Summary
Background
In Japan, vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initiated on 17 February 2021, mainly using messenger RNA vaccines and prioritizing health care professionals. Whereas nationwide vaccination alleviated the coronavirus disease 2019 (COVID-19)-related burden, the population impact has yet to be quantified in Japan. We aimed to estimate the numbers of COVID-19 cases and deaths prevented that were attributable to the reduced risk among vaccinated individuals via a statistical modeling framework.
Methods
We analyzed confirmed cases registered in the Health Center Real-time Information-sharing System on COVID-19 (3 March–30 November 2021) and publicly reported COVID-19-related deaths (24 March–30 November 2021). The vaccination coverage over this time course, classified by age and sex, was extracted from vaccine registration systems. The total numbers of prevented cases and deaths were calculated by multiplying the daily risk differences between unvaccinated and vaccinated individuals by the population size of vaccinated individuals.
Findings
For both cases and deaths, the averted numbers were estimated to be the highest among individuals aged 65 years and older. In total, we estimated that 564,596 (95% confidence interval: 477,020–657,525) COVID-19 cases and 18,622 (95% confidence interval: 6522–33,762) deaths associated with SARS-CoV-2 infection were prevented owing to vaccination during the analysis period (i.e., fifth epidemic wave, caused mainly by the Delta variant). Female individuals were more likely to be protected from infection following vaccination than male individuals whereas more deaths were prevented in male than in female individuals.
Interpretation
The vaccination program in Japan led to substantial reductions in the numbers of COVID-19 cases and deaths (33% and 67%, respectively). The preventive effect will be further amplified during future pandemic waves caused by variants with shared antigenicity.
Funding
This project was supported by the Japan Science and Technology Agency; the Japan Agency for Medical Research and Development; the Japan Society for the Promotion of Science; and the Ministry of Health, Labour and Welfare.
Keywords: Averted burden, COVID-19, Statistical model, Vaccination, Epidemiology, Direct effectiveness
Research in context.
Evidence before this study
The population impact of vaccination is principally evaluated by measuring direct and indirect effectiveness. Direct effectiveness is based on a comparison of risk between vaccinated and unvaccinated individuals whereas indirect effectiveness is focused on measures of reduced opportunities for infection owing to vaccination programs and reduced transmissibility among vaccinated individuals. When evaluating the unprecedented mass vaccination against coronavirus disease 2019 (COVID-19), it is vital to explore the direct effectiveness of vaccination such that the reduced risk of infection and death owing to the vaccination program can be objectively determined. We searched PubMed for research articles written in English from January 2020 to 18 March 2022 using the following keywords: ("SARS-CoV-2"[title] OR "COVID-19"[title]) AND ("direct effect*"[title] OR "averted"[title] OR "prevented"[title]) AND ("vaccination"[title]) AND ("mass" OR "campaign*" OR "program*") AND ("cases" OR "infections" OR "deaths"). Our search revealed eight published articles. Most studies (n = 7) investigated the number of COVID-19 cases, hospitalizations, and deaths averted by COVID-19 vaccination; of these, five evaluated the impact of vaccination in the presence of the Delta variant (B.1.617) of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The estimated values varied across regions and countries and depended on the size of the epidemic. A common finding was that the benefit of vaccination, especially of the first and second doses, was substantial. Other than studies in European countries and the United States, there has only been one related study in Israel and no relevant research has been published from the Western Pacific region. The present study is the first to report the number of COVID-19 cases and deaths averted owing to the vaccination program in Japan, as of 18 March 2022.
Added value of this study
Before widespread circulation of the Omicron (B.1.529) variant of SARS-CoV-2, many countries in the Western Pacific region experienced lower epidemic levels of COVID-19 infections than Western countries. To the best of our knowledge, the present study is the first to estimate the numbers of averted cases of SARS-CoV-2 infection and averted COVID-19-related deaths considered to be attributable to the mass vaccination program in Japan, a Western Pacific country that achieved two-dose vaccination coverage of 82% as of 18 March 2022. Using the national database of COVID-19 patients and publicly available information on the causes of death, we estimated that mass vaccination contributed to reducing the number of cases and deaths by 33% and 67%, respectively, from March to November 2021. Such substantial reductions were observed during sequential epidemic waves mainly caused by the Alpha (B.1.1.7) and Delta (B.1.617) variants. When analyzing estimates by age group, people aged 65 years and older benefitted more from vaccination than younger individuals.
Implications of all the available evidence
Many countries in the Western Pacific region experienced relatively lower epidemic levels than Western countries. Using readily available datasets such as vaccination coverage and surveillance records of cases stratified by age, sex, and vaccination history, we showed that a substantial reduction in the risk of infection and death can be objectively demonstrated in Japan. High coverage of mRNA vaccination (with BNT162b2 [Pfizer/BioNTech] and mRNA-1273 [Moderna] vaccines) led to substantial direct effects, preventing 560,000 cases and 19,000 deaths owing to COVID-19. Quantifying the direct effectiveness of vaccination can provide critical insights in the evaluation of vaccination programs as a whole, and similar methods may be applied to other country settings. In the present study, we mainly evaluated the fifth epidemic wave in Japan, caused by the Delta variant. The population impact of vaccination would be expected to be further amplified as the pandemic continues. Western Pacific countries can explore future epidemic waves by using the proposed framework, additionally accounting for the effect of booster immunization, waning immunity, and infections and deaths caused by an antigenically distinct variant with a different virulence level.
Alt-text: Unlabelled box
Introduction
Shortly after the emergence of severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2), which causes coronavirus disease 2019 (COVID-19), the main interventions in 2020 were non-pharmaceutical, which are presently referred to as public health and social measures (PHSM). PHSM range from social distancing at a local level to widespread restrictions, such as lockdown policies, and have contributed to reducing virus transmission and buying time. However, these restrictions have curbed people's freedom and the adverse impact on social and economic activities has been substantial.1, 2, 3 In this regard, vaccination has been a key player in epidemic control programs. The vaccine rollout against COVID-19 was launched in December 2020, mainly in high-income countries. Soon after, effects of COVID-19 vaccines such as a reduction in the number of cases, hospitalizations, and deaths, were evident in many countries, including Israel and the United Kingdom, among the first countries in the world to start mass vaccination.4,5 A common strategy of vaccine rollout was that health care professionals were prioritized, followed by individuals aged 65 years and older and those with underlying comorbidities. Subsequently, vaccination programs began to allocate vaccines to younger and healthy people.6,7 This particular approach was taken because a primary focus of mass vaccination programs in many countries has been to prevent cases of COVID-19 from becoming severe and to minimize the disease burden on health care systems more so than to prevent the spread of COVID-19.7
Whereas early evaluation of vaccination programs took place in Western countries where the incidence level was substantial, this was not the case in many countries belonging to the Western Pacific region that successfully maintained lower epidemic levels during the early period of the COVID-19 pandemic. As one of these countries, Japan maintained an incidence level lower than those of many European countries and the United States.8 Even so, Japan experienced five large epidemic surges of SARS-CoV-2 infection between January 2020 and November 2021 involving more than 1·7 million cases and 18,000 deaths.9 During this period, Japan used key PHSM to control the spread of COVID-19 based principally on voluntary restriction of contact, which was requested by the government but was not legally binding.10 Whereas such countermeasures against COVID-19 greatly reduced virus transmission, the emergence of SARS-CoV-2 variants of concern, including the Alpha (B.1.529) and Delta (B.1.617) variants with elevated transmissibility compared with wild-type, has proven challenging. The fifth wave in July–September 2021 was mainly caused by the Delta variant, and the number of cases in August 2021 was the highest recorded since the start of the pandemic (Figure 1A). Regarding vaccination rollout, Japan launched a vaccination program, initially prioritizing health care professionals, from 17 February 2021.11 A mass vaccination program then began on 12 April 2021, giving priority to those aged 65 years and older, people with pre-existing medical conditions, and workers in nursing homes.12 From around the middle of June 2021, when vaccination coverage with the first dose among older people had reached approximately 50%, the program targets gradually and sequentially shifted to younger age groups, although the speed of vaccination was dependent on local governments (Figure 1A).13 Initially, the targets of the vaccination program were individuals aged 16 years and older. However, the messenger RNA (mRNA) vaccine BNT162b2 (Pfizer/BioNTech) was approved for use in those aged ≥ 12 years on 1 June 2021; later, the mRNA-1273 (Moderna) vaccine was approved for those aged 12–15 years.14 To boost vaccination coverage, the Japanese government initiated the “vaccination in the workplace” program on 21 June 2021.15 The program invited large companies, initially restricted to those with more than 1000 workers, as well as universities and colleges, to vaccinate employees and students internally. By the end of November 2021, approximately 75% of the Japanese population had been vaccinated with the first dose of a COVID-19 vaccine, predominantly with the mRNA vaccine BNT162b2 (Pfizer/BioNTech) (83·2% of vaccinated individuals), followed by the mRNA-1273 (Moderna) vaccine (16·7% of vaccinated individuals).13,16
Figure 1.
Epidemiological overview in Japan. (A) Confirmed cases of SARS-CoV-2 infection from 3 March to 30 November 2021 by age group (> 14 years) are shown (left-hand side vertical axis). The coverage of first dose vaccination is shown as a black line (right-hand side vertical axis). Dashed lines represent launch dates of the vaccination program, initially prioritizing those aged 65 years and older and the vaccination in the workplace program. Shaded areas highlighted in yellow represent the second, third, and fourth states of emergency, which were declared from 8 January to 21 March, from 25 April to 20 June, and from 12 July to 30 September 2021, respectively. (B) Timeline of the declarations of the state of emergency during the period of analysis. Each state of emergency period corresponds to the area highlighted in yellow in (A).
Vaccination programs have been evaluated in two ways, at the individual level and at the population level. With regard to the former, epidemiological studies in Israel have estimated that the BNT162b2 vaccine had 70%–90% effectiveness against infection, asymptomatic infection, COVID-19-related hospitalization, and death. These studies partially involved the Alpha variant.5,17 Similar studies from the United Kingdom and the United States during the period when the Alpha variant was dominant in those countries have shown the high effectiveness of COVID-19 vaccines, particularly BNT162b2, which was estimated to be at least 80%.18,19 Although the vaccine's effectiveness against the Delta variant appeared to be lower than that against the Alpha variant, the effectiveness of two doses was estimated to be at least around 80% and 60% for the BNT162b2 and ChAdOx1 (Oxford/AstraZeneca) vaccines, respectively.20,21 Assessment of the population impact—the latter evaluation—was initially reported from Israel in September 2021, in which researchers calculated the averted number of infections, hospitalizations, and deaths related to COVID-19 owing to vaccination. The number of cases in Israel was reduced by one-third during the large epidemic wave between August and September 2021, and this reduction was attributed to mass vaccination.22 Other studies showed that more than 445,000 cases and 22,000 deaths from January to September 2021 and approximately 470,000 deaths from December 2020 to November 2021 were prevented owing to COVID-19 vaccination in Italy and in the World Health Organization European region, respectively.23,24 Additionally, other studies from the United States reported substantial numbers of averted cases, hospitalizations, and deaths related to COVID-19.25,26. However, evaluation of vaccination programs has yet to be reported from Western Pacific countries, where the incidence continued to remain low, especially during the period when the Delta variant was dominant. Therefore, quantifying the averted burden resulting from vaccination programs is critical to assessing vaccination policy in countries belonging to this region.
Since the beginning of the vaccination program in Japan, recording the daily number of vaccinated individuals in the registration system and surveillance data was mandated, and physicians were required to report the vaccination history of confirmed cases along with their age and sex. Here, we estimate the number of prevented COVID-19 cases and deaths that are attributable to the reduced risk of infection and death among vaccinated individuals. We used a simple statistical model based on the difference in risk between unvaccinated and vaccinated people, identifying host groups that experienced greater benefits than others.
Methods
Vaccination data
Vaccination coverage data were retrieved from two registration systems—the Vaccination Record System (VRS) and the Vaccination System (V-SYS); the former registers vaccination by age and the latter records the doses of vaccine distributed from central to local governments. Both data are aggregated as nationwide information. In general, people were registered in the VRS after they participated in the standard mass vaccination program. However, there could be a delay in reporting in real-time, and the number of vaccinated people was accumulated according to the date of reporting (the number of vaccinated individuals in the VRS was inconsistently reported, usually on weekdays). After integrating all vaccinated individuals registered in the VRS and V-SYS, we added 14 days to the date of vaccination to obtain the proportions of immunized people on certain days by age and sex. That is, we assumed that the vaccine was not effective after the first dose for the first 14 days, but that then there was an abrupt increase in vaccine-induced immunity. To integrate datasets from two independent systems of vaccination record, vaccinated people were divided into six different age groups: 15–24, 25–34, 35–44, 45–54, 55–64, and ≥ 65 years. People aged 12–14 years were included in the vaccination program, but they received the vaccine at the end of the program and vaccination coverage was far lower than for other age groups. Thus, for our analysis, we focused on those aged 15 years and older. Because prioritized vaccination for health care professionals was launched on 17 February 2021 in Japan, the dataset of immunized people was available 14 days later, that is, from 3 March 2021. The age-specific time-varying vaccination coverages by sex for people who were at least partially vaccinated or fully vaccinated are shown in Figure 2.
Figure 2.
Estimated vaccination coverage among at least partially vaccinated and fully vaccinated people by sex. Vaccination coverage among at least partially vaccinated individuals by age group (> 14 years) from 3 March to 30 November 2021 for male (a) and female (b) individuals is shown. Additionally, vaccination coverage among fully vaccinated individuals by age group (> 14 years) from 3 March to 25 September 2021 for male (c) and female (d) individuals is shown. It is noted that all values take into account the delay in build-up of immunity.
Daily incidence data
As of 30 November 2021, COVID-19 has been designated as an infectious disease requiring special attention in Japan. All individuals with suspected infection must be tested using PCR at a medical facility, followed by testing of their close contacts and movement restriction. Subsequently, all confirmed cases of COVID-19 are registered in the Health Center Real-time Information-sharing System on COVID-19 (HER-SYS) by health care facilities or local health centers in Japan, along with information on age, sex, date of onset, and vaccination status. After the data were de-identified and aggregated nationally, we analyzed confirmed cases reported from 3 March to 30 November 2021, the period corresponding to the observation period of vaccination rollout, and all confirmed cases were divided into the six age groups above. Approximately 8% of confirmed cases did not have information about vaccination status; therefore, we allocated these cases to either unvaccinated, partly vaccinated (only first dose), or fully vaccinated according to the distribution of vaccine status by age and sex, and the vaccination date registered in the HER-SYS, i.e., where c′i,a, b is the reconstructed daily number of cases and ci,a, b is the daily number of confirmed cases or deaths in age group a of sex b, with vaccination status i, i.e., unvaccinated (i = 0), partly vaccinated (only first dose; i = 1), or fully vaccinated (i = 2). That is, a small number of cases without a known history of vaccination was proportionally distributed. COVID-19-related deaths have not been consistently registered in the HER-SYS; thus, we used death records available in the integrated data by the national broadcasting corporation.27 Accounting for a reporting delay for each death, the dataset between 24 March and 30 November 2021 was used. Although the data for deceased cases were collected according to the date of death, the fifth wave of COVID-19 infections was nearly over by October. Thus, we believe that the impact on analysis owing to a reporting delay between infection and death, attributed to the infections in November, was minimal.
Estimation of the number of prevented cases and deaths
Using the abovementioned sets of information, the daily incidence (risk) among unvaccinated people () and those who were at least partially vaccinated () in age group a of sex b is expressed as:
| (1) |
where c′i,a, b is the reconstructed daily number of confirmed cases or deaths in age group a of sex b, with vaccination status i, i.e., unvaccinated (i = 0), partly vaccinated (only first dose; i = 1), or fully vaccinated (i = 2). v≥ 1 dose, a, b represents the vaccination coverage of people who received at least one dose in age group a of sex b. Na,b is the population size of age group a of sex b as of 1 January 2021 in Japan.28 We then estimated ha,b, the daily number of averted cases or deaths in age group a of sex b, given that at least partial vaccination had been received during the analysis period, as
| (2) |
As mentioned earlier, the analysis periods started from 3 March 2021 for averted cases and 24 March for averted deaths, respectively, but the endpoints for both periods were 30 November 2021. We estimated the daily incidence differences, measuring the risk reduction directly attributable to the vaccination program, and subsequently, the total number of averted cases or deaths, as
| (3) |
We also estimated the vaccination coverage for fully vaccinated people (v2 dose) by shifting the one-dose curve right by 14 days; that is, a 21-day gap between the first and second doses minus 7 days of delay required to build up immunity after receiving two-dose vaccination. Using the daily incidence for people who were fully vaccinated (c′2, a, b/Na,bv2 dose, a, b), we then estimated the number of prevented cases and deaths among fully vaccinated people. The 95% confidence intervals (CIs) of the averted number of cases and deaths were calculated based on the uncertainty of the risk difference between unvaccinated and vaccinated. We assumed that those cases and deaths were sufficiently captured by binomial distribution:
| (4) |
where n stands for the denominator of the incidence (r in Eq. 1) and p is the probability interpreted as r in the present study. We calculated the 95% CIs of prevented burdens using 1000 bootstrap iterations. It should noted that the computed uncertainty bounds does not account for serial dependence structure and thus may be conservative.
Role of the funding source
The funders of the present study had no role in study design, data analysis, data interpretation, or writing of the manuscript.
Ethical considerations
The conduct of this study adhered to the principles of the Declaration of Helsinki, and the research was approved by the Ethics Review Committee of Kyoto University (approval number R2673).
Results
As of 30 November 2021, the vaccination coverage among male individuals who were at least partially vaccinated was estimated to be 77·0%, 77·2%, 79·4%, 87·8%, 88·9%, and 94·2% among those aged 15–24, 25–34, 35–44, 45–54, 55–64, and ≥ 65 years, respectively (Figure 2A). By contrast, the vaccination coverage among female individuals who were at least partially vaccinated was estimated to be 81·8%, 81·3%, 83·4%, 91·4%, 91·0%, and 94·3% among those aged 15–24, 25–34, 35–44, 45–54, 55–64, and ≥ 65 years, respectively (Figure 2B). Earlier elevated vaccination coverage among those aged ≥ 65 years, in both male and female individuals, could be observed (Figure 2). The subsequent increase in vaccination coverage among younger people is evident from July 2021.
As a function of calendar month, the median daily incidence differences for confirmed cases of SARS-CoV-2 and COVID-19-related deaths per one million in each age group and sex stratum between unvaccinated and at least partially vaccinated individuals are shown in Table 1. Because the epidemic size of the fifth wave was at a record high level in August 2021, the estimated incidence differences in cases were estimated to be highest around that time. By contrast, the estimates of death risk differences were more evident in September, perhaps owing to a delay from diagnosis to death. Similarly, median daily incidence differences between unvaccinated and fully vaccinated individuals are shown in Table S1. Because most people vaccinated with one dose received a second dose of vaccine, the estimates in Table S1 only differed slightly from those in Table 1. The relationship of the daily incidence difference between unvaccinated and vaccinated individuals with COVID-19 cases over the study period by age group is illustrated in Figure S1.
Table 1.
Daily incidence differences in prevented cases and deaths between unvaccinated and at least partially vaccinated people by age, sex, and analysis period.
| Age group | Overall analysis | Monthly analysis in 2021 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| March | April | May | June | July | August | September | October | November | ||
| Cases infected with SARS-CoV-2 | ||||||||||
| Male | ||||||||||
| 15–24 | 39·15 (15·28–90·75) | 19·97 (12·88–31·89) | 64·56 (47·71–83·24) | 69·09 (56·25–99·9) | 29·53 (24·5–34·65) | 64·19 (35·34–109·35) | 410·51 (296·64–535·75) | 89·42 (45·8–177·1) | 12·41 (7·44–18·62) | 4·65 (2·66–6·83) |
| 25–34 | 37·85 (16·6–85·38) | 17·36 (12·5–23·98) | 56·8 (42·06–71·07) | 60·42 (48·05–82·53) | 27 (20·8–32·11) | 60·37 (38·49–112·93) | 394·13 (304·65–510·42) | 106 (49·27–177·54) | 13·18 (8·74–20·8) | 4·39 (2·31–7·35) |
| 35–44 | 26·29 (10·98–59·26) | 11·95 (9·15–16·95) | 38·09 (26·77–48·54) | 46·26 (35·31–55·36) | 20·14 (14·76–24·06) | 38·77 (25·23–68·49) | 267·19 (187·89–311·03) | 73·25 (37·32–119·33) | 9·75 (5·49–16·82) | 2·72 (1·34–4·35) |
| 45–54 | 21·47 (10·18–49·15) | 11·29 (8·55–15·1) | 33·36 (23·44–42·85) | 36·11 (24·76–48·64) | 15·52 (11·66–18·95) | 28·83 (17·62–53·49) | 213·76 (145·17–256·23) | 71·21 (37·74–114·75) | 10·31 (6·37–14·85) | 2·83 (1·29–4·88) |
| 55–64 | 16·29 (7·6–40·2) | 9·76 (7·55–13·29) | 26·35 (18·84–37·82) | 28·21 (17·71–38·54) | 11·73 (7·12–14·36) | 16·41 (10·55–27·68) | 151·06 (94–189·65) | 58·56 (35·22–103·17) | 7·78 (3·45–12·66) | 1·18 (–0·15–3·67) |
| ≥ 65 | 12·1 (6·09–29·76) | 9·57 (7·52–11·18) | 17·39 (12·62–23·56) | 17·07 (8·48–24·93) | 5·95 (4·32–7·67) | 10·97 (7·37–18·81) | 143·96 (78·75–182·05) | 62·49 (33·2–114·13) | 10·91 (6·36–16·43) | 1·24 (–0·27–3·36) |
| ≥ 15 | 23·96 (9·7–60·98) | 11·98 (8·9–17·62) | 35·08 (21·4–52·99) | 40·19 (23·04–58·59) | 16·62 (9·09–25·55) | 33·26 (15·49–65·55) | 218·13 (147·93–341·63) | 72·94 (39·37–131·15) | 10·71 (6·1–16·65) | 2·88 (0·9–5·21) |
| Female | ||||||||||
| 15–24 | 35·14 (13·82–80·18) | 16·48 (11·55–25·32) | 55·67 (29·06–67·91) | 59·44 (46·68–81·94) | 26·58 (20·8–32·57) | 64·35 (37·47–100·71) | 379·88 (307·38–472·45) | 90·81 (45–173·3) | 10·78 (7·15–15·36) | 4·01 (1·78–6·96) |
| 25–34 | 32·8 (14·48–72·81) | 15·19 (12·01–20·58) | 45·27 (33·48–58·78) | 53·56 (41·26–73·11) | 23·12 (18·59–27·89) | 53·25 (33·62–92·29) | 326·06 (271·35–404·47) | 85·62 (45·43–153·64) | 11·39 (7·55–16·22) | 3·88 (1·74–6·25) |
| 35–44 | 18·79 (8·73–42·48) | 8·99 (6·97–11·77) | 26·82 (19·01–35·94) | 32·41 (25·11–41·57) | 13·17 (9·95–16·48) | 25·27 (14·83–43·6) | 193·19 (141·36–234·95) | 59·94 (30·75–104·24) | 8·11 (4·75–13·15) | 2·15 (0·75–3·8) |
| 45–54 | 18·46 (9·31–41·77) | 9·31 (7·14–12·05) | 27·77 (18·95–37·08) | 31·33 (22·27–39·58) | 11·64 (9·44–14·33) | 21·01 (15·16–39·84) | 192·48 (125·92–236·09) | 64·44 (36·44–110·07) | 10·22 (6·28–16·25) | 4·23 (1·97–6·98) |
| 55–64 | 14·24 (7·11–33·3) | 8·15 (6·46–10·5) | 20·78 (13·14–27·74) | 24·88 (18·29–32) | 9·5 (6·95–12·32) | 13·69 (9·13–24·04) | 130·58 (83·61–167·47) | 61·98 (27·95–96·99) | 8·87 (4·88–14·61) | 1·42 (0–3·87) |
| ≥ 65 | 10·9 (5·57–28·39) | 8·71 (7·28–9·95) | 16·11 (10·66–22·81) | 17·8 (12·2–22·89) | 5·16 (3·26–6·93) | 8·69 (5·93–16·99) | 132·08 (78·63–173·86) | 58·17 (34·12–92·57) | 8·16 (4·56–15·54) | 1·62 (0·23–3·79) |
| ≥ 15 | 20·29 (8·55–51·82) | 10·01 (7·5–14·5) | 27·1 (17·02–41·96) | 32·56 (21·46–48·27) | 12·66 (7·88–21·02) | 27·38 (12·91–55·1) | 188·77 (129·26–285·71) | 67·75 (37·09–114·2) | 9·7 (5·77–15·19) | 2·81 (0·89–5·35) |
| Deaths related to COVID-19 | ||||||||||
| Male | ||||||||||
| 15–24 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 25–34 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 35–44 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0·19) | 0 (0–0·38) | 0 (0–0) | 0 (0–0) |
| 45–54 | 0 (0–0·32) | 0 (0–0) | 0 (0–0·11) | 0·11 (0–0·32) | 0 (0–0·11) | 0 (0–0) | 0·17 (0–0·48) | 0·74 (0·29–1·34) | 0 (0–0·77) | 0 (0–0) |
| 55–64 | 0·21 (0–0·82) | 0 (0–0·13) | 0·13 (0–0·39) | 0·4 (0·13–0·66) | 0·27 (0–0·54) | 0 (0–0·17) | 0·44 (0–1·07) | 2·54 (1·54–3·69) | 0 (0–3·37) | 0 (0–0) |
| ≥ 65 | 1·8 (0·66–3·86) | 1·09 (0·84–1·35) | 0·9 (0·51–1·48) | 2·73 (2·07–3·5) | 1·93 (1·07–2·86) | 0·92 (0·39–1·53) | 3·55 (1·54–6·54) | 10·23 (6·52–15·32) | 2·7 (0–6·48) | 0 (0–1·06) |
| ≥ 15 | 0 (0–0·4) | 0 (0–0·12) | 0 (0–0·25) | 0 (0–0·53) | 0 (0–0·27) | 0 (0–0·13) | 0·14 (0–0·63) | 0·34 (0–2·52) | 0 (0–0·77) | 0 (0–0) |
| Female | ||||||||||
| 15–24 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 25–34 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 35–44 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| 45–54 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0·29) | 0 (0–0) | 0 (0–0) |
| 55–64 | 0 (0–0) | 0 (0–0) | 0 (0–0·13) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0·31) | 0·51 (0–1·63) | 0 (0–0) | 0 (0–0) |
| ≥ 65 | 1·17 (0·41–2·42) | 0·54 (0·4–0·74) | 0·69 (0·45–0·99) | 1·69 (1·2–2·22) | 1·36 (0·89–1·83) | 0·61 (0·2–1·12) | 2 (0·89–3·88) | 5·2 (3·13–8·29) | 1·65 (0–4·32) | 0 (0–0) |
| ≥ 15 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0·19) | 0 (0–0·91) | 0 (0–0) | 0 (0–0) |
Median daily incidence differences per 1,000,000 population are shown. Interquartile ranges are presented in parentheses.
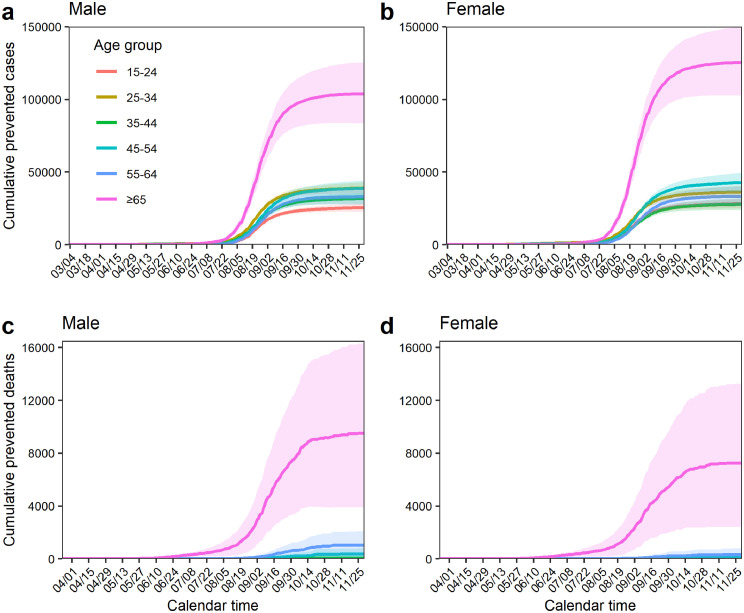
The averted number of COVID-19 cases for the entire observation period was estimated to be 271,300 (95% CI: 230,194–314,632) and 293,297 (95% CI: 246,826–342,892) cases in male and female individuals, respectively. Moreover, we estimated that 10,938 (95% CI: 4174–19,334) male deaths and 7684 (95% CI: 2348–14,428) female deaths were prevented owing to the vaccination program. Figure 3 shows the age-specific cumulative number of averted cases and deaths by sex among at least partially vaccinated people. For both cases and deaths, the estimates of prevented counts were highest among older people. By 30 November 2021, the averted number of cases among people aged 65 years and older was estimated to have been 103,637 (95% CI: 83,427–125,170) and 125,313 (95% CI: 102,679–149,496) in male and female individuals, respectively. With regard to deaths, by 30 November, 9487 (95% CI: 3906–16,281) and 7277 (95% CI: 2379–13,362) deaths in male and female individuals, respectively, were estimated to have been prevented because of the risk reduction among vaccinated people compared with unvaccinated people.
Figure 3.
Cumulative numbers of prevented cases and deaths by sex that are attributed to the reduced risk among vaccinated individuals. Cumulative numbers of averted cases by age group (> 14 years) between 3 March and 30 November 2021 for male (a) and female (b) individuals are shown. Additionally, cumulative numbers of averted deaths by age group (> 14 years) between 24 March and 30 November 2021 for male (c) and female (d) individuals are shown. Each shaded area represents the 95% confidence interval.
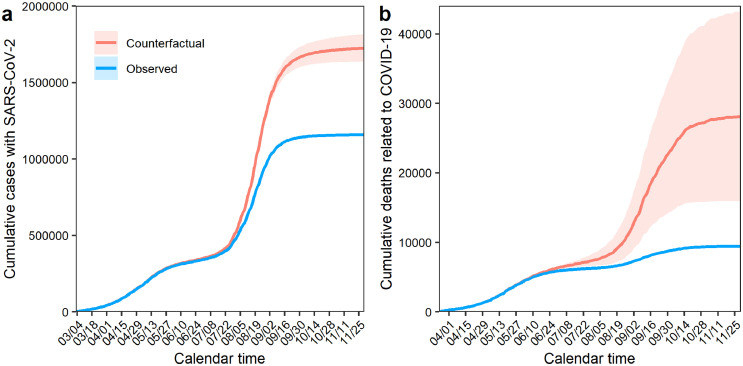
Figure 4 shows the estimated cumulative number of cases and deaths prevented, along with the observed numbers, to illustrate the predicted number of cases and deaths without the vaccination program, that is, the “counterfactual” scenario. Without the vaccination program, an estimated 1,722,437 (95% CI: 1,634,589–1,815,561) cases and 28,059 (95% CI: 16,003–43,122) deaths would have been observed. In other words, 564,596 (95% CI: 477,020–657,525) COVID-19 cases and 18,622 (95% CI: 6522–33,762) deaths were prevented owing to the vaccination program by the end of November 2021. Because the vaccine rollout was particularly accelerated before July (i.e., 1 month before the Tokyo Olympic Games), the discrepancies between the observed confirmed COVID-19 cases and COVID-19-related deaths and the estimated values become evident from around that period. However, the gaps between observed and counterfactual values later plateaued because the incidences during the fifth epidemic wave were greatly reduced. Table 2 summarizes the total number of prevented cases and deaths among at least partially vaccinated individuals, fully vaccinated individuals, and partly vaccinated individuals during the study period.
Figure 4.
Counterfactual scenarios and total numbers of prevented cases and deaths that are attributable to the reduced risk among vaccinated individuals. Total number of averted cases from 3 March to 30 November 2021 (a) and averted deaths related to COVID-19 from 24 March to 30 November 2021 (b) are shown. Red lines represent actual observed COVID-19 cases and deaths, and blue lines represent the estimates of counterfactual scenarios without vaccination. Shaded areas describe 95% confidence intervals.
Table 2.
Total number of prevented cases and deaths by age, sex, and vaccination status that are attributable to reduced risk among vaccinated individuals.
| Age group | Prevented outcomes |
|||
|---|---|---|---|---|
| Cases infected with SARS-CoV-2 | Deaths related to COVID-19 | |||
| At least partially vaccinated people | ||||
| Male | Female | Male | Female | |
| 15–24 | 25,463 (22,700–28,304) | 28,132 (24,918–31,495) | 6 (0–19) | 1 (-4–8) |
| 25–34 | 38,931 (35,162–42,758) | 36,183 (32,138–40,373) | 15 (0–39) | 1 (-15–16) |
| 35–44 | 31,711 (28,001–35,557) | 27,670 (23,718–31,823) | 58 (-3–160) | 20 (0–54) |
| 45–54 | 38,653 (33,546–44,040) | 42,704 (36,380–49,569) | 347 (51–772) | 69 (-4–189) |
| 55–64 | 32,905 (27,358–38,803) | 33,294 (26,994–40,136) | 1023 (219–2062) | 316 (-8–799) |
| ≥ 65 | 103,637 (83,427–125,170) | 125,313 (102,679–149,496) | 9487 (3906–16,281) | 7277 (2379–13,362) |
| ≥ 15 | 271,300 (230,194–314,632) | 293,297 (246,826–342,892) | 10,938 (4174–19,334) | 7684 (2348–14,428) |
| Fully vaccinated people | ||||
| Male | Female | Male | Female | |
| 15–24 | 16,771 (14,992–18,606) | 19,476 (17,256–21,785) | 4 (0–13) | 0 (-5–6) |
| 25–34 | 27,764 (25,164–30,473) | 26,628 (23,679–29,656) | 10 (1–26) | 1 (-9–11) |
| 35–44 | 22,069 (19,581–24,673) | 19,793 (16,997–22,722) | 38 (0–104) | 12 (-1–35) |
| 45–54 | 24,674 (21,493–28,117) | 27,456 (23,262–32,027) | 221 (40–489) | 41 (-2–110) |
| 55–64 | 20,001 (16,465–23,831) | 20,039 (15,943–24,601) | 720 (149–1,451) | 225 (10–569) |
| ≥ 65 | 80,592 (64,535–97,988) | 98,262 (80,048–117,887) | 8097 (3453–13,723) | 6190 (2108–11,241) |
| ≥ 15 | 191,870 (162,230–223,688) | 211,656 (177,185–248,679) | 9090 (3643–15,805) | 6469 (2102–11,971) |
| Partly vaccinated people | ||||
| Male | Female | Male | Female | |
| 15–24 | 8692 (7717–9708) | 8655 (7638–9696) | 2 (0–7) | 1 (-1–3) |
| 25–34 | 11,167 (10,004–12,363) | 9555 (8472–10,720) | 5 (0–12) | 0 (-6–5) |
| 35–44 | 9642 (8409–10,859) | 7877 (6718–9077) | 20 (-2–60) | 8 (2–18) |
| 45–54 | 13,979 (12,068–15,946) | 15,248 (13,117–17,519) | 126 (9–280) | 28 (0–70) |
| 55–64 | 12,905 (10,902–15,057) | 13,254 (11,068–15,549) | 303 (60–628) | 91 (-14–236) |
| ≥ 65 | 23,045 (18,998–27,419) | 27,051 (22,687–31,779) | 1391 (496–2504) | 1087 (290–2078) |
| ≥ 15 | 79,430 (68,098–91,352) | 81,641 (69,699–94,339) | 1847 (564–3491) | 1215 (270–2409) |
The study period of each outcome was from 3 March to 30 November 2021 and from 24 March to 30 November 2021 for cases and deaths, respectively.
The 95% confidence intervals are presented in parentheses.
Discussion
According to the Prime Minister's Office, the total number of available doses of COVID-19 vaccine in Japan reached more than 197 million by the end of November 2021, achieving vaccination coverage of 75% of the population.13,16 In such a highly vaccinated county with widespread use of mRNA vaccines, we estimated that the vaccination program prevented 564,596 (95% CI: 477,020–657,525) COVID-19 cases and 18,622 (95% CI: 6522–33,762) COVID-19-related deaths, correlating with reductions of 33% and 67% in cases and deaths, respectively. The highest numbers of averted cases and deaths were seen among those aged 65 years and older for both men and women. Whereas 103,637 (95% CI: 83,427–125,170) and 125,313 (95% CI: 102,679–149,496) cases in male and female individuals were prevented, the averted number of deaths was 9487 (95% CI: 3906–16,281) and 7277 (95% CI: 2379–13,362) in male and female individuals, respectively.
To the best of our knowledge, the present study is the first to demonstrate the substantial direct benefit of the vaccination program in Western Pacific countries where a relatively low incidence of COVID-19 had been maintained prior to emergence of the Omicron variant. As a result of the rapid vaccine rollout and high vaccination coverage, we showed that more than 30% of COVID-19 cases and two-thirds of COVID-19-related deaths were prevented, especially among older people. During the fifth wave, the Delta variant acted as a trigger for another epidemic wave and incidences rose rapidly, coinciding with the start of the Tokyo Olympic Games. By comparing the observed values and counterfactual estimates, we objectively showed that the vaccination program substantially reduced the epidemiological impact during this period.
An important technical caveat is that the direct effectiveness of the vaccination program can be evaluated by simply comparing the daily incidence between vaccinated and unvaccinated individuals as a function of time, as was done in Israel.22 This can be achieved when the vaccination coverage is available and when the vaccination history of cases is consistently recorded over time. However, it must be noted that, other than the direct effect as presented here, indirect effects have a tremendous impact on the epidemiological dynamics. In many countries with substantially high vaccination coverage, herd immunity was at least temporarily and locally achieved, and the disease incidence was greatly reduced. The total vaccination effect can be broken down into actual observations and counterfactual estimates, i.e., stacked additional cases/deaths if the vaccination program were not implemented. The effects can also be divided into indirect effects and direct effects, as per the following equation:
| (5) |
However, measuring the indirect effect is technically challenging; for example, we may have to compare the observed cumulative number of cases and deaths against theoretically reconstructed epidemic counterfactual scenarios, that is,
| (6) |
where C stands for the cumulative number of cases. The former term on the right-hand side requires very careful study design or simulations and we would need to know how many cases and deaths there would have been without the vaccination program. To compare our estimate of direct effect against possible indirect effect, we conducted an ad hoc analysis using a compartmental model (see Supplementary material). It was shown that indirect effect would lead to prevention of more than hundred times and thirty times greater number of cases and deaths, respectively, than those attributed to direct effect. The result indicates that indirect effect would involve enormously large population impact. For precise estimation, we need to carefully derive the indirect effect adjusting for other countermeasures, and developing an alternative counterfactual model for the estimation is our ongoing future study.
It is obvious that various PHSM including lockdowns have played crucial roles in suppressing the number of COVID-19 cases and deaths in many countries since the pandemic started. During the vaccination program, a state of emergency was declared three times in Japan, with the second state of emergency lasting from 7 January to 21 March 2021, which overlapped with the initial stage of the vaccination program prioritizing health care professionals (Figure 1B). Other states of emergency were declared between 25 April and 20 June and between 12 July and 30 September 2021 in the fourth and fifth epidemic waves, respectively. The coverage of each prefecture depended on the timing of its declaration, but Tokyo and Osaka, which usually bore the highest number of cases, were generally covered under the states of emergency. In the present study, we did not explicitly account for the impact of PHSM on the estimated numbers of averted cases and deaths. In other words, without PHSM, the size of the epidemic would be expected to be greater than that observed, and thus, the prevented cases and deaths would also have been elevated compared with those reported in the present study. Similar observations have been reported in Israel and other countries.22, 23, 24, 25, 26 However, the median daily incidence differences in cases of the present study were estimated to be approximately 5 per 100,000 population, which were much lower than those in Israel (72 per 100,000 population).22 Examining the ratios of the total number of prevented cases/deaths to observed values in both countries, the differences can be explained by the lower incidences themselves rather than waning immunity effects and incidence differences between unvaccinated and vaccinated people. Although Japan is a super-aging society, which imposes a greater COVID-19-related burden, PHSM and other interventions maintained the epidemic level and disease burdens at relatively low levels during the period of study.
Although our study mainly focused on the effects for at least partially vaccinated people, the total numbers of averted cases and deaths were also calculated according to vaccination status. Our estimates indicated that the effect of two doses contributed to reducing COVID-19 cases and deaths by 2·5 and 5·1 times, respectively, compared with one-dose vaccination. However, the effects of the first dose appeared not to be small, implying that we could expect a certain degree of vaccine effectiveness among partly vaccinated people. Furthermore, we explored the difference in outcomes by sex. The total number of cases prevented among female individuals was estimated to be 293,297 (95% CI: 246,826–342,892), which was higher than that among male individuals, estimated to be 271,300 (95% CI: 230,194–314,632). These estimates were influenced not only by the vaccination program but also by other factors such as the volume of the number of COVID-19 cases. Therefore, accounting for high-risk (socially active) behavior, it is reasonable that more cases/deaths were averted among male individuals aged 25–44 years than among female individuals. However, an estimated total 9487 (3906–16,281) deaths in male individuals were prevented, which was higher than this number in their female counterparts, estimated to be 7277 (2379–13,362). The same trends were recognized in all groups aged 15 years and older, which may be explained by the biological mechanisms underlying sex differences.
This study has four limitations. First, the averted number of hospitalizations was not consistently collected over time, precluding explicit estimation of the impact of vaccination on hospitalizations in Japan. Setting up hospitalization surveillance to systematically register and monitor admitted individuals along with their vaccination history would be required for this analysis. Second, we imposed an assumption that all individuals vaccinated with a first dose received a second dose at a constant interval. In Japan, the vaccination program explicitly recommended that people receive a second dose exactly 21 days after the first dose; however, there could have been discrepancies, particularly when using mRNA-1273 vaccine, which adopts a 28-day rather than 21-day interval. In fact, the vaccination rates at the end of 2021 among people who received the first dose and were fully vaccinated were 74·7% and 74·1%, respectively.13 Third, we ignored waning immunity in our analysis. Vaccine-induced immunity gradually wanes, and a reduction in vaccine efficacy against SARS-CoV-2 has been observed in 6-month follow-up research.29, 30 Our study period was approximately 9 months, from March to November; however, the rate of vaccination was accelerated from June to August, and we believe that the impact of ignoring the effect of waning immunity would be minimal. Fourth, geographic heterogeneity in the vaccination rollout was not taken into account. For example, as of the end of October 2021, the lowest vaccination coverage for the first dose was estimated to be 62% in Okinawa, and the highest coverage was 76% in Akita.16 An analysis focused on the impact of such gaps will be addressed in our future studies.
In the current study, we successfully quantified the direct effectiveness of the mass vaccination program in Japan. Substantial numbers of cases and deaths were prevented owing to mRNA vaccination, correlating to reductions of 33% and 67%, respectively, as compared with counterfactual scenarios without vaccination. Vaccine-induced immunity against SARS-CoV-2 is the safest and most effective means of reducing the number of COVID-19 cases and deaths, thereby limiting the burden on health care systems. The preventive effect of vaccination will be further amplified as the pandemic proceeds.
Using a statistical model, in the present study, we estimated the averted number of COVID-19 cases and deaths, by age and sex, that can be attributed to the reduced risk resulting from vaccination in Japan from March to November 2021. The estimated numbers were highest among those aged 65 years and older. For individuals who were at least partially vaccinated, we estimated that 564,596 (95% CI: 477,020–657,525) cases and 18,622 (95% CI: 6522–33,762) deaths were successfully prevented, representing a reduction in risk of 33% and 67%, respectively. Our findings confirm that the vaccination program was highly successful in Japan during the Delta variant epidemic wave. As vaccination continues among all eligible individuals, the preventive effects will be further amplified.
Contributors
All authors participated in the study design. T.K., M.S., T.K., K.Y., and K.O collected and retrieved the data. T.K. analyzed the data and H.N. evaluated the validity of analysis. T.K. drafted the manuscript, including the figures and tables. H.N. edited earlier versions of the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
Data on confirmed cases are available online (Open data, Ministry of Health, Labour and Welfare; https://www.mhlw.go.jp/stf/covid-19/open-data.html). Data on deaths are publicly available elsewhere.27
Declaration of interests
All authors declare no competing interests in this paper.
Acknowledgments
H.N. received funding from Health and Labour Sciences Research Grants (20CA2024, 20HA2007, 21HB1002 and 21HA2016); the Japan Agency for Medical Research and Development (AMED; JP20fk0108140, JP20fk0108535 and JP21fk0108612); Japan Society for the Promotion of Science (JSPS) KAKENHI (21H03198); and Japan Science and Technology Agency (JST) SICORP program (JPMJSC20U3 and JPMJSC2105). T.K. received funding from the JSPS KAKENHI (21K10495). We thank Analisa Avila, MPH, ELS, of Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This project was supported by the Japan Science and Technology Agency; the Japan Agency for Medical Research and Development; the Japan Society for the Promotion of Science; and the Ministry of Health, Labour and Welfare. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100571.
Appendix. Supplementary materials
References
- 1.Bavel JJV, Baicker K, Boggio PS, et al. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav. 2020;4:460–471. doi: 10.1038/s41562-020-0884-z. 2020 45. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. 2020 5847820. [DOI] [PubMed] [Google Scholar]
- 3.Osterrieder A, Cuman G, Pan-Ngum W, et al. Economic and social impacts of COVID-19 and public health measures: results from an anonymous online survey in Thailand, Malaysia, the UK, Italy and Slovenia. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persad G, Peek ME, Emanuel EJ. Fairly prioritizing groups for access to COVID-19 vaccines. JAMA. 2020;324:1601–1602. doi: 10.1001/jama.2020.18513. [DOI] [PubMed] [Google Scholar]
- 7.Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19: who to vaccinate first? Sci Adv. 2021;7 doi: 10.1126/SCIADV.ABF1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw R, Kim Yk, Hua J. Governance, technology and citizen behavior in pandemic: lessons from COVID-19 in East Asia. Prog Disaster Sci. 2020;6 doi: 10.1016/j.pdisas.2020.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health Labour and Welfare of Japan. Situation report on COVID-19. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html. Accessed 19 October 2021.
- 10.Watanabe T, Yabu T. Japan's voluntary lockdown. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health Labour and Welfare of Japan. COVID-19 vaccines.https://www.mhlw.go.jp/stf/covid-19/vaccine.html. Accessed 19 October 2021.
- 12.Prime Minister's Office of Japan. Supply schedule of COVID-19 vaccine. https://www.kantei.go.jp/jp/headline/kansensho/vaccine_supply.html. Accessed 2 November 2021.
- 13.Government CIOs’ Portal Japan. Regarding COVID-19 vaccine. https://cio.go.jp/ Accessed 20 October 2021.
- 14.Ministry of Health Labour and Welfare of Japan. Partial revision of the implementation of vaccinations for COVID-19 (instructions). https://www.mhlw.go.jp/content/000786653.pdf. Accessed 20 October 2021.
- 15.The Japan Times. Japan won't resume accepting workplace vaccination applications. https://www.japantimes.co.jp/news/2021/06/29/national/coronavirus-workplace-vaccinations-applications/ Accessed 20 October 2021.
- 16.Prime Minister's Office of Japan. Daily achievements: COVID-19 vaccine. https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html. Accessed 20 October 2021.
- 17.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L, Hijano DR, Gaur AH, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325:2500–2502. doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernal JL, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin JM, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2021;22:357–366. doi: 10.1016/S1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacco C, Mateo-Urdiales A, Petrone D, et al. Estimating averted COVID-19 cases, hospitalisations, intensive care unit admissions and deaths by COVID-19 vaccination, Italy, January−September 2021. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.47.2101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meslé MMI, Brown J, Mook P, et al. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.47.2101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoukat A, Vilches TN, Moghadas SM, et al. Lives saved and hospitalizations averted by COVID-19 vaccination in New York City: a modeling study. Lancet Reg Heal Am. 2022;5 doi: 10.1016/j.lana.2021.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilches TN, Sah P, Moghadas SM, et al. COVID-19 hospitalizations and deaths averted under an accelerated vaccination program in northeastern and southern regions of the USA. Lancet Reg Heal Am. 2022;6 doi: 10.1016/j.lana.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Japan Broadcasting Corporation. A special content: confirmed cases, deaths and severe cases of COVID-19. https://www3.nhk.or.jp/news/special/coronavirus/data-all/. Accessed 20 October 2021.
- 28.Statistics Bureau: Ministry of Internal Affairs and Communications Japan. Summary of population estimates. https://www.stat.go.jp/data/jinsui/2.html#monthly. Accessed 20 October 2021.
- 29.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Heal - Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas SJ, Edson D, Moreira J, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.