Abstract
Purpose:
The clinical impact of concurrent corticosteroid use (CCU) on enzalutamide-treated patients with metastatic castration-resistant prostate cancer (mCRPC) is unknown. We investigated the association of CCU with overall survival (OS), radiographic progression-free survival (rPFS), and time to prostate-specific antigen progression (TTPP) in post-chemotherapy, enzalutamide-treated patients with mCRPC.
Patients and Methods:
Post hoc analysis of AFFIRM (NCT00974311) with patients (n = 1,199) randomized 2:1 to enzalutamide 160 mg/day or placebo. Treatment group, CCU, and known prognostic factors were evaluated for impact on OS, rPFS, and TTPP using a multivariate Cox proportional hazards model. CCU was defined as “baseline” (use started at baseline) or “on-study” (baseline plus use that was started during the trial).
Results:
Enzalutamide significantly improved OS, rPFS, and TTPP independent of baseline CCU but was associated with inferior clinical outcomes when compared with no baseline CCU, including a shorter OS [10.8 months vs. not reached (NR); HR for use vs. no use, 2.13; 95% confidence interval (CI), 1.79–2.54], rPFS (5.2 months vs. 8.0 months; HR, 1.49; 95% CI, 1.29–1.72], and TTPP (4.6 months vs. 5.7 months; HR, 1.50; 95% CI, 1.25–1.81). These findings held in a multivariate analysis adjusting for baseline prognostic factors wherein baseline CCU was independently associated with decreased OS (HR, 1.71; 95% CI, 1.43–2.04; P < 0.0001) and rPFS (HR, 1.28; 95% CI, 1.11–1.48; P = 0.0007).
Conclusions:
Patients with mCRPC benefited from enzalutamide treatment independent of CCU, but CCU was associated with worse baseline prognostic factors and outcomes.
Translational Relevance.
Enzalutamide is a second-generation, androgen receptor signaling inhibitor that significantly improves clinical outcomes for patients with nonmetastatic and metastatic castration-resistant and metastatic noncastrate prostate cancer. Corticosteroids are frequently used in advanced prostate cancer to manage symptoms and mitigate therapy-related side effects. The true impact of corticosteroid use on clinical outcomes in patients receiving androgen receptor inhibitors is unknown. Here we present a post hoc analysis of the AFFIRM phase III clinical trial which shows that enzalutamide improves overall survival, radiographic progression-free survival, and time to prostate-specific antigen progression of patients with metastatic castration-resistant prostate cancer independent of corticosteroid use. However, the benefit from enzalutamide was less for patients who received concomitant corticosteroids. Further validation is required to determine the causal relationship of the negative effect of corticosteroids on survival and the clinical setting where the short-term benefits of corticosteroid use outweigh the potential for adverse effects and an inferior outcome.
Introduction
Prostate cancers that progress on androgen deprivation therapy are classified as castration-resistant because most remain dependent on androgen receptor (AR) signaling for growth (1, 2). This dependence has been demonstrated clinically in the placebo-controlled Phase III registration trials of second-generation AR signaling inhibitors, such as abiraterone and enzalutamide, both of which showed a significant survival benefit in the experimental arm relative to the control arm for patients with progressing metastatic castration-resistant disease (mCRPC; refs. 3–6).
Abiraterone inhibits androgen production by targeting 17-α-hydroxylase (CYP17), a key enzyme involved in androgen biosynthesis, and was the first androgen receptor signaling directed agent approved for men with mCRPC in both the pre- and post-chemotherapy settings, and more recently for those with metastatic castration-sensitive disease (mCSPC; refs. 3, 4, 7). Safe administration requires concurrent prednisone, which has independent anticancer effects, to counteract the increased mineralocorticoid production induced with abiraterone when given alone (8, 9).
The antitumor effects of corticosteroids on metastatic prostate cancer were first described in the 1950s (10–13). Further, owing to their anti-inflammatory properties, anti-androgenic endocrine and other pleiotropic effects, corticosteroids have also been used in CRPC to palliate pain from osseous metastases, reduce cancer-associated weight loss, fatigue, and chemotherapy-related adverse events (14). However, high-dose or long-term, low-dose corticosteroid use can lead to immune suppression and increased susceptibility to infections, osteoporosis, steroid-induced diabetes, peptic ulcer disease, myopathy, fluid retention, metabolic syndrome, electrolyte instability, weight gain, insomnia, and others (14–16). An analysis of patients from two Phase III abiraterone trials suggested that low-dose prednisone at 5 mg twice a day was generally safe but associated with a corticosteroid-related adverse event in 24.6% of patients, but only 4.5% were grade 3 or greater (15).
Enzalutamide is a rationally designed oral AR inhibitor targeting multiple steps in the AR signaling pathway (17). The drug is approved for the treatment of patients with CRPC based on the AFFIRM, PREVAIL, and PROSPER trials as well as in men with mCSPC based on the ARCHES trial (5, 6, 18–20). In AFFIRM, enzalutamide improved the median overall survival (OS) by 4.8 months (HR, 0.631; P < 0.0001) compared with placebo. Trial entry permitted the continuation of corticosteroids at baseline or to add corticosteroids while on protocol therapy (5).
Research into mechanisms associated with castration resistance led to the discovery that corticosteroids may stimulate prostate cancer cell growth in certain contexts by activating “promiscuous” or mutated ARs, such as an AR with L702H mutation (21–25). Further study showed that CRPC cell lines treated with potent AR signaling inhibitors such as enzalutamide and abiraterone can upregulate glucocorticoid receptor (GR) expression and hijack GR signaling to activate a shared subset of AR-regulated genes to bypass AR blockade (26–29). Clinical credentialing of these findings in correlative studies found both the emergence of an AR L702H mutation and upregulation of GR expression at variable frequencies in patients who developed resistance to these drugs (26, 28, 30), the significance of which is now being studied prospectively in trials evaluating the combination of selective GR antagonists with enzalutamide for their potential to restore sensitivity to AR inhibition in patients with mCRPC (31, 32).
As corticosteroids are commonly used in the management of advanced prostate cancer and have the potential to have agonist growth stimulatory effects on the tumor in certain contexts, we performed an exploratory, post hoc analysis to evaluate the association of corticosteroid use on OS, radiographic progression-free survival (rPFS), and time to prostate-specific antigen (PSA) progression (TTPP) in the AFFIRM trial.
Patients and Methods
Study design and conduct
AFFIRM (5) was a randomized, double-blind, placebo-controlled, multinational phase III study. Patients were randomized 2:1 to enzalutamide 160 mg once every day or placebo. The use of corticosteroids was allowed at study entry and during the trial. Approximately 30% of the study participants were taking corticosteroids at study entry, in most cases first initiated upon receipt of docetaxel, the standard first-line chemotherapy regimen for mCRPC.
For this analysis, corticosteroid use was recorded in two ways: “baseline,” defined as a patient already on a corticosteroid at the time of trial initiation that was continued for 1 day or longer after starting the trial, and “on-study,” those who had taken any corticosteroid concurrently for 1 day or longer at any point during the trial, which included a broader patient population from both baseline users and patients for whom corticosteroid use was initiated during the trial. In both contexts, enzalutamide could be continued until disease progression or initiation of a new systemic antineoplastic therapy, unacceptable toxicity, or withdrawal. The primary endpoint was OS. Secondary endpoints included rPFS, TTPP, and safety.
This study was reviewed and approved by the Institutional Review Boards and Independent Ethics Committees of the participating investigational centers and was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice according to the International Conference on Harmonization guidelines. All patients provided written informed consent to participate.
Statistical analysis
To construct a multivariate model, we started with a total of 15 covariates: age (<65 years vs. ≥65 years), region (North America vs. rest of world), prior chemotherapy regimens (1 vs. ≥2), log of baseline PSA, type of progression at entry (PSA progression only vs. radiographic progression), treatment group (enzalutamide vs. placebo), baseline Eastern Cooperative Oncology Group performance status (ECOG PS; 0–1 vs. 2), presence of visceral disease (no vs. yes), baseline mean pain score (<4 vs. ≥4), oral corticosteroid use (yes vs. no), baseline serum albumin (g/L; continuous variable), baseline hemoglobin (g/L; continuous variable), lactate dehydrogenase [LDH; normal vs. >upper limit of normal (ULN)], alkaline phosphatase (ALP; normal vs. >ULN), and baseline opioid use (no vs. yes). This more extensive list included all the prognostic factors used in a well-validated prognostic nomogram for OS in patients with mCRPC, including ECOG PS, disease site, LDH, opioid analgesic use, albumin, hemoglobin, PSA, and ALP (33). As the decision to continue or start corticosteroid use was made by the treating physician and not based on randomized assignment, statistical analyses were run with these factors to evaluate differences between the groups with baseline corticosteroid use and those without baseline use. Baseline and prognostic characteristics were then evaluated for their effects on OS and rPFS using a univariate Cox regression model. Covariates shown to be statistically significant (P < 0.05) in the univariate analysis were then considered for the multivariate Cox proportional hazards model. Some covariates not significant in the univariate analysis at P < 0.05 (e.g., age <65, region, PSA, and prior number of chemotherapy) were included for completeness, but none proved significant and did not appear in the final multivariate model.
To analyze the interaction of treatment by corticosteroid use, a Cox model was run with terms for treatment, baseline subgroup variable, and the treatment-by-baseline interaction. The HR, along with its 95% confidence interval (CI), and P value of the interactions were derived.
A risk score was derived from a prognostic model (34), including the nine baseline measures of visceral disease, measurable disease, pain, progression on docetaxel, ECOG-PS, time on hormone therapy, hemoglobin, ALP, and PSA. Each measure contributed to the total score for a patient. The sum of the nine components yielded a total score, which then slotted a patient into low or high risk. Cox models with terms for treatment were then run by risk group, separately for with and without corticosteroid use.
HRs for death and rPFS were calculated using a stepwise selection method including only the variables that were independently statistically significant. First, the nonsignificant (P ≥ 0.05) factors were eliminated at entry into the multivariate model. Next, additional factors were eliminated after their contribution was assessed with greater stringency (P ≥ 0.001). Variables that did not contribute independently were eliminated and the next variable demonstrating statistical significance in the stepwise method was added. For instance, mean baseline pain score was eliminated because it co-varied with, but was less significant than, baseline opioid use. The stricter stay criterion of P < 0.001 was intended for a more parsimonious final model. As this was a post hoc exploratory analysis, P values were not adjusted for multiple comparisons nor were endpoints controlled for overall type 1 error. P values should be considered descriptive only. Missing data were not imputed. Missing covariates were present in 10/1,199 (0.834%) patients who were not included in the analysis.
Data sharing statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (i) for indications that have been approved in the United States and/or European Union or (ii) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Results
This analysis included 1,199 randomized patients in the prespecified AFFIRM intent-to-treat analysis. The baseline characteristics and disease burden are grouped by treatment (enzalutamide vs. placebo) and baseline corticosteroid use (with vs. without baseline use; Table 1). Overall, 30% of the patients were taking corticosteroids at baseline in both study arms. Considering any on-study corticosteroid use, including baseline use and corticosteroids newly started during the trial, the percentage was 45% and 48% in the placebo and enzalutamide arms, respectively (Supplementary Table S1). The most commonly used corticosteroids included prednisone/prednisolone, followed by dexamethasone (Supplementary Tables S2 and S3).
Table 1.
Characteristics of patients and their disease burden, according to treatment groups and baseline corticosteroid use.
| Patients with baseline corticosteroid use | Patients without baseline corticosteroid use | Enzalutamide-treated patients with vs. without baseline corticosteroid use | Placebo-treated patients with vs. without baseline corticosteroid use | |||||
|---|---|---|---|---|---|---|---|---|
| Enzalutamide | Placebo | Enzalutamide | Placebo | |||||
| n = 241 (30.1%a) | n = 119 (29.8%b) | P value | n = 559 (69.9%a) | n = 280 (70.2%b) | P value | P value | P value | |
| Baseline characteristics | ||||||||
| Age | ||||||||
| Mean, years | 68.8 | 67.5 | 0.156 | 68.8 | 69.1 | 0.612 | 1.00 | 0.082 |
| % ≥75 years | 26% | 20% | 0.245 | 25% | 29% | 0.205 | 0.715 | 0.080 |
| Gleason score (mean) | 7.7 | 7.8 | 0.476 | 7.5 | 7.5 | 1 | 0.056 | 0.033 |
| ECOG performance status = 2 | 14.5% | 4.2% | 0.003 | 6.3% | 9.6% | 0.078 | <0.001 | 0.067 |
| BPI ≥4 on question 3c | 31.5% | 36.1% | 0.383 | 26.8% | 25.7% | 0.729 | 0.175 | 0.036 |
| Prior musculoskeletal and connective tissue disorders | 68.5% | 64.7% | — | 66.4% | 60.4% | — | — | — |
| Duration of corticosteroid use (mean months, range) | 8.9 (0.0–128.3) | 9.8 (0.0–44.2) | — | — | — | — | — | — |
| Disease burden | ||||||||
| Mean PSA (ng/mL) | 582.6 | 405.8 | 0.104 | 343.6 | 382.4 | 0.596 | <0.001 | 0.847 |
| Mean hemoglobin (g/L) | 116.2 | 117.4 | 0.517 | 120.4 | 120.4 | 1 | <0.001 | 0.090 |
| % abnormal (<125 g/L) | 68.3% | 61.3% | 0.188 | 57.1% | 57.5% | 0.905 | 0.003 | 0.476 |
| Mean alkaline phosphatase (U/L) | 295.5 | 342.5 | 0.428 | 206.2 | 191.6 | 0.527 | 0.002 | 0.001 |
| % abnormal (>125 U/L) | 56.4% | 47.9% | 0.127 | 41.9% | 42.1% | 0.938 | <0.001 | 0.289 |
| Mean LDH (U/L) | 316.0 | 314.5 | 0.954 | 252.5 | 249.7 | 0.880 | <0.001 | <0.001 |
| % abnormal (>234 U/L) | 53.1% | 52.5% | 0.919 | 31.3% | 35.5% | 0.224 | 0 | 0.002 |
| Mean albumin (g/L) | 36.1 | 37.0 | 0.047 | 38.1 | 37.7 | 0.175 | 0 | 0.128 |
| % abnormal <33 (g/L) | 19.1% | 13.4% | 0.182 | 9.3% | 11.2% | 0.399 | <0.001 | 0.517 |
| Any lab abnormality % abnormal | 85.5% | 84.9% | 0.879 | 71.9% | 71.1% | 0.799 | 0 | 0.004 |
| Visceral disease at screening | 29.0% | 18.5% | 0.031 | 22.5% | 21.4% | 0.715 | 0.050 | 0.506 |
| >20 bone metastases at screening | 49.0% | 47.1% | 0.734 | 32.9% | 33.9% | 0.769 | 0 | 0.013 |
| Months from initial diagnosis (mean) | 80.7 | 71.3 | — | 88.4 | 86.4 | — | — | — |
| Baseline opioid use = Yes | 55.6% | 61.3% | 0.230 | 39.0% | 47.1% | 0.024 | 0 | 0.009 |
Note: Significant P values are indicated in bold.
Abbreviation: BPI, Brief pain inventory.
aPercent of enzalutamide patients.
bPercent of placebo patients.
cBPI Short Form Question 3 measures pain on a scale from 0 (no pain) to 10 (pain as bad as you can imagine).
Baseline patient demographics and disease characteristics differed as a function of corticosteroid use, as those who received corticosteroids had more aggressive tumors and a higher disease burden as characterized by higher Gleason scores, median PSA, ALP, LDH, a higher frequency of visceral metastases, >20 osseous metastases, and opioid use (Table 1). The finding is consistent with clinical practice in that patients with more advanced disease were more likely to be symptomatic and to be prescribed corticosteroids.
Overall, patients taking a corticosteroid at baseline experienced an inferior outcome relative to nonusers, irrespective of study drug assignment with a median OS of 10.8 months versus not reached (NR; HR with vs. without corticosteroid use, 2.13; 95% CI, 1.79–2.54; P < 0.0001), median rPFS of 5.2 months versus 8.0 months (HR, 1.49; 95% CI, 1.29–1.72; P < 0.0001), and a more rapid median TTPP of 4.6 months versus 5.7 months (HR, 1.50; 95% CI, 1.25–1.81; P < 0.0001; Table 2).
Table 2.
Outcomes by baseline corticosteroid use irrespective of treatment group.
| Patients with baseline corticosteroid use | Patients without baseline corticosteroid use | |
|---|---|---|
| n = 360 (30%) | n = 839 (70%) | |
| OS | ||
| Median (95% CI), months | 10.8 (10.0–12.5) | NR (18.3–NR) |
| P (stratified log-rank) | <0.0001 | |
| Stratified HR (95% CI) | 2.13 (1.79–2.54) | |
| rPFS | ||
| Median (95% CI), months | 5.2 (4.0–5.5) | 8.0 (6.1–8.3) |
| P (stratified log-rank) | <0.0001 | |
| Stratified HR (95% CI) | 1.49 (1.29–1.72) | |
| TTPP | ||
| Median (95% CI), months | 4.6 (4.6–5.5) | 5.7 (5.6–8.3) |
| P (stratified log-rank) | <0.0001 | |
| Stratified HR (95% CI) | 1.50 (1.25–1.81) | |
Note: Stratification factors are baseline ECOG status (0 and 1 vs. 2) and baseline mean pain score (<4 vs. ≥4). HR is with corticosteroid use vs. without corticosteroid use.
To account for factors that might have affected the OS outcome independent of steroid use, a univariate Cox regression model was developed for OS and rPFS (Table 3A and B, respectively). In the final, stepwise, multivariate model, eight variables emerged as independently significant for OS (Table 3C) and five for rPFS (Table 3D). In both analyses, baseline corticosteroid use was shown to be a strong and independent prognostic factor for OS (HR, 1.71; 95% CI, 1.43–2.04; P < 0.0001; Table 3C) and rPFS (HR, 1.28; 95% CI, 1.11–1.48; P = 0.0007; Table 3D). A small but statistically significant difference in regional OS was observed in the univariate analysis, likely due to the differences in baseline disease characteristics, as it dropped out of the final multivariate model.
Table 3.
(A) Univariate Cox regression analysis of OS and (B) rPFS, and multivariate analysis of (C) OS and (D) rPFS.
| (A) Univariate Cox regression OS analysis (ordered by descending P)a | |||
|---|---|---|---|
| Variable | P | HR (95% CI) | |
| Age group (<65 vs. ≥65) | 0.73 | 0.97 (0.80–1.17) | |
| Region (North America vs. rest of world) | 0.0180 | 1.24 (1.04–1.48) | |
| Prior number of chemotherapy (1 vs. ≥2) | 0.0157 | 0.80 (0.66–0.96) | |
| Baseline PSA (μg/L)d | 0.0007 | 1.00 (1.00–1.00) | |
| Type of progression (PSA only vs. radiographic) | 0.0001 | 0.70 (0.58–0.84) | |
| Treatment (enzalutamide vs. placebo) | <0.0001 | 0.63 (0.53–0.75) | |
| Baseline ECOG performance status (0–1 vs. 2) | <0.0001 | 0.49 (0.38–0.64) | |
| Visceral disease at screening (no vs. yes) | <0.0001 | 0.58 (0.48–0.70) | |
| Baseline mean pain score strata (≤4 vs. >4) | <0.0001 | 0.48 (0.41–0.58) | |
| Baseline alkaline phosphatase (normal vs. >ULN) | <0.0001 | 0.39 (0.33–0.47) | |
| Baseline opioids (no vs. yes) | <0.0001 | 0.38 (0.32–0.45) | |
| Baseline serum albumin (g/L)d | <0.0001 | 0.87 (0.85–0.89) | |
| Baseline hemoglobin (g/L)d | <0.0001 | 0.96 (0.96–0.97) | |
| Baseline LDH (normal vs. >ULN) | <0.0001 | 0.24 (0.20–0.29) | |
| Baseline corticosteroid use (yes vs. no) | <0.0001 | 2.21 (1.85–2.62) | |
| (B) Univariate Cox regression rPFS analysis (ordered by descending P) | |||
|---|---|---|---|
| Variable | P value | HR (95% CI) | |
| Region North America vs. rest of world | 0.18 | 1.10 (0.96–1.27) | |
| Baseline PSA (μg/L)d | 0.16 | 1.00 (1.00–1.00) | |
| Prior number of chemotherapy (1 vs. ≥2) | 0.06 | 0.87 (0.75–1.01) | |
| Age group (<65 vs. ≥65) | 0.03 | 1.17 (1.01–1.36) | |
| Baseline ECOG performance status (0–1 vs. 2) | 0.02 | 0.76 (0.60–0.95) | |
| Baseline mean pain score strata (≤4 vs. >4) | 0.0002 | 0.76 (0.66–0.88) | |
| Type of progression (PSA only vs. radiographic) | <0.0001 | 0.75 (0.66–0.87) | |
| Baseline alkaline phosphatase normal vs. >ULN | <0.0001 | 0.73 (0.64–0.83) | |
| Visceral disease at screening (no vs. yes) | <0.0001 | 0.63 (0.54–0.74) | |
| Baseline opioids (no vs. yes) | <0.0001 | 0.65 (0.57–0.74) | |
| Baseline serum albumin (g/L)d | <0.0001 | 0.95 (0.93–0.96) | |
| Baseline hemoglobin (g/L)d | <0.0001 | 0.99 (0.98–0.99) | |
| Baseline LDH (normal vs. >ULN) | <0.0001 | 0.46 (0.40–0.53) | |
| Treatment (enzalutamide vs. placebo) | <0.0001 | 0.40 (0.35–0.47) | |
| Baseline corticosteroid use (yes vs. no) | <0.0001 | 1.50 (1.30–1.73) | |
| (C) Results of stepwise multivariate analysis of OSb parameter estimates | |||
|---|---|---|---|
| Variable | Coefficient ± SE | P | HR for death (95% CI) |
| Treatment (enzalutamide vs. placebo) | –0.49 ± 0.090 | <0.0001 | 0.61 (0.51–0.73) |
| Type of progression (PSA only vs. radiographic) | –0.33 ± 0.094 | 0.0005 | 0.72 (0.60–0.87) |
| Visceral disease at screening (no vs. yes) | –0.43 ± 0.097 | <0.0001 | 0.65 (0.54–0.79) |
| Baseline serum albumin (g/L)d | –0.06 ± 0.013 | <0.0001 | 0.94 (0.92–0.96) |
| Baseline hemoglobin (g/L)d | –0.01 ± 0.003 | <0.0001 | 0.99 (0.98–0.99) |
| Baseline LDH (normal vs. >ULN) | –1.03 ± 0.098 | <0.0001 | 0.36 (0.30–0.44) |
| Baseline opioids (no vs. yes) | –0.49 ± 0.096 | <0.0001 | 0.61 (0.51–0.74) |
| Baseline corticosteroid use (yes vs. no) | 0.53 ± 0.091 | <0.0001 | 1.71 (1.43–2.04) |
| (D) Results of stepwise multivariate analysis of rPFSc | |||
|---|---|---|---|
| Parameter estimates | |||
| Coefficient ± SE | P | HR for event (95% CI) | |
| Treatment (enzalutamide vs. placebo) | –0.85 ± 0.072 | <0.0001 | 0.43 (0.37–0.49) |
| Type of progression (PSA only vs. radiographic) | –0.28 ± 0.071 | 0.0001 | 0.76 (0.66–0.87) |
| Visceral disease at screening (no vs. yes) | –0.45 ± 0.078 | <0.0001 | 0.64 (0.55–0.75) |
| Baseline LDH (normal vs. >ULN) | –0.65 ± 0.071 | <0.0001 | 0.52 (0.46–0.60) |
| Baseline corticosteroid use (yes vs. no) | 0.25 ± 0.073 | 0.0007 | 1.28 (1.11–1.48) |
aBecause baseline PSA was skewed, log-transformation was used in the multivariate model.
bSurvival for patients who had not died by the time of analysis was censored at the date the patient was last known to be alive.
cProgression for patients who had not yet progressed by the time of analysis was censored at the date of the last radiographic assessment.
dSerum albumin, PSA, and hemoglobin use was continuous.
In AFFIRM (5), the difference in median OS was 4.8 months for enzalutamide (HR, 0.631; P < 0.0001) compared with placebo. The clinically significant and meaningful benefit of enzalutamide in AFFIRM (5) was retained regardless of baseline corticosteroid use (Fig. 1A–C). In patients with baseline concurrent corticosteroid use (CCU), enzalutamide treatment extended the median OS from 9.3 months to 12.3 months (HR, 0.70; P = 0.012; Fig. 1A), median rPFS from 2.9 months to 5.6 months (HR, 0.59; P < 0.001; Fig. 1B), and the TTPP from 3.0 months to 5.5 months (HR, 0.36; P < 0.001), relative to the placebo (Fig. 1C). In patients without baseline corticosteroid use, median OS was extended from 15.8 months to NR after 24 months of follow-up (HR, 0.59; P < 0.001; Fig. 1A), median rPFS from 2.9 months to 10.6 months (HR, 0.33; P < 0.001; Fig. 1B), and median TTPP from 3.0 months to 8.3 months (HR, 0.22; P < 0.001; Fig. 1C).
Figure 1.
OS (A), rPFS (B), and PSA PFS (C), with and without baseline corticosteroid use. NR, not reached.
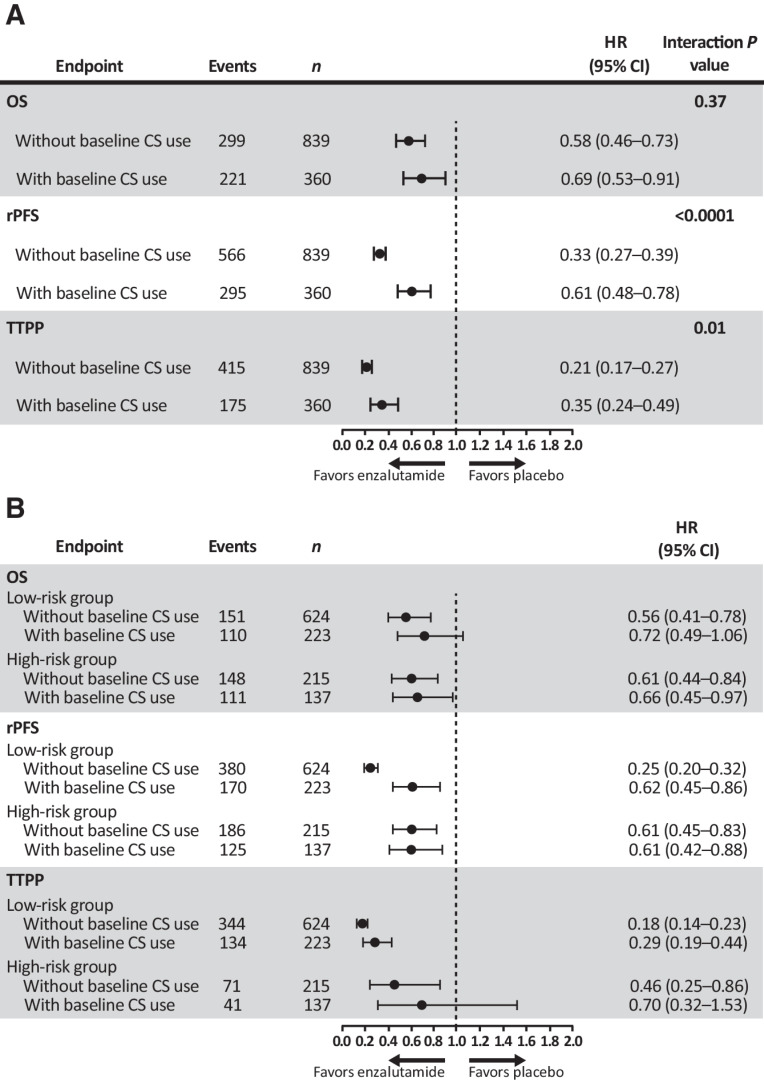
Even though baseline corticosteroid use was shown to be a significant and independent prognostic factor for inferior OS in both treatment arms (Table 2; Fig. 1A), baseline corticosteroid use did not significantly impact rPFS (Fig. 1B) and TTPP (Fig. 1C) in the placebo group where the median rPFS was 2.9 months (Fig. 1B) and median TTPP 3.0 months in both corticosteroid-exposed and unexposed patient groups (Fig. 1C). In contrast, in enzalutamide-treated patients, median rPFS and TTPP were significantly shorter in baseline corticosteroid users versus nonusers (Fig. 1B and C). Consistently, a quantitative treatment-by-baseline corticosteroid use interaction analysis showed a significant interaction between treatment arms and status of baseline corticosteroid use for rPFS (HR, 0.33 vs. 0.61; P < 0.0001) and TTPP (HR, 0.21 vs. 0.35; P = 0.01) but not for OS (HR, 0.58 vs. 0.69; P = 0.37; Fig. 2A).
Figure 2.
Assessment of treatment-by-baseline corticosteroid use interaction analysis (A) and effects of baseline corticosteroid use on OS, rPFS, and TTPP in patients stratified into high-risk or low-risk groups (B).
To further address whether baseline corticosteroid use affected clinical outcomes of patients treated with enzalutamide independent of baseline disease risk characteristics, a clinically validated nomogram for survival in mCRPC was used to risk-stratify patients into high-risk and low-risk groups (33). Further stratification into different risk groups enabled an independent assessment of the effect of corticosteroid use within the same risk group with similar baseline disease characteristics. Risk stratification was based on the presence or absence of visceral disease, measurable disease, pain, time to progression on docetaxel (<6 months or ≥6 months), ECOG PS, time on hormone treatment, and pretreatment levels of hemoglobin, ALP, and PSA. The results suggested that baseline corticosteroid use may have attenuated the enzalutamide treatment benefit, as evidenced by the higher HRs for those with versus without baseline corticosteroid use in OS (0.72 vs. 0.56), rPFS (0.62 vs. 0.25), and TTPP (0.29 vs. 0.18) in the low-risk group. In the high-risk group, HRs with versus without corticosteroid use were similar for OS (0.66 vs. 0.61) and rPFS (0.61 vs. 0.61) and again trended higher for TTPP (0.70 vs. 0.46), although patient numbers were smaller in this cohort (Fig. 2B).
Similar analyses were performed with cohorts defined more broadly by any on-study corticosteroid use, which included both patients receiving corticosteroids at baseline and those for whom oral corticosteroids were newly prescribed during the trial. This increased the percent of patients receiving corticosteroids on study from 30% to 48% in the enzalutamide treatment group and from 30% to 45% in the placebo group, for whom the characteristics and disease burden are listed (Supplementary Table S1). The baseline characteristics of patients receiving corticosteroids at baseline were similar compared with those who received them after the trial started (but without baseline use), with the exception of ECOG PS in placebo-treated patients (4.2% in patients with baseline use had an ECOG PS of 2 vs. 15.3% in patients who received corticosteroids after the trial started; P = 0.01; Supplementary Table S4).
Similar analyses were also performed on a broader population of corticosteroid users defined as any on-study use, which included baseline use and any use that started after the trial onset. The results confirmed that any on-study corticosteroid use was associated with an inferior OS, rPFS, and TTPP (Supplementary Table S5). Although OS, rPFS, and TTPP were improved in the enzalutamide group relative to placebo independent of corticosteroid use, the magnitude of benefit from enzalutamide treatment was smaller for those who had any on-study use as well (Supplementary Figs. S1A–S1C). Consistent with baseline corticosteroid use, the same results were observed for any on-study corticosteroid use in the treatment-by-any corticosteroid use interaction (Supplementary Fig. S2A) and risk stratification (Supplementary Fig. S2B) analyses.
Discussion
The objective of this study was to analyze the potential association of CCU, at baseline and on study, with clinical outcomes in patients receiving enzalutamide or placebo in the AFFIRM trial. The results suggest that although patients with mCRPC benefited from enzalutamide independent of corticosteroid use, those who took concurrent corticosteroids at baseline or at any point during the study experienced shorter PFS and OS compared with those without corticosteroid use. These findings highlight the potential clinical implications of corticosteroid use when combined with a second-generation androgen receptor-targeted therapy in patients with mCRPC.
As early as the 1950s, corticosteroids have been shown to clinically benefit patients with metastatic prostate cancer who had failed androgen deprivation therapies (likely orchiectomy or diethylstilbestrol; refs. 10–13). These drugs are also beneficial for the palliation of symptoms of disease such as fatigue or pain from bone metastases and other noncancer-related skeletal morbidities, and mitigating the nausea, vomiting, and allergic reactions that can occur with chemotherapeutic agents such as docetaxel and cabazitaxel. They can also improve appetite, mood, and depressive symptoms related to prostate cancer.
The abiraterone indication includes the co-administration of prednisone with the CYP17 inhibitor abiraterone acetate, to prevent secondary mineralocorticoid excess characterized by fluid retention, hypertension, and/or hypokalemia. On the other hand, chronic concomitant corticosteroid use is also associated with significant adverse events and toxicities (14, 16). However, it remains unclear whether corticosteroid use affected the clinical outcomes of patients receiving next-generation AR antagonists, such as enzalutamide, favorable or unfavorable.
AFFIRM was a prospectively designed, randomized, phase III, registration trial powered to demonstrate the efficacy of enzalutamide versus placebo in men with mCRPC who had progressed on docetaxel and prednisone. The results indicated a significant OS benefit in favor of enzalutamide that led to its initial FDA approval in 2012 for treatment of mCRPC in the post-docetaxel setting (5). In the AFFIRM trial, a substantial proportion of patients in each treatment group were taking corticosteroids at study entry or were prescribed corticosteroids while on study. Here, in an exploratory, post hoc analysis, baseline corticosteroid use compared with no corticosteroid use was associated with shortened OS, rPFS, and TTPP that remained significant even after adjusting for adverse, baseline, and prognostic factors. The results held in a broader cohort of patients defined by any on-study corticosteroid use that included those taking corticosteroids at trial initiation and those who newly initiated use while on study. Notably, the overall benefit of enzalutamide relative to placebo was retained in both the nonsteroid user and steroid user groups. However, the magnitude of clinical benefits from enzalutamide treatment was smaller in corticosteroid users relative to nonusers. One possible explanation is that the patients requiring corticosteroids had more aggressive disease at baseline, as evidenced by a higher Gleason score, higher median PSA, lower ECOG PS, higher ALP, higher LDH, and higher frequency of visceral disease.
To address the imbalance in baseline patient characteristics between corticosteroid users and nonusers, univariate and multivariate models were developed, which showed that even after adjusting for validated prognostic factors (33–35), baseline corticosteroid use remained a significant independent factor associated with inferior clinical outcomes. Further analysis applied an independent statistical model to stratify patients into low-risk or high-risk groups based on a clinically validated nomogram to determine the effect of corticosteroid on clinical outcomes within the same risk groups (34). Here again, inferior outcomes with corticosteroid use were observed. Further evidence suggesting an agonist/stimulatory interaction of corticosteroids was the shorter time to disease progression among corticosteroid users in the enzalutamide-treated but not the placebo group.
In a preclinical model, Arora and colleagues (26) explored mechanisms of acquired resistance to AR inhibitors and reported that among the most upregulated genes was the GR, which can activate the transcription of a shared set of AR-regulated genes, suggesting that resistance may be associated with a transition from AR- to GR-driven transcriptional activity. In addition, experimental evidence suggests that dexamethasone induces PSA expression in human prostate cancer cell lines, consistent with the GR coopting AR transcription sites, in effect, bypassing enzalutamide-mediated AR blockade (26).
A retrospective analysis assessed the prognostic significance of baseline corticosteroids on survival in patients with mCRPC treated with abiraterone and prednisone in COU-AA-301 (36). COU-AA-301 and AFFIRM differed in that abiraterone-treated and placebo-treated patients in COU-AA-301 all received prednisone during the trial. Not surprisingly, COU-AA-301 reported that baseline corticosteroid use was associated with adverse prognostic features and inferior OS relative to the nonsteroid use group, but in contrast to the current results, did not emerge as an independent prognostic factor for OS in a multivariate analysis. This observation provides indirect support that the more advanced disease status associated with baseline corticosteroid use can be accounted for in the multivariate model that included similar prognostic factors used in this study.
The SWITCH trial investigated the effects of transitioning corticosteroid use from prednisone, 5 mg twice a day, to dexamethasone, 0.5 mg every day, in patients with confirmed biochemical progression with or without limited radiologic progression of mCRPC treated with abiraterone acetate (37). The results showed that in so doing, a ≥50% decline in PSA occurred in more than 30% of patients. Several explanatory mechanisms have been proposed, including: emergence of secondary mutations in the AR itself that could be stimulated by prednisone but not dexamethasone; better suppression of adrenocorticotropic hormone by dexamethasone due to its superior pharmacology; upregulation of GR, which co-opts signaling through the AR when bound by prednisone but not dexamethasone; and secondary activation of the mineralocorticoid receptor, which has higher affinity for prednisone than dexamethasone. These suggest a potentially important interaction between corticosteroids and AR-targeted therapies in the setting of drug resistance.
Model systems provide evidence that mutations in the AR gene result in amino acid substitutions to the ligand binding domain, decreasing ligand specificity and selectivity (38). Mutant AR proteins can bind to glucocorticoids to activate AR transcription and prostate cancer cell growth. In addition, GR is also upregulated in a proportion of enzalutamide-resistant prostate cancers, allowing corticosteroids to bind to GR to activate protumor growth and survival genes shared by the GR and AR, bypassing AR blockade and leading to tumor progression (26–28). This preclinical evidence is further supported by multiple studies that examined the AR mutation status in clinical biopsy specimens or circulating tumor DNAs (ctDNA), and found that increased frequency of AR gene mutations, such as L702H, T878A, W742C, and F877L, were detected in 10% to 30% of the patients with mCRPC who progressed on enzalutamide or abiraterone (23, 25, 30, 39–42). Specifically, Carreira and colleagues (39) showed that AR ligand binding mutations were found in 4 out of 12 (33%) patients with mCRPC, including 2 patients with an L702H mutation that emerged during treatment with enzalutamide or abiraterone administered concurrently with a glucocorticoid. Other studies by Lallous and colleagues and Wyatt and colleagues (30, 40) using ctDNA sequencing identified AR ligand binding mutations in over 20% of patients with mCRPC at baseline. Furthermore, some of these AR mutations, in particular glucocorticoid-sensitive L702H and H875Y and promiscuous T878A mutations, further increased in frequency in ctDNAs with enzalutamide or abiraterone treatment. In addition, elevated GR expression was also observed in a subset of patients with mCRPC who developed resistance to enzalutamide or abiraterone (24, 26–29). In the study by Arora and colleagues (26), GR expression was upregulated in 30% of patient tumor cells after enzalutamide treatment compared with 10% before therapy, and higher levels of GR expression was associated with poor clinical response to enzalutamide. Taken together, corticosteroids may play a potential role in promoting resistance to a second-generation AR inhibitor in a substantial percentage of the patients with mCRPC with certain AR mutations and/or upregulated GR expression.
Limitations of this post hoc analysis are that AFFIRM was not designed to prospectively evaluate the impact of corticosteroid use on outcome, because the potential stimulatory effect of GR upregulation or certain AR mutations was unknown at the time the trial was designed and ultimately accrued. We also recognize that multivariate and risk-stratification analyses suggesting that corticosteroid use was an independent predictor of worse clinical outcome, backed by a plausible biological mechanism, may not account for the entirety of underlying, confounding factors that contribute to suboptimal therapeutic responses. AFFIRM trial did not include the collection of pre- or posttreatment tumor biopsies or ctDNAs for genomic profiling or gene expression analysis, as AFFIRM trial predated the availability to conduct molecular profiling of metastatic tumor or liquid biopsies. Such studies are needed to determine both the frequency of GR upregulation and AR alterations that may be present pretreatment or develop on treatment with abiraterone, enzalutamide, apalutamide, or darolutamide in the mCRPC. Definitive proof of a causal effect of long-term corticosteroid use on the outcomes of men receiving enzalutamide for CRPC will require a prospective, randomized, clinical trial.
Research supporting potential interactions between GR and AR signaling is important given the widespread use of glucocorticoids in patients with prostate cancer who may be concurrently treated with a second-generation AR inhibitor. Furthermore, understanding the resistance mechanism and devising a treatment strategy to overcome drug resistance to the second generation of AR inhibitors remains a significant unmet challenge. The study reported here is also timely given several ongoing trials testing the efficacy of selective GR antagonists combined with enzalutamide for patients with mCRPC (31, 32).
Since AFFIRM, successful clinical trials have been conducted with enzalutamide in patients with chemotherapy-naive mCRPC (6), nonmetastatic disease with a rapid PSA doubling time (18), and metastatic hormone-sensitive disease (20). In each of these settings there are patients for whom a corticosteroid would be considered for symptom management. Although further prospective validation is required to establish a definitive, causal relationship, physicians should consider carefully whether the potential benefit of corticosteroid use outweighs the potential risk of treatment-associated adverse events and the possibility of an inferior clinical outcome.
Supplementary Material
Acknowledgments
We thank the patients who volunteered to participate in this study for their dedication, and the study-site physicians, nurses, and support staff who cared for them. J.L. Zhao and H.I. Scher had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.L. Zhao was supported by Conquer Cancer Foundation of the American Society of Clinical Oncology Jonathan Nebel Young Investigator Award, Prostate Cancer Foundation Young Investigator Award (20YOUN22), and K12 Paul Calabresi Career Development Award for Clinical Oncology (K12CA184746). Medical writing and editorial support funded by both sponsor companies was provided by Ira Mills, PhD, and Dena McWain of Ashfield Healthcare Communications and Lauren Rainer, BSc, and Laura Geuss, PhD, at Onyx (a Prime Global Agency). The AFFIRM trial and analyses presented in this article were sponsored by Pfizer Inc. and Astellas Pharma, Inc., the co-developers of enzalutamide. The AFFIRM trial was supported by grants from the NIH SPORE grant (P50-CA92629) and Cancer support grant (P30 CA008748).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
K. Fizazi reports personal fees from Astellas during the conduct of the study, as well as personal fees from AstraZeneca, Amgen, Bayer, Curevac, Janssen, Orion, and Sanofi outside the submitted work. F. Saad reports grants, personal fees, and nonfinancial support from Astellas during the conduct of the study, as well as grants, personal fees, and nonfinancial support from Janssen and Bayer outside the submitted work. K.N. Chi reports grants and personal fees from Astellas, AstraZeneca, Janssen, Merck, Novartis, Point Biopharma, Roche, and Sanofi, as well as personal fees from Daiichi Sankyo and Pfizer outside the submitted work. M.-E. Taplin reports personal fees from Pfizer outside the submitted work. C. Sternberg reports grants and personal fees from Astellas and Pfizer during the conduct of the study. A.J. Armstrong reports grants and personal fees from Pfizer, Astellas, Janssen, Merck, Dendreon, Bayer, AstraZeneca, Forma, and BMS; personal fees from Clovis; and grants from Amgen, Constellation, Genentech/Roche, and BeiGene outside the submitted work. J.S. de Bono reports personal fees and nonfinancial support from Astellas during the conduct of the study. J.S. de Bono also reports grants and personal fees from AstraZeneca, Bayer, Daiichi Sankyo, MSD, Merck Serono, and Pfizer; personal fees from Amgen, Janssen, and Novartis/AAA; personal fees and nonfinancial support from Astellas; and grants from Harpoon Therapeutics outside the submitted work. W.T. Duggan reports other support from Pfizer outside the submitted work, reports employment with Pfizer, and also owns shares of Pfizer stock. H.I. Scher reports grants from Medivation and Pfizer, as well as nonfinancial support from Medivation and Pfizer during the conduct of the study. H.I. Scher also reports personal fees from Asterias Biotherapeutics, Ambry Genetics Corp/Konica Minolta Inc., Bayer, Pfizer, WCG, and Sun Pharmaceuticals; nonfinancial support from Amgen, Janssen, Menarini Silicon Biosystems, Amgen, Epic Sciences, WCG Oncology, ESSA Pharma Inc., Phosplatin, and Pfizer; and grants from Epic Sciences, Janssen, Menarini Silicon Biosystems, Illumina, and Thermo Fisher Scientific outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
J.L. Zhao: Conceptualization, data curation, formal analysis, writing–original draft, writing–review and editing. K. Fizazi: Conceptualization, data curation, formal analysis, writing–review and editing. F. Saad: Conceptualization, data curation, formal analysis, writing–review and editing. K.N. Chi: Conceptualization, data curation, formal analysis, writing–review and editing. M.-E. Taplin: Conceptualization, data curation, formal analysis, writing–review and editing. C.N. Sternberg: Conceptualization, data curation, formal analysis, writing–review and editing. A.J. Armstrong: Conceptualization, data curation, formal analysis, writing–review and editing. J.S. de Bono: Conceptualization, data curation, formal analysis, writing–review and editing. W.T. Duggan: Conceptualization, data curation, formal analysis, writing–review and editing. H.I. Scher: Conceptualization, data curation, formal analysis, writing–original draft, project administration, writing–review and editing.
References
- 1. Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23:8253–61. [DOI] [PubMed] [Google Scholar]
- 2. Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol 2019;9:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- 6. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- 8. Geynisman DM, Szmulewitz RZ, Plimack ER. Corticosteroids and prostate cancer: friend or foe? Eur Urol 2015;67:874–5. [DOI] [PubMed] [Google Scholar]
- 9. Gill D, Gaston D, Bailey E, Hahn A, Gupta S, Batten J, et al. Efficacy of eplerenone in the management of mineralocorticoid excess in men with metastatic castration-resistant prostate cancer treated with abiraterone without prednisone. Clin Genitourin Cancer 2017;15:e599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller GM, Hinman F Jr. Cortisone treatment in advanced carcinoma of the prostate. J Urol 1954;72:485–96. [DOI] [PubMed] [Google Scholar]
- 11. Plowman PN, Perry LA, Chard T. Androgen suppression by hydrocortisone without aminoglutethimide in orchiectomised men with prostatic cancer. Br J Urol 1987;59:255–7. [DOI] [PubMed] [Google Scholar]
- 12. Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 1989;7:590–7. [DOI] [PubMed] [Google Scholar]
- 13. Venkitaraman R, Lorente D, Murthy V, Thomas K, Parker L, Ahiabor R, et al. A randomised phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol 2015;67:673–9. [DOI] [PubMed] [Google Scholar]
- 14. Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol 2013;24:31–8. [DOI] [PubMed] [Google Scholar]
- 15. Fizazi K, Chi KN, de Bono JS, Gomella LG, Miller K, Rathkopf DE, et al. Low Incidence of corticosteroid-associated adverse events on long-term exposure to low-dose prednisone given with abiraterone acetate to patients with metastatic castration-resistant prostate cancer. Eur Urol 2016;70:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schultz NM, Penson DF, Wilson S, Song Y, Yang H, Ramaswamy K, et al. Adverse events associated with cumulative corticosteroid use in patients with castration-resistant prostate cancer: an administrative claims analysis. Drug Saf 2020;43:23–33. [DOI] [PubMed] [Google Scholar]
- 17. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2018;378:2465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2020;382:2197–206. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2019;37:2974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang CY, Walther PJ, McDonnell DP. Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Res 2001;61:8712–7. [PubMed] [Google Scholar]
- 22. Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res 2012;72:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med 1995;332:1393–8. [DOI] [PubMed] [Google Scholar]
- 24. Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med 2000;6:703–6. [DOI] [PubMed] [Google Scholar]
- 25. Annala M, Taavitsainen S, Khalaf DJ, Vandekerkhove G, Beja K, Sipola J, et al. Evolution of castration-resistant prostate cancer in ctDNA during sequential androgen receptor pathway inhibition. Clin Cancer Res 2021;27:4610–23. [DOI] [PubMed] [Google Scholar]
- 26. Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer 2014;5:72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin Cancer Res 2018;24:927–38. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Alyamani M, Zhang A, Chang KH, Berk M, Li Z, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. Elife 2017;6:e20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, McConeghy B, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol 2016;2:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ClinicalTrials.gov. Study to evaluate CORT125281 in combination with enzalutamide in patients with mCRPC. [cited 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT03437941.
- 32. Shore ND, Efstathiou E, Patel R, Xu R, Johnson A, Multani PS, et al. An open-label phase Ib study of ORIC-101 in combination with enzalutamide in patients with metastatic prostate cancer progressing on enzalutamide. J Clin Oncol 2020;38:Abstract TPS253. [Google Scholar]
- 33. Halabi S, Lin CY, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Halabi S, Lin CY, Small EJ, Armstrong AJ, Kaplan EB, Petrylak D, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst 2013;105:1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scher HI, Graf RP, Schreiber NA, Jayaram A, Winquist E, McLaughlin B, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol 2018;4:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montgomery B, Kheoh T, Molina A, Li J, Bellmunt J, Tran N, et al. Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: exploratory analysis from COU-AA-301. Eur Urol 2015;67:866–73. [DOI] [PubMed] [Google Scholar]
- 37. Romero-Laorden N, Lozano R, Jayaram A, López-Campos F, Saez MI, Montesa A, et al. Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study). Br J Cancer 2018;119:1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin 2015;36:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med 2014;6:254ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lallous N, Volik SV, Awrey S, Leblanc E, Tse R, Murillo J, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jernberg E, Bergh A, Wikström P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr Connect 2017;6:R146–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res 2015;21:2315–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.