Abstract
Background
Data concerning the comparison between transcatheter aortic valve implantation and surgical aortic valve replacement in a real-world setting are scarce and in Central and Eastern Europe no such data exist. In this study, we aimed at analyzing retrospectively the characteristics and outcome of patients with aortic stenosis treated either with surgical aortic valve replacement or transcatheter aortic valve implantation between 2006 and 2016 in the Silesian Province, Poland in a representative real-world cohort.
Methods
In the Silesian Cardiovascular Database we retrospectively identified 5186 patients who received either transcatheter aortic valve implantation or surgical aortic valve replacement in 1 of 3 tertiary cardiovascular centers. Baseline characteristics, including relevant clinical history, and outcomes were compared before and after propensity-score matching of both groups, with 348 pairs of patients constituting the propensity-matched study cohort. The primary end-point was 24-month all-cause mortality.
Results
Preoperative characteristics of propensity-matched groups were similar. There was no difference between transcatheter aortic valve implantation and surgical aortic valve replacement groups with respect to the death rate at 2 years (19.9% vs. 15.6%; P = .479). In the transcatheter aortic valve implantation group, cardiac resynchronization therapy devices were more frequently implanted after the procedure (3.7% vs. 0.0, P < .001). The groups had similar rates of myocardial infarction, stroke, and re-hospitalization. Hospital stay in the matched groups was shorter after transcatheter aortic valve implantation: 14.1 versus 15.7 days (P < .001).
Conclusions
At 24 months, transcatheter aortic valve implantation patients had similar outcomes as surgical aortic valve replacement except for a higher rate of cardiac resynchronization therapy device implantation and shorter hospital stay.
Keywords: Aortic stenosis, transcatheter aortic valve implantation, TAVI, surgical aortic valve replacement, SAVR
HIGHLIGHTS
Of 4747 patients who received SAVR and 439 who received transcatheter aortic valve implantation (TAVI) in 3 tertiary centers across a 10-year period, after propensity-score matching, there were 348 pairs who constituted the study cohort
At 24 months, the risk of death, myocardial infarction, or stroke was similar in both groups
In the TAVI group, hospital stay was significantly shorter, although cardiac resynchronization therapy devices were more frequently implanted after the procedure
Introduction
Transcatheter aortic valve implantation (TAVI) proved to be a viable alternative for patients turned down from surgical aortic valve replacement (SAVR) due to high operative risk.1-3 According to the guidelines published by the European Society of Cardiology and Association for Cardio-Thoracic Surgery, SAVR continues to remain the first choice in severe aortic stenosis (AS) and low-risk patients, those who are <75 years old or asymptomatic but require other heart surgery.4 Transcatheter aortic valve implantation, in turn, is recommended in patients who are not suitable for SAVR as assessed by the Heart Team, hence it is often favored in elderly frail patients, with multiple comorbidities and a history of coronary artery bypass graft surgery (CABG) with patent grafts.4,5 The results of the recently published SURTAVI randomized trial comparing SAVR with TAVI in intermediate-risk patients justify the wider use of TAVI in this subpopulation of AS patients.6
In reality, the groups assigned to valve intervention (SAVR or TAVI) differ substantially with regard to age, clinical profile, and in consequence—operative risk. Despite the growing number of TAVI procedures performed worldwide, data concerning the comparison of TAVI and SAVR outcomes in the real world are scarce. In Central and Eastern Europe, there are no such published data. Therefore, we devised a methodology to compare these 2 invasive treatment modalities of severe AS among the adult population treated in 3 large tertiary cardiology and cardiac surgery centers in the Silesian Province—the only centers performing both SAVR and TAVI.
Methods
The study population was obtained from the Silesian Cardiovascular Database (SILCARD) database comprising all patients admitted and treated due to severe AS in the period from 2006 to 2016. The SILCARD database and applied methodology were described elsewhere.7,8 In brief, the database was created upon agreement between the Silesian Center for Heart Diseases in Zabrze and the Regional Department of National Health Fund in Katowice to perform a comprehensive and complex analysis of all registered patients with cardiovascular diseases in the Upper Silesian Province. The region is the most highly urbanized in the country and is inhabited by approximately 4 500 000 inhabitants.
All patients were enrolled in the study based on the identification codes according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) assigned to the patient at the time of the first hospitalization at any department of cardiology, cardiac surgery, diabetology, vascular surgery, department of internal medicine, or intensive care. The relevant ICD-10 codes pertaining to AS were as follows: I06.0, I06.2, I35.0, and I35.2. Other ICD-10 codes of concomitant cardiovascular diseases for patients with AS were collected and are summarized in Table 1. Tracking the patients with a unique personal identification number (PESEL) and international classification system for surgical, diagnostic, and therapeutic procedures (ICD-9) codes we identified patients with AS who received TAVI or SAVR. Following the patients after the index procedure, applying the same methodology we collected data concerning adverse events, including cardiovascular procedures.
Table 1.
The ICD-10 Codes Assigned to Individual Cardiovascular Diseases
| Disease | ICD-10 Code |
|---|---|
| Heart failure | I50, I51.5, I51.7, J81, R57, I42, I43 |
| Chronic coronary syndrome | I25, I20.1, I20.8, I20.9 |
| Unstable angina | I20.0, I24.0, I24.8, I24.9 |
| Myocardial infarction | I21, I22 |
| Atrial fibrillation | I48 |
| Arterial hypertension | I10, I11, I12, I13, I15 |
| Pulmonary embolism | I26 |
| Infective endocarditis | I33, I38, I39 |
| Grown-up congenital heart disease | Q20-Q28 |
| Valvular heart diseases without aortic stenosis | I05, I06.1, I06.8, I06.9, I07, I08, I34, I35.1, I35.8, I35.9, I36, I37 |
The primary end-point of the study was 24-month all-cause mortality, while the secondary end-points recorded in 24-month follow-up were: length of hospital stay during the index procedure, permanent pacemaker implantation, re-hospitalization due to heart failure, stroke, myocardial infarction, coronary revascularization procedure, and dialysis.
Statistical Analysis
Mortality and repeated hospitalizations in a 24-month follow-up were analyzed according to the first hospitalization of the given patient treated with TAVI or SAVR. Other calculations were performed according to the analysis of all hospitalizations. Descriptive statistics were applied. Compilations were generated directly from the Oracle database using the SQL Developer tool. An Excel spreadsheet was used for graphic data development. The normality of the distribution of continuous variables was verified using the Shapiro–Wilk test.
Continuous variables were compared using the one-way analysis of variance. The differences in the number of patients and mortality over the years were verified using the χ2 test for trend in proportions. Mortality data were adjusted for age (per 10-year increment) and sex using the entire SILCARD population as a reference. A 2-sided P value of less than .05 was considered statistically significant. Because of the significant differences in baseline characteristics and risk factors between patients undergoing TAVI and SAVR, an analytic sample was created using propensity-score-based matching. A logistic regression based on demographic and risk factors was used to generate a propensity score for undergoing TAVI or SAVR for each patient. Propensity-score matching was conducted in a 1 : 1 ratio, by greedy matching, using a caliper of 0.20 SDs in the linear predictor. The multiple analysis was performed with the use of logistic regression, and the results are expressed as odds ratios and 95% CIs. For the comparison of the estimated occurrence of the analyzed outcomes, the Kaplan–Meier method with log-rank test for all patients was performed, before and after matching. All investigated parameters are presented in Supplementary Table.
Results
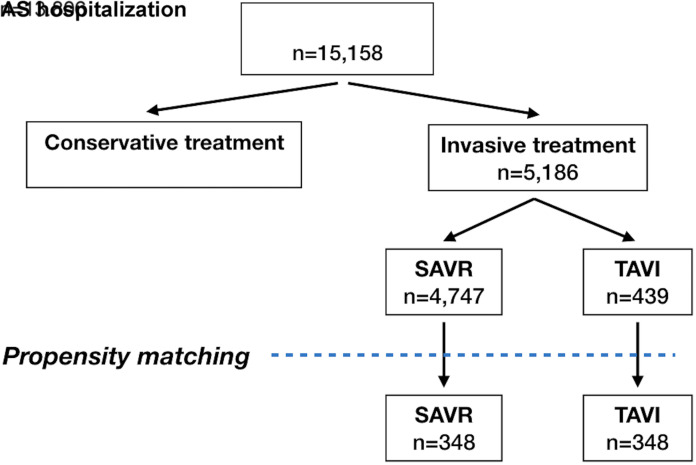
During the analyzed period, the number of patients with AS treated invasively was steadily increasing, mostly due to TAVI procedures, which were initiated in Poland in 2008, whereas the numbers of SAVR remained stable since 2012. Nevertheless, the proportion of SAVR continuously prevailed over TAVI (Figure 1). Out of 15 158 patients hospitalized with the diagnosis of AS over the analyzed period, 5186 (34%) received invasive treatment. The remaining 9972 were assigned to conservative treatment and/or further observation. A flow chart of the treatment strategies is shown in Figure 2.
Figure 1.
Invasive treatment of AS over decade: 2006-2016. AS, aortic stenosis; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Figure 2.
Flow chart of treatment of patients with AS in the years 2006-2016. AS, aortic stenosis; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
The clinical characteristics of both groups before and after matching are presented in Table 2. The TAVI group proved to be much older (over 12 years mean) with numerous comorbidities. Moreover, before the index procedure, they had experienced many more cardiovascular interventions than their counterparts from the SAVR group (Table 3). After the invasive treatment, proportions of percutaneous coronary intervention (PCI), CABG valve surgery, a pacemaker, or ICD implantation were similar except for cardiac resynchronization therapy (CRT), which was more often employed in the TAVI group, both before and after matching (Table 4). In-hospital mortality of patients treated with TAVI was significantly higher than after SAVR before matching (7.1% vs. 4.2%, P = .008) but this difference did not remain significant after matching (6.6% vs. 9.2%, P = .261). The hospital stay was shorter in the TAVI group before matching: 13.8 ± 12.9 vs. 15.6 ± 13.3 days (P < .001) and the difference was still significant in propensity-matched comparison: 14.1±13.1% versus 15.7 ± 10.6 days (P < .001).
Table 2.
Baseline Clinical Characteristics of Analyzed Groups Before (Left Panel) and After Propensity-Matching (Right Panel)
| Variable |
Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| SAVR n = 4747 |
TAVI n = 439 |
P | SAVR n = 348 |
TAVI n = 348 |
P | |
| Age, years, ±SD | 66.5 ± 10.7 | 78.6 ± 7.2 | <.001 | 76.7 ± 6.0 | 77.1 ± 7.2 | .338 |
| Prior myocarditis | 3.4% | 4.1% | .554 | 3.7% | 3.7% | .842 |
| Prior MI | 10.6% | 21.4% | <.001 | 19.5% | 19.3% | 1.000 |
| CCS | 80.7% | 95.0% | <.001 | 94.3% | 94.0% | 1.000 |
| Heart failure | 29.9% | 65.2% | <.001 | 62.1% | 58.9% | .438 |
| Hypertension | 79.0% | 94.5% | <.001 | 93.4% | 93.1% | 1.000 |
| Diabetes | 28.3% | 49.0% | <.001 | 50.6% | 46.6% | .324 |
| Hypercholesterolemia | 21.9% | 28.9% | .001 | 28.7% | 26.4% | .553 |
| Atrial fibrillation | 12.6% | 30.5% | <.001 | 27.6% | 28.5% | .866 |
| Prior pulmonary edema | 2.6% | 6.6% | <.001 | 4.3% | 4.9% | .856 |
| COPD | 10.9% | 24.2% | <.001 | 23.0% | 22.4% | .928 |
| Chronic kidney disease | 3.4% | 9.6% | <.001 | 7.8% | 7.8% | .887 |
| Cancer | 23.1% | 38.3% | <.001 | 39.7% | 35.6% | .309 |
| Prior stroke | 4.4% | 13.4% | <.001 | 10.6% | 9.5% | .705 |
CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVI, transcatheter aortic valve implantation.
Table 3.
History of Cardiovascular Interventions in Analyzed Groups Before (Left Panel) and After Propensity-Matching (Right Panel)
| Prior Intervention |
Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| SAVI n = 4747 |
TAVI n = 439 |
P | SAVR n = 348 |
TAVI n = 348 |
P | |
| Prior PCI | 10.6% | 42.1% | <.001 | 20.1% | 41.7% | <.001 |
| Prior CABG | 0.4% | 10.5% | <.001 | 1.4% | 11.5% | <.001 |
| Prior valve surgery | 0.9% | 3.9% | <.001 | 4.0% | 3.2% | .684 |
| Prior PPM | 2.6% | 12.8% | <.001 | 9.5% | 8.1% | .592 |
| Prior ICD | 0.3% | 1.4% | .005 | 2.3% | 1.2% | .382 |
| Prior CRT | 0.0% | 0.9% | <.001 | 0.3% | 0.9% | .616 |
| Prior ablation | 0.1% | 0.0% | .991 | 0.3% | 0.0% | 1.000 |
| Dialysis | 0.9% | 0.9% | .801 | 0.6% | 1.2% | .682 |
CABG, coronary artery bypass graft surgery; CRT, cardiac resynchronization therapy; ICD, Implantable cardioverter-defibrillator; PCI, percutaneous coronary intervention, PPM, permanent pacemaker; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 4.
Interventions After Invasive Treatment (SAVR or TAVI) Before (left panel) and After Propensity-Matching (Right Panel)
| Intervention |
Unmatched | Matched | ||||
|---|---|---|---|---|---|---|
| SAVR n = 4747 |
TAVI n = 439 |
P | SAVR n = 348 |
TAVI n = 348 |
P | |
| PCI | 4.6% | 3.0% | .138 | 2.9% | 2.9% | .821 |
| CABG | 0.2% | 0.0% | .900 | 0.0% | 0.0% | - |
| Valve surgery | 1.3% | 0.9% | .605 | 0.3% | 0.9% | .616 |
| PPM | 5.0% | 4.8% | .953 | 3.2% | 4.3% | .549 |
| ICD | 0.6% | 0.7% | .854 | 0.6% | 0.6% | .616 |
| CRT | 0.6% | 3.2% | <.001 | 0.0% | 3.7% | <.001 |
| Ablation | 0.3% | 0.0% | .592 | 0.0% | 0.0% | - |
| Dialysis | 1.8% | 1.1% | .435 | 0.9% | 1.4% | .722 |
CABG, coronary artery bypass graft surgery; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; PCI, percutaneous coronary intervention, PPM, permanent pacemaker, SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
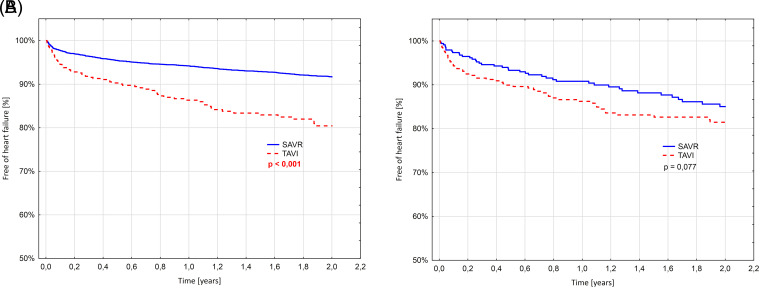
The Kaplan–Meier curves representing the estimates of freedom from all-cause mortality through 24 months are presented in Figure 3. The 24-month survival in the propensity-matched cohort was similar between the groups, which is contrary to the unmatched group revealing significantly higher mortality in the TAVI group (9.3% vs. 22.1%; P < .001). Stroke and myocardial infarction rates were similar at 24-month follow-up in both groups (Figures 4, 5). Interestingly, re-hospitalization rates were much more frequent in unmatched patients in the TAVI group than in SAVR (19.1% vs. 8.3%, P < .01), whereas after propensity-matching the difference was no more statistically significant. However, a trend toward a higher re-hospitalization rate was observed in the TAVI group (18.5% vs. 15.0% P < .077) (Figure 6). In the multiple analysis, the type of intervention for AS was not independently associated with a higher risk of death at 24 months, while implantation of ICD or CRT before the procedure, chronic kidney disease requiring dialysis, and PCI during the index hospitalization were most strongly associated with 24-month mortality (Table 5).
Figure 3.
Kaplan–Meier curves representing freedom from all-cause mortality. (A) Before propensity-matching, (B) Matched groups. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Figure 4.
Kaplan–Meier curves representing freedom from myocardial infarction. (A) Before propensity-matching, (B) Matched group. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Figure 5.
Kaplan–Meier curves representing freedom from stroke. (A) Before propensity-matching, (B) Matched groups. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Figure 6.
Kaplan–Meier curves representing freedom from re-hospitalization due to heart failure. (A) Before propensity-matching, (B) Matched groups. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 5.
Multiple Analysis of Independent Predictors of 24-Month Mortality
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Prior chronic coronary syndrome | 0.70 | 0.54-0.92 | .010 |
| Age (increasing per 5 years) | 1.19 | 1.13-1.26 | <.001 |
| Prior atrial fibrillation | 1.28 | 1.00-1.63 | .050 |
| Prior chronic kidney disease | 1.60 | 1.05-2.45 | .029 |
| Coronary revascularization before or during the index hospitalization | 1.61 | 1.32-1.96 | <.001 |
| Prior heart failure | 1.88 | 1.54-2.29 | <.001 |
| Percutaneous coronary intervention during the index hospitalization | 2.44 | 1.17-5.06 | .017 |
| Prior dialyses | 3.02 | 1.40-6.50 | .005 |
| Implantation of ICD or CRT | 3.10 | 1.60-6.00 | .001 |
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; OR, odds ratio.
Discussion
Since the early 60s of the 20th century, SAVR remains a treatment of choice for the majority of patients with severe symptomatic AS. However, the advent of TAVI in April 2002 revolutionized the contemporary approach to the treatment strategies of patients with severe AS.9 Multiple trials were performed so far to compare these 2 definitive treatment modalities of degenerative AS. Already historical randomized PARTNER trial proved that transcatheter valve implantation was not inferior to surgical replacement in terms of 1-year survival in high-risk patients (24.2% vs. 26.8%; P = .44).1 This result was maintained in a 2-year follow-up (33.9% in the TAVI group and 35.0% in the surgery group; P = .78).10 In an intermediate—risk cohort in PARTNER 2A trial, TAVI was similar to SAVR with respect to death or disabling stroke for up to 2 years.11 In the SURTAVI randomized trial, which also comprised intermediate-risk patients, TAVI proved to be non-inferior to SAVR with respect to death from any cause or disabling stroke at 24 months.6 However, for many reasons, the results from randomized trials usually do not reflect, the “real-world” population.
In our registry, we analyzed all patients with AS treated with TAVI or SAVR in 3 large tertiary cardiovascular centers. As expected, baseline characteristics revealed that the TAVI group was much older and had many more comorbidities and risk factors than the SAVR group. Therefore, only 348 out of 4747 (7.3%) SAVR patients could be matched with patients from the TAVI group, from which 348 out of 439 (79%) were sampled. This reflects the referral process in our centers over the period of more than 10 years: High-risk, patients burdened with multiple comorbidities were assigned to TAVI, whereas only a small portion of high-risk patients was qualified for SAVR. Nonetheless, the 2-year survival rates were similar in the propensity-matched groups: 19.1% versus 15.6% (P = .479) in TAVI and SAVR groups, respectively (Supplementary Figure). Moreover, in the multiple analysis, TAVR or SAVR were not independent predictors of 24-month mortality.
The only significant difference in the outcomes between the analyzed groups was observed in the frequency of CRT device implantations, which occurred more frequently in patients treated with TAVI than with SAVR (3.7% vs. 0.0%, P < .001). Despite the recent publication of consensus papers regarding the necessity for permanent pacing after TAVI, the treatment schemes still very often reflect the experiences and discretions of individual centers.12,13 In our study, the higher prevalence of CRT implantations after TAVI most probably reflects the scenario of complete AV block development after TAVI, which results in the high anticipated percentage of ventricular pacing in the long-term prognosis. Hence, in order to maintain synchronous ventricular contractions, and protect the patient from developing pacing-induced heart failure after right ventricular pacing, the patient could have been offered a CRT.
In a large cohort of 9,464 propensity-matched intermediate- and high-risk patients Brennan et al. reported 17.3% versus 17.9% (P = .5) 1-year death rate in TAVI and SAVR groups, respectively.14 In a 2-year follow-up in analyzed propensity-matched groups, we observed no differences with regard to survival (Figure 3B). Schymik et al. demonstrated a similar probability of survival up to 3 years after TAVI or SAVR in patients with severe symptomatic AS and less than high risk.15 However, in low-risk patients, 3-year follow-up proved to be unfavorable for TAVI. According to Rosato et al. at 3 years, survival was 72.0% after TAVI and 83.4% after SAVR (P = .0015), whereas freedom from major adverse cardiac and cerebrovascular events was 67.3% after TAVI and 80.9% after SAVR (P < .001).16
In a comprehensive meta-analysis of randomized trials and propensity-score matched observational studies, Witberg et al. observed that TAVI is equivalent to SAVR in the low-risk population but only with regard to short-term mortality. At the same time, the authors conclude that TAVI may not be equivalent to SAVR in terms of mortality beyond the short term.17 Interestingly in another large meta-analysis comprising 19 observational studies with a propensity-score analysis of 6,234 patients, the authors found that TAVI was likely to be associated with a 21% increase in the hazard of follow-up all-cause mortality relative to SAVR. The arithmetic mean of 3-year survival rates was 71.3% after TAVI and 77.9% compared to SAVR (HR = 1.21; 95% CI, 1.05-1.39; P = .010).18 Quite recently, the results of 2 large randomized controlled trials comparing TAVI with SAVR in the low-risk groups of patients were published.19,20 Both trials revealed that TAVI is at least non-inferior to SAVR in terms of efficacy and safety in low-risk patients with severe AS, whereas in the PARTNER 3 trial, treatment with TAVI was superior to SAVR in the 12-month follow-up.
Study Limitations
There are several limitations of the study derived from its nature. First, our methodology based on the ICD-10 code forbids us from providing the detailed clinical characteristics comprising various descriptive features such as left ventricular ejection fraction, severity of angina, the duration of QRS, or presence of conduction disturbances before and after the procedure, etc. For the same reason, other continuous variables could not be presented and analyzed. Second, surgical procedures in our region were performed in 3 high-volume academic centers with large experience and established high-quality care. At the same time, the TAVI program in these centers was initiated only in 2008, and due to a small number of the procedures performed in the early years, the learning curve was extended. Nonetheless, it should be noted that the outcomes among patients treated with TAVI did not differ significantly between patients treated in the years 2008-2014 and 2015-2016, as demonstrated in Supplementary Figure 2.
Third, in our analysis, we were not able to establish the operative risk in both cohorts. We can only speculate that after matching the patients were overall of high or, in minority, intermediate-risk because of reimbursement policy at that time. It is based on assumption that in the Silesian Province over the analyzed period of time, the majority of TAVI procedures were performed after deferral from surgery due to a high operative risk or at a much lower rate due to the presence of other factors not comprised in the risk scores (e.g., porcelain aorta, chest deformation, and others). Finally, although it has been previously demonstrated that the short- and mid-term outcomes favor the transfemoral TAVI over the non-transfemoral approach, the administrative data source, on the basis of which our analysis was performed, did not allow to differentiate the access route utilized to perform TAVI procedures.
Conclusions
Our study demonstrated similar in-hospital and 24-month mortality in the propensity-matched comparison of TAVI and SAVR patients who were considered a high-risk population. Moreover, at 24 months, the risk of myocardial infarction, or stroke was similar in patients treated with TAVI and SAVR. The hospital stay of patients was shorter after TAVI regardless of the propensity matching. TAVI and SAVR were not independent predictors of death due to any cause at 24-months. For the lower-risk population of patients with AS such an observational comparison study needs to be performed to provide relevant data.
Declaration of Interests
None declared.
Supplementary Figure 1.
All-cause in-hospital and 24-month mortality in patients treated with TAVI or SAVR. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Supplementary Figure 2.
Kaplan–Meier curves representing survival estimates in patients treated in the years 2008-2014 and 2015-2016.
Supplementary Table.
All Variables Included in the Multiple Analysis
| Variables |
|---|
| Age Aortic stenosis treatment modality (TAVI/SAVR) Aortic valve treatment before the procedure Revascularization after AS treatment Implantation of ICD/CRT before or during index hospitalization Gender PCI before or during index hospitalization CABG before or during index hospitalization Prior myocarditis Prior myocardial infarction Prior chronic coronary syndrome Prior heart failure Prior pulmonary edema Prior cardiomyopathy Prior hypertension Prior diabetes Prior hypercholesterolemia Prior atrial fibrillation Prior non-AF arrhythmias Prior COPD Prior asthma Prior pulmonary airway infection in less than 30 days before AS treatment Prior pneumonia Prior chronic kidney disease Prior malignancies Prior stroke Prior hyperthyroidism Prior pulmonary embolism Prior dementia Prior viral hepatitis type B or C Prior PCI Prior CABG Prior permanent pacemaker implantation Prior ICD implantation Prior CRT implantation Chronic kidney disease requiring dialyses Prior large abdominal surgeries (such as cesarean section) |
AS, aortic stenosis; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; PCI, percutaneous coronary intervention, PPM, permanent pacemaker, SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Footnotes
Ethics Committee Approval: The study was approved by the institutional review board and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent: Due to the retrospective design of the study, no additional patient consent was required.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – K.W, M.H, M.G; Design – K.W, M.G.; Supervision – Z.K, M.G; Funding – None; Materials – K.W, D.C, M.G; Data Collection and/or Processing – D.C.; Analysis and/or Interpretation – K.W, P.C., M.G.; Literature Review – K.W, P.C., M.D.; Writing – K.W, M.D.; Critical Review –W.W., P.B., A.B., M.D., Z.K., M.G., M.Z.
Funding: This study received no funding.
References
- 1. Smith CR, Leon MB, Mack MJ.et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187 2198. 10.1056/NEJMoa1103510) [DOI] [PubMed] [Google Scholar]
- 2. Makkar RR, Fontana GP, Jilaihawi H.et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696 1704. 10.1056/NEJMoa1202277) [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M.et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597 1607. 10.1056/NEJMoa1008232) [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, Falk V, Bax JJ.et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739 2791. 10.1093/eurheartj/ehx391) [DOI] [PubMed] [Google Scholar]
- 5. Falk V, Baumgartner H, Bax JJ.et al. Corrigendum to '2017 ESC/EACTS guidelines for the management of valvular heart disease’ [Eur J Cardiothorac Surg 2017;52:616-664]. Eur J Cardiothorac Surg. 2017;52(4):832. 10.1093/ejcts/ezx363) [DOI] [PubMed] [Google Scholar]
- 6. Reardon MJ, Van Mieghem NM, Popma JJ. Surgical or transcatheter aortic-valve replacement. N Engl J Med. 2017;377(2):197 198. 10.1056/NEJMc1706234) [DOI] [PubMed] [Google Scholar]
- 7. Gąsior M, Pres D, Wojakowski W.et al. Causes of hospitalization and prognosis in patients with cardiovascular diseases. Secular trends in the years 2006-2014 according to the Silesian cardiovascular (SILCARD) database. Pol Arch Med Wewn. 2016;126(10):754 762. 10.20452/pamw.3557) [DOI] [PubMed] [Google Scholar]
- 8. Roleder T, Hawranek M, Gąsior T.et al. Trends in diagnosis and treatment of aortic stenosis in the years 2006-2016 according to the SILCARD registry. Pol Arch Intern Med. 2018;128(12):739 745. 10.20452/pamw.4352) [DOI] [PubMed] [Google Scholar]
- 9. Cribier A, Eltchaninoff H, Bash A.et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006 3008. 10.1161/01.cir.0000047200.36165.b8) [DOI] [PubMed] [Google Scholar]
- 10. Kodali SK, Williams MR, Smith CR.et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686 1695. 10.1056/NEJMoa1200384) [DOI] [PubMed] [Google Scholar]
- 11. Leon MB, Smith CR. Transcatheter aortic-valve replacement. N Engl J Med. 2016;375(7):700 701. 10.1056/NEJMc1606814) [DOI] [PubMed] [Google Scholar]
- 12. Rodés-Cabau J, Ellenbogen KA, Krahn AD.et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. 2019;74(8):1086 1106. 10.1016/j.jacc.2019.07.014) [DOI] [PubMed] [Google Scholar]
- 13. Lilly SM, Deshmukh AJ, Epstein AE, et al. 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76(20):2391 2411. 10.1016/j.jacc.2020.08.050) [DOI] [PubMed] [Google Scholar]
- 14. Brennan JM, Thomas L, Cohen DJ.et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70(4):439 450. 10.1016/j.jacc.2017.05.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schymik G, Heimeshoff M, Bramlage P.et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv. 2015;86(4):738 744. 10.1002/ccd.25866) [DOI] [PubMed] [Google Scholar]
- 16. Rosato S, Santini F, Barbanti M.et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. 2016;9(5):e003326. 10.1161/circinterventions.115.003326) [DOI] [PubMed] [Google Scholar]
- 17. Witberg G, Lador A, Yahav D, Kornowski R. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: a meta-analysis of randomized trials and propensity score matched observational studies. Catheter Cardiovasc Interv. 2018;92(2):408 416. 10.1002/ccd.27518) [DOI] [PubMed] [Google Scholar]
- 18. Takagi H, Umemoto T. ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Worse survival after transcatheter aortic valve implantation than surgical aortic valve replacement: a meta-analysis of observational studies with a propensity-score analysis. Int J Cardiol. 2016;220:320 327. 10.1016/j.ijcard.2016.06.261) [DOI] [PubMed] [Google Scholar]
- 19. Popma JJ, Deeb GM, Yakubov SJ.et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706 1715. 10.1056/NEJMoa1816885) [DOI] [PubMed] [Google Scholar]
- 20. Mack MJ, Leon MB, Thourani VH.et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695 1705. 10.1056/NEJMoa1814052) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a