Introduction
Antiglomerular basement membrane (GBM) disease is caused by the presence of anti-GBM antibodies, usually directed against the noncollagenous domain of α-3 chain of type IV collagen (α3NC1), also called the Goodpasture antigen. This rare life-threatening autoimmune disorder typically presents with a rapidly progressive glomerulonephritis (GN) associated with pulmonary hemorrhage in 30% to 60% of patients. The pathologic hallmark of anti-GBM nephritis is bright linear staining for polyclonal IgG of GBMs by immunofluorescence. Most cases have diffuse crescentic and necrotizing GN on light microscopy without mesangial or endocapillary hypercellularity.1 Atypical anti-GBM nephritis is a rare variant without detectable circulating anti-α3NC1 antibodies by conventional testing. The largest published series of atypical anti-GBM disease included 20 patients, accounting for approximately 10% of anti-GBM cases. By light microscopy, these cases had atypical characteristics, including mesangial and/or endocapillary hypercellularity in all, absence of crescents in 12 cases, and only focal crescents in 8 cases. In contrast to typical anti-GBM disease, most patients presented with mild renal insufficiency, and none had pulmonary involvement.2
Rare cases of anti-GBM disease have been reported following immunotherapy. Clatworthy et al.S1 described, in 2008, 2 patients who developed typical anti-GBM disease after alemtuzumab, an anti-CD52 monoclonal antibody mostly used to treat B-cell chronic lymphocytic leukemia. More recently, similar cases have been reported after introduction of tumor necrosis factor-α antagonists or CTLA-4 and PD-1 checkpoint inhibitors. However, the mechanisms of this association are unclear.S2–S6
We describe a case of atypical anti-GBM disease developing after treatment with an anti–PD-1 monoclonal antibody, pembrolizumab, and discuss the mechanisms potentially involved in the pathophysiology of this rare but probably emergent entity due to the increased use of immune checkpoint inhibitors (ICIs) in various cancers.
Case Presentation
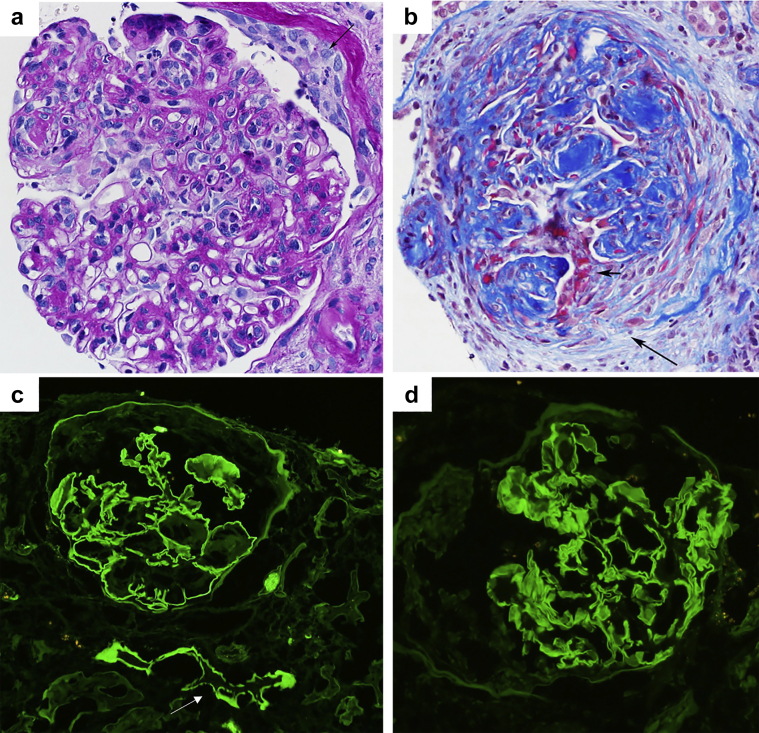
A 74-year-old White male presented with acute kidney injury and proteinuria. He had a long-term history of type 2 diabetes, hypertension, and tobacco use and was recently diagnosed with having metastatic squamous cell lung cancer treated with pembrolizumab, carboplatin, and paclitaxel. He had a good oncologic response to this regimen. However, 2 months after introduction of this therapy, his serum creatinine level jumped from 0.9 to 3.2 mg/dl, and despite stopping cancer therapy, it further increased to 4 mg/dl. Empirical treatment with prednisone (0.5 mg/kg/d) was introduced, and the patient was referred to nephrology. At presentation, proteinuria was 11 g/d with microscopic hematuria and peripheral edema. Serology results for antineutrophil cytoplasmic antibodies, antimyeloperoxidase, anti–proteinase-3 antibodies, HIV, hepatitis B surface antigen, and hepatitis C virus antibodies were negative. No monoclonal immunoglobulin was found on serum and urine immunofixation, and serum free light chain ratio was normal. The patient reports having stopped prednisone due to intolerance. The main biological parameters at the time of kidney biopsy are summarized in Table 1. A kidney biopsy was performed. Of the 22 glomeruli sampled for light microscopy, 10 were globally sclerotic, 5 (22%) exhibited crescents (4 fibrocellular and 1 cellular) including 3 with fibrinoid necrosis, and 1 had endocapillary hypercellularity with leukocyte infiltration (Figure 1). Some glomeruli exhibited nodular mesangial sclerosis. There were diffuse acute tubular injury, erythrocyte casts, and moderate interstitial fibrosis. No arteritis was found. On immunofluorescence, there were linear GBMs and focal tubular basement membrane staining for IgG (2+), lambda (2–3+), kappa (1+), IgG lambda (2+), and IgG kappa (1+). There were 1+ diffuse linear GBMs and tubular basement membrane positivity for albumin and trace granular segmental mesangial positivity for C3. The glomeruli were negative for IgA, IgM, and C1q. One glomerulus had segmental tuft positivity for fibrinogen. Staining for IgG subtypes revealed diffuse linear GBMs and focal tubular basement membrane staining for IgG2 only. Ultrastructurally, no electron-dense glomerular deposits were found. The pathologic findings were consistent with the polytypic variant of atypical anti-GBM nephritis superimposed on nodular diabetic glomerulosclerosis. Search for circulating anti-GBM antibodies was negative using standard testing, and the patient had no evidence of pulmonary hemorrhage.
Table 1.
Laboratory data at the time of kidney biopsy
| Parameters | Result | Reference range |
|---|---|---|
| Hemoglobin, g/dl | 10.1 | 13–17 |
| White blood cell count, ×109/l | 6.9 | 4–10 |
| Platelets, ×109/l | 135 | 150–410 |
| Creatinine, mg/dl | 5.6 | 0.7–1.2 |
| BUN, mg/dl | 47 | 6–24 |
| Albumin, g/dl | 3.3 | 4.1–5.3 |
| Hepatitis B | Negative | |
| Hepatitis C | Negative | |
| HIV | Negative | |
| ANCA | Negative | |
| Anti-GBM | Negative | |
| IgG subclass, mg/dl | ||
| IgG1 | 780 | 341–894 |
| IgG2 | 244 | 171–632 |
| IgG3 | 46.5 | 18.4–106 |
| IgG4 | 65.2 | 2.4–121 |
| Serum/urine protein immunofixation | Negative | |
| Urine studies | ||
| Red blood cell/hpf | >50 | 0–5 |
| White blood cell/hpf | <5 | 0–5 |
| Proteinuria, mg/24 h | 12,000 | 0–300 |
ANCA, antineutrophilic cytoplasmic antibody; anti-GBM, antiglomerular basement membrane antibody; BUN, blood urea nitrogen; hpf, high-power field.
Figure 1.
Kidney biopsy findings. (a) The glomerulus exhibits endocapillary hypercellularity with abundant intracapillary infiltrating neutrophils and monocytes, mesangial sclerosis and hypercellularity, thickening of the GBM, and a tiny cellular crescent (arrow) (periodic acid-Schiff, ×400). An adjacent arteriole has intimal hyalinosis. (b) Another glomerulus reveals nodular mesangial sclerosis and a fibrocellular crescent associated with fibrinoid necrosis (small arrow) and rupture of Bowman’s capsule (long arrow) (trichrome stain, ×200). (c) There is bright, linear staining of the GBM and few tubular basement membranes (arrow) for IgG (immunofluorescent image, ×200). (d) There is bright, linear GBM staining for IgG2 (immunofluorescent image, ×400). The glomeruli were negative for IgG1, IgG3, and IgG4 (not illustrated). GBM, glomerular basement membrane.
The patient was treated with rituximab (two 1 g i.v. infusions 2 weeks apart) and 7 days of plasmapheresis. Prednisone was not restarted due to the previous intolerance. Seven days after starting therapy, he was initiated on hemodialysis due to fluid overload. He remained on chronic hemodialysis at last follow-up, 4 months after biopsy.
Discussion
ICIs are antibodies targeting immune checkpoint proteins allowing the T cells to become activated and exert antitumor activity. The dark side of ICIs is the risk of an exuberant activation of the immune system leading to the development of various organ damage called immune-related adverse events. Acute interstitial nephritis is the most common kidney immune-related adverse event reported in approximately 3% of patients. In such cases, discontinuation of ICIs and empirical treatment with steroids is recommended.3 However, due to the plethora of renal lesions now reported after ICI therapy, other authors argue that empirical treatment with steroids should only be considered after complete nephrologic workup to exclude another plausible etiology for acute kidney injury.4,5 In our patient, creatinine level increased rapidly despite discontinuation of pembrolizumab and introduction of empirical treatment with steroids. Because early stage GN is mostly revealed by urine abnormalities (proteinuria and hematuria), our case highlights that urine dipstick and quantitative measurement of proteinuria may be beneficial in some patients treated by ICIs.
Only 4 cases of anti-GBM disease following ICIs have been previously reported, including 1 case with the atypical variant (Supplementary Table S1). In these cases, the glomerular disease occurred between 4 and 16 months after ICI initiation. All had bright linear polyclonal staining of GBMs for IgG (subclass analysis not done) on immunofluorescence, and circulating anti-GBM antibodies were detected in the 3 cases with typical variant.S3–S6 In our patient, testing for circulating anti-α3NC1 antibodies remained negative by enzyme-linked immunosorbent assay. As previously suggested, another autoantigen than α3NC1, such as α4NC1 or linear type IV collagen epitopes, could be involved in atypical anti-GBM disease.2 Interestingly, we found that IgG2 was the sole positive IgG subclass by immunofluorescence on the kidney biopsy. IgG subclass analysis in classic anti-GBM disease revealed IgG1 or IgG4 deposits, and IgG2 seems mostly involved in atypical anti-GBM disease.2 IgG2 is known to be a weak activator of the complement, which could explain the little C3 deposition found in our patient. A recent study highlighted that high serum IgG2 level is associated with better response to ICIs in metastatic melanoma patients.6 In our patient, serum IgG2 level was normal at the time of kidney biopsy. However, we cannot exclude that a transient increase of IgG2 may have preceded kidney injury, promoting an IgG2-mediated immune-related adverse event.
The pathophysiological link between ICIs and anti-GBM disease is unclear. Autoreactive CD4+ T cells specific for α3NC1 play an important role in the development of autoimmunity to the GBM. The transfer of these activated α3NC1 specific T-cells to naive rats caused crescentic GN.7 Importantly, healthy individuals have detectable α3NC1 specific T-cells suggesting that mechanisms of self-tolerance prevent clinical development of the disease and relapses.8 Among these mechanisms, the protective role of regulatory T-cells, a subset of CD4+ T-cells implicated in the maintenance of immune homeostasis, has been suggested.9 Because ICIs are known to alter Treg functions, the loss of their protective role may have promoted local inflammation and immune system activation against GBM antigens in our patient.
We report a case of atypical anti-GBM disease following treatment with pembrolizumab, highlighting the importance of kidney biopsy in patients treated with ICI who develop acute kidney injury and had evidence of GN on urine sediment (Table 2).
Table 2.
Teaching points
|
|
|
α3NC1, noncollagenous domain of α-3 chain of type IV collagen; GBM, glomerular basement membrane; ICI, immune checkpoint inhibitor.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in this report.
Footnotes
Supplementary References
Table S1. Summary of reported cases of anti-glomerular basement membrane disease following immune checkpoint inhibitor therapy.
Contributor Information
Vincent Javaugue, Email: javaugue.vincent@yahoo.fr.
Samih H. Nasr, Email: nasr.samih@mayo.edu.
Supplementary Material
Supplementary References
Table S1. Summary of reported cases of anti-glomerular basement membrane disease following immune checkpoint inhibitor therapy.
References
- 1.Jennette J.C. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 2.Nasr S.H., Collins A.B., Alexander M.P., et al. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 2016;89:897–908. doi: 10.1016/j.kint.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchlu A., Jhaveri K.D., Wadhwani S., et al. A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep. 2021;6:66–77. doi: 10.1016/j.ekir.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Cortazar F.B., Riella L.V., Leaf D.E. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney360. 2020;1:130–140. doi: 10.34067/KID.0000852019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diem S., Fässler M., Bomze D., et al. Immunoglobulin G and subclasses as potential biomarkers in metastatic melanoma patients starting checkpoint inhibitor treatment. J Immunother. 2019;42:89–93. doi: 10.1097/CJI.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Hicks J., Borillo J., et al. CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest. 2002;109:517–524. doi: 10.1172/JCI13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou J., Hannier S., Cairns L.S., et al. Healthy individuals have Goodpasture autoantigen-reactive T cells. J Am Soc Nephrol. 2008;19:396–404. doi: 10.1681/ASN.2007050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salama A.D., Chaudhry A.N., Holthaus K.A., et al. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int. 2003;64:1685–1694. doi: 10.1046/j.1523-1755.2003.00259.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.