Abstract
Objectives
This study aims to reveal the relationship between serum total antioxidant status (TAS), clinical parameters, and nutrition (dietary total antioxidant capacity [TAC]) in patients with fibromyalgia.
Patients and methods
This cross-sectional study was conducted with a total of 60 female participants (mean age: 44.7±9.7 years; range, 18 to 50 years) at Gaziler Physical Medicine and Rehabilitation Hospital between July 2020 and February 2021. Thirty female patients with fibromyalgia were compared with 30 age-, sex-, and body mass index-matched healthy individuals. The short-form McGill Pain Questionnaire, Fibromyalgia Impact Questionnaire (FIQ), and Pittsburg Sleep Quality Index were used. Total antioxidant status, total oxidant status (TOS), and oxidative stress index (OSI) were measured. Dietary TAC was calculated using the ferric reducing ability of plasma according to a food frequency questionnaire.
Results
Total antioxidant status showed no significant difference between groups (p=0.080). Total oxidant status and OSI were significantly higher in the patient group (p<0.001 and p=0.005, respectively). The mean dietary TAC was 16.5±6.5 in the patient group and 17.2±6.2 in the control group, and it was similar between groups (p=0.492). Pittsburg Sleep Quality Index global score was significantly higher in the patient group than in the control group (p<0.001). Dietary TAC showed a moderate positive correlation with serum TAS in both groups (r=0.373, p=0.042 for the patient group, and r=0.380, p=0.038 for the control group). In the patient group, TOS and OSI showed a moderate positive correlation with FIQ total scores (r=0.420, p=0.021 and r=0.450, p=0.013, respectively). The mean polyunsaturated fatty acid and omega-6 intake of the patient group was significantly lower than the control group (p=0.025 for both). Dietary antioxidant intake from vegetables (p=0.025), legumes/nuts (p=0.049), and meat (p<0.001) was significantly lower, whereas dietary antioxidant intake from cereal and potatoes was significantly higher in the patient group compared to the control group (p=0.028).
Conclusion
The results indicate that oxidative stress can be reduced by increasing dietary antioxidant intake in fibromyalgia.
Keywords: Antioxidants, dietary phytochemical index, fibromyalgia, oxidants
Introduction
Fibromyalgia syndrome (FMS) is a common rheumatic disease characterized by numerous symptoms such as widespread musculoskeletal pain, fatigue, sleep disturbance, anxiety, and stiffness.[1] The etiology and pathogenesis of FMS remain unclear; however, genetic, neuroendocrine, psychosocial, behavioral, and biochemical problems are thought to be responsible. To date, various studies have been conducted to identify a reliable pathogenic factor related to disease severity, and in the last decade, it has been stated that oxidative stress may be a causal factor.[2]
Reactive oxygen species (ROS) are substrates that include unpaired electrons. They attack membrane lipids (particularly polyunsaturated fatty acids), change membrane potentials and integrity, and therefore result in lipid peroxidation. Lipid peroxidation causes membrane rupture and the release of cellular materials to extracellular space.[3] In normal circumstances, the oxidant and antioxidant status of the cellular and extracellular space are in balance. Any disruption of this balance will direct it towards the oxidant or antioxidant side.
Higher quantities of ROS will lower antioxidant capacity and result in high oxidative stress.[4] Previous studies have demonstrated a relationship between oxidative stress and pain perception in chronic fatigue syndrome and other inflammatory diseases.[5] It was established that antioxidant capacity is lower in healthy subjects than in patients with FMS.[4] In addition, oxidative stress due to high lipid peroxidation is also mentioned in FMS.[6] Considering oxidative stress mechanisms and low inflammatory status in FMS, oxidative stress is thought to be related with FMS pathogenesis and disease course.
As mentioned above, the disease manifests with a wide range of symptoms from pain to sleep disturbance. These symptoms have a significant impact on the patients’ quality of life (QoL); therefore, investigators have been trying to define the association between clinical and biochemical parameters. In a previous report, Sendur et al.,[7] found a correlation between antioxidant status, pain, and morning stiffness, while Bagis et al.[8] did not find any correlation.
Accumulating data about diet and FMS revealed that diet may be a contributing factor in the disease pathogenesis of FMS.[9] Chronic pain is thought to be associated with noxious stimulus caused by neurotransmitters such as substance P and glutamate.[10] Substance P increases the permeability of the blood-brain barrier, and increased permeability may permit excess dietary glutamate into the central neural tissues and affect pain perception.[11] Micronutrients are also important in FMS. Low magnesium and zinc levels and deficiency in vitamin B6 and omega-3 fatty acids are known to support excitotoxicity. This effect has been demonstrated in N-methyl-D-aspartate and gamma-aminobutyric acid receptors, and the resulting excitotoxicity can cause oxidative stress.[12] Sufficient antioxidant intake is important to increase the total antioxidant capacity (TAC) of the organism. One of the most well-known measurements that can be used to determine the amount of dietary antioxidant intake is the calculation of dietary TAC. This measurement is a method of summing the amount of antioxidants contained in each food separately and was defined by Carlsen et al.[13]
The aim of this study was to reveal the relationship between serum total antioxidant status (TAS), clinical parameters and nutrition (dietary TAC) in patients with FMS.
Patients and Methods
This cross-sectional study was conducted with 60 female participants (mean age: 44.7±9.7 years; range, 18 to 50 years) at the Gaziler Physical Medicine and Rehabilitation Hospital between July 2020 and February 2021; of the participants, 30 were FMS patients who met the 2010 American College of Rheumatology criteria.[14] A healthy control group was formed of 30 age-, sex-, and body mass index (BMI)-matched healthy individuals. Smokers, alcohol users, nutritional supplement users, patients with additional diseases that may affect oxidative status such as diabetes mellitus, hyperthyroidism, respiratory diseases, malignancy, cardiac, hematological, metabolic or other diseases were excluded. Control group exclusion criteria were the same as FMS group. Demographic data and medical history of the participants were recorded.
The Short-Form McGill Pain Questionnaire (SF-MPQ), Fibromyalgia Impact Questionnaire (FIQ) and Pittsburg Sleep Quality Index (PSQI) were used to evaluate the clinical characteristics of the patients. The SF-MPQ is a valid and reliable scale for the Turkish population and includes three main parts: sensory- affective descriptors, visual pain scale, and present pain inventory. The higher scores show the worse pain.[15]
The FIQ assesses the effect of FMS on QoL and is a valid and reliable questionnaire for the Turkish population.[16] The FIQ includes questions related to physical function, general wellness, number of days the patient was unable to work, pain, fatigue, morning tiredness, stiffness, anxiety, and depression. The total score of the FIQ is 100, and greater scores indicate a greater impact on QoL.
The PSQI is a questionnaire designed to assess seven domains concerning sleep (quality, latency, duration, sleep efficiency, sleep disturbance, medication use for sleep, and daytime dysfunction). The total score (global score) is 21, and scores above 5 indicate significant sleep disturbance. The validity and reliability of the PSQI were studied by Agargun et al.[17]
Body weight (kg), height (cm), and waist and hip circumference (cm) were measured. Body weight of the participants was measured using a calibrated electronic scale (Tanita HD-366; Tanita, Arlington Heights, IL, USA) with an accuracy of 0.1 kg, and the measurements were conducted when the patient was hungry, wearing lightweight clothes, and barefoot. Body height was measured from head to heel with a stadiometer. The BMI was calculated using the formula body weight (kg)/height (m2). Waist circumference was measured at 2 cm distal to the umbilicus, and hip circumference was measured at the widest perimeter. Hip circumference was subsequently measured using a tape measure at the highest point of the hip while standing on the left side of the individual. The waist/hip ratio was calculated by dividing waist circumference by hip circumference.
Blood samples were collected at 08:00-09:00 AM after 8 h fasting for TAS and total oxidant status (TOS). Samples were centrifugated at 3600 rpm for 10 min, and the serum was separated into Eppendorf tubes.
Total antioxidant status (μmol Trolox Eq/L) and TOS (μmol H2O2 Eq/L) analyses were done according to the Erel method.[18,19] Oxidative stress index (OSI) is an indicator of oxidative stress as a combined ratio between oxidants and antioxidants, and it was calculated as the ratio of TOS to TAS. While a lower ratio indicates better antioxidant-oxidant balance, a higher ratio means higher oxidative stress.
Dietary evaluation of the participants was done by two methods: 24-h dietary records and a semiquantitative food frequency questionnaire (FFQ). To determine the daily nutrient intake (energy, macronutrient, and micronutrient intake), 24-h dietary records were collected from all patients and controls. Portions were estimated by a picture booklet including 120 photographs of nutrients compatible with national foods.[20] Data obtained from the 24-h dietary records were entered into the Bebis version 7.2 software (Ebispro, Stuttgart, Germany) to calculate energy, macronutrient, and micronutrient intakes.[21] In addition to the 24-h dietary records, a semiquantitative FFQ was administered to all participants during the last month. The FFQ included a wide list of nutrients and responses about how often each food was eaten in a certain time interval. The dietary TAC was calculated from the FFQ data using the ferric reducing ability of plasma defined by Carlsen et al.[13] A list of nutrients with more than 3,100 items with TAC values (mmol/100 g) was used for calculation, and the sum of all TAC values was presented in mmol/1000 kcal.
Statistical analysis
Statistical analysis was performed with the IBM SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). Categorical variables were presented as numbers and percentages, and continuous variables were presented as means ± standard deviation. The normality of variables was assessed with the Kolmogorov-Smirnov test. The chi-square test was used to compare categorical data, and the Mann- Whitney U test was used to compare continuous data. Correlation analyses were done by the Spearman test. To determine significant predictors, a multiple regression analysis was planned; however, in the univariate analysis, the p value of the parameters BMI, age, waist circumference, hip circumference, and waist/hip ratio was not lower than 0.25. Therefore, a multiple regression model was not formed. The statistical significance threshold was set at p<0.05.
Results
The main findings of the patients and controls are presented in Table 1. Groups were similar in terms of age (p=0.219). The BMI was similar between groups (p=0.056), but waist circumference, hip circumference, and waist/hip ratio were significantly higher in the patient group compared to the control group (p<0.001, p=0.020, and p<0.001, respectively). The mean disease interval was 4.5±4.5 years in the study group.
Table 1. Main findings of the patient and control groups.
| Characteristics | Patient group (n=30) | Control group (n=30) | p | ||||
| n | % | Mean±SD | n | % | Mean±SD | ||
| Age (year) | 46.0±9.8 | 43.5±9.5 | 0.219 | ||||
| Disease interval (year) | 4.5±4.5 | NA | |||||
| Body mass index (kg/m2) | 27.0±4.6 | 25.0±4.3 | 0.056 | ||||
| Waist circumference (cm) | 93.8±13.7 | 77.4±12.5 | <0.001 | ||||
| Hip circumference (cm) | 105.2±11.0 | 98.8±5.9 | 0.020 | ||||
| Waist/hip ratio | 0.9±0.1 | 0.8±1.0 | <0.001 | ||||
| Education | 0.168 | ||||||
| Illiterate | 1 | 3.3 | 0 | 0.0 | |||
| Primary school | 5 | 16.7 | 7 | 23.3 | |||
| Secondary-High school | 9 | 30.0 | 3 | 10.0 | |||
| University or higher | 15 | 50.0 | 20 | 66.7 | |||
| McGill Sensory | 10.2±6.8 | NA | NA | ||||
| McGill Affective | 4.9±3.8 | NA | NA | ||||
| Total Pain Rating Index | 15.1±10.7 | NA | NA | ||||
| McGill present pain intensity | 3.8±0.9 | NA | NA | ||||
| McGill VAS | 75.6±22.3 | NA | NA | ||||
| FIQ total | 63.7±21.4 | NA | NA | ||||
| Global PSQI score | 11.9±3.5 | 3.9±1.8 | <0.001 | ||||
| TAS (pmol Trolox Eq/L) | 1.7±0.2 | 1.6±0.3 | 0.080 | ||||
| TOS (gmol H2O2 Eq/L) | 8.1±3.9 | 5.1±2.1 | <0.001 | ||||
| OSI | 0.4±0.2 | 0.3±0.1 | 0.005 | ||||
| Dietary total antioxidant capacity (mmol/1000 kcal) | 16.4±6.5 | 17.2±6.2 | 0.492 | ||||
| SD: Standard deviation; VAS: Visual Analog Scale; FIQ: Fibromyalgia Impact Questionnaire; PSQI: Pittsburg Sleep Quality Index; TAS: Total antioxidant status; TOS: Total oxidant status; OSI: Oxidative stress index; NA: Not applicable. | |||||||
The mean TAS was 1.7±0.2 μmol Trolox Eq/L in the patient group and 1.6±0.3 μmol Trolox Eq/L in the control group, and there was no significant difference between groups (p=0.080). The mean TOS was 8.1±3.9 μmol H2O2 Eq/L in the patient group and 5.1±2.1 μmol H2O2 Eq/L in the control group. The mean OSI was 0.4±0.2 in the patient group and 0.3±0.1 in the control group. The TOS and OSI were significantly higher in the patient group (p<0.001 and p=0.005, respectively). The mean dietary TAC was 16.5±6.5 in the patient group and 17.2±6.2 in the control group, and it was similar between groups (p=0.492).
The MPQ score, FIQ total score, and PSQI global score are shown in Table 1. The mean PSQI global score was 11.9±3.5 in the patient group and 3.9±1.8 in the control group, and it was significantly higher in the patient group compared to the control group (p<0.001). In correlation analysis (Figures 1 and 2), dietary TAC showed a significant moderate positive correlation with serum TAS in both groups (r=0.373, p=0.042 for the patient group; r=0.380, p=0.038 for the control group). Dietary TAC was not correlated with serum TOS, OSI, BMI, waist circumference, hip circumference, and waist/hip ratio. In the patient group, according to correlation analysis, TOS and OSI showed a moderate positive correlation with FIQ total score (r=0.420, p=0.021 and r=0.450, p=0.013, respectively; Table 2).
Figure 1. Correlation analysis between dietary TAC and TAS (patient group). TAC: Total antioxidant capacity; TAS: Total antioxidant status.
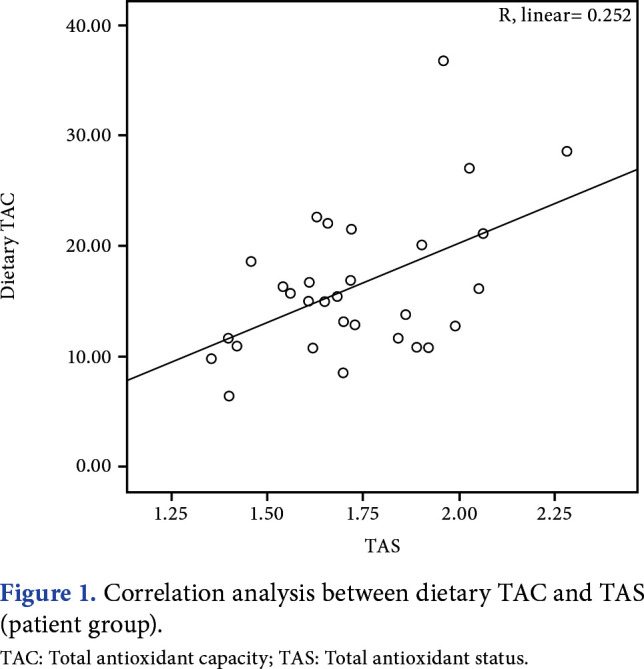
Figure 2. Correlation analysis between dietary TAC and TAS (control group). TAC: Total antioxidant capacity; TAS: Total antioxidant status.
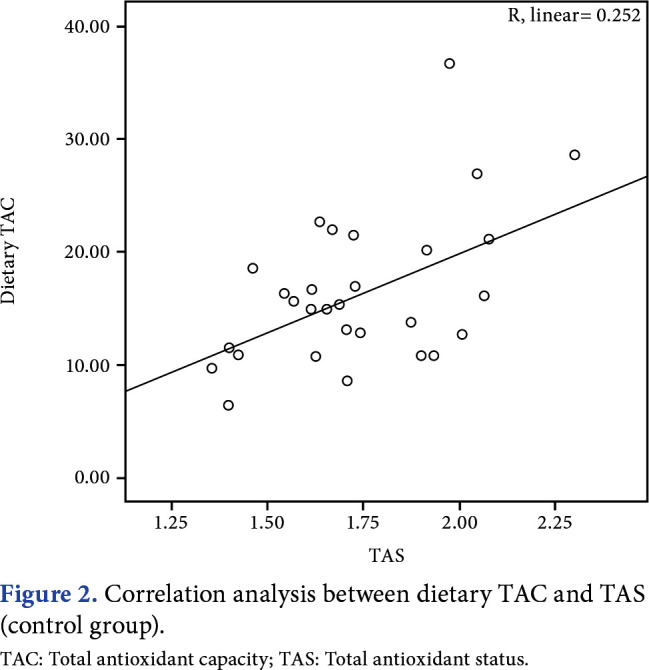
Table 2. Correlations between the McGill Pain Questionnaire, FIQ, total PSQI and TAS, TOS, OSI, and dietary TAC.
| McGill sensory | McGill affective | Total Pain Rating Index | McGill present pain intensity | McGill VAS | FIQ total | PSQI | ||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| TAS | -0.099 | 0.601 | 0.102 | 0.593 | 0.155 | 0.469 | -0.112 | 0.557 | -0.045 | 0.813 | -0.317 | 0.087 | 0.191 | 0.320 |
| TOS | 0.255 | 0.174 | 0.262 | 0.161 | 0.217 | 0.308 | 0.107 | 0.572 | 0.239 | 0.203 | 0.420 | 0.021 | -0.222 | 0.347 |
| OSI | 0.329 | 0.076 | 0.235 | 0.212 | 0.209 | 0.327 | 0.147 | 0.439 | 0.241 | 0.200 | 0.450 | 0.013 | -0.268 | 0.160 |
| Dietary TAC | -0.249 | 0.185 | 0.063 | 0.740 | -0.146 | 0.440 | -0.153 | 0.421 | -0.082 | 0.666 | 0.146 | 0.440 | 0.096 | 0.621 |
| FIQ: Fibromyalgia Impact Questionnaire; PSQI: Pittsburg Sleep Quality Index; TAS: Total antioxidant status; TOS: Total oxidant status; OSI: Oxidative stress index; TAC: Total antioxidant capacity; VAS: Visual Analog Scale. | ||||||||||||||
Daily energy and nutrient intake of the patients and controls was given in Table 3. The mean polyunsaturated fatty acid (PUFA) and omega-6 intake of the patient group was significantly lower than that of the control group (p=0.025 for both).
Table 3. Daily energy and nutrients intake of the patient and control groups.
| Patient group | Control group | p | |
| Mean±SD | Mean±SD | ||
| Energy | 1915.4±416.1 | 1783.9±317.1 | 0.165 |
| Carbohydrates | 215.9±68.3 | 183.4±50.2 | 0.056 |
| Protein (total) | 71.1±23.8 | 68.0±14.1 | 0.994 |
| Animal-based protein | 37.8±23.0 | 37.7±14.9 | 0.506 |
| Plant-based protein | 33.2±10.7 | 30.3±8.7 | 0.264 |
| Fat | 83.1±24.9 | 84.3±18.6 | 0.636 |
| Fiber | 25.5±9.1 | 22.3±6.0 | 0.337 |
| MUFA | 30.9±11.2 | 29.9±10.2 | 0.836 |
| PUFA | 21.6±8.1 | 26.8±9.6 | 0.025 |
| Omega 3 | 1.0±0.3 | 1.5±1.0 | 0.025 |
| Omega 6 | 20.5±8.0 | 25.2±9.5 | 0.053 |
| SFA | 25.1±11.1 | 22.4±3.8 | 0.790 |
| Cholesterol | 252.8±164.6 | 215.3±126.0 | 0.451 |
| Vitamin B12 | 4.0±2.3 | 4.6±2.4 | 0.228 |
| Vitamin A | 910.2±586.6 | 1104.6±1064.8 | 0.848 |
| Vitamin C | 125.5±92.4 | 116.9±84.1 | 0.790 |
| Vitamin E | 22.7±8.5 | 24.4±9.6 | 0.813 |
| Carotenoids | 3.1±3.1 | 3.3±2.8 | 0.906 |
| Vitamin B6 | 1.5±0.5 | 1.6±0.5 | 0.294 |
| Zinc | 10.7±2.9 | 10.0±1.9 | 0.530 |
| SD: Standard deviation; MUFA: Monounsaturated fatty acid; PUFA: Polyunsaturated fatty acid; SFA: Saturated fatty acid. | |||
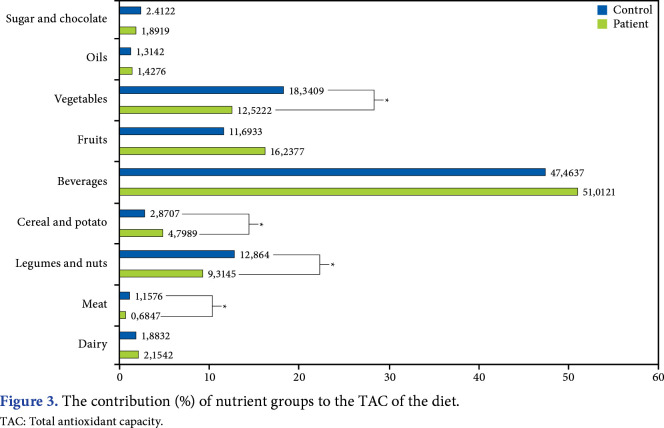
The contribution (%) of nutrient groups to the dietary TAC was calculated and demonstrated in Figure 3. Dietary antioxidant intake from vegetables (p=0.025), legumes/nuts (p=0.049), and meat (p<0.001) was significantly lower in the patient group than in the control group. Dietary antioxidant intake from cereal and potatoes was significantly higher in the patient group compared to the control group (p=0.028).
Figure 3. The contribution (%) of nutrient groups to the TAC of the diet. TAC: Total antioxidant capacity.
Discussion
The end products of cell membrane damage (induced by reactive oxygen substrates) are elevated in patients with FMS.[22] In addition, it is shown that trigger points develop due to local hypoxia, and levels of enzymes, such as superoxide dismutase and catalase, are decreased in FMS.[7] Although research on how oxidative stress contributes to symptoms in FMS has yielded some conclusions, this issue is still unclear. In the light of this information, this study was planned to analyze oxidative stress parameters and their relationship with FMS symptoms and nutrition. The results revealed that oxidative stress in patients with FMS is higher than that of the healthy population, and this is demonstrated by both TOS and OSI levels. These findings were concordant with some results of previous studies.[23] In the current study, TAS was also expected to be lower in the patient group; however, we found no significant difference between groups. In the literature, there are studies indicating similar TAS and TOS levels between patients and healthy subjects.[24] The inconsistency may be due to different patient characteristics, dietary TAC, inflammation status, or laboratory methodologies.
As demonstrated in the main findings of the participants, waist circumference, hip circumference, and waist/hip ratio were significantly higher in the patient group than in the control group. Considering these differences, the difference in TAS and TOS levels between the groups may be due to the anthropometric data, but the current literature does not support this explanation. Mozaffari et al.[25] examined the association between serum antioxidant levels and waist circumference, and they reported no significant association. In another study that aimed to investigate the relationship between body composition and antioxidant defense capacity of cells (total thiol level), while a weak correlation was found between total thiol level and waist circumference, there was no correlation with waist/hip ratio.[26] In addition to the data in the literature, univariate analysis showed that waist circumference, hip circumference, and waist/hip ratio differences between groups did not have significance on oxidative stress parameters. Therefore, although this may be interpreted as a limitation of the study, we suppose that the difference in TAS and TOS between the groups is not due to differences in the anthropometric measurements (waist circumference, hip circumference, and waist/hip ratio).
To date, no pathogenic mechanism alone is thought to be responsible for FMS, and no treatment alone provides widespread symptom relief in FMS. Instead, complex mechanisms play substantial roles in the disease course. In respect, nutrition-related factors were widely investigated. It is known that vitamins and some minerals are major antioxidants for organisms, and they serve as the first defense system against lipid peroxidation, cell membrane degradation, and free radical suppression.[27] In a study investigating the relationship between serum antioxidant vitamins (vitamins A, C, and E) and clinical parameters of FMS, no significant correlation was reported.[28] Bjørklund et al.[29] suggested that inadequate selenium intake was related to pain severity and recommended antioxidant intake for pain reduction. In addition, a previous study reported that energy, carbohydrate, and fat intake were lower in the patient group, and protein intake in patients with chronic fibromyalgia was positively correlated with pain perception.[30] In the current study, there was no difference between groups in terms of macronutrient intake, whereas PUFA and omega-3 intake of patients with FMS were lower than that in the control group. The contradictory results of the studies reveal the necessity of evaluating the entire nutritional pattern rather than focusing on a single nutrient. It is considered that nutrients eventually manifest synergistic effects instead of acting alone.[31] Dietary TAC is indicative of the cumulative abilities of diet antioxidants, rather than a single nutrient. It may demonstrate the synergistic effects of all foods’ antioxidative properties individually. Therefore, in the current study, we aimed to evaluate the dietary TAC, and it was correlated with serum TAS in patient and control groups. However, we did not find any correlation between dietary TAC and clinical assessment parameters. To the best of our knowledge, there is no study directly investigating the dietary relationship between TAC and FMS in the literature, though different diet modalities (e.g., vegan diet, chlorella green algae, extra virgin olive oil consumption, a diet low on oligo-, di-, monosaccharides and polyols) were researched, and links mostly associated with antioxidant-rich food groups have been expressed.[32,33] Here the fruit and vegetable group comes to the fore, particularly with their disease-preventing and QoL-enhancing properties. These food groups display their antioxidant capacity-enhancing properties with certain plant chemicals, such as resveratrol, polyphenol, and anthocyanin.[34] In our patient group, the antioxidant content was mostly caused by beverages, followed by the fruit and vegetable group. In addition, the contribution of foods to dietary TAC was different regarding some food groups between patient and control groups. While the percentage of legumes, nuts, vegetables, and meat were significantly higher in the control group, the percentage of cereal and potato was significantly higher in the patient group.
In the current study, a 24-h dietary record and FFQ were used. The 24-h dietary record is one of the most widely used tools in nutritional epidemiology and helps determine nutrient and energy intake in national nutrition surveys, cross-sectional studies, clinical trials, and cohort studies, as well as individual food intake. Although three-day and 7-day dietary records are used in clinical trials, the 24-h dietary record is also accepted as a valid and reliable tool for the assessment of energy and nutrient intake.[35] The FFQ is a useful tool to investigate the links between diet and disease, and in the current study, it was used to calculate the dietary TAC by the method described by Carlsen et al.[13] The FFQ assesses habitual consumption and is easy to administer. It also has some disadvantages. Respondent memory is required for accurate reporting, and over-or underestimation of certain foods may be encountered due to social factors and bias.[36] To minimize these problems, an experienced dietitian administered the FFQ in this study.
In patients with FMS, symptoms of the disease, such as pain, fatigue, and sleep disorder, may be disruptive. Therefore, it is crucial to detect relationships between these symptoms and biochemical factors that may probably affect the course of the disease. To date, efforts in this direction have shown that oxidative stress may play a number of roles in the pathogenesis and severity of this disease. Shukla et al.[37] reported a strong positive relationship between the FIQ scores and oxidative stress, which was exhibited by nitric oxide and lipid peroxidation. In the same study, a strong negative correlation between the FIQ scores and antioxidant enzymes was observed. Fais et al.[38] also investigated fibromyalgia severity and pain with the FIQ and the Visual Analog Scale. They reported significant correlation between TAS and FIQ. In addition, TAS and platelet-activating factor acetylhydrolase (an antioxidative enzyme) were decreased in the severe patient group. In the current study, TOS and OSI showed a significant moderate positive correlation with the FIQ total score. This result was in concordance with the above-mentioned studies. The MPQ scores were not correlated with laboratory findings and dietary TAC in the current study. We did not encounter any study investigating the relationship between the SF-MPQ and oxidative biomarkers in the literature, though, Sakarya et al.[28] used pain rating scales such as VAS, and they reported no correlation between antioxidant vitamins (vitamins A, C, and E). However, Sendur et al.[7] reported a significant correlation between nitric oxide and VAS score.[7] Nitric oxide is a mediator that plays a key role in pain modulation.[39] We did not use such a biomarker as it is directly associated with pain cascade and may hamper the ability to find a relationship between the two data.
The duration and quality of sleep have consequential effects on daily life, and therefore, impaired sleep is another substantial issue in FMS. Previous reports on FMS convey a high prevalence of sleep disorders. In the study of Andrade et al.,[40] 92.9% of FMS patients in their cohort claimed to have a sleep disorder. Previous studies about nutrition and sleep quality that included healthy subjects reported an association between higher fat intake and sleep disorders.[41] Very long-chain fatty acids are known to play a leading role in melatonin synthesis in the pineal gland. In a study based on this information, it was revealed that fatty acid supplementation for those with chronic sleep disorders did not have a significant effect on sleep and melatonin synthesis.[42] In the current study, impairment of sleep quality was more prominent in the patient group than in the control group, and it was remarkable that PUFA and omega-3 intake were lower in the patient group compared to the control group. We did not find any correlation between omega-3 intake, PSQI, TAS, TOS, OSI, and dietary TAC in the patient group, whereas the moderate negative correlation between PUFA intake and PSQI was intriguing.
The main limitations of this study are the small sample size and the baseline differences in groups in terms of waist circumference, hip circumference, and waist/hip ratio. A more comprehensive clinical assessment of patients with several tender points and a pain threshold would provide additional data.
In conclusion, considering the relationship between dietary oxidant capacity and blood antioxidant level, it can be predicted that oxidative stress can be reduced by increasing dietary antioxidant intake in fibromyalgia. Dietary intervention studies are needed to clarify whether the signs and symptoms of the disease can be controlled with this method.
Footnotes
Ethics Committee Approval: The study protocol was approved by the University of Health Sciences Türkiye Ethics Committee (Decision date: 26 February 2019, Decision no: 19/17). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Conceived and designed the analysis: K.T.A, Ö.K.; Patient selection: K.T.A., Ö.K., A.K.T.; Collected the data: K.T.A, Ö.K.; Prepared blood samples: K.T.A, Ö.K; Contributed data or analysis tools: K.T.A, Ö.K., E.Y., A.K.T., G.S.; Calculation of of dietary: T.A.C, G.S., K.T.A.; Performed statistical analysis: E.Y.; Searched literature and wrote the paper: K.T.A., Ö.K., G.S.; Supervised the paper: E.Y., A.K.T., G.S.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from the participants.
References
- 1.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27–27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helfenstein M Jr, Goldenfum MA, Siena CA. Fibromyalgia: Clinical and occupational aspects. Rev Assoc Med Bras (1992) 2012;58:358–365. [PubMed] [Google Scholar]
- 3.Fatima G, Das SK, Mahdi AA. Oxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: Implications in the pathogenesis of the disease. S128-33Clin Exp Rheumatol. 2013;31(6 Suppl 79) [PubMed] [Google Scholar]
- 4.Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: The potential role of oxidative stress and nitric oxide. Rheumatol Int. 2006;26:585–597. doi: 10.1007/s00296-005-0078-z. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 6.Cordero MD, de Miguel M, Carmona-López I, Bonal P, Campa F, Moreno-Fernández AM. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol Lett. 2010;31:169–173. [PubMed] [Google Scholar]
- 7.Sendur OF, Turan Y, Tastaban E, Yenisey C, Serter M. Serum antioxidants and nitric oxide levels in fibromyalgia: A controlled study. Rheumatol Int. 2009;29:629–633. doi: 10.1007/s00296-008-0738-x. [DOI] [PubMed] [Google Scholar]
- 8.Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, et al. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder. Rheumatol Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 9.Tomaino L, Serra-Majem L, Martini S, Ingenito MR, Rossi P, La Vecchia C, et al. Fibromyalgia and nutrition: An updated review. J Am Coll Nutr. 2021;40:665–678. doi: 10.1080/07315724.2020.1813059. [DOI] [PubMed] [Google Scholar]
- 10.Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Al-Ahmad AJ, Pervaiz I, Karamyan VT. Neurolysin substrates bradykinin, neurotensin and substance P enhance brain microvascular permeability in a human in vitro model. e12931J Neuroendocrinol. 2021;33 doi: 10.1111/jne.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savic Vujovic KR, Vuckovic S, Srebro D, Medic B, Stojanovic R, Vucetic C, et al. A synergistic interaction between magnesium sulphate and ketamine on the inhibition of acute nociception in rats. Eur Rev Med Pharmacol Sci. 2015;19:2503–2509. [PubMed] [Google Scholar]
- 13.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3–3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 15.Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 16.Sarmer S, Ergin S, Yavuzer G. The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheumatol Int. 2000;20:9–12. doi: 10.1007/s002960000077. [DOI] [PubMed] [Google Scholar]
- 17.Agargün MY, Kara H, Anlar O. Pittsburgh uyku kalitesi indeksinin geçerliği ve güvenirliği. Türk Psikiyatri Dergisi. 1996;7:107–115. [Google Scholar]
- 18.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Rakıcıoğlu N, Tek Acar N, Ayaz A, Pekcan G. Yemek ve Besin Fotograf Kataloğu-Ölçü ve Miktarlar. 2. Baskı. Ankara: Ata Ofset Matbaacılık; 2012. [Google Scholar]
- 21.Bebis (Beslenme Bilgi Sistemi) Nutrition Database Software . Database: The German Food Code and Nutrient Data Base (BLS II.3, 1999) with additions from USDA-sr and other sources. Istanbul: 2004. [Google Scholar]
- 22.Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: Etiologic and therapeutic concerns. Rheumatol Int. 2006;26:598–603. doi: 10.1007/s00296-005-0079-y. [DOI] [PubMed] [Google Scholar]
- 23.Fatima G, Das SK, Mahdi AA. Some oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndrome. Int J Rheum Dis. 2017;20:39–45. doi: 10.1111/1756-185X.12550. [DOI] [PubMed] [Google Scholar]
- 24.Akbas A, Inanir A, Benli I, Onder Y, Aydogan L. Evaluation of some antioxidant enzyme activities (SOD and GPX) and their polymorphisms (MnSOD2 Ala9Val, GPX1 Pro198Leu) in fibromyalgia. Eur Rev Med Pharmacol Sci. 2014;18:1199–1203. [PubMed] [Google Scholar]
- 25.Mozaffari H, Daneshzad E, Larijani B, Surkan PJ, Azadbakht L. Association of dietary total antioxidant capacity to anthropometry in healthy women: A cross-sectional study. Nutrition. 2020;69:110577–110577. doi: 10.1016/j.nut.2019.110577. [DOI] [PubMed] [Google Scholar]
- 26.Anusruti A, Jansen EHJM, Gào X, Xuan Y, Brenner H, Schöttker B. Longitudinal associations of body mass index, waist circumference, and waist-to-hip ratio with biomarkers of oxidative stress in older adults: Results of a large cohort study. Obes Facts. 2020;13:66–76. doi: 10.1159/000504711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaźmierczak-Barańska J, Boguszewska K, Karwowski BT. Nutrition can help DNA repair in the case of aging. Nutrients. 2020;12:3364–3364. doi: 10.3390/nu12113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakarya ST, Akyol Y, Bedir A, Canturk F. The relationship between serum antioxidant vitamins, magnesium levels, and clinical parameters in patients with primary fibromyalgia syndrome. Clin Rheumatol. 2011;30:1039–1043. doi: 10.1007/s10067-011-1697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjørklund G, Dadar M, Chirumbolo S, Aaseth J. Fibromyalgia and nutrition: Therapeutic possibilities. Biomed Pharmacother. 2018;103:531–538. doi: 10.1016/j.biopha.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 30.Batista ED, Andretta A, de Miranda RC, Nehring J, Dos Santos Paiva E, Schieferdecker ME. Food intake assessment and quality of life in women with fibromyalgia. Rev Bras Reumatol Engl Ed. 2016;56:105–110. doi: 10.1016/j.rbre.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Rus A, Molina F, Ramos MM, Martínez-Ramírez MJ, Del Moral ML. Extra virgin olive oil improves oxidative stress, functional capacity, and health-related psychological status in patients with fibromyalgia: A preliminary study. Biol Res Nurs. 2017;19:106–115. doi: 10.1177/1099800416659370. [DOI] [PubMed] [Google Scholar]
- 33.Marum AP, Moreira C, Saraiva F, Tomas-Carus P, Sousa-Guerreiro C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand J Pain. 2016;13:166–172. doi: 10.1016/j.sjpain.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30:511–517. doi: 10.1016/j.nut.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Salvador Castell G, Serra-Majem L, Ribas-Barba L. What and how much do we eat? 24-hour dietary recall method. Nutr Hosp. 2015;31 Suppl 3:46–48. doi: 10.3305/nh.2015.31.sup3.8750. [DOI] [PubMed] [Google Scholar]
- 36.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. e2014009Epidemiol Health. 2014;36 doi: 10.4178/epih/e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla V, Kumar DS, Ali MA, Agarwal S, Khandpur S. Nitric oxide, lipid peroxidation products, and antioxidants in primary fibromyalgia and correlation with disease severity. J Med Biochem. 2020;39:165–170. doi: 10.2478/jomb-2019-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fais A, Cacace E, Atzori L, Era B, Ruggiero V. Plasma phospholipase, γ-CEHC and antioxidant capacity in fibromyalgia. Int J Rheum Dis. 2017;20:550–554. doi: 10.1111/1756-185X.12787. [DOI] [PubMed] [Google Scholar]
- 39.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: Effects of training in health and cardiovascular disease. FASEB J. 2000;14:1685–1696. doi: 10.1096/fj.99-0896rev. [DOI] [PubMed] [Google Scholar]
- 40.Andrade A, Vilarino GT, Sieczkowska SM, Coimbra DR, Bevilacqua GG, Steffens RAK. The relationship between sleep quality and fibromyalgia symptoms. J Health Psychol. 2020;25:1176–1186. doi: 10.1177/1359105317751615. [DOI] [PubMed] [Google Scholar]
- 41.Tan X, Alén M, Cheng SM, Mikkola TM, Tenhunen J, Lyytikäinen A, et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res. 2015;24:414–424. doi: 10.1111/jsr.12283. [DOI] [PubMed] [Google Scholar]
- 42.Cornu C, Remontet L, Noel-Baron F, Nicolas A, Feugier-Favier N, Roy P, et al. A dietary supplement to improve the quality of sleep: A randomized placebo controlled trial. BMC Complement Altern Med. 2010;10:29–29. doi: 10.1186/1472-6882-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]