Abstract
Intracellular polysaccharides (IPS) are glycogen-like storage polymers which contribute significantly to Streptococcus mutans-induced cariogenesis. We previously identified and cloned a locus from the S. mutans chromosome which is required for the accumulation of IPS. Sequencing of this locus revealed at least four contiguous open reading frames, all of which are preceded by a common promoter region and are transcribed in the same direction. Analysis of the amino acid sequence deduced from the first of these open reading frames (ORF1) revealed domains which are highly conserved among d-alanine-activating enzymes (DltA) in Lactobacillus rhamnosus (formerly Lactobacillus casei) and Bacillus subtilis. The deduced amino acid sequences derived from ORF2, -3, and -4 also exhibit extensive similarity to DltB, -C, and -D, respectively, in these microorganisms. However, Southern hybridization experiments indicate that this operon maps to a locus on the S. mutans chromosome which is separate from the glgP, glgA, and glgD genes, whose products are known mediators of bacterial IPS accumulation. We therefore assigned a new dlt designation to the locus which we had formerly called glg. We maintain that the dlt genes are involved in S. mutans IPS accumulation, however, since they complement a mutation in trans which otherwise renders S. mutans IPS deficient. In this study, we found that expression of the S. mutans dlt genes is growth phase dependent and is modulated by carbohydrates internalized via the phosphoenolpyruvate phosphotransferase system (PTS). We demonstrated that the S. mutans dlt genes are expressed constitutively when non-PTS sugars are provided as the sole source of carbohydrate. Consistent with a role for the PTS in dlt expression is a similar constitutive expression of the dlt genes in an S. mutans PTS mutant grown in a chemically defined medium supplemented with glucose. In summary, these findings support a novel role for the dlt gene products in S. mutans IPS accumulation and suggest that dlt expression in this oral pathogen is subject to complex mechanisms of control imposed by growth phase, dietary carbohydrate, and other factors present in the plaque environment.
Streptococcus mutans, the chief etiologic agent of dental cavities in humans (13), colonizes the oral cavity shortly after tooth eruption and is found ubiquitously in approximately 98% of the population worldwide (6). The S. mutans-induced caries-forming process is complex, beginning with the attachment of the bacterium to the tooth pellicle and culminating in tooth decay owing to the production of lactic acid as a sole metabolic by-product.
Carbohydrates consumed in the diet are internalized by S. mutans primarily via the phosphoenolpyruvate phosphotransferase transport system (PTS) (7, 20, 25). More recently, Tao et al. described a multiple-sugar metabolism (msm) operon in S. mutans which provides a pathway for the uptake of non-PTS sugars, such as raffinose and melibiose (30). Sugars internalized via PTS or non-PTS pathways are subsequently metabolized, and the lactic acid which is produced demineralizes tooth enamel to form carious lesions.
S. mutans can also metabolize intracellular glycogen-like storage polymers (intracellular polysaccharides [IPS]). Tanzer et al. were the first to suggest that IPS can serve as metabolic substrates for acid production during periods of carbohydrate limitation in the oral cavity (29). The utilization of IPS by S. mutans can therefore exacerbate tooth decay by prolonging the period of exposure of host tissues to organic acids, such as during the periods between meals.
We previously confirmed a significant role for IPS in the S. mutans-induced cariogenic process by constructing genetic mutants altered in their ability to accumulate IPS (27, 28). Specifically, we generated an S. mutans transposon library by using the streptococcal transposon Tn916 (23) and screened it for IPS-altered mutants by iodine staining. Two mutants were identified, and their IPS contents were confirmed by quantitating electron-dense cytosolic glycogen-like granules on transmission electron micrographs (27, 28). One mutant, SMS201, is IPS deficient and significantly less cariogenic than the UA130 wild-type progenitor strain in a germ-free rat model system (28). Southern analysis confirmed a single transposon insertion in SMS201 and revealed that the transposon interrupts a region immediately upstream of an operon on the S. mutans chromosome. Importantly, the generation of a knockout mutation at the site of transposon insertion in S. mutans UA130 gave rise to a mutant which also proved to be IPS deficient (28), thereby ruling out the possibility that polar effects were imposed by the transposon insertion in SMS201.
Northern hybridization studies conducted in our laboratory support transcription of this locus, which we named glg, as a single 6.2-kb polycistronic mRNA (27). However, sequence analysis of this locus revealed at least four contiguous open reading frames (ORFs) which exhibit significant homology to the previously described dlt genes from Lactobacillus rhamnosus (formerly Lactobacillus casei) (9, 11) and Bacillus subtilis (17). Moreover, the results of Southern hybridization experiments indicated that the S. mutans glgP gene, cloned by Smith et al. (26), and the S. mutans glgA and glgD genes, recently cloned in our laboratory, occupy a locus on the S. mutans chromosome which is independent of glg. Consequently, we assigned a new designation, dlt, to the S. mutans glg locus. However, we maintain that it is uniquely involved in IPS accumulation, since the cloned dlt operon is capable of trans complementation in the IPS-deficient mutant SMS201 (28). In the present study, we determined the organization of the S. mutans dlt operon and monitored its expression in the presence of various dietary carbohydrates. Indeed, an improved understanding of the mechanism(s) which regulates expression of the dlt genes could elucidate how dlt affects IPS accumulation and, consequently, how S. mutans persists and causes disease in the oral cavity.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in the present study are described in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| HB101 | supE44 hsdS20 recA13 ara-14 proA2 lacY galK2 rpsL20 xyl-5 mtl-1 | 2 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thiΔ(lac-proAB) | 33 |
| CLEO1 | HB101 transformed with pMC1; Tcr Cmr | This work |
| S. mutans strains | ||
| UA130 | Serotype c; IPS+ | P. Caufield |
| GMS100 | UA130 with dlt::cat fusion from pMC1; Tcr | This work |
| BM71 | PTS+ | 3, 16 |
| DC10 | ptsI::erm PTS− | 8 |
| Plasmids | ||
| pAM620 | pVA891::pAD1EcoRIF′::Tn916 Tcr Emr | 32 |
| pMH109 | Contains promoterless cat gene preceded by pUC13 MCS; Tcr | 12 |
| pNC10 | pMH109 derived; contains promoterless cat gene; Tcr ColE1 | This work |
| pNC12 | pNC10 derived; ColE1 tetM | This work |
| pMC1 | pNC12 derived; ColE1 dlt::cat tetM Cmr | This work |
| pDLT1-2 | 3.1-kb S. mutans amplicon containing dlt1 and dlt2 in pGEM-T Easy; Apr | This work |
| pDLT3 | 0.3-kb S. mutans amplicon containing dlt3 in pGEM-T Easy; Apr | This work |
| pDLT3-4 | 1.7-kb S. mutans amplicon containing dlt3 and dlt4 in pGEM-T Easy; Apr | This work |
| pYA3023 | 6.9-kb BglII fragment containing S. mutans dlt in pUC19; Apr | 28 |
| pYA3031 | 8-kb XbaI fragment containing S. mutans dlt in pUC19; Apr | This laboratory |
Culture conditions.
Escherichia coli HB101 and JM109 were grown with aeration at 37°C in Luria-Bertani (LB) broth supplemented with 0.1% dextrose. S. mutans UA130 (serotype c) and its derivatives were grown in Todd-Hewitt broth (Difco) at 37°C in a 5% CO2 atmosphere. When propagating the GMS100 fusion strain, 5 μg of tetracycline was added to the medium. S. mutans BM71 was grown on Tryptone-yeast extract–0.3% raffinose plates, while the DC10 isogenic mutant was propagated on the same medium supplemented with 8 μg of erythromycin per ml.
For expression studies, S. mutans UA130, GMS100, BM71, and DC10 were grown as described above but in a chemically defined medium (CDM) (31) supplemented with various dietary carbohydrates each to a final concentration of 1%. The carbohydrate-supplemented medium was inoculated with 150 μl of a concentrated S. mutans frozen stock.
For reporter gene studies, an aliquot of a GMS100 overnight culture was diluted 1:10 in prewarmed CDM supplemented with 1% glucose and grown as described above for an additional 2 h. These cultures were designated as the zero time points. Aliquots from the zero time point cultures were then washed in phosphate-buffered saline, inoculated into prewarmed CDM supplemented with the test carbohydrate(s), and grown as usual before harvesting from early-, mid-, and late-logarithmic-phase and stationary-phase cultures.
For dot blot and Northern hybridization experiments, S. mutans was grown, as described above, in CDM supplemented with a PTS sugar (e.g., glucose, fructose, or sucrose) or with a non-PTS sugar (e.g., raffinose or melibiose). Growth phase was determined by periodic measurements of the optical density at 600 nm and comparisons made against previously defined standard growth curves.
For primer extension studies, S. mutans UA130 was grown to mid-logarithmic phase, as described above, in CDM supplemented with either 1% glucose or both 0.5% glucose and 1% fructose.
DNA isolation and digestion.
S. mutans chromosomal DNA was isolated as previously described (14, 28) and purified by ethidium bromide-cesium chloride density gradient centrifugation. Plasmid DNA was isolated from E. coli by the alkaline lysis method of Birnboim and Doly (1). Restriction enzyme digestions were performed as recommended by the enzyme supplier (Promega).
Bacterial transformation.
Electrocompetent E. coli HB101 was transformed with pNC10, pNC12, or pMC1 in an electroporator according to protocols provided by the manufacturer (Bio-Rad). The resulting strains were selected on LB agar supplemented with only tetracycline (15 μg/ml) or with both tetracycline (15 μg/ml) and chloramphenicol (15 μg/ml). Electrocompetent E. coli JM109 was transformed with pDLT1-2, pDLT3, or pDLT3-4 as described above, and the resulting transformants were selected on LB agar supplemented with ampicillin (100 μg/ml).
S. mutans UA130 was transformed with pMC1 (10 μg) by electroporation as previously described (28). Transformants were screened on Todd-Hewitt agar supplemented with tetracycline (5 μg/ml). Chromosomal DNA isolated from tetracycline-resistant transformants was analyzed by Southern blotting with a 0.36-kb HindIII-SphI fragment internal to dlt1 as a probe. This confirmed integration of the plasmid into the chromosome, and the resulting fusion strain was designated GMS100.
Southern blot analysis.
Southern blotting was performed according to standard procedures (21) with a 0.6-kb HindIII-SphI probe which harbors the dlt promoter region or the 0.36-kb HindIII-SphI fragment which is internal to dlt1. Hybridizations were carried out under stringent conditions at 42°C, and filters were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at room temperature with gentle agitation for 30 min; this was followed by two 30-min washes in 0.5× SSC–0.1% SDS at 50°C. The filters were air dried and exposed to Kodak X-OMat XAR5 film at −80°C overnight.
RNA isolation.
Total RNA was isolated from S. mutans cultures grown to early logarithmic, mid-logarithmic, late logarithmic, or stationary phase by a hot phenol extraction method described previously (27).
Northern and dot blot analyses.
For Northern hybridization experiments, total RNA was resolved on 0.8% formaldehyde–agarose gels in 20 mM morpholinepropanesulfonic acid (MOPS) at 40 V overnight. Equal loading was confirmed by densitometric scanning of ethidium bromide-stained gels. The RNA was transferred to nylon membranes (Micron Separations, Inc., Westboro, Mass.) as previously described (21) and cross-linked in an FB-UVXL-1000 cross-linker apparatus (Fisher Scientific, Springfield, N.J.). Dot blots were prepared by spotting equal amounts of total RNA, as determined spectrophotometrically at 260 nm and by densitometric scanning of ribosomal subunits on ethidium bromide-stained gels, onto nitrocellulose membranes (Protran) by the use of a microsample filtration manifold (Schleicher and Schuell, Keene, N.H.). The nitrocellulose membranes were baked in a vacuum oven at 80°C for 2 h. Northern and dot blots were probed with the radiolabeled 0.36-kb HindIII-SphI dlt fragment or a 0.62-kb dlt-specific PCR product, both of which are internal to the dlt1 coding region. Hybridization and wash conditions were as described above. Individual dots were then excised from the membrane with a hole punch, and radioactivity was counted in a Packard (Meriden, Conn.) Tri-Carb model 1900CA liquid scintillation analyzer.
Preparation of radiolabeled probes.
DNA fragments used to probe Southern blots were isolated from 1% low-melting-temperature agarose gels following digestion with HindIII and SphI (Promega). The 0.62-kb fragment used to probe the Northern and dot blots was derived from plasmid pYA3023 by PCR with 5′-CTCATAGGCCGCAAATGCTG-3′ and 5′-GAGCAAGAGACCTTCATCTGTC-3′ as primers. The DNA fragments were purified by spin column chromatography (Qiagen) and subsequently labeled by nick translation (19). Unincorporated nucleotides were removed from the reaction mixture by Sephadex G-25 chromatography (5 Prime→3 Prime, Inc., Boulder, Colo.).
Construction of an S. mutans dlt::cat fusion strain (GMS100).
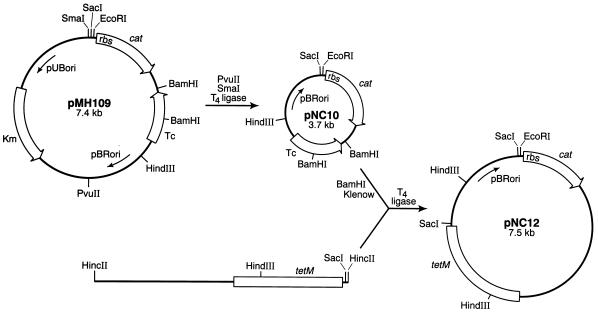
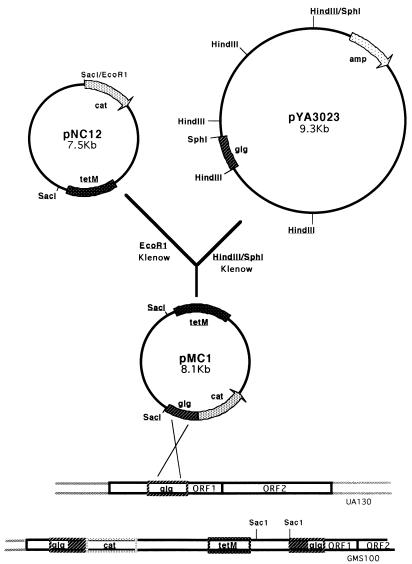
A DNA fragment harboring an E. coli origin of replication, a promoterless cat reporter gene with an appropriate ribosome binding site (RBS), and a gene encoding resistance to tetracycline in E. coli was isolated from plasmid pMH109 (12). Following the addition of T4 DNA ligase (Promega), the resulting 3.7-kb plasmid, designated pNC10, was transformed into E. coli HB101 (2), and transformants were selected on LB agar supplemented with tetracycline (10 μg/ml). The tetM gene, which is expressed in both E. coli and S. mutans, was isolated from Tn916 on a 4.8-kb HincII fragment and ligated into pNC10 as illustrated in Fig. 1. E. coli transformants harboring the new construct, pNC12, were selected as described above. This plasmid was subsequently linearized with EcoRI and made blunt ended with the Klenow fragment of DNA polymerase (Fig. 2). A 0.6-kb HindIII-SphI fragment from pYA3023, which harbors the S. mutans dlt promoter region, was similarly made blunt ended and ligated to pNC12. The resulting operon fusion construct, pMC1, which harbors the dlt promoter region and the cat gene cloned in the same orientation, was transformed into E. coli as described above, and its presence was confirmed by restriction endonuclease mapping and Southern analysis with the radiolabeled 0.6-kb HindIII-SphI fragment as the probe (data not shown). Finally, pMC1 was introduced into the S. mutans chromosome by electroporation, and the Campbell-type insertion illustrated in Fig. 2 was selected by screening transformants for resistance to tetracycline. The single-crossover event was confirmed by Southern blot analysis with a 0.36-kb HindIII-SphI dlt fragment as the probe (data not shown).
FIG. 1.
Construction of integration vector pNC12. Plasmid pMH109 was digested with PvuII and SmaI, and the fragment which harbors pBRori, the promoterless cat reporter gene, and the tetracycline resistance-encoding gene was recircularized with ligase and transformed into E. coli HB101. Transformants were selected on LB agar containing 10 μg of tetracycline per ml, and the recombinant plasmid, pNC10, was isolated and confirmed by restriction enzyme mapping. pNC10 was digested with BamHI to create a deletion in the tetracycline resistance gene, and the ends of the linearized plasmid were filled in with the Klenow fragment of DNA polymerase. The tetM gene, contained on a 4.8-kb HincII fragment purified from plasmid pAM620 (32), was ligated into blunt-ended pNC10, and E. coli transformants were selected on LB agar containing 10 μg of tetracycline per ml. Plasmid pNC12 was isolated by an alkaline lysis protocol (1) and confirmed by restriction enzyme and Southern blot analyses. pBRori, origin of replication functional in E. coli but not in S. mutans; pUBori, origin of replication functional in S. aureus and B. subtilis but not in E. coli; Tc, tetracycline resistance gene selectable in E. coli but not in S. mutans; tetM, gene from Tn916 which encodes resistance to tetracycline in both S. mutans and E. coli; Km, kanamycin resistance gene.
FIG. 2.
Cloning strategy used to generate pMC1 for subsequent chromosomal integration. The S. mutans dlt promoter region, contained on a 0.6-kb HindIII-SphI fragment on plasmid pYA3023, and pNC12 digested with EcoRI were treated with the Klenow fragment of DNA polymerase prior to blunt-end ligation, which was performed overnight at 20°C. The resulting operon fusion construct, pMC1, was electroporated into E. coli DH5α, and transformants were selected on LB agar plates containing tetracycline (15 μg/ml). The operon fusion was confirmed by restriction enzyme mapping and Southern analysis with the dlt promoter fragment as a probe. S. mutans UA130 was then electrotransformed with pMC1, and transformants that had undergone plasmid integration into the chromosome (GMS100) were identified by selection on Todd-Hewitt agar containing tetracycline.
Preparation of S. mutans GMS100 cell lysates and determination of CAT specific activity.
GMS100 cell pellets grown in carbohydrate-supplemented CDM were resuspended in 1 ml of Tris-EDTA buffer, pH 8.0, and combined with 300 μl of 0.1-mm-diameter zirconium beads. The cells were lysed by mechanical disruption in a Mini-Bead Beater (BioSpec) for two 1.5-min intervals at 4°C with intermittent cooling on ice. The beads were then pelleted by centrifugation, and the supernatant was clarified by an additional low-speed centrifugation. Fifty microliters of the lysate was removed from each sample for protein determination with a bicinchoninic acid protein assay kit (Pierce). The remaining lysate was stored at 0°C for subsequent monitoring of chloramphenicol acetyltransferase (CAT) specific activity by the spectrophotometric assay of Shaw (24). CAT specific activities were expressed as nanomoles of chloramphenicol acetylated per minute per milligram of total protein.
Primer extension studies.
Total RNA was isolated from S. mutans UA130 as described above and stored at −80°C. For 5′ primer extension, an oligonucleotide with the sequence 5′-GCAAGCGAATCAGAATCAGC-3′ was used. End labeling and primer extension reactions were performed as previously described (10).
In vitro transcription-translation studies.
The gene products derived from the S. mutans dlt operon were analyzed in a coupled in vitro transcription-translation system (Promega Biotech, Madison, Wis.) according to the recommendations of the supplier. Plasmids pDlt1-2, pDlt3, and pDlt3-4, which were used in this analysis, are described in Table 1. [35S]methionine was used to radiolabel the reaction products, which were subsequently resolved on SDS–12% polyacrylamide gels and visualized by autoradiography.
DNA sequencing and analysis.
DNA was sequenced by the dideoxy chain termination method (22). Double-stranded template DNA was isolated by alkaline lysis (1) and purified by a polyethylene glycol precipitation procedure (21) prior to sequencing. Sequence analyses were carried out with the Macintosh program MacDNAsis 3.0 (Hitachi Software Engineering Co., Ltd.) and the programs BLASTP and BLASTX (National Center for Biotechnology Information Laboratories, Los Alamos, N. Mex.). Alignment of the deduced amino acid sequences was performed with DNASTAR software (DNASTAR Inc., Madison, Wis.).
Nucleotide sequence accession number.
The S. mutans dlt sequence reported herein has been entered into the GenBank database under accession no. AF050517.
RESULTS
Organization and nucleotide sequence of the S. mutans dlt genes.
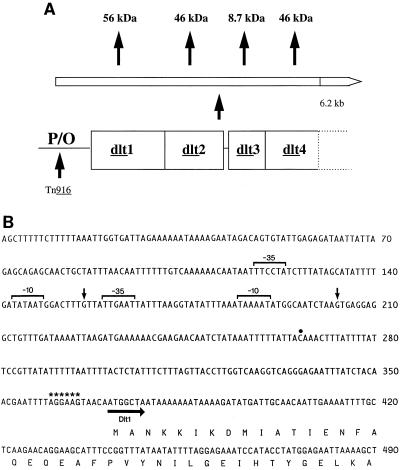
Shown in Fig. 3A is the organization of the S. mutans dlt1, dlt2, dlt3, and dlt4 genes, which are harbored on a 6.9-kb BglII fragment on plasmid pYA3023 (28) and an 8.3-kb fragment on plasmid pYA3031. Nucleotide sequencing of these constructs revealed a 1,545-bp ORF, dlt1, which encodes a putative gene product of 515 amino acids with a predicted molecular mass of 56 kDa. Upstream of dlt1 is a putative RBS (AGGAAG) which precedes the ATG start codon by 5 nucleotides (nt) (Fig. 3B). The site of the transposon insertion which renders S. mutans SMS201 IPS deficient is 94 nt upstream of the RBS. The DNA sequence 3′ of the dlt1 gene contains another ORF (dlt2); the dlt2 initiation codon overlaps the termination codon of dlt1 by 1 nt. The dlt2 ORF is 1,262 nt in length and codes for a predicted protein of 46 kDa with distinct hydrophobic domains. Another ORF (dlt3) is located 15 bp downstream of dlt2 and is preceded by its own putative RBS. This ORF is 237 bp in size and encodes a predicted protein of 8.7 kDa. Finally, the dlt4 gene overlaps dlt3 by 1 nt and, like dlt2, is 1.26 kb in size and gives rise to a predicted protein of 46 kDa.
FIG. 3.
(A) Operon-like arrangement of the S. mutans dlt genes. P/O, promoter-operator. (B) Nucleotide sequence of the S. mutans dlt promoter region. The transcription start sites and −10 and −35 promoter regions are indicated by vertical arrows and brackets, respectively. The putative RBS is designated by asterisks. The dot marks the site of transposon insertion into S. mutans SMS201, which renders it IPS deficient The horizontal arrow indicates the direction of transcription of the dlt operon.
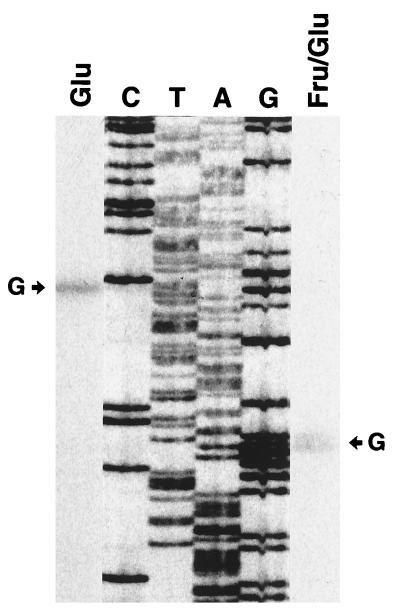
Shown in Fig. 4 are results of primer extension studies, which reveal two possible transcription start sites upstream of the S. mutans dlt operon, at nt 156 and 203. Consequently, we localized the dlt promoter regions to TATAAT at −10 and TTCCTA at −35 for the former and TAAAAT at −10 and TTGAAT at −35 for the latter (Fig. 3B).
FIG. 4.
Primer extension analysis of dlt transcripts. A radiolabeled oligonucleotide was annealed to RNA isolated from S. mutans cultures grown in CDM supplemented with either glucose alone (Glu) or both fructose and glucose (Fru/Glu). The cDNAs resulting from the addition of avian myeloblastosis virus reverse transcriptase were run alongside the dideoxy sequencing reactions involving plasmid pYA3023. The arrows indicate the transcription start sites at guanine residues corresponding to nt 156 (left) and 203 (right).
Comparison of the sequence of the deduced Dlt1 protein with those of ATP-utilizing enzymes from other bacteria revealed significant homology, especially with the d-alanine-activating enzymes (DltA) from L. rhamnosus (11) and B. subtilis (17). These cytosolic enzymes catalyze the biosynthesis of d-alanyl-lipoteichoic acids in L. rhamnosus and B. subtilis, and each has a conserved phosphate binding loop encoded by the consensus sequence GXXGXPK. Two additional domains conserved among this group of enzymes, GRXDXQXKXXGXRXE and PX9PX5KXDX3L, presumably catalyze the transfer of d-alanine to lipoteichoic acids via a thiol ester intermediate. Multiple alignment of the amino acid sequences indicates that all three functional domains are highly conserved in S. mutans Dlt1, L. rhamnosus DltA, and B. subtilis DltA. Comparative analysis of Dlt1 from S. mutans with DltA from L. rhamnosus revealed 95, 70, and 58% identity at the amino acid level within regions 1, 2, and 3, respectively, and an overall identity of 48.5%. A gene product of approximately 56 kDa is derived from dlt1, as determined via in vitro transcription-translation (data not shown).
The S. mutans Dlt2 sequence exhibits 48% amino acid identity to the DltB proteins from L. rhamnosus (9) and B. subtilis (17), respectively. DltB is a putative membrane-associated transporter which presumably mediates the transport and subsequent transfer of cytosolic d-alanine residues to neighboring lipoteichoic acids at the bacterial cell surface. In vitro transcription-translation studies revealed that a protein of approximately 45 kDa is derived from dlt2 (data not shown).
S. mutans Dlt3 exhibits 44% amino acid identity to the L. rhamnosus dltC gene product; the latter encodes a d-alanine carrier protein which exhibits significant similarity to fatty acid acyl carrier proteins of E. coli and Saccharomyces cerevisiae (17). Interestingly, Dlt3 harbors a serine residue at position 35 which is strictly conserved among acyl carrier proteins. The S. mutans dlt3 gene encodes a protein in vitro which approximates 8.7 kDa as predicted (data not shown).
Finally, Dlt4 exhibits 30% amino acid identity to the B. subtilis dltD gene product. Based on the amino acid composition, the presence of an amino-terminal signal peptide is suggested. Specifically, two positively charged residues are followed by a run of hydrophobic residues (MLKRLWLILGPVFCALVLVF). Thus, Dlt4 is likely to be a secreted protein which may be anchored into the cell membrane at its amino-terminal end (17). By in vitro transcription-translation analysis, it was also revealed (data not shown) that a protein of approximately 45 kDa is derived from dlt4.
Expression of dlt in S. mutans GMS100.
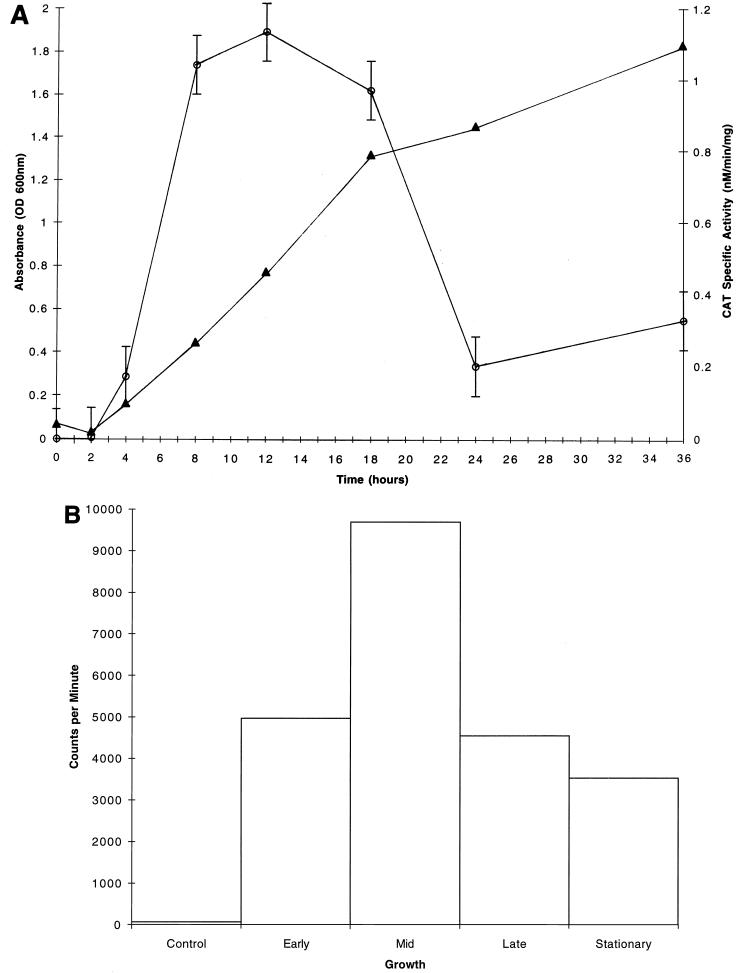
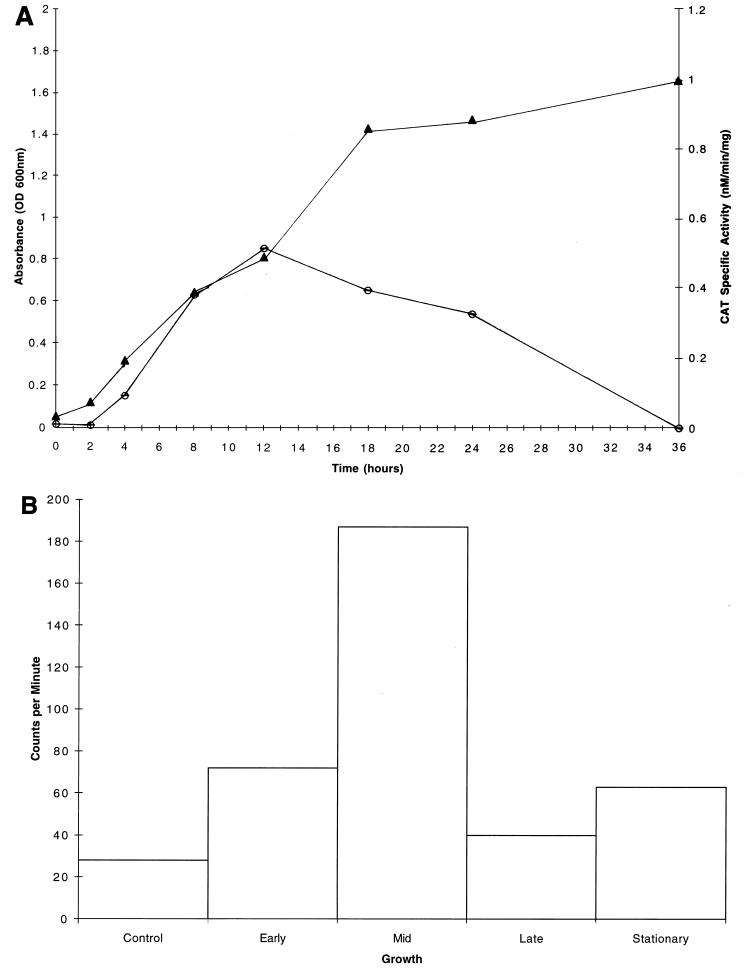
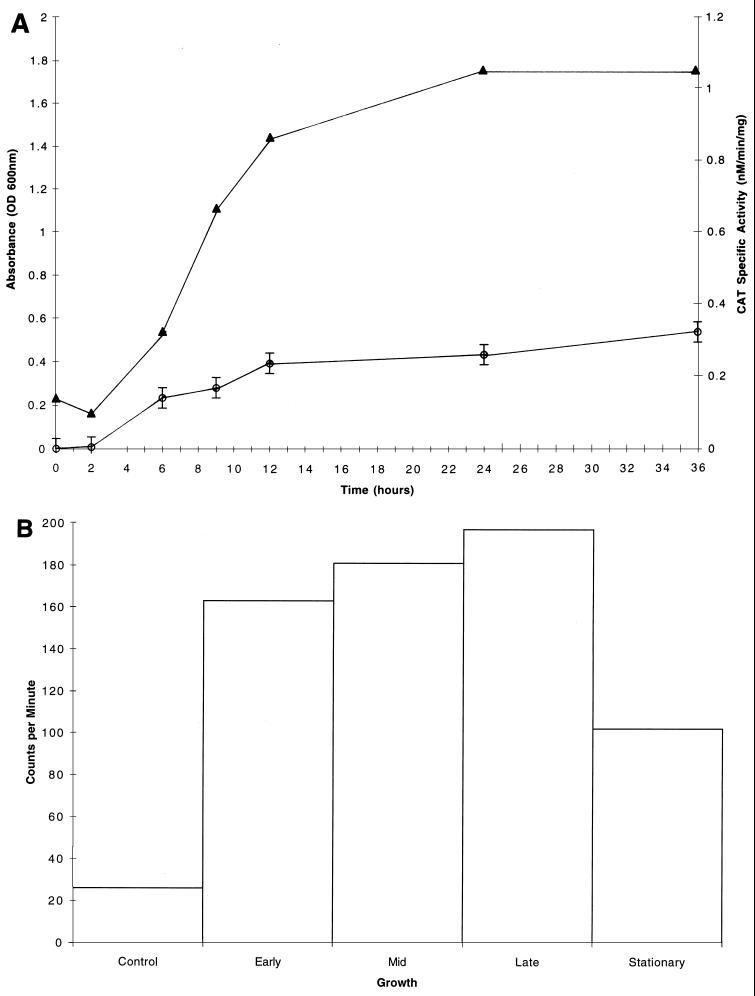
CAT specific activities were determined for the S. mutans GMS100 dlt::cat fusion strain grown in CDM supplemented with glucose, fructose, or sucrose, all of which are carbohydrates internalized by the PTS. Interestingly, the dlt genes were not expressed in early-logarithmic-phase cultures when glucose was provided as the sole source of carbohydrate; however, expression was induced during mid-logarithmic phase, reaching a maximum of 1.1 nmol/min/mg (Fig. 5A). During later stages of growth, the CAT specific activity returned to near baseline levels. Maximal dlt expression (0.5 nmol/min/mg) was also achieved during mid-logarithmic phase for cultures propagated in CDM supplemented with sucrose (Fig. 6A). Other PTS sugars, including fructose as well as glucose and fructose in combination, similarly gave rise to maximal dlt expression during mid-logarithmic phase (data not shown). In contrast, different patterns of dlt expression were observed when S. mutans UA130 was grown in CDM supplemented with a non-PTS sugar, such as raffinose (Fig. 7A) or melibiose (data not shown). These carbohydrates seemed to direct the constitutive expression of dlt during all stages of growth, including the early logarithmic phase.
FIG. 5.
(A) Growth curve (closed triangles) and CAT specific activities (open circles) for S. mutans GMS100 cultures grown in CDM supplemented with 1% glucose. Each CAT specific activity data point represents the mean of values from three independent experiments. Standard deviations are negligible except where indicated by vertical bars. (B) Total RNA (1 μg) isolated from S. mutans UA130 grown to early, mid-, or late logarithmic phase or to stationary phase in CDM supplemented with 1% glucose was dot blotted onto a nitrocellulose membrane and probed with a DNA fragment which is internal to dlt1. Individual dots were excised, and the radioactivity was counted in a Packard Tri-Carb model 1900CA liquid scintillation analyzer. A dot representing nonspecific hybridization was included as a control. Shown are the results of a single representative experiment. Note the maximal expression of dlt during mid-logarithmic phase.
FIG. 6.
(A) Growth curve (closed triangles) and CAT specific activities (open circles) for S. mutans GMS100 cultures grown in CDM supplemented with 1% sucrose. For each CAT specific activity data point, representing the mean of values from three independent experiments, the standard deviation was negligible. (B) Total RNA (1 μg) isolated from S. mutans UA130 grown to early, mid-, or late logarithmic phase or to stationary phase in CDM supplemented with 1% sucrose was dot blotted onto a nitrocellulose membrane and probed with a DNA fragment which is internal to dlt1. Individual dots were excised, and the radioactivity was counted in a Packard Tri-Carb model 1900CA liquid scintillation analyzer. A dot representing nonspecific hybridization was included as a control. Shown are the results of a single representative experiment. Note the maximal expression of dlt during mid-logarithmic phase.
FIG. 7.
(A) Growth curve (closed triangles) and CAT specific activities (open circles) for S. mutans GMS100 cultures grown in CDM supplemented with 1% raffinose. Except where indicated by the vertical bars, the standard deviation was negligible for each data point, representing the mean of values from three independent experiments. (B) Total RNA (1 μg) isolated from S. mutans UA130 grown to early, mid-, or late logarithmic phase or to stationary phase in CDM supplemented with 1% raffinose was dot blotted onto a nitrocellulose membrane and probed with a DNA fragment which is internal to dlt1. Individual dots were then excised, and the radioactivity was counted in a Packard Tri-Carb model 1900CA liquid scintillation analyzer. A dot representing nonspecific hybridization was included as a control. Shown are the results of a single representative experiment. Note the constitutive expression of the S. mutans dlt genes.
Northern blotting of total RNA isolated from the GMS100 fusion strain grown in CDM supplemented with glucose, fructose, or sucrose revealed that dlt expression in GMS100 was identical to that of the UA130 wild-type strain (data not shown). This confirms that dlt expression in the fusion strain is not aberrantly affected by integration of pMC1 into the chromosome.
Dot blot analysis of S. mutans dlt expression.
Dot blotting of total RNA isolated from S. mutans UA130 cultures grown in the presence of various PTS sugars confirmed the expression patterns derived from cat reporter gene assays. That is, maximal expression of dlt-specific mRNA was evident during the mid-logarithmic phase of growth when glucose was provided in the medium (Fig. 5B). This expression declined during the late logarithmic and early stationary phases. The growth of S. mutans cultures supplemented with either fructose alone or both fructose and glucose gave rise to the same pattern of dlt expression (data not shown). When sucrose was provided as the sole carbohydrate source, expression of the dlt-specific transcript was also maximal during mid-logarithmic phase (Fig. 6B). In contrast, we observed constitutive dlt expression during all stages of growth when raffinose (Fig. 7B) or melibiose (data not shown) was present in the medium.
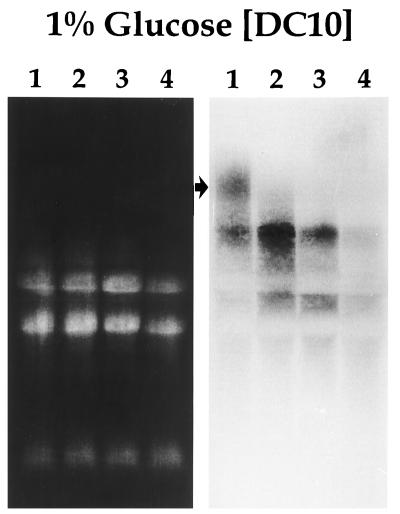
dlt expression in an S. mutans PTS mutant.
The Northern blot shown in Fig. 8 reveals the constitutive dlt expression evident in S. mutans DC10, a PTS mutant which we propagated in CDM supplemented with glucose. Also noteworthy is the unique expression of a large dlt-specific transcript (>10 kb) in S. mutans DC10 during early logarithmic phase which is not apparent in the BM71 progenitor strain (16) under the same growth conditions.
FIG. 8.
Ethidium bromide-stained gel (left panel) and corresponding Northern blot (right panel) of total RNA isolated from S. mutans DC10, a PTS mutant. Cells were grown in CDM supplemented with glucose to early logarithmic phase (lane 1), mid-logarithmic phase (lane 2), late logarithmic phase (lane 3), and stationary phase (lane 4). Hybridization was carried out with a radiolabeled dlt1-specific PCR product as the probe. Note that expression of the S. mutans dlt genes is constitutive for all phases of growth. A unique transcript (>10 kb) which is expressed only during early logarithmic phase is designated by the arrow.
DISCUSSION
IPS are high-molecular-weight glucan polymers which provide S. mutans with a metabolizable source of carbon during periods of nutrient deprivation in the oral cavity (29). The inactivation of S. mutans dlt expression diminishes IPS accumulation (28), resulting in significantly reduced cariogenicity in germ-free rats (15) maintained on a sucrose-containing, caries-promoting diet. Importantly, complementation of the IPS-deficient phenotype in S. mutans SMS201 can be achieved by providing the cloned dlt locus in trans (28). The ability of dlt to restore S. mutans IPS accumulation to wild-type levels in this mutant suggests at least an ancillary role for the dlt locus in IPS biosynthesis, storage, and/or breakdown. In fact, recent reports in the literature reveal novel roles for the streptococcal dlt genes, such as in S. mutans acid tolerance (3) and in Streptococcus gordonii intrageneric coaggregation (5).
In this study, we found that dlt expression in S. mutans is growth phase dependent and that it is regulated at the level of transcription. Specifically, growth of an S. mutans dlt::cat fusion strain in CDM supplemented with glucose, fructose, or sucrose gave rise to maximal cat specific activity during the mid-logarithmic phase. This activity declined markedly during the late logarithmic and early stationary phases despite the presence of excess carbohydrate in the growth medium. Importantly, an increase in cell mass (measured as milligrams of protein) after peak expression during mid-logarithmic phase is insufficient to account for the subsequent decrease in dlt expression. Similar expression of the dlt operon was reported in B. subtilis, in which transcription occurs maximally during the exponential phase of growth and then decreases during stationary phase and before the onset of sporulation (17). In nonsporulating cultures of B. subtilis, however, expression of the dlt operon is not regulated, suggesting that the modulation of dlt transcription in B. subtilis may be stress responsive.
Sugars consumed in the host diet also seem to play a role in the regulation of S. mutans dlt expression. We noted two different primer extension products derived from S. mutans cultures propagated in CDM supplemented with glucose alone or with both glucose and fructose. This suggests that different promoters may be recognized by S. mutans RNA polymerase under these and possibly other carbohydrate conditions. In addition, non-PTS sugars such as raffinose and melibiose gave rise to a unique pattern of S. mutans dlt expression. Unlike the modulated dlt expression of cultures grown in the presence of glucose, fructose, or sucrose (carbohydrates internalized by the PTS system), the constitutive expression of dlt was directed by non-PTS sugars during the early, mid-, and late logarithmic phases and the stationary phase of growth. This expression pattern is presumably due to induction of the msm operon by raffinose and/or melibiose to promote the internalization of these and other sugars via this non-PTS pathway (30).
A similar hybridization pattern was observed on Northern blots of total RNA isolated from S. mutans DC10, a PTS mutant which we propagated in CDM supplemented with glucose. This mutant bears a mutation in the enzyme I component of the PTS, such that glucose is prevented from entering bacterial cells via the conventional PTS pathway. The fact that dlt expression is altered in this mutant suggests a role for the PTS in dlt regulation. Moreover, the absence of a functional PTS system in the DC10 strain necessitates the rerouting of glucose uptake via an alternative pathway. In fact, reports by Cvitkovitch et al. (8) indicate that some glucose uptake in DC10 can occur via a non-PTS pathway that is independent of the msm operon. Additional reports suggest that such a transport pathway may involve a transmembrane carrier protein capable of recognizing glucose (4). Interestingly, the expression of a novel dlt-specific transcript in S. mutans DC10 was evident during early logarithmic phase in cultures grown in the presence of glucose (Fig. 8); this transcript was not expressed, however, by the BM71 progenitor strain. The early onset of expression of this unique transcript in the mutant is consistent with the elaboration of a protein required for glucose recognition so that uptake via a non-PTS route may follow. Studies to determine whether this transcript is absent from S. mutans DC10 cultures propagated in the presence of raffinose and/or melibiose are currently under way.
In conclusion, we assigned a novel dlt designation to the previously described S. mutans glg locus. However, we maintain that this locus is involved in IPS accumulation, which is known to contribute significantly to the S. mutans-induced caries-forming process. We report that the regulated expression of the S. mutans dlt genes is cell density dependent and subject to regulatory control by PTS sugars. One might propose a role for the membrane-associated d-alanyl lipoteichoic acids in sensing the plaque environment and, as part of a two-component signal transducing system, regulating IPS accumulation and possibly other cellular activities. This is consistent with the temporal expression in Staphylococcus aureus of an accessory gene regulator (agr) (18) which indirectly modulates the expression of other target genes in response to pH, cell density, and carbohydrate availability. Exactly how cell density and dietary carbohydrate modulate dlt expression in S. mutans is unclear, and it is not known whether these growth conditions are likely to regulate the coordinate expression of the S. mutans glg genes. Investigations aimed at elucidating putative cross-regulation between the dlt operon and other genes whose products contribute to carbohydrate metabolism and virulence in S. mutans will address this question. Taken collectively, these studies will reveal the regulatory network which is likely to control S. mutans IPS accumulation in the plaque environment and the complexity of events which promote cariogenesis in a niche as dynamic as the oral cavity.
ACKNOWLEDGMENTS
We thank Paula Fives-Taylor, Dennis Cvitkovitch, and Ronald Taylor for critical review of the manuscript and David Boyd for providing S. mutans BM71 and DC10.
Support for this research was provided by grant 1 R29 DE12306 from the National Institute of Dental Research to G.A.S. This work was also supported by Sigma Xi grants-in-aid to D.B. and K.H. and by the Middlebury College Department of Biology.
REFERENCES
- 1.Birnboim H, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolivar F, Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, D. A., I. R. Hamilton, D. G. Cvitokovitch, and A. S. Bleiweis. Unpublished data.
- 4.Buckley N D, Hamilton I R. Vesicles prepared from Streptococcus mutans demonstrate the presence of a second glucose transport system. Microbiology. 1994;140:2639–2648. doi: 10.1099/00221287-140-10-2639. [DOI] [PubMed] [Google Scholar]
- 5.Clemans, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 6.Colby S M, Russell R R B. Sugar metabolism by mutans streptococci. J Appl Microbiol. 1997;83:80–88. doi: 10.1046/j.1365-2672.83.s1.9.x. [DOI] [PubMed] [Google Scholar]
- 7.Cvitkovitch D G, Boyd D A, Hamilton I R. Regulation of sugar uptake via the multiple sugar metabolism operon by the phosphoenolpyruvate-dependent sugar phosphotransferase transport system of Streptococcus mutans. Dev Biol Stand. 1995;85:351–356. [PubMed] [Google Scholar]
- 8.Cvitkovitch D G, Boyd D A, Thevenot T, Hamilton I R. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J Bacteriol. 1995;177:2251–2258. doi: 10.1128/jb.177.9.2251-2258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debabov D V, Heaton M P, Zhang Q, Stewart K D, Lambalot R H, Neuhaus F C. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J Bacteriol. 1996;178:3869–3876. doi: 10.1128/jb.178.13.3869-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli D M, Leblanc D J. Transcriptional analysis of rolling circle replicating plasmid pVT736-1: evidence for replication control by antisense RNA. J Bacteriol. 1995;177:4474–4480. doi: 10.1128/jb.177.15.4474-4480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton M P, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J Bacteriol. 1992;174:4707–4717. doi: 10.1128/jb.174.14.4707-4717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson M C, Stewart G C. Differential utilization of Staphylococcus aureus promoter sequences by Escherichia coli and Bacillus subtilis. Gene. 1986;48:93–100. doi: 10.1016/0378-1119(86)90355-0. [DOI] [PubMed] [Google Scholar]
- 13.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 15.Michalek S M, McGhee J R, Navia J M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975;12:69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milnes A R, Bowden G H. The microflora associated with the developing lesions of nursing caries. Caries Res. 1985;19:287–297. doi: 10.1159/000260858. [DOI] [PubMed] [Google Scholar]
- 17.Perego M, Glaser P, Minutello A, Strauch M A, Leopold K, Fisher W. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis: identification of genes and regulation. J Biol Chem. 1995;270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 18.Regassa L B, Novick R P, Betley M J. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect Immun. 1992;60:3381–3388. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigby P W J, Diekmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick-translation with DNA polymerase I. J Mol Biol. 1977;113:237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- 20.Saier M H. Mechanisms and regulation of carbohydrate transport in bacteria. Orlando, Fla: Academic Press, Inc.; 1985. [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senghas E, Jones J M, Yamamoto M, Gawron-Burke C, Clewell D B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988;170:245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw W V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1979;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 25.Slee A M, Tanzer J M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979;24:821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A J, Quivey R G, Jr, Faustoferri R C. Cloning and nucleotide sequence analysis of the Streptococcus mutans membrane-bound, proton-translocating ATPase operon. Gene. 1996;183:87–96. doi: 10.1016/s0378-1119(96)00502-1. [DOI] [PubMed] [Google Scholar]
- 27.Spatafora G, Rohrer K, Barnard D, Michalek S. A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun. 1995;63:2556–2563. doi: 10.1128/iai.63.7.2556-2563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spatafora-Harris G, Michalek S M, Curtiss R., III Cloning of a locus involved in Streptococcus mutans intracellular polysaccharide accumulation and virulence testing of an intracellular polysaccharide-deficient mutant. Infect Immun. 1992;60:3175–3185. doi: 10.1128/iai.60.8.3175-3185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzer J M, Freedman M L, Woodiel F N, Eifert R L, Rinehimer L A. Association of Streptococcus mutans virulence with synthesis of intracellular polysaccharide. In: Stiles H M, Loesche W J, O’Brien T L, editors. Proceedings in microbiology: aspects of dental caries. London, United Kingdom: Information Retrieval, Inc.; 1976. pp. 597–616. [Google Scholar]
- 30.Tao L, Sutcliffe I S, Russell R R B, Ferretti J J. Transport of sugars, including sucrose, by the msm system of Streptococcus mutans. J Dent Res. 1993;72:1386–1390. doi: 10.1177/00220345930720100701. [DOI] [PubMed] [Google Scholar]
- 31.van de Rijn I, Kessler R E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Jones J M, Senghas E, Gawron-Burke C, Clewell D B. Generation of Tn5 insertions in streptococcal conjugative transposon Tn916. Appl Environ Microbiol. 1987;53:1069–1072. doi: 10.1128/aem.53.5.1069-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanisch-Perron C, Vieira C J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]