Abstract
Data on updated hyperuricemia prevalence in Beijing-Tianjin-Hebei (BTH) region in China, which is one of the world-class urban agglomerations, is sparse. Overweight/obesity, alcohol consumption, smoking and sedentary behavior are modifiable risk factors (MRFs) for elevated serum uric acid (SUA), but their population attributable fractions (PAFs) for hyperuricemia is still unclear. Using baseline data from the BTH Physical Examination General Population Cohort, we calculated the crude- and adjusted-prevalence of hyperuricemia based on the 30,158 participants aged 18–80 years. Hyperuricemia was defined as SUA >420 μmol/L in men and >360 μmol/L in women, or currently use of uric acid lowering drugs. Overweight/obesity, alcohol consumption, smoking and sedentary behavior were considered as MRFs and their adjusted PAFs were estimated. The prevalence of hyperuricemia was 19.37%, 27.72% in men and 10.69% in women. The PAFs and 95% confidence intervals for overweight, obesity were 16.25% (14.26–18.25%) and 12.08% (11.40–12.77%) in men, 13.95% (12.31–15.59%) and 6.35% (5.97–6.74%) in women, respectively. Alcohol consumption can explain 4.64% (2.72–6.56%) hyperuricemia cases in men, but with no statistical significance in women. Cigarette smoking contributed to 3.15% (1.09–5.21%) cases in men, but a much lower fraction in women (0.85%, 0.49–1.22%). Compared with sedentary time <2 h per day, the PAFs of 2–4 h, 4–6 h, and more than 6 h per day were 3.14% (1.34–4.93%), 6.72% (4.44–8.99%) and 8.04% (4.95–11.13%) in men, respectively. Sedentary time was not found to be associated with hyperuricemia in women. These findings concluded that hyperuricemia is prevalent in this representative Chinese adult general population with substantial sex difference. Four MRFs (overweight/obesity, alcohol consumption, cigarette smoking and sedentary behavior) accounted for a notable proportion of hyperuricemia cases. The PAF estimations enable the exploration of the expected proportion of hyperuricemia cases that could be prevented if the MRFs were removed, which warrants the public health significance of life-style intervention.
Keywords: serum uric acid, body mass index, alcohol consumption, cigarette smoking, sedentary behavior, attributable fraction
Introduction
Serum uric acid (SUA) is the catabolic product of exogenous dietetic compounds and endogenous purines (1). Hyperuricemia is defined as the presence of elevated SUA. Hyperuricemia is not only necessary for the development of gout, but is also associated with cardiometabolic disease and kidney disorders (2–4), and even premature mortality (5–7). The prevalence of hyperuricemia is high in China. Results from the China National Health Survey revealed that the prevalence of hyperuricemia in mainland China is around 20% (8). Modifiable risk factors (MRFs), such as overweight/obesity, alcohol consumption, and sedentary behavior, were found to significantly increase the SUA level (8–11) and some major chronic diseases, such as hypertension, chronic kidney disease and dyslipidemia, which are commonly comorbid with hyperuricemia (12–14). Therefore, identifying individuals with high risk of hyperuricemia would benefit disease prevention and health promotion, and help the health professionals determine the priority population for intervention.
In epidemiological studies, relative risk (or odds ratio in a case-control scenario) is often used to assess the strength of health effect of a given exposure on a particular event. However, this measure did not consider prevalence of the exposure and thus cannot estimate the disease burden comprehensively (7). Population attributable fraction (PAF) is the proportion of cases for an outcome within a population that can be attributed to a certain risk factor, related with both exposure prevalence and its effect size (15). The focus on modifiable risk factors (MRFs), such as overweight/obesity, smoking, alcohol consumption and sedentary behavior, could provide real-world evidence on to what level the health hazards would have been avoided if people in high-risk changed their behavior. Furthermore, by calculating PAF, the importance of different MRFs could be estimated in sub-populations, thus helps policy makers to develop more tailored and targeted health promotion strategies. Previous cross-sectional studies have estimated several MRFs' PAF (8, 15, 16), but updated data derived from general adult Chinese is sparse.
The Beijing-Tianjin-Hebei (BTH) area is one of the world-class urban agglomerations in China. As the political center and third largest economy in China, this region accounts for 8.1% of China's population and is experiencing rapid socio-economic development and urbanization (17). Initiated in 2017, supported by the National Key R&D Program of China, the BTH Physical Examination Cohort aimed to explore determinants of non-communicable diseases using multidisciplinary methods, and update the real-world data to enhance health promotion. In recent years, the disease burden of hyperuricemia is increasing with various risk factors contributing to its rapid growth (18). Hence, using baseline data from the BTH Physical Examination Cohort, we aim to explore the hyperuricemia prevalence and the PAFs of MRFs in this general population.
Methods
Data resource and study population
This study was conducted using baseline data of the BTH Physical Examination General Population Cohort, which is a prospective cohort study initiated in 2017, containing over 30,000 adults recruited in the BTH area. The baseline survey was conducted from 2017 to 2018, with a multi-stage stratified cluster sampling method used in enrolling attendees at physical examination centers. The selection and sampling procedure: (1) among records of more than 200 physical examination centers in tertiary care hospitals, we randomly selected two or three medical examination centers by systematic sampling; (2) stable employees in organizations, institutions, and companies were selected with multiple labor categories (white collar, pink collar, or blue collar) from each selected physical examination center in the first stage. Finally, 100–200 registered organizations, institutions and companies for each occupation category were selected, within which individuals aged 18 years and above, living in the local place for at least 1 year, were asked to voluntarily participate in the study. Individuals who were pregnant, with severe mental or physical disorders were excluded.
The study has been carried out in accordance with the Declaration of Helsinki. Ethical approval was obtained from the ethics review boards of Nankai University (NKUIRB2016063), Tianjin First Central Hospital (2017N052KY), Tianjin Union medical center (2018C02) and Hebei Medical University (2016021). Written informed consent was obtained from each participant before the survey.
Measurements
An id card reader has been used to automatically collect the subject's unique ID number, sex, birthday, and living address, and save the information into electronic database.
Sociodemographic and lifestyle information (age, sex, educational level, smoking, alcohol drinking, and daily sedentary time) were collected by standardized questionnaire interview. Immediately after the interview, a scanning software which was designed for this study was used to transform the hand-written information into electronic database in real time and save as excel files.
All subjects had standard physical and anthropometry measurements and provided blood samples for biochemical tests. This information will be linked with their annual physical examination electronic records to facilitate the follow-up.
During the anthropometric measurements, participants wore light clothing and were barefoot. Height (cm) and weight (kg) were measured using the same device (GL-310, Seoul, Korea) in different areas, with the accuracy on a decimal level.
Venous blood samples were drawn after an at least 8 h-overnight fast and were immediately sent to the laboratory of the physical examination centers for biochemical tests, which were performed using the same instrument (Hitachi 7600, Japan). SUA was measured by oxidization with the specific enzyme uricase.
Outcome definition
Hyperuricemia was defined as SUA higher than 360 μmol/L (~6 mg/dl) in women and 420 μmol/L (~7.0 mg/dl) in men (12), or currently use of uric acid lowering drugs.
Assessment of modifiable risk factors and other covariates
Overweight and obesity, ever-smoking, current alcohol drinking and daily sedentary time were considered as MRFs in the study. Underweight was defined as BMI < 18.5 kg/m2, normal weight was BMI between 18.5 kg/m2 and <24 kg/m2, overweight was BMI ≥ 24 kg/m2 but <28 kg/m2, and obesity was BMI ≥ 28 kg/m2, according to the recommendation suitable for the Chinese population (19). Information on smoking, alcohol drinking and daily sedentary time were collected by self-report in the face-to-face questionnaire interview. Participants who reported smoking for over half 1 year were defined as current smokers. Those who ever smoked but quit for at least half 1 year were defined as prior smokers. Individuals who consumed alcohol at least once per week were defined as current drinkers, and who had quit drinking alcohol for at least half 1 year were prior drinkers. Smoking status was grouped as never, prior, and current smoking. Ever-smoking was considered as either prior or current smoking. Alcohol drinking was categorized as never, prior and current drink. Daily sedentary time was classified as four groups: <2 h per day, 2–4 h per day, 4–6 h per day, and more than 6 h per day.
Estimated glomerular filtration rate (eGFR, ml/min per 1.73 m2) was calculated according to the Modification of Diet in Renal Disease equation for Chinese (c-MDRD) (20). Menopausal status was self-reported by female subjects in the face-to-face interview. Participants included in the survey were asked whether they had at least one menstrual bleeding in the past year to define menopause, excluding those caused by medications, hormones, medical conditions, or surgical procedures.
Statistical analyses
The original data contains 31,311 participants. A total of 30,158 subjects were included in the final analyses after excluding subjects aged over 80 (n = 541, to avoid “healthy worker” effect), or with missing values on both serum uric acid level and information on uric-acid-lowering medication use (n = 436) or had an eGFR < 60 ml/min per 1.73 m2 (n = 176) to exclude possible kidney disorders (21).
The difference in basic characteristics between men and women were tested using chi-square test for grouped data or Wilcoxon rank test for non-normally distributed continuous data. The prevalence of hyperuricemia was adjusted using logistic regression models when compared among groups (22). General linear regression models (GLMs) were used to examine the relationship between MRFs and SUA. As odds ratio (OR) may overestimate the health effect when the outcome is not rare (10), prevalence ratio (PR) was used to estimate PAF. PAF was calculated as follow:
Pdi refers to the proportion of cases in the ith exposure level, and PR represents the prevalence risk (which is a simulation of relative risk) comparing the ith exposure level with the unexposed group (I = 0) (23). A modified Poisson regression models were used to estimate the adjusted PAFs, which can be added to yield the integrated fraction that can be attributable to modifiable risk factors for hyperuricemia (24).
Furthermore, we did sensitivity analyses in three ways: (1) stratified analysis by menopause status to explore whether menopause modified the effect of MRFs on hyperuricemia; (2) excluding subjects with diagnosed chronic diseases to avoid possible inverse causality due to behavior change after awareness of disease; and (3) age- and sex- stratified analyses on PAFs estimation to learn whether there was age-varied effect of the MRFs on hyperuricemia.
For all coefficients and PR estimates, we calculated their 95% confidence intervals (CIs). A p-value (two-sided) < 0.05 was considered as statistically significant. SAS (version 9.4, SAS institute, Cary, NC) was used for statistical analyses.
Results
Basic characteristics and hyperuricemia prevalence
The mean age of the study population was 45.33 years (SD = 13.78 years), 45.99 years (SD = 13.79 years) in men and 44.65 years (SD = 13.74 years) in women. More than half (51%) were men. The median SUA level was 319 μmol/L (interquartile range: 121), 368.30 μmol/L (interquartile range: 107.11) and 271.18 μmol/L (interquartile range: 82.06) in men and women, respectively. The overall prevalence of hyperuricemia was 19.37%, 27.72% in men and 10.69% in women, respectively. The hyperuricemia prevalence in sub-groups, stratified by sex, was summarized in Table 1. Either in men or women, people in Tianjin area had lower hyperuricemia prevalence (24.24% in men and 8.98% in women). The patterns of hyperuricemia prevalence with aging were different between sexes, decreasing in men but increasing in women (both P for trend < 0.001). People with higher BMI, ever smoking cigarettes, ever drinking alcohol, with longer daily sedentary time had higher prevalence of hyperuricemia (Table 1).
Table 1.
The prevalence of hyperuricemia in general population from the Beijing, Tianjin, and Hebei areas (n = 30,158).
| Male (n = 15,381) | Female (n = 14,777) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Crude- P | Adjusted- P * and 95% CI | N | Crude- P | Adjusted- P * and 95% CI | |||||
| Demographic characteristics | ||||||||||
| Areas | ||||||||||
| Beijing | 4,854 | 34.84 | 33.15 | 31.81 | 34.52 | 3,709 | 12.91 | 13.16 | 12.09 | 14.31 |
| Tianjin | 4,149 | 24.24 | 23.47 | 22.43 | 24.55 | 7,450 | 8.98 | 9.09 | 8.44 | 9.77 |
| Hebei | 6,378 | 24.73 | 25.02 | 23.70 | 26.39 | 3,618 | 11.94 | 11.92 | 10.89 | 13.04 |
| Age groups | ||||||||||
| 20– | 2,062 | 32.88 | 32.62 | 30.62 | 34.70 | 2,290 | 9.56 | 10.04 | 8.84 | 11.38 |
| 30– | 4,014 | 35.58 | 35.31 | 33.81 | 36.83 | 4,179 | 8.69 | 9.13 | 8.27 | 10.08 |
| 40– | 3,336 | 27.52 | 27.57 | 26.07 | 29.12 | 3,444 | 7.61 | 7.86 | 6.99 | 8.82 |
| 50– | 3,313 | 22.64 | 22.85 | 21.44 | 24.32 | 2,383 | 13.85 | 14.55 | 13.15 | 16.07 |
| 60–80 | 2,656 | 18.41 | 18.94 | 17.47 | 20.50 | 2,481 | 16.36 | 16.90 | 15.45 | 18.45 |
| Ethnicity | ||||||||||
| Han | 14,844 | 27.53 | 26.92 | 26.17 | 27.69 | 14,141 | 10.69 | 11.25 | 10.71 | 11.82 |
| Others | 537 | 32.77 | 29.79 | 26.08 | 33.79 | 636 | 10.85 | 11.78 | 9.39 | 14.68 |
| Educational level | ||||||||||
| Primary/junior high school | 1,805 | 19.56 | 20.52 | 18.62 | 22.57 | 1,166 | 11.49 | 10.32 | 8.70 | 12.21 |
| Senior high school | 2,257 | 23.97 | 24.75 | 22.95 | 26.64 | 1,596 | 14.41 | 12.32 | 10.80 | 14.01 |
| College | 9,003 | 29.98 | 29.13 | 28.16 | 30.13 | 9,036 | 10.98 | 11.76 | 11.06 | 12.48 |
| Postgraduate | 670 | 28.93 | 26.66 | 24.79 | 28.62 | 224 | 7.52 | 9.29 | 8.14 | 10.59 |
| Modifiable risk factors | ||||||||||
| Body mass index (kg/m2) | ||||||||||
| <24 | 5,075 | 18.03 | 17.15 | 16.13 | 18.22 | 8,954 | 6.85 | 7.39 | 6.83 | 7.99 |
| 24- | 6,707 | 27.82 | 28.00 | 26.89 | 29.14 | 4,078 | 14.71 | 15.05 | 13.93 | 16.25 |
| ≥28 | 3,260 | 41.96 | 41.44 | 39.69 | 43.21 | 1,328 | 23.64 | 23.73 | 21.45 | 26.16 |
| Cigarette smoking | ||||||||||
| Never | 8,941 | 27.14 | 25.50 | 24.56 | 26.46 | 14,470 | 10.59 | 11.18 | 10.64 | 11.74 |
| Prior | 1,197 | 27.23 | 32.06 | 29.27 | 34.99 | 55 | 16.36 | 16.58 | 8.81 | 29.02 |
| Current | 5,243 | 28.80 | 28.40 | 27.15 | 29.68 | 252 | 15.48 | 15.70 | 11.64 | 20.84 |
| Alcohol drinking | ||||||||||
| Never | 8,671 | 25.64 | 25.01 | 24.08 | 25.98 | 13,998 | 10.66 | 11.25 | 10.69 | 11.82 |
| Prior | 407 | 24.82 | 29.00 | 24.52 | 33.93 | 48 | 14.58 | 14.68 | 7.12 | 27.85 |
| Current drinking | 6,303 | 30.76 | 29.65 | 28.49 | 30.84 | 731 | 11.08 | 11.52 | 9.34 | 14.12 |
| Daily sedentary time | ||||||||||
| <2 h | 1,685 | 22.20 | 20.96 | 19.06 | 22.99 | 1,305 | 11.11 | 9.80 | 8.33 | 11.48 |
| 2–4 h | 3,130 | 27.41 | 26.39 | 24.85 | 27.99 | 2,605 | 11.36 | 10.43 | 9.32 | 11.66 |
| 4–6 h | 4,548 | 29.64 | 28.95 | 27.61 | 30.33 | 4,169 | 10.70 | 11.33 | 10.36 | 12.38 |
| >6 h | 6,018 | 27.97 | 27.90 | 26.68 | 29.15 | 6,698 | 10.35 | 12.06 | 11.20 | 12.98 |
The adjusted covariates were age and areas.
CI, confidence interval.
Distribution of modifiable risk factors and their effect on SUA
The distribution of MRFs in different age groups and sex were presented in Table 2. The proportions of overweight and obesity in men were much higher than that in women in each age group. Over 60% of men aged 30 and above were overweight. An increasing trend of overweight and obesity with aging was observed in women (P for trend < 0.001). The proportions of other MRFs except for long sedentary time were also higher in men than in women (Table 2).
Table 2.
The distribution of modifiable risk factors in the study population (n = 30,158).
| Age-groups | 20– | 30– | 40– | 50– | 60–80 | Overall | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Men | |||||||||||||
| Body mass index categories | <0.001 | ||||||||||||
| Under/normal weight | 915 | 44.37 | 1,298 | 32.34 | 1,037 | 31.09 | 1,004 | 30.30 | 821 | 30.91 | 5,075 | 33.00 | |
| Overweight | 716 | 34.72 | 1,673 | 41.68 | 1,473 | 44.15 | 1,577 | 47.60 | 1,268 | 47.74 | 6,707 | 43.61 | |
| Obesity | 406 | 19.69 | 941 | 23.44 | 746 | 22.36 | 653 | 19.71 | 514 | 19.35 | 3,260 | 21.19 | |
| Cigarette smoking | <0.001 | ||||||||||||
| Never | 1,368 | 66.34 | 2,603 | 64.85 | 1,906 | 57.13 | 1,602 | 48.35 | 1,462 | 55.05 | 8,941 | 58.13 | |
| Ever | 54 | 2.62 | 130 | 3.24 | 242 | 7.25 | 291 | 8.78 | 480 | 18.07 | 1,197 | 7.78 | |
| Current | 640 | 31.04 | 1,281 | 31.91 | 1,188 | 35.61 | 1,420 | 42.86 | 714 | 26.88 | 5,243 | 34.09 | |
| Alcohol drinking | <0.001 | ||||||||||||
| Never | 1,313 | 63.68 | 2,345 | 58.42 | 1,736 | 52.04 | 1,661 | 50.14 | 1,616 | 60.84 | 8,671 | 56.37 | |
| prior | 18 | 0.87 | 36 | 0.90 | 86 | 2.58 | 121 | 3.65 | 146 | 5.50 | 407 | 2.65 | |
| Current | 731 | 35.45 | 1,633 | 40.68 | 1,514 | 45.38 | 1,531 | 46.21 | 894 | 33.66 | 6,303 | 40.98 | |
| Daily sedentary time | <0.001 | ||||||||||||
| <2 h | 179 | 8.68 | 269 | 6.7 | 370 | 11.09 | 531 | 16.03 | 336 | 12.65 | 1,685 | 10.96 | |
| 2–4 h | 408 | 19.79 | 682 | 16.99 | 645 | 19.33 | 656 | 19.80 | 739 | 27.82 | 3,130 | 20.35 | |
| 4–6 h | 566 | 27.45 | 1,252 | 31.19 | 1,025 | 30.73 | 925 | 27.92 | 780 | 29.37 | 4,548 | 29.57 | |
| >6 h | 909 | 44.08 | 1,811 | 45.12 | 1,296 | 38.85 | 1,201 | 36.25 | 801 | 30.16 | 6,018 | 39.13 | |
| Women | |||||||||||||
| Body mass index categories | <0.001 | ||||||||||||
| Under/normal weight | 1,824 | 79.65 | 2,882 | 68.96 | 1,968 | 57.14 | 1,201 | 50.40 | 1,079 | 43.49 | 8,954 | 60.59 | |
| Overweight | 298 | 13.01 | 867 | 20.75 | 1,054 | 30.60 | 868 | 36.42 | 991 | 39.94 | 4,078 | 27.60 | |
| Obesity | 88 | 3.84 | 281 | 6.72 | 323 | 9.38 | 272 | 11.41 | 364 | 14.67 | 1,328 | 8.99 | |
| Cigarette smoking | <0.001 | ||||||||||||
| Never | 2,254 | 98.43 | 4,095 | 97.99 | 3,391 | 98.46 | 2,328 | 97.69 | 2,402 | 96.82 | 14,470 | 97.92 | |
| Ever | 36 | 1.57 | 84 | 2.01 | 53 | 1.54 | 55 | 2.31 | 79 | 3.18 | 307 | 2.08 | |
| Alcohol drinking | <0.001 | ||||||||||||
| Never/prior | 2,124 | 92.75 | 3,983 | 95.31 | 3,272 | 95.01 | 2,266 | 95.09 | 2,401 | 96.78 | 14,046 | 95.05 | |
| Current | 166 | 7.25 | 196 | 4.69 | 172 | 4.99 | 117 | 4.91 | 80 | 3.22 | 731 | 4.95 | |
| Daily sedentary time | <0.001 | ||||||||||||
| <2,h | 147 | 6.42 | 248 | 5.93 | 335 | 9.73 | 232 | 9.74 | 343 | 13.83 | 1,305 | 8.83 | |
| 2–4 h | 366 | 15.98 | 498 | 11.92 | 567 | 16.46 | 463 | 19.43 | 711 | 28.66 | 2,605 | 17.63 | |
| 4–6 h | 622 | 27.16 | 1,144 | 27.37 | 987 | 28.66 | 723 | 30.34 | 693 | 27.93 | 4,169 | 28.21 | |
| >6 h | 1,155 | 50.44 | 2,289 | 54.77 | 1,555 | 45.15 | 965 | 40.50 | 734 | 29.58 | 6,698 | 45.33 | |
SUA was significantly influenced by the MRFs, especially in men (Table 3). Excess body weight increased the SUA in both sexes. An average of 50.82 (95%CI: 47.30–54.35) μmol/L higher SUA level was observed in obese men, compared with under/normal weight men. Similar result was observed in obese women, with an elevated SUA of 50.04 (95%CI: 46.28–53.80) μmol/L, compared with their normal weight counterparts. Interestingly, longer daily sedentary time seemed to only influence male participants, without positive association observed in women. There were few women currently smoking or drinking alcohol, leading to marginal or non-significant statistical analysis results (Table 3).
Table 3.
The effect of modifiable risk factors on serum uric acid, stratified by sex.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Modifiable risk factors | B | 95% CI | P | B | 95% CI | P | ||||
| BMI categories | ||||||||||
| Under/normal weight | Ref | NA | NA | Ref | NA | NA | ||||
| Overweight | 23.51 | 20.61 | 26.41 | <0.001 | 25.86 | 23.46 | 28.25 | <0.001 | ||
| Obesity | 50.82 | 47.30 | 54.35 | <0.001 | 50.04 | 46.28 | 53.80 | <0.001 | ||
| Cigarette smoking | ||||||||||
| Never | Ref | NA | NA | Ref | NA | NA | ||||
| Prior | 8.77 | 3.68 | 13.87 | <0.001 | 13.07 | −5.90 | 32.03 | 0.177 | ||
| Current | 2.39 | −0.53 | 5.32 | 0.108 | 10.31 | 0.58 | 20.05 | 0.038 | ||
| Alcohol drinking | ||||||||||
| Never | Ref | NA | NA | Ref | NA | NA | ||||
| Prior | 3.98 | −4.1532 | 12.11 | <0.001 | 1.29 | −17.41 | 19.98 | 0.893 | ||
| Current | 8.68 | 5.97 | 11.39 | <0.001 | 2.85 | −2.16 | 7.85 | 0.265 | ||
| Daily sedentary time | ||||||||||
| <2 h | Ref | NA | NA | Ref | NA | NA | ||||
| 2–4 h | 7.19 | 2.45 | 11.93 | 0.003 | −1.31 | −5.60 | 2.98 | 0.550 | ||
| 4–6 h | 12.01 | 7.34 | 16.69 | <0.001 | −0.67 | −4.84 | 3.50 | 0.752 | ||
| >6 h | 11.27 | 6.63 | 15.91 | <0.001 | 0.09 | −3.98 | 4.15 | 0.966 | ||
B, regression coefficient; CI, confidence interval; NA, not applicable; BMI, body mass index, kg/m2.
The covariates adjusted in the regression model were age, geographic areas, body mass index, alcohol consumption, smoking status, daily sedentary time and serum creatinine. Menopausal status was additionally adjusted in women.
The PAF of modifiable risk factors for hyperuricemia
The sex specific PR, PAF of MRFs for hyperuricemia were presented in Table 4. The overall PAF for excess body weight (overweight and obesity) in men was 28.31% (16.25% for overweight and 12.08% for obesity), indicating that 28.31% of the hyperuricemia cases in the male participants can be explained by excess body weight. In women, the PAF of excess body weight was 20.30% (13.95% for overweight and 6.35% for obesity). Consistent with the GLM results, sedentary behavior was only found to be associated with male hyperuricemia, men who had more than 6 sedentary h per day contributed to 8% of cases. Alcohol drinking and smoking had relatively low PAFs both in men and women (Table 4). After stratified by body weight, we found that men who had lower body weight were more likely to be affected by sedentary behavior (see in Supplementary Table S1).
Table 4.
The prevalence ratio, population attributable fraction of modifiable risk factors for hyperuricemia, stratified by sex.
| Modifiable risk factors | % of exposure | PR | 95% CI of PR | PAF (%) | 95%CI or PAF | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| Overweight | 44.59 | 1.57 | 1.46 | 1.68 | 16.25 | 14.26 | 18.25 | <0.001 | ||
| Obesity | 21.67 | 2.26 | 2.10 | 2.42 | 12.08 | 11.40 | 12.77 | <0.001 | ||
| Current drinking | 40.98 | 1.13 | 1.07 | 1.19 | 4.64 | 2.72 | 6.56 | <0.001 | ||
| Ever smoking | 41.87 | 1.08 | 1.02 | 1.14 | 3.15 | 1.09 | 5.21 | 0.003 | ||
| Daily sedentary time | ||||||||||
| 2–4 h | 20.35 | 1.18 | 1.06 | 1.31 | 3.14 | 1.34 | 4.93 | <0.001 | ||
| 4–6 h | 29.57 | 1.29 | 1.17 | 1.42 | 6.72 | 4.44 | 8.99 | <0.001 | ||
| >6 h | 39.13 | 1.26 | 1.13 | 1.38 | 8.04 | 4.95 | 11.13 | <0.001 | ||
| Female | ||||||||||
| Overweight | 28.40 | 1.97 | 1.74 | 2.19 | 13.95 | 12.31 | 15.59 | <0.001 | ||
| Obesity | 9.25 | 3.19 | 2.77 | 3.62 | 6.35 | 5.97 | 6.74 | <0.001 | ||
| Current drinking | 4.95 | 0.84 | 0.63 | 1.04 | −0.01 | −0.02 | 0.005 | 0.195 | ||
| Ever smoking | 2.08 | 1.69 | 1.19 | 2.19 | 0.85 | 0.49 | 1.22 | <0.001 | ||
| Daily sedentary time | ||||||||||
| 2–4 h | 17.63 | 1.06 | 0.86 | 1.26 | 1.00 | −2.17 | 4.16 | 0.538 | ||
| 4–6 h | 28.21 | 1.05 | 0.87 | 1.26 | 1.64 | −3.23 | 6.51 | 0.509 | ||
| >6 h | 45.33 | 1.14 | 0.93 | 1.34 | 5.41 | −1.73 | 12.55 | 0.137 | ||
PR, prevalence ratio; PAF, population attributable fraction; CI, confidence interval.
The covariates adjusted in the regression model were age, geographic areas, body mass index, alcohol consumption, smoking status, daily sedentary time, and serum creatinine. Menopausal status was additionally adjusted in women.
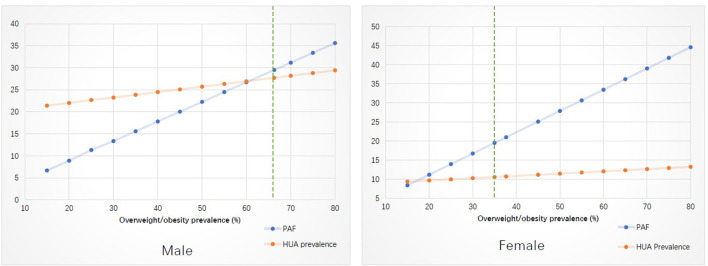
In the simulation analysis (Figure 1), taking overweight/obesity for example, we demonstrated the change of hyperuricemia prevalence due to PAF variation. More details for Figure 1 were available in Supplementary Table S2.
Figure 1.
Stimulation analysis on the effect of changed modifiable risk factors on the prevalence of hyperuricemia. PAF, population attributable fraction; HUA, hyperuricemia.
Sensitivity analyses
The results of sensitivity analyses were shown in Supplementary Tables S3–S5. After stratified by menopausal status, the main findings were consistent with results in Table 4. Post-menopausal women were more likely to be influenced by excess body weight (Supplementary Table S3). After excluding people suffering from chronic diseases, the PAFs estimates kept consistent with primary analyses (Supplementary Table S4). Middle-aged men and elder women had larger PAFs in the age-stratified analyses (Supplementary Table S5).
Discussion
In this representative sample of general population of Chinese adults, we reported the most updated data of hyperuricemia prevalence in central China. Furthermore, we took overweight/obesity, alcohol drinking, cigarette smoking, and sedentary behavior as modifiable risk factors and estimated their populational attributable fractions for hypouricemia cases, thus highlights the life-style intervention priorities in the prospective of public health importance.
Our study revealed a high prevalence of hyperuricemia in BTH area of China. In line with previous studies, there is sex difference in hyperuricemia prevalence. In the Henan Rural Cohort study from 2015 to 2017, the crude hyperuricemia prevalence was 12.80% in men and 8.56% in women, respectively (11). Revealed by the China National Health Survey (2012–2017), the hyperuricemia prevalence was 25.07% in men and 15.94% in women (8). Using baseline data of the China Health and Retirement Longitudinal Study in 2010, Song et al. (25) reported a lower prevalence of hyperuricemia, 7.9 vs. 4.9% for men and women. A cross-sectional survey from 2013 to 2014 in southwest China among middle-aged adults also showed a higher hyperuricemia prevalence in men than in women (17.3 vs. 10.0%) (26). The inconsistence among prevalence may be attributed to the population heterogeneity, disparity on covariates distribution, survey time variation of hyperuricemia as its increasing trend, or geographic difference (8, 9, 27).
In contrary to women, the prevalence of hyperuricemia in men decreased with aging, with the highest prevalence in the younger group (over 30% in the 20–40 age group). The sex difference, therefore, is shrinking as aging, same with the results observed in previous observational study (28). The increasing prevalence among women may be influenced by both aging and menopausal status (29). Menopause has been suggested as a female-specific cardiovascular risk factor by the American Heart Association (30). Higher level of SUA has been observed in post-menopausal women in both European and Asian populations (31–33). Higher prevalence of hyperuricemia with aging in female requires more attention on aged women, especially post-menopausal individuals.
Life-style risk factors, such as excess adiposity, alcohol consumption, cigarette smoking, and sedentary behavior, were found to be associated with hyperuricemia (8, 9, 11, 34–38). Mendelian randomization studies have demonstrated the causal associations among excess adiposity and alcohol consumption with elevated SUA (16). Our study also revealed the positive effect of overweight/obesity in both sexes. Notably, men had a high overweight/obese prevalence, even in the youngest group. Since PAF incorporates both exposure proportion and health effect size, it is not surprising that excess body weight played a predominant role (28.33%) in the overall PAF of hyperuricemia. For women, although their exposure proportion was lower than men, given the greater effect size of exposure, the PAF of excess body weight in women was also substantial (20.3%). Few studies have explored the PAF of excess body weight. Data from the Third National Health and Nutrition Examination Survey (NHANES-III) revealed a 44% PAF of overweight/obesity for hyperuricemia (10); data from three UK cohorts reported 59.0–68.6% PAFs for BMI > 25 kg/m2 among non-gout population (16). Our previous study using data from CNHS showed a similar PAF of overweight/obesity to the present study (20.6 vs. 28.33% in men; 18.1 vs. 20.3% in women). The higher PAFs in the western population are possibly due to their high exposure prevalence or using OR instead of PR to estimate the effect size (which may lead to overestimation when the outcome is not rare) and difference in genetic background.
Previous studies reported a varied PAF of alcohol consumption from 8 to 21.6% (8, 15, 38). Our study revealed a minor effect of current alcohol drinking on hyperuricemia in men. Possible reasons for this inconsistence may be attributed to different consumption dose of alcohol, as there is a dose-response relationship between alcohol intake and SUA level (39), and the lower exposure proportion in the study population (especially in women, <5%). More details on alcohol intake is needed in the follow-up data collection to enable further analysis.
The associations between smoking and hyperuricemia are not consistent among studies (40). In some cross-sectional studies, inverse relationships were observed that current smoking had lower SUA (41, 42). In Han et al.'s study, only passive smoking was found to be associated with elevated SUA, but not for active smoking (37). Our study revealed that ever-smoking was associated with a higher likelihood of hyperuricemia. The possible reason is that ever-smoking maybe related to other health disorders, such as metabolic syndrome or other cardiometabolic conditions which were believed to be associated with hyperuricemia (43, 44). The sensitivity analyses excluding people with chronic disease proved this hypothesis to some extent: ever smoking lost its statistical significance in the association with hyperuricemia. Therefore, the explanation of the contribution of smoking to hyperuricemia should be with caution and further be understood in prospective study.
Our study revealed sex-difference of the effect of sedentary behavior on elevated SUA. Men with longer sedentary time had increased odds of hyperuricemia, about 18% of hyperuricemia cases in men could be attributed to longer sedentary time. Although physical activity has been proved to be able to reduce the increases in mortality risk from high SUA (45), the role of sedentary behavior has received limited attention. Park et al. observed a positive association between sedentary behavior and hyperuricemia among the Korean population (46). In a Chinese population, per hour increased sedentary time was associated with a 5% higher hyperuricemia risk (11). As sedentary behavior may lead to excess body weight (47), we further explore the effect of sedentary time in men stratified by body weight. Interestingly, men who had lower body weight were more likely to be affected by sedentary behavior. The possible reason could be the competing risk among MRFs. As excess adiposity had the strongest effect size among MRFs, the effect of alcohol consumption, smoking or sedentary behavior may be attenuated in obese population. Inconsistent with previous study (48), women, but not men, was observed to have higher risk of hyperuricemia due to sedentary behavior. Possible reasons for this inconsistency may be due to difference in study population and varied exposure levels of MRFs. Hong et al.'s study focused on a population in Yunnan province, Southeast China (48), where our previous research has demonstrated a low prevalence of overweight/obesity, but high prevalence of hyperuricemia (8). As above discussed, competing risk may exist in females where excess adiposity perhaps played predominant role in developing hyperuricemia in the study population.
The prevention strategies for high-risk subjects should be identified based on their personal health profiles. Revealed by our study, obese men currently drinking alcohol and ever smoking cigarettes with long sedentary time, maybe the key population for hyperuricemia prevention.
Our study had some strengths. First, using a representative sample with large sample size, we reported the updated hyperuricemia prevalence in the core area of China where is experiencing fast economic development and lifestyle changing. Second, several essential modifiable risk factors were considered in the health effect estimates. Most importantly, by calculating their PAFs, we can estimate the expected proportion of hyperuricemia cases that could be prevented if the MRFs were removed (23), and this is of great public health significance. Third, we for the first time estimate the PAF of sedentary time on hyperuricemia in a general population, which could provide new evidence for a rapid urbanization area where young adults may be influenced more by sedentary behavior. The limitations of our study should also be acknowledged. First, the baseline data of a cohort study cannot guarantee the causal relationship between MRFs on hyperuricemia, longitudinal observation will be needed when enough follow-up data is available. Second, we did not collect dietary information. Nevertheless, previous research has suggested an indirect effect of diet on hyperuricemia, the adjustment of BMI in the regression model would eliminate this indirect effect (10, 49), and a recent study also revealed that diet has a relatively minor role in determining serum urate levels (16). Third, other obesity measurements, such as waist circumference and hip circumference were not available, thus limited the comprehensive effect estimation of excess adiposity on hyperuricemia. Lastly, the generality of this study may be limited by its survey in specific areas of China.
Conclusions
In summary, using representative samples from a general population in the core area, Beijing-Tianjin-Hebei, of China, our study revealed a high prevalence of hyperuricemia, and indicates that modifiable risk factors (overweight/obesity, alcohol consumption, cigarette smoking and sedentary behavior) contribute greatly to hyperuricemia cases based on their populational attributable fractions. Public health efforts including weight loss, reduction on alcohol consumption and cigarette smoking, and less sedentary time would be helpful for hyperuricemia prevention, especially in men.
Data availability statement
The raw data supporting the conclusions of this article will be made available by reasonable request by sending email to the corresponding author (zhangminying@nankai.edu.cn).
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Review Boards of Nankai University (NKUIRB2016063), Tianjin First Central Hospital (2017N052KY), Tianjin Union Medical Center (2018C02), and Hebei Medical University (2016021). The participants provided their written informed consent to participate in this study.
Author contributions
Concept and data analysis: HH and MZ. Design: HH, MZ, and GS. Definition of intellectual content: PG, HH, JZ, SC, YN, FG, FL, RZ, QL, SM, BZ, and LP. Data acquisition: HH, MZ, PG, JH, JZ, SC, YN, FG, FL, RZ, QL, SM, and BZ. Statistical analysis and manuscript preparation: HH. Manuscript editing: MZ and GS. Manuscript review: PG, JH, JZ, SC, YN, FG, FL, RZ, QL, SM, BZ, and LP. MZ takes responsibility for the integrity of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2016YFC0900600/2016YFC0900604), and the National Natural Science Foundation of China (82003531).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate all staff involved in the BTH Physical Examination Cohort and all the participants.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.936717/full#supplementary-material
References
- 1.Fathallah-Shaykh SA, Cramer MT. Uric acid and the kidney. Pediatr Nephrol. (2014) 29:999–1008. 10.1007/s00467-013-2549-x [DOI] [PubMed] [Google Scholar]
- 2.Feig DI, Kang D, Johnson RJ. Uric acid and cardiovascular risk. New Eng J Med. (2008) 359:1811–21. 10.1056/NEJMra0800885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11:649–62. 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Kang J, Kim G. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol. (2018) 37:2529–38. 10.1007/s10067-018-4130-2 [DOI] [PubMed] [Google Scholar]
- 5.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. (2017) 15:123. 10.1186/s12916-017-0890-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int J Cardiol. (2016) 213:4–7. 10.1016/j.ijcard.2015.08.110 [DOI] [PubMed] [Google Scholar]
- 7.Otaki Y, Konta T, Ichikawa K, Fujimoto S, Iseki K, Moriyama T, et al. Possible burden of hyperuricaemia on mortality in a community-based population: a large-scale cohort study. Sci Rep. (2021) 11:8999. 10.1038/s41598-021-88631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H, Pan L, Ren X, Wang D, Du J, Cui Z, et al. The effect of body weight and alcohol consumption on hyperuricemia and their attributable population fractions: a National health survey in China. Obes Facts. (2022) 15:216–27. 10.1159/000521163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He H, Pan L, Ren X, Wang D, Du J, Cui Z, et al. The effect of body adiposity and alcohol consumption on serum uric acid: a quantile regression analysis based on the China national health survey. Front Nutr. (2022) 8:724497. 10.3389/fnut.2021.724497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HK, McCormick N, Lu N, Rai SK, Yokose C, Zhang Y. Population Impact attributable to modifiable risk factors for hyperuricemia. Arthritis Rheumatol. (2019) 72:157–65. 10.1002/art.41067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong X, Li Y, Zhang L, Liu X, Tu R, Wang Y, et al. Independent and interactive effect of sitting time and physical activity on prevalence of hyperuricemia: the Henan Rural Cohort Study. Arthritis Rheumatol. (2021) 23:7. 10.1186/s13075-020-02385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. (2018) 71:851–65. 10.1053/j.ajkd.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L, Yang Z, Wu Y, Yin RX, Liao Y, Wang J, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. (2016) 248:2–9. 10.1016/j.atherosclerosis.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Du G, Song N, Ma Y, Li X, Gao X, et al. Hyperuricemia and its association with adiposity and dyslipidemia in Northwest China: results from cardiovascular risk survey in Xinjiang (CRS 2008–2012). Lipids Health Dis. (2020) 19:58. 10.1186/s12944-020-01211-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapata-Diomedi B, Barendregt JJ, Veerman JL. Population attributable fraction: names, types and issues with incorrect interpretation of relative risks. Br J Sports Med. (2018) 52:212–3. 10.1136/bjsports-2015-095531 [DOI] [PubMed] [Google Scholar]
- 16.Topless RKG, Major TJ, Florez JC, Hirschhorn JN, Cadzow M, Dalbeth N, et al. The comparative effect of exposure to various risk factors on the risk of hyperuricaemia: diet has a weak causal effect. Arthritis Res Ther. (2021) 23:75. 10.1186/s13075-021-02444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Dong S, Li Z. The economic and environmental effects of the Beijing-Tianjin-Hebei Collaborative Development Strategy— taking Hebei Province as an example. Environ Sci Pollut Res Int. (2020) 27:35692–702. 10.1007/s11356-020-09790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. (2020) 12:3167–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. [PubMed] [Google Scholar]
- 20.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. 10.1681/ASN.2006040368 [DOI] [PubMed] [Google Scholar]
- 21.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. (2010) 5:1388–93. 10.2215/CJN.01580210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roalfe AK, Holder RL, Wilson S. Standardisation of rates using logistic regression: a comparison with the direct method. BMC Health Serv Res. (2008) 8:275. 10.1186/1472-6963-8-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. (1998) 88:15–9. 10.2105/AJPH.88.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. (2004) 159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 25.Song P, Wang H, Xia W, Chang X, Wang M, An L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. (2018) 8:4314. 10.1038/s41598-018-22570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Zhang W, Tang W, Liu Y, Ning Y, Huang C, et al. Prevalence and associated factors of hyperuricemia among urban adults aged 35–79 years in southwestern China: a community-based cross-sectional study. Sci Rep. (2020) 10:15683. 10.1038/s41598-020-72780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Ma ZF, Zhang Y, Wan Z, Li Y, Zhou H, et al. Geographical distribution of hyperuricemia in mainland China: a comprehensive systematic review and meta-analysis. Glob Health Res Policy. (2020) 5:52. 10.1186/s41256-020-00178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi D, Liu J, Wang C, Wang L, Zhang X, Lin Q, et al. Sex-specific differences in the prevalence of and risk factors for hyperuricemia among a low-income population in China: a cross-sectional study. Postgrad Med. (2020) 132:559–67. 10.1080/00325481.2020.1761133 [DOI] [PubMed] [Google Scholar]
- 29.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women–the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. (2008) 10:R116. 10.1186/ar2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 31.Joo JK, Hong GP, Han SE, Lee YJ, Kim SC, Kim CW, et al. The association between serum uric acid level and incidence of metabolic syndrome according to menopausal status in Korean women. J Menopausal Med. (2014) 20:126–32. 10.6118/jmm.2014.20.3.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockl D, Doring A, Thorand B, Heier M, Belcredi P, Meisinger C. Reproductive factors and serum uric acid levels in females from the general population: the KORA F4 study. PLoS ONE. (2012) 7:e32668. 10.1371/journal.pone.0032668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He H, Pan L, Liu F, Ren X, Cui Z, Pa L, et al. The mediation effect of body composition on the association between menopause and hyperuricemia: evidence from China National Health Survey. Front Endocrinol. (2022) 13:879384. 10.3389/fendo.2022.879384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W, Kao Y, Lin H, Li MS, Luo T, Fritz JM, et al. Age difference in the combined effect of soda drinks consumption and body adiposity on hyperuricaemia in US adults. Public Health Nutr. (2021) 24:5756–68. 10.1017/S1368980021000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y, Jia S, Li D, Huang C, Meng Z, Wang Y, et al. Prediction model of random forest for the risk of hyperuricemia in a Chinese basic health checkup test. Biosci Rep. (2021) 41:R20203859. 10.1042/BSR20203859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwabara M, Kuwabara R, Niwa K, Hisatome I, Smits G, Roncal-Jimenez C, et al. Different risk for hypertension, diabetes, dyslipidemia, and hyperuricemia according to level of body mass index in Japanese and American subjects. Nutrients. (2018) 10:1011. 10.3390/nu10081011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Q, Zhang D, Zhao Y, Liu L, Li J, Zhang F, et al. Risk factors for hyperuricemia in Chinese centenarians and near-centenarians. Clin Interv Aging. (2019) 14:2239–47. 10.2147/CIA.S223048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He H, Pan L, Ren X, Wang D, Du J, Cui Z, et al. Joint effect of beer, spirits intake, and excess adiposity on hyperuricemia among chinese male adults: evidence from the China national health survey. Front Nutr. (2022) 9:806751. 10.3389/fnut.2022.806751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura K, Sakurai M, Miura K, Morikawa Y, Yoshita K, Ishizaki M, et al. Alcohol intake and the risk of hyperuricaemia: a 6-year prospective study in Japanese men. Nutr Metab Cardiovasc Dis. (2012) 22:989–96. 10.1016/j.numecd.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 40.Fanning N, Merriman TR, Dalbeth N, Stamp LK. An association of smoking with serum urate and gout: a health paradox. Semin Arthritis Rheum. (2018) 47:825–42. 10.1016/j.semarthrit.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 41.Chen HG, Sheng LT, Wan ZZ, Wang XC, Lin YH, Wang YX, et al. The relationship between smoking and hyperuricemia in Chinese residents. Chin J Epidemiol. (2018) 52:524–9. 10.3760/cma.j.issn.0253-9624.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Zhang Y, Wei J, Zeng C, Li L, Xie X, et al. Relationship between cigarette smoking and hyperuricemia in middle-aged and elderly population: a cross-sectional study. Rheumatol Int. (2017) 37:131–6. 10.1007/s00296-016-3574-4 [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Wang Y, Cheng J, Huangfu N, Zhao R, Xu Z, et al. Hyperuricemia and Cardiovascular Disease. Curr Pharm Des. (2019) 25:700–9. 10.2174/1381612825666190408122557 [DOI] [PubMed] [Google Scholar]
- 44.Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. (2020) 80:1–11. 10.1016/j.ejim.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Wen CP, Wu SB, Lan J, Tsai MK, Tai Y, et al. Attenuating the mortality risk of high serum uric acid: the role of physical activity underused. Ann Rheum Dis. (2015) 74:2034–42. 10.1136/annrheumdis-2014-205312 [DOI] [PubMed] [Google Scholar]
- 46.Park DY, Kim YS Ryu SH, Jin YS. The association between sedentary behavior, physical activity and hyperuricemia. Vasc Health Risk Manag. (2019) 15:291–9. 10.2147/VHRM.S200278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 48.Hong R, Huang J, Xu C, Zhang X, Mi F, Xu F, et al. Association of sedentary behavior and physical activity with hyperuricemia and sex differences: results from the China multi-ethnic cohort study. J Rheumatol. (2022) 49:513–22. 10.3899/jrheum.211180 [DOI] [PubMed] [Google Scholar]
- 49.Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. (2008) 87:1480–7. 10.1093/ajcn/87.5.1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by reasonable request by sending email to the corresponding author (zhangminying@nankai.edu.cn).