Abstract
In recent years, intermittent fasting (IF), including periodic fasting and time‐restricted feeding (TRF), has been increasingly suggested to constitute a promising treatment for cardiometabolic diseases (CMD). A deliberate daily pause in food consumption influences the gut microbiome and the host circadian clock, resulting in improved cardiometabolic health. Understanding the molecular mechanisms by which circadian host‐microbiome interactions affect host metabolism and immunity may add a potentially important dimension to effective implementation of IF diets. In this review, we discuss emerging evidence potentially linking compositional and functional alterations of the gut microbiome with IF impacts on mammalian metabolism and risk of development of hypertension, type 2 diabetes (T2D), obesity, and their long‐term micro‐ and macrovascular complications. We highlight the challenges and unknowns in causally linking diurnal bacterial signals with dietary cues and downstream metabolic consequences and means of harnessing these signals toward future microbiome integration into precision medicine.
Keywords: cardiometabolic disease, circadian rhythms, gut microbiome, intermittent fasting, time‐restricted feeding
Highlights
Time‐specific diet is a novel nutritional approach to prevent and treat cardiometabolic disease.
Feeding‐fasting cycles shape gut microbial composition and metabolite production. These alterations may play a causative role in driving the cardiometabolic benefits of intermittent fasting.
Despite the impressive results in animals, human studies show contradicting results, possibly due to intraindividual variation involved in the host‐microbiome response to diet.
Better mechanistic understanding is required to develop personalized dietary interventions to treat cardiometabolic diseases.
摘要
近年来, 间歇性禁食(IF), 包括周期性禁食和限时进食(TRF), 逐渐被认为是心脏代谢性疾病(CMDS)的潜在治疗方法。每天有意识地停止进食会影响肠道微生物群和宿主生物钟, 从而改善心脏代谢健康。了解昼夜宿主‐微生物组相互作用影响宿主新陈代谢和免疫的分子机制, 可能会为有效实施IF饮食增加一个潜在的重要维度。在这篇综述中, 我们讨论了IF可能改变肠道微生物组的组成和功能, 从而影响哺乳动物的新陈代谢以及高血压, 2型糖尿病(T2D), 肥胖及其长期微血管和大血管并发症的发展风险。我们强调了在将昼夜细菌信号与饮食线索和下游代谢进行因果关联的挑战和未知, 以及利用这些信号实现未来微生物组与精准医疗的整合的手段。
Keywords: 肠道菌群, 心脏代谢性疾病, 间歇性禁食, 限时进食, 昼夜节律
1. INTRODUCTION
Cardiometabolic diseases (CMDs), mainly encompassing cardiovascular diseases (CVDs) and type 2 diabetes ( T2D ), are rapidly increasing in prevalence worldwide. 1 , 2 In parallel, human eating behavior has dramatically changed over the past decades. The traditional breakfast‐lunch‐dinner pattern has been replaced by frequent snacking, large nighttime meals, and breakfast skipping. 3 , 4 Such irregular eating patterns may have an adverse effect on cardiometabolic risk factors, such as obesity, insulin resistance, and hyperglycemia, 4 thus contributing to the rising prevalence of T2D. 1 In contrast, intentional eating with awareness of the timing and frequency of eating occasions could improve lifestyle and cardiometabolic risk factor management, 5 which can protect against the development of T2D and CVDs. 6 , 7
Intermittent Fasting Regimens. Intermittent fasting (IF) is a general term for a predetermined pause in the consumption of food, usually accomplished by restricted eating for 12 hours to a few days per week. 5 During this protocol, calories and macronutrients are consumed at precise times, followed by periods without food ingestion. There are different types of IF diets, ranging from relatively long fasting periods to those spanning only a few daily wake hours 5 (Table 1). One of them is alternate‐day fasting (ADF), which entails alternating between regularly eating one day and refraining from eating the next day. The feeding:fasting ratio of 5:2 days, also known as periodic fasting, is characterized by cycles of extreme limitation or complete abstinence of food for two days a week, whereas food can be eaten without restrictions on the other five days of the week. A variation of periodic fasting is the fasting‐mimicking diet (FMD), which includes cycles of several consecutive days of low‐calorie intake. 8 A less extreme diet is time‐restricted feeding (TRF), in which individuals eat their meals within a particular window of time each day, for example, fasting:feeding of 12:12 hours or 16:8 hours, respectively. The flexibility in the fasting frequency and duration enables individuals to choose their preferential eating pattern. Within the TRF framework, the specific time of the day in which the food is consumed is of importance. Consuming calories during the active phase, in comparison with the resting phase, is typically associated with improved features of cardiometabolic health, probably affecting circadian rhythms. 5 , 9 , 10 , 11 Thus, it is advisable that within the TRF diet, calories should be consumed during the active phase. In obese mice fed with a high‐fat diet, restriction of feeding to the active phase (in the dark) led to reduced body weight and fat mass, improved glycemic control, reduced liver steatosis, and better running endurance compared with mice consuming the same calories and fed ad libitum. 12 In line with these studies, some clinical trials suggest that eating breakfast may be associated with weight loss, improved glycemic control, and cardiovascular outcomes in humans. 4 , 13 , 14 On the other hand, several randomized controlled trials demonstrated that eating breakfast does not significantly affect body fat composition or cardiometabolic parameters in humans compared to skipping breakfast and consuming an equivalent number of calories during a day. 15 , 16 Several clinical studies show that IF diets increase life expectancy and provide a wide range of benefits, including mitigation of obesity, hypertension, T2D, and CVDs. 17 , 18 , 19 However, other studies have found that time‐specific diets are not superior to energy restriction in improving cardiovascular and metabolic outcomes. 20 , 21 , 22 , 23 , 24 More recent meta‐analyses of randomized controlled trials in overweight or obese patients indicated that intermittent energy restriction involving 2–3 days of periodic fasting led to improved weight loss and reduced body fat versus continuous energy restriction. 25 , 26 In summary, there is a lack of agreement between clinical studies on whether IF may lead to improved metabolic outcomes. The conflicts between studies stem from differences in restricted feeding durations (eg, 12:12, 14:10, 16:8, ADF or periodic fasting), feeding time (during the active or resting phase), the amount of calories consumed during the feeding time, as well as the feeding regimens and the caloric content given to the control groups. 17 , 20 , 22 In addition, body mass index, glycemic control, age, sex, physical activity, and geographic and ethnic differences between individuals had major implications on the metabolic outcomes of different IF diets. 17 , 18 , 20 , 21 , 23 , 25 With these limitations withstanding, it is possible that personalized, individually tailored time‐restricted diets will be more feasible, tolerable and sustainable, and will result in more consistent long‐term metabolic improvements.
TABLE 1.
Types of intermittent fasting regimens
| Fasting regimen | Description |
|---|---|
| Alternate‐day fasting (ADF) | Switching between a day of eating regularly followed by a day of fasting. |
| 5:2 periodic fasting diet | Two days of fasting per week. |
|
Fasting‐mimicking diet (FMD) |
Several consecutive days of reduced caloric intake, followed by a normal eating cycle every one to four months or every other week. Most of the FMDs composition is based on plant‐derived compounds. |
| Time‐restricted feeding (TRF) | Limit daily food intake to a 4‐ to 12‐h window. This includes fasting during Ramadan. |
There are several theories hypothesizing about the mechanisms potentially underlying the possible benefits of IF diets compared to ad libitum feeding. A critical factor in many of these benefits is the “metabolic switch,” a phenomenon in which fasting causes the body to preferentially use fatty acids and ketone bodies derived from fatty acids as a fuel instead of glucose. 27 Consequently, ketone bodies can serve as signaling molecules that trigger response against oxidative and metabolic stress, as well as removal of damaged molecules. 18 , 28 Refeeding, on the other hand, results in increased levels of the incretin hormone glucagon‐like peptide 1 (GLP‐1) and insulin, which then enhance glucose uptake and promote tissue‐specific growth and plasticity. 18 , 28 There are ongoing efforts to identify the mechanisms by which the metabolic switch is controlled and influences the body. One of the hypotheses was that the biological clock is required for the TRF impact. 29 However, it appears that TRF also improves the metabolic phenotype in mice with mutations in the biological clock genes. 29 Thus, the mechanisms underlying the metabolic switch during fasting and feeding merits further investigation. More recently, IF has been shown to affect the gut bacteria, 30 , 31 , 32 , 33 , 34 , 35 , 36 which are involved in virtually all aspects of host physiology, suggesting an entirely new mechanism for the physiological impact of IF.
The gut microbiome. The digestive system of most living organisms is colonized by commensal bacteria, viruses, fungi, and parasites, collectively referred to as the gut microbiome. Overall, the gut microbiome consists of an enormous number of cells and functions, regarded by some as the equivalent of an additional organ. 37 Dietary components provide ample substrates for microbial growth and production of bacterial metabolites, which in turn govern many aspects of host physiology, including metabolism, digestion, and immunity. 38 Indeed, it has been shown that the intestinal microbiome has an effect on various diseases, including metabolic diseases, 39 , 40 , 41 liver diseases, 42 , 43 neurodegenerative disorders, 44 , 45 , 46 , 47 , 48 cancer, 49 among others. As for metabolic disorders, multiple studies have suggested that obese individuals have a distinct profile of gut bacterial phyla compared to lean people, and in general obese compared to lean humans and rodents show lower bacterial diversity. 41 , 50 , 51 Over the past decade, increasing evidence has emerged that the diversity, composition, and function of the gut microbiome are altered in individuals with cardiometabolic abnormalities. 52 , 53 Furthermore, CMD patients compared to healthy individuals feature differential microbial‐derived metabolites that can communicate with various remote organs and affect host physiology. 40 , 41 , 54 , 55 These include short‐chain fatty acids (SCFAs; eg, acetate, propionate, and butyrate), lactate, secondary bile acids, lipids and fatty acids, vitamins, flavonoids, byproducts from amino acids and protein metabolism, lipopolysaccharides, and trimethylamine (TMA). 40 , 41 , 56 , 57 , 58 , 59 , 60 , 61 , 62 The microbial compounds may promote health outcomes, depending on the dose of the particular metabolite as well as the specific phenotype being evaluated. 57 For example, the bacterial‐derived secondary bile acids hyodeoxycholate (HDCA) and lithocholate (LCA) show a protective effect against infection by some clinically relevant Clostridium difficile strains, 63 but the same concentrations of HDCA and LCA were found to be toxic to mammalian hepatic cells. 64 Therefore, controlled quantity of a specific type of microbial metabolite is a critical task required for the host physiology. Through the activities of the gut microbiome, metabolites from food and environmental factors are converted into molecules that are precursors for the liver, which in turn form molecules that control the host physiological functions under various circumstances. For example, dimethylglycine and N‐acetylglycine jointly produced by the host and the gut microbiome from dietary choline can regulate weight gain after smoking cessation and may affect obesity beyond the smoking settings. 40 All of these microbial‐associated metabolites can act as signaling molecules that affect the host physiology in metabolic health and disease. A causal role for the gut microbiome in CMDs progression has been demonstrated in studies using germ‐free mice that were colonized with microbes obtained from humans or rodents with cardiometabolic disorders, leading to the development of metabolic complications such as weight gain, 40 , 41 , 65 , 66 aberrant glycemic response, 67 , 68 and hypertension 69 in recipient mice.
Because the gut microbiome shows a significant impact on the host physiology, it is of particular importance to understand which factors shape the gut microbiome or control its function. Dietary composition, 70 , 71 timing, 71 , 72 , 73 , 74 and duration 41 affect the gut microbiome structure, which may persist even after diet discontinuation, 41 , 75 making dietary interventions a powerful tool for treating diseases. Recently, an increasing number of studies show that various IF diets alter the gut microbiome composition and function. 36 , 69 , 72 , 73 , 74 , 76 , 77 , 78 , 79 , 80 , 81 , 82 According to most reports, IF diets are usually accompanied by increased bacterial diversity, enhanced Firmicutes/Bacteroidetes ratio, and higher levels of SCFAs. 83 , 84 , 85 , 86 Past studies have related the Firmicutes/Bacteroidetes ratio to lower weight and improved cardiovascular health, 66 , 87 , 88 , 89 whereas some recent studies have shown conflicting results regarding the association of this bacterial ratio with improved metabolic outcomes. 90 Additional compositional alterations induced by IF diets may include an increased abundance of species belonging to Akkermansia and Lactobacillus and decreased abundance of opportunistic pathogens, such as Alistipes, which were reported in lean and healthy humans and rodents. 34 , 76 , 91 However, not all studies agree on the specific compositional alterations in bacterial communities following IF diet. For example, one study found a decrease in Akkermansia 85 in diabetic mice undergoing ADF, whereas another report showed no difference. 86 In line with these observations, a specific phylum, such as Firmicutes or Bacteroidetes, may contain a different composition of bacterial species, thus changes reported at higher levels, especially at the phylum level, appears to be of limited value. Collectively, although different IF diets induce a prominent impact on the composition and function of the gut bacteria, the effect of this altered microbiome configuration on the host metabolic functions of the host requires more comprehensive studies.
Host‐microbe circadian network. Rhythmic patterns exist at virtually all levels of biological organization and are considered one of the most important features of life. For example, mammals display daily rhythms in the form of sleep–wake cycles, cardiovascular activity, hepatic metabolism, and endocrine system function. 92 A network of circadian clocks controls the timing of food intake by setting daily windows that overlap with the active phase of the host. Circadian clocks set the internal timing by synthesizing clock‐controlled proteins that send intracellular and eventually extracellular signals. These clock genes comprise the transcriptional factors Bmal1 and Clock, whose protein products dimerize, enter the nucleus, and stimulate the transcription of repressor genes Per1, Per2, Cry, and Nr1d1. 93 , 94 The circadian clock is responsible for synchronizing metabolism and food intake with daily cycles 95 and appears to influence microbial homeostasis. The microbiome exhibits daily oscillations, manifesting in dynamic compositional and functional profiles throughout the day derived from the host's eating behavior, which in turn stimulates oral 96 , 97 , 98 and gut 72 , 73 , 74 , 99 , 100 , 101 microbial oscillations. Several studies using indirect perturbation of microbial rhythms or complete abolishment of the microbiome by antibiotics or germ‐free mice models revealed that the microbiome is responsible for the global circadian programming of the host transcriptome, epigenome, and metabolome. 73 , 102 In mice, absence of the microbiome alters rhythmic gene expression in multiple tissues, including colon, small intestine, liver, and adipose tissue. 73 , 102 Host‐microbiome circadian interactions are necessary for a variety of physiological processes, including metabolism and nutrient absorption, 103 , 104 energy expenditure, 105 hepatic detoxification, 73 , 106 sexual growth, 102 and immune function. 71 , 102 , 107 , 108 Observations in mice indicate that circadian programming is primarily derived from the diurnal penetration of commensal microbes through the mucus layer. 73 The diurnal bacterial localization through feeding is likely involved in maintaining the ability of the host to perform various physiological functions, such as nutrient uptake and management of barrier integrity. 71 , 108
In rodents, a dampened gut microbiome oscillation was observed under high‐fat diet, jet lag, and in Per1/2‐deficient mice. 72 , 73 , 74 , 99 Under any of these conditions, mice also exhibit disruptions of biological clock gene expression and arrhythmic behavior and are subsequently prone to metabolic abnormalities. 109 , 110 , 111 Interestingly, limiting the feeding time of these circadian‐disrupted mice with TRF alleviates their metabolic abnormalities 29 , 112 and also restores their gut microbiome rhythm. 72 , 74 , 84 In line with these studies in rodents, it is possible that modulating the diurnal rhythms of the gut microbiome by IF diets could be developed in the future as a valuable tool to promote health and treat metabolic disorders in humans. However, it is challenging to generalize the findings from murine data to humans, because of a large intervariability between humans in clinical studies undergoing IF diets, variations in fasting:feeding duration and variations in the caloric content of the diet given to the participants and their corresponding control groups as mentioned previously. Another major difference between rodent and human studies results from the source of the microbial sampling. Because of accessibility considerations, most human studies are conducted on stool samples. Stool samples, however, do not represent mucosal microbes that have been linked to circadian programming. 73 Nevertheless, a loss of diurnal rhythms in the gut microbiome obtained from stool samples has been reported in both humans and rodents. 85 , 100 , 113 With respect to dietary patterns, it has been reported that IF restructured the stool microbiome of diabetic mice resulting in altered bacterial configuration compared to control mice. 85 Therefore, despite possible discrepancies arising from limited information on mucosal‐associated bacteria in different parts of the gut, stool samples may still offer some valid information. Collectively, the role of the bacterial rhythmicity in cardiometabolic health and diseases should be further investigated.
2. INTERMITTENT FASTING AND MICROBIAL REGULATION IN CARDIOMETABOLIC DISORDERS
Fluctuations in food availability affect the entire ecosystem, including host and symbiotic communities, and have profound implications on cardiometabolic health. In this section, we highlight some of the remarkable discoveries about the microbiome's role in IF, which in turn influence metabolic manifestations related to CMDs such as obesity, hyperglycemia, and hypertension.
Obesity and lipid metabolism. Fat accumulation in the body that causes obesity presents serious health risks to the cardiovascular system. Excessive energy intake coupled with lower energy expenditure leads to a weight gain, which is usually composed of accumulation of body fat. 114 , 115 , 116 Rodents fed with high‐fat diets develop obesity and metabolic disorders and are widely used in research to mimic the human metabolic syndrome. 117 In mice fed with high‐fat diet, TRF was found to reduce body weight and fat mass, independent of total caloric intake and without any change in physical activity. 12 Meta‐analysis of randomized controlled trials in humans showed that intermittent energy restriction by periodic fasting of 2–3 days per week led to improved weight loss and lower body fat in overweight and obese subjects, in comparison with matched chronic calorie restriction, 25 , 26 but this was not the case for TRF or ADF diets 22 or for lean participants subjected to the ADF regimen. 21 A significant contribution to these improvements can be attributed to the impact of the gut microbiome on lipid metabolism and energy balance. 118 One such mechanism involves adipose tissue, a heterogeneous organ sensitive to nutritional stimuli and undergoing dynamic remodeling during IF. Adipose tissues are found in two different forms in mammals: white adipose tissues (WAT) that store energy as triglycerides and brown adipose tissues that burn extra calories to generate heat. 119 Within the WAT, beige adipocytes have similar properties to brown adipose tissue and are formed in response to various stimuli, predominantly cold temperatures. 120 Li et al. 118 reported that ADF modulates the formation of beige adipocytes through microbiome‐dependent mechanisms. In this study, obese mice fed with high‐fat diet and undergoing an ADF regimen exhibited an increased accumulation of beige fat in WAT accompanied by weight loss and altered gut microbiome composition. 118 Notably, antibiotics supplementation to obese mice undergoing ADF abrogated the beneficial metabolic effects of ADF, and fecal transplantation from ADF mice to antibiotics‐treated obese mice improved metabolic health, suggesting a causal role for the gut microbiome in metabolic improvements mediated by ADF. 118 Combining cecal metabolomics with shotgun metagenomics revealed that ADF induced alterations in the composition of the gut microbiome toward lactate‐ and acetate‐producing bacteria, such as Lactobacillus reuteri, which in turn resulted in accumulation of serum lactate and acetate. ADF diet also led to enhanced energy expenditure by promoting beige adipogenesis, and ameliorated weight gain and other metabolic disorders. 118 In another study, Lactobacillus levels were reproducibly elevated upon ADF only in mice fed with normal chow, whereas the genus Allobaculum was identified as exclusively enriched in mice undergoing ADF and fed with high‐fat diets. 121 Interestingly, the Allobaculum genus is an active glucose metabolizer that produces butyrate and lactate. 121 , 122 These findings suggest that ADF induced production of acetate and lactate 118 by various species of the intestinal bacteria. Yet, more studies are needed to define the effect of lactate and SCFAs on WAT browning and on the host thermogenesis and energy expenditure.
In addition to adipocyte thermogenesis, alterations in the gut microbiome may influence lipid uptake during timely eating. There are several mechanisms by which this may occur, including regulation of the nuclear factor interleukin‐3 (NFIL3), a transcription factor that is controlled by the circadian clock and regulates the rhythmic expression of genes involved in the uptake, processing, and storage of lipids in intestinal epithelial cells. 103 The rhythmic oscillations in NFIL3 are driven by the gut microbiome through activation of innate immune cells response. 103 A second factor controlling host circadian lipid uptake is histone deacetylase 3 (HDAC3). 104 Stimulation of rhythmic expression and recruitment of HDAC3 to the chromatin results in synchronized circadian oscillations of histone acetylation in the gut epithelium, which in turn regulate gene expression of nutrient transporters, thus affecting nutrient uptake and lipid absorption. 104 However, the interplay between feeding rhythms, gut microbiome oscillations, NFIL3, HDAC3, and body weight control has not been fully elucidated to date. Collectively, the gut microbiome influences energy metabolism by regulating genes that control the uptake of lipids and nutrients and by production of microbial metabolites affecting adipose tissue browning (Figure 1).
FIGURE 1.
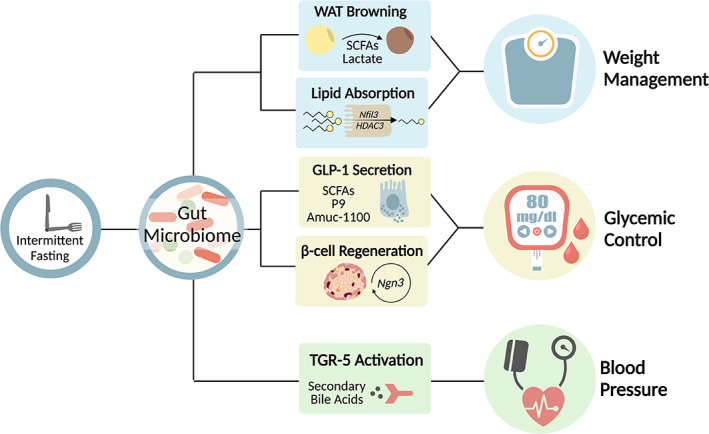
Intermittent fasting mediated changes in the gut microbiome composition and function, which in turn may affect cardiometabolic health. Gut microbiome driven WAT browning and lipid absorption contribute to weight management. Microbiome effects on glycemic control through GLP‐1 secretion and β‐cell regeneration. Microbiome derived secondary bile acids activates TGR‐5 to control blood pressure. GLP‐1, glucagon‐like peptide‐1; HDAC3, histone deacetylase 3; Nfil3, nuclear factor interleukin‐3; Ngn3, Neurogenin‐3; SCFA, short chain fatty acids; TGR‐5, Takeda G protein‐coupled receptor 5; WAT, white adipocyte tissue. Figure created with BioRender (biorender.com)
Glycemic control. Studies in rodents and monkeys demonstrated consistent beneficial effects of IF on glycemic control. 5 , 123 Several human studies suggest that IF diets can lower blood glucose and may lead to better glycemic control in T2D patients. 11 , 18 , 124 However, recent randomized controlled trials did not provide evidence that IF diets are more effective in regulating the glycemic response compared with matched caloric restriction in lean, 20 overweight, and obese individuals 21 , 22 , 26 , 125 and T2D patients. 126 The gut microbiome composition correlates with blood glucose levels 67 , 68 and adapts to fasting and refeeding periods in daily life, including circadian eating patterns and TRF (eg, as part of religious fasting). 91 , 127 In mice, the genus Lactobacillus is highly abundant during fasting, whereas the species Akkermansia muciniphila is highly enriched during feeding. 72 , 128 Apart from diurnal variations, A. muciniphila is significantly expanded in both humans and mice undergoing TRF. 76 , 91 These compositional changes are intriguing as A. muciniphila is inversely correlated with blood glucose levels in rodents and humans. 129 Furthermore, an increase in A. muciniphila is associated with enhanced secretion of GLP‐1, an insulinotropic hormone secreted by enteroendocrine L cells in response to a meal and plays a key role in whole‐body glycemic control. 129 GLP‐1 secretion follows a circadian rhythm, with higher levels of postprandial GLP‐1 following glucose loads at the active phase and lower levels during the resting phase, 130 , 131 , 132 , 133 and is additionally regulated by the clock machinery in L cells. 134 Martchenko et al 128 found that the fluctuating secretion of GLP‐1 was attenuated in obese mice fed with western diet and in microbiome‐depleted mice. This study demonstrated that restoration of GLP‐1 circadian rhythmicity in germ‐free mice was achieved by transfer of fecal microbiome from conventional mice consuming normal chow diet into obese mice. These results suggest that diurnal changes in the microbiome may play a central role in the circadian secretion of GLP‐1 and its subsequent effects on glucose homeostasis. 128 As for mechanisms, recent studies have identified several putative biomolecules produced by A. muciniphila that could trigger the secretion of GLP‐1 from intestinal L cells. Among them, propionate and the proteins P9 and Amuc‐1100 may stimulate GLP‐1 secretion from L cells. 135 It remains unclear how these bacterial molecules control GLP‐1 secretion, by which mechanisms and physiological conditions, and whether microbial modulation of GLP‐1 is driven by IF diets.
IF or FMD given to obese and hyperglycemic mice, or diabetic db/db mice lacking the leptin receptor, led to improved glycemic control and amelioration of T2D, respectively. 136 , 137 , 138 One of the primary underlying mechanisms for the beneficial effect of FMD on glucose homeostasis includes activation of Neurogenin 3 (Ngn3), a transcription factor that is essential for the development of insulin‐producing β cells. 136 Wei et al 138 confirmed that the regeneration of β cells occurs upon exposure to intermittent FMD and showed that it follows a restructuring of the gut microbiome, which was correlated with blood glucose levels in db/db mice. According to 16S rRNA sequencing of stool microbiome, FMD increased the relative abundance of Parabacteroides distasonis and Blautia, and decreased the abundance of Lachnospiraceae NK4A136, Prevotellaceae, Alistipes, and Ruminococcaceae, which correlated with lower blood glucose levels. 138 In this context, Blautia, which had higher abundance in FMD, 138 was found to be relatively increased after T2D medication treatment in diabetic rats. 139 However, a direct causal effect of the gut microbiome on β‐cell proliferation during IF diets has not been defined and merits further investigation. It is noteworthy that the induction of such phenotypes does not necessarily require complete fasting days, because ADF can be combined with alternate days of a leucine‐free diet in order to slow the progression of T2D. 140
Collectively, influences of nutritional timing on the gut microbiome and on GLP‐1 secretion as well as on β‐cell proliferation may contribute to improved glycemic control and insulin sensitivity and thereby provide a rationale for diet and microbiome‐based therapeutic potential in management of T2D (Figure 1). In agreement with the effect of IF diets on β cells regeneration in rodents, human randomized clinical trials suggested a superior effect of periodic fasting (5:2 diet) on fasting insulin compared to a matched group with daily caloric restriction. 23 , 25 , 125
Blood pressure. Hypertension refers to persistently high arterial blood pressure that, if left untreated, can lead to a number of devastating consequences such as heart failure and peripheral vascular disease. 141 Although no cure for primary hypertension is currently available, hypertension is controllable by chronic antihypertensive medical treatment coupled with lifestyle modifications. 142 There are several indications that IF and meal timing may contribute to improved hypertension in mice and humans. 11 , 18 , 143 , 144 , 145 With these observations notwithstanding, the validity of IF on blood pressure has been questioned in a number of randomized controlled studies with large numbers of participants, showing that IF, ADF, or 5:2 diet have no additive effect on blood pressure. 21 , 23 , 25 Although the effectiveness of IF is debatable, the DASH diet, which stands for Dietary Approaches to Stop Hypertension, offers a low sodium diet that may reduce hypertension. 146 , 147 A recent study suggested that a five‐day fast followed by a modified DASH diet reduces systolic blood pressure in patients with hypertensive metabolic syndrome. 147 This fasting regime also altered the gut microbiome, including several bacterial communities and genes associated with SCFA production. 147 Within the IF group in this study, a prediction of sustained systolic blood pressure response was performed using machine‐learning analysis of baseline microbiome data, identifying Desulfovibrionaceae, Hydrogenoanaerobacterium, Akkermansia, and Ruminococcaceae as potential contributors to controlled hypertension. 147 In rats, five weeks of ADF regime significantly reduced blood pressure in hypertensive stroke‐prone animals. 69 This phenotypic change was accompanied by altered microbiome configuration including elevated Bacteroides uniformis, Lactobacillus reuteri, Lactobacillus johnsonii. Mechanistically, the ADF diet was associated with a microbial shift toward bacteria producing secondary bile acids, both conjugated and unconjugated (such as taurolithocholic acid, taurodeoxycholic acid, tauroursodeoxycholic acid [ TUDCA], LCA, glycochenodeoxycholic acid, and more), and activation of the bile acid receptor Takeda G protein‐coupled receptor 5 (TGR5; Figure 1). Moreover, treatment of these hypertensive rats with cholic acid or TGR5 agonist attenuated elevated blood pressure, thus exceeding the need for ADF. 69 Fecal transplantation from ADF‐fed rats to germ‐free rats prevented elevated systolic blood pressure, showing the causal role of the gut microbiome in lowering blood pressure. 69 Further studies need to define the target tissue and cell types that respond to the secondary bile acids as well as the cellular pathways induced by these bile acids, leading to the reduction in blood pressure. In addition, more trials are required to define the efficacy and sustainability of different IF diets in lowering blood pressure in patients with various cardiometabolic derangements.
3. INTERMITTENT FASTING AND MICROBIAL REGULATION IN LONG‐TERM CARDIOMETABOLIC COMPLICATIONS
CMDs are progressive diseases, demonstrating long‐term and devastating consequences. A common manifestation in T2D patients is microvascular complications that include retinopathy and nephropathy as well as macrovascular diseases such as CVDs. IF diets, as part of nutrition therapy, have emerged as a potential intervention for managing several long‐term complications of T2D, including retinopathy, cognitive decline, heart failure, and nephropathy (Figure 2).
FIGURE 2.
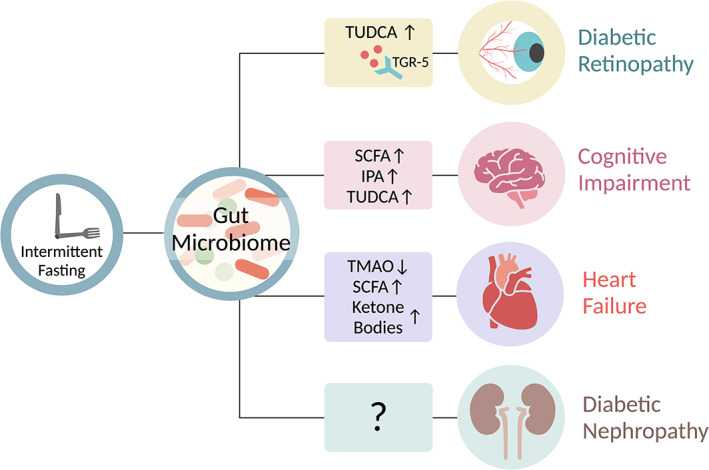
Intermittent fasting mediated gut microbial metabolites may affect cardiometabolic complications. IPA, Indole‐3‐propionic acid; SCFA, short chain fatty acids; TGR‐5, Takeda G protein‐coupled receptor 5; TMAO, trimethylamine N‐oxide; TUDCA, tauroursodeoxycholic acid. Figure created with BioRender (biorender.com)
Retinopathy. Diabetic retinopathy is a complication of T2D that affects the blood vessels of the retina and can lead to blindness in untreated individuals. 148 Fortunately, the risk of vision loss due to diabetic retinopathy can be reduced by early detection and timely treatment to control glucose levels and blood pressure. 149 , 150 It has been suggested that the gut microbiome may be involved in the pathogenesis of diabetic retinopathy. 85 , 151 , 152 , 153 , 154 , 155 According to Das et al, 152 the gut microbiome of patients with diabetic retinopathy differs significantly from healthy individuals as well as from T2D patients without retinopathy. Examples of these differences include decreased abundance of Bifidobacterium and Turicibacter, and increased abundance of Akkermansia in diabetic retinopathy. 152 Following this observational study, Huang et al 154 presented the potential use of the gut microbiome as a discriminating biomarker of diabetic retinopathy and found that Pasteurellaceae, Oxalobacteraceae, and Gallionellaceae bacterial families were the main biomarkers distinguishing patients with T2D and patients with diabetic retinopathy, which could be helpful for diagnosis of retinopathy. Among them, the bacterial family Pasteurellaceae was specifically reduced in T2D patients with retinopathy and loss of this bacteria can serve as a predictive biomarker for the disease. 154 Surprisingly, Das et al 152 did not report similar changes. In relation to the IF diet regimes, a study by Beli et al 85 reported an association between the gut microbiome, dietary patterns, and diabetic retinopathy. The authors use diabetic (db/db) mice to compare the classical markers of diabetic retinopathy in mice fed with an ADF diet compared to those fed ad libitum. They found that the fasting regime prevented the increase in the number of acellular retinal capillaries and reduced the infiltration of inflammatory cells into the retina. 85 Diabetic mice fed with ADF featured a significant expansion of Firmicutes phylum, and more specifically at the genus level, enhanced abundance of Oscillospira, Ruminococcus, and Turicibacter and reduced abundance of Bacteroides, Akkermansia, Bifidobacterium, and Allobaculum. 85 Along with the changes in gut microbiome composition, the authors observed that diabetic mice exhibited differences in diurnal microbial patterns compared to nondiabetic mice, which also altered in response to ADF. 85 Although the functional significance of the microbial oscitations following the ADF diet has not been addressed in depth, this observation is important as it implies that the effect of IF diet is highly dependent on the health status of the host. Notably, ADF enhanced the metabolism of primary to secondary bile acids, such as TUDCA, only in diabetic mice. The receptor for TUDCA, TGR5, is expressed in retinal ganglion cells, suggesting that microbial production of TUDCA can potentially affect the retina. Supplementation of diabetic mice with a potent agonist for TGR5 resulted in diminished diabetic retinopathy, featured by reduced retinal inflammation and lower acellular capillaries. Consequently, it is feasible that ADF may lead to altered gut bacterial production of secondary bile acids, activation of retinal TGR5, and protection against retinal degeneration 85 (Figure 2). Sor far, it is unknown which cells in the retina respond to the secondary bile acids and the signaling pathways induced by the bile acids resulting in protection against retinal degeneration. Taken together, these results suggest that IF interventions such as ADF may lead to a unique modification of the gut microbial communities and metabolites that may contribute to retinopathy diagnosis and may potentially ameliorate diabetic retinopathy.
Cognitive impairment. T2D can cause pronounced central nervous system complications, including structural alterations or brain atrophy, cerebral microvascular damage, neuroinflammation as well as changes in electrophysiological properties of the brain that ultimately result in cognitive deficits. 156 , 157 These alterations in cognition and brain structure may, over time, lead to an acceleration of cognitive decline and an increased risk for age‐related neurodegenerations such as Alzheimer's disease. 158 Multiple factors were proposed to induce cognitive impairments in diabetes, these include brain insulin resistance and lower glucose uptake as well as, disrupted neurotransmitter metabolism. 156 , 159 In animal models, various types of IF diets have been reported to benefit brain health and delay the development of neurodegenerative diseases. 160 , 161 , 162 Notably, the gut microbiome may play a role in modulating cognitive functions induced by IF. 80 , 86 , 163 , 164 Liu et al show a link between the gut microbiome and cognitive functions during ADF treatment of diabetic db/db mice. 86 These diabetic mice exhibited cognitive decline, but a 28‐day ADF regimen improved anxious behavior, locomotor activity, and synapse structure, along with preservation of insulin signaling and mitochondrial biogenesis in the hippocampus. The improvement in brain functions of the db/db‐ADF mice group was accompanied by an increased abundance of genus Lactobacillus and Odoribacter and decreased Enterococcus, Streptococcus, and Enterococcaceae. The protective effect of ADF on the cognitive functions of the diabetic mice was partly abolished upon antibiotic treatment. In order to determine how these microbes might affect cognitive function, the authors used genome‐based functional predictive analysis and metabolomics. 86 They found enrichment of primary and secondary bile acid biosynthesis pathways in the ADF group. In addition, db/db‐ADF mice group exhibited an increased fecal and plasma levels of several microbial‐related metabolites, among these metabolites are SCFAs, TUDCA (secondary bile acid that was also protective against retinopathy), indole‐3‐propionic acid (IPA), and serotonin. Supplementation of these metabolites improved cognitive functions and insulin sensitivity in db/db mice. 86 The signaling pathways induced by the microbial metabolites, the connection to brain insulin sensitivity, and the responding cells in the brain remain to be determined. Collectively, these studies propose that gut bacterial species and metabolites induced by ADF may contribute to alleviation of diabetes‐induced cognitive impairment (Figure 2) and suggest that the bacterial metabolites may modulate features of brain function even in the absence of ADF.
Heart failure. Murine models of insulin resistance have suggested that IF diets such as TRF 12 , 137 and FMD 138 may lead to improvement of hypertension, dyslipidemia, hyperglycemia, and hyperinsulinemia, all constituting general indicators of cardiovascular health. 165 Initially, clinical studies suggested that IF diets, including ADF 21 and TRF, 166 improve cardiac protection in T2D patients. A number of human randomized controlled trials have reported that restricting calories and IF diets are equally effective in lowering hypertension, dyslipidemia, hyperglycemia, and hyperinsulinemia, all of which are risk factors for cardiac events. 20 , 21 , 22 , 23 , 25 , 26 Several studies have shown a compositional alterations of gut microbial communities in patients with heart failure. 167 , 168 , 169 A study in Dahl salt‐sensitive rats, which develop hypertension and are more prone to heart failure, showed that supplementation with the probiotic bacteria Lactobacillus plantarum 299v reduced their susceptibility to heart failure and resulted in better recovery following myocardial infarction. 170 Several mechanisms have been proposed to explain the potential link between the gut microbiome and heart failure, including microbiome‐induced modulation of inflammation, intestinal permeability, and associations made to bacterial overgrowth and bacterial biofilm formation. 167 , 168 , 169 Expansion of pathogenic bacteria was also detected in patients with heart failure in several studies. 169 , 171 A study by Crawford et al 172 sheds light on how IF may benefit the heart, proposing that the gut microbiome can produce SCFAs that provide the heart with adequate energy during fasting. Their study showed that germ‐free mice compared to conventionalized mice exhibited reduced liver production of ketone bodies. According to this study, fasting was associated with higher abundance of Bacteroides that may be responsible for the production of SCFAs, especially acetate, which may be utilized for hepatic ketogenesis, thereby providing a source of energy for the heart. 172 During fasting, the heart of germ‐free mice utilized glucose instead of ketone bodies, which was reverted to ketone body metabolism by feeding a ketogenic diet. Therefore, it is plausible that the beneficial effects of fasting on cardiac function depends, at least in part, on the presence and the ability of intestinal bacteria to enhance the generation of ketone bodies that can be oxidized by the heart 172 (Figure 2). A recent study by Mistry et al 173 suggested that the circadian clock governs the bacterial effects on the heart, including those reflected during TRF. This study reported that mice undergoing myocardial infarction and treated with antibiotics had an increased incidence of heart failure, which was abrogated by cohousing with mice without microbiome depletion. Interestingly, mice with a disrupted circadian rhythm showed perturbation in gut microbiome rhythmicity, as well as impaired ischemic cardiac healing and heart failure, even in the presence of an intact microbiome. 173 With respect to IF dietary patterns, the authors used TRF diet and showed that mice fed only at the dark phase before myocardial infarction had improved cardiac function compared to mice fed only at the light phase. Thus, both the gut microbiome and TRF can individually affect heart failure outcomes. 173 However, it remains to be determined whether the gut microbiome mediates the improved cardiac functions induced by TRF.
An additional mechanism by which the gut microbiome can impact cardiovascular health is through the gut bacterial production of trimethylamine‐N‐oxide (TMAO). A seminal study by Wang et al 174 showed that gut microbial metabolism of dietary choline and L‐carnitine resulted in production of TMA, which was converted by the host liver to TMAO. The TMA‐TMAO pathway was found to be involved in atherosclerosis, platelet hyperactivity, and thrombosis and was used to predict an increased risk of CVDs. 174 , 175 Fasting affects liver metabolism and is associated with decreased TMAO 176 (Figure 2). It has been previously suggested that maintaining low levels of TMAO may be particularly helpful in preventing T2D‐associated cardiomyopathy. 177 Recently, elevated TMAO levels were found in db/db mice during both daytime and nighttime, which was associated with loss of diurnal oscillations of various gut bacteria. 113 The authors suggested that it may be possible to restore the lost diurnal bacterial oscillations by restricting the feeding during the active period. 113 In accordance with findings in rodents, it remains to be determined whether IF diets can harness the gut microbiome, regulate TMAO levels and lead to effective improvements in cardiovascular outcomes in T2D patients.
Nephropathy. Kidney dysfunction or nephropathy as a result of diabetes is the leading cause of chronic kidney disease. 178 In diabetic patients, poor glycemic control and hypertension can lead to glomerular hyperfiltration, albuminuria, nephrotic proteinuria, and development of end‐stage renal disease. 179 , 180 , 181 Several studies have shown alterations in gut bacterial abundance in patients with diabetic nephropathy compared to controls, and overall lower bacterial diversity was correlated with disease progression. 182 , 183 , 184 In rodents, diabetic nephropathy was associated with altered bacterial communities and microbial metabolites like phenyl sulfate 185 , 186 and correlated with activation of the renin‐angiotensin system. 187 , 188 Several observational studies have tested the effect of Ramadan fasting on diabetic nephropathy severity, but none of them showed significant changes in renal functions. These reports were not randomized controlled studies, had small sample sizes, and contained patients with different medications or dialysis treatment. Furthermore, most of the studies were conducted in countries where fasting durations were 12‐14 h during the winter season and thus cannot be generalized to longer fasting durations. 189 Currently, the efficacy of IF on kidney functions and diabetic nephropathy and the involvement of the gut microbiome in this process have not been elucidated.
4. CHALLENGES AND LIMITATIONS
Recent studies report that during multiple IF diets, the intestinal microbiome may act as a signaling hub driving the improvement in cardiometabolic complications. However, there are several technical and conceptual obstacles that still need to be carefully taken into consideration in interpreting these studies. First, IF diets regimens are inconsistently defined across studies and the long‐term effects of IF diets were mostly undetermined. From the perspective of microbiome analyses, some studies are lacking a suitable availability of some microbial datasets and resources. Furthermore, microbiome sampling in most of the human studies analyze fecal samples and disregard regional differences in the gut microbiome composition and function along the gastrointestinal tract. Additionally, some microbiome studies feature technical differences in data acquisition and analysis modalities (eg, sample allocation, extraction, or sequencing methods) differ between studies, making comparisons between their results challenging. There is also a global inability to generalize conclusions on microbiome structural alterations across human studies, due to differences between ethnical, geographical and demographical groups and variability in body mass index, sex, age, physical activity, and other clinical parameters of the participants in different studies. In addition to technical and human individual variabilities, other differences derived from personalized uniqueness of microbiome configurations constitute a formidable challenge in generalizing such results. However, the individualized microbiome heterogeneity is not necessarily a disadvantage, because it may allow for identification and development of personalized responses to various IF interventions and forms a basis (together with a variety of host clinical and laboratory features) for personalized nutrition.
5. FUTURE PERSPECTIVES
The discovery that the gut microbiome may modulate the metabolic outcomes of IF diets may provide mechanistic explanations for the observed phenotypes as well as for the personal response to the IF regimens observed in different individuals exposed to a similar dietary intervention. Indeed, gut microbiome composition and function differ between humans and rodents following IF diets as compared to those following continuous food consumption. In addition to the altered bacterial configuration, IF collectively leads to bacterial production of specific metabolites such as secondary bile acids, SCFAs, and ketone bodies, which may have a distinct downstream impact on host physiology and disease. Although we highlight in this review how changes in microbial configuration and metabolite production may potentially influence the clinical outcomes of different cardiometabolic abnormalities, comprehensive future research is warranted to decipher the molecular and chemical processes that mediate these effects. In addition, further research is needed to address the effect of the microbiome on additional organs such as the lung, skin, muscles, and kidneys, whose functions could also be influenced by IF diets. 190 , 191 , 192 , 193 Moreover, it remains to be determiced what is the role of the IF‐microbiome axis in several T2D‐related complications, such as the susceptibility to fungal infections, 194 diabetic foot ulcers, and diabetic nephropathy, which account for high morbidity and mortality rates in T2D patients. 195 Another question is whether a combination of IF with a defined diet composition or food choice consumed in eating days (eg, the aforementioned 5:2 DASH diet 147 ) can provide targeted benefits for certain CMD‐related conditions. In this respect, it is worth noting that although the nutrient composition of the diet has a tremendous effect on the gut microbiome, 196 the impact on CMD has not been elucidated. Other factors such as digestibility and transit time can be modulated during multiple IF diets. However, their relevance to CMD is unclear.
A major challenge in translating IF regimens into clinical practice is the contradictory results found in human studies investigating the effects of IF diets on human metabolism. 22 , 23 , 24 , 25 , 26 , 197 One reason for these discrepancies stems from the human individual response to IF diets. For example, Berry et al 198 showed that postprandial responses to food vary across individuals when the same meals are given at different hours. Because the gut microbiome is involved in the personal response to food, 70 it would be reasonable to assume that the personal response to IF can also be linked to the gut microbiome. Collectively, more well‐controlled, prospective, longitudinal clinical studies are required to define the microbial alterations in different IF diets that can be further translated into clinical practice.
The idea that the gut microbiome is affected by IF diets and affects normal host physiology and CMD progression allows using these findings as a potential basis for therapeutic intervention. Identification of bioactive microbial‐derived molecules potentially affecting human metabolism can be developed as a bona fide “postbiotic therapy,” bypassing the high interindividual variability of the microbiomes producing such molecules. One such example is SCFAs, which in rodents demonstrated beneficial effects on adipose tissue browning and energy expenditure, 118 secretion of GLP‐1 from L cells and glycemic control, 135 and cardiac functions. 172 Secondary bile acids are another example of bacterial molecules that showed beneficial cardiometabolic effects in mice, as exemplified by secondary bile acids that attenuated hypertension 69 and show protection against diabetic retinopathy 85 and cognitive impairments. 86 On the other hand, specific microbial products can induce worsened metabolic outcomes, such as TMAO that was involved in cardiac dysfunctions and associated with a higher risk for CVDs. 174 , 175 In many cases, these metabolites were modified during implementation of different IF diets (Figure 2) and contributed to the effect of IF on metabolic outcomes. An addition of the secondary bile acid TUDCA or an agonist of the bile acids receptor TGR5 led to protection from cognitive decline and retinopathy in diabetic mice, bypassing the need for IF diet, 85 , 86 whereas microbial enzyme inhibitors for TMAO 169 , 199 may alleviate cardiometabolic derangements. These and other microbiome‐based interventions merit further studies.
CONFLICT OF INTEREST
E.E. is a scientific founder of DayTwo and BiomX, and a paid consultant to Hello Inside GmbH. The remaining authors declare no competing interests.
ACKNOWLEDGEMENTS
We thank the members of the Elinav lab, Weizmann Institute of Science, and members of the DKFZ microbiome and cancer division for insightful discussions. K.R. is supported by The Nehemia Levtzion Scholarship for Outstanding Doctoral Students; H.S. is the incumbent of the Vera Rosenberg Schwartz Research Fellow Chair; K.G. is supported by The Ariane de Rothschild Women Doctoral Program. E.E. is supported by the Leona M. and Harry B. Helmsley Charitable Trust; Adelis Foundation; Ben B. and Joyce E. Eisenberg Foundation; Estate of Bernard Bishin for the WIS‐Clalit Program; Jeanne and Joseph Nissim Center for Life Sciences Research; Miel de Botton; the Vera Rosenberg Schwartz Research Fellow Chair; Swiss Society Institute for Cancer Prevention Research; Belle S. and Irving E. Meller Center for the Biology of Aging; Sagol Institute for Longevity Research; Sagol Weizmann‐MIT Bridge Program; Norman E Alexander Family M Foundation Coronavirus Research Fund; Mike and Valeria Rosenbloom Foundation; Daniel Morris Trust; Isidore and Penny Myers Foundation; Vainboim Family; and by grants funded by the European Research Council; Israel Science Foundation; Israel Ministry of Science and Technology; Israel Ministry of Health; the German‐Israeli Helmholtz International Research School: Cancer‐TRAX (HIRS‐0003); Helmholtz Association‘s Initiative and Networking Fund; Minerva Foundation; Garvan Institute; European Crohn's and Colitis Organization; Deutsch‐Israelische Projektkooperation; IDSA Foundation; WIS‐MIT grant; Emulate; Charlie Teo Foundation; Mark Foundation for Cancer Research, and Welcome Trust. E.E. is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair of immunology; a senior fellow, Canadian Institute of Advanced Research (CIFAR); and an international scholar, The Bill & Melinda Gates Foundation and Howard Hughes Medical Institute (HHMI).
Ratiner K, Shapiro H, Goldenberg K, Elinav E. Time‐limited diets and the gut microbiota in cardiometabolic disease. Journal of Diabetes. 2022;14(6):377‐393. doi: 10.1111/1753-0407.13288
Karina Ratiner, Hagit Shapiro and Kim Goldenberg are equal contributors.
Funding information N/A
REFERENCES
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047‐1053. [DOI] [PubMed] [Google Scholar]
- 2. Aron‐Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12(3):169‐181. [DOI] [PubMed] [Google Scholar]
- 3. Fjellström C. Mealtime and meal patterns from a cultural perspective. Scand Jof Nutr. 2004;48(4):161‐164. [Google Scholar]
- 4. St‐Onge MP, Ard J, Baskin ML, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96‐e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133(6):1921‐1929. [DOI] [PubMed] [Google Scholar]
- 7. Fontana L, Meyer TE, Klein S, Holloszy JO. Long‐term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659‐6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandhorst S, Choi IY, Wei M, et al. A periodic diet that mimics fasting promotes multi‐system regeneration, enhanced cognitive performance, and Healthspan. Cell Metab. 2015;22(1):86‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regmi P, Chaudhary R, Page AJ, et al. Early or delayed time‐restricted feeding prevents metabolic impact of obesity in mice. J Endocrinol. 2021;248(1):75‐86. [DOI] [PubMed] [Google Scholar]
- 10. Jamshed H, Beyl RA, Della MDL, Yang ES, Ravussin E, Peterson CM. Early time‐restricted feeding improves 24‐hour glucose levels and affects markers of the circadian clock, aging, and autophagy in human. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time‐restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212‐1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hatori M, Vollmers C, Zarrinpar A, et al. Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab. 2012;15(6):848‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neil CE, Byrd‐Bredbenner C, Hayes D, Jana L, Klinger SE, Stephenson‐Martin S. The role of breakfast in health: definition and criteria for a quality breakfast. J Acad Nutr Diet. 2014;114(12):S8‐S26. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi M, Ozaki M, Kang MI, et al. Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults. Nutrients. 2018;10(11):1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wicherski J, Schlesinger S, Fischer F. Association between breakfast skipping and body weight—a systematic review and meta‐analysis of observational longitudinal studies. Nutrients. 2021;13(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonnet JP, Cardel MI, Cellini J, Hu FB, Guasch‐Ferré M. Breakfast skipping, body composition, and Cardiometabolic risk: a systematic review and meta‐analysis of randomized trials. Obesity (Silver Spring). 2020;28(6):1098‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541‐2551. [DOI] [PubMed] [Google Scholar]
- 19. Cho Y, Hong N, Kim KW, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta‐analysis. J Clin Med. 2019;8(10):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Templeman I, Smith HA, Chowdhury E, et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. 2021;13(598):eabd8034. [DOI] [PubMed] [Google Scholar]
- 21. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate‐day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowe DA, Wu N, Rohdin‐Bibby L, et al. Effects of time‐restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta‐analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time‐restricted eating in weight loss. N Engl J Med. 2022;386(16):1495‐1504. [DOI] [PubMed] [Google Scholar]
- 25. He S, Wang J, Zhang J, Xu J. Intermittent versus continuous energy restriction for weight loss and metabolic improvement: a meta‐analysis and systematic review. Obesity (Silver Spring). 2021;29(1):108‐115. [DOI] [PubMed] [Google Scholar]
- 26. Patikorn C, Roubal K, Veettil SK, et al. Intermittent fasting and obesity‐related health outcomes: an umbrella review of meta‐analyses of randomized clinical trials. JAMA Netw Open. 2021;4(12):e2139558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: understanding and applying health benefits of fasting. Obesity (Silver Spring). 2018;26(2):254‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time‐restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303‐319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Merwe M, Sharma S, Caldwell JL, et al. Time of feeding alters obesity‐associated parameters and gut bacterial communities, but not fungal populations, in C57BL/6 male mice. Curr Dev Nutr. 2020;4(2):nzz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J, Zhong Y, Luo XM, Ma Y, Liu J, Wang H. Intermittent fasting reshapes the gut microbiota and metabolome and reduces weight gain more effectively than melatonin in mice. Front Nutr. 2021;8:784681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeb F, Wu X, Chen L, et al. Time‐restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition. 2020;78:110797. [DOI] [PubMed] [Google Scholar]
- 33. Pinto FCS, Silva AAM, Souza SL. Repercussions of intermittent fasting on the intestinal microbiota community and body composition: a systematic review. Nutr Rev. 2022;80(3):613‐628. [DOI] [PubMed] [Google Scholar]
- 34. Palomba A, Tanca A, Abbondio M, et al. Time‐restricted feeding induces lactobacillus‐ and Akkermansia‐specific functional changes in the rat fecal microbiota. NPJ Biofilms Microbiomes. 2021;7(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papadopoulou RT, Theodorou MR, Ieong CS, et al. The acute effect of meal timing on the gut microbiome and the Cardiometabolic health of the host: a crossover randomized control trial. Ann Nutr Metab. 2020;76(5):322‐333. [DOI] [PubMed] [Google Scholar]
- 36. Zeb F, Wu X, Chen L, et al. Effect of time restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. 2020;123(11):1216‐1226. [DOI] [PubMed] [Google Scholar]
- 37. Goodrich JK, Davenport ER, Clark AG, Ley RE. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;51:413‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lynch S v., Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375(24):2369–2379. [DOI] [PubMed] [Google Scholar]
- 39. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55‐71. [DOI] [PubMed] [Google Scholar]
- 40. Fluhr L, Mor U, Kolodziejczyk AA, et al. Gut microbiota modulates weight gain in mice after discontinued smoke exposure. Nature. 2021;600(7890):713‐719. [DOI] [PubMed] [Google Scholar]
- 41. Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post‐dieting weight regain. Nature. 2016;540(7634):544‐551. [DOI] [PubMed] [Google Scholar]
- 42. Kolodziejczyk AA, Federici S, Zmora N, et al. Acute liver failure is regulated by MYC‐ and microbiome‐dependent programs. Nat Med. 2020;26(12):1899‐1911. [DOI] [PubMed] [Google Scholar]
- 43. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2):e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474‐480. [DOI] [PubMed] [Google Scholar]
- 45. Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067‐1080.e16. [DOI] [PubMed] [Google Scholar]
- 46. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism Spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600‐1618.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469‐1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241‐255. [DOI] [PubMed] [Google Scholar]
- 49. Cullin N, Antunes CA, Straussman R, Stein‐Thoeringer KC, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39(10):1317‐1341. [DOI] [PubMed] [Google Scholar]
- 50. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541‐546. [DOI] [PubMed] [Google Scholar]
- 51. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261‐273. [DOI] [PubMed] [Google Scholar]
- 53. Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018;9(5):397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torres‐Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747‐756. [DOI] [PubMed] [Google Scholar]
- 55. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079‐1089. [DOI] [PubMed] [Google Scholar]
- 56. Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753‐760. [DOI] [PubMed] [Google Scholar]
- 57. Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi‐kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77‐94. [DOI] [PubMed] [Google Scholar]
- 58. Koh A, Molinaro A, Ståhlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175(4):947‐961.e17. [DOI] [PubMed] [Google Scholar]
- 59. Zhang L, Yang G, Untereiner A, Ju Y, Wu L, Wang R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology. 2013;154(1):114‐126. [DOI] [PubMed] [Google Scholar]
- 60. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716‐724. [DOI] [PubMed] [Google Scholar]
- 61. Koeth RA, Levison BS, Culley MK, et al. γ‐Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L‐carnitine to TMAO. Cell Metab. 2014;20(5):799‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Utzschneider KM, Kratz M, Damman CJ, Hullarg M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2020;101(4):1445‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thanissery R, Winston JA, Theriot CM. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perreault M, Białek A, Trottier J, et al. Role of glucuronidation for hepatic detoxification and urinary elimination of toxic bile acids during biliary obstruction. PLoS One. 2013;8(11):e80994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027‐1031. [DOI] [PubMed] [Google Scholar]
- 67. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181‐186. [DOI] [PubMed] [Google Scholar]
- 68. Gérard C, Vidal H. Impact of gut microbiota on host glycemic control. Front Endocrinol (Lausanne). 2019;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shi H, Zhang B, Abo‐Hamzy T, et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circ Res. 2021;128(9):1240‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079‐1094. [DOI] [PubMed] [Google Scholar]
- 71. Tuganbaev T, Mor U, Bashiardes S, et al. Diet diurnally regulates small intestinal microbiome‐epithelial‐immune homeostasis and enteritis. Cell. 2020;182(6):1441‐1459.e21. [DOI] [PubMed] [Google Scholar]
- 72. Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514‐529. [DOI] [PubMed] [Google Scholar]
- 73. Thaiss CA, Levy M, Korem T, et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167(6):1495‐1510.e12. [DOI] [PubMed] [Google Scholar]
- 74. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20(6):1006‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hu D, Mao Y, Xu G, et al. Time‐restricted feeding causes irreversible metabolic disorders and gut microbiota shift in pediatric mice. Pediatr Res. 2019;85(4):518‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li L, Su Y, Li F, et al. The effects of daily fasting hours on shaping gut microbiota in mice. BMC Microbiol. 2020;20(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reichelt AC, Loughman A, Bernard A, et al. An intermittent hypercaloric diet alters gut microbiota, prefrontal cortical gene expression and social behaviours in rats. Nutr Neurosci. 2020;23(8):613‐627. [DOI] [PubMed] [Google Scholar]
- 78. Zhang L, Xue X, Zhai R, et al. Timing of calorie restriction in mice impacts host metabolic phenotype with correlative changes in gut microbiota. mSystems. 2019;4(6):e00348‐e00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Su J, Wang Y, Zhang X, et al. Remodeling of the gut microbiome during Ramadan‐associated intermittent fasting. Am J Clin Nutr. 2021;113(5):1332‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cignarella F, Cantoni C, Ghezzi L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27(6):1222‐1235.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gabel K, Marcell J, Cares K, et al. Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health. 2020;26(2):79‐85. [DOI] [PubMed] [Google Scholar]
- 82. Costello EK, Gordon JI, Secor SM, Knight R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. 2010;4(11):1375‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu D, Mao Y, Xu G, Liao W, Yang H, Zhang H. Gut flora shift caused by time‐restricted feeding might protect the host from metabolic syndrome, inflammatory bowel disease and colorectal cancer. Transl Cancer Res. 2018;7(5):1282‐1289. [Google Scholar]
- 84. Ye Y, Xu H, Xie Z, et al. Time‐restricted feeding reduces the detrimental effects of a high‐fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front Nutr. 2020;7:596285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beli E, Yan Y, Moldovan L, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67(9):1867‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu Z, Dai X, Zhang H, et al. Gut microbiota mediates intermittent‐fasting alleviation of diabetes‐induced cognitive impairment. Nat Commun. 2020;11(1):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ley RE, BäCkhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070‐11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ley RE, Turnbaugh PJ, Klein S, Gordon IJ. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022‐1023. [DOI] [PubMed] [Google Scholar]
- 89. Canfora EE, Jocken JW, Blaak EE. Short‐chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577‐591. [DOI] [PubMed] [Google Scholar]
- 90. Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut Dysbiosis in obese patients? Nutrients. 2020;12(5):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ozkul C, Yalinay M, Karakan T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: a preliminary study on intermittent fasting. Turk J Gastroenterol. 2020;30(12):1030‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18(3):250‐260. [DOI] [PubMed] [Google Scholar]
- 93. Panda S, Hogenesch JB. It's all in the timing: many clocks, many outputs. J Biol Rhythms. 2004;19(5):374‐387. [DOI] [PubMed] [Google Scholar]
- 94. Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125‐137. [DOI] [PubMed] [Google Scholar]
- 95. Challet E. The circadian regulation of food intake. Nat Rev Endocrinol. 2019;15(7):393‐405. [DOI] [PubMed] [Google Scholar]
- 96. Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. Temporal stability of the salivary microbiota in oral health. PLoS One. 2016;11(1):e0147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Collado MC, Engen PA, Bandín C, et al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 2018;32(4):2060‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Skarke C, Lahens NF, Rhoades SD, et al. A pilot characterization of the human chronobiome. Sci Rep. 2017;7(1):17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Leone V, Gibbons SM, Martinez K, et al. Effects of diurnal variation of gut microbes and high‐fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reitmeier S, Kiessling S, Clavel T, et al. Arrhythmic gut microbiome signatures predict risk of type 2 diabetes. Cell Host Microbe. 2020;28(2):258‐272.e6. [DOI] [PubMed] [Google Scholar]
- 101. Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A. 2015;112(33):10479‐10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weger BD, Gobet C, Yeung J, et al. The mouse microbiome is required for sex‐specific diurnal rhythms of gene expression and metabolism. Cell Metab. 2019;29(2):362‐382.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper L. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 2017;357(6354):912‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuang Z, Wang Y, Li Y, et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. 2019;365(6460):1428‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rong B, Wu Q, Saeed M, Sun C. Gut microbiota‐a positive contributor in the process of intermittent fasting‐mediated obesity control. Anim Nutr. 2021;7(4):1283‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gong S, Lan T, Zeng L, et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018;69(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812‐827. [DOI] [PubMed] [Google Scholar]
- 108. Brooks JF, Behrendt CL, Ruhn KA, et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell. 2021;184(16):4154‐4167.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kohsaka A, Laposky AD, Ramsey KM, et al. High‐fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414‐421. [DOI] [PubMed] [Google Scholar]
- 110. Husse J, Hintze SC, Eichele G, Lehnert H, Oster H. Circadian clock genes Per1 and Per2 regulate the response of metabolism‐associated transcripts to sleep disruption. PLoS One. 2012;7(12):e52983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Desmet L, Thijs T, Segers A, Verbeke K, Depoortere I. Chronodisruption by chronic jetlag impacts metabolic and gastrointestinal homeostasis in male mice. Acta Physiol (Oxf). 2021;233(4):e13703. [DOI] [PubMed] [Google Scholar]
- 112. Schilperoort M, van den Berg R, Dollé MET, et al. Time‐restricted feeding improves adaptation to chronically alternating light‐dark cycles. Sci Rep. 2019;9(1):7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Beli E, Prabakaran S, Krishnan P, Evans‐Molina C, Grant MB. Loss of diurnal oscillatory rhythms in gut microbiota correlates with changes in circulating metabolites in type 2 diabetic db/db mice. Nutrients. 2019;11(10):2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab (Lond). 2004;1(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wells JCK, Siervo M. Obesity and energy balance: is the tail wagging the dog? Eur J Clin Nutr. 2011;65(11):1173‐1189. [DOI] [PubMed] [Google Scholar]
- 117. Hariri N, Thibault L. High‐fat diet‐induced obesity in animal models. Nutr Res Rev. 2010;23(2):270‐299. [DOI] [PubMed] [Google Scholar]
- 118. Li G, Xie C, Lu S, et al. Intermittent fasting promotes white adipose Browning and Decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26(4):672‐685.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Peirce V, Carobbio S, Vidal‐Puig A. The different shades of fat. Nature. 2014;510(7503):76‐83. [DOI] [PubMed] [Google Scholar]
- 120. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Deng Y, Liu W, Wang J, Yu J, Yang LQ. Intermittent fasting improves lipid metabolism through changes in gut microbiota in diet‐induced obese mice. Med Sci Monit. 2020;26:e926789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Herrmann E, Young W, Rosendale D, et al. RNA‐based stable isotope probing suggests Allobaculum spp. as particularly active glucose assimilators in a complex murine microbiota cultured in vitro. Biomed Res Int. 2017;2017:1829685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mattison JA, Colman RJ, Beasley TM, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Furmli S, Elmasry R, Ramos M, Fung J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018;2018:bcr2017221854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. 2018;1(3):e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Secor SM, Carey HV. Integrative physiology of fasting. Compr Physiol. 2016;6(2):773‐825. [DOI] [PubMed] [Google Scholar]
- 128. Martchenko SE, Martchenko A, Cox BJ, et al. Circadian GLP‐1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes. 2020;69(12):2589‐2602. [DOI] [PubMed] [Google Scholar]
- 129. Dao MC, Everard A, Aron‐Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426‐436. [DOI] [PubMed] [Google Scholar]
- 130. Muñoz JSG, Rodríguez DJ, Morante JJH. Diurnal rhythms of plasma GLP‐1 levels in normal and overweight/obese subjects: lack of effect of weight loss. J Physiol Biochem. 2015;71(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 131. Gil‐Lozano M, Hunter PM, Behan LA, Gladanac B, Casper RF, Brubaker PL. Short‐term sleep deprivation with nocturnal light exposure alters time‐dependent glucagon‐like peptide‐1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab. 2016;310(1):E41‐E50. [DOI] [PubMed] [Google Scholar]
- 132. Ahrén B, Carr RD, Deacon CF. Incretin hormone secretion over the day. Vitam Horm. 2010;84:203‐220. [DOI] [PubMed] [Google Scholar]
- 133. Gil‐Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP‐1 by the rodent L cell. Diabetes. 2014;63(11):3674‐3685. [DOI] [PubMed] [Google Scholar]
- 134. Martchenko SE, Martchenko A, Biancolin AD, Waller A, Brubaker PL. L‐cell Arntl is required for rhythmic glucagon‐like peptide‐1 secretion and maintenance of intestinal homeostasis. Mol Metab. 2021;54:101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cani PD, Knauf C. A newly identified protein from Akkermansia muciniphila stimulates GLP‐1 secretion. Cell Metab. 2021;33(6):1073‐1075. [DOI] [PubMed] [Google Scholar]
- 136. Cheng CW, Villani V, Buono R, et al. Fasting‐mimicking diet promotes Ngn3‐driven β‐cell regeneration to reverse diabetes. Cell. 2017;168(5):775‐788.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu H, Javaheri A, Godar RJ, et al. Intermittent fasting preserves beta‐cell mass in obesity‐induced diabetes via the autophagy‐lysosome pathway. Autophagy. 2017;13(11):1952‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wei S, Han R, Zhao J, et al. Intermittent administration of a fasting‐mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr Metab (Lond). 2018;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang X, Zhao Y, Xu J, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high‐fat diet‐induced obesity in rats. Sci Rep. 2015;5:14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wei S, Zhao J, Wang S, Huang M, Wang Y, Chen Y. Intermittent administration of a leucine‐deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon. 2018;4(9):e00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet. 2014;383(9932):1899‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mindikoglu AL, Abdulsada MM, Jain A, et al. Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome. Sci Rep. 2020;10(1):18341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Witbracht M, Keim NL, Forester S, Widaman A, Laugero K. Female breakfast skippers display a disrupted cortisol rhythm and elevated blood pressure. Physiol Behav. 2015;140:215‐221. [DOI] [PubMed] [Google Scholar]
- 145. Deshmukh‐Taskar P, Nicklas TA, Radcliffe JD, O'Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and nutrition examination survey (NHANES): 1999–2006. Public Health Nutr. 2013;16(11):2073‐2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chiavaroli L, Viguiliouk E, Nishi S, et al. DASH dietary pattern and Cardiometabolic outcomes: an umbrella review of systematic reviews and meta‐analyses. Nutrients. 2019;11(2):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Maifeld A, Bartolomaeus H, Löber U, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12(1):1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124‐136. [DOI] [PubMed] [Google Scholar]
- 149. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703‐713. [PMC free article] [PubMed] [Google Scholar]
- 150. The Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 151. Ye P, Zhang X, Xu Y, Xu J, Song X, Yao K. Alterations of the gut microbiome and metabolome in patients with proliferative diabetic retinopathy. Front Microbiol. 2021;12:667632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Das T, Jayasudha R, Chakravarthy SK, et al. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021;11(1):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu W, Wang C, Xia Y, et al. Elevated plasma trimethylamine‐N‐oxide levels are associated with diabetic retinopathy. Acta Diabetol. 2021;58(2):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Huang Y, Wang Z, Ma H, et al. Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front Cell Infect Microbiol. 2021;11:646348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Verma A, Xu K, Du T, et al. Expression of human ACE2 in lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev. 2019;14:161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50(4):711‐719. [DOI] [PubMed] [Google Scholar]
- 157. Takeda S, Sato N, Uchio‐Yamada K, et al. Diabetes‐accelerated memory dysfunction via cerebrovascular inflammation and a deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107(15):7036‐7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jayaraman A, Pike CJ. Alzheimer's disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep. 2014;14(4):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262‐2272. [DOI] [PubMed] [Google Scholar]
- 160. Liu Y, Cheng A, Li YJ, et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun. 2019;10(1):1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Halagappa VKM, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age‐related behavioral deficits in the triple‐transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26(1):212‐220. [DOI] [PubMed] [Google Scholar]
- 162. Singh R, Manchanda S, Kaur T, et al. Middle age onset short‐term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology. 2015;16(6):775‐788. [DOI] [PubMed] [Google Scholar]
- 163. Soares NL, Dorand VAM, Cavalcante HC, et al. Does intermittent fasting associated with aerobic training influence parameters related to the gut‐brain axis of Wistar rats? J Affect Disord. 2021;293:176‐185. [DOI] [PubMed] [Google Scholar]
- 164. Di Giovanni S, Serger E, Chadwick J, et al. The Intermittent Fasting‐Dependent Gut Microbial Metabolite Indole‐3 Propionate Promotes Nerve Regeneration and Recovery after Injury. Research Square. Published online 2020.
- 165. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten‐hour time‐restricted eating reduces weight, blood pressure, and Atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92‐104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16):1561‐1569. [DOI] [PubMed] [Google Scholar]
- 168. Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64(11):1092‐1102. [DOI] [PubMed] [Google Scholar]
- 169. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26(4):1727‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pasini E, Aquilani R, Testa C, et al. Pathogenic gut Flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220‐227. [DOI] [PubMed] [Google Scholar]
- 172. Crawford PA, Crowley JR, Sambandam N, et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Natl Acad Sci U S A. 2009;106(27):11276‐11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mistry P, Reitz CJ, Khatua TN, et al. Circadian influence on the microbiome improves heart failure outcomes. J Mol Cell Cardiol. 2020;149:54‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet Hyperreactivity and thrombosis risk. Cell. 2016;165(1):111‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Washburn RL, Cox JE, Muhlestein JB, et al. Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N‐oxide changes during 24‐hour water‐only fasting in the FEELGOOD trial. Nutrients. 2019;11(2):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Miao J, Ling A v, Manthena P v, et al. Flavin‐containing monooxygenase 3 as a potential player in diabetes‐associated atherosclerosis. Nat Commun. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341(15):1127‐1133. [DOI] [PubMed] [Google Scholar]
- 179. Ritz E. Nephropathy in type 2 diabetes. J Intern Med. 1999;245(2):111‐126. [DOI] [PubMed] [Google Scholar]
- 180. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164‐176. [DOI] [PubMed] [Google Scholar]
- 181. Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41(6):389‐393. [DOI] [PubMed] [Google Scholar]
- 182. Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308‐315. [DOI] [PubMed] [Google Scholar]
- 183. Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr Diab Rep. 2017;17(3):16. [DOI] [PubMed] [Google Scholar]
- 184. Chen W, Zhang M, Guo Y, et al. The profile and function of gut microbiota in diabetic nephropathy. Diabetes Metab Syndr Obes. 2021;14:4283‐4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Li Y, Su X, Gao Y, et al. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165764. [DOI] [PubMed] [Google Scholar]
- 186. Kikuchi K, Saigusa D, Kanemitsu Y, et al. 01 gut microbiome‐derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10(1):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lu CC, Ma KL, Ruan XZ, Liu BC. Intestinal dysbiosis activates renal renin‐angiotensin system contributing to incipient diabetic nephropathy. Int J Med Sci. 2018;15(8):816‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chen Lu C, Bo Hu Z, Wang R, et al. Gut microbiota dysbiosis‐induced activation of the intrarenal renin‐angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. 2020;41(8):1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Malik S, Bhanji A, Abuleiss H, et al. Effects of fasting on patients with chronic kidney disease during Ramadan and practical guidance for healthcare professionals. Clin Kidney J. 2021;14(6):1524‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wang H, van Spyk E, Liu Q, et al. Time‐restricted feeding shifts the skin circadian clock and alters UVB‐induced DNA damage. Cell Rep. 2017;20(5):1061‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lundell LS, Parr EB, Devlin BL, et al. Time‐restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat Commun. 2020;11(1):4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Manella G, Sabath E, Aviram R, et al. The liver‐clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat Metab. 2021;3(6):829‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rojas‐Morales P, León‐Contreras JC, Granados‐Pineda J, et al. Protection against renal ischemia and reperfusion injury by short‐term time‐restricted feeding involves the mitochondrial unfolded protein response. Free Radic Biol Med. 2020;154:75‐83. [DOI] [PubMed] [Google Scholar]
- 194. Rodrigues C, Rodrigues M, Henriques M, Candida SP. Infections in patients with diabetes mellitus. J Clin Med. 2019;8(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Iatcu CO, Steen A, Covasa M. Gut microbiota and complications of Type‐2 diabetes. Nutrients. 2021;14(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742‐753. [DOI] [PubMed] [Google Scholar]
- 197. Soeters MR, Lammers NM, Dubbelhuis PF, et al. Intermittent fasting does not affect whole‐body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009;90(5):1244‐1251. [DOI] [PubMed] [Google Scholar]
- 198. Berry SE, Valdes AM, Drew DA, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26(6):964‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wang Z, Roberts AB, Buffa JA, et al. Non‐lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]


