Abstract
Thrombotic coronary artery occlusions usually manifest as acute coronary syndrome with cardiogenic shock, acute pulmonary edema, cardiac arrest, fatal arrhythmias, or sudden cardiac death. Although it usually occurs based on atherosclerosis, it can also occur without atherosclerosis. There is no predictor of coronary artery thrombosis clinically and no consensus regarding the optimal treatment. In the current literature, treatment options include emergency coronary artery bypass grafting, entrapment of thrombus in vessel wall with stent implantation, intracoronary thrombolysis, glycoprotein IIb/IIIa inhibitors, anticoagulation with heparin, and thrombus aspiration as reperfusion strategies. Here, we reviewed a new treatment strategy based on the literature, and a case series with successful results in hemodynamically stable patients with low-dose slow infusion tissue plasminogen activator (tPA) for thrombotic coronary artery occlusions that allow coronary flow was reported. Prospective randomized studies and common consensus are needed on low-dose, slow-infusion tissue plasminogen activator treatment regimen and optimal treatment management for thrombotic coronary artery occlusions.
Keywords: Thrombosis, coronary artery occlusion, low-dose slow-infusion tPA
Highlights
Thrombotic coronary artery occlusion treatment options include emergency coronary artery bypass grafting, entrapment of thrombus in vessel wall with stent implantation, intracoronary or intravenous thrombolytic agents, anticoagulant agents, potent antiplatelet agents with glycoprotein IIb/IIIa inhibitors, conservative therapy, and thrombus aspiration.
There is no consensus regarding treatment option for thrombotic coronary occlusions that allow coronary flow in hemodynamically stable patients.
Low-dose, slow-infusion tissue plasminogen activator (tPA) regimen may be a new and minimally invasive treatment option for thrombotic coronary occlusions that allow coronary flow in hemodynamically stable patients.
To the best of our knowledge, this is the first case series and a new treatment strategy based on the literature, with successful results of low-dose, slow-infusion tPA therapy for the treatment of coronary thrombi that allow coronary flow.
Introduction
Thrombotic coronary artery occlusions, especially in the left main coronary artery (LMCA) or left anterior descending (LAD) artery thrombosis, usually present with cardiogenic shock, acute pulmonary edema, cardiac arrest, fatal arrhythmias, or sudden cardiac death.1-3 Within the context of atherosclerotic risk factors, the usual cause of coronary artery thrombotic occlusions is atherosclerotic process resulting from plaque rupture and release of thrombogenic components from plaque with subsequent thrombus formation.2 Despite the absence of atherosclerosis, some cases of spontaneous coronary thrombus formation have been described, especially in younger patients. In the literature, other reported major causes of thrombotic coronary artery occlusions are coronary artery embolism, coronary artery ectasia, hypercoagulable state, cocaine use, aortic dissection, hematological or malignant disorders, vasculitis, and vasospasm.2-8
There is no standard treatment approach and clear guideline recommendations. In literature, nonobstructive thrombotic coronary artery occlusion treatment options include emergency coronary artery bypass grafting (CABG), entrapment of thrombus in vessel wall with stent implantation, intracoronary or intravenous thrombolytic agents, anticoagulant agents, potent antiplatelet agents with glycoprotein IIb/IIIa inhibitors, conservative therapy, and thrombus aspiration.9-18 While emergency percutaneous coronary intervention (PCI) or CABG is performed in conditions such as ongoing chest pain, hemodynamic instability, and ST-segment elevation myocardial infarction (STEMI), but there is no consensus on the treatment of thrombotic coronary occlusions that allow coronary flow in hemodynamically stable patients. Recently, a case was reported with successful results with a low-dose, slow-infusion tissue plasminogen activator (tPA) for LMCA thrombosis that allows coronary flow.3
Here, we evaluated our thrombotic coronary cases based on the literature and we presented our case series with successful results with low-dosage, slow-infusion tPA therapy for thrombotic coronary occlusions that allow coronary flow as a new treatment strategy (Table 1). All patients were informed and gave written consent, but an ethics committee application was not made due to a retrospective case evaluation.
Table 1.
Baseline Clinical, Demographic, Angiographic Characteristics of Patients
| Cases |
Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age (years) | 43 | 54 | 45 | 45 | 41 |
| Gender | Male | Male | Male | Male | Male |
| Symptom | Chest pain, dyspnea | Chest pain, presyncope, dyspnea | Chest pain, pain in the arm, dyspnea |
Chest pain | Crushing chest pain |
| ECG | ST depression in anterior and lateral derivations | ST depression in anterior derivations | ST depression in D1, aVL, V5, V6 leads | ST depression in inferior derivations | ST elevation in DII, DIII, aVF leads |
| Troponin I | 4.24 ng/mL | 1.04 ng/mL | 4.2 ng/mL | 2.1 ng/mL | 4.8 ng/mL |
| Diagnosis | Non-STEMI |
Non-STEMI |
Non-STEMI |
Non-STEMI |
Non-STEMI |
| Risk factors of cardiovascular disease | Cigarette Family history |
No | Cigarette Family history |
Cigarette | Cigarette |
| Co-morbidities | No | Psoriasis, history of deep venous thrombosis | History of deep venous thrombosis, Factor V Leiden heterozygote mutation | No | No |
| Coronary angiography, thrombus location | LMCA | LMCA | LAD proximal | LAD proximal | LAD proximal |
| Echocardiography | EF 48%, septal hypokinesia | EF 55%, basal septal wall minimal hypokinesia | EF 50%, basal septal wall minimal hypokinesia | EF 48%, inferior, posterolateral wall minimal hypokinesia | EF 45%, apical wall minimal hypokinesia |
| GP IIb/IIIa inh. | Tirofiban, 80 hours | Absiximab, 72 hours | Tirofiban, 48 hours | Tirofiban, 48 hours | Tirofiban, 24 hours |
| tPA dose | 25 mg, 6 hours | 25 mg, 6 hours 25 mg, 25 hours |
25 mg, 6 hours 25 mg, 25 hours |
25 mg, 6 hours 25 mg, 25 hours |
25 mg, 6 hours 25 mg, 25 hours |
| 1-year follow-up medication | ASA, prasugrel, atorvastatin 40 | Clopidogrel, edoxaban 60 mg, metoprolol, atorvastatin 40 mg, ramipril 2.5 mg | ASA, prasugrel, atorvastatin, ramipril, nebivolol | ASA, clopidogrel, atorvastatin, ramipril, metoprolol | ASA, clopidogrel, atorvastatin 40, metoprolol, ramipril |
| Survey | 72 months, no symptom, no complication, no control angiography |
20 months, no symptom, no complication, no control angiography | 22 months, no symptom, no complication, 1-year control with computed tomographic angiography | 4 months, no symptom, no complication, no control angiography | 31 months, no symptom, no complication, no control angiography |
| Last visit medication | ASA | Clopidogrel, atorvastatin 40 mg, ramipril 2.5 mg, edoxaban 60 mg | Clopidogrel, ASA, atorvastatin | ASA, clopidogrel, atorvastatin, ramipril, metoprolol | ASA, atorvastatin 20 mg, ramipril 2.5 mg |
ASA, acetylsalicylic acid, ECG, electrocardiography, EF, ejection fraction; GP IIb/IIIa inh., platelet glycoprotein IIb/IIIa receptor inhibition; LAD, left anterior descending artery; LMCA, left main coronary artery; Non-STEMI, non-ST-Elevation myocardial infarction; tPA, tissue-type plasminogen activator.
Cases
Case 1
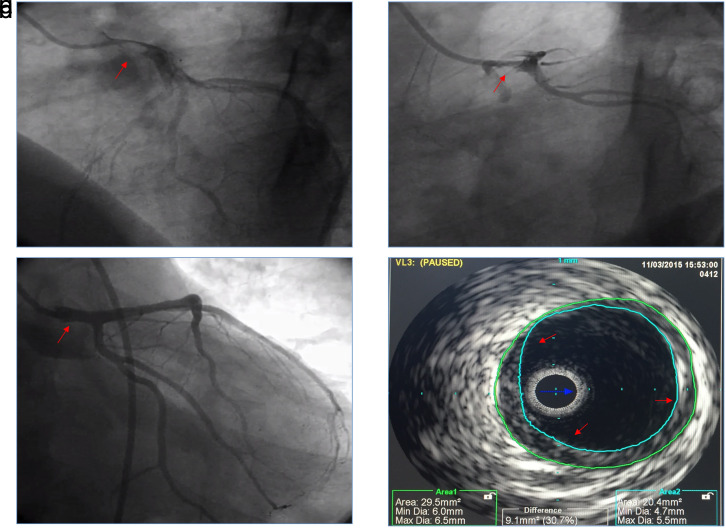
A 43-year-old male patient was referred with intermittent chest pain, a troponin I (TnI) value of 4.24 ng/mL, and non-ST elevation myocardial infarction (NSTEMI). Physical examination and electrocardiography were normal. He had no additional risk factors apart from smoking and family history. Echocardiography showed basal septal wall hypokinesia with an ejection fraction of 48%. In the coronary angiography, semi-mobile thrombus leading to 80-90% stenosis with Thrombolysis in Myocardial Infarction (TIMI) ≤2 flow was detected in LMCA (Figure 1A). The patient was hemodynamically stable and chest pain did not recur. He was given an infusion of the Gp IIb/IIIa inhibitor tirofiban for 80 hours in addition to enoxaparin, acetylsalicylic acid, and clopidogrel treatment. In control coronary angiography, thrombus remained exactly the same (Figure 1b). We gave 25 mg alteplase (tPA) for 6 hours, after heart team evaluation. Repeat coronary angiography after 24 hours revealed that thrombus had disappeared and there was TIMI 3 coronary flow without severe stenosis (Figure 1c). In the follow-up of the patient, chest pain did not recur, electrocardiographic and echocardiographic changes were not observed. The patient was discharged with acetylsalicylic acid, clopidogrel, atorvastatin, ramipril, and metoprolol therapy. A follow-up coronary angiography and intravascular ultrasound (IVUS) revealed an unobstructed plaque in LMCA 1 month later (Figure 1D). The patient was asymptomatic at the 72nd-month telephone visit, was using only acetylsalicylic acid, and was not using other drugs.
Figure 1.
(A) A semi-mobile thrombus was observed in the left main coronary artery in coronary angiography. (B) Imaging the left main coronary artery, after tirofiban infusion. (C) Imaging the left main coronary artery, 24 hours after tPA therapy. (D) A nonobstructing plaque was observed in the left main coronary artery in intravascular ultrasound imaging. tPA, tissue-type plasminogen activator.
Case 2
A 54-year-old male patient was admitted to the emergency department with typical angina, which started recently and lasted for 45 minutes. No pathology was observed in physical examination and electrocardiography. In his medical history, he had deep venous thrombosis 4 years ago, used topical creams for psoriasis, and there was no other cardiovascular risk factor. The TnI value and low-density lipoprotein level were 1.04 ng/mL and 91 mg/dL, respectively. Coronary angiography was performed in the diagnosis of NSTEMI and revealed a semi-mobile thrombus formation leading to 70-80% stenosis in LMCA with TIMI 2 flow (Figure 2A). There was no stenosis or atherosclerotic plaque in other coronary vessels. Abciximab was administered as intracoronary bolus dose and continuous intravenous infusion for 72 hours in addition to appropriate doses of acetylsalicylic acid, clopidogrel, and unfractionated heparin. Antiaggregant and anticoagulant treatment continued for 4 days. The patient was hemodynamically stable during this period. Echocardiography showed minimal hypokinesia in basal septal wall with ejection fraction 55%. In control angiography, the thrombus formation remained the same (Figure 2B). After heart team evaluation, we gave 25 mg tPA for 6 hours to the patient. After giving tPA, we followed up the patient for 12 hours in terms of complications and then we gave a second dose of 25 mg tPA for 25 hours. In the control coronary angiography after 24 hours of the last dose of tPA, the thrombus has dissolved and there was TIMI 3 coronary flow without plaque or stenosis (Figure 2C-D). In the follow-up, clinical, electrocardiographic, and echocardiographic changes were not observed, and complication was not observed except minimal bleeding at the femoral access site. The patient was discharged with low-dose oral anticoagulant therapy (edoxaban 30 mg) in addition to acetylsalicylic acid, clopidogrel, atorvastatin, ramipril, and metoprolol therapy. In the evaluation of patient’s thrombophilia panel, homocysteine, protein C and S, activated protein C impedance, anti-thrombin III levels were within normal limits. At the first month follow-up, the patient was asymptomatic, acetylsalicylic was discontinued, edoxaban dose was increased to 60 mg, and other drugs were continued. The patient was asymptomatic at the 20th-month telephone visit, was using edoxaban, atorvastatin, and ramipril therapy.
Figure 2.
(A) A semi-mobile thrombus in the left main coronary artery in coronary angiography. (B) Imaging the left main coronary artery, after tirofiban infusion. (C-D) Right anterior oblique cranial and left anterior oblique caudal view of the left main coronary artery, 24 hours after tPA therapy. tPA, tissue-type plasminogen activator.
Case 3
A 45-year-old male patient was admitted to the emergency department with typical chest pain that persisted for 2 hours and then disappeared. There was no pathology in physical examination and electrocardiography. In his medical history, he had deep venous thrombosis and Factor V Leiden mutation. He had no additional risk factors apart from smoking 2 pocket/year cigarettes and family history. Troponin I value detected was 4.2 ng/mL. Coronary angiography was performed with the diagnosis of NSTEMI. Coronary angiography revealed a thrombus formation leading to 70-80% stenosis in the middle region of LAD artery with TIMI ≤2 flow (Figure 3A). Tirofiban was administered as intracoronary bolus dose and continuous intravenous infusion for 48 hours in addition to acetylsalicylic acid, clopidogrel, enoxaparin, high-dose statin, metoprolol, and ramipril therapy. Echocardiography showed minimal hypokinesia in basal septal wall with an ejection fraction of 55%. In control angiography, the thrombus formation remained the same (Figure 3B-C). After heart team evaluation, we gave 25 mg tPA for 6 hours to the patient. After giving tPA, we followed the patient for up to 12 hours in terms of complications, and then we gave a second dose of 25 mg tPA for 25 hours. In the follow-up, clinical, electrocardiographic, echocardiographic changes and major or minor complications were not observed. In the control coronary angiography after 24 hours of the last dose of tPA, the thrombus has dissolved and TIMI 3 coronary flow was achieved with noncritical atherosclerotic plaque (Figure 3d). The patient was discharged with acetylsalicylic acid, prasugrel, atorvastatin, ramipril, and metoprolol therapy. There was no pathology except mild ectasia (approximately 4.8 mm) at the mid LAD region, which was detected in the computed tomographic angiography performed 1 year later. At the 22nd-month telephone visit, the patient was asymptomatic under acetyl salicylic acid, clopidogrel, atorvastatin therapy and continued to use cigarette.
Figure 3.
(A-B). Right anterior oblique caudal and anteroposterior cranial view of the left anterior descending artery with a semi-mobile thrombus. (C) Imaging the left anterior descending artery, after tirofiban infusion. (D) Imaging the left anterior descending artery, after tPA therapy. tPA, tissue-type plasminogen activator.
Case 4
A 45-year-old male patient was admitted to the emergency department with typical chest pain that persisted for 2 hours and then decreased. There was no pathology in physical examination and electrocardiography. He has no cardiovascular risk factors except smoking. Troponin I value was 2.1 ng/mL. The patient was admitted to the coronary intensive care unit with the diagnosis of NSTEMI. Acetylsalicylic acid, ticagrelor, enoxaparin, atorvastatin, ramipril, and metoprolol therapy was initially started. Coronary angiography was performed and revealed a thrombus formation leading to 80% stenosis in the middle region of LAD with TIMI 1 flow (Figure 4A). Tirofiban was administered as intracoronary bolus dose and continuous intravenous infusion for 48 hours in addition to other medical therapies. Echocardiography showed anterior and septal wall mild hypokinesia with ejection fraction 48%. In control angiography, the thrombus formation persisted unchanged (Figure 4B). After this angiographic sign, it was decided to administer 25 mg tPA for 6 hours to the patient at the heart team meeting. After 12 hours of monitoring, a second dose of 25 mg tPA was administered for 25 hours. Before thrombolytic therapy, ticagrelor was switched to clopidogrel. In the follow-up, clinical, electrocardiographic, echocardiographic changes, and major or minor complications were not observed. In the control coronary angiography, after 24 hours of the last dose of tPA, the thrombus has dissolved, and there was TIMI 3 coronary flow with noncritical atherosclerotic plaque (Figure 4C-D). The patient was discharged with acetyl salicylic acid, clopidogrel, statin, ramipril, and beta-blocker therapy. He was asymptomatic under the same optimal medical therapy at 4th-month telephone visit.
Figure 4.
(A) Left anterior oblique caudal view of the left anterior descending artery with a semi-mobile thrombus. (B) Imaging the left anterior descending artery, after tirofiban infusion. (C-D) Left anterior oblique caudal and anteroposterior cranial view of the left anterior descending artery, after tPA therapy. tPA, tissue-type plasminogen activator.
Case 5
A 41-year-old male patient was admitted with retrosternal, crushing chest pain. The patient had a history of blunt chest trauma by receiving a blow of fist in a fight 1 hour prior to his admission. On physical examination, he was sweaty and tenderness on left side of the chest wall with palpation was determined. No murmurs were heard and lungs were clear. The brain and chest tomography and extremity radiographs of the patient showed no pathology or bleeding. There was no risk factor other than smoking. Electrocardiography showed transient ST-segment elevation in leads DII, DIII, aVF (Figure 5a). Cardiac TnI was 4.8 ng/mL (0-0.01 ng/mL), and other laboratory parameters were normal. Transthoracic echocardiography demonstrated apical wall minimal hypokinesia with a 45% left ventricular ejection fraction. In the follow-up, ST-segment elevation improved, and acetylsalicylic acid, clopidogrel, and enoxaparin therapy was started with diagnosis of NSTEMI. Coronary angiography demonstrated an intraluminal thrombus leading to 80-90% stenosis in proximal region of LAD with distal TIMI 2 flow (Figure 5b). Tirofiban infusion was started at a 0.15 µg/kg/min intravenous dose for 48 hours after intracoronary bolus dose. 24 hours after end of tirofiban infusion, control coronary angiography revealed the persistence of the thrombus leading to 50-60% stenosis in LAD (Figure 5C). After the literature search and considering the formation of acute thrombus by intimal dissection and subsequent thrombus formation or spontaneous thrombus formation without intimal dissection in the possible etiology, low-dose ultraslow infusion tPA was planned. The patient was informed, and 25 mg tPA was given intravenously within 6 hours. Twelve hours after the first dose, a second dose of 25 mg tPA was given within 25 hours. 48 hours after the end of tPA infusion, control coronary angiography was performed and the thrombus was dissolved completely, TIMI 3 coronary flow was achieved with noncritical irregularity of the vessel wall (Figure 5D). In the follow-up, clinical, electrocardiographic, echocardiographic changes, and major or minor complications were not observed. The patient was discharged uneventfully with acetylsalicylic acid, clopidogrel, atorvastatin, ramipril, and metoprolol therapy 3 days later. One month later, there was no symptom at the follow-up visit. The patient was asymptomatic at the 31st-month telephone visit and was receiving atorvastatin 20 mg, ramipril 2.5 mg, and acetylsalicylic acid therapy.
Figure 5.
(A) The electrocardiography showing the ST-segment elevation in DII, DIII, aVF, and V4-V6 leads. (B) Right anterior oblique caudal view during the diagnostic coronary angiography showing the proximal 80-90% occlusion of left anterior descending artery. (C) Right anterior oblique caudal view during control coronary angiography after tirofiban infusion, showing the proximal 50-60% occlusion of left anterior descending artery. (D) Right anterior oblique caudal view during control coronary angiography after low-dose ultraslow-infusion tPA, showing the patency of left anterior descending artery without thrombus. tPA, tissue-type plasminogen activator.
Literature Review
There is no standard treatment approach and clear guideline recommendations for nonobstructive thrombotic coronary artery occlusion therapy. In literature, treatment options include emergency CABG, entrapment of thrombus in vessel wall with stent implantation, intracoronary or intravenous thrombolytic agents, anticoagulant agents, potent antiplatelet agents with glycoprotein IIb/IIIa inhibitors, conservative therapy, and thrombus aspiration.9-19
Patients with coronary thrombus represent a high-risk subgroup of acute myocardial infarction patients that we can see catastrophic events in the clinic. There are cases reported in the literature that received urgent CABG, PCI, thrombus aspiration, thrombolytic therapy, aggressive anticoagulant, and antiaggregant therapy. There is no study for head-to-head comparison of PCI with CABG, but there are case reports with successful results with PCI.4,9-11 However, distal embolism or no-reflow, slow flow are well-known complications during PCI.2,4 Hernández et al13 reported successful primary PTCA (Percutaneous Transluminal Coronary Angioplasty) and stenting, although distal embolization had occurred, and TIMI 2 flI d stenting, although distal embolization , some publications have suggested intracoronary thrombolysis for widespread thrombus in LMCA and other coronary arteries.14,17 Germing et al18 reported successful treatment with 50 mg tPA for 1 hour in LMCA thrombus, resistant to 48 hours of abciximab plus antiaggregant, anticoagulant therapy. Again, in the literature, tPA and streptokinase, prolonged abciximab infusion with aspirin and heparin have been used effectively in individual cases with angiographic LMCA and other vessel thrombus resolution.12,14,17,20-22 A study showed that Gp IIb/IIIa inhibitors given with ClearWay™ perfusion catheter instead of direct intracoronary infusion was related to a significant reduction in thrombus burden and improvement of coronary flow during PCI.23 Aspiration thrombectomy was used effectively in some cases.2,4,20,24 Garcia et al15 reported a successful case with manual aspiration thrombectomy. Otto et al16 reported a case they have treated mechanically with a self-expanding trapping device after an unsuccessful attempt of thrombus aspiration. Conversely, there are some cases reported in which catheter aspiration was unsuccessful.1,2,8 In a study in the STEMI patients with large thrombus burden, ectatic coronary arteries, technical challenges, or failed thrombus aspiration, giving a low-dose intracoronary thrombolytic therapy (one-third of systemic dose) was safe, reduced thrombus burden, and improved myocardial reperfusion.25-27 Again, in a case series in STEMI patients with large intracoronary thrombus burden, intracoronary thrombolytic agent reduced the thrombus burden and facilitated revascularization.28 Also, studies showed that in STEMI patients with high thrombus burden, intracoronary target vessel thrombolysis with thrombus aspiration compared with thrombus aspiration was more effective and beneficial in improving myocardial microvascular circulation.29 The intracoronary thrombolytic dose was selected as 10 mg alteplase (tPA) directly into the infarct-related artery over 5-10 min based on the protocol from the T-time trial.30,31 In addition to thrombus aspiration, anticoagulant, antiaggregant, or intracoronary thrombolytic therapies, full-dose intravenous tPA is given in cases with high thrombotic burden in the coronary arteries or resistant to these therapies.2,4,17,20,21,32 Aydin et al17 reported successful treatment with full-dose tPA in LMCA thrombus. There are many different treatments available in the literature on isolated thrombotic non-NSTEMI patients that allow coronary flow. However, Akcay et al3 reported successful treatment with 25 mg tPA infusion for 6 hours in a case which was refractory to anticoagulant and antiaggregant therapies that allow coronary flow.
The efficacy and safety of low-dose, slow-, and ultraslow infusion tPA therapies with low complication rates have been previously proven in the management of prosthetic valve thrombosis with TROIA (Comparison of Different TRansesophageal Echocardiography Guided thrOmbolytic Regimens for prosthetIc vAlve Thrombosis) and PROMETEE (PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE) clinical trials.33,34 It has also been shown to be clinically safe and effective in patients with intermediate- to high-risk pulmonary embolism.35 Non-ST elevation myocardial infarction patients with prosthetic heart valves are more likely to be associated with prosthetic valve-derived coronary embolism rather than atherosclerosis. In the management of these patients, low-dose, slow-infusion tPA therapy has shown to be an effective and safe treatment method.36 Again, Karakoyun et al37 have reported prosthetic valve thrombosis complicated with coronary embolism which was successfully treated with low-dose, slow-infusion tPA. Low-dose, slow (6 hours), and ultraslow (25 hours)-infusion tPA therapies instead of full-dose short-term (100 mg, 2 h) tPA are associated with lower complication rates without compromising efficacy. As shown in some cases, low-dose, slow-infusion tPA regime may be a new treatment approach for thrombotic coronary occlusions that allow coronary flow in hemodynamically stable patients. But there is no data or clinical trial about isolated nonobstructive thrombotic coronary artery occlusions; as our case series the low doses, slow infusion tPA may be safety and effective treatment method, should be kept in mind. We need new prospective clinical trials based on this new idea.
The main limitation in our case series is the inability to perform IVUS due to the inability to provide a catheter except in 1 case. In our case series, the etiology of coronary thrombus consisted of a heterogeneous group such as atherosclerotic plaque rupture, susceptibility to thrombosis, and history of trauma, but all of our patients had hemodynamically stable, nonobstructive, flow-permitting coronary thrombi. There was a thrombus resistant to aggressive antiaggregant, anticoagulant therapy, and long-time Gp IIb/IIIa inhibitor infusion. Among the Gp IIb/IIIa inhibitors, the preference for tirofiban or abciximab changed according to drug supply. Two doses of therapy were given to our cases except for 1 case, 25 mg tPA at 6 hours and after 12 hours additional 25 mg tPA at 25 hours. As it was only our first case experience, control angiography was performed after 25 mg tPA at 6 hours and a second dose was not needed.
Conclusion
To the best of our knowledge, this is the first case series and a new treatment strategy based on the literature, with successful results of low-dose, slow-infusion tPA therapy for the treatment of coronary thrombi that allow coronary flow. These patients may seems to be very limited group, but this approach in selected cases may be an effective minimally invasive treatment option. Based on this opinion, we need new prospective clinical trials.
Declaration of Interests
None declared.
Footnotes
Ethics Committee Approval: All patients were informed and gave written consent, but ethics committee approval was not made due to a retrospective case evaluation.
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.A.; Design – M.A.; Supervision – M.A.; Funding – None; Materials – M.A., M.Ç., U.Y., Ö.G., O.G., S.Y., M. M., K. S., Ö.Y., M.Ş.; Data Collection and/or Processing – M.A., M.Ç., U.Y., Ö.G., O.G., S.Y., M. M., K. S., Ö.Y., M.Ş.; Analysis and/or Interpretation – M.A., M.Ç.; Literature Review – M.A., M.Ç., U.Y., Ö.G., O.G., S.Y., M. M., K. S., Ö.Y., M.Ş.; Writing – M.A.; Critical Review – M.A., M.Ç., U.Y., Ö.G., O.G., S.Y., M. M., K. S., Ö.Y., M.Ş.
Acknowledgments: None.
Funding: None.
References
- 1. Akcay M. Evaluation of thrombotic left main coronary artery occlusions; old problem, different treatment approaches. Indian Heart J. 2018;70(4):573 574. 10.1016/j.ihj.2017.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta R, Rahman MA, Uretsky BF, Schwarz ER. Left main coronary artery thrombus: a case series with different outcomes. J Thromb Thrombolysis. 2005;19(2):125 131. 10.1007/s11239-005-1924-2) [DOI] [PubMed] [Google Scholar]
- 3. Akcay M, Soylu K, Yanik A. Acute thrombotic left main coronary artery; treatment with low dose slow infusion tPA. Int J Cardiol. 2016;224:265 266. 10.1016/j.ijcard.2016.09.013) [DOI] [PubMed] [Google Scholar]
- 4. Klein AJ, Casserly IP, Messenger JC. Acute left main coronary arterial thrombosis-a case series. J Invasive Cardiol. 2008;20(8):E243 E246. [PubMed] [Google Scholar]
- 5. Sanchez-Recalde A, Calvo Orbe L, Galeote G. Cardiogenic shock due to complete thrombotic occlusion of the left main coronary ostium in a young female. J Invasive Cardiol. 2006;18(6):E188 E190. [PubMed] [Google Scholar]
- 6. Gottam N, Stewart D, Yamasaki H, Ajjour M. The main coronary artery (LMCA) thrombus presented as an ST Elevation Myocardial infarction in a hypercoagulable patient. Case Rep Vasc Med. 2012;2012:579097. 10.1155/2012/579097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomioka T, Takeuchi S, Ito Y, Shioiri H, Koyama J, Inoue K. Recurrent acute myocardial infarction in a patient with severe coronary artery ectasia: implication of antithrombotic therapy. Am J Case Rep. 2016;17:939 943. 10.12659/ajcr.900474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakazone MA, Tavares BG, Machado MN, Maia LN. Acute myocardial infarction due to coronary artery embolism in a patient with mechanical aortic valve prosthesis. Case Rep Med. 2010;2010:751857. 10.1155/2010/751857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lijoi A, Della Rovere F, Passerone GC.et al. Emergency surgical treatment for total left main coronary artery occlusion. A report of two cases. Tex Heart Inst J. 1993;20(1):55 58. [PMC free article] [PubMed] [Google Scholar]
- 10. Prasad SB, Whitbourn R, Malaiapan Y, Ahmar W, MacIsaac A, Meredith IT. Primary percutaneous coronary intervention for acute myocardial infarction caused by unprotected left main stem thrombosis. Catheter Cardiovasc Interv. 2009;73(3):301 307. 10.1002/ccd.21886) [DOI] [PubMed] [Google Scholar]
- 11. Patel M, Bhangoo M, Prasad A. Successful percutaneous treatment of suspected embolic left main thrombosis in a patient with a mechanical aortic valve. J Invasive Cardiol. 2011;23(11):E263 E266. [PubMed] [Google Scholar]
- 12. Schlaifer JD, Horgan W, Malkowski MJ. Acute thrombotic occlusion of the left main coronary artery in a hypercoagulable patient treated with intracoronary abciximab. Clin Cardiol. 2001;24(12):788. 10.1002/clc.4960241208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernández F, Pombo M, Dalmau R.et al. Acute coronary embolism: angiographic diagnosis and treatment with primary angioplasty. Catheter Cardiovasc Interv. 2002;55(4):491 494. 10.1002/ccd.10122) [DOI] [PubMed] [Google Scholar]
- 14. Lew AS, Weiss AT, Shah PK, Fishbein MC, Berman DS, Maddahi J. Extensive myocardial salvage and reversal of cardiogenic shock after reperfusion of the left main artery by intravenous streptokinase. Am J Cardiol. 1984;54(3):450 452. 10.1016/0002-9149(84)90219-4) [DOI] [PubMed] [Google Scholar]
- 15. Garcia D, Ansari M, Majjul JS, Urbina EM, Ferreira AC, Mendoza CE. Left main coronary embolization after direct current cardioversion for persistent atrial flutter in the absence of detectable intracardiac thrombi. JACC Cardiovasc Interv. 2015;8(2):e17 e18. 10.1016/j.jcin.2014.09.015) [DOI] [PubMed] [Google Scholar]
- 16. Otto S, Mayer TE, Figulla HR. Cryptogenic left main thrombosis: successful mechanical clot retrieval with a self-expanding trapping device. Catheter Cardiovasc Interv. 2014;83(4):553 555. 10.1002/ccd.25162) [DOI] [PubMed] [Google Scholar]
- 17. Aydin M, Ozeren A, Bilge M. Acute incomplete thrombotic occlusion of distal left main coronary artery treated by tissue plasminogen activator. Heart. 2004;90(3):242. 10.1136/hrt.2003.021410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Germing A, Mügge A, Lindstaedt M. Recurrent myocardial ischemia due to riding left main coronary artery bifurcation thrombus--noninterventional therapy with glycoprotein blocker and thrombolysis. Cardiovasc Revasc Med. 2006;7(2):76 80. 10.1016/j.carrev.2005.11.002) [DOI] [PubMed] [Google Scholar]
- 19. Aoun J, Assaker J-P, Faza N, Kleiman NS, Zacca N, Little SH. Mechanical prosthetic aortic valve thrombosis complicated by an acute coronary syndrome during fibrinolysis. JACC Case Rep. 2020;2(14):2186 2190. 10.1016/j.jaccas.2020.08.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oman Z, Khalid N, Boppana S, Helmy T, Abo-Salem E. Management options of acute left main coronary thrombus. Cardiovasc Revasc Med. 2018;19(8S):25 27. 10.1016/j.carrev.2018.05.009) [DOI] [PubMed] [Google Scholar]
- 21. Mischie AN, Nazzaro MS, Sinescu C, Violini R. Successful management of ostial left main thrombus by systemic thrombolysis. Eur Heart J. 2011;32(5):654. 10.1093/eurheartj/ehq416) [DOI] [PubMed] [Google Scholar]
- 22. Gunn J. Intra‐coronary alteplase for extensive coronary artery thrombus. Heart. 2007;93(10):1262. 10.1136/hrt.2006.102392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maluenda G, Sizemore BC, Revtyak G. et al.Intracoronary glycoprotein IIb/IIIa inhibitor infusion via a perfusion coronary catheter to decrease thrombus burden: results from the ClearWay™ Multicenter Registry. Cardiovasc Revasc Med. 2013;14(5):280 283. 10.1016/j.carrev.2012.12.006) [DOI] [PubMed] [Google Scholar]
- 24. Papadimitriou D, Gavrielatos G, Stougiannos P, Kaplanis I, Trikas A. Primary left main coronary artery thrombus aspiration as a standalone treatment: sailing in uncharted waters. Postepy Kardiol Interwencyjnej. 2016;12(3):258 261. 10.5114/aic.2016.61649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boscarelli D, Vaquerizo B, Miranda-Guardiola F.et al. Intracoronary thrombolysis in patients with ST-segment elevation myocardial infarction presenting with massive intraluminal thrombus and failed aspiration. Eur Heart J Acute Cardiovasc Care. 2014;3(3):229 236. 10.1177/2048872614527008) [DOI] [PubMed] [Google Scholar]
- 26. Agarwal SK, Agarwal S. Role of intracoronary fibrinolytic therapy in contemporary PCI practice. Cardiovasc Revasc Med. 2019;20(12):1165 1171. 10.1016/j.carrev.2018.11.021) [DOI] [PubMed] [Google Scholar]
- 27. Lee Yonggu, Kim E, Kim BK, Shin JH. A case of successful reperfusion through a combination of intracoronary thrombolysis and aspiration thrombectomy in ST-segment elevation myocardial infarction associated with an ectatic coronary artery. BMC Cardiovasc Disord. 2017;17(1):94. 10.1186/s12872-017-0527-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higashi H, Inaba S, Nishimura K.et al. Usefulness of adjunctive pulse infusion thrombolysis after failed aspiration for massive intracoronary thrombus. Can J Cardiol. 2011;27(6):869.e1 869.e2. 10.1016/j.cjca.2011.07.005) [DOI] [PubMed] [Google Scholar]
- 29. Xiao Y, Fu X, Wang Y.et al. Effects of different strategies on high thrombus burden in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary catheterization. Coron Artery Dis. 2019;30(8):555 563. 10.1097/MCA.0000000000000743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barua S, Geenty P, Deshmukh T.et al. The role of intracoronary thrombolysis in selected patients presenting with ST-elevation myocardial infarction: a case series. Eur Heart J J Case Rep. 2020;4(5):1 10. 10.1093/ehjcr/ytaa227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCartney PJ, Eteiba H, Maznyczka AM.et al. Effect of low-dose intracoronary alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with acute myocardial infarction: a randomized clinical trial. JAMA. 2019;321(1):56 68. 10.1001/jama.2018.19802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yalta K, Öztürk C, Yalta T, Özkan U. Intracoronary fibrinolysis: an effective yet underutilized therapeutic strategy in clinical practice. Anatol J Cardiol. 2021;25(8):598 599. 10.5152/AnatolJCardiol.2021.297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Özkan M, Gündüz S, Biteker M.et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging. 2013;6(2):206 216. 10.1016/j.jcmg.2012.10.016) [DOI] [PubMed] [Google Scholar]
- 34. Özkan M, Gündüz S, Gürsoy OM.et al. Ultraslow thrombolytic therapy: a novel strategy in the management of PROsthetic MEchanical valveThrombosis and the prEdictors of outcomE: the Ultra-slow PROMETEE trial. Am Heart J. 2015;170(2):409 418. 10.1016/j.ahj.2015.04.025) [DOI] [PubMed] [Google Scholar]
- 35. Güner A, Kalçik M, Aykan AÇ.et al. Clinical safety and efficacy of thrombolytic therapy with low-dose prolonged infusion of tissue type plasminogen activator in patients with intermediate-high risk pulmonary embolism. Blood Coagul Fibrinolysis. 2020;31(8):536 542. 10.1097/MBC.0000000000000960) [DOI] [PubMed] [Google Scholar]
- 36. Yesin M, Karakoyun S, Kalçık M.et al. Status of the Epicardial Coronary arteries in non-ST Elevation Acute Coronary Syndrome in patients with Mechanical Prosthetic Heart Valves (from the TROIA-ACS Trial). Am J Cardiol. 2018;122(4):638 644. 10.1016/j.amjcard.2018.04.045) [DOI] [PubMed] [Google Scholar]
- 37. Karakoyun S, Gürsoy MO, Kalçık M, Yesin M, Özkan M. A case series of prosthetic heart valve thrombosis-derived coronary embolism. Turk Kardiyol Dern Ars. 2014;42(5):467 471. 10.5543/tkda.2014.05031) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a