caspase, was expressed only in euploid embryos that yielded positive implantation. Chi-square analysis with regards to ploidy status and gene expression to implantation outcomes yielded significance: χ2 = 7.72, Φ = 0.635, p = 0.021. While it is important to reiterate that the aims of this pilot analysis are to serve as a proof-of-concept for detecting molecular markers (and not to (1) suggest mechanistic insight or (2) actually posit markers), the determination of a subset of genes of interest in a future larger-scale study would be of significant interest to the broader field.
Discussion
Overall, our findings from this retrospective study collectively show that apoptotic remnants, specifically mRNA, can be detected in blastocoel fluid-conditioned media and that differences in apoptotic gene expression are seen among blastocysts with varying ploidy and implantation status. We conducted the analysis at a wholistic, functional pathway, and gene level, with interesting results present at all three extents of analysis.
We identified specific pro- and anti-apoptotic genes with unique expression patterns in the blastocoel fluid-conditioned media from both euploid embryos and aneuploid embryos. In this study, we found that pro-apoptotic gene expression in this media was higher in implanted embryos when compared to non-implanted embryos (p = 0.045). These expression patterns suggest that there is sophisticated coordination of apoptotic activity within the preimplantation embryo that may regulate self-correction within the preimplantation embryo. A recent study by Yang and colleagues detected selective elimination of aneuploid cells within mosaic human embryos [30]. Additionally, their studies using aneuploid and mosaic gastruloids (3D embryo model using embryonic stem cells) support the hypothesis that a cell elimination mechanism, potentially apoptosis, may be responsible for the removal of aneuploid cells from the early embryo [30]. Interestingly, our study identified a trend of higher expression of pro-apoptotic genes in blastocoel fluid-conditioned media from euploid embryos compared aneuploid embryos (p = 0.053). Given our small sample size, the trend we observed does align with the hypothesis that a self-correction mechanism involving apoptosis of aneuploid cells may occur within euploid (called by PGT-A results) embryos.
Characterizing the gene expression patterns in blastocoel fluid may serve as an additional tool for embryologists to use when selecting which euploid embryo to transfer as a means to improve implantation rates. qPCR serves as a unique methodology which offers rapid, scalable, and accurate quantification of specific mRNA within the blastocoel fluid-conditioned media as shown through our study. A number of studies have emerged recently with the goal of either determining the molecular mechanism of development or developing clinically utilizable analyses to improve the prediction of embryonic outcomes. A recent study used TE biopsy cells for RNASeq analysis to assay for differences in embryos that implanted versus unsuccessful implantation. Though the study had a small samples size, several genes varied between the two groups including the pro-apoptotic gene BAK1 expressed in incompetent blastocysts [31]. Gene expression in the preimplantation human embryo has revealed expression patterns that differ between euploid and aneuploid embryos [32]. In another study, comparing cells from human blastomeres led to the identification of 12 genes with expression patterns that allowed discrimination between aneuploid and euploid human embryos [33]. Generally, assessing gene expression in TE biopsied cells has revealed differences when comparing blastocysts resulting in implantation [34, 35], with advanced maternal age [36] as well as infertility diagnosis [37]. Additionally, profiling the blastocoel fluid for microRNAs revealed their expression as well as the presence of extracellular vesicles suggesting a potential signaling role for the microRNAs within the developing embryo [13]. The existence of this literature demonstrated both the biological and clinical relevance of the methodology delineated within this manuscript.
Despite the limited sample size, this retrospective study demonstrates the possibility and potential for a large-scale analysis of gene expression within blastocoel fluid-conditioned media for those genes that are known to play a role during human development. A larger-scale analysis of this nature may not only lead to the possibility of a clinically utilizable diagnostic for outcomes but may also lead to a greater understanding of the necessary biological expression of specific genes important to canonical human development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Alex Ewing for his statistical analysis recommendations for this dataset.
Author contribution
R. J. C. and W. E. R. designed the experiments. S. Z., T. A. C., R. D. R., and J. D. W. carried out sample collection, pooled patient data records, and contributed to the revision of the manuscript. A. L., A. K., J. B., D. A., A. B, and A. B. carried out the cDNA synthesis and real-time experiments and aided in data analysis. A. L. led figure construction and played an integral role in manuscript writing. L. A. F. performed the statistical analysis on the dataset. A. L. and R. J. C. led the data analysis, interpretation of results, and manuscript writing. All authors contributed to the manuscript drafting process.
Funding
University of South Carolina Magellan Scholar funds supported JB and offset some costs for experiments. University of South Carolina School of Medicine Greenville development funds (to RJC and WER) offset experimental costs.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vidal F, Gimenez C, Rubio C, Simon C, Pellicer A, Santalo J, et al. FISH preimplantation diagnosis of chromosome aneuploidy in recurrent pregnancy wastage. J Assist Reprod Genet. 1998;15:310–313. doi: 10.1023/A:1022552713015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franasiak JM, Scott RT., Jr Embryonic aneuploidy: overcoming molecular genetics challenges improves outcomes and changes practice patterns. Trends Mol Med. 2014;20:499–508. doi: 10.1016/j.molmed.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Balaban B, Gardner DK. Morphological assessment of blastocyst stage embryos: types of grading systems and their reported outcomes. In: Gardner D, Sakkas D, Seli E, Wells D, editors. Human Gametes and Preimplantation Embryos. New York: Springer; 2013. pp. 31–43. [Google Scholar]
- 4.Gonzalez XV, Odia R, Naja R, Serhal P, Saab W, Seshadri S, et al. Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients. J Assist Reprod Genet. 2019;36:1–7. doi: 10.1007/s10815-018-1391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal A, Roudebush WE, Chosed RJ. Embryo biopsy can offer more information than just ploidy status. Front in Cell and Dev Biol. 2020;8. [DOI] [PMC free article] [PubMed]
- 6.Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, et al. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105:225–235. doi: 10.1016/j.fertnstert.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, Magnani M, et al. Genomic DNA in human blastocoele fluid. Reprod Biomed Online. 2013;26:603–610. doi: 10.1016/j.rbmo.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Poli M, Ori A, Child T, Jaroudi S, Spath K, Beck M, et al. Characterization and quantification of proteins secreted by single human embryos prior to implantation. EMBO Mol Med. 2015;7:1465–1479. doi: 10.15252/emmm.201505344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobler K, Zhao Y, Ross R, Benner A, Xu X, Du L, et al. Blastocoel fluid (bf) harbors embryonic DNA that may result from the marginalization of aneuploid cells during embryogenesis. Fertil Steril. 2014;102:e205. doi: 10.1016/j.fertnstert.2014.07.692. [DOI] [Google Scholar]
- 10.Hammond ER, McGillivray BC, Wicker SM, Peek JC, Shelling AN, Stone P, et al. Characterizing nuclear and mitochondrial DNA in spent embryo culture media: genetic contamination identified. Fertil Steril. 2017;107:220–228.e5. doi: 10.1016/j.fertnstert.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Fragouli E, Munne S, Wells D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25:15–33. doi: 10.1093/humupd/dmy036. [DOI] [PubMed] [Google Scholar]
- 12.Brezina PR, Anchan R, Kearns WG. Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J Assist Reprod Genet. 2016;33:823–832. doi: 10.1007/s10815-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battaglia R, Palini S, Vento ME, La Ferlita A, Lo Faro MJ, Caroppo E, et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci Rep. 2019;9:84. doi: 10.1038/s41598-018-36452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond ER, Shelling AN, Cree LM. Nuclear and mitochondrial DNA in blastocoele fluid and embryo culture medium: evidence and potential clinical use. Hum Reprod. 2016;31:1653–1661. doi: 10.1093/humrep/dew132. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li N, Wang L, Sun H, Ma M, Wang H, et al. Molecular analysis of DNA in blastocoele fluid using next-generation sequencing. J Assist Reprod Genet. 2016;33:637–645. doi: 10.1007/s10815-016-0667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy K. Apoptosis in the human embryo. Rev Reprod. 1999;4:125–134. doi: 10.1530/ror.0.0040125. [DOI] [PubMed] [Google Scholar]
- 17.Brill A, Torchinsky A, Carp H, Toder V. The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet. 1999;16:512–519. doi: 10.1023/A:1020541019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128:281–291. doi: 10.1530/rep.1.00241. [DOI] [PubMed] [Google Scholar]
- 19.Jurisicova A, Varmuza S, Casper R. Programmed cell death and human embryo fragmentation. MHR Basic Sci Reprod Med. 1996;2:93–98. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 20.Brison DR. Apoptosis in mammalian preimplantation embryos: regulation by survival factors. Hum Fertil. 2000;3:36–47. doi: 10.1080/1464727002000198671. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs K, Van de Velde H, De Paepe C, Sermon K, Spits C. Mitotic spindle disruption in human preimplantation embryos activates the spindle assembly checkpoint but not apoptosis until Day 5 of development. Mol Hum Reprod. 2017;23:321–329. doi: 10.1093/molehr/gax007. [DOI] [PubMed] [Google Scholar]
- 22.Spanos S, Rice S, Karagiannis P, Taylor D, Becker D, Winston R, et al. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction. 2002;124:353–363. doi: 10.1530/rep.0.1240353. [DOI] [PubMed] [Google Scholar]
- 23.Rule K, Chosed RJ, Chang TA, Wininger JD, Roudebush WE. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. J Assist Reprod Genet. 2018;35:1497–1501. doi: 10.1007/s10815-018-1223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe AD, Hunter HR, Bloor DJ, Lieberman BA, Picton HM, Leese HJ, et al. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev Incorp Gamete Res. 2004;68:35–50. doi: 10.1002/mrd.20055. [DOI] [PubMed] [Google Scholar]
- 25.Wells D, Bermudez M, Steuerwald N, Thornhill A, Walker D, Malter H, et al. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20:1339–1348. doi: 10.1093/humrep/deh778. [DOI] [PubMed] [Google Scholar]
- 26.Bolton H, Graham SJ, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165. doi: 10.1038/ncomms11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capalbo A, Romanelli V, Patassini C, Poli M, Girardi L, Giancani A, Stoppa M, Cimadomo D, Ubaldi FM, Rienzi L. Diagnostic efficacy of blastocoel fluid and spent media as sources of DNA for preimplantation genetic testing in standard clinical conditions. Fertil Steril. 2018;110(5):870–879.e5. doi: 10.1016/j.fertnstert.2018.05.031.Erratum.In:FertilSteril.2019;111(1):194. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Bogale B, Tang Y, Lu S, Xie XS, Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci U S A. 2019;116(28):14105–14112. doi: 10.1073/pnas.1907472116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lal A, Roudebush WE, Mainigi M, Chosed RJ. Fluorescent-dependent comparative Ct method for qPCR gene expression analysis in IVF clinical pre-implantation embryonic testing. Biol Methods Protoc. 2021;6:bpab001. doi: 10.1093/biomethods/bpab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, et al. Depletion of aneuploid cells in human embryos and gastruloids. Nat Cell Biol. 2021;23(4):314–321. doi: 10.1038/s41556-021-00660-7. [DOI] [PubMed] [Google Scholar]
- 31.Ntostis P, Kokkali G, Iles D, Huntriss J, Tzetis M, Picton H, et al. Can trophectoderm RNA analysis predict human blastocyst competency? Syst Biol in Reprod Med. 2019;65:312–325. doi: 10.1080/19396368.2019.1625085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licciardi F, Lhakhang T, Kramer YG, Zhang Y, Heguy A, Tsirigos A. Human blastocysts of normal and abnormal karyotypes display distinct transcriptome profiles. Sci Rep. 2018;8:14906. doi: 10.1038/s41598-018-33279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vera-Rodriguez M, Chavez SL, Rubio C, Reijo Pera RA, Simon C. Prediction model for aneuploidy in early human embryo development revealed by single-cell analysis. Nat Commun. 2015;6:7601. doi: 10.1038/ncomms8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks JC, McCallie BR, Janesch AM, Schoolcraft WB, Katz-Jaffe MG. Blastocyst gene expression correlates with implantation potential. Fertil Steril. 2011;95:1367–1372. doi: 10.1016/j.fertnstert.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Kirkegaard K, Villesen P, Jensen JM, Hindkjaer JJ, Kolvraa S, Ingerslev HJ, et al. Distinct differences in global gene expression profiles in non-implanted blastocysts and blastocysts resulting in live birth. Gene. 2015;571:212–220. doi: 10.1016/j.gene.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 36.McCallie BR, Parks JC, Griffin DK, Schoolcraft WB, Katz-Jaffe MG. Infertility diagnosis has a significant impact on the transcriptome of developing blastocysts. Mol Hum Reprod. 2017;23:549–556. doi: 10.1093/molehr/gax034. [DOI] [PubMed] [Google Scholar]
- 37.McCallie BR, Parks JC, Trahan GD, Jones KL, Coate BD, Griffin DK, et al. Compromised global embryonic transcriptome associated with advanced maternal age. J Assist Reprod Genet. 2019;36:915–924. doi: 10.1007/s10815-019-01438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.