Abstract
Neutral red (NR) functioned as an electronophore or electron channel enabling either cells or membranes purified from Actinobacillus succinogenes to drive electron transfer and proton translocation by coupling fumarate reduction to succinate production. Electrically reduced NR, unlike methyl or benzyl viologen, bound to cell membranes, was not toxic, and chemically reduced NAD. The cell membrane of A. succinogenes contained high levels of benzyl viologen-linked hydrogenase (12.2 U), fumarate reductase (13.1 U), and diaphorase (109.7 U) activities. Fumarate reductase (24.5 U) displayed the highest activity with NR as the electron carrier, whereas hydrogenase (1.1 U) and diaphorase (0.8 U) did not. Proton translocation by whole cells was dependent on either electrically reduced NR or H2 as the electron donor and on the fumarate concentration. During the growth of Actinobacillus on glucose plus electrically reduced NR in an electrochemical bioreactor system versus on glucose alone, electrically reduced NR enhanced glucose consumption, growth, and succinate production by about 20% while it decreased acetate production by about 50%. The rate of fumarate reduction to succinate by purified membranes was twofold higher with electrically reduced NR than with hydrogen as the electron donor. The addition of 2-(n-heptyl)-4-hydroxyquinoline N-oxide to whole cells or purified membranes inhibited succinate production from H2 plus fumarate but not from electrically reduced NR plus fumarate. Thus, NR appears to replace the function of menaquinone in the fumarate reductase complex, and it enables A. succinogenes to utilize electricity as a significant source of metabolic reducing power.
Bacteria utilize many energy sources, including light and diverse organic and inorganic chemicals. Common to the metabolism of these energy sources is the production of an electrochemical gradient that provides an electron donor for metabolism and allows for maintenance of a membrane potential and proton motive force. In microbial metabolism, the energy produced from the driving force of electrons is directly proportional to the ΔE0′ value between the initial electron donor (the first biochemical dehydrogenating reaction) and the final electron acceptor (i.e., the final biochemical hydrogenating reaction) (38).
The specific activities of redox enzymes involved with bacterial catabolism, such as hydrogenase or fumarate reductase, can be measured with their in vivo electron carriers (e.g., NAD or menaquinone) or with artificial redox dyes (e.g., benzyl viologen) (2, 3, 14, 28, 44). In bacteria that produce succinic acid as a major catabolic end product (e.g., Escherichia coli, Wolinella succinogenes, and other species), the fumarate reductase complex catalyzes the fumarate-dependent oxidation of menaquinone. This reaction is coupled to the generation of a transmembrane proton gradient that is used by the organism to support growth and metabolic function (19, 45). The fumarate reductase (FRD) of E. coli is composed of four nonidentical subunits, FRDA, FRDB, FRDC, and FRDD, that are arranged in two domains: (i) the FRDAB catalytic domain and (ii) the FRDCD membrane anchor domain, which is essential for electron transfer and proton translocation reactions involving menaquinone (1, 3, 41).
Investigating the oxidation-reduction characteristics of biological systems by electrochemical techniques is useful for understanding biological energy metabolism (25, 36). Useful redox dyes for bioelectrochemical systems must easily react with both the electrode and the biological electron carriers. Many biological electron carriers, such as NAD (24, 37), c-type cytochromes (47), quinones (33), and many redox enzymes, such as nitrite reductase (42), nitrate reductase (43), fumarate reductase (36), glucose-6-phosphate dehydrogenase (24), ferredoxin-NADP reductase (17), and hydrogenase (34), react electrochemically with the redox dyes. Some redox dyes with lower redox potential than that of NAD, such as methyl viologen (MV) (16, 27, 42), benzyl viologen (4), and neutral red (NR) (8, 15), can alter biological redox reactions in vivo. Hongo and Iwahara (11, 12) discovered that redox dyes with low ΔE0′ values, such as MV, benzyl viologen, and NR, caused a 6% increase in l-glutamate yield during fermentation under cathodic reduction conditions (i.e., electroenergized fermentation); however, they did not show how these dyes functioned biochemically or physiologically. Addition of NR to acetone-butanol fermentations decreased acid and H2 production while enhancing solvent production (8, 15). This NR-dependent metabolic shift from acids to solvents was further enhanced with NR under electroenergized fermentation conditions (7). Viologen dyes have been used as electron mediators for many electrochemical catalytic systems using oxidoreductases in vitro and in vivo (13, 16, 17, 25, 34, 42). A critical factor for the control of end product yields in bacterial fermentations is regulation of electron distribution through the NADH/NAD+ ratio. If additional reducing power (e.g., H2 or electrochemically produced reducing equivalents) is supplied to bacteria, variations in the NADH/NAD+ ratio and metabolism should be expected.
Recent interest has focused on development of succinic acid as a fermentation product because succinic acid has many industrial uses (32). Anaerobiospirillum and Actinobacillus species produce high levels of succinate (35 and 95 g/liter, respectively) during glucose fermentation via the route glucose→ phosphoenol pyruvate→oxaloacetate→malate→fumarate→ succinate (26, 31, 39). Under glucose growth conditions with H2 present, A. succinogenes produces significantly more succinate and less acetate because hydrogen serves as an additional electron donor for metabolism.
The energetics of living systems is driven by electron transfer processes. Electrons are funneled from a source that becomes oxidized to a final acceptor that becomes reduced. In other words, life runs on electricity. This implies that it might be possible to control or alter metabolism by plugging biochemical processes into an external electrochemical system. In one previous report (29) an electrochemical system was used to regenerate reduced iron for growth of Thiobacillus ferrooxidans on electrical reducing power. We show here that electrical reducing power can be utilized to drive fumarate reduction and proton translocation during the growth of A. succinogenes on glucose and electrically reduced NR.
Furthermore, we provide the first biochemical evidence of how NR functions physiologically by showing that (i) the electrical reduction of NR (E0′ = −0.325 V) is chemically linked to NAD reduction and that it is biochemically linked to generation of a proton motive force and succinate production and (ii) that NR appears to function by replacing menaquinone (E0′ = −0.073 V) in the membrane-bound complex.
MATERIALS AND METHODS
Chemicals and reproducibility of results.
All chemicals were reagent grade, and gases were purchased from AGA Chemicals (Cleveland, Ohio). All individual experiments were repeated two to three times with identical results.
ECB systems.
Two types of electrochemical bioreactor (ECB) systems were used. ECB system I (40-ml working volume) was used for enzymatic and chemical reduction tests, and ECB system II (300-ml working volume) was used for electricity-dependent cultivation of cells. The ECB systems, specially designed for maintaining anaerobic conditions and for growing bacteria, were made from Pyrex glass by the Michigan State University Chemistry Department, East Lansing. The ECB system was separated into anode and cathode compartments by a cation-selective membrane septum (diameter [φ] = 22 mm for type I and φ = 64 mm for type II) (Nafion [Electrosynthesis, Lancaster, N.Y.]; 3.5 Ω cm−2 in 0.25 N NaOH). Chemicals and metabolites cannot be transferred across the Nafion membrane; only protons or cations transfer. Both the anode and cathode were made from finely woven graphite felt (6 mm thick; 0.47 m2 g−1 available surface area) (Electrosynthesis). A platinum wire (φ = 0.5 mm; <1.0 Ω cm−2; Sigma, St. Louis, Mo.) was attached to the graphite felt with graphite epoxy (<1.0 Ω cm−2; Electrosynthesis). The electrical resistance between anode and cathode was <1 kΩ. The weight of both electrodes was adjusted to 0.4 g (surface area, 0.188 m2) for system I and 3.0 g (surface area, 1.41 m2) for system II. The current and voltage between anode and cathode were measured by precision multimeter (model 45; Fluke, Everett, Wash.) and adjusted to 0.3 to 2.0 mA and 1.5 V for system I and 1.0 to 10.0 mA and 2.0 V for system II. The electrochemical half oxidation of H2O was coupled to half reduction of NR (100 μM), and the oxidation of reduced NR was coupled to bacteriological reduction of fumarate. H2 was not produced under the electrochemical conditions used to reduce NR or MV. For tests in ECB system I, the cathode compartment contained the cell suspension, membrane suspension, or solubilized membranes and the anode compartment contained 50 mM phosphate buffer (pH 7.2) and 100 mM NaCl. For growth studies in ECB system II, the cathode compartment contained the growth medium inoculated with A. succinogenes and the anode compartment contained 100 mM phosphate buffer (pH 7.0) and 100 mM NaCl.
Organism and growth conditions.
The A. succinogenes type strain, 130Z, is maintained at MBI International (Lansing, Mich.) (10, 39). The bacteria were grown in butyl rubber-stoppered 158-ml serum vials containing 50 ml of medium with a CO2-N2 (20%–80%; 20 lb/in2) gas phase unless otherwise indicated. Growth medium A contained the following (per liter of double-distilled water): yeast extract, 5.0 g; NaHCO3, 10.0 g; NaH2PO4 · H2O, 8.5 g; and Na2HPO4, 12.5 g. The pH of the medium was adjusted to 7.0 after autoclaving. Separately autoclaved solutions of glucose (final concentration, 60 mM) and fumarate (final concentration, 50 mM) were aseptically added to the medium after autoclaving. The media were inoculated with 5.0% (vol/vol) samples of cultures grown in the same medium and incubated at 37°C.
Preparation of cell suspensions.
Cultivation, harvest, and washing of the bacteria were done under a strict anaerobic N2 atmosphere as described previously (39). A 16-h A. succinogenes culture was harvested by centrifugation (5,000 × g; 30 min) at 4°C and washed three times with a 1,500-ml solution of 50 mM Na phosphate buffer (pH 7.2) containing 1 mM dithiothreitol (DTT). The washed bacterial cells were resuspended in 50 mM sodium phosphate buffer with 2 mM DTT. This suspension was used as a catalyst for H2-dependent and electricity-dependent reduction of fumarate to succinate, and it was used for cyclic voltammetry and for NR adsorption to cells.
Electrochemical reduction of NAD.
ECB system I with 1 mM NAD+ and 100 μM NR or MV was used for electrochemical reduction of NAD+. The electrode potential and current were adjusted to 2.0 V and 1.0 to 3.0 mA, respectively. Ag-AgCl and platinum electrodes were used to measure the reactants’ redox potential to check if the reaction was progressing. Generally, the redox potential of a biochemical or electrochemical reaction is measured with an Ag-AgCl electrode (E0′ of [Ag/Ag+], +0.196 V) or a Calomel electrode (E0′ of [Hg/Hg+], +0.244 V) as a reference electrode, but it has to be expressed as the potential versus a natural hydrogen electrode (NHE), which is used for thermodynamic calculation of organic or inorganic compounds (e.g., E0′ of NADH/NAD+ is −0.32 V, and that of H2/2H+ is −0.42 V). A potential measured with an Ag-AgCl electrode is converted to potential versus a NHE by adding +0.196 V to the measured potential (E0′ versus a NHE = E0′ versus Ag-AgCl + 0.196). Oxygen was purged from the reactants and from the redox dye solution in 50 mM Tris-HCl (pH 7.5) by bubbling it with oxygen-free nitrogen for 10 min before supplying electricity. The NADH concentration in the reactant was spectrophotometrically measured at 340 mm and calculated by using the millimolar extinction coefficient 6.23 mM−1 cm−1. NADH production was confirmed by absorption spectra data at each sampling time.
Preparation of purified membranes, solubilized membranes, and membrane-free cell extract.
Cell extracts were prepared at 4°C under an anaerobic N2 atmosphere, as described previously (39). The harvested and washed cells were resuspended in 50 mM phosphate buffer (pH 7.2) containing 1 mM DTT and 0.05 mg of DNase. The cells were disrupted by passing them twice through a French press at 20,000 lb/in2. The cell debris was removed by centrifugation three times at 40,000 × g for 30 min each time. The purified membranes were obtained from the cell extracts by centrifugation at 100,000 × g for 90 min. The supernatant was decanted and saved as the membrane-free cell extract. The clear brown precipitate was washed twice with 50 mM phosphate buffer (pH 7.2) and resuspended in the same buffer by homogenization. Solubilized membranes were obtained from the membrane fraction by Triton X-100 extraction (21). Triton X-100 was added to a final 1% (vol/vol) concentration, and the suspension was incubated for 3 h. Triton-solubilized protein was recovered after removing insoluble debris by centrifugation at 100,000 × g and 4°C for 90 min.
NR binding to cells and membranes.
The adsorption of redox dyes to cells and purified membranes was determined by measuring the residual NR and MV in solution after being mixed with cells or membrane suspensions for 30 min at 37°C. Bacterial cell suspensions (optical density at 660 nm [OD660], between 0 and 3.0) and the purified membrane suspension (0 to 10 mg of protein/ml) were used to analyze redox dye adsorption (i.e., binding). NR solutions (50 and 25 μM) and MV (100 μM) were used for measuring dye binding to intact cells and membranes. MV (100 μM) was used for measuring cell binding. The cells and membranes were removed from the reaction mixture by centrifugation at 12,000 × g for 10 min and by ultracentrifugation at 150,000 × g for 20 min, respectively. The NR concentration was calculated by using a calibration curve spectrophotometrically predetermined at 400 nm and pH 7.2, and MV was measured by using the millimolar extinction coefficient (ɛ578) 9.78 mM−1 cm−1 after reduction by the addition of 1.5 mM dithionite at pH 7.2 (23). The protein concentrations of membrane suspensions were determined by a calibration curve (protein concentration [in milligrams per milliliter] = A595 × 1.3327) with Bradford reagent (Bio-Rad, Hercules, Calif.).
Measurement of proton translocation.
Proton translocation was measured under an anoxic N2 atmosphere. H2-dependent proton translocation by cell suspensions was measured as described by Fitz and Cypionka (6). Electricity-dependent proton translocation was measured in an ECB system designed for measurement of proton translocation. The tube (φ = 10 mm [inside diameter] by 90 mm), with a Vycor tip (ion-exchangeable hard membrane; BAS, West Lafayette, Ind.), was used as an anode compartment, a graphite rod (φ = 7 by 70 mm) was used as an anode, and 0.05-g graphite felt (surface area, 0.0235 m2) was used as a cathode. The pH electrode (Orion 8103; ROSS) was placed in the cathode compartment and was connected to a recorder (Linear) via a pH meter (Corning model 130) that converted the proton pulse into a recordable signal. Cell suspensions were made in KKG solution (pH 7.1), which contains 100 mM KSCN, 150 mM KCl, and 1.5 mM glycylglycin, and placed in the cathode. The anode contained a 50 mM phosphate buffer with 50 mM KCl as an anolyte. The total volumes and working volumes of the cathode and anode compartments were 30 and 5.5 ml, respectively. The working potential and current between anode and cathode were 2.0 V and 0.3 to 0.35 mA for experiments with electrical reducing power and NR. Bacterial cells were cultivated for 16 h in medium A with fumarate-H2 or glucose. The cells were anaerobically harvested by centrifugation at 5,000 × g and 20°C for 30 min and washed twice with 100 mM KCl. The cells were modified with 100 μM NR to measure electricity-dependent proton translocation and washed again with 100 mM KCl. The washed bacteria (OD660, 10) were resuspended in N2-saturated 150 mM KCl. The cell suspensions were allowed to equilibrate for 30 min at room temperature. The incubated cells were centrifuged at 5,000 × g and 20°C for 30 min and resuspended in KKG solution, and then the incubation was continued for 30 min under H2 atmosphere before the measurement of proton translocation. To measure electricity-dependent proton translocation upon fumarate addition, the cell suspension was incubated in the presence or absence of 2-(n-heptyl)-4-hydroxyquinoline N-oxide (HOQNO) in the cathode compartment under an N2 atmosphere and charged with a 2.0-V electrode potential for 20 min.
Enzyme assays.
Enzyme activity measurements were performed under an anaerobic N2 atmosphere, as described previously (39). The membrane-free extract, purified membrane, and solubilized membrane preparations described above were used to assay hydrogenase, diaphorase, and fumarate reductase activities. Fumarate reductase (EC 1.3.) and hydrogenase (EC 2.12.2.2.) activities were measured as described by van der Werf et al. (39) with a Beckman spectrophotometer (model DU-650). Diaphorase activity with benzyl viologen (BV2+) and NR+ was measured under analogous conditions with hydrogenase by using NADH (0.6 mM) instead of H2 as the electron donor (35). The oxidation and reduction of benzyl viologen and NR were spectrophotometrically measured at 578 and 540 nm, respectively, and the oxidation and reduction of NAD(H) were spectrophotometrically measured at 340 nm. Reduced benzyl viologen was prepared as described previously (23). The millimolar extinction coefficient of benzyl viologen (ɛ578), NR (ɛ540), and NAD(H) (ɛ340) were 8.65, 7.12, and 6.23 mM−1 cm−1, respectively.
Enzymatic analysis of fumarate reduction membranes and solubilized membranes.
For enzymatic analysis, membrane suspensions (3.25 mg of protein/ml) and solubilized membranes (3.2 mg of protein/ml) were used as enzyme sources. Serum vials (50 ml) and ECB system I were used for H2-dependent and electricity-dependent reduction of fumarate to succinate, respectively. Anaerobically prepared 50 mM fumarate in 50 mM phosphate buffer (pH 7.2) was used as the reactant and catholyte, and 100 mM phosphate buffer with 100 mM NaCl (pH 7.0) was used as the anolyte. The reaction was started by the addition of enzyme sources, and it was maintained at 37°C. Substrate and product concentrations were analyzed by high-performance liquid chromatography (HPLC) (9).
The influence of HOQNO on fumarate reduction in cell suspensions and membranes was analyzed as follows. Cell suspensions (OD660 = 4.2) and membrane suspension (2.65 mg of protein/ml) were used as enzyme sources. Serum vials (50 ml) and ECB system I were used for H2-dependent and electricity-dependent reduction of fumarate to succinate, respectively. Anaerobically prepared 50 mM fumarate in 50 mM phosphate buffer (pH 7.2) was used as the reactant and catholyte, and 100 mM phosphate buffer with 100 mM NaCl (pH 7.0) was used as the analyte. HOQNO (2 μM) was used as an inhibitor for menaquinone. The reaction was started by the addition of enzyme sources, and it was maintained at 37°C. Substrate and product concentrations were analyzed by HPLC.
Cyclic voltammetry.
A 3-mm-diameter glassy carbon working electrode (BAS), a platinum wire counterelectrode (BAS), and an Ag-AgCl reference electrode (BAS) were used in an electrochemical cell with a working volume of 2 ml. Cyclic voltammetry was performed with a cyclic voltametric potentiostat (BAS model CV50W) linked to an IBM microcomputer data acquisition system. Prior to use, the working electrode was polished with an alumina-water slurry on cotton wool, and the electrochemical cell was thoroughly washed. Oxygen was purged from the cell suspension, membrane suspension, or solubilized membrane solution by bubbling it with oxygen-free N2 for 10 min before electrochemical measurements. Bacterial suspensions (OD660 = 3.0), membrane suspensions (2.54 mg of protein/ml), and solubilized membranes (3.2 mg of protein/ml) were used as enzyme sources. The scan rate used was 25 mV/s over the range −0.3 to −0.8 V. Phosphate buffer (50 mM) containing 5 mM NaCl was used as the electrolyte. NR (100 μM) and 50 mM fumarate were used as the electron mediator and the electron acceptor, respectively.
Growth analysis.
The growth of cells suspended in the medium was determined by measuring the suspensions (OD660), and the growth yield of cells adsorbed onto the electrode was determined by measuring the protein concentration. The protein concentration was converted to OD by using a predetermined calibration curve (bacterial density = protein concentration [in milligrams per milliliter] × 1.7556). The cathode, on which the bacteria were adsorbed, was washed three times by slow agitation in 300 ml of phosphate buffer (50 mM; pH 7.0) for 30 min. The bacterial lysate was obtained from the electrodes by alkaline treatment at 100°C for 10 min with 1 N NaOH. After cell debris was removed from the lysate by centrifugation at 10,000 × g and 4°C for 30 min, the protein concentration of the bacterial lysate was determined with Bradford reagent and a predetermined calibration curve (protein concentration [in milligrams per milliliter] = A595 × 1.3327).
Metabolic analysis.
Glucose, fumarate, succinate, acetate, ethanol, and formate concentrations were determined by HPLC (9). The components were analyzed chromatographically by elution with 0.006 M H2SO4 from a cation-exchange resin in the acid form. A Waters (Marlborough, Mass.) model 660 HPLC system equipped with a Bio-Rad (Richmond, Calif.) HPX-87H column and with a Waters model-410 refractive index detector was used.
RESULTS
Physiological function of NR.
The successful use of a redox dye to transfer electricity’s reducing power into microbial metabolic systems strongly depends on dye toxicity and binding. Experiments were initiated to compare the effectiveness of redox dyes that couple to hydrogenase (5, 18) as suitable electronophore candidates. We tested the effects of three redox dyes on the growth of A. succinogenes in glucose medium. Benzyl viologen at 30 to 90 μM significantly inhibited growth, whereas NR or MV addition was not inhibitory. Next we compared the binding of monocationic NR versus that of dicationic MV to intact cells and purified membranes. NR actively bound to intact cells and cell membranes, whereas MV did not bind significantly. The amount of NR adsorbed to cells and purified membranes was 68 μM per mg of cell protein, and 83 μM NR was adsorbed per mg of membrane protein. MV adsorption was <6 μM dye per mg of cell protein. In separate experiments we compared the hydrophobicity of NR versus that of MV by measuring their transfer rates from the water phase at 100 μM (dye) to the butanol phase. Greater than 95% of the NR, but <5% of MV, was transferred from the water phase to the butanol phase. NR’s higher hydrophobicity and lower charge capacity, when compared to MV, may explain, in part, their different cellular binding capacities. NR is lipophilic and readily incorporates into the membrane, whereas MV does not.
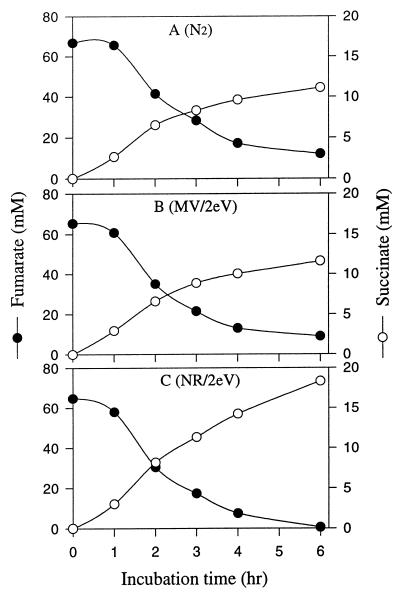
We compared NR (E0′, −0.325 V) and MV (E0′, −0.446 V) as electronophores for electricity-dependent fumarate reduction. Theoretically, reduced MV provides more energy and reducing power than reduced NR because of the difference in the redox potential (ΔEh) of their oxidation-reduction reactions. Figure 1 shows that NR, but not MV, served as an electronophore in the electrically enhanced reduction of fumarate to succinate by whole cells.
FIG. 1.
Influence of redox dyes on utilization of electron reducing power for fumarate reduction to succinate by cell suspensions (OD660, 3.0) of A. succinogenes. The cells were placed in serum vials or ECB system I with a potential of 2.0 V and a current of 0.3 to 2.0 mA. (A) N2; (B) electrically reduced MV; (C) electrically reduced NR.
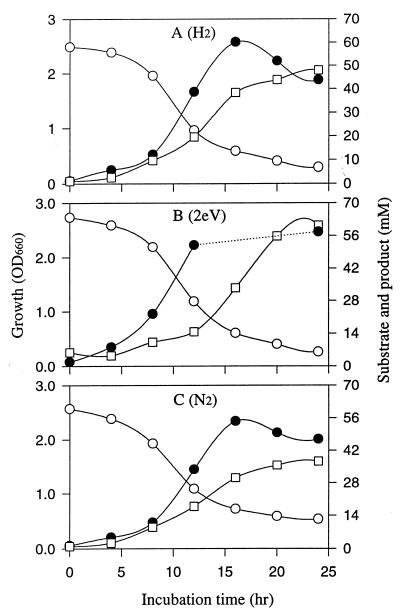
Experiments were initiated to test whether cells fermenting glucose grow faster and produce more succinate in the presence of extra reducing power provided by electrically reduced NR. Figure 2 compares the growth and succinate production of Actinobacillus grown on glucose medium (alone) to its growth and succinate production on glucose medium in an ECB system II with electrically reduced NR. The culture medium pH was kept constant at 6.8. There were distinct increases in the initial rate of growth and in the final yield of succinate produced when electricity served as an additional source of reducing power. It should be noted that the growth data reported before 24 h represents only cells suspended in the medium. These data underestimate the total growth because significant growth occurs on the electrode (26). Table 1 summarizes the chemical yield at the end of the experiment shown in Fig. 2 for growth on glucose versus growth on glucose plus electrically reduced NR. Electrical reducing power significantly enhanced glucose consumption, growth, and succinate and ethanol production while it decreased formate and acetate production.
FIG. 2.
Influence of electrical reducing power on growth and succinate production of A. succinogenes in glucose medium. (A) Normal fermentation conditions plus H2; (B) electrically reduced conditions in ECB system II with 100 μM NR, a current of 1.5 to 10 mA, and a potential of 2.0 V; (C) normal fermentation conditions plus N2. Symbols: ●, growth; □, succinate; ○, glucose.
TABLE 1.
Comparison of glucose fermentation by A. succinogenes in the absence and presence of electrically reduced NRa
| Parameter | Metabolite concn (mM)
|
|
|---|---|---|
| Minus electrical energy | Plus electrical energy | |
| Glucose consumption | 47.33 | 60.44 |
| Cell massb | 45.49 | 57.84 |
| Succinate production | 51.27 | 82.88 |
| Formate production | 36.95 | 9 |
| Acetate production | 6.40 | 3.75 |
| Ethanol production | 5.21 | 21.1 |
| Carbon recovery | 0.984 | 1.001 |
| Electron recovery | 0.932 | 1.036 |
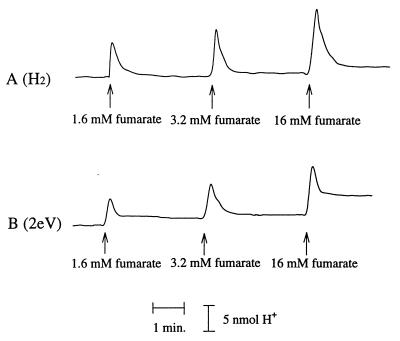
In bacterial fumarate respiration systems, a proton motive force is generated by coupling NADH or hydrogen oxidation with fumarate reduction to succinate (20, 38). Proton translocation was compared in cell suspensions of A. succinogenes producing succinate from fumarate plus hydrogen to that in suspensions producing succinate from fumarate plus electrically reduced NR (Fig. 3). In these experiments, the cells were incubated in the presence of H2 or electrically reduced NR for at least 20 min prior to the addition of fumarate and measurement of proton translocation. Rapid acidification of the cell medium was detected upon addition of fumarate. Proton translocation was no longer detectable 10 to 15 s after the addition of fumarate. Figure 3 shows the dependence of proton translocation on electron acceptor concentration. Proton translocation by cells using H2 or electrically reduced NR was proportionally increased by higher fumarate concentrations.
FIG. 3.
Proton translocation after addition of fumarate to a cell suspension of A. succinogenes. (A) Cells preincubated with H2; (B) cells preincubated in ECB system with electrically reduced NR. The cells (OD660, 10.0; 5.696 mg of protein/ml) were placed in KKG solution at room temperature. The x axis represents time (in minutes), and the y axis represents proton concentration (in nanomoles).
NR oxidoreduction mechanisms.
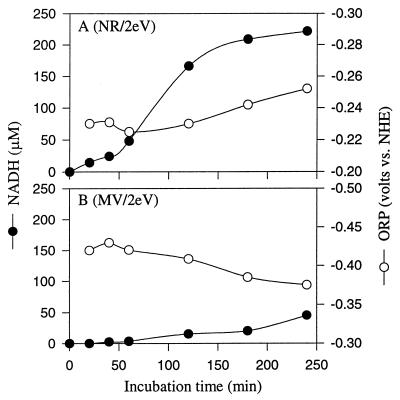
Figure 4 compares the time course for chemical reduction of NAD+ to NADH by electrically reduced NR and MV. During electrochemical reduction, the oxidation-reduction potential (ORP) of the reactant is kept below 0 V to increase the electron donation tendency of the reduced dyes. Figure 4 shows that the ORP of NAD+ with NR and MV was −0.24 and −0.38 V, respectively. These values are influenced by the E0′ of NR and MV, respectively. Significant chemical reduction of NAD occurred with electrically reduced NR but did not occur with reduced MV.
FIG. 4.
Comparison of the effect of electrically reduced NR (A) to that of MV (B) on the chemical reduction of NAD. The experiments were performed in ECB system I. The cathode contained 50 mM Tris-HCl (pH 7.5), 1 mM NAD+, and 100 μM dye. The anode contained 100 mM KPO4 buffer (pH 7.2) and 100 mM NaCl as the electrolyte. The potential was 2.0 V, and the current was 0.8 to 2.4 mA. ORP is an indicator for monitoring how the reactions are going. Decreasing ORP means that the reduction reaction is going very well, but increasing ORP means that the reaction is being disrupted, or the reduced products may be reoxidized.
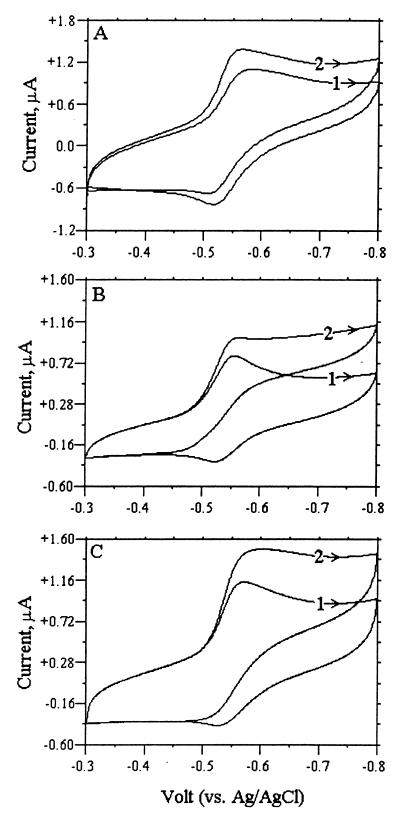
Intact cells cannot directly react with an electrode, but redox dyes can mediate electron transfer from the electrode to the dye and then into cellular metabolism. If redox dyes immobilized in a cell membrane (CM) can bind to a specific redox enzyme (e.g., fumarate reductase), electron flow from the electrode to the redox enzyme (or vice versa) can be measured using cyclic voltammetry. Figure 5 shows the cyclic voltammograms for intact cells (Fig. 5A), purified cytoplasmic membranes (Fig. 5B), and solubilized membranes (Fig. 5C) modified with NR before and after fumarate addition. The oxidation peak (current) of NR completely disappeared when fumarate was added to purified CMs because electrochemically reduced NR is oxidized by fumarate reductase enzymatically. It also significantly decreased in intact cells and solubilized CMs. These results demonstrate that NR bound to the CM or solubilized CM can couple with fumarate reductase, and that electrons can transfer from the electrode to fumarate reductase through NR-linked fumarate reduction to succinate.
FIG. 5.
Cyclic voltammograms measured with a glassy carbon electrode during successive cycles following the introduction of the electrode into a solution containing 50 mM KPO4 buffer (pH 7.2), 100 μM NR, and 5 mM NaCl on either cell suspensions of A. succinogenes (OD660, 3.0; 1.71 mg of protein/ml) (A), purified membrane from A. succinogenes suspension (2.54 mg of protein/ml) (B), or solubilized membrane (3.2 mg of protein/ml) (C). The total working volume was 2.0 ml. 1, before the addition of 50 mM fumarate; 2, after the addition of 50 mM fumarate. The scan rate was 25 mV s−1, the reference electrode was Ag-AgCl, and the counterelectrode was platinum wire.
Fumarate reductase is membrane bound, and it serves as the terminal electron transfer enzyme in succinate-producing bacteria (40). Hydrogenase serves as the initial electron transfer enzyme in bacteria utilizing H2 as an electron donor, and it can be located in the cytoplasm, membrane, or periplasm (46). Table 2 compares the cellular locations and activities versus electron carrier as tested for hydrogenase, fumarate reductase, and diaphorase in A. succinogenes. Hydrogenase and fumarate reductase were both membrane-bound activities that coupled to NAD, benzyl viologen, or NR oxidoreduction. Solubilization of purified membranes inactivated benzyl viologen- and NAD-coupled hydrogenase activities but not the benzyl viologen- or NR-dependent fumarate reductase activity. The loss of NAD-linked fumarate reductase, and perhaps hydrogenase, may be related to the potential loss of menaquinone from the membrane-bound fumarate reductase complex upon solubilization. Notably, NR served as the best electron donor for fumarate reductase. High levels of diaphorase activity were detected in membrane fractions. The A. succinogenes diaphorase activity is similar to the diaphorase associated with NAD-dependent hydrogenases in Nocardia and Alcaligenes, where NADH oxidation is coupled to benzyl viologen reduction (18, 35).
TABLE 2.
Cellular locations and activities of key A. succinogenes oxidoreductases linked to NAD or redox dyes
| Cell fraction | Sp act (mM/min/mg of protein)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Hydrogenase
|
Fumarate reductase
|
Diaphorasea
|
||||||
| NAD | BVb | NR | NADH | BVH | NRH | BV | NR | |
| Membrane-free extract | 0.01 | 0.26 | <0.01 | <0.001 | <0.001 | <0.01 | 8.8 | <0.01 |
| Purified membranes | 0.30 | 12.23 | 1.05 | 4.06 | 13.10 | 24.53 | 109.7 | 0.844 |
| Solubilized membranes | <0.001 | <0.03 | 0.727 | <0.001 | 2.14 | 12.86 | 40.2 | 0.840 |
Measures the oxidation of NADH to NAD coupled to reduction of [2H+ + 2e−] to H2.
BV, benzyl viologen.
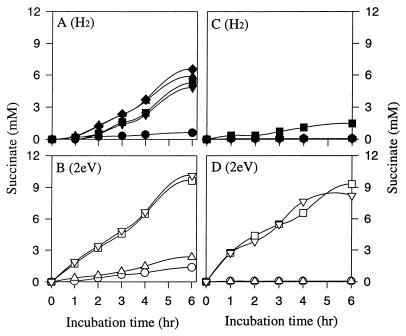
Experiments were initiated to compare H2 and electricity-dependent fumarate reduction by purified membranes and solubilized membranes. Triton X-100 was used to solubilize the fumarate reductase from the membranes (22). Figure 6 shows that the membrane-bound fumarate reductase complex was extremely active at producing succinate from either H2 or electrically reduced NR as the electron donor. Succinate production from fumarate was not significant in controls without H2 or electricity plus NR. The rate and yield of succinate production were significantly higher with electrically reduced NR than H2 as the electron donor for the fumarate reductase complex. Notably, NR addition did not significantly stimulate fumarate reduction from hydrogen and NAD addition did not stimulate fumarate reduction from electrically reduced NR. These observations suggest that NR is not required for H2 oxidation and NAD is not required for NR oxidation by the membrane-bound fumarate reductase complex. Figure 6 also compares H2 to electrically reduced NR as the electron donor for fumarate reductase in solubilized membranes. As expected, hydrogen was not a significant electron donor for the fumarate reductase complex in solubilized membranes because the hydrogenase activity is inactivated by solubilization. On the other hand, electrically reduced NR served as the electron donor for fumarate reductase in solubilized membranes.
FIG. 6.
Comparison of H2 (A and C) to electrical reducing power (2eV) (B and D) as an electron donor for reduction of fumarate to succinate by purified membranes (A and B) and solubilized membranes (C and D) from A. succinogenes. Membranes (3.25 mg of protein/ml) and solubilized membranes (3.2 mg of protein/ml) were suspended in 50 mM KPO4 buffer (pH 7.2), and where indicated, 50 μM NR and 1 mM NAD were added. Symbols: ●, N2 control; ■, H2 alone; ▴, H2 + NAD+; ▾, H2 + NR; ⧫, H2 + NAD+ + NR; ○, control 2eV alone; □, 2eV + NR; ▵, 2eV + NAD+; ▿, 2eV + NAD+ + NR.
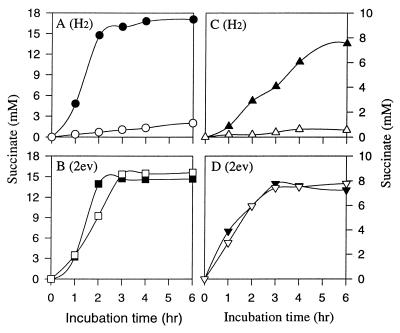
Experiments were initiated to assess whether NR could play menaquinone’s function in the A. succinogenes fumarate reductase complex. Figure 7 compares fumarate reduction by whole cells and membrane fractions with either H2 or electrically reduced NR as the electron donor and in the presence or absence of the quinone inhibitor HOQNO. Fumarate reductase was significantly inhibited by HOQNO when whole cells or membranes were using H2 as the electron donor. Fumarate reductase was not inhibited by HOQNO, however, when electrically reduced NR was used, suggesting that NR can play menaquinone’s function in the fumarate reductase complex. Other experiments (data not shown) indicated that proton translocation by whole cells with electrically reduced NR and fumarate was not inhibited by HOQNO, but it was inhibited when H2 was the electron donor.
FIG. 7.
Influence of HOQNO on fumarate reduction to succinate by intact cells (A and B) or purified membranes (C and D) from A. succinogenes under 2 atm of H2 (A and C) or with electrically reduced NR (B and D). Whole cells (OD660, 4.2) and purified membranes (2.65 mg of protein/ml) were suspended in 50 μM KPO4 buffer (pH 7.2), and where indicated, 50 mM NR was added. Solid symbols, without HOQNO; open symbols, with addition of 2 μM HOQNO.
DISCUSSION
We show here that NR enables A. succinogenes to use electricity as a significant source of reducing power for growth and metabolism. NR functions both in vivo and in vitro as an electronophore and provides a channel enabling Actinobacillus cells, membranes, or solubilized fumarate reductase to utilize electrical reducing power. The biochemical utilization of electrically reduced NR was somewhat analogous to the utilization of hydrogen by Actinobacillus cells and membrane fractions. A. succinogenes contained membrane-bound fumarate reductase complex and hydrogenase, which were shown in vivo to translocate protons in the presence of fumarate and either H2 or electrically reduced NR. NR served as a better electron donor for fumarate reductase than viologen dyes or NAD. The rate and yield of succinate production from fumarate by both whole cells and purified CMs were greater with electrically reduced NR than with hydrogen as an electron donor. Although electrically reduced dyes have previously been shown to alter metabolism they have not been shown to function physiologically and biochemically during growth as electrical mediators for driving cellular energy metabolism.
Several features of NR make it useful as an electron mediator for biological reactions. It shares with MV and benzyl viologen a redox potential more reduced than that of NAD, so it can link to many biochemical redox reactions. However, it is not toxic like benzyl viologen and, unlike MV, it binds to the membrane, it chemically reduces NAD, and an oxygen radical cannot be produced from it with oxygen. These properties make NR an ideal electron mediator for controlling the NADH/NAD ratio in diverse kinds of cells (i.e., bacteria, archaea, and eucaryotes). NR was also shown by cyclic voltammetry to bind and transfer electrons to membrane-bound fumarate reductase. Because HOQNO did not inhibit fumarate reduction from electrically reduced NR, it appears that NR replaces quinones in the fumarate reductase complex and serves as an electron channel for electricity to reduce fumarate and to drive proton translocation. It will be of interest to learn if electrically reduced NR can also drive proton translocation in other microbes that do not contain quinones. In any case, the utilization of electrical reducing power during the NR-mediated fermentation of glucose by A. succinogenes dramatically enhanced the utilization of glucose and the final concentration and yield of both cells and reduced metabolites (i.e., succinate and ethanol) derived from glucose. This would only be expected if electrical energy can both drive a proton motive force for biological energy conservation and serve as an additional electron donor for metabolism.
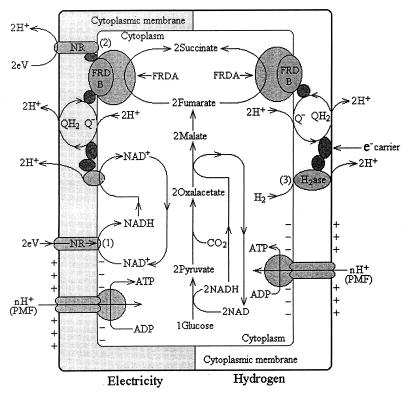
Figure 8 shows a comparative model of the biochemical mechanisms of energy conservation in A. succinogenes grown on glucose plus electrically reduced NR to those of A. succinogenes grown on H2 plus glucose. Although speculative, this model incorporates the following experimental data: (i) NR is a hydrophobic dye that binds to the membrane; (ii) both hydrogenase and fumarate reductase are membrane bound; (iii) proton translocation occurs with either H2 or electrically reduced NR as a donor for fumarate reductase; and (iv) HOQNO does not inhibit fumarate reduction by electrically reduced NR.
FIG. 8.
Hypothetical model depicting two different biochemical functions for NR’s role as an electronophore or electron channel during the growth of A. succinogenes on glucose plus electrical reducing power and those during its growth on glucose plus H2. (1) Electrically reduced NR chemically reduces NAD+ to NADH, generating more reducing equivalents for the reduction of both oxaloacetate and fumarate; and (2) in electricity-dependent fumarate reduction, NR replaces menaquinone in the fumarate reductase complex and electrical reducing power reduces fumarate to succinate while translocating protons, which drives membrane-bound ATP synthesis. During growth on H2 plus glucose, more succinate and cells are formed than on glucose alone but less than with electrically reduced NR because (3) membrane-bound hydrogenase links only to fumarate reductase and not to NADH generation. Symbols: 2eV, electrical reducing power; QH2, reduced menaquinone; FRD, fumarate reductase complex; PMF, proton motive force.
Reduction of NR by electricity occurs by direct contact between cell-bound NR and the cathode. Reduced NR is oxidized by chemical reduction of NAD or by the fumarate reductase complex. Consequently, cells grown on glucose plus electricity gain more reducing power than cells grown on glucose plus H2 which results in additional proton translocation for increased ATP synthesis, metabolism, and growth. Cells grown on H2 plus glucose, in comparison to cells grown on glucose alone, also produced more reduced metabolites and may have gained additional energy, since membrane-bound hydrogenase links to additional proton translocation, but not to the same extent as occurs with electricity and NR because additional NADH is not formed. It should be noted that in the absence of additional reducing power from H2 or electricity, glucose-grown cells must oxidize pyruvate to provide an electron donor for fumarate reduction, and this decreases succinate yield and ATP synthesis via electron transport-mediated phosphorylation.
The fumarate reductase complex can vary in different organisms. For example, the fumarate reductase of E. coli differs in part from that of W. succinogenes because it lacks cytochrome b (19). We need to purify the components of fumarate reductase from A. succinogenes to confirm how NR functions as an electronophore and to characterize its physiological electron acceptor and donor properties. It is of interest to note that the membrane-bound fumarate reductase activity of Actinobacillus was enhanced by hydrogen addition but not by NAD or NR addition.
In bacteria that consume H2, hydrogenases function in the reduction of electron acceptors during energy conservation (46). Hydrogenases are quite diverse, and their cellular localizations vary in conjunction with their physiological electron carriers (30). We need to purify the membrane-bound hydrogenase of A. succinogenes and characterize its physiological electron acceptor. The A. succinogenes hydrogenase appears similar to the membrane-bound hydrogenase of Alcaligenes eutrophus (18), which couples to the respiratory chain and thus contributes to the generation of free energy.
Electrically reduced redox dyes were shown to alter microbial “electroenergized” fermentations and to increase reduced end product chemical yields (e.g., glutamate) by some unknown biochemical mechanism (11). It was previously shown that addition of NR to Clostridium acetobutylicum fermentations with or without electrical reducing power (7, 15) decreased hydrogen production and stimulated solvent production. It was suggested that NR served as an electron carrier, replacing ferredoxin, and that reduced NR served as an electron donor for NAD(P) reduction that coupled to solvent production (8, 15). We have shown here that NR is an electronophore and that it functions by enabling microbes to use electricity for direct chemical reduction of NAD and for membrane-linked translocation of protons and transfer of electrons. This has important potential applications for enhancing the yield and rate of many fermentations (e.g., ethanol, propionate, succinate, glutamate, and citrate) and for enhancing reductive microbial transformation processes, such as dechlorination of aromatic compounds or desulfurization of oil.
ACKNOWLEDGMENT
This research was supported by U.S. Department of Energy Grant DE-F602-93ER20108.
REFERENCES
- 1.Cecchini G, Seces H, Schröder I, Gunsalus R P. Aerobic inactivation of fumarate reductase from Escherichia coli by mutation of the [3Fe-4S]-quinone binding domain. J Bacteriol. 1995;177:4587–4592. doi: 10.1128/jb.177.16.4587-4592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cecchini G, Thompson C R, Ackrell B A, Westenberg D J, Dean N, Gunsalus R P. Oxidation of reduced menaquinone by the fumarate reductase complex in Escherichia coli requires the hydrophobic FrdD peptide. Proc Natl Acad Sci USA. 1986;83:8898–8902. doi: 10.1073/pnas.83.23.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickie P, Weiner J H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979;57:813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- 4.Emde R, Schink B. Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Appl Environ Microbiol. 1990;56:2771–2776. doi: 10.1128/aem.56.9.2771-2776.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewart, G., and G. D. Smith. Purification and properties of soluble hydrogenase from the cyanobacterium Anabaena cylindrical. Arch. Biochem. Biophys. 268:327–337. [DOI] [PubMed]
- 6.Fitz R M, Cypionka H. A study on electron transport-driven proton translocation in Desulfovibrio desulfuricans. Arch Microbiol. 1989;152:369–376. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh B K, Zeikus J G. Abstracts of Papers, 194th ACS National Meeting. American Chemical Society; 1987. Electroenergization for control of H2 transformation in acetone butanol fermentations, abstr. 79. [Google Scholar]
- 8.Girbal L, Vasconcelos I, Silvie Saint A, Soucaille P. How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol Rev. 1995;16:151–162. [Google Scholar]
- 9.Guerrant G O, Lambert M A, Moss C W. Analysis of short chain acid from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol. 1982;16:355–360. doi: 10.1128/jcm.16.2.355-360.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guettler, M. V., M. K. Jain, and B. K. Soni. April 1996. U.S. patent 5,504,004.
- 11.Hongo M, Iwahara M. Application of electro-energizing method to L-glutamic acid fermentation. Agric Biol Chem. 1979;43A:2075–2081. [Google Scholar]
- 12.Hongo M, Iwahara M. Determination of electro-energizing conditions for L-glutamic acid fermentation. Agric Biol Chem. 1979;43B:2083–2086. [Google Scholar]
- 13.James E W, Kell D B, Lovitt R W, Morris J G. Electrosynthesis and electroanalysis using Clostridium sporogenes. Electrochem Bioenerg. 1988;20:21–32. [Google Scholar]
- 14.Kemner J M, Zeikus J G. Purification and characterization of membrane-bound hydrogenase from Methanosarcina barkeri MS. Arch Microbiol. 1994;161:47–54. [Google Scholar]
- 15.Kim B H, Zeikus J G. Hydrogen metabolism in Clostridium acetobutylicum fermentation. J Microbiol Biotechnol. 1992;2:2771–2776. [Google Scholar]
- 16.Kim T S, Kim B H. Electron flow shift in Clostridium acetobutylicum fermentation by electrochemically introduced reducing equivalent. Biotechnol Lett. 1988;10:123–128. [Google Scholar]
- 17.Kim Y, Ikebukuro K, Muguruma H, Karube I. Photogeneration of NADPH by oligothiophenes coupled with ferredoxin-NADP reductase. J Biotechnol. 1998;59:213–220. [Google Scholar]
- 18.Kortlüke C, Friedrich B. Maturation of membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6290–6293. doi: 10.1128/jb.174.19.6290-6293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Körtner C, Lauterbach F, Tripier D, Unden G, Kröger A. Wolinella succinogenes fumarate reductase contains a dihaem cytochrome b. Mol Microbiol. 1990;4:855–860. doi: 10.1111/j.1365-2958.1990.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 20.Kröger A, Geisler V, Lemma E, Theis F, Lenger R. Bacterial fumarate respiration. Arch Microbiol. 1992;158:311–314. [Google Scholar]
- 21.Lemire B D, Robinson J J, Weiner J H. Identification of membrane anchor polypeptides of Escherichia coli fumarate reductase. J Bacteriol. 1982;152:1126–1131. doi: 10.1128/jb.152.3.1126-1131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemire D L, Robinson J J, Bradley R D, Scraba D G, Weiner J H. Structure of fumarate reductase on the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1983;155:391–397. doi: 10.1128/jb.155.1.391-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lissolo T, Pulvin S, Thomas D. Reactivation of the hydrogenase from Delsulfovibrio gigas by hydrogen. J Biol Chem. 1984;259:11725–11729. [PubMed] [Google Scholar]
- 24.Miyawaki O, Yano T. Electrochemical bioreactor with regeneration of NAD+ by rotating graphite disk electrode with PMS absorbed. Enzyme Microb Technol. 1992;14:474–478. doi: 10.1016/0141-0229(92)90140-j. [DOI] [PubMed] [Google Scholar]
- 25.Moreno C, Costa C, Moura I, LeGall J, Liu M Y, Payne W J, Van Portugal C, Moura J J G. Electrochemical studies of the hexaheme nitrite reductase from Desulfovibrio desulfuricans ATCC 27774. Eur J Biochem. 1993;212:79–86. doi: 10.1111/j.1432-1033.1993.tb17635.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakasono S, Matsumoto N, Saiki H. Electrochemical cultivation of Thiobacillus ferrooxidans by potential control. Bioelectrochem Bioenerg. 1997;43:61–66. [Google Scholar]
- 27.Pequin S, Delorme P, Goma G, Soucaille P. Enhanced alcohol yields in batch cultures of Clostridium using a three-electrode potentiometric system with methyl viologen as electron carrier. Biotechnol Lett. 1994;16:269–274. [Google Scholar]
- 28.Petrov R R, Utkin I B, Munilla R, Fernández V M, Popov V O. Effect of redox potential on the catalytic properties of the NAD-dependent hydrogenase from Alcaligenes eutrophus. Arch Biochem Biophys. 1989;268:306–313. doi: 10.1016/0003-9861(89)90592-4. [DOI] [PubMed] [Google Scholar]
- 29.Robinson J J, Weiner J H. Molecular properties of fumarate reductase isolated from the cytoplasmic membrane of Escherichia coli. Can J Biochem. 1982;60:811–816. doi: 10.1139/o82-101. [DOI] [PubMed] [Google Scholar]
- 30.Rohde M, Fürstenau U, Mayer F, Przybyla A E, Peck H D, Jr, LeGall J, Choi E S. Localization of membrane-associated (NiFe) and (NiFeSe) hydrogenases of Desulfovibrio vulgaris using immunoelectron microscopic procedures. Eur J Biochem. 1990;191:389–396. doi: 10.1111/j.1432-1033.1990.tb19134.x. [DOI] [PubMed] [Google Scholar]
- 31.Samuelov N, Lamed R, Lowe S, Zeikus J G. Influence of CO2-HCO3 levels and pH on growth, succinate production and enzyme activities of Anaerobiospirillum succiniciproducens. Appl Environ Microbiol. 1991;57:3013–3019. doi: 10.1128/aem.57.10.3013-3019.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelov N S, Datta R, Jain M K, Zeikus J G. Microbial decarboxylation of succinate to propionate: kinetic studies. Ann N Y Acad Sci. 1990;589:697–704. [Google Scholar]
- 33.Sánchez S, Arratia A, Córdova R, Gómez H, Schrebler R. Electron transport in biological processes. II. Electrochemical behavior of Q10 immersed in a phospholipidic matrix added on a pyrolytic graphite electrode. Bioelectrochem Bioenerg. 1995;36:67–71. [Google Scholar]
- 34.Schlereth D D, Fernández V M. Direct electron transfer between Alcaligenes eutrophus Z-1 hydrogenase and glassy carbon electrodes. Bioelectrochem Bioenerg. 1992;28:473–482. [Google Scholar]
- 35.Schneider K, Cammack R, Schlegel G. Content and localization of FMN, Fe-S clusters and nickel in the NAD-linked hydrogenase of Nocardia opaca 1b. Eur J Biochem. 1984;142:75–84. doi: 10.1111/j.1432-1033.1984.tb08252.x. [DOI] [PubMed] [Google Scholar]
- 36.Sucheta A, Cammack R, Weiner J H, Armstrong F A. Reversible electrochemistry of fumarate reductase immobilized on an electrode surface. Direct voltammetric observation of redox centers and their participation in rapid catalytic electron transport. Biochemistry. 1993;32:5455–5465. doi: 10.1021/bi00071a023. [DOI] [PubMed] [Google Scholar]
- 37.Surya A, Murthy N, Anita S. Tetracyanoquinodimethane (TCNQ) modified electrode for NADH oxidation. Bioelectrochem Bioenerg. 1994;33:71–73. [Google Scholar]
- 38.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1997;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Werf M J, Guettler M V, Jain M K, Zeikus J G. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentations by Actinobacillus sp. 130Z. Arch Microbiol. 1997;167:332–342. doi: 10.1007/s002030050452. [DOI] [PubMed] [Google Scholar]
- 40.Van Hellemond J J, Tielens A G M. Expression and functional properties of fumarate reductase. Biochem J. 1994;304:321–331. doi: 10.1042/bj3040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westenberg D J, Gunsalus R P, Ackrell B A, Cecchini G. Electron transfer from menaquinol to fumarate. J Biol Chem. 1990;265:19560–19567. [PubMed] [Google Scholar]
- 42.White H, Lebertz H, Thanos I, Simon H. Clostridium thermoaceticum production of methanol from carbon monoxide in the presence of viologen dyes. FEMS Microbiol Lett. 1987;43:173–176. [Google Scholar]
- 43.Willner I, Katz E, Lapidot N. Bioelectrocatalysed reduction of nitrate utilizing polythiophene bipyridium enzyme electrodes. Bioelectrochem Bioenerg. 1992;29:29–45. [Google Scholar]
- 44.Wissenbach U, Kröger A, Unden G. The specific functions of menaquinone and demethylmenaquinone in anaerobic respiration with fumarate, dimethylsulfoxide, trimethylamine N-oxide and nitrate by Escherichia coli. Arch Microbiol. 1990;154:60–66. doi: 10.1007/BF00249179. [DOI] [PubMed] [Google Scholar]
- 45.Wissenbach U, Thernes D, Unden G. An Escherichia coli mutant containing only demethylmenaquinone, but not menaquinone: effects on fumarate, dimethylsulfoxide, trimethylamine N-oxide and nitrate respiration. Arch Microbiol. 1992;158:68–73. doi: 10.1007/BF00249068. [DOI] [PubMed] [Google Scholar]
- 46.Wu L F, Mandrand M A. Microbial hydrogenase: primary structure, classification, signature and phylogeny. FEMS Microbiol Rev. 1993;104:243–270. doi: 10.1111/j.1574-6968.1993.tb05870.x. [DOI] [PubMed] [Google Scholar]
- 47.Xie Y, Dong S. Effect of pH on the electron transfer of cytochrome c on a gold electrode modified with bis (4-pyridyl) disulphide. Bioelectrochem Bioenerg. 1992;29:71–79. [Google Scholar]