Abstract
Background
No-reflow phenomenon after primary percutaneous coronary intervention is a common condition affecting the outcomes; therefore, studying its predictive factors is helpful in identifying patients at high risk. Our objective was to investigate the impact of the total ischemia time on no-reflow phenomenon and its correlation to thrombolysis in myocardial infarction flow grade after primary percutaneous coronary intervention.
Methods
This study was conducted on 545 patients with ST-elevation myocardial infarction who underwent PPCI; the patients were divided into two groups according to the incidence of no-reflow, TIMI flow ≤2 was considered no-reflow. The time interval from chest pain onset to balloon dilatation was assessed and correlated to thrombolysis in myocardial infarction flow grade.
Results
The incidence of no-reflow was 13.9%; thrombolysis in myocardial infarction flow ≤2 occurred in 76 patients. Multivariate regression analysis showed that advanced age >65 years, the total ischemia time ˃6 h, high thrombus burden, and cardiogenic shock were the independent predictors of no-reflow phenomenon. Spearman’s correlation analysis showed a significant negative correlation between the total ischemia time and thrombolysis in myocardial infarction flow grade (r = −351 and P-value = .001).
Conclusion
The time delay is the main limitation of achieving thrombolysis in myocardial infarction 3 flow after primary percutaneous coronary intervention. The total ischemia time has a significant negative correlation with thrombolysis in myocardial infarction flow grade after primary percutaneous coronary intervention.
Keywords: Impact, the total ischemia time, no-reflow phenomenon, ST-elevation myocardial infarction, primary percutaneous coronary intervention
Highlights
The total ischemia time >6 h signifies the worst impact on no-reflow phenomenon.
Advanced age >65 years, high thrombus burden, and cardiogenic shock were the independent predictors of no-reflow phenomenon.
There is a significant negative correlation between the total ischemia time and thrombolysis in myocardial infarction flow grade after primary percutaneous coronary intervention.
Introduction
Primary percutaneous coronary intervention (PPCI) is the most efficient and highly recommended method to restore antegrade blood flow rapidly in the occluded coronary artery after acute myocardial infarction (AMI), with reducing myocardial necrosis and improving overall survival.1,2 Despite the recent advances in PPCI, no-reflow phenomenon is still a big challenge to the interventionists during the procedure. Restoration of epicardial coronary perfusion in infarct-related artery is not necessarily followed by myocardial perfusion in its territory and this condition is defined as no-reflow phenomenon.3-5
A pathological classification of no-reflow was proposed: (i) structural no-reflow—microvessels within the necrotic myocardium exhibit (a) loss of capillary integrity with endothelial swelling and edema and (b) microvascular obstruction, lesion extension depends upon the severity and duration of ischemia (it is usually irreversible)—and (ii) functional no reflow—patency of microvasculature is compromised due to distal thrombo-embolization, spasm, ischemic injury, reperfusion injury, and accumulation of neutrophils and platelets with activation of neurohumoral system. It may be reversible to a varying degree.6-8
No-reflow phenomenon affects PPCI outcomes, patients with increased risk for developing left ventricular dysfunction, and progressive myocardial necrosis with worse clinical prognosis.3,9 After about 6 h of AMI, myocardial necrosis occurs, which leads to capillary bed edema, myocardial cell swelling, neutrophil plugging, alterations of capillary integrity, and microvascular dysfunction which contribute to no-reflow phenomenon.10,11 Therefore, avoiding this condition would improve the long-term prognosis of patients with AMI.6-9 In the current study, our aim was to investigate impact of the total ischemia time on no-reflow phenomenon and its correlation to thrombolysis in myocardial infarction (TIMI) flow grade after PPCI.
Methods
This is a cohort prospective study of patients with ST elevation myocardial infarction (STEMI), who underwent revascularization by PPCI in our cardiovascular department. The study was conducted on 545 patients with STEMI who were classified into two groups according to the incidence of no-reflow phenomenon: group I—patients with no-reflow (TIMI 0-2 flow) and group II—patients with normal flow (TIMI 3 flow). An informed consent was obtained from all patients in this research. Every patient in the study had a code number pointed to his name, address, and telephone number. The study was approved by the Local Ethical Committee and in accordance with the principles of declaration of Helsinki II. ST-elevation myocardial infarction was defined by the classic symptoms of typical chest pain, as well as by a 1-mm ST-segment elevation in inferior leads or 2-mm ST-segment elevation in the anterior chest leads occurring in two contiguous leads, or a new or presumably new left bundle branch block.12 Patients with non-STEMI and patients with STEMI who received thrombolysis or underwent coronary artery bypass grafting (CABG) or presented later after 24 h were excluded from the study.
All patients were subjected to full history taking emphasizing on the presence of diabetes mellitus, hypertension, dyslipidemia, and current smoking. History of prior myocardial infarction was interrogated and the onset of chest pain before admission was determined, then the total ischemia time (time interval from chest pain onset to balloon dilatation) was calculated. Full clinical examination and 12 leads surface ECG were done for all patients. Transthoracic echocardiography and routine laboratory investigations including random blood glucose, serum creatinine, and creatine kinase-myocardial band (CK-MB) were done for all the patients. On admission, patients received 4 chewable aspirin tablets 300 mg, clopidogrel 600 mg, or ticagrelor 180 mg, as well as unfractionated heparin intravenously. Primary percutaneous coronary intervention was done through trans-femoral or trans-radial artery approach according to operator’s preference. Standard left and right coronary angiograms with at least two projections were done for each patient. Two experienced interventionists assessed a set of parameters including the culprit vessel, angiographic features of the target lesion, TIMI flow grade before and after PPCI, and the target lesion length. Angiographic data of the lesion responsible for the infarction were recorded: (i) thrombus burden (mild, moderate, or high); (ii) use of aspiration catheter; (iii) use of glycoprotein IIb/IIIa inhibitors; and (iv) reperfusion type (balloon angioplasty, direct stenting, or stenting after pre-dilatation). Successful primary PCI was defined as a less than 20% residual stenosis in the absence of residual dissection in the epicardial coronary artery.13,14
Thrombolysis in myocardial infarction flow score was defined by the degree of flow into the epicardial coronary artery. Thrombolysis in myocardial infarction grades are as follows: grade 0 = complete absence of flow beyond the point of obstruction; grade 1 = some contrast material flows distal to the obstruction, but complete arterial visualization is not achieved; grade 2 = delayed opacification of the entire artery; and grade 3 = full prompt visualization of the entire artery.15 Thrombolysis in myocardial infarction flow 3 was considered (normal flow). No-reflow phenomenon was considered if TIMI flow in the artery ≤2, despite the successful dilatation and the absence of mechanical complications such as dissection, spasm, or evident distal embolization seen angiographically after completing of the procedure.16
Statistical Analysis
Statistical analysis was done using SPSS 23, IBM, Armonk, NY, USA. Quantitative data were expressed as mean ± standard deviation. Qualitative data were expressed as frequency and percentage. Student’s t-test was used to test the significance in quantitative data. Chi-square (χ2) test was used to compare categorical variables. P-value <.05 was considered statistically significant. Multiple binary logistic regression analysis was performed to detect the independent predictors of no-reflow. Spearman’s correlation analysis was performed to test the correlation between the total ischemia time and TIMI flow grade. Kaplan-Meier survival analysis was done to estimate the survival function for the two groups.
Results
The present study was conducted on 545 patients presented with STEMI and who underwent PPCI. Patients were divided into 2 groups: group I—76 patients (13.9%) with no-reflow (TIMI 0-2 flow) and group II—496 patients (86.1%) with normal flow (TIMI 3 flow). There was no statistically significant difference between the two groups regarding sex distribution, presence of hypertension, dyslipidemia, current smoking, and routine lab except CK-MB that was significantly higher in group I than group II (96.62 ± 45.8 vs. 77.04 ± 16.2 U/L, respectively P = .001). The mean age of the patients was higher in group I than group II (62.8 ± 10.49 vs. 59.9 ± 10.94 years, respectively P = .033), the percentage of diabetic patients was significantly higher in group I than group II [28 (36.8%) vs. 118 (25.2%), respectively P = .033]. However, the number of cases who had prior MI was lower in group I than group II [4 (5.3%) vs. 62 (13.2%) respectively, P = 0.049]. As regarding mortality and MACE, mortality was significantly higher in group I [4 (5.3%) vs. 7 (1.5%) with P-value = .030], also cardiogenic shock and re-infarction were significantly higher in this group with P-value = .013 and .010, respectively, as shown in Table 1.
Table 1.
Demographic, Clinical Characteristics, and Outcomes of All Patients in the Two Groups
| Group I (No-Reflow) (n = 76) (13.9%) | Group II (Normal Flow) (n = 469) (86.1%) | P | |
|---|---|---|---|
| Age, years | 62.8 ± 10.49 | 59.9 ± 10.94 | .033* |
| Male gender, n (%) | 36 (47.4%) | 253 (53.9%) | .287 |
| Hypertension, n (%) | 29 (38.2%) | 136 (29.0%) | .107 |
| Diabetes mellitus, n (%) | 28 (36.8%) | 118 (25.2%) | .033* |
| Smoking, n (%) | 31 (40.8%) | 164(35.0%) | .326 |
| Dyslipidemia, n (%) | 27 (35.5%) | 197 (42.0%) | .287 |
| Prior myocardial infarction, n (%) | 4 (5.3%) | 62 (13.2%) | .049* |
| Systolic BP, mm Hg | 103.0 ± 21.9 | 106.6 ± 15.8 | .090 |
| Diastolic BP, mm Hg | 66.5 ± 13.78 | 65.9 ± 9.54 | .601 |
| LVEF (%) | 46.49 ± 5.89 | 47.07 ± 5.23 | .387 |
| Random blood sugar, mg/dL | 172.1 ± 47.6 | 187.5 ± 75.4 | .084 |
| Serum creatinine, mg/dL | 1.06 ± 0.27 | 1.03 ± 0.24 | .311 |
| CK-MB, U/L | 96.62 ± 45.8 | 77.04 ± 16.2 | .001* |
| Mortality, n (%) | 4 (5.3%) | 7 (1.5%) | .030* |
| Major bleeding, n (%) | 2 (2.6%) | 5 (1.1%) | .261 |
| Cardiogenic shock, n (%) | 11 (14.5%) | 30 (6.4%) | .013* |
| Cardiac arrest, n (%) | 3 (3.9%) | 7 (1.5%) | .139 |
| Heart failure, n (%) | 6 (7.9%) | 26 (5.5%) | .419 |
| Contrast-induced nephropathy, n (%) | 8 (10.5%) | 56 (11.9%) | .722 |
| Cerebral stroke, n (%) | 1 (1.3%) | 1 (0.2%) | .140 |
| Re-infarction, n (%) | 5 (6.6%) | 8 (1.7%) | .010* |
BMI, body mass index; BP, blood pressure; LVEF, left ventricular ejection fraction; CK-MB, Creatine kinase-myocardial band.
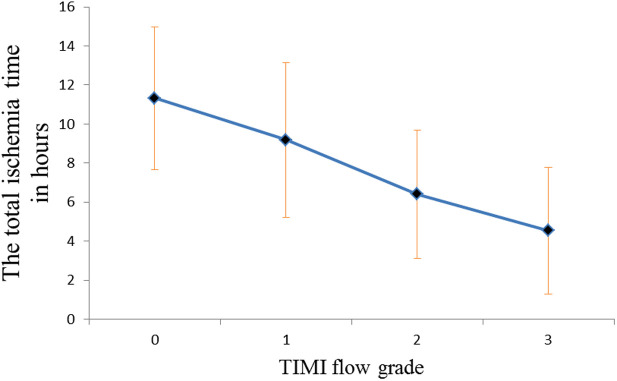
Concerning angiographic results, the total ischemia time was significantly higher in group I than group II (8.17 ± 4.02 vs. 4.54 ± 3.24 h, respectively, P = .001), as regarding thrombus burden of the lesion in the culprit vessel, there was a significant number of patients with high thrombus burden in group I than group II [24 (31.6%) vs. 59 (12.6%) respectively, P = .001]. Moreover, the need for aspiration catheter was significantly higher in group I than group II [23 (30.3%) vs. 44 (9.4%), respectively, P = .001], also the use of glycoprotein IIb/IIIa inhibitors was significantly higher in group I than group II [51 (67.1%) vs. 93 (19.8%), respectively, P = .001]. There was no significant difference between the two groups regarding initial TIMI flow, the length of the lesion, or the culprit vessel as shown in Table 2 and Figure 1.
Table 2.
Angiographic Results of All Patients in the Two Groups
| Group I (No-Reflow) (n = 76) (13.9%) | Group II (Normal Flow) (n = 469) (86.1%) | P | |
|---|---|---|---|
| The total ischemia time, h | 8.17 ± 4.02 | 4.54 ± 3.24 | .001* |
| Initial TIMI flow | |||
| 0-2 | 68 (89.5%) | 408 (87.0%) | .546 |
| 3 | 8 (10.5%) | 61 (13.0%) | |
| Post-procedural TIMI flow | |||
| 0 | 12 (15.8%) | 0 (0.0%) | .001* |
| 1 | 27 (35.5%) | 0 (0.0%) | |
| 2 | 37 (48.7%) | 0 (0.0%) | |
| 3 | 0 (0.0%) | 469(100.0%) | |
| Thrombus burden | |||
| Low | 23 (30.3%) | 270 (57.6%) | .001* |
| Moderate | 29 (38.2%) | 140 (29.9%) | |
| High | 24 (31.6%) | 59 (12.6%) | |
| Aspiration catheter | 23 (30.3%) | 44 (9.4%) | .001* |
| Glycoprotein IIb/IIIa inhibitors | 51 (67.1%) | 93 (19.8%) | .001* |
| Reperfusion type | |||
| Balloon angioplasty | 14 (18.4%) | 11 (2.3%) | .001* |
| Direct stenting | 11 (14.5%) | 98 (20.9%) | |
| Stenting after pre-dilatation | 51 (67.1%) | 360 (76.8%) | |
| Length of the lesion, mm | 21.3 ± 5.99 | 20.7 ± 5.16 | .390 |
| Culprit vessel | |||
| LM coronary artery, n (%) | 3 (3.9%) | 5 (1.1%) | .053 |
| LAD coronary artery, n (%) | 27 (35.5%) | 199 (42.4%) | .257 |
| CX coronary artery, n (%) | 23 (30.3%) | 136 (29.0%) | .822 |
| Right coronary artery, n (%) | 21 (27.6%) | 130 (27.7%) | .987 |
TIMI, thrombolysis in myocardial infarction; BD, balloon dilatation; LM, left main; LAD, left anterior descending; CX, circumflex.
Figure 1.
Relationship between the total ischemia time and TIMI flow grade. TIMI, thrombolysis in myocardial infarction.
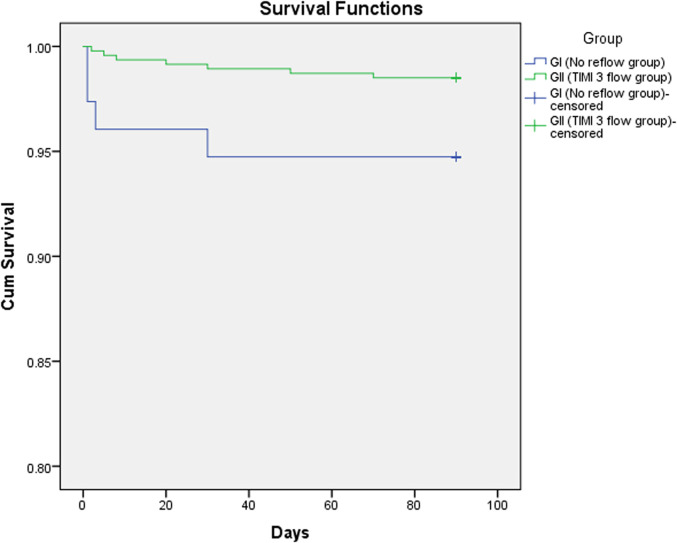
Multiple binary logistic regression analysis for the independent predictors of no-reflow phenomenon as presented in the Table 3 with the following results: age >65 years OR 1.887 (95% CI 1.113–3.198, P = .018), the total ischemia time >6 hours OR 4.655 (95% CI 2.666–8.126, P =.001), high thrombus burden OR 3.424 (95% CI 1.857–6.312, P = .001), and cardiogenic shock OR 3.049 (95% CI 1.318–7.053, P = .009). Spearman’s correlation analysis was performed to test the correlation between the total ischemia time and TIMI flow grade and showed a significant negative correlation (r = −351 and P = .001). Kaplan Meir curve was performed displaying cumulative survival of patients in the two groups as shown in Figure 2.
Table 3.
Multiple Binary Logistic Regression Analysis for the Independent Predictors of No-Reflow
|
Multivariate Analysis |
OR | (95% CI) | P
|
|---|---|---|---|
| Age >65 years | 1.887 | 1.113-3.198 | .018* |
| Diabetes Mellitus | 1.547 | 0.890-2.686 | .122 |
| Prior Myocardial Infarction | 2.830 | 0.941-8.512 | .064 |
| The Total Ischemia Time >6 h | 4.655 | 2.666-8.126 | .001* |
| High Thrombus Burden | 3.424 | 1.857-6.312 | .001* |
| Cardiogenic Shock | 3.049 | 1.318-7.053 | .009* |
*Significant P value
Figure 2.
Kaplan-Meier curve showing cumulative survival of patients in group I (no-reflow group) and group II (TIMI 3 flow). TIMI, thrombolysis in myocardial infarction.
Discussion
Complete restoration of myocardial reperfusion after PPCI doesn’t necessarily occurs after adequate reopening of the infarct-related artery, this condition is defined as no-reflow phenomenon.3,16 Complete coronary blood flow resumption is the ultimate goal for achieving a full clinical benefit.17 No-reflow phenomenon can occur in up to one-third of patients treated by PPCI according to the previous studies.3,4 In the current study, the incidence of no-reflow phenomenon was 13.9% which is relatively small in comparison to the incidence reported in the previous studies. This can be explained by the relatively small total ischemic time reported in our patients in this study versus other studies, in addition to low thrombus burden, use of glycoprotein IIb/IIIa inhibitors, and aspiration catheters which helped us to reach this low rate of no-reflow phenomenon. Door to balloon, first medical contact to balloon, and door to needle time intervals were heavily studied due to their critical importance in the setting of management of AMI and hence the prognosis.18-20 However, the total ischemia time and its impact on outcomes of PPCI wasn’t much studied, so the present study was undertaken to study the impact of the total ischemia time on no-reflow phenomenon after PPCI.
In the current study, patients with prolonged total ischemia time had a significantly higher incidence of no-reflow phenomenon, in agreement to our results, Brosh et al18 reported a significant difference in door to balloon time for maintaining the blood flow in patients with and without the no-reflow phenomenon (P-value = .000). Moreover, Yip et al21 demonstrated that the rate of no-reflow was lower in patients presented with AMI, who had reperfusion in less than 4 h. Kirma et al16 studied a series of 382 patients with AMI who underwent PPCI within 12 h of symptom onset and found that delayed reperfusion >6 h was correlated with no-reflow (P-value < .05) that comes in agreement to our results. In the early stages of AMI, thrombus is rich in thrombocytes and it is easier to be treated with adjunctive pharmacotherapy. However, with delayed reperfusion, the thrombus becomes more rigid and tends to fragment with balloon dilatation, which leads to distal embolization. Furthermore, delayed reperfusion results in a well-organized intracoronary thrombus and this reduces the likelihood of achieving TIMI 3 flow.22-24
Concerning the mean age of patients, it was significantly higher in no-reflow group. Advanced age of the patients tends to be associated with more coronary calcification, diffuse atherosclerosis, distal microembolization, dysfunction in microcirculation, atrial fibrillation that may lead to more hemodynamic compromise,25 and increased comorbidities are contributing factors for no-reflow phenomenon. In agreement to our results, Kirma et al noticed advanced age >60 years was correlated with no-reflow (P-value < .05).16,26 Recurrent attacks of ischemia may have protective effect on the heart via their action on the mitochondrial permeability pores; this is defined as ischemic pre-conditioning,27 so patients with prior MI may exhibit better clinical outcomes and smaller infarct size, which comes in agreement to the results of the current study that showed a significant higher percentage of patients with prior MI achieved TIMI 3 flow.
The incidence of diabetic patients in no-reflow group was significantly higher in our study that comes in agreement to Wang et al28 who demonstrated that hyperglycemia was correlated with more incidence of no-reflow phenomenon. Acute hyperglycemia increases the level of intercellular adhesion molecule-1 (ICAM-1) and P-selectin, which increases the adhesion of leukocytes to capillaries along with increased levels of catecholamine secretion with its harmful effect on fatty acid and glucose metabolism.29,30 Low thrombus burden of the culprit vessel was significantly lower in group II (TIMI 3 flow), so the use of aspiration catheter and glycoprotein IIb/IIIa inhibitors was less frequent in this group. In agreement to our results, De Luca et al31 found that good TIMI flow prior to PCI suggests a lower thrombus burden, spontaneous thrombolysis, vasospasm resolution, and smaller infarct size.
Conclusions
Although PPCI is superior to thrombolytic therapy in achieving a TIMI 3 flow, its main limitation is the delayed time. Advanced age >65 years, the total ischemia time >6 h, high thrombus burden, and cardiogenic shock were the independent predictors of no-reflow phenomenon. There is a significant negative correlation between the total ischemia time and TIMI flow grade after PPCI. Consequently by decreasing the total ischemia time, we can reduce the incidence of no-reflow phenomenon with further improvement of other outcomes.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Tanta University.
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.K.; Design – M.K., D.M.; Supervision – M.K., A.A.; Funding – None.; Materials – M.K., D.M.; Data Collection and/or Processing – M.K., D.M.; Analysis and/or Interpretation – M.K., A.A.; Literature Review – M.K., D.M.; Writing – M.K., A.A.; Critical Review – M.K., D.M.
Acknowledgments: We thank our colleagues from Tanta University Hospital, who provided insight and expertise that greatly assisted the research.
Declaration of Interests: None declared.
Funding: No funding sources.
References
- 1. Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Lessard D, Gore JM. Trends in community mortality due to coronary heart disease. Am Heart J. 2006;151(2):501 507. 10.1016/j.ahj.2005.04.024) [DOI] [PubMed] [Google Scholar]
- 2. Armstrong PW, Granger CB, Adams PX.et al.. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. APEX AMI Investigators, Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D Jr, O’Neill WW, Todaro TG, Vahanian A, Van de Werf F. JAMA. 2007;297(1):43 51. [DOI] [PubMed] [Google Scholar]
- 3. Galiuto L, DeMaria AN, Iliceto S. Microvascular damage during myocardial ischemia-reperfusion: pathophysiology, clinical implications and potential therapeutic approach evaluated by myocardial contrast echocardiography. Ital Heart J. 2000;1(2):108 116. [PubMed] [Google Scholar]
- 4. Danesh Sani HD, Eshraghi A, Sahri B, Vejdanparast M. No-reflow phenomenon in patients with ST-elevation acute myocardial infarction, treated with primary percutaneous coronary intervention: a study of predictive factors. J Cardio Thorac Med. 2014;2(4):221 226. [Google Scholar]
- 5. Resnic FS, Wainstein M, Lee MK.et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003;145(1):42 46. 10.1067/mhj.2003.36) [DOI] [PubMed] [Google Scholar]
- 6. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37(13):1024 1033. 10.1093/eurheartj/ehv484) [DOI] [PubMed] [Google Scholar]
- 7. Bekkers SC, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55(16):1649 1660. 10.1016/j.jacc.2009.12.037) [DOI] [PubMed] [Google Scholar]
- 8. Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34(23):1714 1722. 10.1093/eurheartj/eht090) [DOI] [PubMed] [Google Scholar]
- 9. Wang CH, Chen YD, Yang XC.et al. A no-reflow prediction model in patients with ST-elevation acute myocardial infarction and primary drug-eluting stenting. Scand Cardiovasc J. 2011;45(2):98 104. 10.3109/14017431.2011.558209) [DOI] [PubMed] [Google Scholar]
- 10. Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992;26(2):101 108. 10.1093/cvr/26.2.101) [DOI] [PubMed] [Google Scholar]
- 11. Ali A, Cox D, Dib N.et al. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48(2):244 252. 10.1016/j.jacc.2006.03.044) [DOI] [PubMed] [Google Scholar]
- 12. Menown IB, Mackenzie G, Adgey AA. Optimizing the initial 12-lead electrocardiographic diagnosis of acute myocardial infarction. Eur Heart J. 2000;21(4):275 283. 10.1053/euhj.1999.1748) [DOI] [PubMed] [Google Scholar]
- 13. Dubey G, Verma SK, Bahl VK. Primary percutaneous coronary intervention for acute ST elevation myocardial infarction: outcomes and determinants of outcomes: a tertiary care center study from North India. Indian Heart J. 2017;69(3):294 298. 10.1016/j.ihj.2016.11.322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13 20. 10.1016/S0140-6736(03)12113-7) [DOI] [PubMed] [Google Scholar]
- 15. TIMI Study Group. The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932-936. [DOI] [PubMed] [Google Scholar]
- 16. Kirma C, Izgi A, Dundar C.et al. Clinical and prodedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J. 2008;72(5):716 721. 10.1253/circj.72.716) [DOI] [PubMed] [Google Scholar]
- 17. Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105(5):656 662. 10.1161/hc0502.102867) [DOI] [PubMed] [Google Scholar]
- 18. Brosh D, Assali AR, Mager A.et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol. 2007;99(4):442 445. 10.1016/j.amjcard.2006.08.054) [DOI] [PubMed] [Google Scholar]
- 19. Khalfallah M, Abdalaal M, Adel M. Contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction: is it affected by treatment strategy? Glob Heart. 2019;14(3):295 302. 10.1016/j.gheart.2019.07.001) [DOI] [PubMed] [Google Scholar]
- 20. Menees DS, Peterson ED, Wang Y.et al. Door to Balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369(10):901 909. 10.1056/NEJMoa1208200) [DOI] [PubMed] [Google Scholar]
- 21. Yip HK, Chen MC, Chang HW.et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-flow. Chest. 2002;122(4):1322 1332. 10.1378/chest.122.4.1322) [DOI] [PubMed] [Google Scholar]
- 22. Khalfallah M, Elsheikh A, Abdalaal M. Very early versus early percutaneous coronary intervention after successful fibrinolytic therapy in Pharmacoinvasive strategy. Glob Heart. 2018;13(4):261 265. 10.1016/j.gheart.2018.06.003) [DOI] [PubMed] [Google Scholar]
- 23. Iwakura K, Ito H, Kawano S.et al. Prediction of the no-reflow phenomenon with ultrasonic tissue characterization in patients with anterior wall acute myocardial infarction. Am J Cardiol. 2004;93(11):1357 61, A5. 10.1016/j.amjcard.2004.02.030) [DOI] [PubMed] [Google Scholar]
- 24. Khalfallah M, Abdelmageed R, Allaithy A. Very early versus early percutaneous coronary intervention in patients with decreased e-GFR after successful fibrinolytic therapy. Glob Heart. 2020;15(1):34. 10.5334/gh.794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalfallah M, Elsheikh A. Incidence, predictors, and outcomes of new-onset atrial fibrillation in patients with ST-elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2020;25(4):e12746. 10.1111/anec.12746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowe GD, Rumley A, Woodward M.et al. Epidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow Monica Survey. I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97(4):775 784. 10.1046/j.1365-2141.1997.1222936.x) [DOI] [PubMed] [Google Scholar]
- 27. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124 1136. 10.1161/01.cir.74.5.1124) [DOI] [PubMed] [Google Scholar]
- 28. Wang JW, Zhou ZQ, Chen YD, Wang CH, Zhu XL. A risk score for no reflow in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention. Clin Cardiol. 2015;38(4):208 215. 10.1002/clc.22376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280(6):E848 E856. 10.1152/ajpendo.2001.280.6.E848) [DOI] [PubMed] [Google Scholar]
- 30. Marfella R, Esposito K, Giunta R.et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101(19):2247 2251. 10.1161/01.cir.101.19.2247) [DOI] [PubMed] [Google Scholar]
- 31. De Luca G, Ernst N, Zijlstra F.et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2004;43(8):1363 1367. 10.1016/j.jacc.2003.11.042) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a