Abstract
Introduction
Primary focal segmental glomerular sclerosis (FSGS) is a rare, likely immune-mediated disease. Rituximab (RTX) may play a role in management, although data in adults are scanty.
Methods
We collected cases of RTX-treated primary FSGS within the Italian Society of Nephrology Immunopathology Working Group and explored response rate (24-hour proteinuria <3.5 g and <50% compared with baseline, stable estimated glomerular filtration rate).
Results
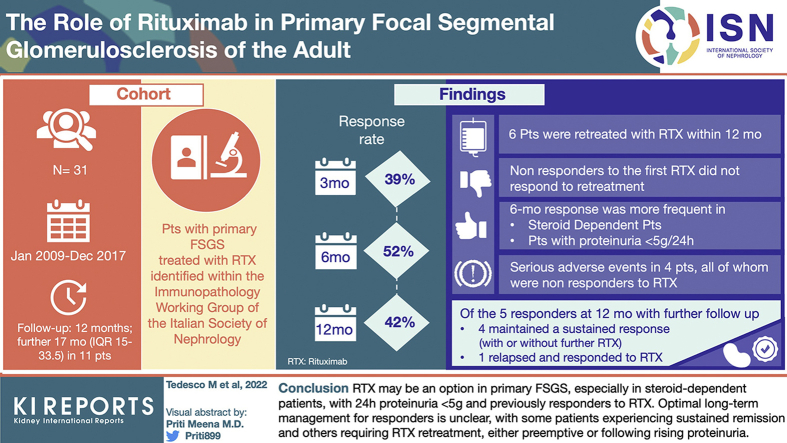
A total of 31 patients were followed for at least 12 months; further follow-up (median 17 months, interquartile range [IQR] 15–33.5) was available for 11. At first RTX administration, median creatinine and 24-hour proteinuria were 1.17 mg/dl (IQR 0.83–1.62) and 5.2 g (IQR 3.3–8.81), respectively. Response rate at 3, 6, and 12 months was 39%, 52%, and 42%, respectively. In the first 12 months, creatinine level remained stable whereas proteinuria and serum albumin level improved, with an increase in the proportion of patients tapering other immunosuppressants. There were 6 patients who were retreated with RTX within 12 months, either for proteinuria increase or refractory disease; only the 2 responders to the first RTX course experienced a further response. At univariate analysis, 6-month response was more frequent in steroid-dependent patients (odds ratio [OR] 7.7 [95% CI 1.16–52.17]) and those with proteinuria <5 g/24 h (OR 8.25 [1.45–46.86]). During long-term follow-up, 4 of 5 responders at 12 months maintained a sustained response, either without further immunosuppression (2 of 4) or with pre-emptive RTX (2 of 4); 1 relapsed and responded to RTX retreatment.
Conclusion
RTX may be an option in primary FSGS, especially in steroid-dependent patients, with 24-hour proteinuria <5 g and previously responders to RTX. Optimal long-term management for responders is unclear, with some patients experiencing sustained remission and others requiring RTX retreatment, either preemptive or after rising proteinuria.
Keywords: focal segmental glomerulosclerosis, rituximab
Graphical abstract
FSGS is the leading cause of nephrotic syndrome in the United States, and its prevalence has been increasing in the last decades worldwide.1 Of note, FSGS is an umbrella definition including several etiologies, ranging from primary to genetic forms, with cases that may be secondary to several conditions. The primary form usually presents with full-blown nephrotic syndrome, characterized by severe proteinuria and hypoalbuminemia, leading to an increased risk of end-stage renal disease (ESRD), acute coronary syndrome, heart failure, and death.2 The pathogenesis of primary FSGS is unclear; a role for an abnormal crosstalk between B cells and autoreactive T lymphocytes, including a still undefined circulating permeabilizing factor, has been proposed.3 Remarkably, the identification of autoantibodies targeting nephrin in patients with minimal change disease4 further supports the role of the B-cell compartment in the pathogenesis of podocytopathies. Because an “immunologic” pathogenesis is likely, immunosuppressive drugs have historically been used in the management of this disease.3 However, despite immunosuppression, partial or complete remission is obtained in only approximately 50% of patients, with significant variability across different studies, reflecting heterogeneous inclusion criteria and therapeutic approaches.5 As a result, the long-term prognosis of FSGS is poor, with approximately half of the affected patients reaching ESRD in 5 to 10 years.6
Glucocorticoids represent the first line of treatment for primary FSGS. On the basis of steroid responsiveness, patients may be classified as steroid sensitive or steroid resistant (SR); the former may be further subclassified as steroid-dependent (SD) or frequently relapsing if they tend to experience disease flares when glucocorticoid dose is reduced, or within 6 months of glucocorticoid withdrawal, respectively.7 The SR group is usually refractory to multiple treatments, which portends a poor prognosis3,5,7; of note, the diagnosis of primary FSGS should always be challenged for this group of patients and secondary or genetic forms considered.3
Independently of steroid sensitivity, the clinical history of patients with FSGS is usually characterized by prolonged exposure to immunosuppression, in the attempt to minimize complications of nephrotic syndrome and reduce the risk of progression to ESRD. Calcineurin inhibitors (CNIs) play a central role in the management of FSGS, being the first line of steroid-sparing medications.7 Despite that, commercially available CNIs have frequent and not always negligible side effects and, even when remission is obtained, relapses are frequent after drug withdrawal.8,9
RTX is an anti-CD20 chimeric monoclonal antibody that is widely used in the management of immunologic diseases with dysregulated activation of the B-cell compartment.10, 11, 12 A role for this drug in pediatric cohorts of SD patients with nephrotic syndrome has been found.13,14 Data on RTX in the management of FSGS of the adult are scanty,15 and response rates in SD/CNI-sensitive FSGS range from 50% to 100%.16, 17, 18, 19, 20, 21 Importantly, when focusing on patients with SR FSGS or with FSGS likely to have a secondary etiology, response rate is reduced to 13% to 38%.22,23 These published cohorts have significant limitations, such as small sample size, with the largest case series consisting of 21 patients,18 significant heterogeneity in terms of inclusion criteria, patient characteristics and remission definition, and the retrospective nature of the observations.
The current study aimed to assess the efficacy of RTX-based regimens in the induction and maintenance of remission in a larger cohort of adults with primary FSGS.
Methods
Patient Cohort
All patients affected by primary FSGS and treated with RTX, with a follow-up of at least 12 months, were identified in the Italian centers belonging to the Italian Society of Nephrology Immunopathology Working Group during the time period 2009 to 2017.
Inclusion criteria were as follows: (i) biopsy-proven FSGS; (ii) exclusion of secondary forms of FSGS after a thorough diagnostic workup (Supplementary Methods)24; (iii) age at diagnosis > 14 years; and (iv) age at first RTX administration > 18 years. Genetic testing was not required for study inclusion. Both SD and SR patients were included (Supplementary Methods). All schedules of RTX administration in 4 weeks were considered as RTX induction therapy (e.g., 375 mg/m2/wk for 4 weeks, 2 doses of 1000 mg each administered 2 weeks apart, a single dose of 1000 mg). At the first RTX administration, patients were classified in the following 3 groups based on the indication for treatment: relapse, persistent disease activity, or drug sparing (Supplementary Methods).
Patients retreated with RTX within the first year of follow-up were censored for the assessment of response at the time of retreatment and then analyzed separately. Patients with a follow-up longer than 12 months were included in the long-term follow-up assessment, independently of whether they received additional RTX infusions or did not. The study protocol was approved by the Ethics Committee of the ASST Fatebenefratelli-Sacco.
Response to Treatment
The study primarily aimed to assess the effectiveness of RTX-based regimens for the achievement of remission in patients with primary FSGS. Response to treatment was evaluated at 3, 6, and 12 months after RTX and during the subsequent follow-up. Response was subclassified as complete response and partial response (PR), in keeping with the standard definitions used in nephrotic syndrome studies (Supplementary Methods)25; moreover, the tapering of other immunosuppressive drugs was a criterion required to classify the patient as responder. Lack of response was classified as no response (NR) (Supplementary Methods).
We also assessed changes over time in serum proteins, serum albumin, serum creatinine, 24-hour proteinuria, and dosing of glucocorticoid and other immunosuppressants, including changes in the peripheral blood CD20+ B-cell counts (with CD20+ B-cell repopulation defined as a count >0 × 106 cells/l).12 Severe adverse events (SAEs) were defined as any event requiring hospitalization, need for i.v. therapies for any reason (e.g., antibiotics, diuretics), diagnosis of cancer, or death. The study also focused on identifying clinical, laboratory, and histologic characteristics associated with response to RTX.
Statistical Analysis
Data were reported as absolute count (percentage) for categorical variables and median (IQR) for continuous variables. Changes over time in serum albumin, total serum protein, serum creatinine, and proteinuria were compared through Wilcoxon matched-pair signed rank test or paired t test when appropriate.
Univariate logistic regression was used to assess clinical, laboratory, and histopathologic factors associated with response at 6 months after RTX administration; results were expressed as OR with 95% CIs. P < 0.05, 2 sided, were considered statistically significant. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA) and SAS statistical software version 9.4 (SAS Institute, Cary, NC).
Results
Study Population
A total of 31 patients were included. Baseline clinical characteristics at the first RTX administration are found in Table 1. The median time between diagnosis and RTX administration was 87 months (IQR 54–196), and the immunosuppressive drugs used before RTX included prednisone in 29 of 31 patients (94%), CNIs in 23 of 31 (74%), mycophenolate mofetil in 9 of 31 (29%), and cyclophosphamide in 7 of 31 (23%). At the time of RTX administration, 18 of 31 patients (58%) were classified as SD and 11 of 31 (35%) as SR, whereas 2 patients (6%) had no previous exposure to glucocorticoids due to high risk of steroid-related complications, as assessed by the treating physicians (1 patient recently had a severe infection and the other had severe osteoporosis). Of 31 patients, 14 (45%) were treated with RTX for disease relapse and 13 of 31 (42%) for persistent disease, with the remaining 4 of 31 (13%) for drug-sparing purposes.
Table 1.
Main characteristics of 31 patients with primary FSGS at disease onset and at the time of the first RTX administration
| Characteristics | Disease onset | First RTX administration |
|---|---|---|
| Age (yr) | 37 (26–42) | 40 (32–50) |
| Male sex | 18 (58) | |
| Weight (kg) | 69.50 (59.50–78.25) | 66.00 (59.00–75.00) |
| Systolic blood pressure (mm Hg) | 125 (120–150) | 120 (110–130) |
| Diastolic blood pressure (mm Hg) | 85 (74–90) | 78 (70–85) |
| Serum creatinine (mg/dl) | 1.07 (0.70–1.30) | 1.17 (0.83–1.62) |
| eGFRa (ml/min) | 80.30 (62.18–120.75) | 72.00 (49.80–100.04) |
| Proteinuria (g/24 h) | 7.20 (4.70–10.48) | 5.20 (3.30–8.81) |
| Serum total protein (g/dl) | 4.50 (4.00–5.40) | 4.95 (4.60–5.85) |
| Serum total albumin (g/dl) | 2.23 (1.89–3.00) | 2.78 (2.17–3.60) |
| Total cholesterol (mg/dl) | 317.00 (259.00–383.00) | 237.00 (200.00–323.00) |
| Triglycerides (mg/dl) | 158.50 (110.25–197.75) | 169.00 (94.50–215.00) |
| Relapse rate before RTX (n/100 patient-yr) | 47 | |
| Schemes of RTX dosing | ||
| 2 × 1000 mg 2 wk apart | 10 (32) | |
| 4 × 375 mg/m2 weekly | 8 (26) | |
| 2 × 500 mg 2 wk apart | 6 (19) | |
| 1 × 375 mg/m2 | 4 (13) | |
| 1 × 1000 mg | 2 (6) | |
| 1 × 500 mg | 1 (3) | |
| FSGS histologic variants | ||
| NOS | 22 (73) | |
| Tip | 3 (10) | |
| Collapsing | 3 (10) | |
| Cellular | 2 (7) | |
eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerular sclerosis; MDRD, Modification of Diet in Renal Disease; NOS, not otherwise specified; RTX, rituximab.
Using the MDRD equation.
Results are expressed as n (%) for categorical variables and median (interquartile range) for continuous variables.
The different RTX regimens used for induction are reported in Table 1. At the time of RTX administration, 18 of 31 patients (58%) were on prednisone at a median dose of 15 mg/d (IQR 12.5–25); 7 of 31 (23%) were on CNIs; 28 of 31 (90%) were taking angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers at the highest tolerated dose.
Response to RTX
Response to therapy at 3, 6, and 12 months could be assessed in 28, 27, and 24 patients, respectively; for 3, 4, and 6 patients, response to treatment was analyzed separately because they were retreated with RTX before the prespecified 3-, 6-, and 12-month intervals. For 1 patient, response at month 12 was not available (lost to follow-up at month 10 while having a persistent lack of response). Response rates at 3, 6, and 12 months (excluding patients retreated with RTX) were 39%, 52%, and 42%, respectively (Table 2); these changed to 35%, 45%, and 43% if patients retreated with RTX within the first 12 months were included in the analysis as well (Supplementary Table S1). Of note, all 14 patients who had a response did so within 6 months of RTX, with no further responses observed later than this time point. Response rates differed among different subgroups of patients: at 6 months, response was achieved by 69% of patients with proteinuria <5 g/d and by 69% of the SD subgroup (Supplementary Table S2).
Table 2.
Response rates at months 3, 6, and 12 after RTX in a cohort of 31 patients with primary FSGS
| Response | 3 mo | 6 mo | 12 moa |
|---|---|---|---|
| Response | 11/28 (39) | 14/27 (52) | 10/24 (42) |
| CR | 5/11 (45) | 7/14 (50) | 5/10 (50) |
| PR | 6/11 (55) | 7/14 (50) | 5/10 (50) |
| No response | 17/28 (61) | 13/27 (48) | 14/24 (58) |
CR, complete response; FSGS, focal segmental glomerular sclerosis; PR, partial response; RTX, rituximab.
Response at 12 months was not available for 1 patient, having been lost to follow-up at month 10; the patient experienced a persistent lack of response.
Results are expressed as n/N (%).
Before RTX, a relapse rate of 47 events per 100 patient-years was observed in the entire cohort. Throughout all the follow-up after the first RTX cycle, 9 relapses were detected in 8 patients who had achieved response at any point (relapse rate = 17 events per 100 patient-years; median time to first relapse 11 months, IQR 7.5–12 months).
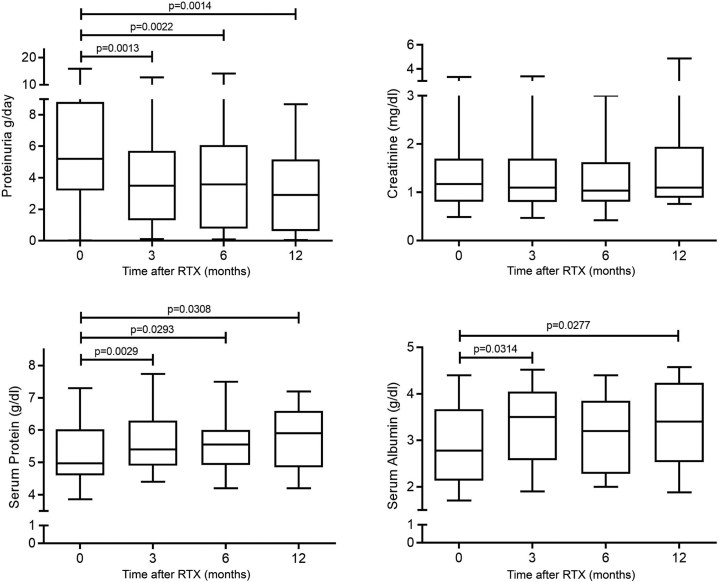
At 3, 6, and 12 months, serum protein level significantly increased compared with baseline (P = 0.003, P = 0.03, and P = 0.03, respectively), from a baseline median value of 4.95 g/dl (IQR 4.60–5.85) up to a maximum median value of 5.90 g/dl (IQR 4.93–6.50) at 12 months. The increase in serum albumin was significantly different from baseline only at months 3 and 12 (P = 0.03 and P = 0.03, respectively), with the maximum value detected at month 3 (3.5 g/dl). Proteinuria was significantly lower at all time points relative to baseline (P = 0.001, P = 0.002, and P = 0.002, respectively), with the lowest value reached at 12 months (median 2.91 g/24 h, IQR 0.66–4.88). Serum creatinine level remained stable throughout the follow-up (Figure 1). Of note, although patients classified as responders to RTX at 6 months experienced a significant improvement in proteinuria, with nearly a normalization of albumin levels throughout the follow-up (Supplementary Figure S1), some degree of improvement at 3 months was detected also in the group of patients classified as NR at 6 months (Supplementary Figure S2).
Figure 1.
Changes in proteinuria and serum creatinine. Serum albumin and serum proteins in a cohort of 31 patients with primary FSGS in the 12 months after therapy with RTX. The line represents median values, the box IQRs, and the whiskers maximum and minimum values. FSGS, focal segmental glomerular sclerosis; IQR, interquartile range; RTX, rituximab.
The percentage of patients free from glucocorticoids increased from 42% at baseline to 46% at month 3, 48% at month 6, and 54% at month 12. Among patients on glucocorticoids, the median dose of prednisone decreased to 10 mg/d (IQR 5.3–13.8) at month 3 and remained stable throughout the first 12 months of follow-up (10 mg/d, IQR 5–11.9, and 10 mg/d, IQR 5–22.5, at 6 and 12 months, respectively); when restricting the analysis only to patients with a response at 6 months, the median prednisone dose at 3, 6, and 12 months was further reduced to 5.6 mg/d (IQR 0–10), 5 mg/d (IQR 0–9.4), and 1.3 mg/d (0–5.3), respectively. The percentage of patients on CNIs was 18% at 3 months, 11% at 6 months, and 21% at 12 months (Supplementary Figure S3).
CD20+ B-cell counts were available in 50% of patients at 3 and 12 months and in 55% at 6 months. The number of patients having B-cell repopulation was 2 of 14 (14%), 7 of 15 (47%), and 8 of 12 (67%) at 3, 6, and 12 months, respectively. No association was found between CD20+ B-cell kinetics and response to RTX (Supplementary Table S3) and between RTX dosing regimens and response at 6 months (Supplementary Table S4).
At univariate analysis, predictors of response to RTX at 6 months were SD status (OR 7.7, 95% CI 1.16–51.17; P = 0.0347) and baseline proteinuria <5 g/24 h (OR 8.25, 95% CI 1.45–46.86; P = 0.0173); a trend toward better response was observed in the subgroup with serum IgG levels >500 mg/dl at RTX when the data were available (Table 3).
Table 3.
Factors associated with response at 6 months at univariate analysis in a cohort of 31 patients with primary FSGS treated with RTX
| Parameters | OR (95% CI) | P value |
|---|---|---|
| Steroid-dependent | 7.7 (1.16–51.17) | 0.0347 |
| Indication to RTX | 0.0910 | |
| - Relapse vs. persistent activity | 8.00 (1.24–51.51) | |
| - Drug sparing vs. persistent activity | 2.67 (0.25–28.44) | |
| RTX infusion schedulea | 0.7328 | |
| - 4 × 375 mg/m2 weekly | 1.67 (0.23–12.22) | |
| - 2 × 500 mg 2 wk apart | 0.50 (0.06–4.47) | |
| - Other schedules | 1.50 (0.16–14.42) | |
| Serum creatinine at RTX <1 mg/dl | 3.00 (0.62–14.61) | 0.1739 |
| Proteinuria at RTX <5 g/24 h | 8.25 (1.45–46.86) | 0.0173 |
| Serum albumin at RTX >3 g/dl | 3.37 (0.60–19.00) | 0.1679 |
| IgG at RTX >500 mg/dl (n = 16) | 10.00 (0.78–128.77) | 0.0774 |
| B-cell count at 3 mob (n = 15) | 0.50 (0.04–5.51) | 0.5714 |
FSGS, focal segmental glomerular sclerosis; OR, odds ratio; RTX, rituximab.
Compared with RTX 1000 mg × 2.
0 × 106/l vs. >0 × 106/l.
RTX Retreatment
There were 6 patients who were retreated with RTX within 12 months after the first RTX administration (data are summarized in Supplementary Table S5). The median time from first RTX to retreatment in this group was 5 months (IQR 3.25–7.5). The dose of RTX at retreatment was 1000 mg in 2 of 6 patients, 1000 mg × 2 in 1 patient, 700 mg in 1 patient, 700 mg × 2 in 1 patient, and 500 mg in the remaining one.
In 2 patients, originally classified as SD and responders to the first RTX cycle, RTX was readministered at months 8 and 10 for an increase in proteinuria (up to 4.8 g/d from 2.27 g/d in 1 patient and up to 3.6 g/d from 0.29 g/d in the second patient). In both cases, retreatment led to PR with proteinuria dropping, after 6 months, to 2.66 g/24 h and 1.31 g/24 h, respectively; changes in prednisone dosing were minor (dose increased from 2.5 to 5 mg in the first patient and no variation in the second one). Both patients were therefore considered to be in PR at 12 months.
In the remaining 4 patients (2 SR and 2 SD), RTX was redosed for refractory disease at months 2, 3, 4, and 6, respectively; NR was recorded in all of these cases. At month 12, only the 2 SD patients reached a 24-hour proteinuria <3.5 g/d after an increase in prednisone dose and addition of tacrolimus and mycophenolate mofetil, respectively.
Follow-up After Month 12
Eleven patients had a follow-up longer than 12 months after the first RTX course (median 17 months, IQR 15–33.5). Of them, 6 were classified as NR at month 12: 5 experienced NR throughout the first 12 months and none was retreated with RTX during this time. After month 12, 4 of 6 maintained a nephrotic-range proteinuria despite retreatment with RTX in 2 of 4 (month 14, 1000 mg, and month 15, 1000 mg × 2). Furthermore, 1 of 6 had persistent nephrotic-range proteinuria up to month 23, having been retreated with RTX at months 14 and 22 (500 mg each time); at month 23, the patient experienced PR but was then lost to follow-up. Moreover, 1 of 6, despite being recorded as NR up to month 12, experienced PR from month 12 to month 27, having been started on cyclosporin 3 months after the first RTX.
Of 11 patients, 5 were classified as responders at month 12 (3 complete response and 2 PR), with 2 having been retreated during the first 12 months. Among them, 4 of 5 maintained remission throughout the long-term follow-up; 2 of these 4 patients did not receive any further immunosuppressive treatment, whereas the remaining 2 were retreated pre-emptively with RTX, without having experienced a proper relapse (1 patient was treated 4 times at months 10, 24, 31, and 43 for increasing proteinuria and CD20 repopulation; 1 patient at month 18 for pre-emptive maintenance of remission). Of the 5 patients classified as responders at month 12, 1 experienced a relapse at month 8, when he received further RTX, achieving remission again; the same patient experienced a further relapse at month 15, was retreated, and had PR. She was thereafter retreated pre-emptively with 500 mg RTX every 6 months, maintaining PR up to month 38 (Supplementary Figure S4).
SAEs
Overall, 9 SAEs were recorded in 4 patients. The most frequent event was hospitalization for fluid overload (4 of 9, 44%), followed by infections (3 of 9, 33%), need of i.v. therapy (1 of 9, 11%), and ESRD (1 of 9, 11%). Of 9 SAEs, 7 occurred during the first year after RTX, whereas the remaining 2 of 9 were recorded during the extended follow-up.
All 4 patients experiencing SAEs never had a response to RTX in the entire follow-up: the first patient, who already had advanced chronic kidney disease at baseline (estimated glomerular filtration rate 31 ml/min), developed progressive worsening of kidney failure, requiring renal replacement therapy at month 99. In addition, 2 patients were admitted to hospital for urinary tract infection and cholecystitis at 3 and 12 months, respectively. The fourth patient was hospitalized twice: the first time for persistent nephrotic syndrome refractory to therapy and then for acute kidney injury and pneumonia. No deaths occurred during the entire follow-up.
Discussion
The management of primary FSGS in adults remains a challenge for nephrologists, because of its heterogeneous clinical-pathologic manifestations and the incomplete understanding of the underlying etiopathogenetic mechanisms.26 The goal of management is to achieve at least a PR, which portends a reduced risk of developing ESRD.27 Novel antiproteinuric drugs are providing further options for treating this insidious disease.28,29 However, immunosuppressive approaches are frequently required in the primary form, where an immune-mediated pathogenesis with a role for B cells is likely. In this context, RTX is an attractive therapeutic option, with a favorable safety profile. However, although data on the effectiveness of RTX in SD pediatric nephrotic syndrome are convincing,14 the role of this drug in the management of primary FSGS in adults remains relatively unclear.
Our study describes the largest multicenter cohort of adult patients with primary FSGS, including both SD and SR forms, currently available in the literature. Our results confirm a role for RTX, especially in the SD subgroup, with response rates in keeping with what published so far. Of note, during the first 12 months of observation, renal function remained stable, whereas proteinuria, serum albumin, and serum proteins improved. Interestingly, all clinical responses were observed within 6 months of RTX. This can inform future trial design, suggesting response at 6 months can be an appropriate outcome to assess efficacy of RTX in FSGS.
In line with other reports, RTX treatment led to a reduction in relapse rate (from 47 to 17 per 100 patient-years).16,17
Our study also aimed to identify factors associated with response to RTX. Patients with signs of less severe nephrotic syndrome were more likely to benefit from RTX: a baseline 24-hour proteinuria <5 g was associated with better response rates and a trend for improved response was observed in patients with higher basal IgG levels, when such data were available. Provided that this association is not driven by the lower baseline proteinuria per se, the mechanisms underlying this observation are to be clarified; putative factors include milder glomerular damage or better RTX pharmacokinetic profiles in patients with lower proteinuria.30,31 This finding may also lead to speculations as to whether RTX dose may affect response, as already questioned in other glomerulonephritides.25,32 In our study, where heterogeneous RTX dosing regimens were used, no clear dose-response association emerged, although in the context of an underpowered analysis.
To be clarified is also whether higher RTX doses may provide some benefits for SR patients, where response is poor.33 Importantly, in the case series of Fernandez-Fresnedo et al.,23 the 3 SR patients who experienced remission after RTX received the highest cumulative dose. Furthermore, our data support a trend toward a transient and mild response at 3 months also in patients classified as NR at 6 months, leaving unanswered the question as to whether or not RTX dose may play a role in this context.
Another matter of debate regarding the use of RTX in FSGS is whether patients may benefit from further therapies aiming at maintaining remission or from RTX retreatment. Moreover, in the case of RTX retreatment, the ideal strategy is indeed still unclear (pre-emptive vs. at the time of proteinuria increase/nephrotic syndrome relapse). One report in a cohort of 13 patients with FSGS suggested a possible role for B-cell–driven RTX retreatment in SD- or CNI-dependent patients, with high remission rates at 12 months (77%).18 Cortazar et al.19 described 20 patients with podocytopathy (13 minimal change disease and 7 SD or SR FSGS) who were treated with an intensified regimen, consisting of RTX induction (1 g 2 weeks apart), followed by 1 g every 4 months for 2 years, to be then reduced to 1 g every 6 months.19 With this approach, all patients achieved at least PR. Of note, in this study, 5 SD patients withdrew RTX after a median of 28 months of treatment and remained relapse free for a median of 20 months despite B-cell repopulation, suggesting a subgroup of patients exposed to prolonged RTX maintenance may enter a sustained remission.19 In a pediatric cohort, maintenance with mycophenolate mofetil after RTX reduced the risk of flares compared with lack of maintenance treatment.34
In our cohort, 6 patients were retreated with RTX within 12 months from the first administration. In the 2 cases that had initially responded to RTX, retreatment was driven by an increase in proteinuria to the nephrotic range and led to a further response, with no need of significant changes in glucocorticoid dose. Further doses of RTX, however, did not improve proteinuria in patients who were refractory to the first administration. Of note, all the patients classified as responders at month 12 maintained the status of complete response or PR during the remaining follow-up, with no further immunosuppression in 2 of 5 or after RTX retreatment in 3 of 5, either pre-emptively or after an increase in proteinuria/full-blown relapse. Our data therefore suggest that a previous response to RTX is the main determinant of response to further RTX treatment, and retreatment may be successful also in the context of rising proteinuria, without significant increases in glucocorticoid doses.
Striking the right balance between controlling disease activity and limiting drug-induced toxicity can be challenging in the management of FSGS. In our cohort, an overall improvement in nephrotic syndrome (such as rising serum albumin and decreasing proteinuria) was observed after RTX, with a concomitant reduction in glucocorticoid exposure. Only 9 SAEs were reported during the entire observation period, consisting mainly of infections and hospitalizations for fluid overload. Of note, all the 4 patients who experienced SAEs were nonresponders to RTX.
This report has several strengths: it is the largest cohort available so far, and it allowed comparisons, within the same cohort, of response rates in SD and SR patients, including an exploratory analysis of factors associated with response to treatment. The largest series available before the one described in this manuscript consisted of 21 patients of Indian ancestry, all steroid or CNI sensitive and with non-nephrotic proteinuria at the time of RTX administration (mean 0.75 g/24 h [0.21–1.56]), revealing in this context high rates of complete and partial remission.21 Our study, including a higher number of patients with more heterogeneous characteristics, has allowed a refined understanding of clinical features predictive of response to RTX.
Limitations need to be acknowledged as well: although larger compared with other studies, this case series remains relatively small. Moreover, most patients had a long disease course before receiving RTX, and the findings may therefore not necessarily apply to other stages of the disease. Of note, the retrospective and multicentric nature of the work, including the need to rely on patients’ referred medical history for some items (e.g., low birth weight), may raise questions on data quality and adherence to the workup used to rule out secondary forms of FSGS. Although this may be a potential issue of our manuscript, it has to be underlined that all the centers involved are experienced in the management of FSGS and tightly linked within the Italian Society of Nephrology Immunopathology Working Group, thus reducing, in our opinion, the risks of such bias. Further limitations are the lack of genetic analysis in the group of nonresponders, the heterogeneous RTX dosing, and the absence of a standardized glucocorticoid dosing and tapering scheme, including the unavailability of granular details regarding glucocorticoid exposure before RTX administration.
In conclusion, our study confirms a potential role for RTX in the management of primary FSGS, especially in the subset of SD patients with less severe nephrotic syndrome; in this context, RTX improved disease control and allowed some degree of steroid sparing, more significant in patients responders to treatment. In patients responding to RTX, pre-emptive retreatment and repeat dosing at the time of rising proteinuria enabled prolonged remission with no significant increases in glucocorticoid exposure. Further studies, ideally prospective, are needed to define the optimal dosing and retreatment strategies, including the potential role of biomarkers in guiding therapy.
Disclosure
LDV advisory board for Astellas; consultancy fees from Vifor. RAS consultancy fees from GlaxoSmithKline, Otsuka, and Roche. MG, consultancy fees from Amicus, AstraZeneca, Baxter, BBraun, BD Bard, Fresenius, Menarini, Medtronic, and Sanofi Genzyme; advisory board for Vifor Pharma. FA, consultancy fees from Baxter, AstraZeneca, and Otsuka; advisory board for Trevere Therapeutics, AstraZeneca, and GlaxoSmithKline. All the other authors declared no competing interests.
Acknowledgments
The authors thank the Italian Society of Nephrology Immunopathology Working Group for contributing to patients’ identification.
Footnotes
Supplementary Methods.
Figure S1. Changes in proteinuria, serum creatinine, serum albumin, and serum proteins in a cohort of 14 patients with primary FSGS treated with RTX and classified as responders at 6 months. The line represents median values, the box IQRs, and the whiskers maximum and minimum values.
Figure S2. Changes in proteinuria, serum creatinine, serum albumin, and serum proteins in a cohort of 13 patients with primary FSGS treated with RTX and classified as non-responders at 6 months. The line represents median values, the box IQRs, and the whiskers maximum and minimum values.
Figure S3. Percentages of patients off therapy and receiving immunosuppressive drugs in the first 12 months of follow-up in a cohort of 31 patients with primary FSGS treated with RTX.
Figure S4. Summary of the clinical course of 11 patients with primary FSGS and treated with RTX for whom long-term follow-up after the 12-month assessment mark was available.
Table S1. Response rates at 3, 6, and 12 months in different subgroups in a cohort of 31 patients with primary FSGS treated with RTX (patients retreated with RTX within the first 12 months have been included).
Table S2. Response rates at 3, 6, and 12 months in different subgroups in a cohort of 31 patients with primary FSGS treated with RTX.
Table S3. CD20+ B-cell repopulation rates and association with response to RTX.
Table S4. RTX schedule regimens and response to therapy at 6 months.
Table S5. Clinical characteristics of the 6 patients retreated with RTX within 12 months after the first administration.
Contributor Information
Federica Mescia, Email: federica.mescia@gmail.com.
The Italian Society of Nephrology Immunopathology Working Group:
Stefania Affatato, Leonardo Caroti, Elena Mancini, Luca Semeraro, Rossella Siligato, Matthias Arnaldo Cassia, Pietro Napodano, Marta Calatroni, Cosimo Distratis, and Andrea Campo
Appendix
Members of The Italian Society of Nephrology Immunopathology Working Group
Stefania Affatato: Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy and Nephrology Unit, Spedali Civili Hospital, ASST Spedali Civili of Brescia, Brescia, Italy.
Leonardo Caroti: Nephrology, Dialysis and Transplantation Unit, Careggi University Hospital, Florence, Italy.
Elena Mancini: Division of Nephrology, Dialysis and Hypertension, Policlinico S. Orsola-Malpighi Hospital, Bologna, Italy.
Luca Semeraro: Department of Internal Medicine and Medical Therapy, University of Pavia, Pavia, Italy.
Rossella Siligato: Unit of Nephrology and Dialysis, Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy.
Matthias Arnaldo Cassia: Department of Health Sciences, University of Milano, Milan, Italy and Nephrology Unit and Immunology Clinic, ASST Santi Paolo e Carlo, Milan, Italy.
Pietro Napodano: Nephrology Unit and Immunology Clinic, ASST Santi Paolo e Carlo, Milan, Italy.
Marta Calatroni: Nephrology Unit, Humanitas Clinical and Research Center, Milano, Italy.
Cosimo Distratis: Nephrology and Dialysis Unit, M. Giannuzzi Hospital, Manduria, Italy.
Andrea Campo: Nephrology Unit, San Lazzaro Hospital, Alba, Italy.
Supplementary Material
Supplementary Methods.
Figure S1. Changes in proteinuria, serum creatinine, serum albumin, and serum proteins in a cohort of 14 patients with primary FSGS treated with RTX and classified as responders at 6 months. The line represents median values, the box IQRs, and the whiskers maximum and minimum values.
Figure S2. Changes in proteinuria, serum creatinine, serum albumin, and serum proteins in a cohort of 13 patients with primary FSGS treated with RTX and classified as nonresponders at 6 months. The line represents median values, the box IQRs, and the whiskers maximum and minimum values.
Figure S3. Percentages of patients off therapy and receiving immunosuppressive drugs in the first 12 months of follow-up in a cohort of 31 patients with primary FSGS treated with RTX.
Figure S4. Summary of the clinical course of 11 patients with primary FSGS and treated with RTX for whom long-term follow-up after the 12 month assessment mark was available.
Table S1. Response rates at 3, 6, and 12 months in different subgroups in a cohort of 31 patients with primary FSGS treated with RTX (patients retreated with RTX within the first 12 months have been included).
Table S2. Response rates at 3, 6, and 12 months in different subgroups in a cohort of 31 patients with primary FSGS treated with RTX.
Table S3. RTX schedule regimens and response to therapy at 6 months.
Table S4. CD20+ B-cell repopulation rates and association with response to RTX.
Table S5. Clinical characteristics of the 6 patients retreated with RTX within 12 months after the first administration.
References
- 1.Kitiyakara C., Eggers P., Kopp J.B. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825. doi: 10.1016/S0272-6386(04)01081-9. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Tan T.C., Chertow G.M., et al. Primary nephrotic syndrome and risks of ESKD, cardiovascular events, and death: the Kaiser Permanente nephrotic syndrome study. J Am Soc Nephrol. 2021;32:2303–2314. doi: 10.1681/ASN.2020111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronbichler A., Leierer J., Oh J., et al. Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. BioMed Res Int. 2016;2016:1–5. doi: 10.1155/2016/2150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts A.J.B., Keller K.H., Lerner G., et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol. 2022;33:238–252. doi: 10.1681/ASN.2021060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer A., Mayer G., Kronbichler A. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. BioMed Res Int. 2016;2016:1–9. doi: 10.1155/2016/4192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korbet S. Clinical picture and outcome of primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 1999;14(suppl 3):68–73. doi: 10.1093/ndt/14.suppl_3.68. [DOI] [PubMed] [Google Scholar]
- 7.Rovin B.H. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Braun N., Schmutzler F., Lange C., et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2008;3:CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattran D.C., Appel G.B., Hebert L.A., et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 10.Alberici F., Jayne D.R.W. Impact of rituximab trials on the treatment of ANCA-associated vasculitis. Nephrol Dial Transplant. 2014;29:1151–1159. doi: 10.1093/ndt/gft318. [DOI] [PubMed] [Google Scholar]
- 11.Scolari F., Delbarba E., Santoro D., et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32:972–982. doi: 10.1681/ASN.2020071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassia M.A., Alberici F., Jones R.B., et al. Rituximab as maintenance treatment for systemic lupus erythematosus: a multicenter observational study of 147 patients. Arthritis Rheumatol. 2019;71:1670–1680. doi: 10.1002/art.40932. [DOI] [PubMed] [Google Scholar]
- 13.Basu B., Sander A., Roy B., et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172:757–764. doi: 10.1001/jamapediatrics.2018.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravani P., Magnasco A., Edefonti A., et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/CJN.09421010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauckler P., Shin J.I., Alberici F., et al. Rituximab in adult minimal change disease and focal segmental glomerulosclerosis—what is known and what is still unknown? Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102671. [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P., Ruggiero B., Cravedi P., et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25:850–863. doi: 10.1681/ASN.2013030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren H., Lin L., Shen P., et al. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget. 2017;8:93438–93443. doi: 10.18632/oncotarget.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran R., Bharati J., Rao I., et al. Persistent CD-19 depletion by rituximab is cost-effective in maintaining remission in calcineurin-inhibitor dependent podocytopathy. Nephrology (Carlton) 2019;24:1241–1247. doi: 10.1111/nep.13554. [DOI] [PubMed] [Google Scholar]
- 19.Cortazar F.B., Rosenthal J., Laliberte K., Niles J.L. Continuous B-cell depletion in frequently relapsing, steroid-dependent and steroid-resistant nephrotic syndrome. Clin Kidney J. 2019;12:224–231. doi: 10.1093/ckj/sfy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DaSilva I., Huerta A., Quintana L., et al. Rituximab for steroid-dependent or frequently relapsing idiopathic nephrotic syndrome in adults: a retrospective, multicenter study in Spain. BioDrugs. 2017;31:239–249. doi: 10.1007/s40259-017-0221-x. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran R., Bharati J., Nada R., et al. Rituximab in maintaining remission in adults with podocytopathy. Nephrology (Carlton) 2020;25:616–624. doi: 10.1111/nep.13717. [DOI] [PubMed] [Google Scholar]
- 22.Roccatello D., Sciascia S., Rossi D., et al. High-dose rituximab ineffective for focal segmental glomerulosclerosis: a long-term observation study. Am J Nephrol. 2017;46:108–113. doi: 10.1159/000477944. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fresnedo G., Segarra A., González E., et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:1317–1323. doi: 10.2215/CJN.00570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scolari F., Alberici F., Mescia F., et al. Therapies for membranous nephropathy: a tale from the old and new millennia. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.789713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogo A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol. 2015;11:76–87. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troyanov S., Wall C.A., Miller J.A., et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler D.C., Jongs N., Stefansson B.V., et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trachtman H., Nelson P., Adler S., et al. DUET: A Phase 2 study evaluating the efficacy and safety of Sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allinovi M., Trivioli G., Lugli G., et al. Proteinuria selectivity index predicts response to rituximab in adults with minimal change disease and focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2022;37:789–791. doi: 10.1093/ndt/gfab323. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs R., Langer-Jacobus T., Duong M., et al. Detection and quantification of rituximab in the human urine. J Immunol Methods. 2017;451:118–121. doi: 10.1016/j.jim.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Chan E.Y., Webb H., Yu E., et al. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020;97:393–401. doi: 10.1016/j.kint.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Hladunewich M.A., Cattran D., Sethi S.M., et al. Efficacy of rituximab in treatment-resistant focal segmental glomerulosclerosis with elevated soluble urokinase-type plasminogen activator receptor and activation of podocyte β3 integrin. Kidney Int Rep. 2022;7:68–77. doi: 10.1016/j.ekir.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iijima K., Sako M., Oba M., et al. Mycophenolate mofetil after rituximab for childhood-onset complicated frequently relapsing or steroid-dependent nephrotic syndrome. J Am Soc Nephrol. 2022;33:401–419. doi: 10.1681/ASN.2021050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.