Abstract
Purpose
To investigate the potential genetic cause in a primary infertility patient with multiple morphological abnormalities of sperm flagella (MMAF).
Methods
The patient’s sperm was observed by light and electron microscopy. Whole-exome sequencing (WES) was carried out to identify candidate genes. Then, the mutation found by WES was verified by Sanger sequencing. The proteins interacting with ARMC2 were revealed by co-immunoprecipitation (co-IP) and mass spectrometry. Intracytoplasmic sperm injection (ICSI) was carried out to achieve successful pregnancy.
Results
Typical MMAF phenotype (absent, short, coiled, bent irregular flagella) was shown in the patient’s sperm. A novel homozygous mutation in ARMC2 (c.1264C > T) was identified. The proteins interacting with ARMC2 we found were CEP78, PGAM5, RHOA, FXR1, and SKIV2L2. The ICSI therapy was successful, and boy-girl twins were given birth.
Conclusion
We found a novel mutation in ARMC2 which led to MMAF and male infertility. This is the first report of ICSI outcome of patient harboring ARMC2 mutation. The interacting proteins indicated that ARMC2 might be involved in multiple processes of spermatogenesis.
Keywords: Infertility, ARMC2, Teratozoospermia, Intracytoplasmic sperm injection
Introduction
Male infertility is a worldwide problem which cannot be ignored. Among the complicated problems, asthenoteratozoospermia is a clinically common condition whose causes vary. The flagella ultrastructure defects are responsible for many asthenoteratozoospermia cases. Multiple morphological abnormalities of sperm flagella (MMAF) is a type of uncommon but severe asthenoteratozoospermia which is characterized by the multiple flagellar malformations: short, absent, coiled, angulation, irregular caliber [1].
MMAF is thought to be caused by genetic defects. So far, lots of genes were reported to be related with MMAF, such as DNAH1 (MIM: 603332) [2], CFAP43 (MIM: 617558), CFAP44 (MIM: 617559) [3], CFAP65 (MIM: 614270) [4], CFAP251 (MIM: 618146) [5], TTC21A (MIM: 611430) [6], and TTC29 (MIM: 618735) [7]. ARMC2 (also termed SPGF38, MIM: 618424) is a gene located on chromosome 6 and encodes an 867-amino-acid testis specifically expressing protein. It was first reported related to MMAF in a cohort of 168 infertile men by Coutton C, et al., using whole-exome sequencing [8]. Benefited from the development and wide application of whole-exome sequencing (WES), novel mutations in ARMC2 have been identified in a series of MMAF cases. However, only about 60% of patients suffering from MMAF are caused by previously identified mutations [9]; the reveal of new mutations is still necessary.
Here, we used WES and identified a novel nonsense mutation (c.1264C > T) in a patient with MMAF. The patient harboring the ARMC2 mutation presented a severe impair in sperm motility and flagella morphology. The results of co-immunoprecipitation (co-IP) showed the potential interaction between ARMC2 and various proteins. The ICSI outcome of patients with the ARMC2 mutations was reported for the first time, and it presented satisfactory. Our finding provided new experiment fundamental and insight to the diagnosis and therapy of the MMAF.
Materials and methods
Study participants
The patient with primary male infertility was recruited from the First Affiliated Hospital of Xinjiang Medical University. The proband was a 28-year-old Han Chinese male who suffered from 5 years of infertility. It is worth noting that according to the proband dictation, his paternal and maternal grandmothers are sisters. The physical examinations and hormone examinations of the patient displayed normal results. No obvious abnormalities were detected in the bilateral spermatic veins upon palpation. This study was approved by the Ethics Committees of the Affiliated Suzhou Hospital of Nanjing Medical University and the First Affiliated Hospital of Xinjiang Medical University. Signed informed consent was provided by the patient and his family.
Semen parameter and sperm morphology analysis
Semen parameter and sperm morphology analysis were carried out according to the WHO laboratory manual for the examination and processing of human semen (5th edition). The Papanicolaou stained sperm slides were photographed by a Nikon Eclipse CI microscope (Nikon, Japan) for the sperm morphological images.
Electron microscopy evaluation
The seminal plasma was removed after centrifugation for 400 g × 15 min while the sperm cells were rinsed and fixed routinely. Samples for scanning electron microscopy (SEM) were sputter coated by an ionic sprayer meter (ACE200; Leica, Germany) and analyzed by SEM (Nova NanoSEM 450, FEI, USA) with an accelerating voltage of 5 kV. For transmission electron microscopy (TEM), the specimens were embedded in Epon 812 (SPI, USA); ultrathin sections were stained with uranyl acetate and lead citrate and observed and photographed by TEM (TECNAI-10, Philips, Netherlands) with an accelerating voltage of 80 kV.
Whole-exome sequencing, Sanger sequencing validation, and data processing
Genomic DNA was extracted by QIAamp DNA Blood Mini Kit (Qiagen, Germany). A minimum of 3 μg DNA of the patient was used to create the DNA libraries enriched by xGen Exome research panel v1.0 (Integrated DNA Technologies, Coralville, IA, USA). After bioinformatic analysis, we filtrated and analyzed the data. Taking into account the phenotypes and modes of inheritance, one homozygous mutation in ARMC2 came into sight. Then, a direct Sanger sequencing was conducted to validate putative mutations. The sequence was amplified by polymerase chain reaction (PCR) with the primers of ARMC2. The sequences of the primers are listed in Table 1. PCR products were verified by agarose gel electrophoresis and subsequently sequenced by an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Table 1.
Sperm parameters of the proband with several semen analyses
| Primer | Sequence | Tm | |
| ARMC2-F | 5ʹ-CAGGATTTAGTCGTCCGTGTTG -3ʹ | 58 °C | |
| ARMC2-R | 5ʹ-GGAAAGTTGGTTGTGTCTGCTG -3ʹ | ||
| Program | Cycles | Target (°C) | Hold (hh:mm:ss) |
| Initial-denature | 1 | 95 | 3 mm |
| Denature | 35 | 95 | 15 ss |
| Annealing | 55 | 15 ss | |
| Extension | 72 | 30 ss | |
| Final extension | 1 | 72 | 5 mm |
Expression plasmid construction
ARMC2 cDNA was amplified from the plasmid expressing ARMC2 (Sino Biological, China, HG25854-UT) and inserted into pcDNA 3.1/V5-His plasmid (Thermo Fisher, K4800-01), which encodes a C-terminal V5 tag. PCR-based site-directed mutagenesis was used to make construct expressing ARMC2 mutant R422X using ClonExpress One Step Cloning kit (Vazyme, China, C112). Both plasmids were verified by DNA sequencing.
Cell culture and transfection
HEK293 cells (ATCC, STR profiling) were culture in Dulbecco’s modified Eagle’s medium (HyClone, SH30243.01) with 10% fetal bovine serum (FBS) (Gibco, 10099–141) at 37 °C with 5% CO2. Plasmids were transfected into the cells at ∼80% confluence using ExFect Transfection Reagent (Vazyme, T101) based on the manufacturer’s instructions. After 6 h of incubation, the cells were switched to fresh medium and incubated for 24 h before being used for Western blotting and proteomic analysis.
Western blotting
After 24 h of incubation, the cells were lysed in 25 mM Tris–HCl (PH 7.4), 150 mM NaCl, 1% NP-40, 5% glycerol, and 1 mM phenylmethylsulfonyl fluoride (PMSF, NCM Biotech). Cell lysates were mixed with 5 × loading buffer, and analyzed by SDS-PAGE and Western blotting using a horseradish peroxidase (HRP)–conjugated anti-V5 antibody (1:5000, Invitrogen, R96125). After incubation with a solution with an enhanced chemiluminescent substrate (ECL) (Vazyme, E411-04), Western blots were exposed to a chemiluminescent imager (Tannon).
co-IP and mass spectrometry
The extracted proteins from WT and mutant ARMC2 plasmids were incubated with 3 μg of target antibodies at 4 °C overnight, followed by adding 50 μL of protein A/G magnetic beads (LSKMAGAG10, Millipore) to each sample and incubating for 1 h at room temperature. The beads were washed with PBS for three times. Then, the mass spectrometry (MS) analyses were carried out. The peptides were resuspended in 0.1%formic acid and analyzed using a LTQ Orbitrap Velos mass spectrometer (Thermo Finnigan) as described before [10]. The minimum peptide length required was six. The mass tolerance for MS/MS fragment ions was set to 0.5 Da. Statistical significance was investigated by the unpaired two-tailed Student t test. Proteins with a P value less than 0.05 and a fold change greater than 1.5 were considered as differentially expressed between each group.
ICSI procedure
The patient’s wife underwent a long protocol to induce ovulation. The total Gn dose was 3000 IU. A total of 0.2 mg of triptorelin acetate (Ferring Pharmaceuticals) was injected while the diameter of dominant follicle reached 18 mm, and 35 h later, the oocytes were retrieved. The obtained cumulus oocyte complex was washed and placed in a protein-containing fertilization fluid (Vitrolife, Sweden). After 5 h, the patient’s sperms were injected into the prepared oocytes.
Results
The patient’s sperm showed a typical MMAF phenotype
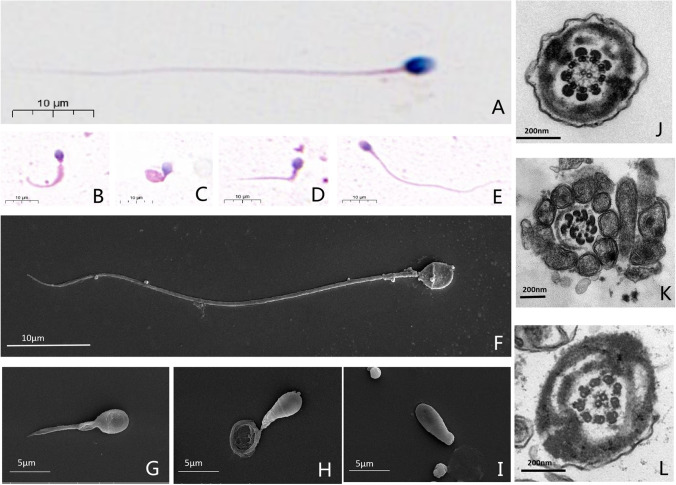
In the 4 times of routine clinical semen tests, the patient’s sperms all showed low concentration with severe low motility, while the semen volumes were normal (Table 2). Under light microscopy, by contrast to the normal control, the sperms of the patient showed a typical MMAF phenotype. The sperm flagella demonstrated a variety of morphological abnormalities (short, coiled, bent, etc.), but it was worth noting that there were still few sperms with normal flagella in the man harboring ARMC2 mutation. The results of SEM were consistent with the light microscopy. The sperm head, compared with flagella, showed much more normal. TEM was used to investigate the ultrastructure of sperm flagella and the central microtubule pairs were found absent in the sperm of the patient, and corresponding to the previous results, normal ultrastructure flagella also existed in the patient’s sperms (Fig. 1).
Table 2.
Sperm parameters of the proband with several semen analyses
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Sperm volume (mL) | 3.5 | 4.0 | 4.0 | 4.8 |
| Concentration (106/mL) | 1.4 | 0.9 | 1.2 | 1.4 |
| Total motility (%) | 4.2 | 0 | 0* | 0 |
| Progressive motility (%) | 3.2 | 0 | 0 | 0 |
| Normal sperm morphology (%) | 0 | 0 | 0 | 0 |
*Only one non-forward motile sperm was observed
Fig. 1.
Sperm morphology analyses for men with ARMC2 mutation. Normal morphology spermatozoa from a healthy man were revealed by light microscopy (A) and SEM (F). While in sperm from the man harboring homozygous ARMC2 mutation, multiple malformations can be observed, including short and irregular caliber (B, G), coiled (C, H), and absent (I) and bent (D) flagella. In addition, sperm with normal length can also be found in the ARMC2-deficient patient’s sperm (E). Under TEM, the cross-sections of the sperm from normal control show a typical “9 + 2” structure of microtubules and the regularly arranged outer dense fibrous sheath (J). Most of the sperm from the man with ARMC2 mutations show a severe disorder in the arrangement of the flagellar ultrastructure, especially the loss of the central pair of microtubules (K), and few are normal (L)
Novel mutations in ARMC2
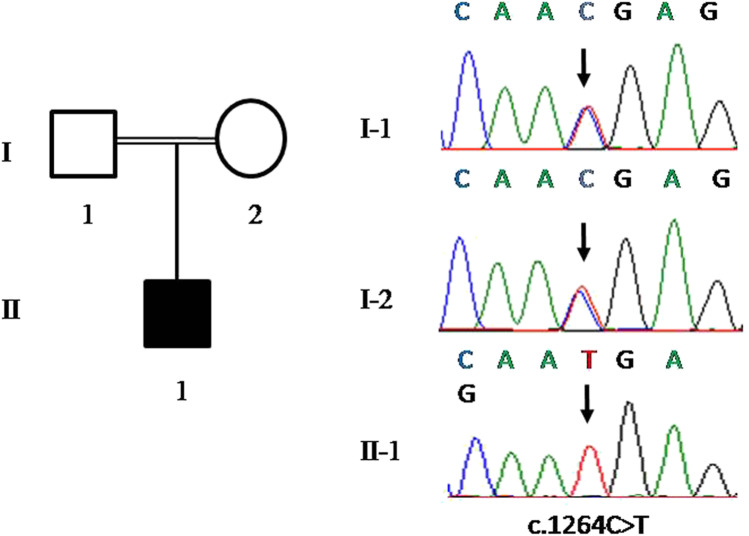
The mutation identified in the patient was a nonsense mutation c.1264C > T (p.R422X) located in exon 13. Meanwhile, we examined other MMAF-related genes (DNAH1, CFAP43, CFAP44, CFAP69, FSIP2, TTC21A, TTC29, and WDR66) and other loci in the ARMC2 gene in the raw data, but there were no positive findings. Sanger sequencing revealed that the patient’s parents both carried a heterozygous mutation in the same site (Fig. 2). The c.1264C > T mutation was classified as pathogenic according to the ACMG mutation classification guideline [11] with 1 very strong (PVS1) and 2 supporting (PM2 and PM3_Supporting) evidences. The c.1264C > T mutation was not recorded in the dbSNP (http://www.ncbi.nlm.nih.gov/SNP/). The allele frequency of this mutation shown in Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/) was 0.00003229, and in Genome Aggregation Database (gnomAD) (http://gnomad.broadinstitute.org/) was 0.0000279.
Fig. 2.
Identification of novel mutations of ARMC2 in a man with MMAF. The pedigree of the family showing the father (I-1) and mother (I-2) with their offspring numbered II-1; black-filled squares indicate the male individuals with MMAF. Sanger sequencing results are shown below and the mutation positions are indicated by black arrows
The ARMC2 protein interacted with multiple proteins
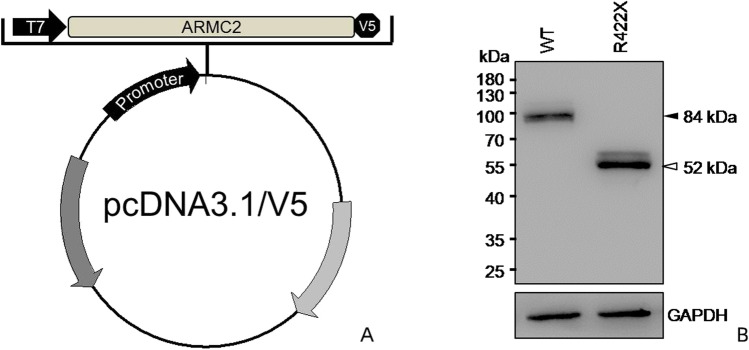
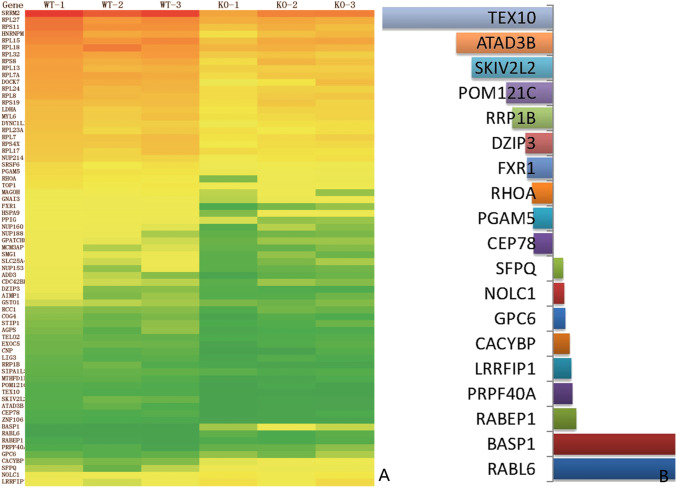
The construction scheme and effect verification of the plasmid are shown in Fig. 3. After co-IP and mass spectrometry, 1912 differential proteins of WT and ARMC2 mutant plasmid groups were detected in the precipitated products, and finally 69 of them were found to be different between the WT and ARMC2 mutant groups. As shown in Fig. 4, the most downregulated protein in mutant groups was TEX10. Among the downregulated proteins, 5 proteins might be involved in the sperm function, namely CEP78, PGAM5, RHOA, FXR1, and SKIV2L2, which was indicating the ARMC2 might participate in multiple processes of spermatogenesis.
Fig. 3.
Construction and expression detection of ARMC2 mutant plasmid. A WT ARMC2 cDNA and ARMC2 mutant R422X were inserted into pcDNA 3.1/V5-His plasmid, which encodes a C-terminal V5 tag. B The result of WB shows the expression product of mutant plasmid was shorter than WT
Fig. 4.
ARMC2 interacts with multiple proteins. A Heat map from three independent proteomic analyses of proteins extracted from wild-type and ARMC2-mutant HEK293 cells. Red: proteins upregulated; green: proteins downregulated. B The top up (left) and down (right) regulated proteins
Successful ICSI outcome achieved
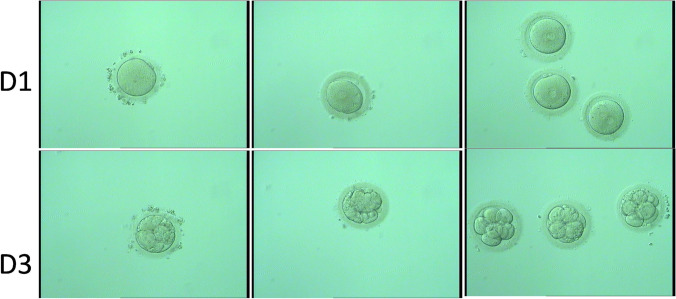
Eight oocytes were obtained, and 5 were used for ICSI. On day 1, three 2PN, one 1PN, and one 0PN embryos were observed. On day 3, three level 2 8-cell embryos were obtained and the other two also divided (Fig. 5). Two level 2 8-cell embryos were transplanted on day 3, and the remaining embryo was frozen. Ultrasound at 28 days showed clinical pregnancy. Finally, a successful birth was given to boy and girl twins in February 2021. The detailed data of ICSI treatment outcome is shown in Table 3.
Fig. 5.
Images of the embryos of the ICSI cycles. Line 1 shows that the 3 embryos (right) carried 2 PN; the other 2 were 0PN (left) and 1PN (middle). Line 2 shows the 3-day embryos; 3 embryos were qualified as good quality (right) and other 2 were also divided
Table 3.
Clinical outcomes of ICSI cycles using spermatozoa from men with homozygous ARMC2 mutation
| Subject | 1 |
| Male age (year) | 28 |
| Female age (year) | 28 |
| Number of ICSI cycles | 1 |
| Number of oocytes obtained | 8 |
| Number of oocytes injected | 5 |
| Number (and rate) of fertilized oocytes | 3 (60%) |
| Number (and rate) of cleavage embryos | 3 (100%) |
| Number (and rate) of high-quality embryos | 2 (66.67%) |
| Number of transfer cycles | 1 |
| Number of embryos transferred per cycle | 2 |
| Implantation rate | 100% |
| Clinical pregnancy rate | 100% |
| The live birth rate | 100% |
| The gender and weight of the newborns (kg) | ♂3.5/♀3.0 |
Discussion
MMAF, termed as dysplasia of fibrous sheath (DFS) before [12], is a set of flagellogenesis dysfunction caused by several genes (e.g., CFAP43, CFAP44) [3]. Several ARMC2 mutations (c.421 C > T, c.1023 + 1G > A, c.1284_1288delAA, c.2279 T > A, c.2353_2354delTT, c.182C > G) had been reported with MMAF in previous studies [8, 13], but no ICSI outcomes of these cases were revealed. Here, we identified a novel homozygous nonsense mutation in ARMC2 from a Chinese patient with a typical MMAF phenotype. Notably, although the flagella of the patient harboring ARMC2 mutation showed multiple abnormalities, there were still a few sperms with normal morphology, which could explain why motile sperm could still be found.
ARMC2, also termed as SPGF38, is a testis specifically expressing gene [14], which encodes a protein containing several armadillo (ARM) repeats [15]. ARM repeat was first characterized in the Drosophila segment polarity protein [16]. It was involved in lots of biological processes, including intracellular signaling, cytoskeletal regulation, and protein degradation or folding [17]. Among all the ARM repeat proteins, there were a subset especially associated with the flagella structure, such as SPGF6, which regulated the motility of flagella by stabilizing the central pair of microtubules in the classic “9 + 2” arrangement [18]. Mutations in these ARM repeat proteins, such as SPAG6 and ARMC3, had been reported related to the male infertility caused by sperm flagella malformations [19, 20]. The localization of ARMC2 was presumed to be on the axonemal central pair complex (CPC), because in sperm from an individual harboring ARMC2 mutations, staining of SPAG6, a CPC protein, was totally absent from the flagellum, while staining for AKAP4, DNALI1, DNAH5, RSPH1, and GAS8 showed no difference from normal control, which suggested other flagellar structures like fibrous sheath, dynein arms, or radial spokes were affected by mutations in ARMC2 [8]. In our observation of the sperm in the patient harboring ARMC2 mutation, the most common flagellar ultrastructure defect was the absence of central pair microtubules, which was consistent with previous conjectures on the location of ARMC2.
The co-IP and mass spectrum analysis revealed the interaction between ARMC2 and multiple proteins, such as CEP78, PGAM5, RHOA, FXR1, and SKIV2L2. CEP78 is a centrosomal protein implicated in ciliogenesis and ciliary length control, which is also important for sperm flagella. The mutations in the CEP78 would result in retinal cone-rod dystrophy associated with hearing loss [21], and sometimes male infertility [22]. But in the study of Giulia Ascar et al. [22], no obvious ultrastructural abnormalities of cilia were observed in sperm of patients harboring CEP78 mutations. PGAM5 is a mitochondrial protein which expresses in the testis and plays an important role in the function of mitochondria [23]. It was reported the cigarette smoking would significantly increase the DNA methylation level in more than one CpG in PGAM5, which could be potentially related to the impacts of smoking on the spermatogenesis [24]. RHOA was identified interacting with proteins involved in sperm capacitation and acrosome reaction [25]. FXR1 is widely distributed in human and mouse tissues and localized to mature spermatocytes. It was found to be associated with microtubules assembly [26], which might interact with ARMC2 in sperm flagella. SKIV2L2 had both the RNA-binding and ATPase activities and was mainly localized in the nuclei of round spermatids. Its coding gene SKIV2L2 was shown to be highly expressed in the spermatocytes at stages I to VI [27]. Nevertheless, we had not continued to explore the interaction mechanism of these proteins and their potential impact on spermatogenesis. Further experiments are still needed to observe the exact axonemal localization and explore the specific role of ARMC2 in spermatogenesis.
Nowadays, ICSI has become the most efficient, even the only approach to achieve a successful pregnancy for MMAF patients. The previous studies showed the MMAF patients harboring mutations in other genes, such as DNAH1 and CFAP251, had a good prognosis after ICSI [28, 29]. However, there were still several failure ICSI cases that were reported, for example, of patients with CEP135 [30] or CFAP65 [31]. The impacts of the affected genes on ICSI outcome are supposed depended on the gene’s function on fertilization and embryonic development. CEP135 is a centrosomal protein taking part in centriole biogenesis. The injection of sperms from patients harboring CEP135 mutations might lead to irregular cleavage during embryo development or chromosomal aberrations, causing delays or stagnation of embryo development. Meanwhile, CFAP65 was recently found related with the sperm head shaping and acrosome, which made its defect severely harmful to the fertility of the sperm [32]. The main function of ARMC2 was supposed to be one component of CPC, and the deficiency in ARMC2 seemed not harmful to the integrity of the sperm nuclear. So the ICSI outcomes of the patients harboring ARMC2 mutations were predicted to be ideal, which was proved by our clinical observation. Here, the first successful ICSI outcome of a patient with the ARMC2 mutation was reported, which indicated that ICSI was still the most effective approach to help MMAF patients, especially the patients harboring ARMC2 mutations, to achieve successful fertilization.
In summary, we identified a novel bi-allelic mutation of ARMC2 which resulted in male infertility via abnormal flagella formation. Our finding will provide a theoretical basis for genetic counseling and clinical treatment of MMAF caused by ARMC2 mutations.
Acknowledgements
We would like to thank Li Wang and Dandan Song in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for their technical support. This work was supported by the General Project of Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01C297), Suzhou Health Talent Cultivation Project (GSWS2019053), Suzhou Science and Technology Development Plan Project (SYSD2020129), Innovative and Entrepreneurial Doctor grant from Jiangsu Province (JSSCBS20211586).
Author contribution
J.W. conceived and designed the experiments and wrote the manuscript. X.L. and J.Z. performed genetic analysis. C.Z. and W.W. performed bioinformatic analyses. Y.X., H.L., and S.Y. contributed to the discussion of the data. All authors have read and agreed to the published version of the manuscript.
Declarations
Ethics approval
This study was approved by the Affiliated Suzhou Hospital of Nanjing Medical University and the First Affiliated Hospital of Xinjiang Medical University.
Consent to participate
Written informed consent was obtained from all of the subjects and their family members participating in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxiong Wang, Xiaoran Liu and Ce Zhang contributed equally to this work.
Contributor Information
Shenmin Yang, Email: drim2004@126.com.
Jing Zhao, Email: littlemili@126.com.
References
- 1.Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–485. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 2.Ben Khelifa M, Coutton C, Zouari R, Karaouzène T, Rendu J, Bidart M, Yassine S, Pierre V, Delaroche J, Hennebicq S, Grunwald D, Escalier D, Pernet-Gallay K, Jouk PS, Thierry-Mieg N, Touré A, Arnoult C, Ray PF. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang S, Wang X, Li W, Yang X, Li Z, Liu W, Li C, Zhu Z, Wang L, Wang J, Zhang L, Sun X, Zhi E, Wang H, Li H, Jin L, Luo Y, Wang J, Yang S, Zhang F. Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2017;100:854–864. doi: 10.1016/j.ajhg.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Wu H, Li F, Tian S, Kherraf ZE, Zhang J, Ni X, Lv M, Liu C, Tan Q, Shen Y, Amiri-Yekta A, Cazin C, Zhang J, Liu W, Zheng Y, Cheng H, Wu Y, Wang J, Gao Y, Chen Y, Zha X, Jin L, Liu M, He X, Ray PF, Cao Y, Zhang F. Biallelic mutations in CFAP65 cause male infertility with multiple morphological abnormalities of the sperm flagella in humans and mice. J Med Genet. 2020;57:89–95. doi: 10.1136/jmedgenet-2019-106344. [DOI] [PubMed] [Google Scholar]
- 5.Li W, He X, Yang S, Liu C, Wu H, Liu W, Lv M, Tang D, Tan J, Tang S, Chen Y, Wang J, Zhang Z, Wang H, Jin L, Zhang F, Cao Y. Biallelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J Hum Genet. 2019;64:49–54. doi: 10.1038/s10038-018-0520-1. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, He X, Yang S, Zouari R, Wang J, Wu H, Kherraf ZE, Liu C, Coutton C, Zhao R, Tang D, Tang S, Lv M, Fang Y, Li W, Li H, Zhao J, Wang X, Zhao S, Zhang J, Arnoult C, Jin L, Zhang Z, Ray PF, Cao Y, Zhang F. Bi-allelic mutations in TTC21A induce asthenoteratospermia in humans and mice. Am J Hum Genet. 2019;104:738–748. doi: 10.1016/j.ajhg.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, He X, Liu W, Yang S, Wang L, Li W, Wu H, Tang S, Ni X, Wang J, Gao Y, Tian S, Zhang L, Cong J, Zhang Z, Tan Q, Zhang J, Li H, Zhong Y, Lv M, Li J, Jin L, Cao Y, Zhang F. Bi-allelic mutations in TTC29 cause male subfertility with asthenoteratospermia in humans and mice. Am J Hum Genet. 2019;105:1168–1181. doi: 10.1016/j.ajhg.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutton C, Martinez G, Kherraf ZE, Amiri-Yekta A, Boguenet M, Saut A, He X, Zhang F, Cristou-Kent M, Escoffier J, Bidart M, Satre V, Conne B, Fourati Ben Mustapha S, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Pernet-Gallay K, Bonhivers M, Hennebicq S, Rives N, Dulioust E, Touré A, Gourabi H, Cao Y, Zouari R, Hosseini SH, Nef S, Thierry-Mieg N, Arnoult C, Ray PF. Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet. 2019;104:331–40. doi: 10.1016/j.ajhg.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu S, Gu Y, Wu Y, Yang S, Li C, Meng L, Yuan W, Jiang T, Zhang X, Li Y, Wang C, Liu M, Ye L, Guo X, Shen H, Yang X, Tan Y, Hu Z. Bi-allelic variants in human WDR63 cause male infertility via abnormal inner dynein arms assembly. Cell Discov. 2021;7:110. doi: 10.1038/s41421-021-00327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y, Zhou T, Guo X, Cui Y, Shen B, Wang Z, Hu Z, Zhou Z, Sha J, Prunskaite-Hyyrylainen R, Yu Z, Ramirez-Solis R, Ikawa M, Matzuk MM, Liu M. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci U S A. 2017;114:E5370–5370E5378. doi: 10.1073/pnas.1621279114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–669. doi: 10.1016/S0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 13.Khan I, Dil S, Zhang H, Zhang B, Khan T, Zeb A, Zhou J, Nawaz S, Zubair M, Khan K, Ma H, Shi Q. A novel stop-gain mutation in ARMC2 is associated with multiple morphological abnormalities of the sperm flagella. Reprod Biomed Online. 2021;43:913–919. doi: 10.1016/j.rbmo.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Darde TA, Lecluze E, Lardenois A, Stévant I, Alary N, Tüttelmann F, Collin O, Nef S, Jégou B, Rolland AD, Chalmel F. The ReproGenomics Viewer: a multi-omics and cross-species resource compatible with single-cell studies for the reproductive science community. Bioinformatics. 2019;35:3133–3139. doi: 10.1093/bioinformatics/btz047. [DOI] [PubMed] [Google Scholar]
- 15.Teves ME, Nagarkatti-Gude DR, Zhang Z, Strauss JF., 3rd Mammalian axoneme central pair complex proteins: broader roles revealed by gene knockout phenotypes. Cytoskeleton (Hoboken) 2016;73:3–22. doi: 10.1002/cm.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 17.Tewari R, Bailes E, Bunting KA, Coates JC. Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 2010;20:470–481. doi: 10.1016/j.tcb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zhang L, Li W, Huang Q, Yuan S, Li Y, Liu J, Zhang S, Pin G, Song S, Ray PF, Arnoult C, Cho C, Garcia-Reyes B, Knippschild U, Strauss JF, Zhang Z. The sperm-associated antigen 6 interactome and its role in spermatogenesis. Reproduction. 2019;158:181–197. doi: 10.1530/REP-18-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Wang J, Cheng H, Gao Y, Liu W, Zhang Z, Jiang H, Li W, Zhu F, Lv M, Liu C, Tan Q, Zhang X, Wang C, Ni X, Chen Y, Song B, Zhou P, Wei Z, Zhang F, He X, Cao Y. Patients with severe asthenoteratospermia carrying SPAG6 or RSPH3 mutations have a positive pregnancy outcome following intracytoplasmic sperm injection. J Assist Reprod Genet. 2020;37:829–840. doi: 10.1007/s10815-020-01721-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pausch H, Venhoranta H, Wurmser C, Hakala K, Iso-Touru T, Sironen A, Vingborg RK, Lohi H, Söderquist L, Fries R, Andersson M. A frameshift mutation in ARMC3 is associated with a tail stump sperm defect in Swedish Red (Bos taurus) cattle. BMC Genet. 2016;17:49. doi: 10.1186/s12863-016-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonçalves AB, Hasselbalch SK, Joensen BB, Patzke S, Martens P, Ohlsen SK, Quinodoz M, Nikopoulos K, Suleiman R, Damsø Jeppesen MP, Weiss C, Christensen ST, Rivolta C, Andersen JS, Farinelli P, Pedersen LB. CEP78 functions downstream of CEP350 to control biogenesis of primary cilia by negatively regulating CP110 levels. Elife. 2021;10:e63731. doi: 10.7554/eLife.63731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascari G, Peelman F, Farinelli P, Rosseel T, Lambrechts N, Wunderlich KA, Wagner M, Nikopoulos K, Martens P, Balikova I, Derycke L, Holtappels G, Krysko O, Van Laethem T, De Jaegere S, Guillemyn B, De Rycke R, De Bleecker J, Creytens D, Van Dorpe J, Gerris J, Bachert C, Neuhofer C, Walraedt S, Bischoff A, Pedersen LB, Klopstock T, Rivolta C, Leroy BP, De Baere E, Coppieters F. Functional characterization of the first missense variant in CEP78, a founder allele associated with cone-rod dystrophy, hearing loss, and reduced male fertility. Hum Mutat. 2020;41:998–1011. doi: 10.1002/humu.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, Duan L, Wang X, Liu L, Liu X, Shen Y, Zhu Y, Chen Q. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Alkhaled Y, Laqqan M, Tierling S, Lo Porto C, Amor H, Hammadeh ME. Impact of cigarette-smoking on sperm DNA methylation and its effect on sperm parameters. Andrologia. 2018. 10.1111/and.12950. [DOI] [PubMed]
- 25.Fiedler SE, Bajpai M, Carr DW. Identification and characterization of RHOA-interacting proteins in bovine spermatozoa. Biol Reprod. 2008;78:184–192. doi: 10.1095/biolreprod.107.062943. [DOI] [PubMed] [Google Scholar]
- 26.Huot ME, Mazroui R, Leclerc P, Khandjian EW. Developmental expression of the fragile X-related 1 proteins in mouse testis: association with microtubule elements. Hum Mol Genet. 2001;10:2803–2811. doi: 10.1093/hmg/10.24.2803. [DOI] [PubMed] [Google Scholar]
- 27.Osman BA, Kawashima A, Tamba M, Satoh E, Kato Y, Iki A, Konishi K, Matsuda M, Okamura N. Localization of a novel RNA-binding protein, SKIV2L2, to the nucleus in the round spermatids of mice. J Reprod Dev. 2011;57:457–467. doi: 10.1262/jrd.10-179N. [DOI] [PubMed] [Google Scholar]
- 28.Wambergue C, Zouari R, Fourati Ben Mustapha S, Martinez G, Devillard F, Hennebicq S, Satre V, Brouillet S, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Amblard F, Arnoult C, Ray PF, Coutton C. Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection. Hum Reprod. 2016;31:1164–72. doi: 10.1093/humrep/dew083. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Zhang C, Tang H, Zheng A, Li H, Yang S, Xiang J. Successful results of intracytoplasmic sperm injection of a Chinese patient with multiple morphological abnormalities of sperm flagella caused by a novel splicing mutation in CFAP251. Front Genet. 2022;12:783790. doi: 10.3389/fgene.2021.783790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sha YW, Xu X, Mei LB, Li P, Su ZY, He XQ, Li L. A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF) Gene. 2017;633:48–53. doi: 10.1016/j.gene.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Tu C, Nie H, Meng L, Li Y, Yuan S, Zhang Q, Du J, Wang J, Gong F, Fan L, Lu GX, Lin G, Tan YQ. Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J Med Genet. 2019;56:750–757. doi: 10.1136/jmedgenet-2019-106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Tian S, Nie H, Tu C, Liu C, Li Y, Li D, Yang X, Meng L, Hu T, Zhang Q, Du J, Fan L, Lu G, Lin G, Zhang F, Tan YQ. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum Mol Genet. 2021;30:2240–2254. doi: 10.1093/hmg/ddab185. [DOI] [PubMed] [Google Scholar]