Abstract
Erwinia carotovora subsp. carotovora produces extracellular pectate lyase (Pel), polygalacturonase (Peh), cellulase (Cel), and protease (Prt). The concerted actions of these enzymes largely determine the virulence of this plant-pathogenic bacterium. E. carotovora subsp. carotovora also produces HarpinEcc, the elicitor of the hypersensitive reaction. We document here that KdgREcc (Kdg, 2-keto-3-deoxygluconate; KdgR, general repressor of genes involved in pectin and galacturonate catabolism), a homolog of the E. chrysanthemi repressor, KdgREch and the Escherichia coli repressor, KdgREco, negatively controls not only the pectinases, Pel and Peh, but also Cel, Prt, and HarpinEcc production in E. carotovora subsp. carotovora. The levels of pel-1, peh-1, celV, and hrpNEcc transcripts are markedly affected by KdgREcc. The KdgREcc− mutant is more virulent than the KdgREcc+ parent. Thus, our data for the first time establish a global regulatory role for KdgREcc in E. carotovora subsp. carotovora. Another novel observation is the negative effect of KdgREcc on the transcription of rsmB (previously aepH), which specifies an RNA regulator controlling exoenzyme and HarpinEcc production. The levels of rsmB RNA are higher in the KdgREcc− mutant than in the KdgREcc+ parent. Moreover, by DNase I protection assays we determined that purified KdgREcc protected three 25-bp regions within the transcriptional unit of rsmB. Alignment of the protected sequences revealed the 21-mer consensus sequence of the KdgREcc-binding site as 5′-G/AA/TA/TGAAA[N6]TTTCAG/TG/TA-3′. Two such KdgREcc-binding sites occur in rsmB DNA in a close proximity to each other within nucleotides +79 and +139 and the third KdgREcc-binding site within nucleotides +207 and +231. Analysis of lacZ transcriptional fusions shows that the KdgR-binding sites negatively affect the expression of rsmB. KdgREcc also binds the operator DNAs of pel-1 and peh-1 genes and represses expression of a pel1-lacZ and a peh1-lacZ transcriptional fusions. We conclude that KdgREcc affects extracellular enzyme production by two ways: (i) directly, by inhibiting the transcription of exoenzyme genes; and (ii) indirectly, by preventing the production of a global RNA regulator. Our findings support the idea that KdgREcc affects transcription by promoter occlusion, i.e., preventing the initiation of transcription, and by a roadblock mechanism, i.e., by affecting the elongation of transcription.
Erwinia carotovora subsp. carotovora causes tissue-macerating or soft-rotting disease in plants or plant organs (10, 42). The elicitation of this disease requires the production of extracellular enzymes, especially pectinases such as pectate lyase (Pel), polygalacturonase (Peh), and pectin lyase (Pnl), which are responsible for degrading plant cell wall components (2, 3). The genes for exoenzymes are subject to transcriptional as well as posttranscriptional regulation (28, 56). A number of transcriptional factors, including, for example, AepA (36), HexA (17), HexY (50), Hor (54), RpoS (34), Rpf (16), ExpAB (14), and RdgAB (26, 29, 30), have been identified. Expression of pel, peh, cel, and prt is also influenced by plant signals as well as the cell density (quorum) sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone (OHL) (5, 8, 24, 36, 44). How these transcriptional factors and signals interact to modulate the expression of these exoenzyme genes has not yet been elucidated.
RsmA-rsmB constitutes a novel regulatory pair responsible for posttranscriptional regulation of exoenzyme genes (28). RsmA, an RNA-binding protein, promotes the decay of the transcripts of many genes (5, 12). rsmB, formerly known as aepH (35), encodes a unique RNA regulator which is presumed to affect the levels of RsmA, neutralize the RsmA action, or both (28). This regulatory system controls many traits, including the synthesis of OHL, extracellular enzymes, elicitors of the hypersensitive reaction, phytohormones, and extracellular polysaccharides, as well as other traits such as pathogenicity factors, bacterial motility, and various secondary metabolites. The elegant and extensive work of Romeo and associates in Escherichia coli have characterized a homologous system comprising CsrA and csrB (25, 48). This regulatory pair controls glycogen accumulation, cell surface properties, and cell size in E. coli (25, 49).
The current model (28) postulates that RsmA and rsmB act in concert to modulate the expression of many genes, particularly those that are expressed in a growth-phase-dependent manner. Since rsmA specifies an RNA-binding protein which promotes message decay, it is reasonable to assume that RsmA levels and RsmA activity are probably rigorously controlled by bacteria to prevent the extensive decay of transcripts of genes for growth and housekeeping functions. In addition to rigorous regulation of rsmA expression (33, 34), the modulation of the RsmA effect is mainly accomplished by the production of rsmB RNA (28). It therefore follows that factors controlling the production of rsmB RNA could have a profound effect on exoenzyme and other metabolite production.
Extensive studies in E. chrysanthemi (3, 21) have established that KdgR negatively regulates the genes involved in pectin degradation (Kdg, 2-keto-3-deoxygluconate; KdgR, general repressor of genes involved in pectin and galacturonate catabolism). In fact, KdgREch has been found to affect the expression of at least 13 operons of E. chrysanthemi involved in pectin catabolism and enzyme export via the type II secretion pathway (21). Through systematic analysis of the KdgREch binding to operators, Nasser and associates have elucidated the consensus KdgREch binding site (KDGR box) for the E. chrysanthemi genes (38). Although putative KdgR-binding sequences have been detected within several E. carotovora subsp. carotovora pectinase genes (6, 20, 27), as well as in rsmB (28, 35), to our knowledge there has been no report documenting the regulatory effects of KdgREcc. In this work we (i) show that kdgREcc has high homology with the corresponding genes of E. chrysanthemi and E. coli; (ii) document overproduction and purification of KdgREcc from E. coli; (iii) establish that KdgREcc is a DNA binding protein; (iv) localize the KdgR-binding sites; and (v) show that the production of exoenzymes, HarpinEcc, and rsmB transcripts is derepressed in a KdgREcc− mutant constructed by marker exchange and that kdgREcc+ DNA exerts a negative trans-dominant effect. The findings reported here for the first time demonstrate that KdgR affects the levels of Cel, Prt, and HarpinEcc, in addition to the pectinases, and that in E. carotovora subsp. carotovora KdgR regulates the structural genes for some of the exoenzymes directly, as well as indirectly by controlling the expression of a global regulator. Furthermore, we present data that support the hypothesis that KdgREcc affects gene expression in E. carotovora subsp. carotovora by interfering with the initiation of transcription as well as by preventing the elongation of transcription by a roadblock mechanism.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used here are listed in Table 1. Recipes of Luria-Bertani (LB) medium and minimal salts medium have been described (5, 9). When required, antibiotics were supplemented as follows (μg/ml): ampicillin (Ap), 100; kanamycin (Km), 50; spectinomycin (Sp), 50; and tetracycline (Tc), 10. Media were solidified by the addition of 1.5% (wt/vol) agar.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strain | ||
| E. carotovora subsp. carotovora | ||
| Ecc71 | Wild type | 57 |
| AC5073 | KdgREcc− mutant of Ecc71, Spr | This work |
| E. chrysanthemi EC16 | Wild type | 7 |
| E. coli | ||
| DH5α | φ80lacZΔM15 Δ(lacZYA-argF) U169 hsdR17 recA1 endA1 thi-1 | Gibco-BRL |
| JM109(DE3) | endA1 recA1 gyrA96 hsdR17 supE44 relA1 thi Δ(lac-pro) F′ (traD36 proAB+ lacIqlacZΔM15) λcI857 ind1 Sam7 lacU5-T7 gene 1 | Promega Biotec |
| Plasmid | ||
| pBluescript SK(+) | Apr | Stratagene |
| pCRII | Apr | Invitrogen |
| pDK6 | Kmr | 23 |
| pET-28b(+) | Kmr | Novagen |
| pGEM-T Easy | Apr | Promega Biotec |
| pHP45Ω | Source of Spr omega fragment | 45 |
| pLARF5 | Tcr | 22 |
| pMP220 | Tcr | 52 |
| pRK415 | Tcr | 22 |
| pRK2013 | Mob+ Tra+ Kmr | 15 |
| pT7-7 | Apr | 53 |
| pAKC781 | Apr, peh-1+ | 27 |
| pAKC783 | Apr, pel-1+ | 27 |
| pAKC923 | Apr, hrpNEcc+ DNA in pBluescript SK(+) | 13 |
| pAKC924 | Apr, hrpNEcc DNA in pBluescript SK(+) | 13 |
| pAKC1002 | Tcr, rsmB-lacZ fusion | 28 |
| pAKC1014 | Apr, rsmB DNA in pBluescript SK(+) | 28 |
| pAKC1018 | Tcr, rsmB-lacZ fusion | 28 |
| pAKC1020 | Tcr, rsmB-lacZ fusion | 28 |
| pAKC1021 | Tcr, rsmB DNA | 28 |
| pAKC1023 | Apr, PCR product of EC16 kdgREch in pCRII | This work |
| pAKC1024 | Tcr, strain Ecc71 kdgREcc+ DNA in pLARF5 | This work |
| pAKC1025 | Tcr, kdgREcc+, 7.35-kb ClaI fragment from pAKC1024 in pRK415 | This work |
| pAKC1026 | Tcr, 1.2-kb EcoRI DNA containing part of kdgREcc from pAKC1025 cloned in pRK415 | This work |
| pAKC1027 | Spr Tcr, Ω-Sp insertion in the BstEII site of pAKC1026 | This work |
| pAKC1028 | Apr, pT7-kdgREcc in pT7-7 | This work |
| pAKC1029 | Kmr, pT7-kdgREcc-6His in pET-28b(+) | This work |
| pAKC1030 | Apr, 188-bp pel-1 DNA fragment in pBluescript SK(+) | This work |
| pAKC1031 | Tcr, 188-bp pel-1 DNA fragment from pAKC1030 in pMP220 | This work |
| pAKC1032 | Apr, 383-bp peh-1 DNA in pBluescript SK(+) | This work |
| pAKC1033 | Tcr, 383-bp peh-1 DNA fragment from pAKC1032 in pMP220 | This work |
| pAKC1034 | Apr, 200-bp DNA fragment of celV from strain Ecc71 in pGEM-T Easy | This work |
| pAKC1035 | Kmr, ptac-kdgREcc in pDK6 | This work |
Extracellular enzyme assays.
The compositions of agarose media for semiquantitative plate assay for extracellular cellulase (Cel), Pel, Peh, and protease (Prt) were previously described (5). The preparation of enzyme samples and quantitative Pel assays were carried out according to the method of Murata et al. (36).
PCR techniques.
The EasyStart kit (MßP, San Diego, Calif.) was used according to the manufacturer’s specifications for all PCR amplifications, which were performed on a OmniGene thermal cycler (Midwest Scientific, St. Louis, Mo.). The primer sequences are given in Table 2. All PCR products were electrophoresed through low-melting-point SeaPlaque agarose gel (Midwest Scientific). The appropriate bands were excised and purified by using the QIAquick gel extraction kit (Qiagen, Inc., Chatsworth, Calif.) prior to restriction endonuclease treatment and cloning.
TABLE 2.
Synthetic oligonucleotide primers
| Primer | Sequencea (5′ to 3′) | Positionb |
|---|---|---|
| KDGRP1 | TCAGCTKGAYCTGGTGCGYCAGCAG | +684 to +708 |
| KDGRM1 | GTCGGGAARGAGATRCTCAGRCCDG | +818 to +794 |
| KDGRS1 | TCAGCAGAACACAGCGTGTGCG | +596 to +575 |
| KDGRS2 | TCTGATATTCCCTATCAATGCCTG | +842 to +865 |
| KDGRS3 | ACGCAGGTTATACATCGAGTC | +447 to +427 |
| KDGRS4 | TCATCGGCTGCAAGAAGCG | +220 to +202 |
| KDGRS5 | ACGGACGACACGGAATCG | +51 to +31 |
| KDGRS6 | ACGGACGACACGGAATCG | +101 to +84 |
| KDGRS7 | GACATATGGCTAGTGCAGATTTAGA | +55 to +74 |
| KDGRS8 | AGAAGCTTGATAGGGAATATCAGAAAG | +859 to +839 |
| KDGRS10 | ATGTTGCGCAGGAAGGTG | +233 to +250 |
| KDGRS11 | AGAGGTTCTGTCGACTGTCG | +537 to +553 |
| KDGRS12 | ACACGAATATGTGGCTATG | +768 to +786 |
| KDGRS13 | ATTGTTTCCCGCGTTAGTGCAG | +377 to +357 |
| KDGRP2 | ATACCATGGCTAGTGCAGATTTAGATA | +55 to +76 |
| KDGRP3 | ATACTCGAGGAAAGGATAATCGTGGTAACCCA | +843 to +821 |
| PEL1P1 | ATGTTTCATCCGCAATACATTTAAC | −69 to −45 |
| PEL1P2 | TATTTCATTATCACTGTCTCCTTG | +119 to +96 |
| PEH1P1 | AGAAAAGCTTACCACCCGCTG | −101 to −81 |
| PEH1P2 | AAAGGATCCGTTCGGGAATCAGATGCAAATGC | +287 to +263 |
| CELVP1 | CAGCATTATCCGCCACGCCAGTA | +83 to +105 |
| CELVP2 | CATGGCGACGCGGAATACGTTA | +282 to +261 |
D = A or T or G; K = G or T; R = A or G; Y = C or T.
Corresponding to base positions relative to the transcriptional start site, except for primers KDGRP1, KDGRM1, CELVP1, and CELVP2. The base positions of these four primers are given relative to the translational start site.
Cloning and nucleotide sequence analysis of kdgREcc.
The 135-bp kdgREch probe was amplified by PCR with primers KDGRP1 and KDGRM1 (Table 2) from chromosomal DNA of E. chrysanthemi EC16. The primers were designed based on the published sequence of the E. chrysanthemi kdgREch gene (46). PCR product was cloned into pCRII vector to produce pAKC1023, and the nucleotide sequence was confirmed by sequencing analysis. We subsequently used the kdgREch DNA to screen a genomic library of E. carotovora subsp. carotovora Ecc71. Southern hybridizations showed that the kdgREch DNA hybridized with a 331-bp SalI fragment of pAKC1024 (Fig. 1A), a pLARF5 derivative carrying Ecc71 kdgREcc+ DNA (Table 1). The 331-bp SalI fragment was cloned into pBluescript SK(+), and nucleotide sequence (Fig. 1B) was determined by using the universal T3 and T7 primers (Stratagene, La Jolla, Calif.). Starting with this sequence, successive primers (Table 2) were synthesized to sequence the kdgREcc gene in pAKC1025. The GenBank accession number for kdgREcc is AF103871.
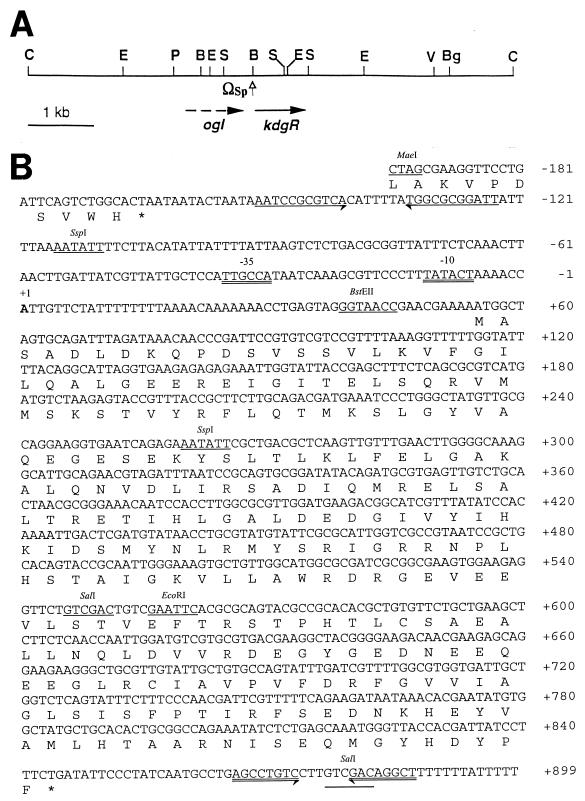
FIG. 1.
(A) Physical map of the 7.35 kb of ClaI DNA segment of strain Ecc71 containing the kdgREcc gene. The location and direction of the gene are indicated by an arrow. The ogl gene is located upstream of kdgREcc, as indicated by the broken arrow. The omega (Ω) fragment (Sp resistance cassette) was introduced at the BstEII site. B, BstEII; Bg, BglII; C, ClaI; E, EcoRI; P, PstI; S, SalI; V, EcoRV. (B) Nucleotide sequence of kdgREcc and the 3′-terminal region of the ogl gene of strain Ecc71. The deduced amino acid sequence of KdgREcc is also given. Palindromic sequences in between the ogl and kdgREcc genes are indicated by inverted arrows. Sequences similar to the −10 and −35 consensus sequences are double underlined, and the transcriptional start site is indicated by “+1”. Transcriptional termination sequences represented by an inverted repeat beyond the 3′ end of kdgREcc are indicated by double-lined inverted arrows. Several restriction endonuclease sites are also shown. Numbers on the right refer to the positions of the nucleotides.
Plasmids.
To construct ptac-kdgREcc, the coding region of kdgREcc was amplified by PCR from pAKC1025 with primers KDGRS7 and KDGRS8 (Table 2). PCR products were digested with NdeI and HindIII and cloned into pT7-7 to yield pAKC1028. The XbaI-HindIII fragment of pAKC1028 was subcloned into pDK6 to produce pAKC1035. For the KdgREcc-6His overexpressing plasmid, the coding region of kdgREcc was amplified by PCR with primers KDGRP2 and KDGRP3 (Table 2). PCR products were digested with NcoI and XhoI and cloned into the vector pET-28b(+) to yield pAKC1029. In pAKC1029, additional eight amino acid residues (Leu-Glu-6His) have been added to the C-terminal region of the 263 amino acid residues of KdgREcc.
The 188-bp pel-1 DNA from −69 to +119 (6) was amplified by PCR with primers PEL1P1 and PEL1P2 (Table 2) and cloned into the EcoRV site of pBluescript SK(+) to produce plasmid pAKC1030. The BamHI-XbaI fragment of pAKC1030 was inserted into the BglII-XbaI sites of pMP220 to yield pAKC1031. To construct peh1-lacZ fusion, the 383-bp peh-1 DNA from −97 to +286 (27) was amplified by PCR with primers PEH1P1 and PEH1P2 (Table 2) and cloned into the HindIII and BamHI sites of pBluescript SK(+) to produce plasmid pAKC1032. The KpnI-XbaI fragment of pAKC1032 was inserted into the KpnI-XbaI sites of pMP220 to yield pAKC1033. The celV DNA was amplified from strain Ecc71 chromosomal DNA by PCR with primers CELVP1 and CELVP2 (Table 2) based on nucleotide sequence of celV of E. carotovora SCRI193 (11) and cloned into pGEM-T Easy.
Construction of KdgREcc− strains by marker exchange.
The 1.2-kb EcoRI fragment from pAKC1025 was subcloned into pRK415 to produce plasmid pAKC1026. The Omega-Sp cassette from pHP45Ω was inserted at the BstEII site of kdgREcc DNA fragment (Fig. 1A) in pAKC1026 to produce pAKC1027. pAKC1027 was transferred into Ecc71 by using the helper plasmid, pRK2013. Transconjugants were selected on minimal salts agar containing sucrose (0.2% [wt/vol]) and supplemented with Sp. Isolates that were Spr and Tcs were selected for further studies. The marker exchange was confirmed by Southern blot hybridization as well as by Northern blot analysis.
β-Galactosidase assay.
The β-galactosidase assays were carried out according to the method of Miller (31).
Purification of KdgREcc-6His recombinant protein.
E. coli JM109(DE3) carrying pAKC1029 was grown at 37°C in LB medium containing Km. When the culture reached an A600 value of 0.7, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to yield a final concentration of 0.5 mM. After an additional 3-h incubation, cells were collected by centrifugation and frozen at −80°C. KdgREcc-6His was purified from sonicated cell extracts by using Ni-nitriloacetic acid (NTA) resin essentially according to the protocol provided by Qiagen, Inc. Fractions collected from the Ni-NTA affinity column were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and those containing KdgREcc protein were pooled. The purified KdgREcc protein was stored at −20°C in 50% glycerol. The protein concentration was determined by the bicinchononic acid (Pierce Corp., Rockford, Ill.) method, with bovine serum albumin (BSA) as a standard.
Gel mobility shift assay.
The DNA fragments were prepared as follows: the 188-bp pel-1 DNA was prepared from pAKC1030 (Table 1), the 383-bp peh-1 DNA was prepared from pAKC1032 (Table 1), and the 284-bp rsmB was prepared from pAKC1021 (28). The plasmid DNAs were digested with the appropriate endonucleases, and the desired fragments were purified from low-melting-point SeaPlaque agarose gel. The DNA fragments were end labeled with [α-32P]dATP and Klenow fragment and then purified by the Sephadex G-50 spin-column chromatography.
Protein-DNA interaction was assayed in 20 μl of binding buffer (12 mM HEPES-NaOH, pH 7.9; 4 mM Tris-HCl, pH 7.9; 75 mM KCl; 10 mM MgCl2; 5 mM CaCl2; 1.0 mM dithiothreitol) containing 1 μg of salmon sperm DNA, 2 μg of BSA, and purified KdgREcc-6His protein. After incubation for 20 min at room temperature, the reaction mixtures were subjected to PAGE in a 5% (wt/vol) polyacrylamide gel. The gel was dried and exposed to X-ray film.
DNase I protection analysis.
PCR labeling of DNA probes, chemical sequence analysis, and DNase I protection assays were carried out according to the method of Liu et al. (29, 30), except that the DNA binding buffer described above was used. The rsmB primers, P13 and P16, and purified SacI fragment from pAKC1020 (28) were used to produce the probe by PCR.
RNA assays.
Total RNA was obtained from E. carotovora subsp. carotovora strains. Bacteria were grown at 28°C in minimal salts medium plus sucrose (0.5% [wt/vol]) or in this medium supplemented with Tc. Total RNA was extracted by the method of Aiba et al. (1).
Primer extension assay was performed according to the manufacturer’s instructions (Promega Biotec, Madison, Wis.) with primer KDGRS6 (Table 2) and 10 μg of RNA.
Northern blot hybridization experiments were performed by following the procedure of Liu et al. (28). The 517-bp BstEII-EcoRI kdgREcc DNA fragment from pAKC1025 (Table 1) was used as a DNA probe. The other DNA probes used in this work were the 314-bp EcoRV-KpnI DNA fragment of pel-1 from pAKC783 (27), the 743-bp HindIII fragment of peh-1 from pAKC781 (27), the 308-bp BamHI-HindIII DNA fragment of rsmB from pAKC1014 (28), the 200-bp EcoRI fragment of celV from pAKC1034 (Table 1), and the 779-bp EcoRV-SmaI DNA fragment of hrpNEcc from pAKC924 (13). DNA probes were labelled with [α-32P]dATP by random priming according to the manufacturer’s instructions (Promega Biotec). Prehybridization (4 h at 65°C) and hybridization (18 h at 65°C) were performed in prehybridization buffer (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 2× Denhardt’s solution, 0.1% (wt/vol) SDS, and 100 μg of denatured salmon sperm DNA per ml). After hybridization, membranes were washed twice for 30 min at 65°C in 2× SSC–0.5% (wt/vol) SDS and then for 30 min at 65°C in 0.1× SSC–0.5% (wt/vol) SDS and finally were examined by autoradiography with X-ray film (Kodak, Rochester, N.Y.). The densities of the hybridization bands were quantified by using the QS30 optically enhanced densitometry system (Fisher Scientific, Pittsburgh, Pa.).
Western blot analysis.
Bacterial strains were grown at 28°C in minimal salts medium containing sucrose to an A600 of 2.0. Western blot analysis of cell extracts was carried out according to the method of Mukherjee et al. (32). The antibodies raised against HarpinEch (4) were used as the probe.
RESULTS
Cloning and nucleotide sequence of kdgREcc of strain Ecc71.
To identify the kdgREcc gene, we amplified a 135-bp segment of the kdgR DNA from E. chrysanthemi EC16 by PCR with the degenerate primers KDGRP1 and KDGRM1 (Table 2). The nucleotide sequence of the PCR product was 88.1% identical to the corresponding sequences of kdgREch of strain 3937 (47). The plasmid pAKC1024 (Table 1), obtained by colony hybridization with the 135-bp PCR product of kdgREch as the probe, repressed Cel, Pel, Peh, and Prt production in strain Ecc71 as indicated by agarose plate assays (data not shown; also see below). The restriction map of the 7.35-kb ClaI DNA fragment containing kdgREcc is shown in Fig. 1A.
The deduced amino acid sequence of kdgREcc (Fig. 1B) shows that the coding region of kdgREcc could specify a polypeptide of 263 amino acid residues with a molecular mass of 29,676 Da. An ogl gene is located upstream of kdgREcc (Fig. 1B), as previously reported in E. chrysanthemi (47). A palindromic structure, consisting of a GC-rich stem-loop and an AT-rich tail (Fig. 1B), is localized between ogl and kdgREcc. Since this stem loop is 11 bp downstream of the stop codon of ogl, it is likely that the palindrome functions as a rho-independent terminator of ogl transcription.
The transcriptional start site of kdgREcc was localized by primer extension analysis to the adenine residue 54 nucleotides upstream of the putative start codon (Fig. 1B). The sequences of the putative −35 box (TTGCCA) and the −10 box (TATACT) of kdgREcc are very similar to those of the E. coli sigma-70 promoter (Fig. 1B). However, we have not found any other regulatory element in the vicinity of the kdgREcc promoter region, such as the consensus sequences for the binding of KdgREch (aaTg/aAAAc/tNNt/cg/aTTTc/tA [38]), CRP (TGTGAnnnnnnTCACA [37]), or IclR (TGGAAATna/gTTTCCa/g [41]).
Analysis of the 3′ sequence of kdgREcc revealed a palindromic structure 19 bp downstream of the kdgREcc coding region, which is connected to 11 T residues, giving rise to a poly(T) structure (Fig. 1B). If this structure functions as the transcriptional terminator of kdgREcc, as would be expected, the kdgREcc mRNA would comprise a 900-base transcriptional unit. Indeed, the results of the Northern blotting assay confirmed this prediction (data not shown).
The deduced amino acid sequence of KdgREcc has the highest similarities to KdgREch of E. chrysanthemi (47) and KdgREco of E. coli (GenBank accession number D90826). An alignment of these sequences is presented in Fig. 2. KdgREcc is 90 and 88% similar to KdgREch and KdgREco, respectively. While KdgREcc and KdgREco each consist of 263 amino acid residues, KdgREch has 43 additional amino acid residues at the N-terminal end. KdgREcc is 57% similar to the IclR repressor of E. coli (40), as well as to the GylR repressor of Streptomyces coelicolor (51), which is a member of the IclR family. Sequence comparisons between these bacterial transcriptional regulators and KdgREcc revealed two regions of high similarity. Near the NH2 terminus of the KdgREcc protein, the 34-ITELSQRVMMSKSTVYRFIQ-53 stretch of residues match the helix-turn-helix (HTH) structural motif in GylR, IclR, and KdgREch transcriptional regulators. Near the COOH terminus of the KdgREcc protein, residues 194-GYGEDNEEQEEGLRCIAVPVFD-215 match the PROSITE pattern (PS01051, “IclR family signature”) found in members of the IclR family of regulators, such as IclR and GylR. These observations strongly suggest that KdgREcc is a member of the IclR transcriptional regulator family.
FIG. 2.
Alignment of the deduced amino acid sequence of KdgREcc of strain Ecc71 with those of E. chrysanthemi EC3937 (KdgREch) and E. coli (KdgREco). The HTH motif is shown. Identical amino acids are not identified. Dots indicate conserved substitutions.
Effects of KdgREcc on extracellular enzyme and HarpinEcc production and pathogenicity.
To determine the effects of KdgREcc on exoenzyme production, the KdgREcc− mutant, strain AC5073, and its parent strain, Ecc71, were grown in minimal salts medium plus sucrose, and culture supernatants were assayed to determine their enzymatic activities. The cells from these cultures were used for the isolation of total RNA for transcript assays. The levels of Pel, Peh, Cel, and Prt were higher in AC5073 than in Ecc71 (Fig. 3A; Table 3). Similarly, the levels of transcripts in AC5073 were higher than in strain Ecc71 (Fig. 3C): the pel-1 transcript was fivefold higher; the peh-1 transcript was twofold higher; and the celV transcript was threefold higher.
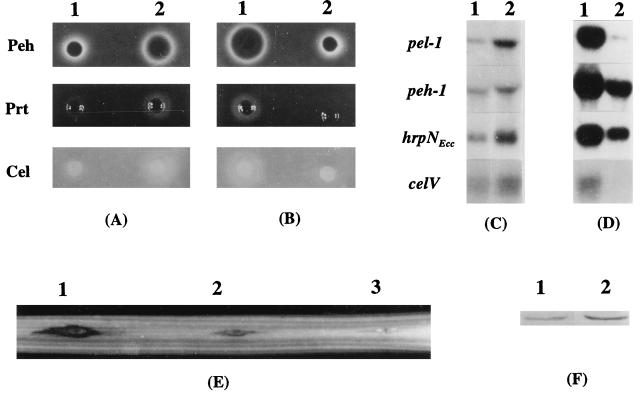
FIG. 3.
Effects of KdgREcc on the production of exoenzymes and HarpinEcc, on the transcription of exoenzyme genes and hrpNEcc, and on pathogenicity. (A and B) Agarose plate assays for Peh, Prt, and Cel activities of E. carotovora subsp. carotovora strains. Strains Ecc71 (KdgREcc+, column A1) and AC5073 (KdgREcc−, column A2) were grown at 28°C in minimal salts medium plus sucrose to an A600 of 2.3, and the culture supernatants (20 μl) were used for the assays of enzymatic activities. AC5073 carrying pLAFR5 (cloning vector, column B1) or pAK1024 (KdgREcc+, column B2) was grown in minimal salts medium plus sucrose and Tc to an A600 of 2.3, and the culture supernatants (20 μl) were used for the assays of enzymatic activities. The plates were scored for activities after incubation for 24 h at 28°C. Halos around the wells are due to enzymatic activities. (C and D) Levels of transcripts of pel-1, peh-1, hrpNEcc, and celV. Bacteria were grown at 28°C in minimal salts medium plus sucrose or in this medium supplemented with Tc to an A600 of 1.0 for RNA extraction. Total RNAs from strains Ecc71 (column C1), AC5073 (column C2), AC5073 carrying pLAFR5 (column D1), and AC5073 carrying pAKC1024 (column D2) were used for Northern blot analysis. Lanes 1 and 2 in parts C and D contained 10 and 20 μg of total RNA, respectively. (E) Plant tissue maceration induced by strain Ecc71 (site 2) and its KdgREcc− mutant, strain AC5073 (site 1). Each inoculation site of this celery petiole was injected with 2 × 108 cells. Water was used as a control (site 3). The inoculated petiole was incubated in a moist chamber at 25°C for 24 h. (F) Western blot analysis of HarpinEcc produced by strain Ecc71 (lane 1) and its KdgREcc− derivative, strain AC5073 (lane 2). Each lane contained 20 μg of total bacterial protein.
TABLE 3.
Levels of Pel produced by E. carotovora subsp. carotovora
| Straina | Relevant phenotype | Pel sp actb |
|---|---|---|
| Ecc71 | KdgREcc+ | 0.05 |
| AC5073 | KdgREcc− | 0.43 |
| AC5073(pLARF5) | KdgREcc−(KdgREcc−) | 0.34 |
| AC5073(pAKC1024) | KdgREcc−(KdgREcc+) | 0.02 |
E. carotovora subsp. carotovora strains Ecc71 and AC5073 were grown at 28°C in minimal salts medium plus sucrose (0.5% [wt/vol]), and strains AC5073(pLARF5) and AC5073(pAKC1024) were grown in minimal salts medium plus sucrose (0.5% [wt/vol]) and Tc to an A600 of 2.3. Pel activity was assayed as described by Murata et al. (36).
Expressed as units per milliliter per A600 unit.
As a further proof for the negative regulation of exoenzymes by KdgR, AC5073 carrying the KdgREcc+ plasmid, pAKC1024, or the cloning vector was grown in minimal salts medium plus sucrose and Tc, and culture supernatants and cells were collected for assays of enzymatic activities and transcripts, respectively. While the KdgREcc− mutant carrying the cloning vector produced substantial levels of Pel, Peh, Cel, and Prt, these activities were undetectable or barely detectable in the mutant carrying multiple copies of KdgREcc+ DNA (Fig. 3B and Table 3). The levels of pel-1, peh-1, and celV transcripts also were considerably lower in the mutant carrying the KdgR+ plasmid than in the mutant carrying the vector (Fig. 3D).
To obtain additional evidence that KdgREcc inhibits transcription of pel-1 and peh-1, we examined the expression of pel1-lacZ and peh1-lacZ transcriptional fusions in the E. coli kdgREcc-overexpressing strain, DH5α (pAKC1035). The data in Table 4 show that the levels of β-galactosidase produced by the E. coli kdgREcc-overexpressing strain carrying the pel1-lacZ and peh1-lacZ fusions were considerably lower than the levels produced by DH5α carrying the pDK6 vector and the same lacZ constructs. We attribute this repression of transcription to binding of KdgREcc to the KdgREcc-binding sites localized in the 5′ regions of pel-1 and peh-1 transcription units (see below).
TABLE 4.
Levels of β-galactosidase activity of transcriptional pel1-lacZ, peh1-lacZ, and rsmB-lacZ fusions in the kdgREcc-overexpressing E. coli strain DH5α(pAKC1035, ptac-kdgREcc)
| Plasmids | β-Galactosidase activity (Miller units) |
|---|---|
| pAKC1031(pel1-lacZ) + pDK6a | 326 |
| pAKC1031(pel1-lacZ) + pAKC1035a | 65 |
| pAKC1033(peh1-lacZ) + pDK6a | 969 |
| pAKC1033(peh1-lacZ) + pAKC1035a | 303 |
| pAKC1002(rsmB-lacZ) + pAKC1035b | 612 |
| pAKC1018(rsmB-lacZ) + pAKC1035b | 279 |
E. coli constructs were grown at 28°C in LB plus Km plus Tc plus 10 μM IPTG, and cells for β-galactosidase assay were collected at the A600 value of 2.0.
E. coli constructs were grown at 28°C in LB plus Km plus Tc plus 50 μM IPTG, and cells for β-galactosidase assay were collected at the A600 value of 1.0.
Previous studies have established a positive correlation between the levels of exoenzymes and the virulence of E. carotovora subsp. carotovora (3, 5, 12, 13, 19, 34, 43). As exoenzyme production was derepressed in the KdgREcc− mutant, it was deemed of interest to compare the degree of virulence of the KdgREcc− and the KdgREcc+ strains. The data presented in Fig. 3E demonstrate that AC5073 caused more extensive maceration of celery petioles than strain Ecc71.
We have shown that hrpNEcc expression and HarpinEcc levels in strain Ecc71 are coregulated, along with exoenzymes, OHL, RsmA, and rsmB RNA (28, 32). Thus, in light of the effects of KdgREcc on pectinases, as well as on Cel and Prt, it was of interest to examine the influence of KdgR on hrpNEcc expression. The results of Western and Northern analyses (Fig. 3C and F) show that HarpinEcc and hrpNEcc mRNA levels were higher in the KdgREcc− mutant than in the KdgREcc+ parent, Ecc71. In addition, multiple copies of KdgREcc+ DNA severely repressed the production of hrpNEcc mRNA (Fig. 3D). To our knowledge, this is the first report of a negative effect of KdgR on the expression of a hrp gene.
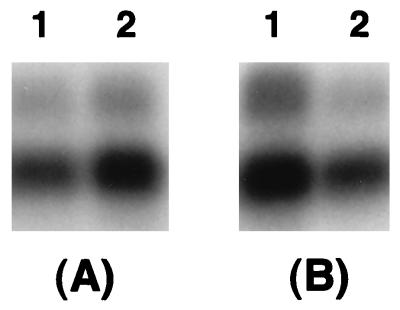
kdgREcc reduces the levels of rsmB RNA.
As stated above, rsmB RNA was recently shown to activate extracellular enzyme and HarpinEcc production in Ecc71. rsmB contains three potential KdgR-binding sites within the 5′ transcribed region (28, 35), suggesting that KdgR may bind rsmB DNA and interrupt elongation of rsmB transcription. The following lines of evidence support this hypothesis. (i) The amount of rsmB RNA is about twofold higher in the KdgREcc− mutant than in the Ecc71 wild-type strain carrying a chromosomal copy of kdgREcc (Fig. 4A). (ii) The results (Fig. 4B) also reveal a >75% reduction in the level of rsmB RNA in strain Ecc71 carrying pAKC1024 compared to the level in strain Ecc71 carrying the cloning vector. Thus, multiple copies of kdgREcc in strain Ecc71 reduce the level of rsmB RNA.
FIG. 4.
Effect of kdgREcc on the transcription of rsmB in E. carotovora subsp. carotovora. Bacteria were grown in minimal salts medium plus sucrose or in this medium supplemented with Tc at 28°C to an A600 of 1.0. Total RNAs were isolated and used for Northern blot analysis. Lanes: A1, Ecc71 (KdgREcc+); A2, AC5073 (KdgREcc−); B1, AC5073 carrying pLAFR5 (cloning vector); B2, AC5073 carrying pAKC1024 (KdgREcc+). Each lane contained 5 μg of total RNA.
To rigorously establish the role of KdgR-binding sites on rsmB transcription, we examined the expression of lacZ operon fusions. Two such fusions were used: pAKC1018 contains 488 bp of rsmB DNA and includes all three KdgR-binding sites (see below), as well as the promoter-regulator region; pAKC1002, on the other hand, contains 221 bp of rsmB DNA, which includes the promoter-regulator region but not the KdgR-binding sites. These plasmids were transferred into E. coli DH5α carrying pAKC1035, wherein kdgREcc expression is controlled by the tac promoter (see Table 1). Bacteria were grown in LB containing Km, Tc, and IPTG (50 μM), and culture samples were assayed for β-galactosidase activity. The data in Table 4 show that β-galactosidase levels were about twofold higher with the rsmB-lacZ construct lacking the KdgR-binding sites than with the construct containing the three KdgR-binding sites.
Purification of the KdgREcc-6His recombinant protein.
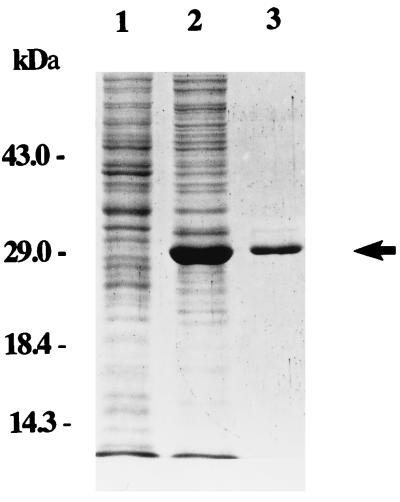
To characterize KdgREcc, we purified KdgREcc-6His recombinant protein overproduced in E. coli. For this, we amplified the coding region of kdgREcc by PCR and cloned it into the T7 promoter expression vector, pET-28b(+), to produce plasmid pAKC1029. After IPTG induction, a protein of approximately 29 kDa was overproduced by JM109(DE3) carrying pAKC1029 (Fig. 5, lane 2), but not by JM109(DE3) carrying pET-28b(+) (Fig. 5, lane 1). The apparent molecular mass of 29 kDa matches well with the mass of 29,676 Da of the polypeptide deduced from the kdgREcc sequence, further indicating that this overproduced protein is encoded by kdgREcc. The one-step purification protocol produced recombinant KdgREcc-6His protein of about 95% purity, as judged by the SDS-PAGE analysis (Fig. 5, lane 3).
FIG. 5.
Overexpression and purification of KdgREcc-6His. Crude extracts and fractionated KdgREcc-6His were analyzed by SDS-PAGE in a 12% (wt/vol) polyacrylamide gel. Lanes: 1, lysate of JM109(DE3) carrying the cloning vector, pET28b(+); 2, lysate of JM109(DE3) carrying pAKC1029; and 3, purified KdgREcc-6His protein. Lanes 1 and 2 contained 10 μg of protein, whereas lane 3 contained 2 μg of protein.
Identification of KdgREcc-binding site.
Gel mobility shift assays were carried out to determine interaction of purified KdgREcc-6His protein with several DNA segments that contain potential KdgR-binding sites. Purified KdgREcc-6His protein and labeled rsmB, peh-1, and pel-1 fragments were incubated in the binding buffer and electrophoresed in 5% (wt/vol) polyacrylamide gels. Figure 6 shows that the KdgREcc-6His protein binds DNA segments of rsmB, peh-1, and pel-1, in each case producing a single retarded band. The extent of band shift was proportional to the concentration of KdgREcc (Fig. 6C), indicating that KdgREcc binding was specific. This was also supported by the competition experiment, wherein the excess of cold rsmB DNA abolished the retarded band (Fig. 6C).
FIG. 6.
Gel mobility shift assays for binding of KdgREcc-6His to the pel-1 (A), peh-1 (B), and rsmB (C) DNAs. 32P-labeled rsmB (1 ng), pel-1 (2 ng), or peh-1 (2 ng) DNA was used. Lanes A1, B1, and C1, no protein was added; lanes A2 and B2, reaction mixtures contained 300 ng of KdgREcc-6His; lanes C2, C3, C4, and C5, reactions were carried out with 300, 400, 500, or 600 ng of purified KdgREcc-6His protein, respectively; lane C6, reaction was performed with 300 ng of KdgREcc-6His in the presence of 200 ng of excess of cold rsmB DNA.
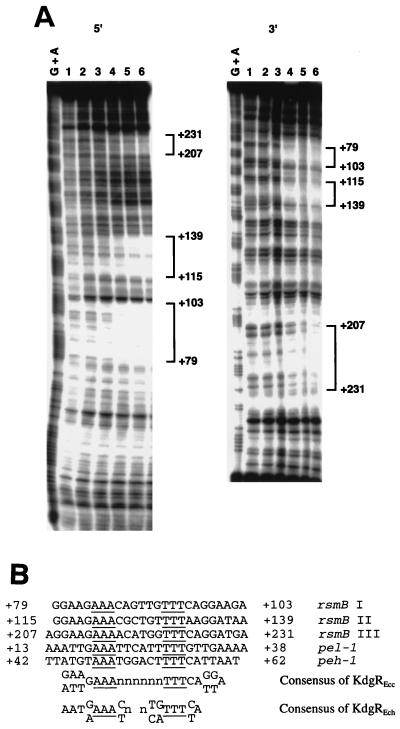
DNase I protection experiments were performed to precisely localize the binding sites of KdgREcc on the rsmB DNA. The upper strand and the lower strand of the rsmB fragment were specifically labeled with [γ-32P]ATP and then incubated in the presence of increasing amounts of purified KdgREcc-6His. These DNA-protein complexes were subjected to partial DNase I digestion, and the resulting products were separated on 8% (wt/vol) polyacrylamide sequencing gels and visualized by autoradiography. Three 25-bp protected regions were detected within the nucleotides +79 and +103, +115 and +139, and +207 and +231 (Fig. 7A), relative to the rsmB transcriptional start site (28).
FIG. 7.
(A) DNase I protection analysis of the rsmB DNA fragment by KdgREcc. 5′ and 3′ refer to the 32P-end-labeled portion of rsmB DNA. In the 50 μl of binding reaction mixture, an 11.7 nM concentration of the sense strand probe (5′) or a 5.0 nM concentration of the antisense strand probe (3′) was incubated with 0 (lane 1) or with 0.16, 0.32, 0.64, 0.96, and 1.28 μM purified KdgREcc-6His protein (lanes 2 to 6, respectively). The G+A chemical sequence of the same labeled DNA fragments is shown in the leftmost lanes. Brackets indicate nucleotide positions relative to the transcriptional start site, which were protected from DNase I digestion by KdgREcc-6His. (B) Nucleotide sequence alignment of the protected regions of rsmB and putative KdgREcc-binding sites of pel-1 and peh-1.
The nucleotide sequence alignment of the three protected regions of rsmB revealed the binding sequence of 5′-GGAAGAAA[N6]TTTCAGGAA/TG/AA-3′ (Fig. 7B). This sequence is highly similar to the known consensus sequence of KdgREch-binding site (Fig. 7B). The putative KdgREcc-binding sites are also present within the 5′ regions of pel-1 and peh-1 transcription units (Fig. 7B), and this observation explains our findings that KdgREcc binds pel-1 and peh-1 DNA fragments in vitro (Fig. 6A and B) and represses transcriptional fusions in vivo (Table 4).
DISCUSSION
In this report we have established that KdgREcc functions as a global regulator in that it controls not only pectinases such as Pel and Peh, but also Cel, Prt, and HarpinEcc production. Our findings suggest that this is brought about by affecting the expression of at least some of the structural genes as well as rsmB, an RNA regulator that in turn controls exoenzymes and HarpinEcc. To our knowledge, these data provide the first experimental evidence for this dual role of KdgREcc (depicted in Fig. 8). Whether this also is true for the other KdgR species, such as those from E. chrysanthemi and E. coli, remains to be determined, although it is reasonable to predict, based upon genetic homology, that they have similar functions as well. That KdgREcc is a repressor is clearly demonstrated by the derepressed phenotypes of the KdgREcc− mutant and the negative trans-dominant effect of multiple copies of the kdgREcc+ DNA. The effects on transcripts (Fig. 3C and D), when considered along with the binding of purified KdgREcc to the putative operator DNAs within the 5′ regions of pel-1 and peh-1 transcription units, indicate that KdgREcc interferes with the initiation of transcription, as previously reported for some of the E. chrysanthemi pel and pectate catabolic genes (37, 39).
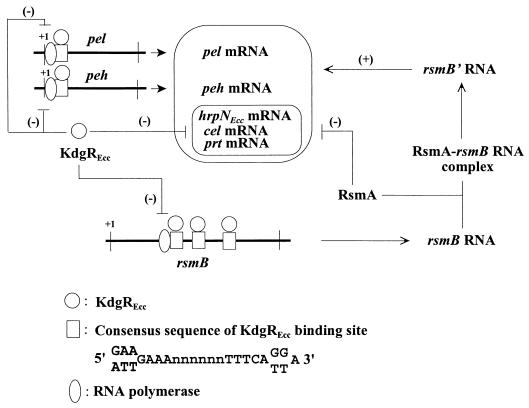
FIG. 8.
A speculative model depicting the regulatory effects of KdgREcc on the production of exoenzymes and HarpinEcc. The proposed scheme postulates KdgREcc to function via two different pathways: by directly repressing the transcription of the exoenzyme genes, i.e., pel and peh, and by affecting the transcription of rsmB, a global RNA regulator, which controls pel, peh, cel, prt, and hrpNEcc expression (28). While we have documented the inhibition of pel and peh transcription by kdgREcc, we do not have similar evidence for a direct effect of kdgREcc on the transcription of hrpNEcc, cel, or prt genes. However, the data presented here show that rsmB transcription is affected by a roadblock mechanism. We propose that as the level of active KdgREcc drops, rsmB transcription is stimulated, producing RNA which binds RsmA. Since RsmA promotes transcript decay, the decrease in the free RsmA pool could contribute to mRNA stability. The formation of RsmA-rsmB RNA complex also facilitates rsmB RNA processing. The processed rsmB RNA (rsmB′) then activates Pel, Peh, Cel, Prt, and HarpinEcc production, although the mechanism by which this is brought about is not yet fully understood.
KdgREcc binds to three KDGR boxes located within the transcriptional unit of rsmB, 79 bases downstream of the transcriptional initiation site. In fact, KdgREcc binding sequences are not present within the promoter region of rsmB. While the details of rsmB transcription are not yet known, it would appear, based upon the characteristics of the promoter DNA and the expression of rsmB-lacZ fusions, that sigma-70 RNA polymerase holoenzyme can by itself activate the rsmB promoter (28). Thus, KdgREcc binding to rsmB DNA, starting at sites 79 bases downstream of the promoter, may not interfere with the initiation of transcription but instead may affect the elongation of transcription. Precedence exists for this sort of regulation in both eukaryotic and prokaryotic systems (55), wherein DNA binding proteins exert their effects by binding the DNA templates. For example, the purine repressor, PurR, was shown to regulate the transcription elongation of the E. coli purB operon by a roadblock mechanism (18). The binding of PurR to the purB operator, 242 bp downstream of the transcriptional start site, blocked the polymerase during elongation. The effect on elongation was independent of the purB promoter and also did not require cotranslation (18). Since rsmB encodes a RNA regulator and not a protein product (28), cotranslation is certainly not required in the regulation of transcription by KdgREcc. Therefore, it is possible that in rsmB the KdgREcc binds the three operators downstream of transcription start site and halts the movement of RNA polymerase by a roadblock mechanism similar to that in E. coli. Our observations also raise the possibility that conformational change of rsmB template DNA is triggered by KdgREcc binding and that this alteration is responsible for the inhibition of transcription elongation. Although rsmB DNA contains three KdgREcc-binding sites, in gel shift assays only one retarded band appeared in a concentration-dependent manner (Fig. 6C). Since no intermediate complexes were detected in these assays, we assume that a highly ordered and cooperative binding occurs between KdgREcc protein and rsmB DNA. It is conceivable that after three KdgREcc dimers bind rsmB double-stranded DNA, polymerization of KdgREcc proteins could produce a looped or bent conformation of the rsmB DNA, giving rise to a template nonpermissive for transcription elongation. Additional detailed analysis of interactions between KdgREcc and variously modified rsmB DNA should clarify the physical and biological consequences of the occurrence of multiple binding sites within the transcriptional unit.
The rationale for regulating gene expression by interfering with elongation of transcription but not of initiation, if that indeed is the case, is hard to appreciate unless we consider the possibility that this allows the bacterium to very rapidly adjust the levels of rsmB RNA upon the relief of repression. According to this hypothesis, once the level of active KdgREcc drops, transcripts already initiated will immediately elongate, allowing rapid production of rsmB RNA. Such a rapid response would certainly be an advantage, even a requirement, if rsmB were to perform a vital function. Indeed, several lines of indirect evidence point to such a role of rsmB. For example, rsmB RNA neutralizes the negative effects of the RNA-binding protein RsmA, which promotes message decay (28). In the absence of rsmB RNA, RsmA may induce a nonspecific decay of transcripts which could have an extremely detrimental effect on cell physiology and cell viability. Our inability to obtain stable rsmB null mutants (33) also points to an important role of this RNA.
The negative regulation of Cel, Prt, and HarpinEcc by KdgR has not been reported previously and merits comment. We do not yet know if there are binding sites for KdgREcc within the promoter region of the structural gene for Prt. We are, however, certain that KdgREcc binding sites are not present within 475 bp upstream of the translational start site of hrpNEcc (32); this DNA region includes a 75-bp untranslated sequence and a 400-bp sequence upstream of the transcriptional start site. Thus, it is highly unlikely that the KdgREcc effect on hrpNEcc transcripts (Fig. 3D) is due to the binding of KdgREcc to operator DNA, thereby preventing promoter activation by RNA polymerase holoenzyme. Not eliminated is the rare possibility that KdgREcc binds hrpNEcc DNA far upstream of the transcriptional start site, binding that may somehow negatively interfere with promoter activation and initiation of transcription. A more plausible hypothesis is that the KdgREcc effect on hrpNEcc expression is directed via its effect on expression of rsmB or another regulator of hrpNEcc. We have shown here that the KdgREcc− mutant produces higher levels of rsmB and hrpNEcc transcripts compared to the KdgREcc+ parent (Fig. 3C and Fig. 4A). Previous studies (28) have established that overexpression of rsmB is invariably accompanied by overexpression of hrpNEcc, as well as the genes for several exoenzymes. It is therefore likely that in the absence of KdgREcc a higher level of rsmB expression results in the activation of hrpNEcc transcription (Fig. 8). Since KdgREcc binding sites are not found within the 490-bp sequence upstream of the translational start site of celV, we suggest that at least part of the KdgREcc effect on Cel is due to the regulation of rsmB by KdgREcc. Production of Prt may also be similarly affected by KdgREcc. In light of the global regulatory role of OHL in E. carotovora subsp. carotovora (5, 24, 44), we tested the possibility that KdgREcc represses OHL production and that this, in turn, affects exoenzyme and HarpinEcc levels. However, our comparative studies with KdgREcc+ and KdgREcc− strains did not support this hypothesis. Studies have been initiated to identify another global regulator that is affected by KdgREcc and to determine whether this presumed KdgREcc-mediated repression, in conjunction with the negative effect on rsmB transcription, actually accounts for the inhibition of hrpNEcc, cel, and prt expression.
ACKNOWLEDGMENTS
Our work was supported by the National Science Foundation (grant MCB-9728505) and the Food for the 21st Century program of the University of Missouri.
We thank Alan Collmer for the anti-HarpinEch antibodies and J. D. Wall for reviewing the manuscript.
Footnotes
Journal series 12,848 of the Missouri Agricultural Experiment Station.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Alfano J R, Collmer A. Bacterial pathogens in plants: life up against the wall. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barras F, van Gijsegem F, Chatterjee A K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–234. [Google Scholar]
- 4.Bauer D W, Wei Z-M, Beer S V, Collmer A. Erwinia chrysanthemi harpinEch: an elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol Plant-Microbe Interact. 1995;8:484–491. doi: 10.1094/mpmi-8-0484. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol. 1995;61:1959–1967. doi: 10.1128/aem.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee A, Liu Y, Chatterjee Arun K. Nucleotide sequence of a pectate lyase structural gene, pel1 of Erwinia carotovora subsp. carotovora strain 71 and structural relationship of pel1 with other pel genes of Erwinia species. Mol Plant-Microbe Interact. 1995;8:92–95. doi: 10.1094/mpmi-8-0092. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A K, Buchanan G E, Behrens M K, Starr M P. Synthesis and excretion of polygalacturonic acid trans-eliminase in Erwinia, Yersinia, and Klebsiella species. Can J Microbiol. 1979;25:94–102. doi: 10.1139/m79-014. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee A K, McEvoy J L, Murata H, Collmer A. Regulation of the production of pectinases and other extracellular enzymes in the soft-rotting Erwinia spp. In: Patil S S, Ouchi S, Mills D, Vance C, editors. Molecular strategies of pathogens and host plants. New York, N.Y: Springer-Verlag; 1991. pp. 45–58. [Google Scholar]
- 9.Chatterjee A K, Ross L M, McEvoy J L, Thurn K K. pULB113, an RP4::mini-Mu plasmid, mediates chromosomal mobilization and R-prime formation in Erwinia amylovora, Erwinia chrysanthemi, and subspecies of Erwinia carotovora. Appl Environ Microbiol. 1985;50:1–9. doi: 10.1128/aem.50.1.1-9.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collmer A, Keen N T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. [Google Scholar]
- 11.Cooper C J C, Salmond G P C. Molecular analysis of the major cellulase (CelV) of Erwinia carotovora: evidence for an evolutionary “mix-and-match” of enzyme domains. Mol Gen Genet. 1993;241:341–350. doi: 10.1007/BF00284687. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y, Chatterjee A, Liu Y, Dumenyo C K, Chatterjee A K. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, Madi L, Mukherjee A, Dumenyo C K, Chatterjee A K. The RsmA− mutants of Erwinia carotovora subsp. carotovora strain Ecc71 overexpress hrpNEcc and elicit a hypersensitive reaction-like response in tobacco leaves. Mol Plant-Microbe Interact. 1996;9:565–573. doi: 10.1094/mpmi-9-0565. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson R B, Andersson A R, Pirhonen M, Palva E T. Two-component regulators involved in the global control of virulence in Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1998;11:743–752. doi: 10.1094/MPMI.1998.11.8.743. [DOI] [PubMed] [Google Scholar]
- 15.Figurski D H, Helinski D R. Replication of an origin-containing derivative of a plasmid RK2 depend on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederick R D, Chiu J, Bennetzen J L, Handa A K. Identification of a pathogenicity locus, rpfA, in Erwinia carotovora subsp. carotovora that encodes a two-component sensor-regulator protein. Mol Plant-Microbe Interact. 1997;10:407–415. doi: 10.1094/MPMI.1997.10.3.407. [DOI] [PubMed] [Google Scholar]
- 17.Harris S J, Shih Y-L, Bentley S D, Salmond G P C. The hexA gene of Erwinia carotovora encodes a LysR homologue and regulates motility and the expression of multiple virulence determinants. Mol Microbiol. 1998;28:705–717. doi: 10.1046/j.1365-2958.1998.00825.x. [DOI] [PubMed] [Google Scholar]
- 18.He B, Zalkin H. Repression of Escherichia coli purB is by a transcriptional roadblock mechanism. J Bacteriol. 1992;174:7121–7127. doi: 10.1128/jb.174.22.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinton J C D, Salmond G P C. Use of TnphoA to enrich for extracellular enzyme mutants of Erwinia carotovora subsp. carotovora. Mol Microbiol. 1987;1:381–386. doi: 10.1111/j.1365-2958.1987.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 20.Hinton J C D, Sidebotham J M, Gill D R, Salmond G P C. Extracellular and periplasmic isoenzymes of pectate lyase from Erwinia carotovora subspecies carotovora belong to different gene families. Mol Microbiol. 1989;3:1785–1795. doi: 10.1111/j.1365-2958.1989.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 21.Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol. 1996;50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 22.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner D, Paul W, Merrick M J. Construction of multicopy expression vectors for regulated overproduction of proteins in Klebsiella pneumoniae and other enteric bacteria. J Gen Microbiol. 1988;134:1779–1784. doi: 10.1099/00221287-134-7-1779. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M Y, Gui G, Wei B, Preston III J F, Oakford L, Yüksel Ü, Giedroc D P, Romeo T. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem. 1997;272:17502–17510. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Chatterjee A, Chatterjee A K. Nucleotide sequence, organization and expression of rdgA and rdgB genes that regulate pectin lyase production in the plant pathogenic bacterium Erwinia carotovora subsp. carotovora in response to DNA damaging agents. Mol Microbiol. 1994;14:999–1010. doi: 10.1111/j.1365-2958.1994.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Chatterjee A, Chatterjee A K. Nucleotide sequence and expression of a novel pectate lyase gene (pel-3) and a closely linked endopolygalacturonase gene (peh-1) of Erwinia carotovora subsp. carotovora 71. Appl Environ Microbiol. 1994;60:2545–2552. doi: 10.1128/aem.60.7.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Cui Y, Mukherjee A, Chatterjee A K. Characterization of a novel RNA regulator of Erwinia carotovora subsp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol Microbiol. 1998;29:219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Cui Y, Mukherjee A, Chatterjee A K. Activation of the Erwinia carotovora subsp. carotovora pectin lyase structural gene pnlA: a role for RdgB. Microbiology. 1997;143:705–712. doi: 10.1099/00221287-143-3-705. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wang X, Mukherjee A, Chatterjee A K. RecA relieves negative autoregulation of rdgA, which specifies a component of the RecA-Rdg regulatory circuit controlling pectin lyase production in Erwinia carotovora subsp. carotovora. Mol Microbiol. 1996;22:909–918. doi: 10.1046/j.1365-2958.1996.01537.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Mukherjee A, Cui Y, Liu Y, Chatterjee A K. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Mol Plant-Microbe Interact. 1997;10:462–471. doi: 10.1094/MPMI.1997.10.4.462. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee, A., Y. Cui, Y. Liu, and A. K. Chatterjee. Unpublished data.
- 34.Mukherjee A, Cui Y, Ma W, Liu Y, Ishihama A, Eisenstark A, Chatterjee A K. RpoS (sigma-S) controls the expression of rsmA, a global regulator of secondary metabolites, Hairpin, and extracellular proteins in Erwinia carotovora. J Bacteriol. 1998;180:3629–3634. doi: 10.1128/jb.180.14.3629-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata H, Chatterjee A, Liu Y, Chatterjee A K. Regulation of the production of extracellular pectinase, cellulase, and protease in the soft rot bacterium Erwinia carotovora subsp. carotovora: evidence that aepH of Erwinia carotovora subsp. carotovora 71 activates gene expression in Erwinia carotovora subsp. carotovora, E. carotovora subsp. atroseptica, and Escherichia coli. Appl Environ Microbiol. 1994;60:3150–3159. doi: 10.1128/aem.60.9.3150-3159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata H, McEvoy J L, Chatterjee A, Collmer A, Chatterjee A K. Molecular cloning of an aepA gene that activates production of extracellular pectolytic, cellulolytic, and proteolytic enzymes in Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1991;4:239–246. [Google Scholar]
- 37.Nasser W, Robert-Baudouy J, Reverchon S. Antagonistic effect of CRP and KdgR in the transcription control of the Erwinia chrysanthemi pectinolysis genes. Mol Microbiol. 1997;26:1071–1082. doi: 10.1046/j.1365-2958.1997.6472020.x. [DOI] [PubMed] [Google Scholar]
- 38.Nasser W, Reverchon S, Condemine G, Robert-Baudouy J. Specific interactions of Erwinia chrysanthemi KdgR repressor with different operators of genes involved in pectinolysis. J Mol Biol. 1994;236:427–440. doi: 10.1006/jmbi.1994.1155. [DOI] [PubMed] [Google Scholar]
- 39.Nasser W, Reverchon S, Robert-Baudouy J. Purification and functional characterization of the KdgR protein, a major repressor of pectinolysis genes of Erwinia chrysanthemi. Mol Microbiol. 1992;6:257–265. doi: 10.1111/j.1365-2958.1992.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 40.Negre D, Cortay J C, Old I A, Galinier A, Richaud C, Saint-Girons I, Cozzone A J. Overproduction and characterization of the iclR gene product of Escherichia coli K12 and comparison with that of Salmonella typhimurium LT2. Gene. 1991;97:29–37. doi: 10.1016/0378-1119(91)90006-w. [DOI] [PubMed] [Google Scholar]
- 41.Pan B, Unnikrishnan I, Laporte D C. The binding site of the IclR repressor protein overlaps the promoter of aceBAK. J Bacteriol. 1996;178:3982–3984. doi: 10.1128/jb.178.13.3982-3984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérombélon M C M, Kelman A. Ecology of the soft rot Erwinias. Annu Rev Phytopathol. 1980;18:361–387. [Google Scholar]
- 43.Pirhonen M, Saarilahti H, Karlsson M-B, Palva E T. Identification of pathogenicity determinants of Erwinia carotovora subsp. carotovora by transposon mutagenesis. Mol Plant-Microbe Interact. 1991;4:276–283. [Google Scholar]
- 44.Pirhonen M, Flego D, Heikinheimo R, Palva E T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 46.Reverchon S, Huang Y, Bourson C, Robert-Baudouy J. Nucleotide sequences of the Erwinia chrysanthemi ogl and pelE genes, negatively regulated by the kdgR gene product. Gene. 1989;85:125–134. doi: 10.1016/0378-1119(89)90472-1. [DOI] [PubMed] [Google Scholar]
- 47.Reverchon S, Nasser W, Robert-Baudouy J. Characterization of kdgR, a gene of Erwinia chrysanthemi that regulates pectin degradation. Mol Microbiol. 1991;5:2203–2216. doi: 10.1111/j.1365-2958.1991.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 48.Romeo T M. Global regulation by a small RNA-binding protein CsrA and the noncoding RNA molecule CsrB. Mol Microbiol. 1998;29:1321–1330. doi: 10.1046/j.1365-2958.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- 49.Romeo T, Gong M, Liu M Y, Brun-Zinkernagel A M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175:4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shih Y L, Harris S, Bentley S, Salmond G P C. 7th International Congress of Plant Pathology, Edinburgh, Scotland. 1998. Coordinate regulation of exoenzymes and motility in Erwinia carotovora, abstr. 1.8.52. [Google Scholar]
- 51.Smith C P, Chater K F. Structure and regulation of controlling sequences for the Streptomyces coelicolor glycerol operon. J Mol Biol. 1988;204:569–580. doi: 10.1016/0022-2836(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 52.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 53.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson N R, Cox A, Bycroft B W, Stewart G S A B, Williams P, Salmond G P C. The Rap and Hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol Microbiol. 1997;26:531–544. doi: 10.1046/j.1365-2958.1997.5981976.x. [DOI] [PubMed] [Google Scholar]
- 55.Uptain S M, Kane C M, Chamberlin M J. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 56.Wharam S D, Mulholland V, Salmond G P C. Conserved virulence factor regulation and secretion systems in bacterial pathogens of plants and animals. Eur J Plant Pathol. 1995;101:1–13. [Google Scholar]
- 57.Zink R T, Kemble R J, Chatterjee A K. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J Bacteriol. 1984;157:809–814. doi: 10.1128/jb.157.3.809-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]