Abstract
Objective
To investigate the safety and efficacy of a percutaneous revascularization strategy that is based on the use of drug-coated balloon for the treatment of patients with acute coronary syndrome and de novo Medina type 0,1,0 or 0,0,1 left main stem bifurcation lesions.
Methods
: In this multicenter, prospective, proof-of-concept study, patients fulfilling the above criteria were enrolled and received treatment with drug-coated balloon combined with provisional drug-eluting stent implantation in the proximal major branches of the left main stem. Patients who declined this revascularization approach were treated with drug-eluting stent implantation 1-2 mm distally to the left anterior descending or left circumflex artery ostium followed by drug-coated balloon therapy for the ostial disease. The primary endpoint of the study was the calculation of percent diameter stenosis on quantitative coronary angiography post-procedure as well as event rate at 8 months follow-up.
Results
A total of 30 patients were enrolled in the study; their mean age was 60.3 ± 7.8 years, while 22 (73.3%) were male. Twenty-two patients were treated only with drug-coated balloon and provisional drug-eluting stent implantation and 8 had drug-eluting stent implantation followed by drug-coated balloon therapy of the ostium of the left main stem major branch. All the procedures were successful with no immediate complications. The percent diameter stenosis of lesion decreased significantly post-procedure from 87.5% (80.0-90.0) to 20% (17.5-30.0), P < .001. During the follow-up period, no major adverse cardiac events were reported.
Conclusions
This proof-of-concept study indicates that ostial drug-coated balloon therapy of the left main stem major branches is safe and effective. Larger clinical data and longer follow-up are needed before advocating its regular use in clinical practice.
Keywords: Acute coronary syndrome, drug-coated balloon, left main bifurcation lesion, percutaneous coronary intervention
HIGHLIGHTS
A novel drug-coated balloon (DCB)-based percutaneous coronary intervention (PCI) technique, DCB combined with provisional drug-eluting stent, or drug-eluting stent combined with DCB techniques provide excellent acute angiographic results in Medina 0,0,1 and 0,1,0 left main bifurcation lesions.
The techniques introduced not only provide a safe and effective alternative in the treatment of these challenging lesions but also keep the procedure simple without compromising the disease-free side branch.
The short-term follow-up outcomes were associated with a low major adverse cardiovascular event rate; however, large-scale randomized studies with a long-term follow-up are required to robustly evaluate the safety and efficacy of our novel techniques against conventional DES PCI.
Introduction
Percutaneous coronary intervention (PCI) for coronary bifurcation lesions (CBLs) represents one of the most challenging procedures in interventional cardiology because of lower angiographic success rate and increased risk of procedural complications.1
Currently, the single stent strategy has been considered as the default approach for the treatment of CBL2; however, the optimal therapy for Medina type 0,1,0 or 0,0,1 left main stem (LMS) bifurcation lesions remains unclear. For these lesions, precise ostial stent placement and cross-over stenting techniques have been proposed. Nevertheless, precise stent placement is known to be challenging, and there is no established technique for perfect ostial stent deployment despite the fact that different strategies and/or devices have been tested.3 Several studies have shown that the cross-over stenting approach is superior to the ostial stenting with a lower rate of major adverse cardiovascular event (MACE) during long-term clinical follow-up.4,5 However, data indicate that this procedure may be complicated by a significant stenosis of the other major branch of the LMS even if its ostium is disease-free pre-procedure requiring a switch to a two-stent strategy.6
Drug-coated balloon (DCB) has been established as an effective alternative for the treatment of de novo coronary artery disease (CAD) in small and large vessels as well as in bifurcation lesions. The updated international expert consensus on DCB for treatment of CAD highlighted that they are non-inferior to drug-eluting stents (DES) in small vessel lesions.7 Moreover, a recent study has demonstrated the safety and efficacy of SeQuent® Please DCB in lesions with a reference diameter exceeding 3.0 mm,8 while reports underscored the value of DCB for treating de novo bifurcation lesions.9-11 However, data regarding its performance in LMS bifurcation lesions are lacking.
In this study, we aimed to investigate for the first time the feasibility and short-term efficacy of DCB therapy for de novo LMS bifurcation lesions Medina type 0,1,0 and 0,0,1 in patients presenting with an acute coronary syndrome (ACS).
Methods
Study Population
Between January 2019 and October 2020, patients aged 18-75 years, presented with ACS and angiographic evidence of a de novo culprit lesion in the ostium of left anterior descending (LAD) or left circumflex artery (LCx) were recruited at 4 hospitals in the city of Huaihai, China.
Study exclusion criteria included patients with cardiogenic shock, left ventricular ejection fraction <35%, severe renal insufficiency (estimated glomerular filtration rate ≤30 mL/min/1.73 m2), previous stent implantation in the LMS, and life expectancy <1 year. The study protocol complied with the Declaration of Helsinki and was approved by the Xuzhou Cancer Hospital (Approval Date: April 15, 2019; Approval Number: IEC-C-008-A07-V1.0; 2019-02-002-K01); all enrolled patients gave written informed consent.
Interventional Procedures and Devices
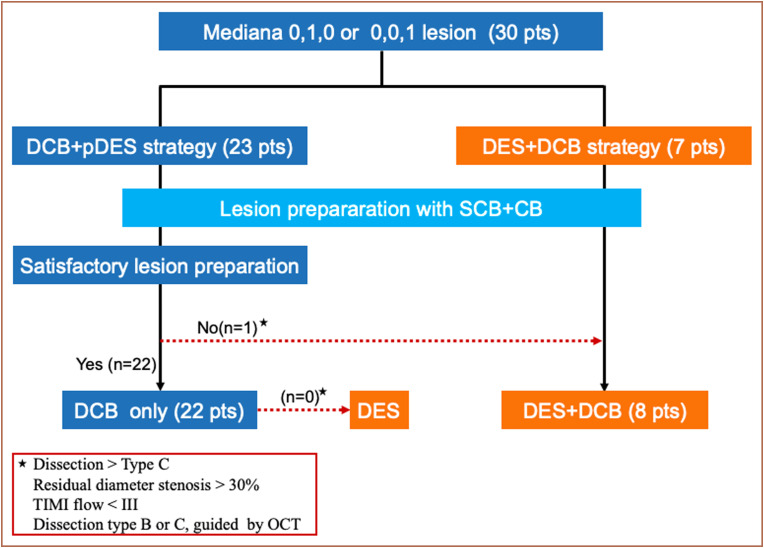
In view of the potential risks of using DCB alone in the treatment of de novo LMS bifurcation lesions, the following 2 treatment strategies were proposed (Figure 1, Supplementary file):
Figure 1.
Study diagram of the present study. CB, cutting balloon; DCB, drug-coated balloon; OCT, optical coherence tomography; pDES, provisional drug-eluting stent; SCB, semi-compliant balloon; TIMI, thrombolysis in myocardial infarction.
Drug-coated balloon combined with provisional DES implantation 1-2 mm distally to the LAD or LCx ostium whenever this was required (DCB+pDES strategy) (Figure 2).
Drug-eluting stent implantation 1-2 mm distally to the LAD or LCx ostium followed by DCB to treat the ostial lesion (DES+DCB strategy) (Figure 3).
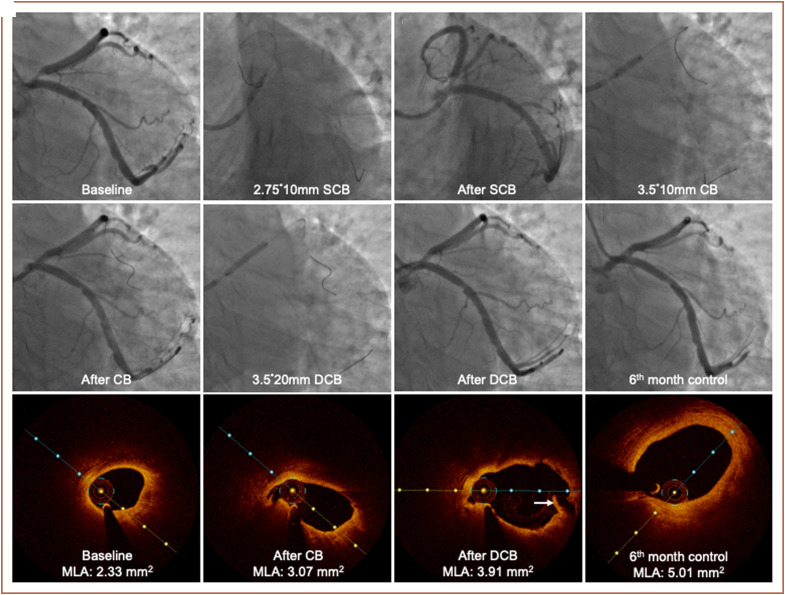
Figure 2.
A case example of DCB+pDES strategy. (A) Initial angiogram with LAD ostial 95% visual stenosis; (B) pre-dilation with 2.75 × 10 mm SCB; (C) result after dilation with SCB, 40% residual stenosis; (D) pre-dilation with 3.5 × 10 mm CB; (E) 20% residual stenosis with A-type dissection is observed after CB implantation; (F) treatment with 3.5 × 20 mm DCB implantation; (G) final result with 20% residual stenosis after DCB treatment; (H) 6-month follow-up angiography showed no obvious stenosis; a, pre-procedure OCT showed LAD ostium, MLA = 2.33 mm2; b, localized dissection in the proximal LAD after CB, MLA = 3.07 mm2; c, localized dissection in the proximal LAD after DCB treatment (white arrow), MLA = 3.91 mm2; d, 6-month follow-up, endothelial repair is seen, lumen size was significantly increased, MLA = 5.01 mm2. CB, cutting balloon; DCB, drug-coated balloon; LAD, left anterior descending artery; MLA, minimal luminal area; OCT, optical coherence tomography; pDES, provisional drug-eluting stent; SCB, semi-compliant balloon.
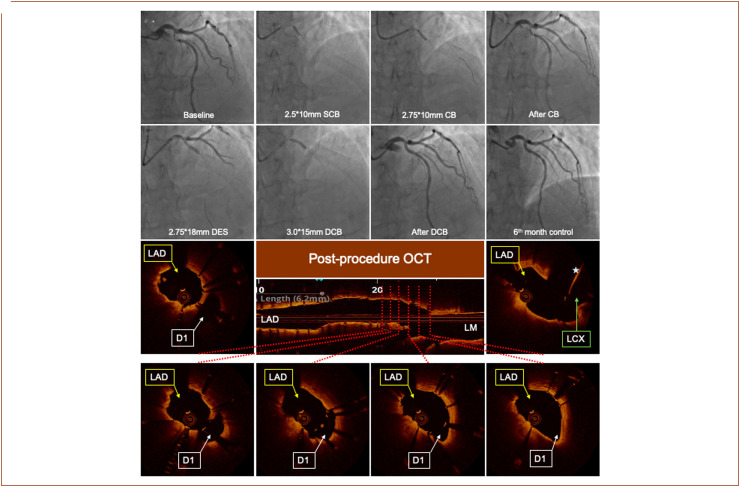
Figure 3.
A case example of DES+DCB strategy. (A) Initial angiogram shows a visual 30% LM end stenosis and LAD ostial and proximal 90% stenosis; (B) pre-dilation with 2.5 × 10 mm CB; (C) pre-dilation with 2.75 × 10 mm CB; (D) X-ray angiography post-CB pre-dilatation, a type-A dissection and visually residual stenosis of >60% was observed; (E) a 2.75 × 18 mm DES was implanted, located 1-2 mm distally from the LAD ostium; (F) 3.0 × 15 mm DCB implantation covering the LAD ostium; (G) final result after DES+DCB treatment, visual residual stenosis is <20%; (H) 6-month follow-up angiography shows no obvious stenosis; post-procedure OCT images, the stent positioned and expanded well, and the proximal stent strut is about 1 mm away from the LAD ostium. Left circumflex artery wire is seen (white star). CB, cutting balloon; D1, 1. diagonal artery; DCB, drug-coated balloon; DES, drug-eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; OCT, optical coherence tomography; SCB, semi-compliant balloon.
Before the procedure, the 2 different treatment options, potential risks and benefits of these approaches and also the conventional approaches (cross-over technique and ostial stenting) were fully discussed with the patients and their families. The first strategy, that is, DCB+pDES strategy was recommended for all patients, while the DES+DCB strategy was performed in those patients who declined DCB+pDES in view of the possible post-procedure risks associated with DCB treatment alone such as an acute occlusion of the target vessel.
Drug-coated balloon therapy was performed in line with the recommendations proposed in the consensus documents on DCB treatment in CAD.7,12 In this study, paclitaxel-coated balloons were used including Sequent® Please (Braun, Germany) and Swide DCB (Shenqi Medical, China), while the FIREHAWK DES (Shanghai MicroPort Medical Group, Shanghai, China) was implanted in the cases requiring stenting procedure.13
Quantitative Coronary Angiography Analysis
Coronary angiograms at baseline, after the procedures (N = 29), and at follow-up (N = 8) were analyzed using the QAngio XA version 7.3 (Medis Medical Imaging System Inc., Leiden, the Netherlands) by 2 experienced independent investigators. Quantitative coronary angiography analyses were undertaken in corresponding end-diastolic angiographic frames acquired pre- and post-device implantation and at 6 months follow-up. Angiographic measurements were performed in the segment defined by the target lesion and 5 mm proximal and distal to the lesion segments. For each lesion, the reference vessel diameter (RVD), the lesion length, the minimum lumen diameter (MLD), and the percent diameter stenosis (DS%) were estimated in the coronary angiographies performed at pre-, post-procedure, and follow-up. The acute gain was defined as the difference in the MLD at post- and pre-procedure, while the late lumen loss (LLL) was estimated as post-procedural MLD minus follow-up MLD.
Medication and Follow-Up
All patients enrolled in the study received aspirin (100 mg/day), ticagrelor (180 mg loading dose followed by 90 mg twice daily), and statins before procedure according to the current revascularization guidelines.14 Heparin (100 U/kg) was administered intravenously at the beginning of the procedure and then the activated clotting time ≥250 s was maintained during the procedure. In patients who received treatment with a DCB, dual antiplatelet therapy (DAPT) was prescribed for 3 months, whereas in those treated with DES+DCB, it was prescribed for at least 12 months. All patients were reviewed in outpatient clinics or had a telephone consultation at 8 months whereas repeat coronary angiography was scheduled at 6-month follow-up.
Clinical Endpoints and Definitions
Procedural success was defined as DS <30% in the target lesion and absence of immediate complications [i.e., vessel perforation, new LMS stenosis (DS >30%), or new stenosis on the untreated branch (DS >30%) requiring a switch to a two-stent strategy].
Major adverse cardiovascular event was defined as cardiac death, myocardial infarction, and target lesion revascularization (TLR). Death was considered to have a cardiac cause unless a non-cardiac cause was identified. Myocardial infarction was defined according to historical definitions used in stent studies.15 Target lesion revascularization was defined as a repeated intervention (percutaneous or surgical) due to >50% DS within the treated segment or 5 mm proximally or distally.
Statistical Analysis
Continuous variables were expressed as mean and standard deviation or median and interquartile range (IQR) and compared using a t-test or Wilcoxon rank-sum test depending on data distribution. Categorical variables were shown as counts and percentages. A two-sided P value <.05 was considered statistically significant. Statistical analysis was conducted using the SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Studied Population
A total of 30 patients were enrolled in the study; 26 (86.7%) of these were admitted with a non-ST elevation myocardial infarction and 4 (13.3%) with an ST elevation myocardial infarction. The mean age of the studied patients was 60.3 ± 7.8 years, and 22 patients (73.3%) were male. Approximately half of the patients had hypertension, a quarter of them suffered from diabetes mellitus, while 36.6% of the patients had a history of prior PCI (Table 1).
Table 1.
Baseline Clinical and Angiographic Characteristics of the Studied Patients
| Studied Patients (n = 30) |
|
|---|---|
| Age (years) | 60.3 ± 7.8 |
| Gender (male) | 22 (73.3%) |
| Smoking history | 14 (46.7%) |
| Family history of CAD | 6 (20%) |
| Co-morbidities | |
| Diabetes mellitus | 8 (26.7%) |
| Hypertension | 17 (56.7%) |
| Hypercholesterolemia | 6 (20%) |
| Renal failure* | 0 (0%) |
| Anemia | 0 (0%) |
| Previous PCI | 11 (36.6%) |
| Previous bypass surgery | 0 (0%) |
| LV function ** | 59.0 ± 4.1 |
| Normal LV function | 29 (96.7%) |
| Impaired LV function | 1 (3.3%) |
| Clinical presentation | |
| NSTEMI | 26 (86.7%) |
| STEMI | 4 (13.3%) |
| Periprocedural medications | |
| DAPT (ticagrelor + aspirin) | 30 (100%) |
| Statins | 30 (100%) |
| β-blockers | 22 (73.3%) |
CAD, coronary artery disease; DAPT, dual antiplatelet treatment; LV, left ventricle; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
*Renal failure was defined as an estimated glomerular filtration rate of <30ml/min/1.73m2.
**Impaired LV function was defined as LV ejection fraction <50%.
Lesion and Procedural Characteristics
Twenty-two (73.3%) patients had ostial lesion in the LAD and 8 (26.7%) patients had ostial disease in the LCx. The mean RVD was 2.81 ± 0.60 mm, and the median lesion length was 10.0 mm (IQR 8.4-12.0).
Overall, 23 patients (76.7%) were treated with the DCB+pDES strategy, while the DES+DCB strategy was performed in 7 cases (23.3%). In the former group, 22 patients had PCI with DCB, whereas 1 patient underwent DES implantation plus DCB treatment due to a type C dissection after lesion preparation. Of note, in 10 patients, PCI was performed under optical coherence tomography (OCT) guidance.
Lesion preparation was performed using semi-compliant balloons in all the cases and cutting balloons in 96.7% of the patients. One DCB was used for each lesion; the size of the DCB, the expansion pressure, and the inflation duration are shown in Table 2. After lesion preparation, angiographically detectable dissections were noted in only 5 patients (Table 2) of which only 1 had type C dissection. Optical coherence tomography was used in this case to assess the lesion and then to guide PCI with DES implantation 2 mm distally to the ostium of the vessel followed by DCB to treat ostial disease. No stent implantation was required for the treatment of the 4 cases with type A or B dissection.
Table 2.
Procedural Characteristics
|
|
Studied Patients (n = 30) |
|---|---|
| Balloon types for lesion preparation | |
| Semi-compliant balloon | 30 (100%) |
| Cutting balloon | 29 (96.7%) |
| Dissection after lesion preparation | |
| No | 25 (83.3%) |
| Type A | 3 (10%) |
| Type B | 1 (3.3%) |
| Type C and above | 1 (3.3%) |
| DCB types | |
| SeQuent® Please | 21 (70%) |
| Swide | 9 (30%) |
| DCB size (mm) | |
| Diameter | 3.00 (2.75-3.50) |
| Length | 20.0 (15.0-20.0) |
| DCB procedure | |
| Expansion pressure (atm) | 7.5 (6.8-9.0) |
| Expansion time (s) | 46.0 (30.0-58.5) |
| DES size (mm) | |
| Diameter | 3.2 ± 0.3 |
| Length | 18.6 ± 9.4 |
DCB, drug-coated balloon; DES, drug-eluting stent.
Quantitative Coronary Angiography Analysis
The QCA analysis before intervention and post-procedure is shown in Table 3. The MLD of the treated vessel increased at the end of the procedure to 2.25 ± 0.5 mm, while the %DS decreased from 62.9 ± 14.6 to 13.3 ± 7.5, P < .001. The acute gain was estimated as 1.26 ± 0.45 mm after PCI in the treated vessel did not affect the lumen dimensions in the LMS or the untreated branch.
Table 3.
Quantitative Coronary Angiography Analysis at Pre-procedure, Post-procedure, and Follow-Up
|
Pre-procedure
(n = 29) |
Post-procedure
(n = 29) |
P | 6 Months Follow-Up (n = 8) | |
|---|---|---|---|---|
| Left main stem | ||||
| RVD (mm) | 3.7 ± 0.59 | 3.68 ± 0.45 | .843 | 3.77 ± 0.50 |
| %DS (%) | 8.1 ± 7.4 | 8.4 ± 7.8 | .861 | 4.03 ± 4.49 |
| Treated vessel | ||||
| RVD (mm) | 2.81 ± 0.60 | 2.64 ± 0.48 | .251 | 3.01 ± 0.21 |
| MLD (mm) | 1.00 ± 0.39 | 2.25 ± 0.50 | <.001 | 2.75 ± 0.19 |
| %DS (%) | 62.9 ± 14.6 | 13.3 ± 7.5 | <.001 | 7.77 ± 3.39 |
| Acute gain (mm) | 1.26 ± 0.45 | |||
| Lesion length (mm) | 11.36 ± 5.04 | |||
| Untreated branch | ||||
| RVD (mm) | 2.90 ± 0.55 | 2.87 ± 0.55 | .852 | 3.20 ± 0.60 |
| %DS (%) | 9.3 ± 6.4 | 10.6 ± 9.9 | .555 | 7.38 ± 5.50 |
MLD, minimum lumen diameter; RVD, reference vessel diameter; %DS, percent diameter stenosis.
Only 8 patients accepted to have repeat coronary angiography at 6-month follow-up. For this subgroup of patients, the LLL was −0.34 ± 0.48 (Figure 2- -panel H and panel D).
One-third of the studied patients had OCT imaging during PCI. From this subgroup, 4 patients underwent OCT examination at 6-month follow-up, which showed a complete vessel wall repair and numerical higher minimum lumen area compared to post-procedure OCT.
Clinical Outcomes
The procedural success rate was 100%, and no MACE was recorded during the index hospitalization. Clinical follow-up data were available for all the patients with a mean follow-up period of 7.7 ± 6.0 months; during this period, none of the patients experience an adverse event.
Discussion
This study investigated for the first time the feasibility, clinical safety, and short-term efficacy of a new revascularization strategy that relies on the use of DCB to treat de novo Medina type 0,1,0 or 0,0,1 LMS bifurcation lesions in patients admitted with an ACS. We demonstrated that (1) DCB+pDES strategy was safe and effective with the DCB alone intervention providing satisfactory results in 95.6% of the cases; (2) the DES+DCB strategy which was adopted in 7 patients (23.3%), who did not wish to undergo DCB+pDES therapy was also effective in providing excellent final angiographic results in all the cases; and (3) reassuringly no MACE was reported in the studied population at 8 months follow-up.
Optimal T reatment of Medina 0 ,1,0 and 0,0, 1 Left Main Stem Coronary Bifurcation L esions
Optimal management of Medina type 0,1,0 and 0,0,1 LMS bifurcation lesions is an unresolved issue.16 The 15th consensus document from the European Bifurcation Club recommends ostial or cross-over stenting to treat these lesions.16
The ostial stenting is often used in clinical practice; however, the accurate positioning of the stent can be challenging due to difficulty in identifying the optimal bifurcation angle.17 Therefore, ostial stenting is recommended only in the presence of a rectangular angle between LAD-LCx and perfect visualization of SB take-off; in all the other cases, cross-over stenting should be preferred.16 A recent study comparing ostial LAD stenting with cross-over technique showed the feasibility of ostial stenting4; however, a high restenosis rate was observed in this group.4 Moreover, Medina et al18 showed that the floating stent technique for the treatment of ostial LAD disease provides excellent mid-term results, but it can compromise LCx ostium in 26% and cause significant stenosis in 10% due to carina displacement. Our DCB+pDES or DES+DCB techniques overcome the limitations of ostial stenting as the use of DCB maintains the original anatomy of the carina and hence diminishes the risk of abnormal flow patterns into the SB.
The cross-over stenting technique has better clinical efficacy for these types of lesions compared to ostial stenting.4 Nevertheless, it has been shown that the stent struts suspended the side branch ostium, which can easily lead to thrombosis after discontinuing DAPT or fenestrated restenosis of SB ostium.16,19 Additionally, the value of SB dilation after cross-over stenting is debatable and may be challenging. SB intervention is recommended whenever the lumen dimensions of the untreated branch are compromised; however, today there are no established cutoffs for considering PCI to the disease-free side branch and a switch to a two-stent strategy.5
Conversely, our technique is consistent with the “KISS” (keep it simple and safe) principle recommended by guidelines16 because it not only minimizes the risk of SB stenosis but also does not require SB rewiring to perform the final kissing balloon when needed.
Technical Considerations
In the treatment of de novo lesions in large vessels, adequate preparation of the lesions is essential to ensure optimal short- and long-term results after DCB treatment. In this study, cutting balloons were used in 96.7% of the lesions, which resulted in a large MLD and small %DS. However, this approach can increase the risk of dissection. Therefore, we advocate the use of provisional DES implantation whenever this is deemed necessary to minimize the risk of acute occlusion or dissection extension after DCB treatment. The risk of acute vascular occlusion in type A and type B dissections is extremely low7; however, our previous study found that type B dissection after DCB treatment may progress to type C dissection leading to cardiac events during follow-up.8 Therefore, in our study, OCT was recommended to better assess lumen pathology whenever a type B dissection was noted, and in case of a major dissection (dissection extending to the medial with intimal flap >60˚ or ≥3 mm length), stent implantation was recommended.
In our study, we combined the use of DCB and DES only in the case who had a type C dissection as was recommended in the recently published consensus document.7 Similarly, a DES+DCB strategy may be a safe and effective alternative as it safeguards the patency of the vessel, keeping at the same time the procedure simple without compromising the disease-free side branch.
Dual Antiplatelet Therapy Regimen After Drug-Coated Balloon Only Treatment
Recently, TWILIGHT20 and TICO21 trials have successfully revealed that 3 months of DAPT is safe after DES implantation in ACS patients. A previous study on the use of DCB in large lesions showed no thrombotic events in patients receiving DAPT for 3 months.8 In our study, patients who underwent DCB treatment alone (73.3%) received DAPT for only 3 months and none has experienced a MACE; however, further evidence is needed from a large number of patients before advocating this strategy.
Limitations
This was a small-scale single-arm feasibility study including a small number of patients. Therefore, it lacks power and a control group that will allow us to robustly assess its efficacy compared to the currently used ostial or cross-over stenting approaches. Moreover, we included patients admitted with an ACS that have soft plaques that respond well to balloon angioplasty. It is therefore unclear whether this approach has a value in patients with stable angina having calcific-rich lesions. Additionally, the follow-up period was short and did not allow us to assess the long-term safety and efficacy of this strategy. Although clinical follow-up data were available for all the patients, angiographic follow-up in the study was performed in a small number of patients; thus, it was not possible to accurately quantify the incidence of lesion restenosis. Finally, all patients were treated with paclitaxel DCBs; hence, our results cannot be adopted for patients treated with other sirolimus-eluting DCBs.
Conclusions
This proof-of-concept study demonstrated that DCB combined with provisional DES implantation may provide excellent acute angiographic results and is associated with a low MACE rate at short-term follow-up in patients admitted with an ACS having LMS bifurcation lesion Medina type 0,0,1 or 0,1,0. Further large-scale randomized studies with longer follow-up periods are needed to robustly assess its safety and efficacy against conventional DES PCI and establish this approach as an effective alternative in the treatment of these challenging lesions.
Ethics Committee Approval
Ethics committee approval was received from the Xuzhou Cancer Hospital (Approval Date: April 15, 2019; Approval Number: IEC-C-008-A07-V1.0; 2019-02-002-K01).
Informed Consent
All enrolled patients gave written informed consent.
Author Contributions
Author contributions: Concept – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y., C.V.B., Y.J.Z.; Design – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y., C.V.B., Y.J.Z.; Supervision – C.V.B., Y.J.Z.; Fundings – None; Materials – E.E., Z.L., Y.X.Z., V.T., B.L., Q.H.Z., N.A.L.Y., C.V.B., Y.J.Z.; Data collection &/or processing – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y., C.V.B., Y.J.Z.; Analysis &/or interpretation – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y.; Literature search – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y.; Writing – E.E., Z.L., Y.X.Z., V.T., S.L.F., Q.L., L.L., S.C., L.T.B., B.L., Q.H.Z., N.A.L.Y., C.V.B.; Critical review – C.V.B., Y.J.Z.
Funding Statement
None.
Footnotes
Peer-review: Internally peer-reviewed.
Conflict of Interest: None of the other authors have a conflict of interest.
References
- 1. Kornowski R. The complexity of stenting in bifurcation coronary lesions. JACC Cardiovasc Intv United States. 2013;6(7):696 697. 10.1016/j.jcin.2013.04.005) [DOI] [PubMed] [Google Scholar]
- 2. Behan MW, Holm NR, de Belder AJ.et al. Coronary bifurcation lesions treated with simple or complex stenting: 5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J. 2016;37(24):1923 1928. 10.1093/eurheartj/ehw170) [DOI] [PubMed] [Google Scholar]
- 3. Kwan TW, James D, Huang Y, Liou M, Wong S, Coppola J. Perfection of precise ostial stent placement. J Invasive Cardiol. 2012;24(7):354 358. [PubMed] [Google Scholar]
- 4. Rigatelli G, Zuin M, Baracca E.et al. Long-term clinical outcomes of isolated ostial left anterior descending disease treatment: ostial stenting Versus left main cross-over stenting. Cardiovasc Revasc Med. 2019;20(12):1058 1062. 10.1016/j.carrev.2019.01.030) [DOI] [PubMed] [Google Scholar]
- 5. Capranzano P, Sanfilippo A, Tagliareni F.et al. Long-term outcomes after drug-eluting stent for the treatment of ostial left anterior descending coronary artery lesions. Am Heart J. 2010;160(5):973 978. 10.1016/j.ahj.2010.07.002) [DOI] [PubMed] [Google Scholar]
- 6. Burzotta F, Talarico GP, Trani C.et al. Frequency-domain optical coherence tomography findings in patients with bifurcated lesions undergoing provisional stenting. Eur Heart J Cardiovasc Imaging. 2014;15(5):547 555. 10.1093/ehjci/jet231) [DOI] [PubMed] [Google Scholar]
- 7. Jeger RV, Eccleshall S, Wan Ahmad WA.et al. Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13(12):1391 1402. 10.1016/j.jcin.2020.02.043) [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Zhang YJ, Deng LX.et al. 12-month clinical results of drug-coated balloons for de novo coronary lesion in vessels exceeding 3.0 mm. Int J Cardiovasc Imaging. 2019;35(4):579 586. 10.1007/s10554-018-1505-z) [DOI] [PubMed] [Google Scholar]
- 9. Her AY, Ann SH, Singh GB.et al. Serial morphological changes of side-branch ostium after paclitaxel-coated balloon treatment of de novo coronary lesions of main vessels. Yonsei Med J. 2016;57(3):606 613. 10.3349/ymj.2016.57.3.606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleber FX, Rittger H, Ludwig J.et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol. 2016;105(7):613 621. 10.1007/s00392-015-0957-6) [DOI] [PubMed] [Google Scholar]
- 11. Vaquerizo B, Fernández-Nofreiras E, Oategui I.et al. Second-generation drug-eluting balloon for ostial side branch lesions (001-bifurcations): mid-term clinical and angiographic results. J Interv Cardiol. 2016;29(3):285 292. 10.1111/joic.12292) [DOI] [PubMed] [Google Scholar]
- 12. Her AY, Shin ES, Bang LH.et al. Drug-coated balloon treatment in coronary artery disease: recommendations from an Asia-pacific consensus group. Cardiol J. 2021;28(1):136 149. 10.5603/CJ.a2019.0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lansky A, Wijns W, Xu B.et al. Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET all comers): a multicentre, open-label, randomised non-inferiority trial. Lancet. 2018;392(10153):1117 1126. 10.1016/S0140-6736(18)31649-0) [DOI] [PubMed] [Google Scholar]
- 14. Valgimigli M, Bueno H, Byrne RA.et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European. Eur Heart J. 2017;39:213 260. [DOI] [PubMed] [Google Scholar]
- 15. Vranckx P, Cutlip DE, Mehran R.et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention J Eur Collab Work Gr Interv Cardiol Eur Soc Cardiol. 2010;5:871 874. [DOI] [PubMed] [Google Scholar]
- 16. Burzotta F, Lassen JF, Lefèvre T.et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16(16):1307 1317. 10.4244/EIJ-D-20-00169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Çayli M, Elbasan Z, Gür M.et al. Modified flower petal technique in the treatment of Medina type 0,0,1 or 0,1,0 lesions. EuroIntervention. 2015;11(7):772 779. 10.4244/EIJV11I7A154) [DOI] [PubMed] [Google Scholar]
- 18. Medina A, Martín P, Suárez de Lezo J.et al. Vulnerable carina anatomy and ostial lesions in the left anterior descending coronary artery after floating-stent treatment. Rev Esp Cardiol. 2009;62(11):1240 1249. 10.1016/s1885-5857(09)73351-1) [DOI] [PubMed] [Google Scholar]
- 19. Murasato Y, Shibao K, Meno K, Takenaka K. A case of very late stent thrombosis on the protruded struts at the left main coronary bifurcation. J Cardiol Cases. 2020;22(1):40 43. 10.1016/j.jccase.2020.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehran R, Baber U, Sharma SK.et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032 2042. 10.1056/NEJMoa1908419) [DOI] [PubMed] [Google Scholar]
- 21. Kim BK, Hong SJ, Cho YH.et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323(23):2407 2416. 10.1001/jama.2020.7580) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a