Abstract
Aims
ST-segment elevation myocardial infarction (STEMI) is associated with an intense acute inflammatory response and an increased risk of death and heart failure (HF). In this study, we sought to evaluate the effect of anakinra, a recombinant interleukin-1 receptor antagonist, on the incidence of HF.
Methods and results
We performed a pooled analysis of three early phase randomized clinical trials. The endpoints included the composite of all-cause death and new-onset HF, and the composite of all-cause death and hospitalization for HF at 1-year follow-up. Safety events, including injection site reaction and serious infections, were also recorded. We analysed 139 patients with STEMI from three separate trials: VCUART (N = 10), VCUART2 (N = 30), and VCUART3 (N = 99). Of these, 84 (60%) patients were randomized to anakinra and 55 (40%) to placebo. Treatment with anakinra significantly reduced the incidence of all‐cause death or new-onset HF (7 [8.2%] vs. 16 [29.1%], log-rank P = 0.002) and of all-cause death or HF hospitalization (0 [0] vs. 5 [9.1%], log-rank P = 0.007). Patients treated with anakinra had significantly higher injection site reactions (19 [22.6%] vs. 3 [5.5%], P = 0.016) without a significant difference in the incidence of serious infections (11 [13.1%] vs. 7 [12.7%], P = 0.435). Treatment with anakinra significantly reduced the area under the curve for high-sensitivity C-reactive protein between baseline and 14 days (75.48 [41.7–147.47] vs. 222.82 [117.22–399.28] mg day/L, P < 0.001).
Conclusion
IL-1 blockade with anakinra for 14 days in patients with STEMI reduces the incidence of new-onset HF or hospitalization for HF at 1 year following STEMI.
Keywords: Anakinra, Interleukin-1, STEMI, Heart failure, Inflammation
Introduction
ST-segment elevation myocardial infarction (STEMI) remains a significant source of morbidity and mortality despite many advances in treatments and prevention strategies. Prompt reperfusion with primary percutaneous coronary intervention (PCI) has dramatically decreased mortality following acute myocardial infarction (MI).1–5 As survivorship has increased, however, so too has the number of patients who are at risk for developing post-MI heart failure (HF), including 10% who are hospitalized with HF within 2 years of the event.1–7 This suggests that despite the advances in current treatment paradigms (anti-platelet/anti-coagulation agents, cholesterol-lowering agents, neurohumoral blockade), additional pathophysiological mechanisms are adversely contributing to patient outcomes and there is a growing unmet clinical need for pharmacotherapy strategies aimed at preventing HF in the post-MI population.
It is well known that following STEMI, an intense inflammatory response occurs, the degree of which predicts adverse cardiac remodelling and overall risk of HF or premature death.8–10 While attempts with broad anti-inflammatory agents have failed to improve outcomes in this population, more targeted approaches have shown promise.11–18 Interleukin-1 (IL-1) has been identified as key pro-inflammatory mediator and a potential therapeutic target.12–18 The Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial showed prevention of recurrent atherothrombotic events12 as well as prevention of hospitalizations for HF13 in patients with prior MI treated with canakinumab, an IL‐1β blocking antibody, at three dose levels given every 3 months for 3 months. Two pilot clinical trials14,15 and a more recent phase 2 study17 were completed as part of the Virginia Commonwealth University Anakinra Response Trial (VCUART) programme, each of which has been individually published previously and showed encouraging findings regarding the acute inflammatory response and the risk of HF after STEMI. The purpose of this study was to perform a patient-level pooled analysis of the patients enrolled in all three studies as part of the VCUART programme to further explore the therapeutic potential suggested in these trials.
Methods
Trials’ designs
The designs of the individual studies are registered in www.clinicaltrials.gov (NCT00789724, NCT00175018, and NCT01950299) and published separately.14,15,17 Briefly, all three studies were purposefully designed with overlapping inclusion and exclusion criteria of patients with STEMI, defined as chest pain (or equivalent) with an onset within 12 h and ST-segment elevation (>1 mm) in two or more anatomically contiguous leads on electrocardiogram that is new or presumably new, adult age, presenting within 12 h of pain onset, and enrolled within 12 h of reperfusion. The exclusion criteria shared among the three studies included cardiac arrest, unsuccessful PCI, haemodynamic instability, pre-existing severe congestive heart and/or severe left ventricular dysfunction [left ventricular ejection fraction (LVEF) <20%], severe aortic or mitral valve disease, pregnancy, chronic infections, autoinflammatory or autoimmune disease, or cancer. As the VCUART and VCUART2 studies incorporated cardiovascular magnetic resonance studies, individuals with contraindications to magnetic resonance imaging were excluded from those studies. VCUART3 was unable to include magnetic resonance imaging.
Patients were enrolled at the Virginia Commonwealth University (Richmond, VA) for VCUART (from November 2008 to February 2009) and VCUART2 (September 2010 to May 2012) and at three clinical sites for VCUART3 (July 2014 to December 2017), including Virginia Commonwealth University, Virginia Cardiovascular Specialists (Richmond, VA), and Medstar Washington Hospital Center (Washington, DC).
Investigational treatment
Patients in VCUART and VCUART2 were randomly assigned in a 1:1 ratio to anakinra 100 mg/day (Kineret; Swedish Orphan Biovitrum, Stockholm, Sweden) in 0.67 mL or matching NaCl (0.9%) placebo injected subcutaneously for 14 days. Patients in VCUART3 were randomized 1:1:1 to anakinra 100 mg twice daily, anakinra 100 mg once daily, placebo once daily, or placebo twice daily. The two anakinra arms of VCUART3 were pooled together for this analysis, as they individually reduced high-sensitivity C-reactive protein (hsCRP) vs. placebo without significant differences between the two anakinra arms.17
Event adjudication and follow-up
Patients had in-person clinical follow-up at weeks 2 and 12 in all three studies, and at 6 and 12 months for VCUART3.14–18 For VCUART and VCUART2, the clinical events during the conduct of the study were adjudicated by the study team based on the in-person assessment prior to unblinding treatment allocation,14,15,17 while the events occurring after the last visit were reviewed and adjudicated by two independent cardiologists who were unaware of treatment allocation or CRP kinetics in the study and based on the documentation collected via chart review.16 For VCUART3, the clinical event adjudication was performed by an independent committee, blinded to treatment allocation and not involved in the conduct of the study, using pre-specified criteria and prior to the treatment allocation and CRP unblinding.17 Events for all three studies were censored at 12 months for this analysis.
HF outcomes
The clinical outcomes for this patient-level pooled analysis were the composite of all-cause death and new-onset HF, and of all-cause death and hospitalization for HF, using criteria established by a consensus document on the definition of HF after MI19:
-
incidence of HF (not hospitalized) defined as new or worsening dyspnoea and meeting one of the two of the following criteria:
○ physical signs of HF—including two or more of the following: oedema, crackles/rales, jugular vein distention, hepatojugular reflex, tachypnoea, rapid weight gain, S3 gallop, abdominal distension/ascites, radiologic evidence of worsening oedema, pulmonary artery occlusive pressure (wedge) >18 mmHg, or cardiac output <2.2 l/min/m2;
○ need for additional/increased HF therapy—including one of the following:
– initiation or significant increase of oral diuretics, requirement of intravenous diuretics,
– inotropes or vasodilators, need for ultrafiltration due to decompensated HF;
hospitalization for HF defined as a hospitalization with HF being the primary diagnosis and meeting all the diagnostic requirements for HF listed previously for outpatient HF.
Safety events, namely injection site reactions (defined as local reaction at or near the injection site) and serious infections (defined as sepsis or some other serious infection requiring antibiotic therapy) were also pooled and analysed.
Inflammatory response after STEMI
hsCRP was measured at baseline, 72 h, and 14 days in all three studies. The area under the curve of hsCRP (hsCRP-AUC) after 14 days was estimated using the linear trapezoidal method for each subject and compared between the anakinra group (all arms combined) and placebo group.
Statistical analysis
Continuous variables are reported as median and interquartile range (IQR), and were compared between groups using a Mann–Whitney U test. Categorical data are reported as number and percentage and were compared using the χ2 test or Fisher's exact test as appropriate. Kaplan–Meier curves for event-free survival were constructed for the time-dependent composite endpoints and compared using the log-rank (Mantel–Cox) test. The analyses were completed using SPSS, version 24.0 (SPSS; Chicago, IL).
Results
Baseline characteristics
A total of 139 patients with qualifying STEMI were enrolled across the three trials. Of these, 110 (79%) were males, 87 (63%) were White, and 52 (37%) were Black, with an age of 56 [49.0–63.0] years. In total, 84 (60%) patients were randomized to anakinra and 55 (40%) to placebo. Clinical, laboratory, angiographic, and echocardiographic characteristics were well balanced, without statistically significant differences between the two groups (Table 1). The median duration of patient follow-up was 365 [240–365] days.
Table 1.
Demographic characteristics
| Placebo (n = 55) | Anakinra (n = 84) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (median, IQR) | 57 [51–65] | 55 [48–61] | 0.801 |
| Male (%) | 48 (87.3) | 62 (73.8) | 0.056 |
| Female (%) | 7 (12.7) | 22 (26.2) | 0.056 |
| White (%) | 35 (63.6) | 52 (61.9) | 0.837 |
| Black (%) | 20 (36.4) | 32 (38.1) | 0.837 |
| BMI (median, IQR) | 29 [27.0–34.8] | 31 [25.1–34.3] | 0.390 |
| Diabetes mellitus (%) | 19 (34.5) | 20 (23.8) | 0.168 |
| Hypertension (%) | 37 (67.3) | 45 (53.6) | 0.108 |
| Tobacco use (%) | 29 (52.7) | 51 (60.7) | 0.352 |
| Dyslipidaemia (%) | 25 (45.5) | 46 (54.8) | 0.283 |
| Previous history of CABG (%) | 2 (3.6) | 4 (4.8) | 1 |
| Peripheral vascular disease (%) | 3 (5.5) | 7 (8.3) | 0.740 |
| Chronic obstructive pulmonary disease (%) | 5 (9.1) | 3 (3.6) | 0.264 |
| Medication at admission | |||
| Beta blocker (%) | 9 (16.4) | 21 (25) | 0.232 |
| Aspirin (%) | 14 (25.5) | 25 (29.8) | 0.595 |
| ACEi/ARB (%) | 14 (25.5) | 21 (25) | 0.935 |
| Statin (%) | 16 (29.1) | 27 (32.1) | 0.721 |
| Aldactone (%) | 0 (0.0) | 2 (2.4) | 0.519 |
| Metformin (%) | 11 (20) | 8 (9.5) | 0.076 |
| Plavix (%) | 4 (7.3) | 3 (3.6) | 0.436 |
| Ticagrelor (%) | 0 (0) | 0 (0) | — |
| Prasugrel (%) | 0 (0) | 0 (0) | — |
| Insulin (%) | 7 (12.7) | 6 (7.2) | 0.279 |
| NSAIDs (%) | 5 (9.1) | 7 (8.3) | 0.893 |
| Clinical presentation | |||
| Thrombolysis (%) | 7 (12.7) | 7 (8.3) | 0.400 |
| Killip class (%) | 0.176 | ||
| I | 43 (78.5) | 75 (89.3) | — |
| II | 3 (5.5) | 1 (1.2) | — |
| III | 5 (9.1) | 5 (6) | — |
| IV | 4 (7.3) | 3 (3.6) | — |
| Symptoms to balloon time (min) | 180 [111.5–360] | 172.5 [106.25–333.5] | 0.856 |
| Angiographic data | |||
| Culprit vessel (%) | 0.569 | ||
| LAD | 17 (30.9) | 30 (35.7) | — |
| Cx | 10 (18.2) | 16 (19.0) | — |
| RCA | 28 (50.9) | 36 (42.9) | — |
| SVG | 0 (0.0) | 2 (2.4) | — |
| TIMI flow 0/1 pre-PCI (%) | 43 (78) | 74 (88) | 0.117 |
| TIMI flow 3 post-PCI (%) | 53 (96) | 80 (95) | 1 |
| Therapy (%) | 0.059 | ||
| PTCA | 52 (94.5) | 84 (100.0) | — |
| Balloon | 0 (0.0) | 8 (9.5) | — |
| DES | 39 (70.9) | 54 (64.3) | — |
| BMS | 16 (29.1) | 21 (25) | — |
| Manual aspiration thrombectomy (%) | 10 (18.2) | 11 (13.1) | 0.584 |
| Laboratory data at baseline (median IQR) | |||
| WBC (×103/L) | 11.20 [8.25–14.52] | 11.05 [8.60–13.27] | 0.918 |
| Creatinine (mg/L) | 0.98 [0.83–1.09] | 0.96 [0.75–1.41] | 0.902 |
| hsCRP (mg/L) | 4.95 [2.22, 8.54] | 4.70 [3.00, 8.96] | 0.500 |
| Peak CK-MB (ng/mL) | 101.70 [49.37–231.52] | 134.10 [47.60–239.30] | 0.595 |
| Echocardiographic data | |||
| LV ejection fraction, % (median, IQR) | 53.7 [41.1–64.4] | 51.5 [40.1–59.3] | 0.871 |
| Medication at discharge | |||
| Beta blockers (%) | 47 (85) | 74 (88) | 0.650 |
| Aspirin (%) | 55 (100) | 84 (100) | 1 |
| ACEi/ARB (%) | 47 (85) | 69 (82) | 0.607 |
| Statin (%) | 54 (98) | 83 (99) | 1 |
| Spironolactone (%) | 3 (5) | 5 (6) | 1 |
| Sacubitril–valsartan (%) | 0 (0) | 0 (0) | — |
| Sodium–glucose cotransporter-2 inhibitors (%) | 0 (0) | 0 (0) | — |
| Plavix (%) | 27 (49) | 29 (34) | 0.087 |
| Prasugrel (%) | 12 (22) | 22 (26) | 0.557 |
| Ticagrelor (%) | 16 (29) | 33 (39) | 0.217 |
ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; BMS, bare metal stent; CABG, coronary artery bypass graft; CK-MB, creatine kinase-myocardial band; DES, Drug eluted stent; hsCRP, high-sensitivity C-reactive-protein; IQR, interquartile range; LAD, left anterior discending artery; LV, left ventricle; NSAID, non steroidal anti-inflammatory drug; PTCA, Percutaneous transluminal coronary angioplasty; RCA, right coronary artery; SVG, saphenous venous graft; SD, standard deviation; TIMI, thrombolysis in myocardial infarction; WBC: white blood cell count.
Anakinra effect on HF outcomes
Compared with placebo, treatment with anakinra was associated with a significant reduction in the combined endpoint of new-onset HF or death (7 [8.2%] vs. 16 [29.1%], P = 0.002) and the composite of HF hospitalization or death (0 [0] vs. 5 [9.1%], P = 0.007) (Figure 1 and Table 2). Only two deaths occurred during the year follow-up, both in the placebo group. Additional parameters were used to define HF events such as brain natriuretic peptide serum levels and invasively measured left ventricular (LV) end-diastolic pressures: a breakdown of the HF event criteria and effect of anakinra on HF endpoints according to the definition used is shown in Table 2 and in the Supplementary material online, Table S1.
Figure 1.
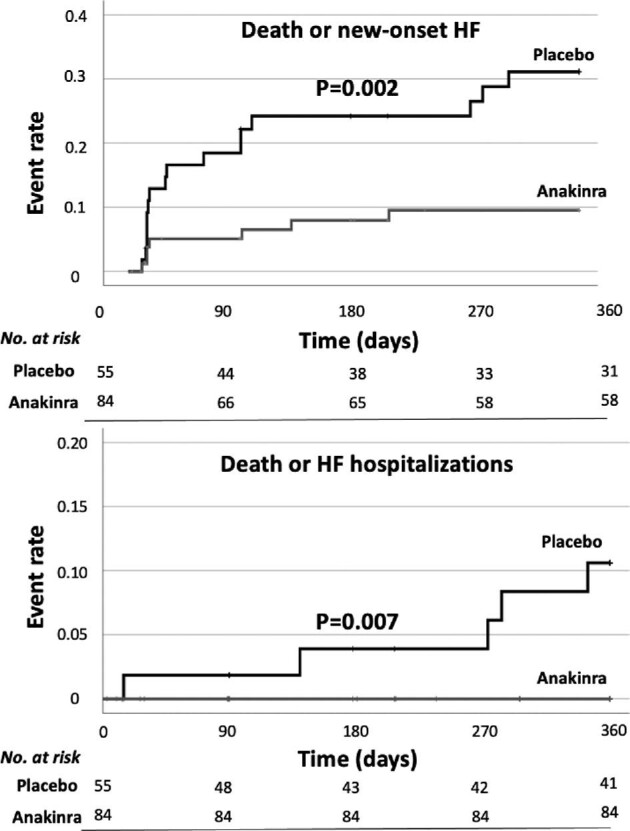
Kaplan–Meier curves for the incidence of the composite of all-cause death and new-onset heart failure and for the composite of all-cause death and heart failure hospitalizations, with a comparison between groups by log-rank test.
Table 2.
Effect of anakinra on heart failure outcomes using different definitions
| Placebo (n = 55) | Anakinra (n = 84) | P-value | |
|---|---|---|---|
| New-onset HF or death (%) | 16 (29%) | 7 (8%) | 0.002 |
| Limited to cases with BNP > 100 pg/ml | 15 (27%) | 4 (5%) | <0.001 |
| Limited to cases with LVEDP > 18 mmHg | 7 (13%) | 1 (1%) | 0.006 |
| With LVEF ≤ 40% (HFrEF) | 8 (15%) | 3 (4%) | 0.026 |
| With LVEF > 40% (HFmrEF/HFpEF) | 8 (15%) | 4 (5%) | 0.063 |
| HF hospitalizations or death (%) | 5 (9%) | 0 (0) | 0.007 |
| Death (%) | 2 (4%) | 0 (0) | 0.155 |
BNP, brain natriuretic peptide, HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heat failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction.
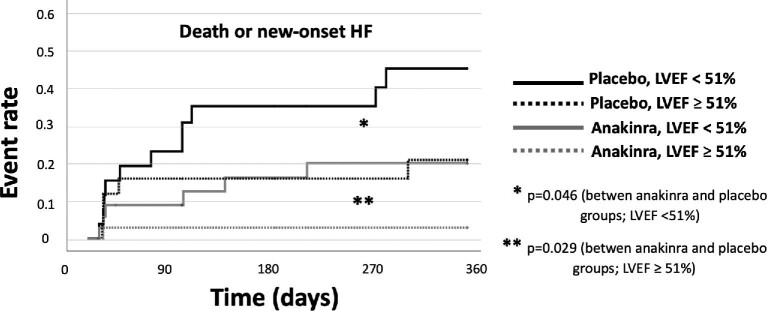
LVEF during the acute phase was available in 126 (91%) patients: those who experienced new-onset HF or death had a lower LVEF during the acute phase (40 [32–52%] vs. 52 [44–58%], P = 0.001) compared with those who did not. As a sensitivity analysis, we evaluated the effects of anakinra on new-onset HF or death in patients stratified by LVEF: in patients with LVEF < 51% (median), new-onset HF or death occurred in 11 (42%) patients in the placebo group and in 6 (16%) in the anakinra group (P = 0.046), whereas in those with LVEF ≥ 51%, the incidence of HF or death was 5 (19%) in the placebo group as compared with 1 (3%) in the anakinra group (P = 0.029) (Figure 2).
Figure 2.
Kaplan–Meier curves for the incidence of the composite of new-onset heart failure or death according to treatment (placebo or anakinra) stratified by left ventricular ejection fraction (above or below the median), with a comparison between groups by log-rank test.
All patients with HF events had LVEF assessment at follow-up. In the placebo group, 8 (50%) patients with HF had LVEF < 40% [reduced LVEF, HF with reduced ejection fraction (HFrEF)], 4 (25%) had LVEF of 40–50% [mildly reduced LVEF, HF with mildly reduced ejection fraction (HFmrEF)], and 4 (25%) had LVEF > 50% (preserved EF, HF with preserved ejection fraction (HFpEF)] (Supplementary material online, Table S1). In the anakinra group, of the patients with HF, 3 (43%) had HFrEF, 1 (14%) had HFmrEF, and 3 (43%) HFpEF (Supplementary material online, Table S1).
Safety data
Patients receiving anakinra had a significantly higher rate of injection site reactions (19 [22.6%] vs. 3 [5.5%], P = 0.016), which nonetheless did not result in a higher rate of drug discontinuation due to these reactions (6 [7.1%] vs. 1 [1.8%] for anakinra and placebo, respectively, P = 0.160). Serious infections occurred in 11 [13.1%] patients in the anakinra group and 7 [12.7%] in placebo (P = 1.0) (Table 3).
Table 3.
Safety events
| Placebo (n = 55) | Anakinra (n = 84) | P-value | |
|---|---|---|---|
| Serious infections (%) | 7 (13) | 11 (13) | 1.0 |
| Injection site reaction (%) | 3 (5) | 19 (23) | 0.016 |
| Injection site reaction leading to discontinuation (%) | 1 (2) | 6 (7) | 0.160 |
| Death (%) | 2 (4) | 0 (0) | 0.155 |
Inflammatory response after STEMI
The hsCRP-AUC values during the first 14 days were available in 48 (87%) and 67 (80%) of the placebo and anakinra combined groups, respectively, without significant differences between missing data in the two groups (P = 0.252). No missing data imputation was used. Hs-CRP levels were well matched at baseline. Treatment with anakinra significantly reduced the AUC for hsCRP between baseline and 14 days (75.48 [41.7–147.47] vs. 222.82 [117.22–399.28] mg day/L, P < 0.001) (Figure 3).
Figure 3.
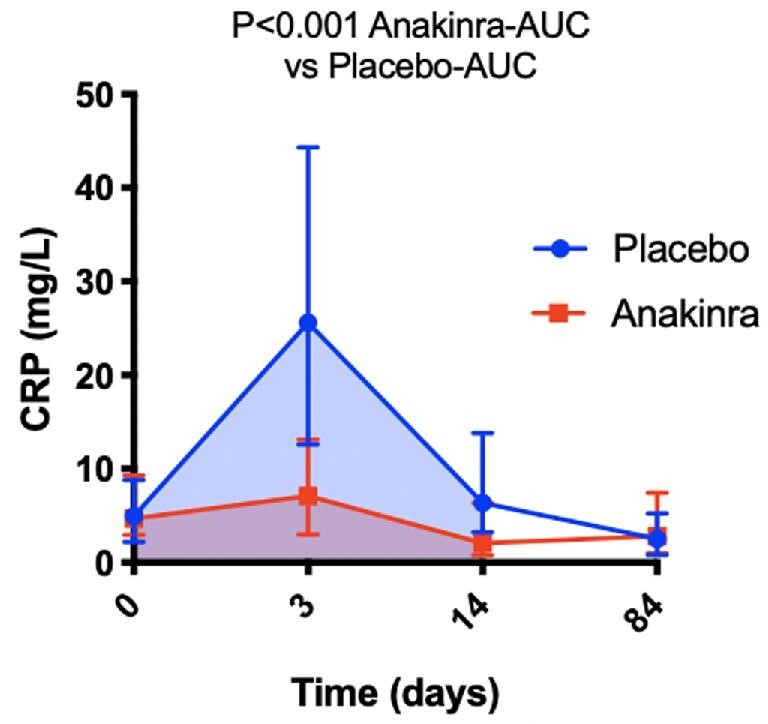
Effects of anakinra on high-sensitivity C-reactive protein. Anakinra (combined arms, n = 67) significantly reduced the area under the curve for high-sensitivity C-reactive protein at 14 days (shaded areas) compared to placebo (n = 48). Data are presented as median and interquartile range.
Discussion
The results of this pooled analysis of three clinical trials showed that treatment with anakinra, an IL-1 receptor antagonist, for 14 days in patients with STEMI is associated with a lower incidence of HF at follow-up. Anakinra is associated with a small increase in injection site reactions that infrequently lead to drug discontinuation. There was no evidence for excess risk of serious infections with anakinra.
Our results also confirm that, despite the low mortality, patients with STEMI remain at significant risk of HF, with approximately 1 in 4 patients experiencing a new HF event at 1 year, and 1 in 11 being hospitalized for HF. Treatment with anakinra for 14 days significantly reduced the incidence of HF events at follow-up, thus supporting the notion that IL-1 blockade may interfere with one of the pathophysiological mechanisms leading to HF after STEMI. Of note, lower LVEF during the acute phase was associated with a higher risk of HF-related events in the follow-up, and anakinra showed a protective effect in patients independent of whether they had reduced or preserved LVEF.
Recently, the CANTOS trial showed that targeting inflammation can confer cardiovascular benefit in high-risk patients with established atherosclerotic disease who had already survived an MI and had residual inflammatory risk.12 However, despite the increasing interest in the role of inflammation in MI, few clinical trials have tested anti‐inflammatory drugs in patients with acute MI, and no drugs are currently approved for use in the acute setting.14,15,17,20,21
In the individual trials within the VCUART programme, anakinra blunted the acute systemic inflammatory response without significant effects on infarct size or cardiac remodelling.14,15,17 The benefit of IL-1 blockade may be therefore independent of the classic paradigm of infarct sparing for the prevention of adverse remodelling. IL-1 is a soluble cardiodepressant factor, known to affect cardiac performance and promote the incidence of HF.22 We previously showed that treating patients with HF with anakinra can improve cardiac function and cardiorespiratory fitness.22–27 In line with our findings, the CANTOS trial showed IL-1β blockade with canakinumab to significantly reduce HF hospitalizations and HF-related mortality13—and in patients with systolic HF, to improve cardiorespiratory fitness.24
The assessment of the safety profile of anakinra also confirmed that anakinra was not associated with an increased rate of infections. This differs from the significant yet small increase in risk observed with canakinumab in the CANTOS trial12: the difference may reflect a lack of power in the current study to detect the signal for harm, or the shorter duration of treatment, or the pharmacodynamic differences between anakinra and canakinumab.
We acknowledge several limitations of this study. This was a post-hoc analysis of three small, randomized control trials. The two anakinra arms of VCUART3 were pooled together for the purpose of this analysis and the three trials had limited power for detecting differences in clinical outcomes; therefore, our results should be interpreted as hypothesis-generating only. The relatively high rate of new-onset HF or death (29% in the placebo group at 1-year follow-up) observed in our cohort is likely due to the broad definition of HF used and the accurate adjudication of all HF events.19 Many of the events were diagnosed based on symptoms and signs and need for loop diuretics in the outpatient settings, and approximately half had preserved or mildly reduced LVEF at follow-up. Nevertheless, the beneficial effects of anakinra were apparent across a variety of criteria used to define HF, including more stringent criteria such as LVEF < 40% or invasive measurement of elevated LV end-diastolic pressure, or hospitalization for HF.
An additional limitation of these trials is that they were conducted before the implementation of new pharmacological strategies (i.e. sacubitril–valsartan, sodium–glucose cotransporter-2 inhibitors) that may affect the risk of HF after STEMI.28
Finally, there were a small number of females in the control group, as well as in the anakinra group, with a slight imbalance between groups likely reflecting a limitation of the small sample size.
Conclusion
In conclusion, modulation of the inflammatory response with interleukin‐1 blockade is a promising therapeutic strategy to prevent HF in patients with STEMI. The positive signal of anakinra on HF events requires confirmation in adequately powered clinical trials and further investigation into the mechanisms of action.
Supplementary Material
Contributor Information
Antonio Abbate, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
George F Wohlford, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Marco Giuseppe Del Buono, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Juan Guido Chiabrando, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Roshanak Markley, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Jeremy Turlington, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Dinesh Kadariya, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Cory R Trankle, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Giuseppe Biondi-Zoccai, Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy; Mediterranea Cardiocentro, Napoli, Italy.
Michael J Lipinski, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Benjamin W Van Tassell, VCU Pauley Heart Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
Funding
The VCUART2 study was supported by an American Heart Association Scientist Development grant 10SDG 3030051 and a Presidential Research Incentive Program of the Virginia Commonwealth University to Dr Abbate and by an Institutional National Institute of Health K12 award KL2RR031989 to Dr Van Tassell. The VCUART3 study was supported by a grant from the National Institutes of Health (1R34HL121402-01) to Dr Abbate and Dr Van Tassell. Swedish Orphan Biovitrum provided anakinra and matching placebo for VCUART3. Dr Abbate received support from the ‘Sapienza Visiting Professor Programme 2020’ of Sapienza Università di Roma, Italy.
Conflict of interest: Dr Abbate and Dr Van Tassell have served as consultants to Swedish Orphan Biovitrum LLC in the past. Dr Biondi-Zoccai has consulted for Cardionovum, CrannMedical, InnovHeart, Meditrial, Opsens Medical, and Replycare. The remaining authors have no disclosures to report.
References
- 1. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Randé JL, Unterseeh T, Le Breton H, Béard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 2. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation 2013;128:2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, Galiñanes M, Good MC, Hartikainen J, Hauskeller C, Janssens S, Kala P, Kastrup J, Martin J, Menasché P, Sanz-Ruiz R, Ylä-Herttuala S, Zeiher A; BAMI Group . The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 2020;41:3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mewton N, Roubille F, Bresson D, Prieur C, Bouleti C, Bochaton T, Ivanes F, Dubreuil O, Biere L, Hayek A, Derimay F, Akodad M, Alos B, Haider L, El Jonhy N, Daw R, De Bourguignon C, Dhelens C, Finet G, Bonnefoy-Cudraz E, Bidaux G, Boutitie F, Maucort-Boulch D, Croisille P, Rioufol G, Prunier F, Angoulvant D. Effect of colchicine on myocardial injury in acute myocardial infarction. Circulation 2021;144:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 7. Cavender MA, O'Donoghue M, Abbate A, Aylward P, Fox KAA, Glaser RX, Park JG, Lopez-Sendon J, Steg PG, Sabatine MS, Morrow DA. Inhibition of p38 MAP kinase in patients with ST-elevation myocardial infarction—findings from the LATITUDE TIMI 60 trial. Am Heart J 2021, doi: 10.1016/j.ahj.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 8. Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014;63:1593–1603. [DOI] [PubMed] [Google Scholar]
- 9. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 10. Seropian IM, Sonnino C, Van Tassell BW, Biasucci LM, Abbate A. Inflammatory markers in ST-elevation acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2016;5:382–395. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 13. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 14. Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW; VCU-ART Investigators. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction [Virginia Commonwealth University anakinra remodeling trial (VCU-ART) pilot study]. Am J Cardiol 2010;105:1371–1377e1. [DOI] [PubMed] [Google Scholar]
- 15. Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol 2013;111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, Oddi C, Carbone S, Trankle CR, Roberts CS, Mueller GH, Gambill ML, Christopher S, Markley R, Vetrovec GW, Dinarello CA, Biondi-Zoccai G. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 2015;115:288–292. [DOI] [PubMed] [Google Scholar]
- 17. Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, Canada JM, Carbone S, Roberts CS, Abouzaki N, Melchior R, Christopher S, Turlington J, Mueller G, Garnett J, Thomas C, Markley R, Wohlford GF, Puckett L, Medina de Chazal H, Chiabrando JG, Bressi E, Del Buono MG, Schatz A, Vo C, Dixon DL, Biondi-Zoccai GG, Kontos MC, Van Tassell BW. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc 2020;9:e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Tassell BW, Lipinski MJ, Appleton D, Roberts CS, Kontos MC, Abouzaki N, Melchior R, Mueller G, Garnett J, Canada J, Carbone S, Buckley LF, Wohlford G, Kadariya D, Trankle CR, Oddi Erdle C, Sculthorpe R, Puckett L, DeWilde C, Shah K, Angiolillo DJ, Vetrovec G, Biondi-Zoccai G, Arena R, Abbate A. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): a randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol 2018;41:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eapen ZJ, Tang WH, Felker GM, Hernandez AF, Mahaffey KW, Lincoff AM, Roe MT. Defining heart failure end points in ST-segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ Cardiovasc Qual Outcomes 2012;5:594–600. [DOI] [PubMed] [Google Scholar]
- 20. Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA heart study. Eur Heart J 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ørn S, Manhenke C, Ueland T, Damås JK, Mollnes TE, Edvardsen T, Aukrust P, Dickstein K. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. Eur Heart J 2009;30:1180–1186. [DOI] [PubMed] [Google Scholar]
- 22. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res 2020;126:1260–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckley LF, Carbone S, Trankle CR, Canada JM, Erdle CO, Regan JA, Viscusi MM, Kadariya D, Billingsley H, Arena R, Abbate A, Van Tassell BW. Effect of interleukin-1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol 2018;72:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi-Erdle C, Kadariya D, Christopher S, Viscusi M, Del Buono M, Kontos MC, Arena R, Van Tassell B, Abbate A. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol 2018;122:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail 2017;10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, Shah K, Canada J, Voelkel NF, Dinarello CA, Abbate A. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One 2012;7:e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2209–2225. [DOI] [PubMed] [Google Scholar]
- 28. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021:ehab368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.