Abstract
The pursuit to improve the TB control program comprising one approved vaccine, M. bovis Bacille Calmette-Guerin (BCG) has directed researchers to explore progressive approaches to halt the eternal TB pandemic. Mycobacterium tuberculosis (M.tb) was first identified as the causative agent of TB in 1882 by Dr. Robert Koch. However, TB has plagued living beings since ancient times and continues to endure as an eternal scourge ravaging even with existing chemoprophylaxis and preventive therapy. We have scientifically come a long way since then, but despite accessibility to the standard antimycobacterial antibiotics and prophylactic vaccine, almost one-fourth of humankind is infected latently with M.tb. Existing therapeutics fail to control TB, due to the upsurge of drug-resistant strains and increasing incidents of co-infections in immune-compromised individuals. Unresponsiveness to established antibiotics leaves patients with no therapeutic possibilities. Hence the search for an efficacious TB immunization strategy is a global health priority. Researchers are paving the course for efficient vaccination strategies with the radically advanced operation of core principles of protective immune responses against M.tb. In this review; we have reassessed the progression of the TB vaccination program comprising BCG immunization in children and potential stratagems to reinforce BCG-induced protection in adults.
Keywords: adjunct vaccination strategies against tuberculosis, vaccine, BCG, host directed therapy, immunotherapy, memory T cells
Introduction
Tuberculosis (TB) is caused by the facultative intracellular pathogen Mycobacterium tuberculosis (M.tb). Since the 1800s, TB was the leading cause of health menace worldwide. Despite being declared a global health emergency in 1993 by World Health Organization (WHO), TB continues to be the leading cause of morbidity and mortality amongst bacterial infections (1). The majority of individuals remain asymptomatically and latently infected with M.tb owing to confiscation of the pathogen by immune cell populations and this does not lead to disease. Upon serious immunosuppression, around 10% of latently infected individuals develop active TB. Owing to the indefinability of the disease, explosive TB epidemics are hardly encountered which results in underestimated harm caused by M.tb worldwide (2). The host immune responses can restrict the pathogen but fail to accomplish complete bacterial sterility. The overwhelming progression of the development of new therapeutics and the emergence of resistant pathogenic strains can be prevented by the enhancement of population-wide immunity against M.tb (3).
M.tb was originally identified in 1882 by Dr. Robert Koch as the causative agent of TB however, it has lurked among living beings since ancient times. 139 years post-discovery of this pathogen, it still endures as an eternal scourge ravaging globally. Current strategies fail to control TB, due to the upsurge of multidrug-resistant strains, increasing incidents of co-infections in immune-compromised individuals, and the emergence of TB-IRIS (Immune Responsive Inflammatory Syndrome). Globally in 2020, approximately 10 million people were disease-ridden with TB and an aggregate of 1.3 million lives were lost (together with 208000 people with HIV). Furthermore, almost one-fourth of humankind is infected asymptomatically (latently) with M.tb, with a 5-15% risk of progressing into clinical manifestations (1). An effective vaccine is indispensable to enhance population-wide immune protection and reduce disease burden. Vaccines operate by stimulating a cascade of immunological responses and ensuing the institution of immune memory against subsequent infections (4). Immune memory was originally described by the Greek historian Thucydides while observing survivors of the plague of Athens and comprehended that survivors have conferred life-long resistance to disease. Hence, he stated that “this disease never took any man the second time” (5). This feature of the immune system is a requisite evolutionary trait. Since historic eras for infectious diseases like smallpox, it is distinctly demonstrated that while initial infections had a fatality rate of 20% to 60% subsequently affected individuals were eternally immune to infection. Edward Jenner was the first to employ this hallmark feature of immunity to treat smallpox and provided a foundation for the development of vaccines (6). Secondary immune responses are refined protective responses mounted by sub-populations of memory cells on subsequent encounters which impart endurance to combat recurrent infections caused by pathogens in the environment. A series of events following primary exposure establishes a pool of long-lasting antigen-specific immune cells that mount quantitatively and/or qualitatively improved immune response upon reinfection. Documented from the times of ancient Greeks but still, many components of immune memory are still debatable (5). Immune memory is the cardinal property of the adaptive immune system and exclusively lymphocytes were known to mediate these responses. However, organisms that lack T and B lymphocytes similarly possess heightened proficiency to combat recurring infections caused by the same pathogen, demonstrating the existence of innate immune memory (7). Numerous studies in simpler living beings have reported that cells of the innate immune system can mount heightened secondary responses upon reinfection. Thus, the conventional characterization of immunological memory is continuously advancing (8). Better insights into the generation of immunological memory are fundamental to foster progressive vaccination strategies.
Despite pre-exposure vaccination with BCG, a large extent of latent TB infections urges the need for an efficacious complementary TB control strategy. It is evident that the most extensively used vaccine, Bacille Calmette-Guerin (BCG) which has existed for 100 years fails to impart long-term immunity and has limited efficacy against adult pulmonary TB (9). BCG immunization has a limited impact on M.tb transmission since it cannot inhibit primary infection or recrudescence of latent TB (10). Failure to develop a significantly effective vaccine has constrained the phasedown of the global TB burden (9). Furthermore, safety concerns regarding BCG immunization in immune-compromised individuals including HIV-TB coinfected individuals necessitate a vaccine that is safer and more efficient than BCG and can ameliorate infections (11). The efficacy of BCG against pulmonary TB is even more disappointing in tropical regions with a high TB burden (12). Escalating TB cases worldwide despite BCG administration further demands the advancement of the existing vaccination strategy. In the past decade, various research groups have utilized pioneering technologies to improve the current scenario. Progressive vaccine design strategies have been implemented and vaccine candidates have been evaluated in different clinical trials (13). It is challenging to discover more efficacious vaccine candidates for TB that can substitute BCG. It is highly critical to comprehend the shortcomings and prospects of novel vaccination strategies for better implementation and amendment of TB control measures. Table 1 summarizes key desirable characteristics of improved vaccination strategy. In the current scenario, COVID-19 and TB are the top two causes of death from contagious diseases (1). The similarity in symptoms, risk factors, and primary organ affected further exacerbates the circumstances. TB-COVID-19 co-infection certainly intensifies the severity and threat of death. Even though both diseases primarily affect the lungs, due to disparities in modes of transmission and pathogenesis, distinct therapeutic measures are required (14). Nevertheless, with extraordinary scientific endeavour, information regarding the pathogenesis of SARS-CoV-2 was channelled to develop diagnostics, therapeutics, and vaccines for COVID-19. Further, years of implementation of TB control programs were utilized to implement control strategies to constrain the pandemic (15). This prompts the necessity to retrospect and harnesses the paramount knowledge for progressive solutions to counteract the syndemic of COVID-19 and TB. Lessons learned from BCG vaccination for TB have been operative to control the COVID-19 pandemic owing to the broad-spectrum immunomodulatory potential of BCG (16). The emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the consequent COVID-19 pandemic has negatively influenced the years of progress in tuberculosis (TB) control (17). Advancement in the direction to put an end to TB was hit hard by the ongoing COVID-19 pandemic. Reports of the World Health Organization (WHO) corroborate that advent of COVID-19 has caused a diminution in TB diagnosis and escan alation in mortality. To augment protective immune responses against TB in adults, massive scientific and economic attempts have been made globally. Aspiration to attain complete bacterial sterility to counteract active TB and restrict the transmission with next-generation vaccines has been actively trailed. Even with several vaccine candidates in different phases of clinical trials, we are yet to uncover vaccination strategies that can efficaciously restrict escalating TB burden worldwide (17). Hence, in this review, we have discussed strategies to augment the existing vaccination approach. Immune responses induced by BCG vaccination have been studied comprehensively in past and we are still uncovering new information regarding host responses stimulated by BCG that impede the establishment of effective memory responses (18). To improve the immunotherapeutic efficacy of BCG it is vital to completely understand the mechanism of BCG-induced immune responses. Modulation of host immunity via immunomodulators along with vaccination can be employed as a stratagem to incline immune responses to attain ever-lasting immunity against M.tb infections. We will reassess the radical approaches utilized by the researchers to limit the prevailing TB cases and how a better understanding of BCG is prime for progress in the TB vaccination program.
Table 1.
Desired characteristics for novel Tuberculosis immunization approaches.
| S. No | Desired characteristics |
|---|---|
| 1. | Safe to be administered in immunocompromised individuals at risk of developing active TB |
| 2. | Expenses associated with regimen and dosage should be reasonable for high burdened developing countries. |
| 3. | Immunization strategy must lower the risk of developing active pulmonary TB in adults previously vaccinated with BCG |
| 4. | Must protect against M.tb infections for more than 10 years subsequent to immunization |
| 5. | Minimum administrations requisite to elicit host protective responses |
| 6. | Evaluation of protective immune correlates by employing established assays |
| 7. | Must offer greater than 50% protective efficacy against established pulmonary TB |
Expedition From Virulent M. bovis to the BCG Vaccine:
Albert Calmette and Camille Guérin commenced the pursuit to develop a vaccine against TB in 1900 at the Pasteur Institute (19). They began by cultivating a virulent bovine strain of bacillus which was isolated from a tuberculous cow by Nocard. Initially, the bacilli were grown on glycerine, potato medium supplemented with ox bile to limit the clumping and attain homogenous bacterial suspension. This effort to minimize bacterial clumping additionally lowered the virulence of pathogen upon sub-culturing. This scientific observation provoked the scientists further to focus on using the attenuated strain of bacilli for the generation of TB vaccine (20). Till 1919, they successfully sub-cultured the bacilli more than 230 times. This strain failed to infect and cause TB in animals such as guinea pigs, cattle, and rabbits. Firstly named “Bacille Bilie Calmette-Guerin” this is now the most widely administered vaccine worldwide Bacille Calmette-Guerin (BCG) (20). BCG was first utilized in 1921 to immunize a new-born via oral route after which it was mass vaccinated to protect infants from disseminated forms of TB. To justify the escalating demand for vaccine strain worldwide, several laboratories around the world began sub-culturing BCG owing to which individuals around the world are vaccinated with characteristically distinct BCG strains (21). Based on existing knowledge, it is evident that diverse BCG strains have variable efficaciousness (22) and immunogenicity (23) but the most efficient BCG strain is yet to be established (23). As little as 1% augmented efficaciousness can rescue around 18,000 individuals and limit 83,000 TB cases in a year (24). Hence, to better understand the protective co-relates of BCG vaccination should be the top priority to upgrade the vaccination strategy.
BCG Induced Protection Against TB
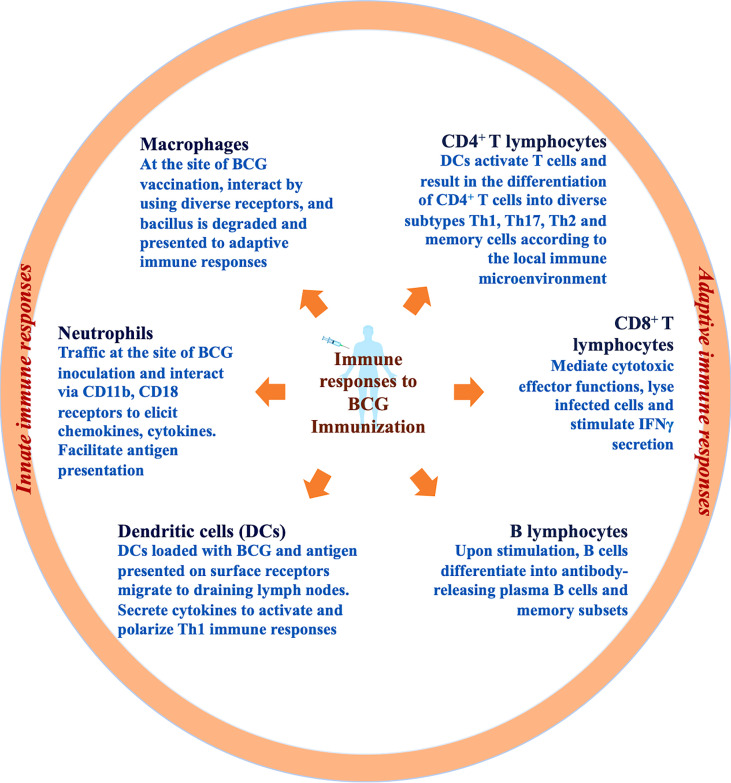
Since the launch of BCG in TB immunization programs, numerous lives have been saved owing to the only existent TB vaccine (19). The percentage decline in disease that can be attributed to vaccination outlines the clinical effectiveness of a vaccine. Mainly, the BCG vaccine is administered to protect against TB. However, the protective efficacy of BCG is assessed distinctively in the case of disseminated TB in children and adult pulmonary TB on the account of colossal discrepancy (25). One of the multifaceted explanations can be the intricate biology of TB establishment and progression in humans (26). In the majority of M.tb infections, the immune system can proficiently restrict the progression of the pathogen to cause active TB. However, the endeavour to eliminate the bacilli completely is rarely achieved and can culminate into escalated inflammatory responses that direct distinct phases of disease such as latent TB and associated immunopathogenesis (27). The aim is to strike a balance for the resolution of M.tb infection exclusive of detrimental inflammation. For more than a century, researchers have attempted to ascertain correlates of BCG-induced defenses in humans through various animal models (10) and clinical trials (28). Additional to M.tb infections, broad-spectrum immunomodulatory characteristics of BCG are utilized to treat bladder cancer (29), asthma (30), leishmaniasis (31) and warts (32). While our understanding of key immunological aspects is continuously expanding (33), we have attained substantial knowledge regarding innate and adaptive immune responses to M.tb infection and BCG immunization which is briefly depicted in Figure 1 and will be reviewed thoroughly in this section.
Figure 1.
Immune responses to BCG immunization. Immune responses to the BCG vaccine initiate at the site of inoculation by induction of innate immune cells such as resident macrophages, neutrophils, and dendritic cells. Innate immune cells internalize, degrade and present antigen of bacilli via surface receptors to further activate adaptive immune cells. Chiefly, DCs loaded with bacilli drain to the lymph nodes and result in lymphocyte stimulation and activation. T and B lymphocytes further differentiate into diverse subtypes including effector and memory cells.
Innate immune system function as the first line of defense in confronting M.tb infections (34). The innate immune responses are the key component of the host immune responses engaged promptly at the site of infection (35). Even in the course of intradermal BCG immunization, early immune responses are elicited by resident epidermal macrophages (36), neutrophils (37), and dendritic cells (DCs) (38). BCG comprises pathogen-associated molecular patterns (PAMPs) such as cellular components (mycolic acids, peptidoglycans, and arabinogalactans) which are recognized by diverse PAMP recognizing receptors (PRRs) present on the surface of innate immune cells. Diverse PRRs abundantly expressed on innate subsets such as complement receptor 3 (CR3) (39), TLR2/4/9 (40), mannose receptor (41), Ca2+-dependent lectin (MINCLE) receptors on macrophages (42), nucleotide-binding oligomerization domain (NOD)-like receptors NOD2 on monocytes, CD18, FcγRII, and FcγRIII on neutrophils and DC-SIGN, CD11c, and CD205 on dendritic cells initiate the prompt innate immune responses upon BCG immunization (43). Disparities in the cellular composition of BCG and M.tb has been linked with recognition by different PRRs which further influences the uptake, processing and representation of antigens to other immune cells. Since the receptor involved determines the fate of downstream signaling, the variation in receptor utilization can be further associated with relatively inefficient immune responses in the case of BCG (44). Examination of skin biopsies demonstrated that BCG blister point majorly comprises CD15+ neutrophils, a small proportion of CD14+ monocytes, and an infinitesimal population of CD3+ T lymphocytes (45). However, in whole blood culture experiments, CD56+ NK cells, γδ T cells, NKT cells, and cells from MAIT were found to be associated with BCG-induced immunity (46). In response to BCG vaccination, innate immune responses such as ROS/RNI generation by neutrophils, the release of monocytic chemokines like IL-6, TNF-α, MIP-1α, MIP-1β, IL-8, and IL-1α within 1-3 hours is initiated to direct systemic immune responses (45). BCG is known to effectively activate monocytic populations (47). In animal models, subsequent to BCG immunization mycobacterial extermination by macrophages was demonstrated independent of adaptive immune responses (48). Deficit immune responses induced by macrophages subsequent to BCG vaccination downgrade bacterial clearance. Furthermore, guinea pigs immunized with BCG upon H37Rv infection demonstrated enhancement of phagosome-lysosomal fusion with a considerable reduction in mycobacterial burden (47).
Dendritic cells (DCs) function as a nexus between innate and adaptive immune responses by presenting processed antigens to T lymphocytes post BCG immunization via IL-1R, MyD88 pathway (49). BCG immunization is known to enhance DC maturation and activation by upregulating the expression of markers implicated in antigen presentation such as MHC-II, CD40, CD80, and CD86 (35). Nonetheless, BCG immunization is also linked with the stimulation of IL-10 and IL-4 cytokines by DCs which can bias the differentiation of T lymphocytes toward the TH2 subtype and can be the cause of weakened BCG effectiveness (49). Nonetheless, the majority of information concerning the role of DCs in the case of BCG inoculation is from in vitro studies. The observation that in vitro BCG stimulation, initiates aggregation of DCs, upregulates antigen presentation with reduced endocytosis, and stimulation of TNF-α infers that DCs contribute to the initiation of immune responses. However, it is concerning that compared to M.tb infection these responses are inadequate to impart requisite protection (50). Apart from the major innate immune cell populations, innate lymphoid cells (ILCs) and mucosal-associated invariant T cells (MAIT) have been connected with BCG-induced innate protection (36). However, fragmentary information is accessible regarding these subsets and further exploration is necessitated.
It is now well-known that analogous to antigen-specific responses elicited by adaptive immunity, subsequent to pathogenic insults cells of innate immunity elicit heterologous memory responses (7). Distinct reports have demonstrated that natural killer (NK) cells and macrophages which have formerly encountered pathogens through epigenetic remodeling are trained to respond to distinct pathogens (51). It has been observed that epigenetic modifications such as H3K4me1, H3K4me3, and H3K27ac have been associated with the reprogramming of monocytic populations owing to unfastening of chromatin positions at the promoters of pro-inflammatory cytokines (52). BCG immunization leads to the expansion of Hematopoietic stem cells (HSCs), drives myelopoiesis and via epigenetic reprograming enhances host protective immune responses (53). Furthermore, it is established that macrophages interact with NK cells and bring about the refinement of innate immune responses against pathogenic insults (54). With a deeper insight into trained immunity, it was observed that BCG vaccination in healthy individuals stimulates NK cells and macrophages to uphold cytokine generation in response to ex vivo stimulation (55). The broad-spectrum immunomodulatory potential of BCG has been employed for the treatment of diverse ailments including the COVID-19 disease caused by SARS-CoV-2 (56). It has been experimentally proved that BCG via epigenetic reprogramming of immune cells exhibits cross-protection against diverse pathogens (57). The research demonstrates the impact of BCG vaccination on the induction of genome-wide histone modifications in trained monocytes which participate in IL-1β generation and the reduction of the yellow fever virus (YFV) burden (58). BCG-induced protection against YFV infection substantiates the broad-spectrum effectiveness of the vaccine against diverse viral infections such as influenza A (H1N1) virus, herpes simplex virus (HSV), and human papillomavirus (HPV) (59). Based on this information, BCG vaccination was evaluated initially during the COVID-19 pandemic. Preliminary ecological studies demonstrated that COVID-19 cases and deaths per population were fewer in countries with BCG vaccination schedules (60). The notion of inducing anti-viral immunity by employing BCG was based on the generation of heterologous immune responses (61). Since it was found that the envelope protein of the SARS-CoV-2 virus shared certain homology with strains of Mycobacterium species (62). It was inferred that the homology was associated with the induction of host-protective Th1/Th17 responses. The concept of trained immunity and heterologous responses were utilized to exploit BCG vaccination in the era of COVID-19. Since the majority of individuals are already vaccinated with BCG, it is judicious to keep BCG in reflection while developing new vaccination strategies. The fight to halt TB must continue progressively while dealing with the ongoing COVID-19 pandemic (17).
Till now adequate information is established regarding protective correlates against TB. Adaptive immune responses play a vital role in eliciting pathogen-specific immune responses with superior efficacy (26). The protective role of T lymphocytes was primarily demonstrated by the adoptive transfer of CD4+ and CD8+ T cells from BCG immunized mice to T and B cell-deficient (Rag1-/-) knockout mice (63). T lymphocytes contribute significantly against M.tb infections upon activation by components of innate immunity. The induction of Th1/Th17 immune responses and IFN-γ secretion is positively linked with augmented clinical outcomes in TB patients (26). Several studies have demonstrated mechanistic insights of BCG-induced defenses as a consequence of Th1 cells through IFN-γ secretion (64). The paramount contribution of Th1 responses was also demonstrated in infants vaccinated with BCG wherein Th1 responses prevailed for more than a year contrary to pronounced Th2 responses in unvaccinated infants (65). Furthermore, for the next few years, BCG-induced protection was attributable to IFN-γ releasing T lymphocytes (65). However, the outcomes of BCG immunization are still disputable and not strongly concurrent with specific immune responses. In IFN-γ deficient mice, BCG immunization demonstrated considerable protection against M.tb infection that vanished after depletion of CD4+ T lymphocytes (66). Comparable outcomes were achieved in a study involving humans vaccinated with BCG wherein restricted M.tb progression and protection were linked with IFN-γ independent CD8+ immunological responses (67). It is established now that polyfunctional CD4+ T lymphocytes play a vital role in enhancing defenses against M.tb by secreting cytokines in different combinations to amend the microenvironment at the site of infection (68). It was confirmed that BCG immunization in infants does not elicit polyfunctional immune responses linked with effective protection against M.tb (12). However, immunization with a booster dose of MVA85A (modified vaccinia virus Ankara expressing antigen 85A) in BCG vaccinated adults confirmed induction of polyfunctional T cell responses (69). This gave rise to the hypothesis of heterologous boosting of BCG immunization for robust protective immunity against M.tb. Thereafter, failure of MVA85A heterologous boosting in infants to induce efficacious immune responses even with induction of polyfunctional T responses blurred the resolution of protective efficacy (70). Similarly, CD8+ T cells exhibit antimycobacterial activity and are directly involved in M.tb killing, cytotoxic extermination of infected cell populations, and IFN-γ secretion (71). In human samples, CD8+ T cells have been shown to distinctively identify and kill infected macrophages along with internalized M.tb with granular discharge comprising perforin and granulysin (63). Therefore, subsiding the bacterial burden and effectively enhancing defenses against M.tb. The vitality of MHC class I-restricted CD8+ T cells was demonstrated in β2-microglobulin (β2m) deficient mice incapable of restricting M.tb infection (72). It is not feasible to achieve sterilizing immunity against reinfections with mycobacteria subsequent to pathogen clearance with antimycobacterial drugs. Several studies have emphasized the significance of IL-17 generating CD4+ T lymphocytes in mediating protection from reinfections and improving clinical outcomes upon vaccination (73). Consistent with these reports, in BCG immunized mice, Th1 responses in the lungs were shown to be reliant on IL-17A and IL-23 secreted by antigen-specific Th17 cells (73). In non-human primates (NHPs) administering a high dose of intradermal or intravenous BCG was linked with augmented protection from M.tb as a consequence of CD4+ T lymphocytes with Th1/Th17 phenotypic characteristics (74). Thus, augmenting Th1/Th17 responses induced by BCG offer prospective solutions against TB (74). BCG immunization has been linked with the enhancement of regulatory T cells (Tregs) via alteration of immune metabolic pathways which consequently reduces the protective efficacy against TB. Depletion of Tregs can result in enrichment of Th1, cytotoxic T cell responses with enhanced bacterial extermination upon infection (75). Boosting BCG with a novel vaccine candidate comprising Ag85B-Mpt64 (76–84)-Mtb8.4 (AMM) along with composite adjuvant lowers the Treg population which was associated with enhanced protection in the mice model (85). Nonetheless, few studies have also demonstrated unaltered outcomes in BCG immunization upon prior Tregs depletion (86). Hence, further analysis is necessitated to validate the prospects for utilization in clinical operation. Regardless, the potential of the BCG vaccine to enrich Tregs and inhibit detrimental inflammation has been employed to treat diverse disorders including SARS-CoV-2 infection-induced cytokine storm in the COVID-19 pandemic (87). Additionally, BCG administration has been linked with increased IL-10 secretion in animal models which consequently restricts anti-mycobacterial pro-inflammatory responses induced by vaccination (88). Furthermore, obstruction of IL-10 signal transduction in the course of BCG immunization improved protective immune responses by mounting Th1, and Th17 responses (89). Hence, striking the immunological balance to achieve affirmative outcomes is requisite to alleviate BCG-induced protection.
Approaches to Amend Inadequacies of BCG Vaccine
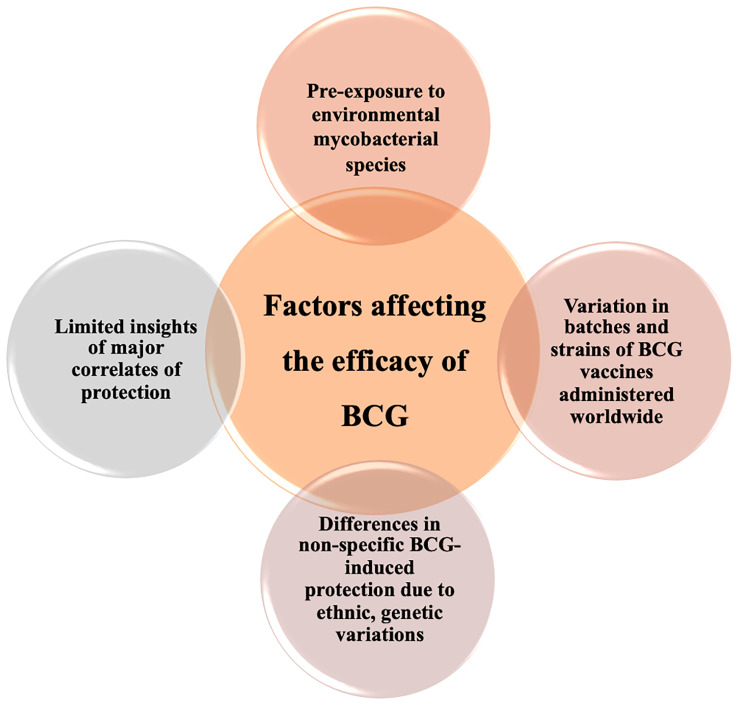
Despite numerous limitations of the BCG vaccine, it is still challenging to stumble upon more effective vaccine candidates for TB (90). BCG is unquestionably the most reliant vaccine for the prevention of disseminated forms of TB in children (91). Hence, it is critical to comprehend the shortcomings of BCG to extemporize protection by progressive approaches. It is widely proclaimed that wearying BCG immunity is a consequence of the non-existent T cell epitopes of M.tb in BCG vaccine strains (92). It is also established that expansion and differentiation of effector T cells declines in age-dependent manner upon BCG inoculation inferring toward weakened central memory responses (93). The contracted pool of antigen-specific memory populations is the basis for short-term protection against M.tb infections (93). Furthermore, the variable efficaciousness of BCG-induced defenses is associated with multifold factors such as ecological aspects, genetics, and differences in nutritional profiles amongst populations, listed in Figure 2 . A major rationale for highly variable BCG efficacy in adults is exposure to environmental non-tuberculous mycobacteria (NTM). Prevalence of NTMs in tropical regions has been linked with low efficacy of BCG and consequent high TB burden due to pre-immunization exposure induced variation in protective efficacy (94). In the regions nearby the equator, UV exposure has been connected with a reduction in BCG efficacy which was further demonstrated in animal models with alterations in cytokine profiles when exposed to UV at the time of BCG immunization (60). Furthermore, variations in handling protocols and numerous passages of BCG vaccine strains have given rise to alterability in immunogenicity of BCG vaccine strains worldwide (95). Despite the wavering protective efficacy of BCG vaccine is deemed to be safe and is administered worldwide is numerous vaccination programs. However, with the raising concerns in immune-compromised HIV-TB co-infected individuals, WHO has addressed disputes regarding the utility of live vaccine in diverse risk groups. In immune-deficient children seldomly BCG vaccination can lead to systemic BCGosis. Atypical adverse reactions were detected in children with chronic granulomatous disease, Di George syndrome and severe combined immune deficiency (SCID) which can result in deadly consequences if unmanaged (96). In immunocompromised individuals especially neonates vaccination can also result in BCG lymphadenitis and disseminated BCG infection which is one of the most detrimental consequence of BCG vaccination (97). Hence, due to the overabundance of factors contributing to undermining protection elicited by BCG progressive approaches have been employed to improvise BCG against M.tb.
Figure 2.
Diverse factors associated with variable efficacy of BCG. Inconsistent efficacy of BCG vaccination can be linked to numerous host factors including genetics, geographical representation, ethnicity, and fragmentary immunological insight addition to variation in BCG vaccine strains with distinct characteristics.
Replacing or Reclaiming BCG
The major TB burden worldwide accountable for morbidity and mortality is due to adult pulmonary TB cases (1). The interval of weakening of BCG-induced defenses overlaps with an escalated incidence of M.tb infections in adults. On the surface foremost justification for the incompetence of the BCG vaccine appears to be immunization in the early years of life which imparts limited protection (90). The prospects of the End TB Strategy hence seem bleak without an improvised vaccination stratagem. Since BCG is the most widely utilized vaccine in the world and imparts protection in infants against disseminated forms of TB, it is judicious to reclaim BCG-induced protection rather than displacing it with a replacement. Fundamental strategy to amend BCG efficacy is by utilizing prime boost vaccination approach (98). Since one of the many desired characteristics for upcoming vaccine candidates is to efficaciously improvise the existing TB control approach i.e. prophylactic BCG immunization (99). Alternatives to strengthen the existing TB control program comprises developing a booster vaccine to augment the protective efficacy of BCG or supplementing immunotherapeutic as an adjunct to strengthen BCG-induced immunity. Our research group has endeavored and designed a novel vaccine comprising TLR2 and TLR9 agonist along with collective of 7 overlapping immunogenic M.tb peptides, packed together in a liposome (PTL). We have demonstrated that intranasal immunization with PTL along with BCG drastically condensed the bacterial burden, enhanced host protective T cell responses with expansion of polyfunctional T cells as well as memory T cell subsets. Furthermore, host protective immune responses were M.tb specific owing to which spectrum of host protective signaling pathways critical to control TB were activated in response to PTL BCG co-immunization (100). Further assessment in higher animal models like Non-human primates (NHPs) is coveted to further validate our findings and progress the research to higher phases. We and various groups worldwide have actively devised and pursued strategies to develop new vaccine candidates, booster vaccines and immunotherapeutic to augment BCG efficacy against M.tb infection. However, in this review we have discussed about new TB vaccines in brief and have focused on host immunotherapeutic approaches comprehensively.
New Vaccines Against TB
The necessity of an alternate vaccination strategy for TB control has not been overlooked and researchers around the world are actively pursuing diverse vaccine candidates to improve existing circumstances (99). On the account of immense attempts, several groups have developed vaccines by employing diverse approaches to control TB. Some classic examples include the attenuated M.tb bacilli strains (101) with high immunogenicity (102), the generation of genetically modified BCG strains for better immune responses (70), sub-unit vaccines incorporating immunogens absent in BCG (103), and adjuvants with enhanced potency. Major TB vaccine candidates in advanced clinial trials are tabulated in Table 2 . A myriad of challenges is liable for slow progress in vaccine development against TB. One of which is the necessity for a vaccine that protects against adult pulmonary TB, in individuals who are presently vaccinated with BCG. Ideally, the development of vaccine candidates that can efficaciously impart protection to individuals previously exposed to mycobacteria including BCG, M.tb and environmental mycobacterial species would be enviable. So as to boost the immune responses thereby limiting adult pulmonary TB infections (19). Diverse studies incorporating booster vaccinations are under evaluation in animal models (104). With the advancement in technology and knowledge regarding host protective defense mechanisms, especially antigen-specific immune responses accountable for effective responses against M.tb we are surpassing conventional vaccination approaches. With improved understanding regarding known correlates of protection, research groups are assessing strategies to enhance long-lasting protective immune responses by employing progressive targets. What we have learned in the past decade from BCG trials as well as recent COVID-19 trials can be employed in the future to better interpret the lacunae which can be resolved for an improved TB vaccination program (16).
Table 2.
Major TB vaccine candidates in clinical trials:.
| Vaccine candidate | Composition | Clinical Trial | Clinical trial Identifier | Ref. |
|---|---|---|---|---|
| Inactivated whole-cell vaccines | ||||
| DAR-901 | Inactivated Mycobacterium obusense | Phase 2, randomized, placebo-controlled, double-blind study to evaluate the efficacy of DAR-901 TB booster to prevent TB in adolescents. | NCT02712424 | (1) |
| MIP | Inactivated Mycobacterium indicus pranii | Phase 3, randomized, double-blind, interventional study to determine the efficacy and safety of MIP as an adjunct in Category I pulmonary TB patients | NCT00341328 | (2) |
| RUTI® | Detoxified, fragmented M.tb contained in liposomes | Phase 2, randomized, double-blind, placebo-controlled interventional trial to assess the therapeutic vaccine, RUTI against TB |
NCT01136161, NCT04919239 |
(3, 4) |
| Vaccae™ | Heat-inactivated Mycobacterium vaccae | Phase 3, randomized, double-blind, interventional trial to assess the safety and efficacy to prevent TB in high-risk groups of TB infection | NCT01979900 | (5) |
| Live attenuated vaccines | ||||
| MTBVAC | Live attenuated M.tb vaccine with PhoP and FadD26 deletions | Phase 3, randomized, quadruple masking intervention to determine safety, efficacy and immunogenicity in newborns | NCT04975178 | (6) |
| VPM1002 | Live recombinant BCG vaccine strain with urease C deletion engineered to express listeriolysin rather than urease C | Phase 3, multicenter, double-blind, randomized, active-controlled trial to examine the safety, efficacy and immunogenicity to prevent M.tb infection | NCT04351685 | (7) |
| Subunit vaccines | ||||
| M72/ASO1E | Fusion protein subunit vaccine based on 32A and 39A prepared in AS01E adjuvant | Phase 2, randomized, interventional clinical trial to determine the efficacy of TB vaccine candidate in Adults | NCT01755598 | (8) |
| H56:IC31 | Recombinant vaccine comprising proteins of M.tb (85B, ESAT6, Rv2660c) and IC31 adjuvant | Phase 2, randomized (1:1), double-blind, placebo-controlled trial to determine efficacy of H56:IC31 in preventing rate of TB recurrence | NCT03512249 | (9) |
| GamTBvac | Recombinant subunit vaccine formulation comprising modified Ag85a and ESAT6-CFP10 M.tb antigens and CpG ODN adjuvant | Phase 3, randomized, multicentered, double-blind, placebo-controlled intervention to determine safety and efficaciousness of GamTBvac against pulmonary TB | NCT04975737 | (10) |
| ID93/GLA-SE | ID93 is a recombinant fusion protein comprising 4 antigens from virulence-associated proteins in GLA-SE i.e., oil-in-water emulsion | Phase 2a, randomized, placebo-controlled, double-blind intervention to evaluate safety and effectiveness of ID93/GLA-SE in TB patients | NCT02465216 | (11) |
Host-Directed Strategies to Improve BCG Efficiency
The outcome of M.tb infection is determined not just by the action of the pathogen but also by the host response. So as to achieve the goal of the End TB strategy, progressive efforts are being made to establish efficacious therapeutics (17). In an attempt to achieve this goal, host-directed therapeutics with the potential to reprogram host defenses for better clinical outcomes are under consideration. Since it is known that in the majority of individuals, the immune system can self-reliantly eradicate the pathogen. Augmenting this phenomenon so as to achieve complete sterility can offer benefits to the existing global TB burden. TB is a chronic disease with a spectrum of pathologies (26). Hence, we need to move ahead from conventional antibiotics toward host-directed therapies for improved clinical outcomes. HDT has found a niche in the treatment of various diseases (105). However, we need more efforts to establish a standard HDT as an adjunct to conventional ATT for the augmentation of disease burden. So as to achieve this target it is a prerequisite to determine key host immune targets for better outcomes. HDTs aim at diverse pathways critical for determining the fate of the infection. HDTs work by restricting pathways exploited by pathogens or by ameliorating host protective immune responses (105). With the advancement in understanding, diverse factors contributing to the establishment of infection have been identified. The primary goal of TB drug discovery is to exterminate both active and persistent bacteria so as to attain complete sterility. Challenge is to aim at heterogenous M.tb populations that respond distinctly to therapeutics. It is essential to be reminiscent of the fact that M.tb infection can instigate a continuum of host responses owing to distinctive physiologies of heterogeneous bacterial populations. Furthermore, a profound assessment of stochastically and phenotypically drug-resistant persisting populations of M.tb subsequent to drug therapy is requisite to cope with TB relapse and reactivation (106). Better insight into mechanisms targeted to exterminate persistent populations is necessitated since it is not conclusive whether targeting bacterial membrane or prime respiratory components will eradicate latent bacteria. It is the need of the hour to get hold of innovative drugs to establish efficacious therapy to attain the goals of the End TB strategy. To accelerate the search for the right drugs, United States Food and Drug Administration (FDA) approved compounds are also under evaluation and operation (107).
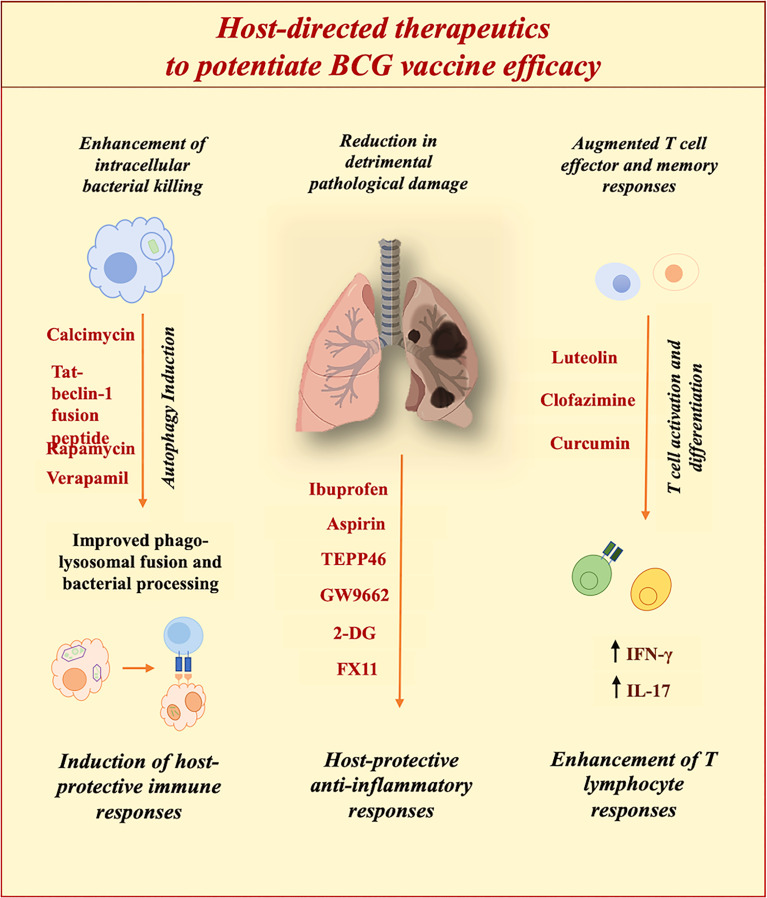
Futuristic therapeutics should aim to shorten the duration of conventional ATT by proficient elimination of persistent bacterial populations, which also result in drug-resistant strains. With the constant expansion of drug resistance, options for treatment are continuously depleting. Chiefly, in terms of drug-resistant TB, HDTs can be employed to augment antimicrobial host defences or to restrain detrimental inflammation instigated by infection. Some of the prospective HDTs against M.tb are listed in Table 3 , summarised in Figure 3 and further mechanism of protection is elaborated in different sections. Furthermore, HDTs that can limit the hepatotoxicity associated with conventional antibiotics are desired to subside unfavourable outcomes of extensive therapies. Theoretically HDTs surpass diverse issues associated with pathogen-directed therapeutics. HDTs augment host immune responses with sufficient proficiency to restrict the progression of the disease. Furthermore, targeting host components provide the advantage of reducing the generation of antibiotic resistance (129). Therapeutics targeting host components avoid the chances of drug resistance which is a global health concern. However, if not chosen wisely targeting host components can lead to off-target binding and might accelerate the chances of detrimental side effects. Thus, our knowledge regarding targeted mechanisms is vital to developing therapies to reprogram host defences for efficacious TB treatment.
Table 3.
Potential host directed immunotherapeutic approaches against M.tb infection.
| S. No. | Therapeutic candidates | Host protective immunological characteristics | References |
|---|---|---|---|
| 1. | Ibuprofen | Inhibits neutrophil infiltration and detrimental inflammation at the site of infection | (108, 109) |
| 2. | Acetylsalicylic acid (Aspirin) | Anti-inflammatory responses reduce detrimental pathology | (108) |
| 3. | Monosodium Urate (MSU) | Activation of immune responses to augment antimycobacterial efficacy of BCG | (110) |
| 4. | Calcimycin | Induction of autophagy by binding to P2X7 receptors | (111) |
| 5. | Verapamil | Inhibits LTCC channels thereby induces autophagy by increasing Ca2+ levels. | (112) |
| 6. | Clofazimine | Enrichment of stem cell memory T memory responses upon BCG revaccination | (113) |
| 7. | Luteolin | Inhibition of Kv1.3 K+ channels, enhancement of antimycobacterial and T cell memory immune response | (114, 115) |
| 8. | Rapamycin (Sirolimus) | Enhances antigen processing and presentation and directs Th1 immunity | (116) |
| 9. | Tat-beclin-1 fusion peptide | Autophagy induction and reduction in progression of pathogens | (117) |
| 10. | Gefitinib | Enhances lysosomal biogenesis, action and bacterial degradation | (118) |
| 11. | 2-deoxyglucose (2-DG) | Metabolic reprograming induced reduction in pathological damage | (119) |
| 12. | Ritonavir (Norvir) | Glucose transporter agonist induces protection against HIV as well as M.tb | (120) |
| 13. | FX11 | Lactate dehydrogenase inhibitor reduces oxidative stress and downgrade iNOS | (121) |
| 14. | TEPP46 | Limits inflammation by reducing PKM2 activation | (122) |
| 15. | Metformin | Induces AMPK mediated signaling, induction of ROS and intracellular bacterial killing | (123) |
| 16. | AICAR | Stimulate anti-microbial immune responses by via (PPARGC1) linked pathways | (124) |
| 17. | C75 | Inhibits lipid derived droplets biogenesis, enhances ROS, NO production and polarizes macrophages from M1 to M2 | (125) |
| 18. | Cerulenin | Inhibition of fatty acid synthase, uncouples UCP2 and promotes NLRP3 activation | (126) |
| 19. | GW9662 | PPARγ antagonist can regulate inflammation and disease progression by altering metabolism in macrophages. | (127) |
| 20. | AGK2 | Inhibits host sirtuin2 (SIRT2) and enhances bacterial clearance, host protective immune responses | (128) |
Figure 3.
Potential of host-directed therapies (HDTs) to improve BCG efficacy. Diverse HDTs aiming at distinct pathways are under evaluation to improve clinical outcomes. HDTs restrict pathogen-induced subversion strategies to ameliorate host defenses against M.tb.
Established Host-Directed Strategies to Potentiate BCG Vaccination
In an attempt to achieve the targets of End TB strategy, diverse host-directed therapeutics with the potential to reprogram host defences for better clinical outcomes are under consideration. Since it is known that in the majority of individuals, the immune system can self-reliantly eradicate the pathogen. Augmenting host defences so as to achieve complete sterility can offer benefits in reducing existing global TB burden. To achieve superior effectiveness against M.tb infections, researchers have evaluated the administration of immunomodulators along with antibiotics and vaccines. This was widely employed in cancer therapies wherein inhibition of anti-inflammatory cytokines, and inhibitory signaling receptors such as PD-1 and CTLA-4 were found effective in augmenting tumor recession (130). Likewise, therapeutics known for inhibition of detrimental immune responses were found effective in improving tumor vaccine efficacies. Constructive outcomes were detected against breast cancer and pancreatic adenocarcinoma by the utilization of COX-2 inhibitors (131). In HIV-infected individuals, therapy with COX-2 inhibitor augmented effector and memory responses induced by T cell targeting vaccine; tetanus toxoid (132). Selected clinical trials that have evaluated immunomodulatory strategies adjunct to BCG immunization for improved clinical results have been listed in Table 4 (133). Furthermore, modulation of monocytic cell populations has been exploited as a prospective strategy for augmenting efficacy of BCG vaccination. Abundance of uric acid crystals namely monosodium urate (MSU) have been linked with bone inflammation and associated immune responses (110). Presence of MSU crystals was further linked with coexisting M.tb joint infection in patients suffering from gout (134). MSU treatment in the THP-1 cell line brings about a generation of ROS, stimulation of phagosome-lysosome fusion, and via NOD-like receptor signaling enhanced BCG clearance (135). MSU alone has no anti-bacterial activity inferring potential to promote bacterial clearance by immunomodulation. MSU therapy in adjunct to BCG vaccination in vivo led to a reduction in bacterial burden in draining lymph nodes. However, MSU treatment did not affect the viability of BCG. As compared to BCG alone, MSU therapy significantly reduced the bacterial burden in the lungs and spleens of M.tb infected mice (110). Based on affirmative evidences of Vitamin D supplementation in restraint of M.tb infections (136), several research groups have correlated protective efficacy of BCG immunization in infants with Vitamin D levels (137). One of the research group has observed increase in Vitamin D levels in infants vaccinated with BCG and have linked the upsurge with non-specific immune responses detected subsequent to vaccination (138). In another study, infants supplemented with Vitamin D were expected to elicit protective IFN-γ responses against M.tb infection (137). However, this clinical trial waned to provide evidences of Vitamin D induced protection in augmenting BCG efficacy. Further assessment is requisite to explore prospects of Vitamin D supplementation along with BCG immunization. Potential of established immunomodulators with BCG have been improvised for better clinical outcomes. One such study demonstrated induction of superior host protective T cell responses upon co-administering curcumin nanoparticles along with BCG immunization in murine model (139). Clofazimine (CLOF), an authorised therapeutic for leprosy treatment and second-line drug used in combinations against drug-resistant M.tb strains has also demonstrated affirmative outcomes in mice model. BCG revaccination along with CLOF administration significantly augmented T cell memory responses comprising enhancement of stem cell-like memory T cell responses (TSCM) along with successive effector and memory T cell populations (113). So as to further resolve the prospects of above-mentioned strategies in enhancement of BCG efficacy, further studies in higher animal models such as non-human primates (NHPs) and clinical trials is necessitated without delay.
Table 4.
List of clinical trials evaluating BCG immunization along with diverse immunotherapeutic for efficient medical utility against diverse disease conditions.
| BCG vaccine and immunotherapeutic regimen | Clinical trial | Trial identifier | Diseased condition | Ref. |
|---|---|---|---|---|
| Intravesical hyaluronic acid (HA) with BCG | Phase 2, randomized, pilot study to examine effect of HA in reducing BCG induced local cytotoxicity | NCT02207608 | Bladder urothelial cell carcinoma | (12) |
| Tislelizumab in Combination with BCG | Phase 2, open-label, single-arm, single center trial to evaluate safety and effectiveness of Tislelizumab along with BCG (TACBIN-01) | NCT04922047 | High risk urinary bladder cancers | (13) |
| Vitamin D supplementation in adjunct to BCG immunization in infants | Randomized, double masked, interventional study to evaluate impact of vitamin D supplement in infants prior to BCG vaccination | NCT01288950 | Tuberculosis | (14) |
| Vitamin A with BCG Vaccine | Phase 4, randomized, double-masked intervention to evaluate the utility of high-dose vitamin A supplementation in infants along with BCG vaccine at birth | NCT00168597 | Mortality and morbidity in infants | (15) |
| Monoclonal Antibody A1G4 and BCG | Phase 1 intervention to evaluate the efficacy of monoclonal antibody A1G4 along with BCG in cancer patients | NCT00003023 | * Neuroblastoma, Sarcoma | (16) |
Modulation of Immune Responses to Improve Protective Efficacy
With the rise of genomics, researchers have utilized the genomic information of M.tb and M. bovis BCG vaccine strains for assessing variations that can be benefitted to develop better vaccination strategies (95). The revelation of the entire M.tb genome revolutionized TB research by expanding the knowledge of central immunomodulatory components (140). To achieve superior effectiveness against M.tb infections, researchers have evaluated the administration of immunomodulators along with vaccines. This was widely employed in cancer therapies wherein inhibition of anti-inflammatory cytokines, and inhibitory signaling receptors such as PD-1 and CTLA-4 were found effective in augmenting tumor recession (130). Likewise, therapeutics known for inhibition of detrimental immune responses were found effective in improving tumor vaccine efficacies. Constructive outcomes were detected against breast cancer and pancreatic adenocarcinoma by the utilization of COX-2 inhibitors (131). In HIV-infected individuals, therapy with COX-2 inhibitor augmented effector and memory responses induced by T cell targeting vaccine; tetanus toxoid (132). Inhibition of neutrophil infiltration at the site of M.tb infection by Ibuprofen (IBP) resulted in improved clinical outcomes and a reduction in bacterial burden in C3HeB/FeB mice (109). IBP is also known to possess specific antitubercular characteristics (141). Furthermore, IBP along with another drug; acetylsalicylic acid was evaluated to be repurposed as an adjunct therapy in TB patients (108). In murine model of TB, drugs were associated with enhancement of pyrazinamide (PYZ) antimycobacterial efficacy (142). This approach can be further exploited to amend BCG-induced responses. These studies indicate that treatment with immunomodulatory compounds or enriching host protective responses along with BCG might can effectively enhance protection induced by BCG vaccination.
Targeting Host Ion Channels
Another strategy is to aim at host ion channels, that orchestrate physiological features of various cell populations by operating ions facilitated currents throughout cellular and subcellular membranes (143). Intracellular calcium levels are known to regulate key immune responses in the host, directly or by directional alteration of other vital ions such as potassium (K+), sodium (Na+), and chloride (Cl-) ions within immune cell populations (144). Obstruction of ion channels by employing diverse blockers has been assessed as a therapeutic target for diseases like hypertension (145). Research groups have also examined ion channel blockers for boosting anti-microbial immune responses. Intracellular calcium (Ca2+) levels play a vital role in the regulation of antimycobacterial mechanisms such as autophagy, maturation of phagosome, and induction of apoptosis (146). However, the impact of Ca2+ levels on processes like autophagy additionally depends on the involvement of diverse ion channels contributing to the maintenance of current (147). For instance, it has been observed that Ca2+ currents via Voltage-gated calcium channels (VGCCs) impede induction of autophagy (147) while Ca2+ currents through P2X purinoceptor 7 (P2X7) receptor heighten autophagy induction and intracellular extermination of M. bovis BCG in macrophages. Calcimycin, an ionophore binds to P2X7 receptor which leads to rise in intracellular Ca2+ levels and exerts antimycobacterial activity against M. bovis BCG (111) by stimulation of autophagy (148). Administering Ca2+ ion channel blockers was linked with a 32% reduction in risk of progression into a diseased state, in a clinical study in TB patients with heart and cerebrovascular diseases (149). Diverse Ca2+ ion channel blockers evaluated in the investigation exhibited variable consequences. L-type calcium channel (LTCC) blocker – verapamil, an FDA approved drug is utilized to treat abnormalities in heart rhythms, angina (150), and hypertension (151). In macrophages, LTCCs attenuate Ca2+ discharge from the endoplasmic reticulum (ER) leading to inhibition of macrophage activation. So as to bypass host immune responses M.tb upregulates the expression of VGCCs in APCs. Verapamil administration inhibits LTCC currents thereby escalating Ca2+ concentration in the cytosol which upregulates autophagy and bacterial clearance in M.tb (143). Additionally, LTCC ion channel blockers can alter iron-associated metabolic pathways thereby impeding iron accessibility which lowers intracellular bacterial survival (143). Furthermore, verapamil acts synergistically with first-line anti-TB drugs- INH (152), and RIF (153) in lowering the bacterial burden in cultures, macrophages, and murine models of TB. In another study, antimycobacterial activity of verapamil was confirmed with several TB drugs including bedaquiline (BDQ) and clofazimine (CFZ) and decreased bacterial load was linked with induction of membrane stress responses as a consequence of verapamil induced membrane function disruption (154). In the murine model, adjunctive verapamil administration augmented efficacy of recently approved MDR-TB drug; bedaquiline at lower doses and diminished the emergent resistant strains (155). Furthermore, progressive approaches such as assessment of inhalable verapamil-rifapentine particles has been assessed by researchers for utility as ATT (156). Even with significant pieces of evidence regarding anti-TB activity and negligible toxicity, verapamil has not transitioned into clinical setup. Further assessment of the anti-TB potential of verapamil along with BCG immunization is necessitated to evaluate the impact on modulating immunological responses. Similarly, Numerous K+ ion channel blockers have been assessed as prospective anti-TB therapeutics owing to their physiochemical ability to activate macrophages. Clofazimine (CFZ) is a conventional first-line drug used for leprosy treatment along with RIF and dapsone (157). It was initially developed against M.tb but was found not as efficacious as INH and RIF. However, with emergent drug-resistant strains, it has been recently employed as a second-line anti-TB drug (158). Numerous clinical trials (BEAT-TB (159), endTB-Q (160), and TB-PRACTECAL (161)) are investigating the efficaciousness of regimens comprising clofazimine. Furthermore, phase-2 clinical trial CLO-FAST is examining 3-month ATT comprising clofazimine and rifapentine against drug-sensitive M.tb (162). In addition to antimycobacterial activity, clofazimine is a potent immunomodulator. It inhibits Kv1.3 K+ channels which are expressed in various immune cells (163). Clofazimine-induced inhibition of Kv1.3 K+ channels abundantly present on T effector memory cells (TEM) enhances the efficacy of BCG vaccination in a murine model of TB by specifically promoting the expansion of the T central memory cell population (TCM). Furthermore, CFZ enriches stem cell memory T cell responses upon BCG revaccination (113). In a similar manner, we have demonstrated the efficacy of less toxic, phytochemical namely Luteolin an established Kv1.3 K+ channel blocker in augmenting BCG-induced immune responses by enriching TCM memory responses, improving TCM : TEM ratio and enhances host protective Th1 and Th17 immune responses against M.tb infection in the murine model of TB (115). Furthermore, immune-protective properties of luteolin condensed the time period of bacterial clearance with INH owing to augmented Th1 and Th17 immune responses and eased pathological damage and TB associated hepatotoxity in vivo (114).
Improving Anti-Microbial Immune Responses
Therapeutic modulation of host immune responses to achieve complete sterility is another approach that has been pursued by several research groups (129). M.tb utilizes complex artillery to evade immune cell populations and associated defense mechanisms. M.tb owing to mycobacterial virulence factors such as cell wall component- mannose-capped lipoarabinomannan is known to inhibit phagolysosome fusion in macrophages. It is a vital phenomenon critical for curbing infection at an early stage (26). However, this can be enforced by autophagy induction, which is another cellular mechanism by which detrimental cytosolic molecules and organelles are targeted to lysosomes for degradation. Early secreted antigen 6 secretion system-1 (ESX-1) of M.tb is known to permeabilize the phagosome to escape degradation (164). However, this facilitates processing by components of ubiquitin-mediated autophagy mechanism and results in a reduction in M.tb persistence (165). Furthermore, stimulation of autophagy sequesters and degrades bacterial components and can also contribute to fostering antigen presentation and moderating pathology (166). The most widely studied autophagy inducer – rapamycin (Sirolimus) is known to inhibit the mammalian target of rapamycin (mTOR) which negatively regulates autophagy. It is majorly employed in organ transplantation owing to the immunosuppressive nature of the drug (167). Researchers have attempted re-purposing of Rapamycin, an autophagy inducer to heighten antigen processing and presentation in murine antigen-presenting cells (APCs) (168). It is well established that confiscation of BCG within phagosome and inability to fuse with lysosome reduces the efficacy of antigenic peptide presentation on DCs. Dendritic cells (DCs) treated with autophagy inducer, rapamycin enhanced Th1 responses against M.tb (116). Improvement in DC activation and Th1 responses against M. with concurrent rapamycin and BCG administration offers prospective approach to autophagy mediated enhancement of bacterial clearance (116). Similarly, the efficacy of BCG can be augmented by simultaneous treatment to direct host responses toward bacterial extermination to achieve complete sterility (116). Few piecemeal studies have questioned the immunotherapeutic strategies to enhance vaccine efficiency against TB, however a great deal is yet to be explored (169). However, side effects associated with rapamycin administration such as interstitial pneumonitis can be alarming in TB patients with substantial pathology (170). Furthermore, metabolization of rapamycin by hepatic enzyme CYP3A4 limits its utility in TB patients since CYP3A4 is intensely stimulated by standard ATT antibiotic – INH (171). Due to the mentioned limitations rapamycin has not been further evaluated as HDT against M.tb. Vadimezan (also known as DMXAA) is another prospective autophagy inducer. It is an established antitumor agent as well as in mice it triggers, a stimulator of IFN genes (STING) dependent autophagy mechanism (172). However, it was found inefficacious in humans (173). Research groups have further examined the utility of fusion peptides for the induction of autophagy. Tat-beclin-1 fusion peptide an autophagy inducer (174) was found to restrict the proliferation of diverse pathogenic strains and heightened survival rates in infected mice (117). Though, restrictions like regular administration by injection constrain the clinical utility of HDT. Alternatively, an inhibitor of epidermal growth factor receptor (EGFR) was found to limit M.tb proliferation in macrophages and reduces bacterial burden in the lungs of infected mice via autophagy induction (175). In M.tb infected macrophages treated with Gefitinib; tyrosine kinase inhibitor, lysosomal biogenesis, function and targeting of bacteria to lysosome for degradation is increased thereby decreasing bacterial burden via EGFR signaling in macrophages (118). However, there is a need to further analyse pieces of evidence and peripheral markers of autophagy for certainty. With the advancement in technologies, sophisticated approaches to evaluate the induction of autophagy can evolve the quest for superior autophagy inducers that can be employed to enhance bacterial killing by limiting dissemination.
Targeting Host Metabolism
Research focus has shifted radically in the past decade towards metabolic shifts in response to infections. Immunometabolism is an emerging field that focuses on the impact of the metabolic state of immune cell populations to provide better insight into disease progression and pathogenesis (176). In the initial course of M.tb infection, metabolic shift is observed to defend the host. Immune protective responses such as stimulation of pro-inflammatory cytokines, and nitric oxide (NO) release is directed via HIF-1-dependent glycolytic pathways (177). However, M.tb is known to stimulate the Warburg effect so as to inhibit anti-microbial host immune responses (178). Host metabolism is utilized by M.tb to survive and proliferate by escaping host protective immune mechanisms (179). This infers that metabolic reprogramming is vital for defense against M.tb so as to augment efficacious sterilization mechanisms. 2-deoxyglucose (2-DG) an inhibitor of hexokinase enzyme, can limit the IL1-β generation in LPS-activated macrophages and result in succinate accumulation (180). 2-DG stimulated glycolysis inhibition can additionally result in a reduction in lung damage induced by LPS (181) via moderating nuclear PKM2-STAT3 signaling. Further, prospects of 2-DG to restrict pathological damage in TB cases can be assessed for augmented clinical outcomes. Similarly, ritonavir (Norvir), a protease inhibitor widely used as antiretroviral medication to treat HIV infections (120), is additionally known for capability to act as an glucose transporter agonist (182). Researchers have evaluated combinations of HIV drugs including ritonavir along with ATT so as to effectively counter HIV-TB coinfections (183). Strategic arrangement by utilizing characteristics of ritonavir to inhibit host glucose transporters can be assessed further in case of HIV-TB patients to better understand mechanism of protection. Inhibitor of pyruvate dehydrogenase kinase- dichloroacetate, is a small molecule that increases pyruvate flux into mitochondria and skews metabolism toward glucose oxidation rather than glycolysis (184). Inhibition of pyruvate dehydrogenase kinase was established as a host target to counter infection of Salmonella enterica serovar typhimurium via metabolic reprogramming of M1 macrophages. However, intracellular burden for M.tb was not reduced upon dichloroacetate treatment, alternate inhibitors can be explored with similar objective (185). Another small molecule and lactate dehydrogenase inhibitor – FX11 (3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid]) is known for induction of oxidative stress and reduction in tumour advancement (121). Downregulation of iNOS and cytokine generation was achieved in LPS-activated RAW 264.7 macrophages upon FX11 induced lactate dehydrogenase inhibition (186). Similarly, the inhibitor of pyruvate kinase– TEPP46 significantly reduced PKM2 activation in LPS-induced macrophages which led to a lowering of IL-1β generation (122). Hence, small molecule inhibitors can be employed to direct metabolic flux for desired clinical outcomes to resolve immunopathology of TB by regulating host metabolism.
M.tb manipulates lipid and fatty acid metabolic pathways of the host for its persistence and proliferation (187). Foamy macrophages recruited around M.tb infected phagocytes, supply nutrition and support in the course of infection. M.tb manipulates host cells to synthesize lipids and fatty acids. Hence, components of lipid synthesis pathways manipulated by M.tb for survival can be targeted as HDT (188). Metabolic energy sensors such as AMP-activated protein kinase (AMPK) play a vital role in the regulation of key host protective mechanisms against infections (189). An approved type 2 diabetes drug, Metformin which activates the AMPK-mediated signaling mechanism has been evaluated for TB (190). Metformin induces the generation of mitochondrial reactive oxygen species (ROS) resulting in restriction in intracellular growth of M.tb and limiting the activation of the inflammatory gene (191). In M.tb infected guinea pigs, metformin acts synergistically with conventional ATT drugs – INH and ETH (192). Metformin administration causes a significant reduction in latent TB incidences in prone diabetic individuals (193). This HDT can be evaluated proficiently at advanced clinical stages. Another AMPK activator, 5-aminoimidazole-4-carboxamide-1-1-β-D-ribofuranoside (AICAR) stimulates anti-microbial responses by activating autophagic pathways in macrophages. AMPK activation by AICAR further controls the biogenesis of mitochondria and metabolic state in macrophages by inducing peroxisome proliferator-activated receptor gamma coactivator-1 (PPARGC1) associated pathways (124). Components of host machinery that curb the metabolism of lipids can reduce detrimental inflammation thereby establishing a balanced immune state. Fatty acid synthase inhibitors such as C75 and cerulenin are prospective targets for the augmentation of efficacious immune responses. Inhibition of lipid-derived droplets by C75 can lead to polarization of macrophages from the M1 to M2 subset causing enhancement of ROS and NO production (125). C75 and cerulenin-mediated inhibition of fatty acid synthase lead to uncoupling protein-2 (UCP2) mediated NLRP3 inflammasome activation (126). PPARγ antagonist, GW9662 is known to regulate vital processes such as metabolism, inflammation, and disease progression (127) in macrophages infected with M. bovis BCG (76). Link between inflammation and lipid metabolism associated PPARγ signaling can be exploited to potentiate BCG-induced protection (76). This infers that reprogramming key components of lipid metabolism can be a prospective target for progressive TB therapeutics. Sirtuins (SIRTs) are another prospective target with the potential to be targeted for the augmentation of host defences (77). SIRTs are deacetylases that regulate cellular mechanisms like inflammatory responses, regulation of lipid metabolism by modulating components of NF-κB immune signaling, and anti-inflammatory responses by regulation of Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (78). It has been observed that SIRT-1 expression is diminished drastically in M.tb infected THP-1 cells. SIRT-1 downregulates RelA/p65 unit of NF-κB so as to modulate inflammation (79). SIRT-6 is also known to diminish pro-inflammatory and anti-microbial responses in the early course of M.tb infection (80). Furthermore, SIRT2 has been established as an immunotherapeutic target against M.tb infection in mice model of TB (128). It was observed that subsequent to M.tb infection, SIRT2 expression increases along with translocation to nucleus to induce immune dampening epigenetic modifications. However, chemical inhibition of SIRT2 using established inhibitor, AGK2 dramatically augmented bacterial clearance and host protective immune responses. Since, existing literature is available regarding SIRT2 induced metabolic programming (81) and signal transduction (82). Further progressive approaches can be shaped by targeting key physiological factors of host.
Targeting microRNAs
miRNAs are non-coding RNAs that are involved at post-transcriptional levels to regulate array of genes decisive of immune responses (83). Over 2000 functional miRNAs are encoded by human genome (84) which regulate diverse protein-coding transcripts (194). It is now well-established that miRNAs distinctly regulate host immune responses against M.tb infection (195). Differential expression of miRNAs can signify the advancement of disease from latent to an active infection (196). Furthermore, miRNAs play a significant role in the moderation of apoptotic and autophagic responses during M.tb infection (196). Owing to advancements in technology, miRNA delivery is being employed to treat diverse diseases. This further pave way for the application of miRNAs as HDT against TB (197). Several studies have assessed mechanisms by which M.tb temper host immune responses for survival in an antimicrobial milieu. Diverse immune mechanisms such as phagolysosome maturation in APCs, cytokine stimulation by immune cell populations, and antigen processing and presentation are dynamically manipulated by M.tb. These cellular processes are strictly regulated by an assortment of miRNAs in the host (198). M.tb is additionally known to alter miRNA expression associated with key biological responses to escape host immune responses (199). Additionally, expectations of combined regulation of transcriptional network by miRNAs and transcription factors represent miRNAs linked with diseases as a novel category of therapeutics.
miRNAs involved in immune pathways are extensively studied for mycobacterial infections (200). It is well-known that during M.tb infection miR-125b inhibits TNF biosynthesis in human alveolar macrophages (201). Several research groups have observed that M.tb infection results in differential expression of miRNAs which determines the fate of immune responses (202). However, comprehensive knowledge is requisite for progressive considerations. Participation of miRNAs in M.tb induced autophagy (203) and apoptosis has provoked further interest to maneuver miRNAs for HDTs. Upon M.tb infection, diverse cell populations respond varyingly in conjunction with variations in miRNA expression. Upregulation of miR-155 has been observed in bone marrow-derived macrophages of mice infected with M.tb (204). In contrast, downregulation of miR-155 has been observed in peripheral blood mononuclear cells (PBMCs) derived macrophages upon M.tb infection (201). Mycobacterial infection in human monocyte-derived macrophages results in overexpression of miR-29a, miR-886-5p, and let-7e, which further target caspase 3 and caspase 7 as predicted by the integrated analysis. Differential expression of miR155, miR-146a, miR145, miR222, miR-27a, and miR-27b was observed in human macrophages infected with virulent M.tb H37Rv and avirulent strain M. bovis BCG. Downregulation of miRNA involved in the regulation of inflammation and lipid metabolism was observed. Furthermore, miR-145 known for induction of apoptosis has been reported to be downregulated upon infection with a virulent M.tb strain, which results in overexpression of targets which inhibit apoptosis (205). Based on microarray analysis, global changes in miRNA expression screened nine miRNA genes which were differentially expressed in M.tb H37Rv and M.tb H37Ra infected THP-1 cells. These differentially expressed miRNAs such as miR-30a, miR-30e, miR-155, miR-1275, miR-3665, miR3178, miR-4484, miR-4668-5p, and miR-4497 contribute in diverse physiological aspects. miR-155 interacts with negative regulators involved in TNF- α generation (201). Evaluation of PBMCs and pleural fluid mononuclear cells (PFMCs) linked miRNA expression with levels of IL-6 cytokine. Additionally, it has been observed that the highly-virulent Beijing/W TB strain represses plenty of miRNA in human macrophages as compared to non-Beijing/W TB strains. Alterations in miRNAs have been observed in patients with active TB. The functional assessment demonstrated that miR-144 restrains T-cell expansion and generation of key cytokines, INF-γ and TNF-α. In RAW264.7 cells, upon BCG inoculation, miRNA-144-3p overexpression is linked with inhibition of autophagy and antimycobacterial activity (206). Elevation in miR-424 and miR-365 levels has been detected in active TB patients. Diverse miRNA contributes to determining the fate of infection. Regulation of immune cell activation by miR-155, miR-146a, miR-21, and miR-9 (207), TLR signaling is positively regulated by miR155 (208). Subsequent to M. bovis BCG infection significant increase in miR-155 expression is observed in macrophages, which modulates diverse innate immune responses including ROS generation (209). It additionally plays role in apoptosis induction in macrophages upon M. bovis BCG inoculation which modulates cellular physiology and immune responses (210). In M.tb infected human alveolar macrophages, miR-125b inhibits TNF generation (201). miR-29 targets IFN-γ and regulate immune responses linked to M.tb infection (211). miR-223 targets several chemo-attractants such as CXCL2, CCL3 and contributes to directing immune response (212). It has been evaluated that endogenous block of miR-29 in transgenic mice, augmented resistance to M.tb infection (211). miR-27a targets IRAK4 and restrict immune response in TB (213). Another study demonstrated that BCG infection in RAW264.7 cells upregulates miR-17-5p which was linked with augmented BCG dissemination and enhanced autophagosome related protein expression (214). As revealed diverse miRNAs are under evaluation owing to gene modulatory potentials. However, our knowledge regarding role of specific miRNA overexpression upon BCG inoculation is still fragmentary. Utilization of miRNAs to augment host immune responses, paves way for advancement in therapeutics for various diseased conditions. Differential expression of miRNAs can be moulded in such a way to achieve better clinical outcomes in TB patients. Progressive therapeutics are employing miRNA-mimics (215), antisense oligonucleotides (216) to manipulate immune responses. Although several research groups have evaluated the utility of miRNA manipulation as HDT against M.tb, further assessment is necessitated to establish the prominence of miRNA as therapeutic. We required more studies to comprehend the contribution of miRNA in host-pathogen interactions and a progressive strategy for augmenting conventional therapies.
Conclusions and Future Perspective
Despite incessant debates on the variable protective efficiency of BCG, it prevails as the only vaccine for TB prevention. Owing to considerable protection in children against disseminated forms of TB, it remains a key component of TB control programs in various countries (90). Throughout our review, we have mentioned several shortcomings and lacunae linked with the failure of the TB vaccination strategy. One of which is the complexity of the disease itself, as we are envisaging resolutions that can impart complete sterility, which is seldomly accomplished in the natural course of infection (26). In this aspect, TB diverges from the diseases that are preventable via vaccination. Hence, progressive approaches were pursued with the utilization of known immune correlates of protection. To surpass the previously failed attempts, it is vital to redirect focus on immunological pathways that can be augmented for better clinical implications (217). Since adult pulmonary TB mainly accounts for M.tb transmission, advances to tackle the inadequacies of BCG to impart long-lasting immunological memory should be highlighted as a better immunization stratagem. With extraordinary scientific efforts to counteract the most fatal pathogens known to humankind, we have progressed to a situation wherein we can harness establish knowledge and immunological concepts to deal with the shortcomings of existing approaches and improvise for robust clinical trajectories. Since the most of individuals worldwide are already vaccinated with BCG, it is judicious to keep BCG in reflection while developing new vaccination strategies. Scientific communities are attempting stratagems to prevent millions of deaths from escalating infectious diseases by investing on immunotherapeutic approaches to augment immunological responses.
Author Contributions
KN, VD wrote the manuscript. AB edited the manuscript. VD conceived the hypothesis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. World Health Organization . Global Tuberculosis Report 2021. Geneva: World Health Organization; (2021). Available at: https://apps.who.int/iris/handle/10665/346387. [Google Scholar]
- 2. Sutherland I, Svandová E, Radhakrishna S. The Development of Clinical Tuberculosis Following Infection With Tubercle Bacilli. 1. A Theoretical Model for the Development of Clinical Tuberculosis Following Infection, Linking From Data on the Risk of Tuberculous Infection and the Incidence of Clinical Tuberculosis in the Netherlands. Tubercle (1982) 63(4):255–68. doi: 10.1016/S0041-3879(82)80013-5 [DOI] [PubMed] [Google Scholar]
- 3. Onozaki I, Raviglione M. Stopping Tuberculosis in the 21st Century: Goals and Strategies. Respirology (2010) 15(1):32–43. doi: 10.1111/j.1440-1843.2009.01673.x [DOI] [PubMed] [Google Scholar]
- 4. Elsevier_Vaccine_Immunology. Available at: https://www.vacunashnrg.com.ar/archivosInteres/Elsevier_Vaccine_immunology.pdf.
- 5. Holladay AJ, Poole JCF. Thucydides and the Plague of Athens. Classical Quarterly (1979) 29(2):282–300. doi: 10.1017/S0009838800035928 [DOI] [PubMed] [Google Scholar]
- 6. Riedel S. Edward Jenner and the History of Smallpox and Vaccination. Proc (Bayl Univ Med Cent) (2005) 18(1):21–5. doi: 10.1080/08998280.2005.11928028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netea MG, Quintin J, van der Meer JWM. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe (2011) 9(5):355–61. doi: 10.1016/j.chom.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 8. Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe (2019) 25(1):13–26. doi: 10.1016/j.chom.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 9. BCG . Available at: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/bcg.
- 10. Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. LTBI: Latent Tuberculosis Infection or Lasting Immune Responses to M. tuberculosis? A TBNET Consensus Statement. Eur Respir J (2009) 33(5):956–73. doi: 10.1183/09031936.00120908 [DOI] [PubMed] [Google Scholar]
- 11. Ottenhoff THM, Kaufmann SHE. Vaccines Against Tuberculosis: Where Are We and Where Do We Need to Go? PloS Pathog (2012) 8(5):e1002607. doi: 10.1371/journal.ppat.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moliva JI, Turner J, Torrelles JB. Immune Responses to Bacillus Calmette–Guérin Vaccination: Why Do They Fail to Protect Against Mycobacterium Tuberculosis? Front Immunol 8:407. doi: 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatherill M, White RG, Hawn TR. Clinical Development of New TB Vaccines: Recent Advances and Next Steps. Front Microbiol (2020) 10:3154. doi: 10.3389/fmicb.2019.03154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song WM, Zhao J, Zhang QY, Liu S, Zhu XH, An Q, et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front Med (2021) 8:657006. doi: 10.3389/fmed.2021.657006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO Information Note . COVID-19 Considerations for Tuberculosis (TB) Care . Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-TB-care-2021.1.
- 16. Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG Vaccine for COVID-19: First Verdict and Future Directions. Front Immunol (2021) 12:632478. doi: 10.3389/fimmu.2021.632478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Global Tuberculosis Programme. Available at: https://www.who.int/teams/global-tuberculosis-programme/covid-19.
- 18. Does the Efficacy of BCG Decline With Time Since Vaccination? Available at: https://www.ingentaconnect.com/content/iuatld/ijtld/1998/00000002/00000003/art00005. [PubMed]
- 19. BCG: To Face an Ancient Enemy. Available at: https://www.nature.com/articles/d42859-020-00010-x.
- 20. History-Of-BCG-Vaccine. Available at: https://association-camille-guerin.com/chabonnes/wp-content/uploads/History-of-BCG-Vaccine.pdf.
- 21. Kaufmann SH. Vaccine Development Against Tuberculosis Over the Last 140 Years: Failure as Part of Success. Front Microbiol 12:750124. doi: 10.3389/fmicb.2021.750124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Genome Plasticity of BCG and Impact on Vaccine Efficacy. PNAS. doi: 10.1073/pnas.0700869104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N. Influence of BCG Vaccine Strain on the Immune Response and Protection Against Tuberculosis. FEMS Microbiol Rev (2008) 32(5):821–41. doi: 10.1111/j.1574-6976.2008.00118.x [DOI] [PubMed] [Google Scholar]
- 24. Kaufmann SH. Vaccination Against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Immunology. doi: 10.3389/fimmu.2020.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Issues Relating to the Use of BCG in Immunization Programmes: A Discussion Document. Available at: https://apps.who.int/iris/handle/10665/66120.
- 26. Flynn JL, Chan J. Immunology of Tuberculosis. Annu Rev Immunol (2001) 19:93–129. doi: 10.1146/annurev.immunol.19.1.93 [DOI] [PubMed] [Google Scholar]
- 27. De Martino M, Lodi L, Galli L, Chiappini E. Immune Response to Mycobacterium Tuberculosis: A Narrative Review. Front Pediatr 7:350. doi: 10.3389/fped.2019.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuberculosis Vaccine Development: Progress in Clinical Evaluation. Clin Microbiol Rev. doi: 10.1128/CMR.00100-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamm DL, Morales A. A BCG Success Story: From Prevention of Tuberculosis to Optimal Bladder Cancer Treatment. Vaccine (2021) 39(50):7308–18. doi: 10.1016/j.vaccine.2021.08.026 [DOI] [PubMed] [Google Scholar]
- 30. El-Zein M, Parent ME, Benedetti A, Rousseau MC. Does BCG Vaccination Protect Against the Development of Childhood Asthma? A Systematic Review and Meta-Analysis of Epidemiological Studies. Int J Epidemiol (2010) 39(2):469–86. doi: 10.1093/ije/dyp307 [DOI] [PubMed] [Google Scholar]
- 31. Pereira LIA, Dorta ML, Pereira AJCS, Bastos RP, Oliveira MAP, Pinto SA, et al. Increase of NK Cells and Proinflammatory Monocytes are Associated With the Clinical Improvement of Diffuse Cutaneous Leishmaniasis After Immunochemotherapy With BCG/Leishmania Antigens. Am J Trop Med Hyg (2009) 81(3):378–83. doi: 10.4269/ajtmh.2009.81.378 [DOI] [PubMed] [Google Scholar]
- 32. Rao AG, Haqqani R. Study of BCG Immunotherapy in the Management of Multiple, Extensive Non-Genital Cutaneous Common Warts. Indian Dermatol Online J (2020) 11(5):784–8. doi: 10.4103/idoj.IDOJ_461_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ON IMMUNOLOGICAL MEMORY. Annu Rev Immunol. doi: 10.1146/annurev.immunol.14.1.333 [DOI] [Google Scholar]
- 34. Chai Q, Wang L, Liu CH, Ge B. New Insights Into the Evasion of Host Innate Immunity by Mycobacterium Tuberculosis. Cell Mol Immunol (2020) 17(9):901–13. doi: 10.1038/s41423-020-0502-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu CH, Liu H, Ge B. Innate Immunity in Tuberculosis: Host Defense vs Pathogen Evasion. Cell Mol Immunol (2017) 14(12):963–75. doi: 10.1038/cmi.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bickett TE, McLean J, Creissen E, Izzo L, Hagan C, Izzo AJ, et al. Characterizing the BCG Induced Macrophage and Neutrophil Mechanisms for Defense Against Mycobacterium Tuberculosis. Front Immunol (2020) 11:1202. doi: 10.3389/fimmu.2020.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJM, Tanguy M, et al. Neutrophils Rapidly Migrate via Lymphatics After Mycobacterium Bovis BCG Intradermal Vaccination and Shuttle Live Bacilli to the Draining Lymph Nodes. Blood (2005) 106(5):1843–50. doi: 10.1182/blood-2005-03-1281 [DOI] [PubMed] [Google Scholar]
- 38. Khader SA, Divangahi M, Hanekom W, Hill PC, Maeurer M, Makar KW, et al. Targeting Innate Immunity for Tuberculosis Vaccination. J Clin Invest (2019) 129(9):3482–91. doi: 10.1172/JCI128877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sendide K, Reiner NE, Lee JSI, Bourgoin S, Talal A, Hmama Z. Cross-Talk Between CD14 and Complement Receptor 3 Promotes Phagocytosis of Mycobacteria: Regulation by Phosphatidylinositol 3-Kinase and Cytohesin-1. J Immunol (2005) 174(7):4210–9. doi: 10.4049/jimmunol.174.7.4210 [DOI] [PubMed] [Google Scholar]
- 40. Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, et al. TLR2 and TLR4 Serve Distinct Roles in the Host Immune Response Against Mycobacterium Bovis BCG. J Leukoc Biol (2003) 74(2):277–86. doi: 10.1189/jlb.0103026 [DOI] [PubMed] [Google Scholar]
- 41. The Mannose Receptor is Expressed by Subsets of APC in non-Lymphoid Organs. BMC Immunol. doi: 10.1186/1471-2172-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Human and Mouse Macrophage-Inducible C-Type Lectin (Mincle) Bind Candida Albicans. [DOI] [PubMed]
- 43. Moliva JI, Turner J, Torrelles JB. Immune Responses to Bacillus Calmette–Guérin Vaccination: Why Do They Fail to Protect Against Mycobacterium Tuberculosis? Front Immunol (2017) 8:407. doi: 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mycobacteria Use Their Surface-Exposed Glycolipids to Infect Human Macrophages Through a Receptor-Dependent Process. [DOI] [PubMed]
- 45. Sugisaki K, Dannenberg AM, Abe Y, Tsuruta J, Su WJ, Said W, et al. Nonspecific and Immune-Specific Up-Regulation of Cytokines in Rabbit Dermal Tuberculous (BCG) Lesions. J Leukoc Biol (1998) 63(4):440–50. doi: 10.1002/jlb.63.4.440 [DOI] [PubMed] [Google Scholar]
- 46. Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman JC, et al. Mycobacterium Bovis BCG-Infected Neutrophils and Dendritic Cells Cooperate to Induce Specific T Cell Responses in Humans and Mice. Eur J Immunol (2008) 38(2):437–47. doi: 10.1002/eji.200737905 [DOI] [PubMed] [Google Scholar]
- 47. Jeevan A, Majorov K, Sawant K, Cho H, McMurray DN. Lung Macrophages From Bacille Calmette-Guérin-Vaccinated Guinea Pigs Suppress T Cell Proliferation But Restrict Intracellular Growth of M. Tuberculosis After Recombinant Guinea Pig Interferon-Gamma Activation. Clin Exp Immunol (2007) 149(2):387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ly LH, Barhoumi R, Cho SH, Franzblau SG, McMurray DN. Vaccination With Bacille-Calmette Guérin Promotes Mycobacterial Control in Guinea Pig Macrophages Infected In Vivo . J Infect Dis (2008) 198(5):768–71. doi: 10.1086/590436 [DOI] [PubMed] [Google Scholar]
- 49. Kapsenberg ML. Dendritic-Cell Control of Pathogen-Driven T-Cell Polarization. Nat Rev Immunol (2003) 3(12):984–93. doi: 10.1038/nri1246 [DOI] [PubMed] [Google Scholar]
- 50. Thurnher M, Ramoner R, Gastl G, Radmayr C, Böck G, Herold M, et al. Bacillus Calmette-Guérin Mycobacteria Stimulate Human Blood Dendritic Cells. Int J Cancer (1997) 70(1):128–34. doi: [DOI] [PubMed] [Google Scholar]
- 51. Ferreira AV, Domiguéz-Andrés J, Netea MG. The Role of Cell Metabolism in Innate Immune Memory. J Innate Immun (2022) 14(1):42–50. doi: 10.1159/000512280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Heijden CDCC, Noz MP, Joosten LAB, Netea MG, Riksen NP, Keating ST. Epigenetics and Trained Immunity. Antioxid Redox Signal (2018) 29(11):1023–40. doi: 10.1089/ars.2017.7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity Against Tuberculosis. Cell (2018) 172(1–2):176–190.e19. doi: 10.1016/j.cell.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 54. Michel T, Hentges F, Zimmer J. Consequences of the Crosstalk Between Monocytes/Macrophages and Natural Killer Cells. Front Immunol (2012) 3:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moorlag SJCFM, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep (2020) 33(7):108387. doi: 10.1016/j.celrep.2020.108387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG Vaccine Protection From Severe Coronavirus Disease 2019 (COVID-19). PNAS (2020) 117(30):17720–6. doi: 10.1073/pnas.2008410117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trained Immunity Confers Broad-Spectrum Protection Against Bacterial Infections . The Journal of Infectious Diseases. Oxford Academic; [Internet]. Available from: https://academic.oup.com/jid/article/222/11/1869/5691195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG Vaccination Protects Against Experimental Viral Infection in Humans Through the Induction of Cytokines Associated With Trained Immunity. Cell Host Microbe (2018) 23(1):89–100. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 59. Adesanya OA, Uche-Orji CI, Adedeji YA, Joshua JI, Adesola AA, Chukwudike CJ. Bacillus Calmette-Guerin (BCG): The Adroit Vaccine. AIMS Microbiol (2021) 7(1):96–113. doi: 10.3934/microbiol.2021007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An Ecological Study to Evaluate the Association of Bacillus Calmette-Guerin (BCG) Vaccination on Cases of SARS-CoV2 Infection and Mortality From COVID-19. PloS One (2020) 15(12):e0243707. doi: 10.1371/journal.pone.0243707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Neill LAJ, Netea MG. BCG-Induced Trained Immunity: Can it Offer Protection Against COVID-19? Nat Rev Immunol (2020) 20(6):335–7. doi: 10.1038/s41577-020-0337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nuovo G, Tili E, Suster D, Matys E, Hupp L, Magro C. Strong Homology Between SARS-CoV-2 Envelope Protein and a Mycobacterium Sp. Antigen Allows Rapid Diagnosis of Mycobacterial Infections and may Provide Specific Anti-SARS-CoV-2 Immunity via the BCG Vaccine. Ann Diagn Pathol (2020) 48:151600. doi: 10.1016/j.anndiagpath.2020.151600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium Tuberculosis. Front Immunol (2014). doi: 10.3389/fimmu.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, et al. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front Immunol (2019) 10:2806. doi: 10.3389/fimmu.2019.02806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, et al. Newborns Develop a Th1-Type Immune Response to Mycobacterium Bovis Bacillus Calmette-Guérin Vaccination. J Immunol (1999) 163(4):2249–55. [PubMed] [Google Scholar]
- 66. Moliva JI, Turner J, Torrelles JB. Immune Responses to Bacillus Calmette-Guérin Vaccination: Why Do They Fail to Protect Against Mycobacterium Tuberculosis? Front Immunol (2017) 8:407. doi: 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, et al. Bacillus Calmette Guerin Vaccination of Human Newborns Induces a Specific, Functional CD8+ T Cell Response. J Immunol (2006) 177(8):5647–51. doi: 10.4049/jimmunol.177.8.5647 [DOI] [PubMed] [Google Scholar]
- 68. Lewinsohn DA, Lewinsohn DM, Scriba TJ. Polyfunctional CD4+ T Cells As Targets for Tuberculosis Vaccination. Front Immunol (2017) 8:1262. doi: 10.3389/fimmu.2017.01262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beveridge NER, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, et al. Immunisation With BCG and Recombinant MVA85A Induces Long-Lasting, Polyfunctional Mycobacterium Tuberculosis-Specific CD4+ Memory T Lymphocyte Populations. Eur J Immunol (2007) 37(11):3089–100. doi: 10.1002/eji.200737504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and Efficacy of MVA85A, a New Tuberculosis Vaccine, in Infants Previously Vaccinated With BCG: A Randomised, Placebo-Controlled Phase 2b Trial. Lancet (2013) 381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lin PL, Flynn JL. CD8 T Cells and Mycobacterium Tuberculosis Infection. Semin Immunopathol (2015) 37(3):239–49. doi: 10.1007/s00281-015-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major Histocompatibility Complex Class I-Restricted T Cells are Required for Resistance to Mycobacterium Tuberculosis Infection. Proc Natl Acad Sci USA (1992) 89(24):12013–7. doi: 10.1073/pnas.89.24.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the Establishment of Protective Pulmonary CD4+ T Cell Responses After Vaccination and During Mycobacterium Tuberculosis Challenge. Nat Immunol (2007) 8(4):369–77. doi: 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- 74. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, et al. Prevention of Tuberculosis in Macaques After Intravenous BCG Immunization. Nature (2020) 577(7788):95–102. doi: 10.1038/s41586-019-1817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jaron B, Maranghi E, Leclerc C, Majlessi L. Effect of Attenuation of Treg During BCG Immunization on Anti-Mycobacterial Th1 Responses and Protection Against Mycobacterium Tuberculosis. PloS One (2008) 3(7):e2833. doi: 10.1371/journal.pone.0002833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Almeida P, Silva A, maya-monteiro C, Törőcsik D, D’Avila H, Dezso B, et al. Mycobacterium Bovis Bacillus Calmette-Guerin Infection Induces TLR2-Dependent Peroxisome Proliferator-Activated Receptor Expression and Activation: Functions in Inflammation, Lipid Metabolism, and Pathogenesis. J Immunol (Baltimore Md : 1950) (2009) 183:1337–45. doi: 10.4049/jimmunol.0900365 [DOI] [PubMed] [Google Scholar]
- 77. Jayashankar L, Hafner R. Adjunct Strategies for Tuberculosis Vaccines: Modulating Key Immune Cell Regulatory Mechanisms to Potentiate Vaccination. Cell Mol Immunol (2020) 17(9):901–13 doi: 10.3389/fimmu.2016.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Transcriptional Targets of Sirtuins in the Coordination of Mammalian Physiology - ScienceDirect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng CY, Gutierrez NM, Marzuki MB, Lu X, Foreman TW, Paleja B, et al. Host Sirtuin 1 Regulates Mycobacterial Immunopathogenesis and Represents a Therapeutic Target Against Tuberculosis. Sci Immunol (2017) 2(9):eaaj1789. doi: 10.1126/sciimmunol.aaj1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Emerging Therapeutic Potential of SIRT6 Modulators. J Medicinal Chem. doi: 10.1021/acs.jmedchem.1c00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hamaidi I, Zhang L, Kim N, Wang MH, Iclozan C, Fang B, et al. Sirt2 Inhibition Enhances Metabolic Fitness and Effector Functions of Tumor-Reactive T Cells. Cell Metab (2020) 32(3):420–36. doi: 10.1016/j.cmet.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nguyen P, Lee S, Lorang-Leins D, Trepel J, Smart DK. SIRT2 Interacts With β-Catenin to Inhibit Wnt Signaling Output in Response to Radiation-Induced Stress. Mol Cancer Res (2014) 12(9):1244–53. doi: 10.1158/1541-7786.MCR-14-0223-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and Pathological Roles for microRNAs in the Immune System. Nat Rev Immunol (2010) 10(2):111–22. doi: 10.1038/nri2708 [DOI] [PubMed] [Google Scholar]
- 84. Kozomara A, Birgaoanu M, Griffiths-Jones S. Mirbase: From microRNA Sequences to Function. Nucleic Acids Res (2019) 47(D1):D155–62. doi: 10.1093/nar/gky1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luo Y, Jiang W, Da Z, Wang B, Hu L, Zhang Y, et al. Subunit Vaccine Candidate AMM Down-Regulated the Regulatory T Cells and Enhanced the Protective Immunity of BCG on a Suitable Schedule. Scand J Immunol (2012) 75(3):293–300. doi: 10.1111/j.1365-3083.2011.02666.x [DOI] [PubMed] [Google Scholar]
- 86. Quinn KM, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, et al. Accelerating the Secondary Immune Response by Inactivating CD4+CD25+ T Regulatory Cells Prior to BCG Vaccination Does Not Enhance Protection Against Tuberculosis. Eur J Immunol (2008) 38(3):695–705. doi: 10.1002/eji.200737888 [DOI] [PubMed] [Google Scholar]
- 87. Kamat S, Kumari M. BCG Against SARS-CoV-2: Second Youth of an Old Age Vaccine? Front Pharmacol (2020) 11:1050. doi: 10.3389/fphar.2020.01050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Madura Larsen J, Stabell Benn C, Fillie Y, van der Kleij D, Aaby P, Yazdanbakhsh M. BCG Stimulated Dendritic Cells Induce an Interleukin-10 Producing T-Cell Population With No T Helper 1 or T Helper 2 Bias In Vitro . Immunology (2007) 121(2):276–82. doi: 10.1111/j.1365-2567.2007.02575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 Signaling During Bacillus Calmette-Guérin Vaccination Enhances and Sustains Th1, Th17, and Innate Lymphoid IFN-γ and IL-17 Responses and Increases Protection to Mycobacterium Tuberculosis Infection. J Immunol (2012) 189(8):4079–87. doi: 10.4049/jimmunol.1201061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Andersen P, Doherty TM. The Success and Failure of BCG - Implications for a Novel Tuberculosis Vaccine. Nat Rev Microbiol (2005) 3(8):656–62. doi: 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 91. Pang Y, Zhao A, Cohen C, Kang W, Lu J, Wang G, et al. Current Status of New Tuberculosis Vaccine in Children. Hum Vaccin Immunother (2016) 12(4):960–70. doi: 10.1080/21645515.2015.1120393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang W, Zhang Y, Zheng H, Pan Y, Liu H, Du P, et al. Genome Sequencing and Analysis of BCG Vaccine Strains. PloS One (2013) 8(8):e71243. doi: 10.1371/journal.pone.0071243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whittaker E, Nicol MP, Zar HJ, Tena-Coki NG, Kampmann B. Age-Related Waning of Immune Responses to BCG in Healthy Children Supports the Need for a Booster Dose of BCG in TB Endemic Countries. Sci Rep (2018) 8(1):15309. doi: 10.1038/s41598-018-33499-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. Non-Tuberculous Mycobacteria Have Diverse Effects on BCG Efficacy Against Mycobacterium Tuberculosis. Tuberc (Edinb) (2014) 94(3):226–37. doi: 10.1016/j.tube.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Behr MA. BCG–different Strains, Different Vaccines? Lancet Infect Dis (2002) 2(2):86–92. doi: 10.1016/S1473-3099(02)00182-2 [DOI] [PubMed] [Google Scholar]
- 96. WHO-IVB-18.06-Eng.Pdf (2022). Available at: http://apps.who.int/iris/bitstream/handle/10665/273089/WHO-IVB-18.06-eng.pdf?ua=1.
- 97. Elsidig N, Alshahrani D, Alshehri M, Alzahrani M, Alhajjar S, Aljummah S, et al. Bacillus Calmette–Guérin Vaccine Related Lymphadenitis in Children: Management Guidelines Endorsed by the Saudi Pediatric Infectious Diseases Society (SPIDS). Int J Pediatr Adolesc Med (2015) 2(2):89–95. doi: 10.1016/j.ijpam.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Andersen P, Kaufmann SHE. Novel Vaccination Strategies Against Tuberculosis. Cold Spring Harb Perspect Med (2014) 4(6):a018523. doi: 10.1101/cshperspect.a018523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. New TB Vaccine Research (2022). Available at: https://www.who.int/teams/global-tuberculosis-programme/research-innovation/vaccines.
- 100. Kumar S, Bhaskar A, Patnaik G, Sharma C, Singh DK, Kaushik SR, et al. Intranasal Immunization With Peptide-Based Immunogenic Complex Enhances BCG Vaccine Efficacy in a Murine Model of Tuberculosis. JCI Insight (2021) 6(4):145228. doi: 10.1172/jci.insight.145228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. MTBVAC: Attenuating the Human Pathogen of Tuberculosis (TB) Toward a Promising Vaccine Against the TB Epidemic. Immunology. doi: 10.3389/fimmu.2017.01803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jensen K, Ranganathan UDK, Van Rompay KKA, Canfield DR, Khan I, Ravindran R, et al. A Recombinant Attenuated Mycobacterium Tuberculosis Vaccine Strain Is Safe in Immunosuppressed Simian Immunodeficiency Virus-Infected Infant Macaques. Clin Vaccine Immunol (2012) 19(8):1170–81. doi: 10.1128/CVI.00184-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Woodworth JS, Clemmensen HS, Battey H, Dijkman K, Lindenstrøm T, Laureano RS, et al. A Mycobacterium Tuberculosis-Specific Subunit Vaccine That Provides Synergistic Immunity Upon Co-Administration With Bacillus Calmette-Guérin. Nat Commun (2021) 12(1):6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dalmia N, Ramsay AJ. Prime-Boost Approaches to Tuberculosis Vaccine Development. Expert Rev Vaccines (2012) 11(10):1221–33. doi: 10.1586/erv.12.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. Host-Directed Therapies for Bacterial and Viral Infections. Nat Rev Drug Discov (2018) 17(1):35–56. doi: 10.1038/nrd.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The Challenge of New Drug Discovery for Tuberculosis. Nature (2011) 469(7331):483–90. doi: 10.1038/nature09657 [DOI] [PubMed] [Google Scholar]
- 107. Commissioner O of the. FDA Approves New Drug for Treatment-Resistant Forms of Tuberculosis That Affects the Lungs (2022). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-resistant-forms-tuberculosis-affects-lungs.
- 108. Fundació Institut Germans Trias I Pujol. Phase 2b Randomized Double-Blind, Placebo-Controlled Trial to Estimate the Potential Efficacy and Safety of Two Repurposed Drugs, Acetylsalicylic Acid and Ibuprofen, for Use as Adjunct Therapy Added to, and Compared With, the Standard WHO-Recommended TB Regimen (SMA-Tb). Available at: https://clinicaltrials.gov/ct2/show/NCT04575519. [DOI] [PMC free article] [PubMed]
- 109. Muefong CN, Sutherland JS. Neutrophils in Tuberculosis-Associated Inflammation and Lung Pathology. Front Immunol 11:962. doi: 10.3389/fimmu.2020.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Monosodium Urate Crystals Promote Innate Anti-Mycobacterial Immunity and Improve BCG Efficacy as a Vaccine Against Tuberculosis (2022). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4449037/. [DOI] [PMC free article] [PubMed]
- 111. Calcimycin Mediates Mycobacterial Killing by Inducing Intracellular Calcium-Regulated Autophagy in a P2RX7 Dependent Manner (2022). [DOI] [PubMed]
- 112. Role of Calcium Channels in Cellular Antituberculosis Effects: Potential of Voltage-Gated Calcium-Channel Blockers in Tuberculosis Therapy - ScienceDirect. Available at: https://www.sciencedirect.com/science/article/pii/S1684118214002102. [DOI] [PubMed]
- 113. Ahmad S, Bhattacharya D, Gupta N, Rawat V, Tousif S, Van Kaer L, et al. Clofazimine Enhances the Efficacy of BCG Revaccination via Stem Cell-Like Memory T Cells. PloS Pathog (2020) 16(5):e1008356. doi: 10.1371/journal.ppat.1008356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Singh DK, Tousif S, Bhaskar A, Devi A, Negi K, Moitra B, et al. Luteolin as a Potential Host-Directed Immunotherapy Adjunct to Isoniazid Treatment of Tuberculosis. PloS Pathogens (2021) 17(8):e1009805. doi: 10.1371/journal.ppat.1009805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Singh DK, Dwivedi VP, Singh SP, Kumari A, Sharma SK, Ranganathan A, et al. Luteolin-Mediated Kv1.3 K+ Channel Inhibition Augments BCG Vaccine Efficacy Against Tuberculosis by Promoting Central Memory T Cell Responses in Mice. PloS Pathogens (2020) 16(9):e1008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jagannath C, Bakhru P. Rapamycin-Induced Enhancement of Vaccine Efficacy in Mice. Methods Mol Biol (2012) 821:295–303. doi: 10.1007/978-1-61779-430-8_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nikouee A, Kim M, Ding X, Sun Y, Zang QS. Beclin-1–Dependent Autophagy Improves Outcomes of Pneumonia-Induced Sepsis. Front Cell Infect Microbiol (2021). doi: 10.3389/fcimb.2021.706637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sogi KM, Lien KA, Johnson JR, Krogan NJ, Stanley SA. The Tyrosine Kinase Inhibitor Gefitinib Restricts Mycobacterium Tuberculosis Growth Through Increased Lysosomal Biogenesis and Modulation of Cytokine Signaling. ACS Infect Dis (2017) 3(8):564–74. doi: 10.1021/acsinfecdis.7b00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tan SY, Kelkar Y, Hadjipanayis A, Shipstone A, Wynn TA, Hall JP. Metformin and 2-Deoxyglucose Collaboratively Suppress Human CD4+ T Cell Effector Functions and Activation-Induced Metabolic Reprogramming. J Immunol (2020) 205(4):957–67. doi: 10.4049/jimmunol.2000137 [DOI] [PubMed] [Google Scholar]
- 120. Ritonavir - an Overview | ScienceDirect Topics (2022). Available at: https://www.sciencedirect.com/topics/medicine-and-dentistry/ritonavir.
- 121. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of Lactate Dehydrogenase A Induces Oxidative Stress and Inhibits Tumor Progression. Proc Natl Acad Sci U S A (2010) 107(5):2037–42. doi: 10.1073/pnas.0914433107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate Kinase M2 Regulates Hif-1α Activity and IL-1β Induction, and is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab (2015) 21(1):65–80. doi: 10.1016/j.cmet.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yu X, Li L, Xia L, Feng X, Chen F, Cao S, et al. Impact of Metformin on the Risk and Treatment Outcomes of Tuberculosis in Diabetics: A Systematic Review. BMC Infect Dis (2019) 19:859. doi: 10.1186/s12879-019-4548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. AICAR Inhibits Nfκb DNA Binding Independently of AMPK to Attenuate LPS-Triggered Inflammatory Responses in Human Macrophages. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Classical Activation of Macrophages Leads to Lipid Droplet Formation Without De Novo Fatty Acid Synthesis. Immunology. doi: 10.3389/fimmu.2020.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, et al. UCP2-Induced Fatty Acid Synthase Promotes NLRP3 Inflammasome Activation During Sepsis. J Clin Invest (2015) 125(2):665–80. doi: 10.1172/JCI78253 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127. Seargent JM, Yates EA, Gill JH. GW9662, a Potent Antagonist of Pparγ, Inhibits Growth of Breast Tumour Cells and Promotes the Anticancer Effects of the Pparγ Agonist Rosiglitazone, Independently of Pparγ Activation. Br J Pharmacol (2004) 143(8):933–7. doi: 10.1038/sj.bjp.0705973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bhaskar A, Kumar S, Khan MZ, Singh A, Dwivedi VP, Nandicoori VK. Host Sirtuin 2 as an Immunotherapeutic Target Against Tuberculosis. eLife (2020) 9:e55415. doi: 10.7554/eLife.55415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Young C, Walzl G, Du Plessis N. Therapeutic Host-Directed Strategies to Improve Outcome in Tuberculosis. Mucosal Immunol (2020) 13(2):190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined With Tumor Vaccine Effectively Restores T Cell Rejection Function in Tumors. Cancer Res (2013) 73(12):3591–603. doi: 10.1158/0008-5472.CAN-12-4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and Cancer Treatment: Understanding the Risk Should Be Worth the Reward. Clin Cancer Res (2010) 16(5):1384–90. doi: 10.1158/1078-0432.CCR-09-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pettersen FO, Torheim EA, Dahm AEA, Aaberge IS, Lind A, Holm M, et al. An Exploratory Trial of Cyclooxygenase Type 2 Inhibitor in HIV-1 Infection: Downregulated Immune Activation and Improved T Cell-Dependent Vaccine Responses▿. J Virol (2011) 85(13):6557–66. doi: 10.1128/JVI.00073-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Home - ClinicalTrials.Gov (2022). Available at: https://www.clinicaltrials.gov/.
- 134. Lorenzo JP, Csuka ME, Derfus BA, Gotoff RA, McCarthy GM. Concurrent Gout and Mycobacterium Tuberculosis Arthritis. J Rheumatol (1997) 24(1):184–6. [PubMed] [Google Scholar]
- 135. Jhang JJ, Cheng YT, Ho CY, Yen GC. Monosodium Urate Crystals Trigger Nrf2- and Heme Oxygenase-1-Dependent Inflammation in THP-1 Cells. Cell Mol Immunol (2015) 12(4):424–34. doi: 10.1038/cmi.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D Supplements for Prevention of Tuberculosis Infection and Disease. New Engl J Med (2020) 383(4):359–68. doi: 10.1056/NEJMoa1915176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Abdelgawad AA, Saoud HAEA, Mohamed AG, Fathi MA, Mokhtar ER. Evaluation of BCG Vaccine Immunogenicity in Relation to Vitamin D Status in a Group of Egyptian Children. Open J Pediatr (2020) 10(2):320–31. doi: 10.4236/ojped.2020.102033 [DOI] [Google Scholar]
- 138. Lalor MK, Floyd S, Gorak-Stolinska P, Weir RE, Blitz R, Branson K, et al. BCG Vaccination: A Role for Vitamin D? PloS One (2011) 6(1):e16709. doi: 10.1371/journal.pone.0016709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ahmad S, Bhattacharya D, Kar S, Ranganathan A, Van Kaer L, Das G. Curcumin Nanoparticles Enhance Mycobacterium Bovis BCG Vaccine Efficacy by Modulating Host Immune Responses. Infect Immun (2019) 87(11):e00291–19. doi: 10.1128/IAI.00291-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the Biology of Mycobacterium Tuberculosis From the Complete Genome Sequence. Nature (1998) 393(6685):537–44. doi: 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 141. Guzman JD, Evangelopoulos D, Gupta A, Birchall K, Mwaigwisya S, Saxty B, et al. Antitubercular Specific Activity of Ibuprofen and the Other 2-Arylpropanoic Acids Using the HT-SPOTi Whole-Cell Phenotypic Assay. BMJ Open (2013) 3(6):e002672. doi: 10.1136/bmjopen-2013-002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Byrne ST, Denkin SM, Zhang Y. Aspirin and Ibuprofen Enhance Pyrazinamide Treatment of Murine Tuberculosis. J Antimicrob Chemother (2007) 59(2):313–6. [DOI] [PubMed] [Google Scholar]
- 143. Mitini-Nkhoma SC, Chimbayo ET, Mzinza DT, Mhango DV, Chirambo AP, Mandalasi C, et al. Something Old, Something New: Ion Channel Blockers as Potential Anti-Tuberculosis Agents. Front Immunol (2021). doi: 10.3389/fimmu.2021.665785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ion Channels in Innate and Adaptive Immunity. [DOI] [PMC free article] [PubMed]
- 145. Baker EH. Ion Channels and the Control of Blood Pressure. Br J Clin Pharmacol (2000) 49(3):185–98. doi: 10.1046/j.1365-2125.2000.00159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Inhibition of Ca2+ Signaling by Mycobacterium tuberculosisIs Associated With Reduced Phagosome–Lysosome Fusion and Increased Survival Within Human Macrophages(2022). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2195750/. [DOI] [PMC free article] [PubMed]
- 147. Kondratskyi A, Kondratska K, Skryma R, Klionsky DJ, Prevarskaya N. Ion Channels in the Regulation of Autophagy. Autophagy (2017) 14(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mawatwal S, Behura A, Ghosh A, Kidwai S, Mishra A, Deep A, et al. Calcimycin Mediates Mycobacterial Killing by Inducing Intracellular Calcium-Regulated Autophagy in a P2RX7 Dependent Manner. Biochim Biophys Acta Gen Subj (2017) 1861(12):3190–200. doi: 10.1016/j.bbagen.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 149. Lee CC, Lee MTG, Hsu WT, Park JY, Porta L, Liu MA, et al. Use of Calcium Channel Blockers and Risk of Active Tuberculosis Disease: A Population-Based Analysis. Hypertension (2021) 77(2):328–37. doi: 10.1161/HYPERTENSIONAHA.120.15534 [DOI] [PubMed] [Google Scholar]
- 150. Vohra J. Verapamil in Cardiac Arrhythmias: An Overview. Clin Exp Pharmacol Physiol Suppl (1982) 6:129–34. [PubMed] [Google Scholar]
- 151. Fahie S, Cassagnol M. Verapamil. In: StatPearls. Treasure Island (FL: StatPearls Publishing; (2022). Available at: http://www.ncbi.nlm.nih.gov/books/NBK538495/. [PubMed] [Google Scholar]
- 152. de Souza JVP, Murase LS, Caleffi-Ferracioli KR, Palomo CT, de Lima Scodro RB, Siqueira VLD, et al. Isoniazid and Verapamil Modulatory Activity and Efflux Pump Gene Expression in Mycobacterium Tuberculosis. Int J Tuberc Lung Dis (2020) 24(6):591–6. doi: 10.5588/ijtld.19.0458 [DOI] [PubMed] [Google Scholar]
- 153. Demitto F de O, Amaral RCR do, Maltempe FG, Siqueira VLD, Scodro RB de L, Lopes MA, et al. In Vitro Activity of Rifampicin and Verapamil Combination in Multidrug-Resistant Mycobacterium Tuberculosis. PloS One (2015) 10(2):e0116545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen C, Gardete S, Jansen RS, Shetty A, Dick T, Rhee KY, et al. Verapamil Targets Membrane Energetics in Mycobacterium Tuberculosis. Antimicrob Agents Chemother (2018) 62(5):e02107–17. doi: 10.1128/AAC.02107-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gupta S, Tyagi S, Bishai WR. Verapamil Increases the Bactericidal Activity of Bedaquiline Against Mycobacterium Tuberculosis in a Mouse Model. Antimicrob Agents Chemother (2015) 59(1):673–6. doi: 10.1128/AAC.04019-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Parumasivam T, Chan JGY, Pang A, Quan DH, Triccas JA, Britton WJ, et al. In Vitro Evaluation of Inhalable Verapamil-Rifapentine Particles for Tuberculosis Therapy. Mol Pharmaceutics (2016) 13(3):979–89. doi: 10.1021/acs.molpharmaceut.5b00833 [DOI] [PubMed] [Google Scholar]
- 157. 9789290226383-Eng.Pdf. Available at: https://apps.who.int/iris/bitstream/handle/10665/274127/9789290226383-eng.pdf.
- 158. Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. Systematic Review of Clofazimine for the Treatment of Drug-Resistant Tuberculosis. Int J Tuberc Lung Dis (2013) 17(8):1001–7. doi: 10.5588/ijtld.12.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Conradie F. An Open Label, Randomized Controlled Trial to Establish the Efficacy and Safety of a Study Strategy Consisting of 6 Months of Bedaquiline (BDQ), Delamanid (DLM), and Linezolid (LNZ), With Levofloxacin (LVX) and Clofazimine (CFZ) Compared to the Current South African Standard of Care (Control Strategy) for 9 Months for the Treatment of Rifampicin Resistant Tuberculosis (RR-Tb) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04062201.
- 160. endTB Clinical Trials . Available at: http://www.endtb.org/clinical-trial.
- 161. Medecins Sans Frontieres, Netherlands . A Randomised, Controlled, Open-Label, Phase II-III Trial to Evaluate the Safety and Efficacy of Regimens Containing Bedaquiline and Pretomanid for the Treatment of Adult Patients With Pulmonary Multidrug Resistant Tuberculosis (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT02589782. [DOI] [PMC free article] [PubMed]
- 162. National Institute of Allergy and Infectious Diseases (NIAID) . A Phase IIc Trial of Clofazimine- and Rifapentine-Containing Treatment Shortening Regimens in Drug-Susceptible Tuberculosis: The CLO-FAST Study. Available at: https://clinicaltrials.gov/ct2/show/NCT04311502.
- 163. Ren YR, Pan F, Parvez S, Fleig A, Chong CR, Xu J, et al. Clofazimine Inhibits Human Kv1.3 Potassium Channel by Perturbing Calcium Oscillation in T Lymphocytes. PloS One (2008) 3(12):e4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mycobacterial ESX-1 Secretion System Mediates Host Cell Lysis Through Bacterium Contact-Dependent Gross Membrane Disruptions. PNAS. doi: 10.1073/pnas.1620133114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. ESX-1 Dependent Impairment of Autophagic Flux by Mycobacterium Tuberculosis in Human Dendritic Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Levine B, Mizushima N, Virgin HW. Autophagy in Immunity and Inflammation. Nature (2011) 469(7330):323–35. doi: 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Li J, Kim SG, Blenis J. Rapamycin: One Drug, Many Effects. Cell Metab (2014) 19(3):373–9. doi: 10.1016/j.cmet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Eissa NT. Autophagy Enhances the Efficacy of BCG Vaccine by Increasing Peptide Presentation in Mouse Dendritic Cells. Nat Med (2009) 15(3):267–76. doi: 10.1038/nm.1928 [DOI] [PubMed] [Google Scholar]
- 169. Schaible UE, Linnemann L, Redinger N, Patin EC, Dallenga T. Strategies to Improve Vaccine Efficacy Against Tuberculosis by Targeting Innate Immunity. Front Immunol (2017). doi: 10.3389/fimmu.2017.01755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Alkhunaizi AM, Al-Khouzaie TH, Alsagheir AI. Sirolimus-Induced Interstitial Lung Disease and Resolution After Conversion to Everolimus. Respir Med Case Rep (2020) 30:101109. doi: 10.1016/j.rmcr.2020.101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A Enzymes are Responsible for Biotransformation of FK506 and Rapamycin in Man and Rat. Drug Metab Dispos (1992) 20(5):753–61. [PubMed] [Google Scholar]
- 172. Baguley BC, Siemann DW. Temporal Aspects of the Action of ASA404 (Vadimezan; DMXAA). Expert Opin Investig Drugs (2010) 19(11):1413–25. doi: 10.1517/13543784.2010.529128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Daei Farshchi Adli A, Jahanban-Esfahlan R, Seidi K, Samandari-Rad S, Zarghami N. An Overview on Vadimezan (DMXAA): The Vascular Disrupting Agent. Chem Biol Drug Des (2018) 91(5):996–1006. doi: 10.1111/cbdd.13166 [DOI] [PubMed] [Google Scholar]
- 174. Levine BC, SHOJI-KAWATA S. Autophagy-Inducing Peptide (2013). Available at: https://patents.google.com/patent/WO2013119377A1/en.
- 175. Kim YS, Silwal P, Kim SY, Yoshimori T, Jo EK. Autophagy-Activating Strategies to Promote Innate Defense Against Mycobacteria. Exp Mol Med (2019) 51(12):151. doi: 10.1038/s12276-019-0290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kominsky DJ, Campbell EL, Colgan SP. Metabolic Shifts in Immunity and Inflammation. J Immunol (2010) 184(8):4062–8. doi: 10.4049/jimmunol.0903002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nitric Oxide Orchestrates Metabolic Rewiring in M1 Macrophages by Targeting Aconitase 2 and Pyruvate Dehydrogenase. Nat Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Infection With Mycobacterium Tuberculosis Induces the Warburg Effect in Mouse Lungs. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chai Q, Wang L, Liu CH, Ge B. New Insights Into the Evasion of Host Innate Immunity by Mycobacterium Tuberculosis. Cell Mol Immunol 17(9):901–13. doi: 10.1038/s41423-020-0502-z192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-Mannose Suppresses Macrophage IL-1β Production. Nat Commun 11(1):1–2. doi: 10.1038/s41467-1409020-20164-6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhong WJ, Yang HH, Guan XX, Xiong JB, Sun CC, Zhang CY, et al. Inhibition of Glycolysis Alleviates Lipopolysaccharide-Induced Acute Lung Injury in a Mouse Model. J Cell Physiol (2019) 234(4):4641–54. doi: 10.1002/jcp.27261 [DOI] [PubMed] [Google Scholar]
- 182. Vyas AK, Koster JC, Tzekov A, Hruz PW. Effects of the HIV Protease Inhibitor Ritonavir on GLUT4 Knock-Out Mice. J Biol Chem (2010) 285(47):36395–400. doi: 10.1074/jbc.M110.176321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pharmacokinetic Evaluation of Rifabutin in Combination With Lopinavir-Ritonavir in Patients With HIV Infection and Active Tuberculosis. Clin Infect Dis. https://academic.oup.com/cid/article/49/9/1305/299191?login=false. doi: 10.1086/606056 [DOI] [PubMed] [Google Scholar]
- 184. Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a Potential Metabolic-Targeting Therapy for Cancer. Br J Cancer (2008) 99(7):989–94. doi: 10.1038/sj.bjc.6604554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. van Doorn CLR, Schouten GK, van Veen S, Walburg KV, Esselink JJ, Heemskerk MT, et al. Pyruvate Dehydrogenase Kinase Inhibitor Dichloroacetate Improves Host Control of Salmonella Enterica Serovar Typhimurium Infection in Human Macrophages. Front Immunol (2021) 12:739938. doi: 10.3389/fimmu.2021.739938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Song YJ, Kim A, Kim GT, Yu HY, Lee ES, Park MJ, et al. Inhibition of Lactate Dehydrogenase A Suppresses Inflammatory Response in RAW 264.7 Macrophages. Mol Med Rep (2019) 19(1):629–37. [DOI] [PubMed] [Google Scholar]
- 187. Intracellular Mycobacterium Tuberculosis Exploits Host-Derived Fatty Acids to Limit Metabolic Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Foamy Macrophages and the Progression of the Human TB Granuloma. [Google Scholar]
- 189. Hardie DG. AMP-Activated Protein Kinase—An Energy Sensor That Regulates All Aspects of Cell Function. Genes Dev (2011) 25(18):1895–908. doi: 10.1101/gad.17420111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang M, He JQ. Impacts of Metformin on Tuberculosis Incidence and Clinical Outcomes in Patients With Diabetes: A Systematic Review and Meta-Analysis. Eur J Clin Pharmacol (2020) 76(2):149–59. doi: 10.1007/s00228-019-02786-y [DOI] [PubMed] [Google Scholar]
- 191. Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Metformin Enhances Protection in Guinea Pigs Chronically Infected With Mycobacterium Tuberculosis. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Magee MJ, Salindri AD, Kornfeld H, Singhal A. Reduced Prevalence of Latent Tuberculosis Infection in Diabetes Patients Using Metformin and Statins. Eur Respir J (2019) 53(3). doi: 10.1183/13993003.01695-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bartel DP. Metazoan MicroRNAs. Cell (2018) 173(1):20–51. doi: 10.1016/j.cell.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Agarwal RG, Sharma P, Nyati KK. microRNAs in Mycobacterial Infection: Modulation of Host Immune Response and Apoptotic Pathways. Immune Netw (2019) 19(5):e30. doi: 10.4110/in.2019.19.e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sabir N, Hussain T, Shah SZ, Peramo A, Zhao D, Zhou X. miRNAs in tuberculosis: new avenues for diagnosis and host-directed therapy. Front Microbiol (2018) 9:602. doi: 10.3389/fmicb.2018.00602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sampath P, Periyasamy KM, Ranganathan UD, Bethunaickan R. Monocyte and Macrophage miRNA: Potent Biomarker and Target for Host-Directed Therapy for Tuberculosis. Front Immunol (2021). doi: 10.3389/fimmu.2021.667206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yang T, Ge B. miRNAs in Immune Responses to Mycobacterium Tuberculosis Infection. Cancer Lett (2018), 431:22–30. doi: 10.1016/j.canlet.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 199. Das K, Garnica O, Dhandayuthapani S. Modulation of Host miRNAs by Intracellular Bacterial Pathogens. Front Cell Infect Microbiol (2016) 6:79. doi: 10.3389/fcimb.2016.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mehta MD, Liu PT. microRNAs in Mycobacterial Disease: Friend or Foe? Front Genet (2014) 5:231. doi: 10.3389/fgene.2014.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mycobacterium Tuberculosis Lipomannan Blocks TNF Biosynthesis by Regulating Macrophage MAPK-Activated Protein Kinase 2 (MK2) and microRNA miR-125b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Harapan H, Fitra F, Ichsan I, Mulyadi M, Miotto P, Hasan NA, et al. The Roles of microRNAs on Tuberculosis Infection: Meaning or Myth? Tuberc (Edinb) (2013) 93(6):596–605. doi: 10.1016/j.tube.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, et al. Mycobacterium Tuberculosis Induces the miR-33 Locus to Reprogram Autophagy and Host Lipid Metabolism. Nat Immunol (2016) 17(6):677–86. doi: 10.1038/ni.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, et al. MicroRNA Let-7 Modulates the Immune Response to Mycobacterium Tuberculosis Infection via Control of A20, an Inhibitor of the NF-κb Pathway. Cell Host Microbe (2015) 17(3):345–56. doi: 10.1016/j.chom.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 205. Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as Mediators of the 5q– Syndrome Phenotype. Nat Med 16(1):49–58. doi: 10.1038/nm.2054 [DOI] [PubMed] [Google Scholar]
- 206. Guo L, Zhou L, Gao Q, Zhang A, Wei J, Hong D, et al. MicroRNA-144-3p Inhibits Autophagy Activation and Enhances Bacillus Calmette-Guérin Infection by Targeting ATG4a in RAW264.7 Macrophage Cells. PloS One (2017) 12(6):e0179772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Belver L, Papavasiliou NF, Ramiro AR. MicroRNA Control of Lymphocyte Differentiation and Function. Curr Opin Immunol (2011) 23(3):368–73. doi: 10.1016/j.coi.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. He X, Jing Z, Cheng G. MicroRNAs: New Regulators of Toll-Like Receptor Signalling Pathways. BioMed Res Int (2014) 2014:945169. doi: 10.1155/2014/945169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wang J, Wu M, Wen J, Yang K, Li M, Zhan X, et al. MicroRNA-155 Induction by Mycobacterium Bovis BCG Enhances ROS Production Through Targeting SHIP1. Mol Immunol (2014) 62(1):29–36. doi: 10.1016/j.molimm.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 210. Ghorpade DS, Leyland R, Kurowska-Stolarska M, Patil SA, Balaji KN. MicroRNA-155 Is Required for Mycobacterium Bovis BCG-Mediated Apoptosis of Macrophages. Mol Cell Biol (2012) 32(12):2239–53. doi: 10.1128/MCB.06597-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 Controls Innate and Adaptive Immune Responses to Intracellular Bacterial Infection by Targeting Interferon-γ. Nat Immunol (2011) 12(9):861–9. doi: 10.1038/ni.2073 [DOI] [PubMed] [Google Scholar]
- 212. MicroRNA-223 Controls Susceptibility to Tuberculosis by Regulating Lung Neutrophil Recruitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Wang J, Jia Z, Wei B, Zhou Y, Niu C, Bai S, et al. MicroRNA-27a Restrains the Immune Response to Mycobacterium Tuberculosis Infection by Targeting IRAK4, a Promoter of the NF-κb Pathway. Int J Clin Exp Pathol (2017) 10(9):9894–901. [PMC free article] [PubMed] [Google Scholar]
- 214. Duan X, Zhang T, Ding S, Wei J, Su C, Liu H, et al. microRNA-17-5p Modulates Bacille Calmette-Guerin Growth in RAW264.7 Cells by Targeting Ulk1. PloS One (2015) 10(9):e0138011. doi: 10.1371/journal.pone.0138011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Inhibition of microRNA Function by antimiR Oligonucleotides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Di Fusco D, Dinallo V, Marafini I, Figliuzzi MM, Romano B, Monteleone G. Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease. Front Pharmacol (2019). doi: 10.3389/fphar.2019.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Young C, Walzl G, Du Plessis N. Therapeutic Host-Directed Strategies to Improve Outcome in Tuberculosis. Mucosal Immunol (2020) 13(2):190–204. doi: 10.1038/s41385-019-0226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]