Abstract
CD47 is expressed in all human cancer cells, including head and neck cancer, and initiates a signaling cascade to inhibit macrophage phagocytosis. However, the mechanism underlying CD47 overexpression has not been elucidated in radioresistant head and neck cancer. The present study demonstrated that decreased Tristetraprolin (TTP) expression induced a sustained overexpression of CD47 using reverse transcription-quantitative PCR and western blotting, and that CD47 overexpression prevented phagocytosis using a phagocytosis assay in a radioresistant HN31R cell line. Subsequently, using TTP transfection, RNA interference, duel-luciferase assay and EMSA, it was revealed that TTP transfection enhanced phagocytosis through degradation of CD47 mRNA by directly binding to CD47 AREs within the CD47 3'UTR. Based on our previous study, methylation-specific PCR and western blotting revealed that DNMT1 was overexpressed in radioresistant HN31R cell line and TTP expression was decreased epigenetically by DMNT1 associated DNA methylation. Overall, these findings provided novel insight into the role of TTP as a biomarker of CD47-positive head and neck cancer patients.
Keywords: head and neck cancer, radioresistance, CD47, phagocytosis, tristetraprolin, post-transcriptional modification, AU-rich elements, DNA methyltransferase
Introduction
Radioresistance has important role in local recurrence and distant metastases of head and neck cancer (HNC). Radiation-induced cytokines in the tumor and its microenvironment collectively contribute to the tumor response to radiation (1-3). CD47 is expressed on the surface of human cancer cells including head and neck cancer and has been implicated in various pathophysiologic processes including tumor cell apoptosis, survival, proliferation, migration, adhesion, and metastasis. Cancer cells expressing CD47, which evade the innate immune system initiates a signaling cascade to inhibit macrophage phagocytosis (4-6). Previously, CD47 has been regarded as an attractive radio-therapeutic target because blocking CD47 signal can increase to radiation response with protection of normal tissues (7). Furthermore, CD47 is frequently overexpressed in HNC and radiation causes a decrease of CD47 expression in a dose-dependent manner (3). However, the mechanism of CD47 overexpression has not been elucidated in radioresistant HNC.
The RNA-binding protein, Tristetraprolin (TTP), is encoded by the ZFG36 gene and is one of the most well-characterized AU-rich element (ARE)-binding proteins within the 3'UTR. It enhances decay of ARE-containing transcripts and plays an important role in various processes including cellular differentiation, proliferation, tumorigenesis, and immunity (8-12). Because of its ability to bind and target ARE-containing mRNAs for rapid degradation, numerous studies have shown that TTP exhibits as tumor-suppressor. Thus, loss of TTP expression or function is closely associated with tumor progression or poor outcome in malignant tumors (13,14). It was known that some tumor-associated genes with ARE sequences can be subject to TTP-mediated mRNA degradation. The data indicate that target mRNAs of TTP are mainly oncogenes in tumorigenesis (12).
Previously, it was reported that a regulatory pathway for the IL-23, IL-27, and IL-33 cytokines by TTP could be important role in regulation of antitumor immunity (15-17). However, there are no convincing reports that identify the molecular mechanism for the regulation of CD47 by TTP in cancer cells exhibiting radioresistance. In the present study, we demonstrate that decreased TTP expression induced sustained CD47 overexpression and prevented phagocytosis in radioresistant HNC cells. Thus, TTP transfection can enhance phagocytosis through its derogation activity by directly binding to CD47 AREs within the CD47 3'UTR in radioresistant HNC cells. Based on our previous study in which DNMT1 was overexpressed in radioresistant HNC (18), we found that DNMT1 regulated TTP expression by DNA methylation, epigenetically.
Materials and methods
Cell culture
We used HN31 cells provided by Dr Jeffrey N. Myers (University of Texas, MD Anderson Cancer Center). The HNC were cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Invitrogen) and 100 µg/ml of penicillin/streptomycin and incubated at 37˚C and 5% CO2. HNC cells were cultured to ~50% confluence in vented 75 cm2 culture flasks. An isogenic model of successively irradiated HN31R cells was established as our protocols designed to investigate radioresistance (19).
In vitro phagocytosis assay
Macrophages (1x105) were seeded into glass bottom cell culture dishes (NEST Biotechnology, 801002). HN31 or HN31R cells were labeled with CFDA SE according to the manufacturer's protocol. Before the adding 2x105 of CFDA SE-labeled tumor cells, macrophages were incubated in serum-free medium for 2 h. Macrophages were repeatedly washed and subsequently images were captured using a confocal microscope. The phagocytic index was estimated as the number of phagocytosed CFSE+ cells per 100 macrophages.
Quantitative real-time PCR (qRT-PCR) analysis for RNA kinetics
For RNA kinetic analysis, actinomycin D was used and CD47 mRNA expression assessed by quantitative PCR. Total RNA was isolated with the PureLink™ RNA Mini Kit (Thermo Fisher Scientific, Inc.) and cDNA synthesized with a first-strand cDNA synthesis kit by reverse transcription-PCR (iNtRON Biotechnology). We used SYBR®-Green master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) for qRT-PCR using a PRISM®-7500 sequence detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). All reactions were performed in triplicate in 96-well plates and the mean values used to estimate mRNA expression. The primer sequences were as follows: CD47 forward, 5'-TATCCTCGCTGTGGTTGGACTG-3' and reverse, 5'-TAGTCCAAGTAATTGTGCTAGAGC-3'; TTP forward, 5'-CGCTACAAGACTGAGCTAT-3' and reverse, 5'-GAGGTAGAACTTGTGACAGA-3'; GAPDH forward, 5'-ACATCAAGAAGGTGGTGAAG-3' and reverse, 5'-CTGTTGCTGTAGCCAAATTC-3'.
Transfection of TTP
TTP overexpressing HN31R cells were generated using the pcDNA6/V5 vector (Invitrogen; Thermo Fisher Scientific, Inc.). We cloned the full-length human cDNA of TTP using RT-PCR from the RNA of HN31 cells with forward primer 5'-CCGTGAATTCATGGATCTGACTGCCAT-3' and reverse primer 5'-CACTCTCGAGCTCAGAAACAGAGATGC-3' The product was subcloned into the pcDNA6/V5 vector. Approximately 1.5x107 cells were electroporated using 20 µg of pcDNA6/V5-TTP at 500 V, 975 lF using a Gene Pulser electroporator II (Bio-Rad). After transfection, transfected HN31R/pcDNA6/V5-TTP cells with human TTP were selected with 10 µg of Blasticidin/ml (Invitrogen; Thermo Fisher Scientific, Inc.) at 3 days after transfection. Stable TTP overexpression was tested by western blots using anti-human TTP polyclonal antibody (ab33058; Abcam). A control cell line, HN31R/pcDNA6/V5, was made by transfection with the pcDNA6/V5 vector.
RNA interference
HN31 cells were pated into 6-well plates (3x105 cells per ml) 24 h before transfection. HN31 cells was transfected with 45 nM of TTP-siRNA (sc-36760; Santa Cruz Biotechnology, Inc.), 50 nM of CD47 siRNA (h) (sc-35006), 30 nM of EphA3 siRNA (sc-39934; Santa Cruz Biotechnology, Inc.) or control siRNA-A (sc-37007; Santa Cruz Biotechnology, Inc.) with Lipofectamine™ RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.). The cells were grown for 24 h before western blotting analysis. TTP expression was evaluated at 3, 7 days and every experiment.
Dual-luciferase assay
Analysis of human CD47 3'UTR revealed the presence of thirteen AREs (http://rna.tbi.univie.ac.at/AREsite2/welcome). A various deletion mutant of the CD47 3'-UTR were PCR-amplified from the cDNA of HN31R cells by PCR using the following primer sets: CD47 Frag-ARE-1-7, CCGCTCGAGACGTGATTGTTAGTT and ATTTGCGGCCGCCTGATTTAAAGAGA; CD47 Frag-ARE-8-13, CCGCTCGAGCAAATTCCATCACAT and ATTTGCGGCCGCACAATCATTTCTCC; CD47 Frag-ARE-10-13, CCGCTCGAGGCTTAGTTCTATTAG and ATTTGCGGCCGCACAATCATTTCTCC; CD47 Frag-ARE-8-13(No9 mut AUUUA-AGCA), GACTCTTCCATTCAGTTTTAGCATTGTGTGTTCTCACAGTGACAC and GTGTCACTGTGAGAACACACAATGCTAAAACTGAATGGAAGAGTC.
The PCR products were inserted into the XhoI/NotI sites a psiCHECK2 Renilla/firefly Dual-Luciferase expression vector (Promega). Luciferase assays, HN31R cells were co-transfected with thepsiCHECK-CD47 3'-UTR constructs and pcDNA6/V5-TTP with the TurboFect™. Transfected cells were collected at 24 h for RNA extraction and at 48 h for measurement of luciferase activity using Wallac Victor 1420 multilabel counter (EG&G Wallac). Firefly luciferase activity of psiCHECK2/CD47 3'UTR was normalized to Renilla luciferase for each sample and Luciferase assays represent at least three independent experiments.
Electrophoretic mobility shift assay (EMSA)
The biotinylated RNA probes for wild-type (wtCD47-EMSA, 5'-UUGACUCUUCCAUUCAGUUUUAUUUAUUGUGUGUUCUCACAGUGACACCAUU-3') and mutant (mutCD47-EMSA, 5'UUGACUCUUCCAUUCAGUUUUAGCAUUGUGUGUUCUCACAGUGACACCAUU-3') were synthesized by BIONEER Co. (Daejeon, Korea). RNA EMSA was performed with Lightshift® Chemoluminescent EMSA Kit (Pierce) as previously described protocols (20).
Western blot analysis
Total protein was extracted with RIPA buffer (Thermo Fisher Scientific, Inc.). Protein concentrations were calculated using the Bradford assay kit (Bio-Rad Laboratories). Equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes (Amershan International). Membranes were incubated with primary antibodies to Tristetraprolin (ab33058; Abcam), CD47 (ab175388; Abcam), DNMT1 (#5032; Cell Signaling Technology), EphA3 (sc-920; Cell Signaling Technology), phospho-AKT Ser473 (#4060; Cell Signaling Technology) and β-actin (sc-47778; Sigma-Aldrich; Merck KGaA). Membranes were then incubated with secondary antibodies (anti-mouse or anti-rabbit IgG HRP conjugate; Bethyl Laboratories), and specific binding was detected with a SuperSignal West Pico Trial kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions (Cayman Chemical). Western blotting analysis was performed at least three times, and representative figures are presented. The expression of the protein was quantified as compared to the beta-actin in the Western blot.
Methylation-specific PCR (MSP) for TTP
Genomic DNA (1 µg) was denatured with sodium bisulfite using the EpiTect Bisulfite kit (Qiagen). This treatment resulted in the conversion of unmethylated cytosine to thymine, but methylated cytosine remained not changed. Primer sequences of TTP were as follows: (5'-to-3'): TTP, ATTGGGTAGGTGTTT thymidine's TATTTGT (unmethylated sense), TAGTTTAGGGTTAGTTAGGTTGCGT (methylated sense), TAACCCTAAACTAATTCCCTTCCA (unmethylated antisense), AAATATCGACCGAAAATAAAAACG (methylated antisense). And PCR amplification was conducted with primers that specifically amplify methylated or unmethylated DNA using an EpiTect MSP kit (Qiagen). PCR was performed as following thermal conditions: 1 cycle of 95˚C for 5 min and 35 cycle of 94˚C for 45 sec, 56˚C (Unmethylation) or 57.5˚C (Methylation) for 30 sec, and 72˚C for 30 sec, and then one cycle of 72˚C for 10 min. Each amplified product was loaded onto 2% agarose gels, visualized using ethidium bromide, and images were captured.
Statistical analysis
For statistical comparisons, P-values were determined using unpaired Student's t-test or one-way ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
CD47 expression and phagocytosis is correlated with TTP expression in radioresistant HNC cell line
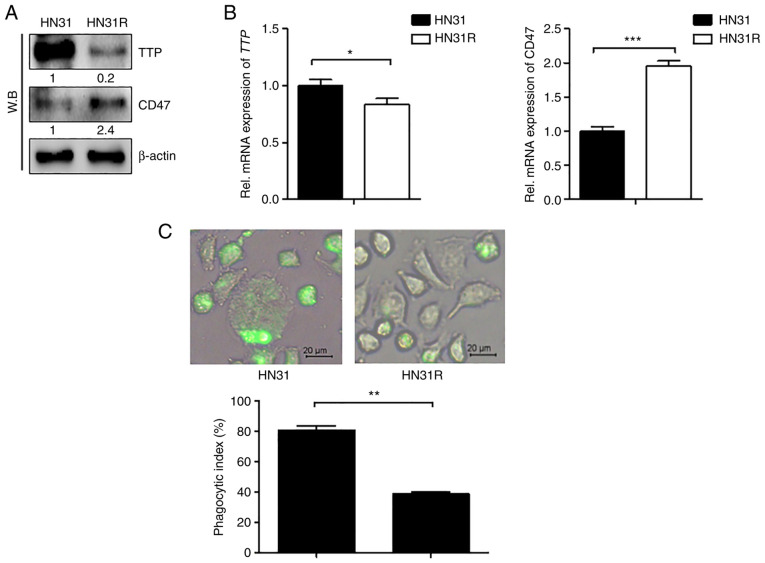
TTP and CD47 expression levels were first examined by RT-PCR and western blot analysis in HN31 and HN31R cells. TTP levels were high in HN31 cells, but low in the radioresistant HN31R cell line. In contrast, CD47 levels were low in HN31 cells compared with that in the HN31R cell line (Fig. 1A and 1B). We next determined whether CD47 phagocytosis is mediated by CD47 expression in HN31 and HN31R cells. In HN31R with CD47 overexpression, phagocytosis was inhibited compared with that in the HN31 cell line (Fig. 1C).
Figure 1.
CD47 expression and phagocytosis is associated with TTP expression in radioresistant HNC cell lines. (A) TTP and CD47 protein levels in HN31 and HN31R cancer cell lines were investigated using western blotting. (B) TTP and CD47 mRNA levels in HN31 and HN31R cancer cell lines were determined using reverse transcription-quantitative PCR. (C) Phagocytosis index was evaluated using a phagocytosis assay in HN31 and HN31R cell lines (scale bar, 20 µm). Values are presented as the mean ± SD (n=3). *P<0.05, **P<0.01, ***P<0.001. n.s., not significant; TTP, tristetraprolin.
TTP decreases expression of CD47 mRNA and increases phagocytosis in radioresistant HNC cell line
We investigated whether silencing or overexpression of TTP regulates CD47 expression in HN31 and HN31R cells. TTP silencing by siRNA increased the levels of CD47 protein in HN31 cells (Fig. 2A). When TTP was expressed in HN31R cells by transfection of TTP significantly inhibited the level of CD47 protein (Fig. 2B). To identify the effect on phagocytosis, a phagocytosis assay was performed following TTP overexpression in HN31R cells. The results indicated that the CD47 suppression-mediated phagocytosis index increased significantly after TTP overexpression (Fig. 2C).
Figure 2.
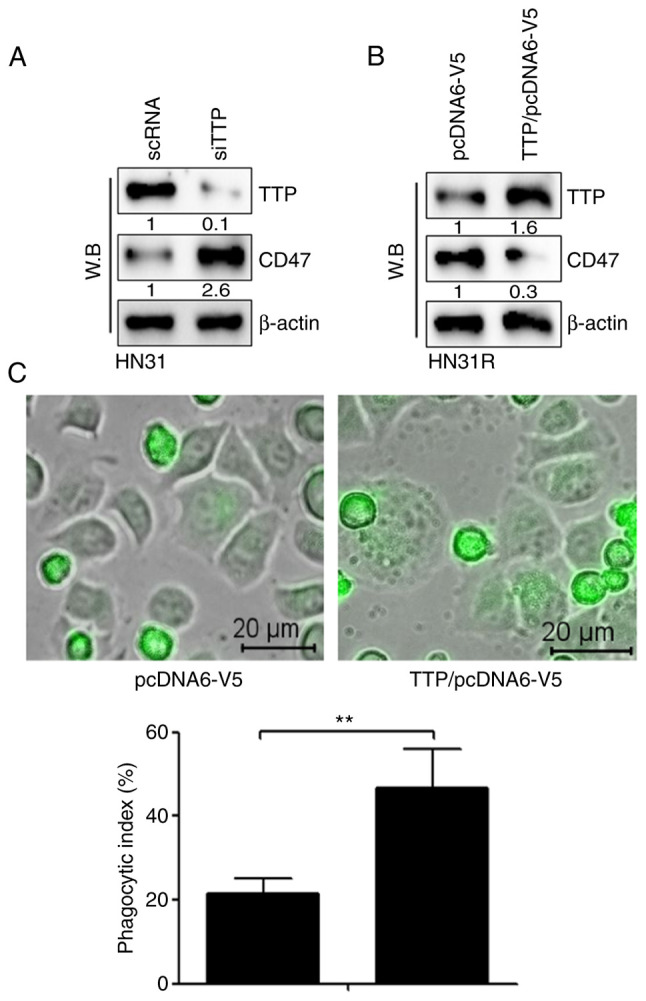
TTP suppresses the expression of CD47 and increases phagocytosis in radioresistant HNC cells. (A) After inhibition of TTP by small interfering RNA, expression of CD47 was measured at protein level in HN31 cells using western blotting. (B) After overexpression of TTP in HN31R cells through transfection with the TTP expression vector (pcDNA6/V5-TTP), the expression of CD47 protein was measured using western blotting. (C) To identify the effect on phagocytosis, a phagocytosis assay was performed after TTP overexpression in the HN31R cell line (scale bar, 20 µm). Values are presented as the mean ± SD (n=3). **P<0.01. n.s., not significant; TTP, tristetraprolin; HNC, head and neck cancer.
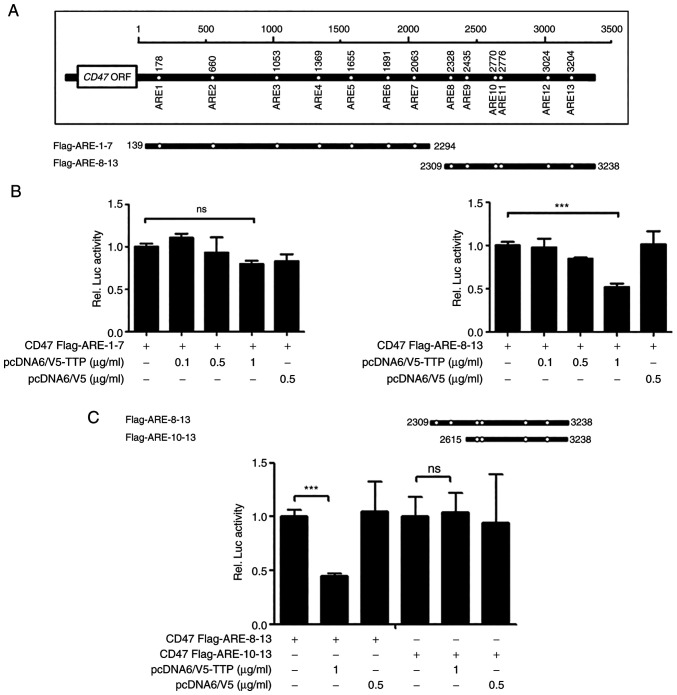
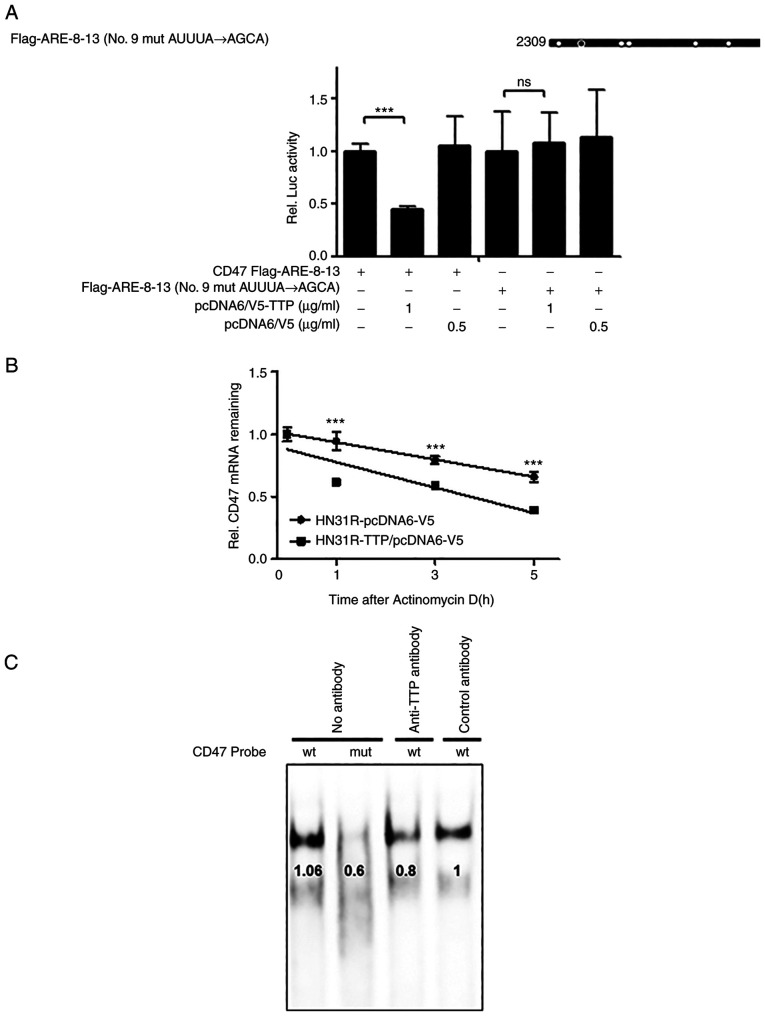
TTP interacts to ARE 9 in the 3'UTR of CD47 mRNA and increases CD47 mRNA degradation
Human CD47 3'UTR has the presence of thirteen AREs (Fig. 3A). To investigate whether suppression of CD47 expression by TTP was mediated through interaction in the 3'UTR of CD47 mRNA, luciferase reporter gene linked to the full-length CD47 3'UTR divided into two constructs (Flag AREs 1-7 and Flag AREs 8-13) in the psiCHECK2 plasmid was used. Next, we investigated which AREs within the CD47 3'UTR were necessary for TTP activity. A luciferase reporter gene linked to oligonucleotides containing Flag AREs 1-7 and AREs 8-13 within the CD47 3'UTR were prepared using the psiCHECK2 plasmid. Whereas the luciferase activity of Flag AREs 1-7 was not changed by TTP expression, that of Flag AREs 8-13 was significantly inhibited (Fig. 3B). We then subdivided Flag AREs 8-13 further to prepare a Flag AREs 10-13 construct and measured luciferase activity of CD47 by TTP. Because the luciferase activity of Flag AREs 10-13 was not affected by TTP expression, we concluded that TTP binds to ARE 8-9 in the CD47 mRNA 3'UTR (Fig. 3C). Because AREs 8-9 was closely located in CD47, AREs 8-13 with single mutation in the ARE 9 motif (AUUUA→AGCA) were used to confirm whether ARE 9 was responsible for the TTP binding (Fig. 4A). A single ARE 9 mutant in CD47 was prevented the TTP inhibitory effect compared with the full ARE 8-13 construct. Although these results were determined using overexpressed TTP protein ectopically, the significance of CD47 ARE 9 for TTP binding was demonstrated. Then, to investigate if suppression of CD47 by TTP resulted from CD47 mRNA stability, the half-life of the mRNA was estimated by qRT-PCR. After the transfection with pcDNA6/V5-TTP (HN31R/TTP) or the pcDNA6/V5 control vector (HN31R/pcDNA), the half-life of TTP overexpressing CD47/TTP cells was significantly shorter compared with control HN31R/pcDNA cells in actinomycin D treatment (Fig. 4B). To confirm the association between endogenous TTP and ARE in the CD47 3'UTR, RNA EMSA was performed with a biotinylated RNA probe containing a wild-type or mutant ARE9 of CD47. Cytoplasmic extracts which prepared from TTP transfection HN31R cells were incubated with the biotinylated RNA. When the wild-type CD47 ARE 9 probe was mixed with the cytoplasmic extracts of TTP transfection HN31R cells, we found a dominant probe-protein complex (Fig. 4C). However, the mutant CD47 ARE 9 probe s could not make the probe-protein complex. When the reaction mixture was preincubated with anti-TTP antibody, the formation of the CD47 ARE 9 probe-protein complex was reduced but not with the control. These results indicate that TTP can combine at the 9th ARE of CD47 and enhances decay of CD47 mRNA.
Figure 3.
TTP binds to ARE 8-9 in CD47 mRNA 3'UTR. (A) Analysis of the human CD47 3'UTR identified the presence of 13 AREs. (B) A luciferase reporter gene linked to oligonucleotides with Flag AREs 1-7 and AREs 8-13 within the CD47 3'UTR were prepared in the psiCHECK2 plasmid, and the luciferase activity of Flag AREs 1-7 were evaluated depending on TTP expression. (C) Flag AREs 8-13 were divided into a Flag AREs 10-13 construct and luciferase activity of CD47 was measured depending on TTP expression. Values are presented as the mean ± SD (n=3). ***P<0.001. n.s., not significant; UTR, untranslated region; ARE, AU-rich element; TPP, tristetraprolin.
Figure 4.
TTP enhances CD47 mRNA degradation by binding to ARE-9 of the CD47 mRNA 3'UTR. (A) Luciferase assay was performed with AREs 8-13 with single mutants of the 9 ARE motif (AUUUA→AGCA) (B) After the transfection of pcDNA6/V5-TTP or pcDNA6/V5 into HN31R cells for 24 h, expression of CD47 was estimated using reverse transcription-quantitative-PCR at the indicated times following the addition of 5 mg/ml actinomycin D. (C) To confirm the association between endogenous TTP and ARE in the CD47 3'UTR, RNA EMSA was performed after mixing cytoplasmic extracts containing 4 µg of total protein from pcDNA6/V5-TTP-transfected HN31R cells with a 20 fmol biotinylated RNA probe containing wt or mut. After adding control antibody or anti-TTP to the reaction mixtures, binding reactions of biotinylated wt or mut probe was measured. Values are presented as the means ± SD (n=3). ***P<0.001. n.s., not significant; wt, wild-type; mut, mutant; UTR, untranslated region; ARE, AU-rich element; TPP, tristetraprolin.
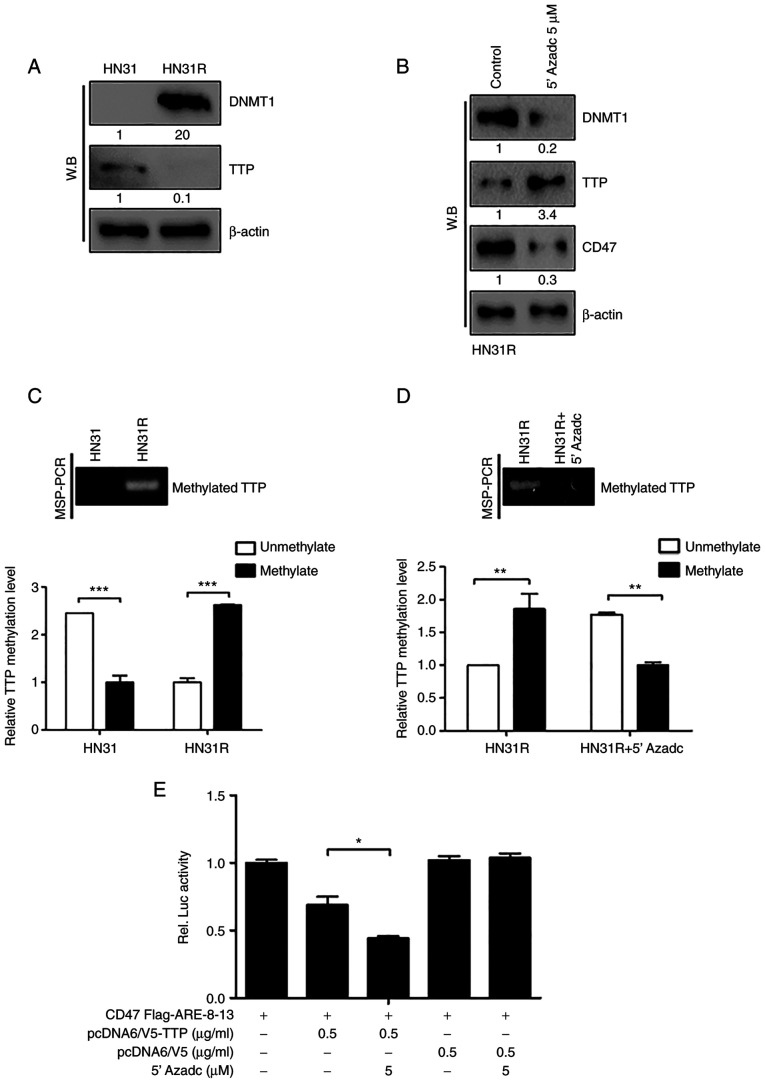
Sustained TTP suppression and CD47 activation is maintained through DNA methylation in radioresistant HNC cell lines
Based on our previous study in which DNMT1 (DNA methyltransferase) was found to be overexpressed in radioresistant HNC (10), we examined the regulation of TTP by DNA methylation in radioresistant HNC cells. DNMT1 expression was increased significantly in HN31R cells compared with parent HN31 cells (Fig. 5A). To determine the DNA methylation of TTP by DNMT1 in HN31R cells, 2 successive days with 5 µM 5-AZAdC (DNA demethylating agent) was treated to HN31R cells and the protein level of TTP and CD47 protein was checked in HN3R cells using Western blotting. When DNMT1 protein in whole-cell lysates was reduced, TTP expression was increased significantly with decreased CD47 expression at the protein level (Fig. 5B).
Figure 5.
Sustained TTP suppression and CD47 activation is maintained through DNA methylation in radioresistant HNC cell lines. (A) Protein levels of DNMT1 and TTP in HN31 and HN31R cancer cell lines were investigated using western blotting. (B) To determine the DNA methylation of TTP by DNMT1 in HN31R cells, the cells were treated for 2 days with 5 µM 5-AZAdC (DNA demethylating agent) and the protein levels of TTP and CD47 protein were checked in HN3R cells using western blotting. (C) To determine change of TTP expression by the DNA methylation effect, MSP assay was performed. (D) MSP was performed after 5 µM 5-AZAdC on HN31R cells to confirm the regulation of TTP by DNMT1 in HN31R cells. (E) Luciferase activity of CD47 AREs 8-13 was measured following treatment with 5-AZAdC to confirm whether DNMT1-mediated DNA methylation of TTP could regulate binding with the CD47 AREs. *P<0.05, **P<0.01, ***P<0.001. DNMT1, DNA methyltransferase; TPP, tristetraprolin; MSP, methylation-specific PCR; W.B, western blotting.
To determine change of TTP expression by the DNA methylation effect we performed an MSP assay. Basically, HN31 cells showed unmethylation status of the TTP promoter, however, HN31R cells aberrant, hypermethylated DNA in this region. (Fig. 5C). To confirm the regulation of TTP by DNMT1 in HN31R cells, MSP was performed after 5 µM 5-AZAdC on HN31R cells. Following treatment with 5-AZAdC, TTP methylation was decreased (Fig. 5D). These results suggest that TTP is suppressed by DNA methylation in radioresistant HNC cells.
We confirmed whether DNMT1-mediated DNA methylation of TTP could regulate binding with CD47 AREs using a luciferase activity and DMNT1 inhibition could induce phagocytosis After treatment with 5-AZAdC, the activity of CD47 AREs 8-13 significantly decreased compared with that of the control through TTP expression (Fig. 5E). And following treatment with 5-AZAdC, we identified the significant increase of phagocytosis (Fig. 6A and B). These findings suggest that TTP suppression by DNMT1-mediated DNA methylation maintains sustained CD47 activation in radioresistant HNC cells.
Figure 6.
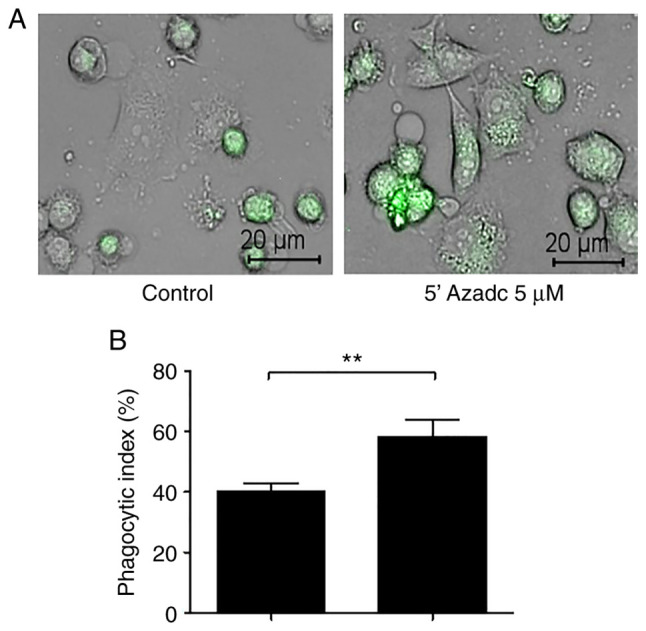
DMNT1 inhibition induces phagocytosis in radioresistant HNC cell lines. (A) Phagocytosis assay was performed. (B) Phagocytosis index was evaluated using phagocytosis assay after treatment with 5 µM 5-AZAdC in HN31R cells (scale bar, 20 µm). Values are presented as the means ± SD (n=3). **P<0.01. n.s., not significant.
Additionally, we investigated the CD47 related signal pathway in radioresistant HNC. We investigated the relationship of EphA3, CD47 and AKT activation, mediate tumor aggressiveness and radioresistance based on previous our study (18). We found that CD47 silencing decreased phosphor-AKT expression on western blot (Fig. S1). And we assessed which EphA3 could regulate CD47 and phosphor-AKT expression. The decrease of CD47 and phosphor-AKT was detected following EphA3 silencing compared to that in the control cells. These results suggest that CD47 antibody may have dual effect in increase of phagocytosis and suppression of AKT activation although these results are preliminary data in radioresistant HNC.
Discussion
In this study, we demonstrated that DNMT1 suppressed TTP expression through DNA methylation in radioresistant HNC cells, which in turn, prevented binding to the ARE within the CD47 3'UTR and inhibited CD47 mRNA decay. Thus, up-regulation of TTP can induce CD47 mRNA degradation and increase phagocytosis in radioresistant HNC cells.
It was known that the overexpression of TTP can induce inhibition of tumor growth, implicating TTP as a tumor suppressor (8). Specifically, TTP can regulate the posttranscriptional regulation of various inflammatory mediators and immune gene expression thus exert anti-cancer effects (8,17,21). Based on these findings, we studied the role of TTP in CD47 expression. In previous study, it was known that NF-κB and Hypoxia-inducible factor-1 (HIF-1) can regulate CD47 expression by binding to at transcription level (22,23). However, little is known about the regulation of CD47 expression through ARE motifs present in 3'UTR of mRNA, post-transcriptionally. We discovered that the CD47 3'UTR contained AREs and overexpression of TTP enhanced degradation of CD47 mRNA through ARE binding in HNC cells, in turn increased phagocytosis. Moreover, we demonstrated that TTP targeted CD47 directly by binding to the ARE flag 9 of CD47 mRNA, and CD47 expression was downregulated by TTP. Our results also indicated that TTP enhances phagocytosis through repression of CD47. To our knowledge, this is the first report suggesting the regulation of CD47 expression mediated by TTP post-transcriptionally.
Currently, several CD47-blocking antibodies are being investigated in clinical trials, alone or as combination therapy (5,24-26). However, the clinical limitation of current CD47-blocking antibodies is the expression of CD47 on normal cells such as myeloid cells which forms a ‘antigen sink’ that may restrict sufficient antibody acceleration at therapeutic sites (26,27). Thus, combination therapy with SIRPα-blocking agents or other target therapeutic agents can help a smaller antigen sink. Combination with TTP overexpression agents can help to reduce antigen sink of CD47 antibody, but currently, there is no drugs which can induce TTP overexpression. These findings suggest that TTP has important role in development of radioresistance in HNC and as biomarker to predict efficacy of CD47 antibody in recurrent HNC patients after radiation therapy.
Recently, one open-label, multicenter, phase 1 study with evorpacept (CD47 antibody) in advanced solid tumors including HNC showed a favorable safety with stable disease control (27). Evorpacept is being studied in combination with other drugs for both solid and hematological malignancies and can be a new approach to CD47 targeted therapies in advanced or recurrent HNC.
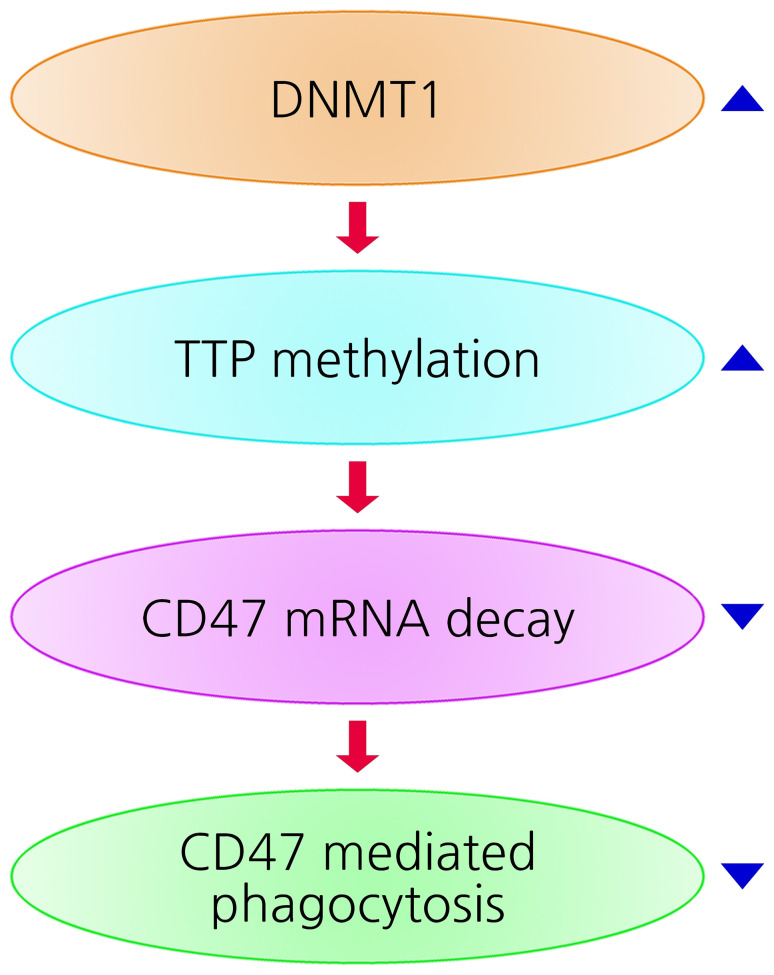
Finally, we investigated the mechanism of TTP suppression in radioresistant HNC cells. A various signaling pathways have been reported which regulate TTP expression at the transcription, post-transcriptional, or post-translational levels (11). Here, we demonstrated that TTP can be regulated epigenetically. Previously, we reported DNMT1 overexpression in radioresistant HNC (18) and found that TTP suppression was regulated by DNA methylation through DNMT1 overexpression in radioresistant HNC cells Our findings suggest a model (Fig. 7) in which suppression of CD47 mRNA decay through DNMT1-mediated TTP methylation induces sustained CD47 activation and inhibition of phagocytosis in radioresistant HNC. Thus, the present study demonstrates a previously unrecognized CD47 mRNA regulation by TTP in radioresistant HNC. The relationship of EphA3 and CD47/Akt pathway remains unclear in radioresistance. Our results indicate that EphA3 may regulate the CD47 and AKT signaling pathway but, further studies are needed to analyze the exact association in the signaling pathway, additionally.
Figure 7.
The present hypothetical model shows the upregulation of CD47 expression in radiation-resistant cells. TTP expression is downregulated by CpG hypermethylation in the TTP promoter (DNMT1-mediated DNA methylation), with constitutive activation of the CD47 by suppression of CD47 mRNA decay. The CD47 activation could inhibit phagocytosis. DNMT1, DNA methyltransferase; TPP, tristetraprolin.
In conclusion, we demonstrated that TTP could regulate CD47 gene expression in HNC, post-transcriptionally. We discovered that TTP could decay its mRNA by binding to the 9th ARE of the CD47 mRNA. As a result, TTP-mediated suppression of CD47 results in increased phagocytosis. TTP suppression by DNA methylation mediates sustained CD47 activation in radioresistant HNC. These findings suggest that TTP has important role in development of radioresistance in HNC. Our findings provide novel insight into role as biomarker of TTP to predict efficacy of CD47 antibody in recurrent HNC patients after radiation therapy.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by the National Research Foundation (NRF) grant funded by the Korea government (MSIT grant nos. MRC-2018R1A5A2020732, NRF-2020R1A2C1011879, NRF-2019R1A2C1006788 and NRF-2021R1A2C1010046) and Ulsan University Hospital Research Grant (grant no. UUH -2020-06).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SHK, WHL and HJC made substantial contributions to conception and design of experiments, and to the acquisition, analysis and interpretation of data. JHA and HWC made substantial contributions to the design of experiments, and to the acquisition, analysis and interpretation of data. TKK made substantial contributions to acquisition, analysis and interpretation of data. MWH made substantial contributions to conception and design of experiments, the acquisition, analysis and interpretation of data and drafted and revised the manuscript critically for important intellectual content. SYK and SWK made substantial contributions to conception and design of experiments, the analysis and interpretation of data and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript. MWH, SYK, SHK and WHL confirm the authenticity of the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9:1215–1231. doi: 10.7150/thno.32648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L, Wang X, Hu B, Wang D, Ren Z. miR-222 enhances radiosensitivity of cancer cells by inhibiting the expression of CD47. Int J Clin Exp Pathol. 2019;12:4204–4213. [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong B, Wang M. CD47 is a novel potent immunotherapy target in human malignancies: Current studies and future promises. Future Oncol. 2018;14:2179–2188. doi: 10.2217/fon-2018-0035. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Jin S, Guo X, Qian W. Targeting the CD47-SIRPα signaling axis: Current studies on B-cell lymphoma immunotherapy. J Int Med Res. 2018;46:4418–4426. doi: 10.1177/0300060518799612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Muz B, Alhallak K, Markovic M, Gurley S, Wang Z, Guenthner N, Wasden K, Fiala M, King J, et al. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers (Basel) 2020;12(305) doi: 10.3390/cancers12020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD. CD47 receptor globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem. 2015;290:24858–24874. doi: 10.1074/jbc.M115.665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks SA, Blackshear PJ. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829:666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WH, Han MW, Kim SH, Seong D, An JH, Chang HW, Kim SY, Kim SW, Lee JC. Tristetraprolin posttranscriptionally downregulates TRAIL death receptors. Cells. 2020;9(1851) doi: 10.3390/cells9081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross CR, Brennan-Laun SE, Wilson GM. Tristetraprolin: Roles in cancer and senescence. Ageing Res Rev. 2012;11:473–484. doi: 10.1016/j.arr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanduja S, Blanco FF, Young LE, Kaza V, Dixon DA. The role of tristetraprolin in cancer and inflammation. Front Biosci (Landmark Ed) 2012;17:174–188. doi: 10.2741/3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JM, Lee TH, Kang TH. Roles of tristetraprolin in tumorigenesis. Int J Mol Sci. 2018;19(3384) doi: 10.3390/ijms19113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Zhou Z, Yang R, Zhang S, Zhang B, Tan Y, Chen L, Li T, Tu J. Tristetraprolin, a potential safeguard against carcinoma: Role in the tumor microenvironment. Front Oncol. 2021;11(632189) doi: 10.3389/fonc.2021.632189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang W, Zhu D, Wang C, Zhu Y. Tumor suppressing effects of tristetraprolin and its small double-stranded RNAs in bladder cancer. Cancer Med. 2021;10:269–285. doi: 10.1002/cam4.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HH, Yang SS, Vo MT, Cho WJ, Lee BJ, Leem SH, Lee SH, Cha HJ, Park JW. Tristetraprolin down-regulates IL-23 expression in colon cancer cells. Mol Cells. 2013;36:571–576. doi: 10.1007/s10059-013-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng K, Wang H, Shan T, Chen Y, Zhou H, Zhao Q, Xia J. Tristetraprolin inhibits gastric cancer progression through suppression of IL-33. Sci Rep. 2016;6(24505) doi: 10.1038/srep24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Ning H, Peng H, Wei L, Hou R, Hoft DF, Liu J. Tristetraprolin inhibits macrophage IL-27-induced activation of antitumour cytotoxic T cell responses. Nat Commun. 2017;8(867) doi: 10.1038/s41467-017-00892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Kang BC, Seong D, Lee WH, An JH, Je HU, Cha HJ, Chang HW, Kim SY, Kim SW, Han MW. EPHA3 contributes to epigenetic suppression of PTEN in radioresistant head and neck cancer. Biomolecules. 2021;11(599) doi: 10.3390/biom11040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Lee WH, Kim SW, Je HU, Lee JC, Chang HW, Kim YM, Kim K, Kim SY, Han MW. EphA3 maintains radioresistance in head and neck cancers through epithelial mesenchymal transition. Cell Signal. 2018;47:122–130. doi: 10.1016/j.cellsig.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee JC, Lee WH, Min YJ, Cha HJ, Han MW, Chang HW, Kim SA, Choi SH, Kim SW, Kim SY. Development of TRAIL resistance by radiation-induced hypermethylation of DR4 CpG island in recurrent laryngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1203–1211. doi: 10.1016/j.ijrobp.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Tu Y, Wu X, Yu F, Dang J, Wang J, Wei Y, Cai Z, Zhou Z, Liao W, Li L, Zhang Y. Tristetraprolin specifically regulates the expression and alternative splicing of immune response genes in HeLa cells. BMC Immunol. 2019;20(13) doi: 10.1186/s12865-019-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y, Leeper NJ, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8(14802) doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Xavy S, Mihardja S, Chen S, Sompalli K, Feng D, Choi T, Agoram B, Majeti R, Weissman IL, Volkmer JP. Targeting macrophage checkpoint inhibitor SIRPα for anticancer therapy. JCI Insight. 2020;5(e134728) doi: 10.1172/jci.insight.134728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriks M, Ploeg EM, Koopmans I, Britsch I, Ke X, Samplonius DF, Helfrich W. Bispecific antibody approach for EGFR-directed blockade of the CD47-SIRPα ‘don't eat me’ immune checkpoint promotes neutrophil-mediated trogoptosis and enhances antigen cross-presentation. Oncoimmunology. 2020;9(1824323) doi: 10.1080/2162402X.2020.1824323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veillette A, Chen J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39:173–184. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Lakhani NJ, Chow LQM, Gainor JF, LoRusso P, Lee KW, Chung HC, Lee J, Bang YJ, Hodi FS, Kim WS, et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): A first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1740–1751. doi: 10.1016/S1470-2045(21)00584-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.