Abstract
The periplasmic molecular chaperone Caf1M of Yersinia pestis is a typical representative of a subfamily of specific chaperones involved in assembly of surface adhesins with a very simple structure. One characteristic feature of this Caf1M-like subfamily is possession of an extended, variable sequence (termed FGL) between the F1 and subunit binding G1 β-strands. In contrast, FGS subfamily members, characterized by PapD, have a short F1-G1 loop and are involved in assembly of complex pili. To elucidate the structural and functional significance of the FGL sequence, a mutant Caf1M molecule (dCaf1M), in which the 27 amino acid residues between the F1 and G1 β-strands had been deleted, was constructed. Expression of the mutated caf1M in Escherichia coli resulted in accumulation of high levels of dCaf1M. The far-UV circular dichroism spectra of the mutant and wild-type proteins were indistinguishable and exhibited practically the same temperature and pH dependencies. Thus, the FGL sequence of Caf1M clearly does not contribute significantly to the stability of the protein conformation. Preferential cleavage of Caf1M by trypsin at Lys-119 confirmed surface exposure of this part of the FGL sequence in the isolated chaperone and periplasmic chaperone-subunit complex. There was no evidence of surface-localized Caf1 subunit in the presence of the Caf1A outer membrane protein and dCaf1M. In contrast to Caf1M, dCaf1M was not able to form a stable complex with Caf1 nor could it protect the subunit from proteolytic degradation in vivo. This demonstration that the FGL sequence is required for stable chaperone-subunit interaction, but not for folding of a stable chaperone, provides a sound basis for future detailed molecular analyses of the FGL subfamily of chaperones.
Over 30 different operons, encoding virulence-associated surface structures of gram-negative bacteria, have now been identified as members of a family using the chaperone-usher protein-assisted assembly pathway (21). In contrast to the apparent complexity of the general secretory (type II) (13) and contact-dependent (type III) pathways (18), the chaperone-usher pathway appears to be rather simple. In addition to the structural subunits, the latter operons encode only two proteins involved in export and assembly. One is a periplasmic chaperone which shows specificity for the structural subunit(s). The other is a large outer membrane protein which is required for translocation across the outer membrane and which may form a large gated channel. The prototype of this pathway has been the PapD chaperone-PapC usher-mediated assembly of Pap pili in Escherichia coli (21). The three-dimensional structure of PapD has been solved (7). It has two domains, each with a β-barrel and an immunoglobulin-like fold. The crystal structure of PapD complexed with the C-terminal 19 residues of PapG revealed that the carboxyl terminus of the peptide was anchored in the interdomain cleft with the peptide bound to the G1 β-strand of the chaperone via a parallel β-strand (or β-zipper interaction) (15). Studies indicate that this interaction may occur at the level of the inner membrane and may be required for correct folding prior to release of the subunit from the inner membrane (10). Less is known about the specificity of the subsequent interaction of chaperone-subunit complex with the outer membrane protein.
The caf operon, responsible for production and assembly of the capsule-like Caf1 surface antigen of Yersinia pestis, belongs to this family. It encodes a 26.5-kDa periplasmic chaperone (Caf1M) and a 90.4-kDa outer membrane protein (Caf1A), which together can mediate surface assembly of Caf1 antigen in recombinant E. coli (5, 11, 12). The steric structure of Caf1M has been constructed by computer modelling using the atomic coordinates of PapD (26). The most striking differences between these two proteins are possession by Caf1M of (i) two Cys residues close to the putative subunit binding pocket and (ii) an additional 18 residues between the F1 and G1 β-strands. Possession of these conserved Cys residues together with an extended variable sequence between the F1 and G1 β-strands was used to identify a Caf1M-like subfamily of seven chaperones (26). Moreover, it was noted that all members of the Caf1M-like subfamily are involved in assembly of simple structures (26). For example, Y. pestis F1 capsule and pH 6.0 antigen are each composed of a single subunit that polymerizes to form a granule-like capsule and flexible fibrillae, respectively (17). This is in contrast to assembly of Pap pili, which involves interaction of the PapD chaperone with six different subunits to form the structurally more complex rigid pili. Hung et al. (8) have named these two families the FGL family (possessing a long sequence between the F1 and G1 strands, e.g., Caf1M) and the FGS family (with a short F1-G1 loop, e.g., PapD). In the Caf1M model, it has been suggested that this additional sequence may form two short β-strands that extend into the binding site cleft of the chaperone (26). While the intensively studied Pap system represents an excellent basic model for chaperone-mediated pilus assembly, a detailed comparison with the more distant relatives of the FGL subfamily will provide a more comprehensive picture of this assembly pathway.
A previous study addressed the significance of the conserved Cys residues of the FGL family (23). Evidence was presented to show that the Cys residues of Caf1M form a disulfide bond, the formation of which is important to in vivo folding but not maintenance of the overall structure of native chaperone. The reduction and alkylation of Caf1M significantly increased the dissociation constant of the Caf1M-Caf1 complex [Kd = (4.77 ± 0.50) × 10−9 M for native Caf1M to (3.68 ± 0.68) × 10−8 M for the modified protein]. Thus, the oxidation state of Caf1M may affect the efficiency of chaperone-subunit interaction and capsule assembly in vivo and offers a potential point of control of capsule assembly. To further investigate the importance of the unique properties of the FGL family of chaperones, we have now studied the role of the FGL sequence of Caf1M in the structure and function of this chaperone.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used were E. coli DH5α [supE44 recA1 gyrA relA1 ΔlacU169 deoR (Φ80 lacZ ΔM15)] (2) and JCB570 [MC1000 phoR zih12::Tn10] (3). Cultures carrying the appropriate plasmid were routinely grown at 37°C in Luria-Bertani (LB) broth, which contained ampicillin (100 μg/ml) and tetracycline (10 μg/ml) as required. Cultures of E. coli cells carrying the plasmid were routinely maintained in the presence of 0.6% glucose. For induction, overnight cultures were diluted 1 in 50 and grown to the mid-exponential phase (optical density at 600 nm [OD600] of 0.4 to 0.5) prior to induction with 0.75 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h.
Plasmids.
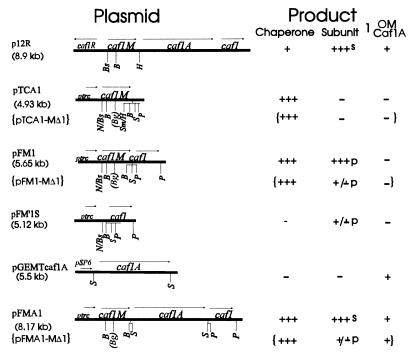
The plasmids p12R (11), pTCA1 (24), and pFM1 (24) have been described previously. pFM′1S, formerly called pFM1S (23), encodes a short nonfunctional fragment of Caf1M upstream of the complete caf1 gene. To reconstruct the whole system in the pTrc99A derivative, caf1A was amplified from pFS2 (6) by PCR. Primers 5′gtcgacGGGACGGGAAATAATGAGGTATTC and 5′gtcgacTCAGTTATTTAAGATGCAGGTTG corresponded to the beginning and end of caf1A, respectively (EMBL accession no. X61996). (Start and stop codons are in boldface, and SalI sites used in subsequent subcloning are in lowercase.) An additional A residue (underlined) was included between the Shine-Dalgarno sequence and start codon in the forward primer. PCR was performed by using Taq Plus polymerase (Stratagene), 0.5 μg of DNA, and 400 ng of each primer with 10 cycles of 93°C for 1 min, 55°C for 1 min, and 72°C for 2 min, with a 10-s extension time for each cycle. The 2.52-kb PCR fragment was gel purified and initially cloned in pGEM-T vector (Promega) to give pGEM-Tcaf1A. The caf1A gene was excised from pGEM-Tcaf1A by SalI digestion and ligated into the unique SalI site of pFM1 to give pFMA1. DNA sequencing of amplified caf1A in pGEM-Tcaf1A identified a single silent PCR-induced mistake (X61996; 2323 C→T). A summary diagram of the structure and products of all plasmids is shown below in Fig. 1.
FIG. 1.
Structure and products of recombinant caf plasmids. Details of plasmids are given in Materials and Methods. With the exception of p12R, which carries the original subcloned caf operon in pUC19, and pGEM-Tcaf1A, all plasmids are pTrc99A (Pharmacia) derivatives and carry lacIq. Only restriction sites used in manipulation of caf genes are shown. B, BamHI; Bg, BglII; Bs, BspHI; H, HpaI; N, NcoI; P, PstI; S, SalI; Sm, SmaI. Brackets ({}) indicate corresponding plasmids encoding the deletion mutant chaperone, dCaf1M, and the level of product from each respective plasmid. The unique BglII site (Bg in parentheses) created at the site of deletion within caf1M is also shown. The Product columns summarize the amount of each caf gene product detectable following induction in E. coli DH5α. Levels were estimated from Coomassie blue-stained SDS-PAGE of periplasmic fractions (chaperone and subunit), whole cells (chaperone and subunit), and outer membrane (OM) preparations (4) (Caf1A). For periplasmic chaperone and subunit, +++ corresponds to approximately 20 to 40 μg/ml/OD600 culture in the final osmotic shock fraction. l, location of subunit. Surface location, s, was identified by a positive reaction in the quantitative immunofluorescence assay, with only low levels present in the osmotic shock fraction; periplasmic location, p, was identified by a negative reaction (i.e., no higher than background, or <3% of the value of E. coli/pFMA1) in the quantitative immunofluorescence assay, and the subunit was recovered in the osmotic shock fraction from E. coli DH5α or E. coli JCB570.
Caf1MΔ105–133 (pFM1-MΔ1 and related plasmids).
DNA (81 bp) encoding amino acids Lys-105 to Ala-133 of Caf1M was removed from pFM1 by inverse PCR. Primers 5′TTCGagatctATTAATAATTGCATTAAG and 5′ATCCTTagatctTGGTGGAATCCCTTTTAC corresponded to nucleotides 1697 to 1720 and 1621 to 1598, respectively, of the caf operon (EMBL accession no. X61996). Taq Plus polymerase (Stratagene) was used for amplification with 100 ng of template DNA, 100 ng of each primer, and 1.5 mM MgCl2 in a 100-μl final volume and the following conditions: 95°C for 10 s; 30 cycles of 95°C for 1 min, 50°C for 2 min, and 72°C for 3 min; and a final 5-min extension at 72°C. The 5.2-kb PCR product was gel purified, digested with BglII (inserted BglII sites in primer sequences are underlined), and ligated at approximately 500 ng of DNA/50 μl of buffer prior to transformation of E. coli DH5α. Plasmids of the correct size were screened for possession of the unique BglII site, and the DNA sequence of the mutated caf1M gene and wild-type caf1 gene of one isolate (pFM1-MΔ1) was confirmed. pTCA1-MΔ1 (encoding dCaf1M alone) was constructed by PstI digestion and religation at a low DNA concentration to remove caf1. pFMA1-MΔ1 (encoding Caf1A and Caf1 in addition to dCaf1M) was created by insertion of the pGEM-Tcaf1A-derived 2.5-kb SalI fragment into the SalI site of pFM1-MΔ1.
Isolation of periplasmic fraction by osmotic shock.
Routinely, induced cells (1 OD600 unit) were suspended in 100 μl of 20% (wt/vol) sucrose in 20 mM Tris-HCl (pH 8.0)–5 mM EDTA and subjected to osmotic shock as previously described (23).
Purification of recombinant Caf1M and mutant dCaf1M.
Caf1M was isolated from the periplasmic fraction of induced E. coli DH5α/pTCA1 by MonoQ and Superose 12 H/R chromatography as previously described (24). dCaf1M was isolated from induced cultures of E. coli JCB570/pTCA1-MΔ1. The periplasmic fraction was adjusted to pH 5.65 with 0.1 N HCl and applied to a MonoS (5/5) column equilibrated with 10 mM ammonium acetate (pH 5.65). dCaf1M was eluted at 15 mM NaCl by using a linear gradient to 1 M NaCl in the same buffer. Fractions containing dCaf1M were pooled, diluted with 20 mM Tris-HCl (pH 8.5) to a total protein concentration of 0.1 mg/ml, and applied to a MonoQ column equilibrated with 20 mM Tris-HCl (pH 8.5). dCaf1M, recovered in the unbound fraction, was precipitated with ammonium sulfate (70% [wt/vol] saturation) and stored at 4°C. Before use, the recovered pellet containing dCaf1M was passed through a PD-10 column in the appropriate buffer. As judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and isoelectric focusing, purified Caf1M and dCaf1M proteins were 95 to 97% pure.
FPLC fractionation and ELISA quantitation of periplasmic Caf1M and Caf1.
Periplasmic samples (50 μl) were fractionated on a Superose 12 H/R 10/30 column in 20 mM Tris-HCl buffer (pH 7.2) containing 0.14 M NaCl (TS buffer) at 0.5 ml/min with a standard fast-performance liquid chromatography (FPLC) system (Pharmacia). Fractions of 0.25 ml were collected from 12.5 to 32 min and assayed for Caf1M and Caf1 by enzyme-linked immunosorbent assay (ELISA). ELISA was basically performed as described previously (23) with Flow microtiter plates coated with 10 μl of sample diluted to 100 μl in 0.02 M phosphate buffer (pH 7.2), blocking buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2% bovine serum albumin, 0.05% Tween 20), mouse monoclonal anti-Caf1M immunoglobulin G (IgG [2 μg/ml]) or rabbit monospecific anti-Caf1 IgG (10 μg/ml) and the appropriate peroxidase conjugate (Sigma).
Pulse-chase experiments and immunoprecipitation.
Exponentially growing cells (OD600 = 0.5 to 0.6) in 10 ml of LB broth with 0.6% glucose were recovered and resuspended in 10 ml of M9 medium containing 50 μg of each amino acid per ml (except methionine and cysteine) and 6% glycerol (2). After a 30-min incubation at 37°C, IPTG was added and cultures were incubated for a further 30 min at 37°C, at which time, cells were concentrated 10-fold in the same medium and incubated for 5 min at 26°C. Cultures (1 ml) were then labelled for 30 s at 26°C with [35S]methionine (50 μCi/ml, >1,000 Ci/mmol; Amersham). Following addition of l-methionine (200 μg/ml) and chloramphenicol (125 μg/ml), samples were taken at the indicated times and subjected to trichloroacetic acid precipitation followed by immunoprecipitation, basically as described previously (9), by using 20-μl of anti-Caf1 serum and 200 μl of Pansorbin cells (Calbiochem)/ml of solubilized antigen suspension. Immunoprecipitated Caf1 was subjected to SDS-PAGE and quantitated with a PhosphorImager S1 and ImageQuant software (Molecular Dynamics).
CD and fluorescence spectroscopy.
Circular dichroism (CD) spectra were recorded on a J-500A dichrograph (Jasco, Tokyo, Japan) with a 1-mm-diameter temperature-controlled cell. Concentration of Caf1M samples was measured on a Shimadzu UV-2100 spectrophotometer (Japan) with an extinction coefficient of E280 = 1.4 M−1 cm−1. Fluorescence spectra were recorded on an MPF-44A spectrofluorimeter (Perkin-Elmer, Norwalk, Conn.) with a 3-mm-diameter temperature-controlled cell.
Molecular modelling.
The three-dimensional models were constructed by using the molecular modelling software packages Chem-X (Chemical Design, Ltd., Oxford, United Kingdom) and MOLMOL (14) on a Hewlett-Packard Vectra workstation.
DNA manipulation and sequencing procedures.
DNA manipulations were performed according to standard procedures (2). DNA sequencing was performed by the AMS sequencing service, University of Reading, Reading, United Kingdom, by using an automatic ALFexpress DNA sequencer (Pharmacia) and cycle sequencing of Qiagen-purified plasmid DNA.
Other protein procedures.
Concentrations of purified proteins were estimated with the Bio-Rad protein assay kit with bovine serum albumin as a standard. N-terminal sequencing of tryptic fragments was performed by P. Barker (Microchemical Facility, IAPGR Cambridge Research Station, Cambridge, United Kingdom) as described previously (4). SDS-PAGE (16% polyacrylamide) was performed by the basic Laemmli procedure (2). Samples were heated at 100°C for 5 min in SDS sample buffer with 0.1 M dithiothreitol (DTT), unless otherwise indicated, prior to electrophoresis. Nondenaturing gels contained 15% acrylamide and Laemmli buffer with no SDS. Samples were incubated in sample buffer (without SDS) for 5 min at 37°C prior to electrophoresis at 200 V for 2.5 h. Caf1 was visualized on immunoblots with an ECL (enhanced chemiluminescence) kit (Amersham) by using a 1:20,000 dilution of both rabbit anti-Caf1 serum and peroxidase conjugate. To quantitate surface-assembled Caf1, cells from induced cultures were incubated sequentially with a 1:1,000 dilution of anti-Caf1 serum and a 1:500 dilution of antirabbit IgG-fluorescein conjugate (Sigma) and fluorescence quantitated with a BioLumin fluorescence plate reader (Molecular Dynamics).
RESULTS
Expression of individual caf genes in E. coli.
The basic plasmids required for this study, constructed as described in Materials and Methods, are shown in Fig. 1. The set of plasmids permitted analyses and isolation of high levels of periplasmic chaperone (pTCA1), periplasmic chaperone-subunit complex (pFM1), periplasmic subunit (pFM′1S), and surface-assembled subunit (pFMA1). Expression of periplasmic chaperone and complex was optimized in E. coli DH5α. Induction with 0.75 mM IPTG for 2 to 3 h was optimal, at which time, chaperone or complex represented 60 to 80% of total protein in osmotic shock fractions (periplasm). Higher concentrations of IPTG and longer induction times led to precursor accumulation and cell death. Surprisingly, expression of chaperone alone (pTCA1) was as toxic to E. coli DH5α as the subunit (pFM′1S). In the absence of chaperone (pFM′1S), the subunit was largely degraded in E. coli JCB570, but in E. coli DH5α, Caf1 appeared to be slightly more stable and could be recovered in the periplasmic fraction (Fig. 1 [and see Fig. 6B and C below]). Whether this was due to differences in proteases present in the two strains or to limited cross-talk with a related specific periplasmic chaperone of DH5α is not known. Caf1 subunit was only localized to the cell surface in constructs (pFMA1 or p12R) expressing caf1A.
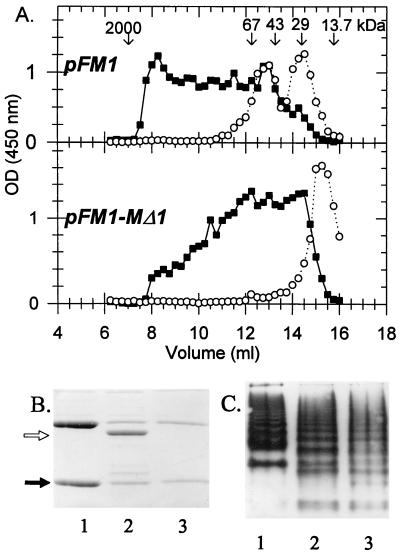
FIG. 6.
Identification of chaperone-subunit complex and polymerization of the Caf1 subunit. (A) Periplasmic fractions from induced cultures of E. coli DH5α/pFM1 or E. coli DH5α/pFM1-MΔ1 fractionated by FPLC on a Superose 12 H/R column. The column was calibrated with RNase A (13.7 kDa), β-lactamase (29 kDa), ovalbumin (43 kDa), bovine serum albumin (66 kDa), and blue Dextran 2000 (2,000 kDa) (Pharmacia). Caf1 (■) and Caf1M (○) were quantitated by ELISA. (B) SDS-PAGE of periplasmic fractions from E. coli DH5α carrying pFM1 (lane 1), pFM1-MΔ1 (lane 2), or pFM′1S (lane 3). Open arrow, dCaf1M; solid arrow, subunit (confirmed by immunoblotting with anti-Caf1 serum). The gel depicted in panel C is the same as for panel B, except that samples were separated on a nondenaturing PAGE gel and visualized by immunoblotting with anti-Caf1 serum. Under these conditions, Caf1M was only detected at the top of the separation gel by immunoblotting with anti-Caf1M serum.
Accessibility of the FGL sequence of Caf1M to trypsin.
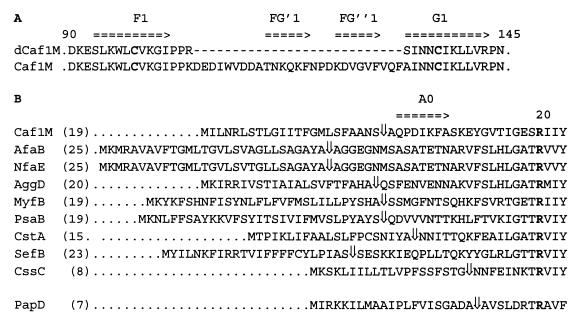
The FGL sequence of Caf1M, as identified in previous alignments (8, 26), is shown in Fig. 2A. Interestingly, following prediction of putative signal sequence cleavage sites, it was noted that members of the FGL family of chaperones also possess a longer sequence N terminal to the conserved Arg residue (Arg-20 in Caf1M) (Fig. 2B). The average length of this sequence was 19 residues in Caf1M-like proteins, compared to N-terminal sequences of only 7 to 9 residues for mature PapD-like chaperones. The only exception was the most distant relative of the FGL subfamily, CssC.
FIG. 2.
FGL sequence of Caf1M and N-terminal sequences of the FGL subfamily. (A) FGL sequence of Caf1M, between the end of the F1 β-strand and the beginning of the G1 β-strand. Arrows indicate proposed β-strands (see Fig. 7 below for further details). Conserved cysteine residues involved in disulfide bond formation are in boldface. The sequence of the corresponding region of the deletion mutant, dCaf1M, is shown for comparison. (B) Alignment of N-terminal sequences of the FGL subfamily. PapD is shown as an example of the FGS subfamily (more than 20 thus far sequenced). ⇓, signal sequence (SS) cleavage site predicted by the SignalP program (19). For Caf1M, the SS cleavage site was confirmed by N-terminal sequencing; numbering of amino acid residues has been accordingly altered from the earlier system (5). For PsaB, the SS cleavage site was predicted by using the ATG start codon at position 2664 rather than 2740 to give a recognizable SS. EMBL accession numbers are as follows: Caf1M, X61996; PsaB, M86713; MyfB, Z21953; AfaB, X76688; NfaE, S61968; AggD, U12984; CS3-1 (CstA), X16944; SefB, L11009; CssC, U04846; and PapD, X61239. Numbers in parentheses give the number of residues in the predicted mature protein N terminal to the absolutely conserved Arg (R in boldface).
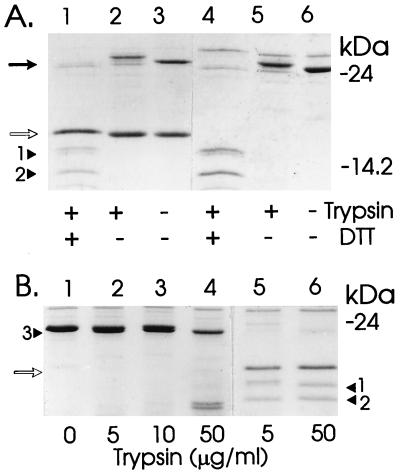
On trypsin treatment of periplasmic fractions containing Caf1M chaperone, Caf1M was cleaved within the FGL sequence (Fig. 2 and 3). Comparison of the SDS-PAGE profiles with and without reducing agent confirmed that a disulfide bond between Cys-98 and Cys-137 held the two tryptic fragments (approximately 12 and 14 kDa) together (Fig. 3A, lanes 4 to 6). N-terminal sequencing identified a single susceptible bond within this region—that between Lys-119 and Phe-120. At 5 μg of trypsin per ml, a low level of cleavage also occurred at the extreme N terminus. This was complete only at higher trypsin concentrations—50 μg/ml (compare Fig. 3B, lanes 5 and 6). The tryptic fragment pattern was the same for the chaperone-subunit complex (Fig. 3A, lanes 1 to 3). Although only approximately 50% of chaperone is complexed to the subunit in isolated periplasmic fractions (see Fig. 6A), all chaperone was cleaved by trypsin. Thus, at least the immediate region around Lys-119 and Phe-120 must remain accessible on interaction with the Caf1 subunit.
FIG. 3.
Sensitivity of Caf1M and dCaf1M to trypsin. Periplasmic fractions from E. coli DH5α expressing chaperone alone or subunit plus chaperone were treated with or without trypsin for 30 min at 37°C in 50 mM Tris (pH 7.5) and then heated at 100°C in sample buffer for 5 min prior to SDS-PAGE. Coomassie blue-stained gels are shown. (A) Caf1M plus Caf1 (lanes 1 to 3) and Caf1M chaperone alone (lanes 4 to 6) treated with (+) or without (−) trypsin (5 μg/ml) and prepared in SDS-PAGE sample buffer with (+) or without (−) DTT. (B) dCaf1M (lanes 1 to 4) and native Caf1M-Caf1 complex (lanes 5 and 6). The trypsin concentrations are indicated. (A and B) Solid arrow, Caf1M chaperone; open arrows, Caf1 subunit; arrowheads 1, 2, and 3, typical profile of tryptic fragments sequenced. The N-terminal sequences following digestion with 5 μg of trypsin per ml were as follows: purified Caf1M (profile similar to that with 50 μg of trypsin per ml [B, lane 6]), arrowhead 1, N-terminal fragment, FASKEY (12.78 kDa)/EYGVTI, 3:2 ratio; arrowhead 2, C-terminal fragment, FNPDKD (12.89 kDa), dCaf1M; arrowhead 3, N terminus, AQPDIK (23.48 kDa), FASKEY. (Sizes of tryptic products were calculated from the primary sequences, assuming there was no additional C-terminal cleavage.) The anomalous slow migration of the Caf1M N-terminal fragment must be attributed to unusual properties of the FGL sequence, because (i) the cleaved polypeptide migrated more slowly under nonreducing conditions and (ii) both fragments of dCaf1M migrated close to their expected sizes of 11.15 kDa (N terminal) and 11.41 kDa (C terminal).
Caf1MΔLys-105–Ala-133 (dCaf1M) folds into a stable conformation.
As an initial step in assessing the importance of the FGL region of Caf1M to the structure and function of this chaperone, caf1MΔ105–133 (encoded by pTCA1-MΔ1), was constructed. In this mutant, the entire sequence between proposed F1 and G1 strands was deleted, leaving only sufficient residues to form a β-turn (see Fig. 2 and Fig. 7B below). Lys-105 and Ala-133 of Caf1M were replaced by Arg and Ser, respectively. Expression of caf1MΔ105–133 in E. coli DH5α or JCB570 resulted in accumulation of high levels of periplasmic dCaf1M (molecular mass of 23.5 kDa [Fig. 3B]), indicating that the dCaf1M protein lacking the accessory sequence folded into a stable conformation. Interestingly, dCaf1M was much less toxic than Caf1M, for example, following 2 h of induction, cultures expressing dCaf1M typically had an OD650 of 1.2 compared to an OD650 of 0.6 for similarly induced cultures expressing caf1M.
FIG. 7.
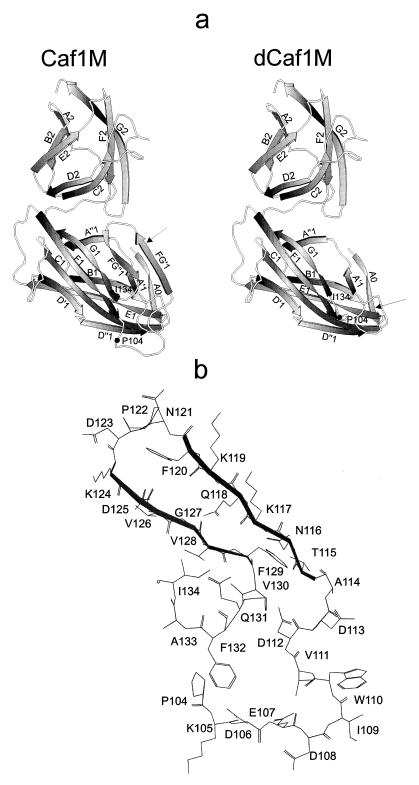
Predicted molecular models of three-dimensional structure of Caf1M and dCaf1M. (a) Models are based on PapD coordinates (Brookhaven Protein Data Base; identification no. 3DPA). β-Strands are shown by arrows. The predicted β-strands A0, FG′, and FG" of domain 1 of Caf1M are absent from PapD (see text for details); all other β-strands are present in PapD. In the models of Caf1M and dCaf1M, the positions of Pro-104 and I-134, connected via the additional Arg and Ser residues in dCaf1M, are indicated. Arrows indicate peptide bonds most sensitive to trypsin. (b) Predicted structure of Caf1M FGL sequence (between the F1 and G1 β-strands of domain 1) showing the side chains of all amino acid residues. Predicted FG′ and FG" β-strands are shown in boldface. (a and b) The numbering of residues of Caf1M has been modified in accordance with identification of the N terminus of mature Caf1M as AQPD (see Fig. 2).
N-terminal sequencing confirmed that dCaf1M retained the native, mature N terminus, Ala-Gln-Pro. The experimental isoelectric point of dCaf1M had increased from a pI of 8.7 to 9.65. This can be attributed to the large number of Asp residues present in the accessory segment of Caf1M and thus lost in dCaf1M. dCaf1M was relatively resistant to low concentrations of trypsin (5 μg/ml). Approximately 50% of dCaf1M was cleaved, but only at the extreme N terminus after Lys-5 (Fig. 3B). At higher levels of trypsin (50 μg/ml), dCaf1M was further cleaved and fragment sizes were consistent with cleavage at the newly created PPRS site that replaced the entire FGL sequence. SDS-PAGE in the absence of DTT confirmed that, as with Caf1M (Fig. 3A, lanes 4 and 5), the dCaf1M tryptic fragments were linked by a disulfide bond (not shown). Formation of the disulfide bond and the similarity in the tryptic profiles of Caf1M and dCaf1M provide supportive evidence that the mutant protein was folded in a conformation close to the native conformation.
Comparison of purified Caf1M and dCaf1M by CD and fluorescence spectroscopy.
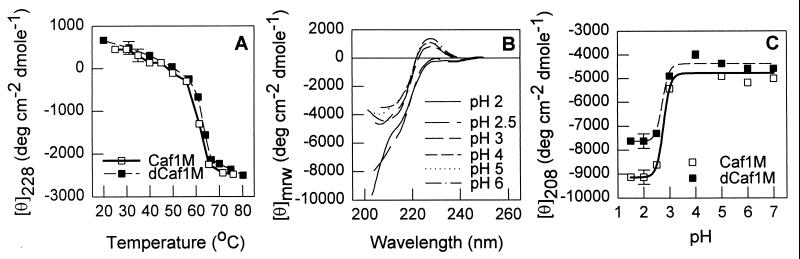
The secondary structures of native Caf1M and dCaf1M were compared by CD spectroscopy. No significant difference was observed between the previously published far-UV spectra or the near-UV spectra of Caf1M (23) and the corresponding spectra of dCaf1M. This demonstrates the absence of any major difference in the overall secondary structure content of Caf1M (estimated at 50% β-structure, 3% α-helix) and dCaf1M. The profile of the temperature dependence of the CD spectra of dCaf1M also looked very similar to that previously published for Caf1M (23). Figure 4A shows the temperature dependence of ellipticity at 228 nm for Caf1M and dCaf1M. For both samples, a highly cooperative transition was observed in the range 55 to 70°C with effectively the same transition temperature (Td) (Td = 63.5 ± 1°C for dCaf1M and 63.0 ± 1°C for Caf1M). The pH dependence properties of the CD spectra of dCaf1M and Caf1M were also very similar (shown only for Caf1M [Fig. 4B]). Figure 4C shows the pH dependence of ellipticity at 208 nm for Caf1M and dCaf1M. Despite the difference in pIs of the native and mutant proteins, the two proteins exhibited similar properties with respect to acid denaturation. There was no significant change in CD spectra down to a pH of 3.0. Further acidification induced an abrupt transition indicative of acid denaturation for both proteins. The similarity in the heat and acid denaturation curves of Caf1M and dCaf1M indicates an absence of any significant difference in the stabilities of the three-dimensional structures of the two proteins. The fluorescence spectra of Caf1M and dCaf1M were typical for fluorescence of tryptophan (20) (spectra not shown). There was a small, but statistically valid shift in the maximum of fluorescence from 336.5 ± 0.25 nm for Caf1M to 335 ± 0.25 nm for dCaf1M. Caf1M contains five tryptophan residues, one of which (Trp-110) is located in the FGL sequence that is absent in dCaf1M. This slight shift might reflect a more hydrophilic microenvironment of Trp-110 than the mean of the other Trp residues.
FIG. 4.
Comparison of conformational properties of Caf1M and dCaf1M by CD measurements. (A) Temperature dependency of ellipticity at 228 nm (wavelength at which the most pronounced differences at different temperatures are observed for Caf1M and dCaf1M). The protein concentration was 0.3 mg/ml in 20 mM Na-phosphate buffer (pH 7.2). (B) pH dependency of the CD spectrum of Caf1M. (C) pH dependency of ellipticity of Caf1M and dCaf1M at 208 nm. (B and C) Protein concentrations were 0.25 to 0.26 mg/ml in 20 mM Na-phosphate buffer (pH 7.2), in 0.1 M Na acetyl buffer (pH 6, 5, and 4), and 0.1 M glycine-HCl buffer (pH 3, 2.5, 2, and 1.5).
Deletion of Lys-105→Ala-133 abolishes in vivo function of the Caf1M chaperone.
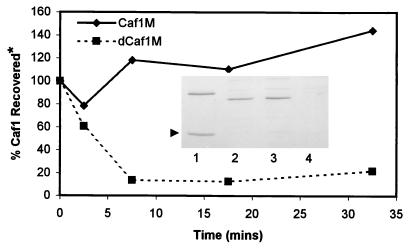
dCaf1M and the subunit were coexpressed from pFM1-MΔ1 in E. coli JCB570. In contrast to the Caf1M chaperone, dCaf1M was unable to protect the subunit from proteolytic degradation. Following 2 h of induction, levels of Caf1 subunit in periplasmic fractions of JCB570/pFM1-MΔ1 were as low as that seen from JCB570/pFM′1S (subunit alone) (Fig. 5). The rate of degradation of subunit was monitored in a pulse-chase experiment with [35S]Met-labelled JCB570 carrying pFM1 or pFM1-MΔ1 (Fig. 5). In the presence of Caf1M, there was an initial slight increase in Caf1 subunit recovery with time, presumably due to continued processing of pre-Caf1. In contrast in the presence of dCaf1M, Caf1 was rapidly degraded (half-life of <5 min). By 7.5 min, cultures of JCB570/pFM1-MΔ1 possessed only 10% of the expected subunit level, as extrapolated from levels of Caf1 in JCB570/pFM1. In the presence of dCaf1M, processing of precursor continued, but the mature Caf1 polypeptide was rapidly degraded shortly after or during translocation across the bacterial inner membrane. Using quantitative immunofluorescence, there was no evidence of surface-localized Caf1 subunit on either E. coli DH5α/pFM1-MΔ1 or E. coli JCB570/pFM1-MΔ1 (i.e., on strains expressing the outer membrane Caf1A usher as well as the subunit and mutant chaperone) (Fig. 1).
FIG. 5.
Kinetics of Caf1 subunit degradation in the presence of Caf1M or dCaf1M. E. coli JCB570 cells carrying pFM1 or pFM1-MΔ1 were induced and radiolabelled, and [35S]Met-labeled Caf1 was immunoprecipitated and quantitated as described in Materials and Methods. *, percent Caf1 recovered relative to the time zero value. In a preliminary experiment, the profile of Caf1 degradation in JCB570/pFM′1S (subunit alone) was similar to that seen in JCB570/pFM1-MΔ1. (Insert) Coomassie blue-stained SDS-PAGE gel of periplasmic fractions (10 μl) following a 2-h induction of pFM1 (lane 1 [Caf1M]), pFM1-MΔ1 (lanes 2 and 3 [dCaf1M]), and pFM′1S (lane 4 [no chaperone]) in E. coli JCB570. An arrowhead indicates the subunit.
With E. coli DH5α as the host bacterium, some periplasmic Caf1 subunit remained even in the absence of functional chaperone (Fig. 6B). Therefore, periplasmic fractions of E. coli DH5α/pFM1 and E. coli DH5α/pFM1-MΔ1 were analyzed by gel filtration chromatography to assess whether the mutant dCaf1M was capable of forming a stable complex with the Caf1 subunit (Fig. 6A). Following 2 h of induction, approximately half of Caf1M eluted as a complex with the subunit (estimated size, 47.3 kDa), while the other half eluted close to the monomer size of 26.6 kDa. dCaf1M eluted at approximately 23 kDa, and there was no indication of complex (expected molecular mass, 44 kDa). In addition, it was evident from the gel filtration experiment (Fig. 6A) that multimerization of Caf1 occurred in both samples, and in the presence of functional chaperone, multimerization appeared to be more efficient. This was confirmed by analyses of the samples by nondenaturing PAGE (Fig. 6C). Nondenaturing PAGE also revealed that while the periplasmic profile from DH5α/pFM1-MΔ1 (dCaf1M) was quite different from that of E. coli DH5α/pFM1, it was virtually identical to the periplasmic profile of DH5α/pFM′1S (no chaperone). These differences may have reflected differences in the conformation of the subunit following interaction with Caf1M chaperone or may simply have reflected differences in the total subunit concentration in the different strains. It is clear, however, that dCaf1M was unable to form a stable complex with the subunit and that the subunit remained in the same state in strains expressing dCaf1M as in strains with no chaperone.
Model of the three-dimensional structure of Caf1M.
The studies of trypsin digestion of Caf1M presented above indicated that the Lys-119–Phe-120 peptide bond was more efficiently cleaved than the bond between Lys-6 and Phe-7 and that the Lys-6–Phe-7 bond was accessible in dCaf1M. To test if this might be attributed to inaccessibility of Lys-6 in the presence of the FGL sequence, a Lys-117–Ala–Lys-119–Ala Caf1M mutant was similarly treated with trypsin. This mutant was more resistant to trypsin than the wild-type chaperone, with eventual partial cleavage (presumably at Lys-105) at 25 μg of trypsin/ml. Despite this there was no detectable N-terminal cleavage of the intact chaperone analogous to that observed with dCaf1M (Fig. 3B, lanes 2 to 4) (data not shown). In light of these experimental data, a modification of the previously suggested model of the three-dimensional structure of Caf1M (23, 25) is presented in Fig. 7a. In the new version of the Caf1M model, both predicted β-strands in the FGL sequence (FG′ and FG") form part of an FG′FG"GFCD β-sheet of domain 1. As a result, the more hydrophobic FG" β-strand is proximal to the functionally important G β-strand, while the hydrophilic FG′ β-strand is located relatively far from the subunit binding site. The predicted A0 β-strand in the extended N terminus forms part of an A0ABE β-sheet and interacts both with the A and the FG′ β-strands of the two different β-layers. Side chains of the amino acid residues forming the predicted structure of the FGL sequence are shown in Fig. 7b.
DISCUSSION
The Y. pestis chaperone Caf1M is a typical representative of the FGL subfamily of periplasmic chaperones characterized by possession of two cysteine residues and a variable, long sequence between the F1 and G1 β-strands (8, 26). Because these two features lie immediately adjacent to the subunit binding G1 β-strand, clarification of their role in chaperone structure and subunit binding, folding, and export is important. We have previously demonstrated that DsbA interaction is essential for folding of Caf1M (23). Recent studies with a Cys-98–Ser mutant have confirmed that this disulfide bond is essential for folding of the chaperone in vivo (27), although it is not essential for maintenance of the overall finally folded structure (23). In contrast, the data presented here with the deletion mutant Caf1M Δ105–133 (dCaf1M) demonstrate that the Caf1M FGL sequence does not significantly affect the folding of the cooperative three-dimensional structure of the protein.
Alignment of the amino acid sequences of the nine members of this family of Caf1M-like chaperones revealed that as well as the characteristic long, variable sequence between the F1 and G1 β-strands (8, 26), eight of these proteins have an N-terminal extension to the A1 β-strand compared to the classic PapD structure. The studies of trypsin accessibility of the Lys-6–Phe-7 peptide bond of Caf1M and two mutant derivatives indicate that the N-terminal extension is shielded from trypsin in the presence of the FGL sequence. Possession of this N-terminal extension may simply reflect the close evolutionary relationship of members of the FGL family of chaperones (22). In addition, it may also be important for stabilization of the FGL sequence and correct topology of the binding site cleft. The latter possibility and the trypsin data are consistent with the predicted arrangement of the Caf1M FGL sequence presented in Fig. 7. In this tentative model, the accessory sequence forms a loop followed by two short β-strands (FG′ and FG"), one of which (FG′) interacts with the A0 β-strand, formed by the extended N terminus. Notably, the conserved Arg-20, which by analogy with the Pap system is almost certainly involved in anchoring the carboxyl terminus of the Caf1 subunit, lies within the A β-strand. In the model, the extended N terminus (A0) also interacts with the A β-strand and could influence the positioning of Arg-20 within the cleft. Detailed information on the structural relationship of the FGL sequence to the rest of the protein will most likely be clarified on resolution of the crystal structure of Caf1M chaperone (currently in progress).
PapD-like chaperones, involved in assembly of complex rigid pili, must recognize and bind a number of different subunits. In contrast, Caf1M and most other members of the FGL subfamily interact with only a single species of subunit (8, 26). Thus, ordered binding of subunit by chaperone and interaction at the outer membrane (21) are not essential in F1 capsule assembly. Possession of the FGL sequence may confer a more specific interaction of chaperone with subunit. dCaf1M (lacks FGL sequence) was unable to function as a chaperone in vivo. There might be several explanations for this. Caf1M evidently binds the COOH terminus of the Caf1 subunit via a β-zipper interaction with the G1 strand (8, 15, 23). It has been proposed that the putative FG"1 β-strand of Caf1M may also directly interact with the Caf1 subunit in the complex, whereas the rest of the FGL sequence is exposed to solvent (23). The trypsin accessibility of Lys-119 in the complex is in agreement with this. Loss of such an interaction might decrease the affinity of the chaperone for the subunit to a level insufficient for in vivo binding and function. dCaf1M lacks 27 residues including a more conserved region immediately following the F1 β-strand (Lys-105–Asp-112). A smaller deletion mutant, Caf1MΔAsp-113–Phe-129, was also inactive in subunit chaperoning activity (3a). Thus, the presence of the FGL sequence (Asp-113–Phe-129) is essential for formation of functional chaperone. Whether this is due to stabilization of the complex via direct interaction of the FGL sequence with the subunit or is due to a requirement of the FGL sequence for correct structural topology of the subunit binding cleft should be further clarified by future detailed mutagenesis and resolution of the crystal structure. The demonstration that the FGL sequence is not required for formation of stable chaperone provides a sound basis for such analyses and determination of the role of this region in export as well as subunit binding.
A detailed understanding of the role of the Caf1M chaperone in the folding and assembly pathway of Caf1 antigen also has significant implications for the detection and prevention of Y. pestis-induced plague. Caf1 (F1) antigen is a major constituent of both recombinant subunit and whole-cell vaccines against Y. pestis (16). It is also the antigen of choice in diagnostic kits (1). Elucidation of any conformational differences between Caf1 multimer produced in the presence and absence of chaperone will aid in further development of these products.
ACKNOWLEDGMENTS
Mary Leonard is acknowledged for her excellent technical support. We thank Irina Zyrianova for the Caf1M Lys-117–Ala–Lys-119–Ala mutant, Alexander Denesyuk for helpful discussions on molecular modelling and Timo Korpela for use of modelling facilities at Turku, Finland. This work was supported by grants of the European Community (INCO-COPERNICUS), International Science and Technology Centre (United States and the European Community), The Royal Society (United Kingdom), International Science Foundation, National Aeronautic and Space Agency (United States), and the Russian Foundation on Basic Research.
REFERENCES
- 1.Agarwal G S, Batra H V. Passive hemagglutination tests for Y. pestis infection in Surat pneumonic patients. Curr Sci. 1996;71:792–793. [Google Scholar]
- 2.Ausbel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1994. [Google Scholar]
- 3.Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulphide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 3a.Chapman, D. A. G., T. V. Chernovskaya, and S. MacIntyre. Unpublished data.
- 4.Costello G M, Vipond R, MacIntyre S. Aeromonas salmonicida possesses two genes encoding homologs of the major outer membrane protein, OmpA. J Bacteriol. 1996;178:1623–1630. doi: 10.1128/jb.178.6.1623-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galyov E E, Karlishev A V, Chernovskaya T V, Dolgikh D A, Smirnov O Y, Volkovoy K I, Abramov V M, Zav’yalov V P. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of the Caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 1991;286:79–82. doi: 10.1016/0014-5793(91)80945-y. [DOI] [PubMed] [Google Scholar]
- 6.Galyov E E, Smirnov O Y, Karlishev A V, Volkovoy K I, Denesyuk A J, Nazimov I V, Rubtsov K S, Abramov V M, Dalvadyanz S M, Zav’yalov V P. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein putative T-cell and B-cell epitopes. FEBS Lett. 1990;277:230–232. doi: 10.1016/0014-5793(90)80852-a. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A, Branden C I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature (London) 1989;342:248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- 8.Hung D L, Knight S D, Woods R M, Pinkner J S, Hultgren S J. Molecular basis of 2 subfamilies of immunoglobulin-like chaperones. EMBO J. 1996;15:3792–3805. [PMC free article] [PubMed] [Google Scholar]
- 9.Ito K, Bassford P J, Beckwith J. Protein localisation in E. coli: is there a common step in the secretion of periplasmic and outer membrane proteins? Cell. 1981;24:707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- 10.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlyshev A V, Galyov E E, Smirnov O, Abramov V, Zav’yalov V P. Structure and regulation of a gene cluster involved in capsule formation of Yersinia pestis. In: Op den Kamp JAF, editor. Biological membranes: structure, biogenesis and dynamics. NATO ASI series. H 82. Berlin, Germany: Springer-Verlag; 1994. pp. 321–330. [Google Scholar]
- 12.Karlyshev A V, Galyov E E, Smirnov O Y, Guzayev A P, Abramov V M, Zav’yalov V P. A new gene of the F1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 1992;297:77–80. doi: 10.1016/0014-5793(92)80331-a. [DOI] [PubMed] [Google Scholar]
- 13.Karlyshev A V, MacIntyre S. Cloning and study of the genetic organization of the exe gene cluster of Aeromonas salmonicida. Gene. 1995;158:77–82. doi: 10.1016/0378-1119(95)00139-w. [DOI] [PubMed] [Google Scholar]
- 14.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structure. J Mol Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuehn M J, Ogg D J, Kihlberg J, Slonim L N, Flemmer K, Bergfors T, Hultgren S J. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993;262:1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- 16.Leary S E C, Griffin K F, Garmory H S, Williamson E D, Titball R W. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb Pathog. 1997;23:167–179. doi: 10.1006/mpat.1997.0141. [DOI] [PubMed] [Google Scholar]
- 17.Lindler L E, Tall B D. Yersinia pestis pH-6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol Microbiol. 1993;8:311–324. doi: 10.1111/j.1365-2958.1993.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 18.Lory S. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr Opin Microbiol. 1998;1:27–35. doi: 10.1016/s1369-5274(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of procaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Schmid F X. Spectral methods of characterising protein conformation and conformational changes. In: Creighton T E, editor. Protein structure. A practical approach. Oxford, United Kingdom: IRL Press; 1990. pp. 251–286. [Google Scholar]
- 21.Thanassi D G, Saulino E T, Hultgren S J. The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1998;1:223–231. doi: 10.1016/s1369-5274(98)80015-5. [DOI] [PubMed] [Google Scholar]
- 22.Van Rosmalen M, Saier M H. Structural and evolutionary relationships between 2 families of bacterial extracytoplasmic chaperone proteins which function cooperatively in fimbrial assembly. Res Microbiol. 1993;144:507–527. doi: 10.1016/0923-2508(93)90001-i. [DOI] [PubMed] [Google Scholar]
- 23.Zav’yalov V P, Chernovskaya T V, Chapman D A G, Karlyshev A V, MacIntyre S, Zavialov A V, Vasiliev A M, Denesyuk A I, Zav’yalova G A, Dudich I V, Korpela T, Abramov V M. Influence of the conserved disulphide bond, exposed to the putative binding pocket, on the structure and function of the immunoglobulin-like molecular chaperone Caf1M of Yersinia pestis. Biochem J. 1997;324:571–578. doi: 10.1042/bj3240571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zav’yalov V P, Chernovskaya T V, Navolotskaya E V, Karlyshev A V, MacIntyre S, Vasiliev A M, Abramov V M. Specific high affinity binding of human interleukin 1β by Caf1A usher protein of Yersinia pestis. FEBS Lett. 1995;371:65–68. doi: 10.1016/0014-5793(95)00878-d. [DOI] [PubMed] [Google Scholar]
- 25.Zav’yalov V P, Zavyalova G A, Denesyuk A I, Gaestel M, Korpela T. Structural and functional homology between periplasmic bacterial molecular chaperones and small heat-shock proteins. FEMS Immunol Med Microbiol. 1995;11:265–272. doi: 10.1111/j.1574-695X.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 26.Zav’yalov V P, Zav’yalova G A, Denesyuk A I, Korpela T. Modeling of steric structure of a periplasmic molecular chaperone Caf1M of Yersinia pestis, a prototype member of a subfamily with characteristic structural and functional features. FEMS Immunol Med Microbiol. 1995;11:19–24. doi: 10.1111/j.1574-695X.1995.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 27.Zyrianova, I. M., M. Leonard, and S. MacIntyre. Unpublished data.