Abstract
Study Objectives
Traumatic brain injuries (TBIs) cause persistent cerebral damage and cognitive deficits. Because sleep may be a critical factor for brain recovery, we characterized the sleep of patients with TBI from early hospitalization to years post-injury and explored the hypothesis that better sleep during hospitalization predicts more favorable long-term cognitive outcomes.
Methods
We tested patients with moderate-to-severe TBI in the hospitalized (n = 11) and chronic (n = 43) stages using full-night polysomnography, with 82% of the hospitalized group being retested years post-injury. Hospitalized patients with severe orthopedic and/or spinal cord injury (n = 14) and healthy participants (n = 36) were tested as controls for the hospitalized and chronic TBI groups, respectively. Groups had similar age and sex and were compared for sleep characteristics, including slow waves and spindles. For patients with TBI, associations between sleep during hospitalization and long-term memory and executive function were assessed.
Results
Hospitalized patients with TBI or orthopedic injuries had lower sleep efficiency, higher wake after sleep onset, and lower spindle density than the chronic TBI and healthy control groups, but only hospitalized patients with brain injury had an increased proportion of slow-wave sleep. During hospitalization for TBI, less fragmented sleep, more slow-wave sleep, and higher spindle density were associated to more favorable cognitive outcomes years post-injury, while injury severity markers were not associated with these outcomes.
Conclusion
These findings highlight the importance of sleep following TBI, as it could be a strong predictor of neurological recovery, either as a promoter or an early marker of cognitive outcomes.
Keywords: traumatic brain injury, TBI, moderate to severe, sleep, slow-wave sleep, cognition, memory, polysomnography, orthopedic injury, spinal cord injury
Statement of Significance.
Sleep is a modifiable factor that could affect recovery following traumatic brain injury, a neurological disorder causing very persistent sequelae. However, how sleep is disrupted during the hospitalization phase of a traumatic brain injury and how it could predict long-term cognitive outcomes remains unclear. In this novel longitudinal study, we have found highly fragmented sleep and elevated slow-wave sleep in patients hospitalized with traumatic brain injury, which was associated with cognitive outcomes years post-injury. These findings reinforce sleep as a potential predictor of neurological recovery, either as a promoter or an early marker of cognitive outcomes, which should be investigated in future intervention studies.
Introduction
Moderate-to-severe traumatic brain injury (TBI) is the main cause of disability in young adults worldwide [1, 2], and most patients develop persistent cognitive deficits that will restrict their quality of life and return to work or school [3–6]. Considering that, substantial efforts are being made in the acute stage of injury to improve long-term cognitive and functional outcomes. Using actigraphy, previous studies have found that sleep is disrupted following TBI in regular care or inpatient rehabilitation units, characterized by high fragmentation, low sleep efficiency, and partial loss of regular activity–rest cycles [7–12]. Some recent studies in moderate-to-severe TBI patients have also shown that more consolidated sleep–wake cycles were associated with better short-term functional outcomes such as recovery of consciousness, post-traumatic amnesia resolution, and motor abilities [7, 10, 12]. These findings brought a new interest for studying sleep during this period when the majority of TBI recovery occurs, and when sleep may be a critical factor in how the brain mends and therefore, have a significant impact on long-term cognitive outcomes. Indeed, sleep is essential to brain health, being notably involved in neuroplasticity [13, 14], neurogenesis [15, 16], global cognitive functioning [17, 18], and the glymphatic clearance of neurotoxic waste [19–21]. In a preliminary study, we demonstrated the feasibility of using polysomnography, the gold standard of objective measurements, to measure sleep at bedside in hospitalized TBI patients [22]. This opens the possibility of directly measuring sleep architecture in the earliest phases of TBI recovery.
Here, using polysomnography in a prospective longitudinal design, we aim to detail the sleep of moderate-to-severe TBI patients from early hospitalization to years after the injury and to explore the hypothesis that better sleep during hospitalization is associated with more favorable long-term cognitive outcomes.
Methods
Participants
Acute TBI patients.
Eleven patients with acute moderate-to-severe TBI (28.9 ± 13.4 years old; 55% male) were recruited during hospitalization at Hôpital du Sacré-Coeur de Montréal, a tertiary trauma center of the Centre intégré universitaire de santé et de services sociaux du Nord de l’Île-de-Montréal, between 2010 and 2015. The extensive recruitment protocol for this patient group, including details of recruitment rate, was previously described [7, 22]. All patients were between 18 and 60 years of age and required hospitalization in the intensive care unit (ICU) for their injury. Diagnosis of moderate-to-severe TBI, defined as an alteration in brain function, or other evidence of brain pathology caused by an external force [23], was confirmed by a practicing TBI neurosurgeon according to standard criteria [24, 25]: Glasgow Coma Scale (GCS) score between 3 and 12 at hospital admission, post-traumatic amnesia longer than 24 h, and loss of consciousness longer than 30 min. In addition, all patients had abnormal cerebral scans. Exclusion criteria included prior TBI of any severity, diagnosed sleep disorders, history of neurological, psychiatric or substance abuse disorders, pregnancy, quadriplegia, body mass index (BMI) over 30 kg/m2, severe eye injuries affecting the perception of light, and temporary skull bone flap removal at the time of study. After a review of hospital charts according to inclusion and exclusion criteria, patients’ families were contacted (n = 65). Following early discharges, medical complications, inability to tolerate research materials, inability to speak English or French, refusals to participate, and other reasons, 11 polysomnography recordings could be performed.
Acute orthopedic and spinal cord injury controls.
Fourteen patients with acute severe orthopedic and spinal cord injury (OSCI) (39.9 ± 17.1 years old; 79% male) were recruited to serve as controls for the acute TBI group. They were comparable in terms of sociodemographic characteristics and were hospitalized in a similar environment. All of them sustained severe orthopedic or spinal cord traumatic injuries requiring admission to the hospital and interventions by a specialized team. The exclusion criteria used for the acute TBI group were also applied for this group. Two patients had mild TBI and one had suspected but unconfirmed mild TBI in addition to their OSCI. Other patients had no TBI.
Chronic TBI participants.
Forty-three participants with chronic moderate-to-severe TBI (31.9 ± 13.5 years old; 67% male) were recruited from patients previously admitted to Hôpital du Sacré-Coeur de Montréal between 2010 and 2016. Of those, nine were part of the acute TBI group. The other two remaining acute TBI patients could not be recruited for this phase. The extensive recruitment protocol for this cohort was previously described [26–28]. All participants were between 18 and 60 years of age and had sustained a moderate-to-severe TBI in the past 1–4 years (2.00 ± 0.85 years, 1.36 ± 0.42 years for the subset of patients previously tested in the acute stage) at the time of testing. Exclusion criteria included history of another TBI of any severity, history of diagnosed sleep disorders or use of sleep medication, history of neurological, psychiatric or substance abuse disorders, pregnancy, quadriplegia, BMI over 30 kg/m2, jetlag due to recent trans-meridian travel, and night-shift work leading to atypical sleep schedules.
Healthy controls.
Thirty-six healthy participants (30.5 ± 12.7 years old; 69% male) were recruited through local and newspaper advertisements as a control group for the chronic TBI group, with similar age and sex distribution. The exclusion criteria used for the chronic TBI group were also applied for this group.
Inclusion in previous studies.
Seven acute TBI patients and six acute OSCI controls were included in a previous preliminary study by our group [22]. Forty-two chronic TBI participants and 36 healthy controls were included in a least one previous publication by our group [26–29].
Ethical approval.
The study was approved by the Research Ethics Board of the Centre intégré universitaire de santé et de services sociaux du Nord de l’Île-de-Montréal (#2011-690). Written and informed consent was obtained for each participant, in compliance with the Declaration of Helsinki. For participants who were transitorily inapt to consent in the acute stage, the written and informed consent was obtained from the immediate family or legal guardians.
Experimental protocol overview
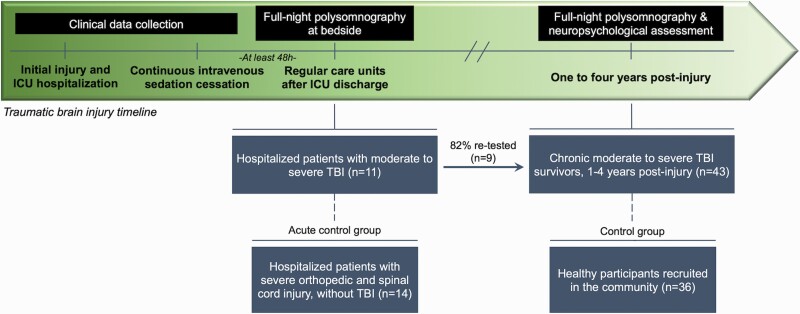
A prospective longitudinal design and a double cross-sectional case–control design were used concurrently (Figure 1). We investigated TBI in both the acute and chronic stages, with most of the acute group (82%) being tested again at follow-up in the chronic stage. Acute OSCI and healthy control groups were used as controls for the acute TBI and chronic TBI groups, respectively.
Figure 1.
Experimental protocol overview. A prospective longitudinal design and a double cross-sectional case–control design were used concurrently. We investigated TBI in both the acute and chronic stages, with most of the acute group being tested again at follow-up. Hospitalized OSCI and healthy control groups were used as controls for the acute TBI and chronic TBI groups, respectively. Sleep in hospitalized TBI and OSCI patients was recorded at bedside in regular care units after ICU discharge with a full-night of polysomnography. Clinical data related to the injury were collected from their hospital charts. Sleep in chronic TBI and healthy control participants was recorded with a full-night of in-laboratory polysomnography and a neuropsychological assessment was performed the following morning. Clinical data related to the injury were retrospectively collected from their hospital charts.
Acute TBI and OSCI patients’ sleep was recorded at bedside with polysomnography in regular care units after ICU discharge. All patients were extubated and free from continuous intravenous sedation and analgesia for at least 48 h, with normal intracranial pressure and no active infections. Clinical data related to the injury (e.g. mechanism of injury, GCS at hospital admission, ICU and hospital lengths of stay, duration of post-traumatic amnesia) were collected from their hospital charts. All medication taken during testing and the day before was documented.
Chronic TBI and healthy control participants underwent a full-night of in-laboratory polysomnography and performed a neuropsychological assessment the following morning. Cognitive domains frequently affected by TBI and associated with sleep were assessed with the following tests: (1) the Hopkins Verbal Learning Test (HVLT) and the Brief Visuospatial Memory Test (BVMT) immediate and delayed recalls to assess learning and memory; (2) the Trail-Making Test (TMT) part B-minus-A, the Tower of London total moves, total correct, and total time, and the Stroop part 3-minus-1 and 4-minus-2 to assess executive functioning (inhibition, flexibility, planning). Clinical data related to the injury were retrospectively collected from their hospital charts, and participants were asked to stop medication affecting sleep one week prior to testing, when possible.
Polysomnography
Acute recording at bedside.
The polysomnography protocol we used with the ambulatory Siesta system (Compumedics Ltd, Charlotte, NC) for the acute TBI and OSCI patients was previously described [22]. Briefly, the recordings took place in the regular neurologic or orthopedic units after ICU discharge. A montage comprising EEG (F4, C3, C4, P4) with a mastoid (M1) reference, chin electromyogram, and bilateral electrooculogram was installed at bedside by two sleep technologists in the late afternoon. EEG acquisition parameters included a 128 Hz sampling rate, a 60 Hz notch filter, and a 35 Hz low-pass filter. No schedule restrictions were placed on the patients for bedtime and wake time; the sleep period started with the first period of continuous nocturnal sleep (>10 minutes) after 20:00 and ended with the first awakening longer than 15 minutes after 06:30 in the morning. A trained research assistant stayed next to the patient’s room and monitored the polysomnography signals throughout the night to notify the on-call sleep technologist in the hospital of any problems, such as the occasional need to reinstall one electrode.
In-laboratory recording.
The sleep schedule of chronic TBI participants and healthy controls was determined according to their usual schedule, with bedtime restricted between 22:00 and 23:30. Their sleep schedule was assessed by continuous actigraphy and daily sleep diaries the week prior to the polysomnography. The in-laboratory polysomnography montage was comprised of a full EEG array (FP1, FP2, Fz, F3, F4, F7, F8, Cz, C3, C4, Pz, P3, P4, O1, O2, T3, T4, T5, T6) with mastoid calculated reference [((electrode-M1)–(M2–M1))/2], electrocardiogram, chin and tibia electromyogram, and bilateral electrooculogram. EEG acquisition parameters included a 256 Hz sampling rate, a 0.3 Hz high-pass filter, and a 100 Hz low-pass filter. Seven chronic TBI participants were recorded with a supplementary 60 Hz notch filter. Additional measurements included blood oxygen saturation (finger pulse oximeter), airflow (pressure transducer), and thoracic and abdominal belts.
Sleep scoring and analysis.
Sleep stages and events were scored using the standard criteria from the American Academy of Sleep Medicine [30]. Both automatic and visual detection were used to mark artifactual epochs to be excluded from all further analyses. The following macroarchitecture sleep variables were derived for the nighttime sleep recording: total sleep time, wake after sleep onset, number of awakenings, apnea–hypopnea index, sleep efficiency, and time spent in each sleep stage (in percentage of the whole nighttime sleep period). Sleep onset latency was only measured for the in-laboratory polysomnography, as patients recorded at bedside in hospital units had no “light-off” protocol and were bedridden, which limited the estimation of sleep onset latency.
Slow-wave and spindle detection.
Slow waves and spindles were detected automatically during the N2 and N3 stages of non-rapid eye movement sleep for all sleep cycles, using methods previously described [27, 28]. A single central derivation (C3) was stable enough for detection in all eleven acute TBI patients and was therefore used for all further analyses on sleep oscillations for each group. For slow waves, EEG signal was band-pass filtered (0.3–4.0 Hz) with a linear phase finite impulse response filter (−3 dB), and previously published detection criteria were used (negative peak lower than −40 µV, peak-to-peak amplitude higher than 75 µV, negative phase duration between 125 and 1500 ms, and positive phase duration lower than 1000 ms) [31]. We measured their density (events per minute) and the following morphological characteristics: peak-to-peak amplitude (µV), slope (µV/s), frequency (Hz), and positive and negative phase durations (seconds). For spindles, EEG signal was band-pass filtered (11.0–14.9 Hz) using a linear phase finite impulse response filter (−3 dB), and the root-mean-square amplitude of the filtered signal was then calculated over 0.25-second epochs. The threshold for spindle detection was set at the 95th percentile and at a minimum duration of 0.5 second. We measured their density (events per minute) and the following morphological characteristics: peak-to-peak amplitude (µV), frequency (Hz), and duration (s).
Statistical analyses
Group differences in demographic and clinical characteristics were assessed with one-way analyses of variance (ANOVAs) or chi-squared tests when applicable, followed by Tukey post-hoc analyses. Group differences in sleep architecture, slow-wave, and spindle characteristics were assessed with one-way ANOVAs, followed by Tukey's post-hoc analyses. The chronic TBI group included a fraction (9/43) of participants who were previously tested as acute TBI patients. To account for this intra-individual variance, additional sensitivity analyses were performed while excluding those participants from the chronic TBI group.
Associations with cognitive outcomes were explored in the subset of TBI patients who were tested with polysomnography at both the acute and chronic stages. Two separate principal component analyses with varimax rotation were first computed to create outcome composite scores representing the two cognitive domains of interest (learning and memory; executive functioning), with a priori selected cognitive test variables for each score. The cutoff for variable inclusion in the components was set at a loading coefficient (LC) >0.3. The learning and memory composite score included the following tests: HVLT immediate recall (LC = 0.58), HVLT delayed recalls (LC = 0.84), BVMT immediate recall (LC = 0.95), and BVMT delayed recalls (LC = 0.89). The executive functioning score included the following tests: TMT part B-minus-A (LC = 0.86), Tower of London total moves (LC = 0.97), total correct (LC = 0.93), total time (LC = 0.51), and Stroop part 3-minus-1 (LC < 0.3) and 4-minus-2 (LC < 0.3). The Stroop part 3-minus-1 and 4-minus-2 were therefore not included in this component as their LC was under the predetermined cutoff. All components were expressed as higher scores representing better cognition.
Pearson correlations were then performed for significant sleep architecture and sleep oscillation characteristics in the acute stage with cognitive composite scores in the chronic stage. Sensitivity analyses to account for variables that could affect these associations included Pearson correlations of cognitive outcome variables with age, injury-related variables, and hospital-related variables, as well as Pearson correlations of acute sleep variables with hospital-related variables. As a post-hoc analysis, because sleep spindles are known to be trait-like, we investigated whether any significant association with cognition involving them would also be present in the chronic stage in addition to the acute stage. All statistical analyses were performed with SPSS Statistics 25 (IBM Corp., 2019), with statistical significance set at p <0.05. Effect sizes were interpreted according to established criteria [32, 33].
Results
Participant characteristics
Demographic and clinical data for the four groups are described in Table 1. All groups were comparable for age and sex. Acute and chronic TBI groups had similar injury severity markers, namely the GCS score at first hospital admission and duration of post-traumatic amnesia. Acute and chronic TBI groups had longer ICU stays than the acute OSCI group, but similar total hospital length of stay. Time since injury at moment of testing was also similar between the acute TBI and OSCI groups. Medication intake was prevalent among the acute hospitalized groups; briefly, a bigger proportion of acute OSCI patients took pain medication such as opioids, while a bigger proportion of acute TBI patients took psychoactive medication such as psychostimulants, anticonvulsants, or antipsychotics.
Table 1.
Demographic and clinical characteristics
| Acute TBI [1] (n =11) |
Acute OSCI [2] (n = 14) |
Chronic TBI [3] (n = 43) |
Healthy controls [4] (n = 36) |
One-way ANOVA/chi-square | Tukey's post-hoc | |
|---|---|---|---|---|---|---|
| Age, years | 28.9 (13.4) | 39.9 (17.1) | 31.9 (13.5) | 30.5 (12.7) | p = 0.138 | — |
| Sex, m:f | 6:5 | 11:3 | 29:14 | 25:11 | p = 0.642 | — |
| GCS at hospital admission | 6.3 (3.0) | — | 8.4 (3.3) | — | p = 0.062 | — |
| Duration of post-traumatic amnesia, days | 14.1 (18.0) | — | 14.8 (15.4) | — | p = 0.896 | — |
| ICU length of stay, days | 13.9 (11.1) | 3.5 (4.7) | 11.3 (9.9) | — | p = 0.002 | 2 < 1,3 |
| Hospital length of stay, days | 31.7 (20.1) | 28.4 (11.6) | 30.7 (17.6) | — | p = 0.866 | — |
| Time since injury at moment of testing, days | 22.0 (14.8) | 17.4 (7.3) | 730.6 (308.5) | — | p < 0.0001 | 1,2 < 3 |
| Medication intake | ||||||
| Opioids, n (%) | 4 (36.4) | 12 (85.7) | 0 (0.0) | 0 (0.0) | ||
| Acetaminophen, n (%) | 6 (54.5) | 13 (92.9) | 1 (2.3) | NA | ||
| Other analgesics and muscle relaxants, n (%) | 0 (0.0) | 7 (50.0) | 0 (0.0) | NA | ||
| Anti-inflammatories, n (%) | 0 (0.0) | 2 (14.3) | 3 (7.0) | NA | ||
| Psychostimulants, n (%) | 4 (36.4) | 0 (0.0) | 2 (4.7) | 0 (0.0) | ||
| Antidepressants, n (%) | 1 (9.1) | 0 (0.0) | 6 (14.0) | 0 (0.0) | ||
| Anxiolytics, n (%) | 1 (9.1) | 3 (21.4) | 0 (0.0) | 0 (0.0) | ||
| Anticonvulsants, n (%) | 3 (27.3) | 0 (0.0) | 2 (4.7) | 0 (0.0) | ||
| Antipsychotics, n (%) | 3 (27.3) | 1 (7.1) | 1 (2.3) | 0 (0.0) |
Medication intake for all groups is defined as any intake at any point during testing or the day prior. Data are presented as mean (SD) unless specified otherwise. NA, not available.
Bold indicates statistically significant values.
Sleep architecture
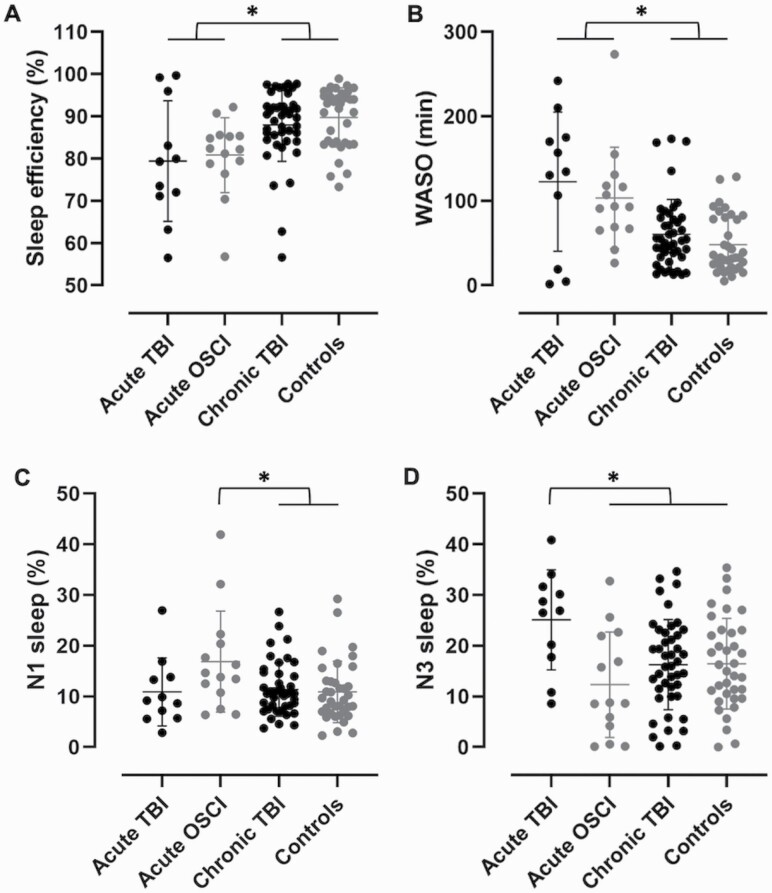
All four groups were compared with one-way ANOVAs for sleep architecture characteristics measured from a full-night of polysomnography (Table 2). While we found no difference in total sleep time, number of awakenings, and apnea–hypopnea index, there were group effects in sleep efficiency (F(3, 100) = 6.2, p = 0.001) and duration of wake after sleep onset (F(3, 100) = 9.8, p = 0.00001). Post-hoc analyses revealed that the acute TBI group had lower sleep efficiency and higher wake after sleep onset than the chronic TBI (p = 0.035, d = 0.85; p = 0.002, d = 1.15, respectively) and healthy control (p = 0.008, d = 1.11; p = 0.0002, d = 1.46, respectively) groups, with large to very large effect sizes (Figure 2, A and B). The acute OSCI group also had lower sleep efficiency and higher wake after sleep onset than the chronic TBI (p = 0.040, d = 0.84; p = 0.011, d = 0.98, respectively) and healthy control (p = 0.007, d = 1.21; p = 0.0009, d = 1.37, respectively) groups, with similar effect sizes. Concerning the percentage of time spent in each sleep stage, we found no group difference in stage N2 or rapid eye movement sleep. On the other hand, stage N1 (F(3, 100) = 2.8, p = 0.042) and N3 (F(3, 100) = 4.0, p = 0.010) sleep were different between groups. Post-hoc analyses revealed that only the acute OSCI group had more N1 sleep than the chronic TBI (p = 0.053, d = 0.75) and healthy control (p = 0.035, d = 0.75) groups, with medium effect sizes, while only the acute TBI group had more N3 sleep than all other groups (vs acute OSCI, p = 0.005, d = 1.25; vs chronic TBI, p = 0.025, d = 1.01; vs healthy controls, p = 0.033, d = 0.99), with large to very large effect sizes (Figure 2, C and D). Taken together, although both the acute OSCI and TBI groups had more fragmented sleep, the OSCI group had lighter sleep while the TBI group had deeper sleep. Of note, the chronic TBI group was no different from the healthy control group.
Table 2.
Differences in sleep architecture
| Acute TBI [1] |
Acute OSCI [2] |
Chronic TBI [3] |
Healthy controls [4] |
One-way ANOVA | Tukey's post-hoc | |
|---|---|---|---|---|---|---|
| Total sleep time, min | 458.5 (75.9) | 434.3 (69.3) | 452.5 (68.3) | 429.5 (59.0) | p = 0.367 | — |
| WASO, min | 121.6 (86.7) | 105.5 (58.4) | 60.4 (41.3) | 48.1 (33.7) | p = 0.00001 | 1,2 > 3,4 |
| Number of awakenings | 35.2 (29.9) | 39.4 (20.7) | 33.0 (12.3) | 28.3 (13.0) | p = 0.150 | — |
| AHI, events/h | NA | NA | 3.5 (5.5) | 2.0 (2.4) | p = 0.141 | — |
| Sleep efficiency, % | 79.4 (15.0) | 80.8 (8.5) | 88.0 (8.6) | 89.8 (7.0) | p = 0.001 | 1,2 < 3,4 |
| Stage N1 sleep, % | 11.0 (7.1) | 16.4 (9.8) | 11.4 (5.3) | 10.9 (6.1) | p = 0.042 | 2 > 3,4 |
| Stage N2 sleep, % | 47.4 (8.2) | 54.3 (10.2) | 54.0 (7.9) | 54.1 (7.0) | p = 0.105 | — |
| Stage N3 sleep, % | 25.6 (10.3) | 12.8 (10.2) | 16.3 (8.9) | 16.5 (8.9) | p = 0.010 | 1 > 2,3,4 |
| REM sleep, % | 15.9 (4.7) | 16.4 (5.6) | 18.3 (5.4) | 18.5 (5.5) | p = 0.371 | — |
Data are presented as mean (SD). AHI, apnea–hypopnea index; REM, rapid eye movement; NA, not available; WASO, wake after sleep onset.
Bold indicates statistically significant values.
Figure 2.
Significant sleep differences in hospitalized and chronic TBI compared to hospitalized and healthy controls. Sleep data were acquired from a full-night of bedside (hospitalized acute TBI and OSCI) or in-laboratory (chronic TBI and healthy controls) polysomnography. Hospitalized TBI and OSCI had more fragmented sleep than chronic TBI and healthy controls, with (A) lower sleep efficiency and (B) higher wake after sleep onset. (C) Hospitalized OSCI had more stage N1 light sleep than chronic TBI and healthy controls. (D) Hospitalized TBI had more stage N3 deep sleep than all other groups. Significant differences at p < 0.05 are shown with an asterisk (*). WASO, wake after sleep onset.
As sensitivity analyses to account for nine patients from the acute TBI group who were also tested at follow-up in the chronic TBI group, we performed these analyses again while excluding those participants from the chronic TBI group. The same differences were found in sleep efficiency (F(3, 91) = 5.6, p = 0.001), wake after sleep onset (F(3, 91) = 9.1, p = 0.00003), percentage of time in stage N1 sleep (F(3, 91) = 3.0, p = 0.036), and percentage of time in stage N3 sleep (F(3, 91) = 4.0, p = 0.010). Minor differences in post-hoc analyses only included the chronic TBI group no longer being significantly different in sleep efficiency from the acute TBI group (p = 0.07), in sleep efficiency from the acute OSCI group (p = 0.13), and in stage N1 sleep from the acute OSCI group (p = 0.06).
Slow waves and spindles
All four groups were compared with one-way ANOVAs for slow-wave and spindle characteristics. For slow waves, differences were only found for the negative phase duration (F(3, 98) = 3.8, p = 0.012), with post-hoc analyses showing longer duration in the acute TBI group compared to the healthy control group (p = 0.017, d = 1.10), with large effect sizes. For spindles, differences were found in density (F(3, 98) = 8.5, p = 0.00005), with post-hoc analyses showing that the acute TBI group had lower spindle density than the chronic TBI (p = 0.0002, d = 1.46) and healthy control (p = 0.0008, d = 1.49) groups, with very large effect sizes. The acute OSCI group also had lower spindles density than the chronic TBI (p = 0.020, d = 0.85) and healthy control (p = 0.048, d = 0.89) groups, with large effect sizes. Differences were also found in spindle amplitude (F(3, 98) = 3.8, p = 0.012), with post-hoc analyses showing lower amplitude in the acute OSCI group compared to the chronic TBI (p = 0.020, d = 0.91) and healthy control groups (p = 0.049, d = 0.91), with large effect sizes. No other differences were found.
As sensitivity analyses to account for nine patients from the acute TBI group who were also tested at follow-up in the chronic TBI group, we performed these analyses again while excluding those participants from the chronic TBI group. The same differences were found in slow-wave negative phase duration (F(3, 89) = 4.1, p = 0.009), spindle density (F(3, 89) = 8.1, p = 0.00008), and spindle amplitude (F(3, 89) = 3.4, p = 0.020). Minor differences in post-hoc analyses only included the healthy control group no longer being significantly different from the acute OSCI group in spindle density (p = 0.06) and spindle amplitude (p = 0.06).
Association between sleep during hospitalization and cognitive outcomes in patients with TBI
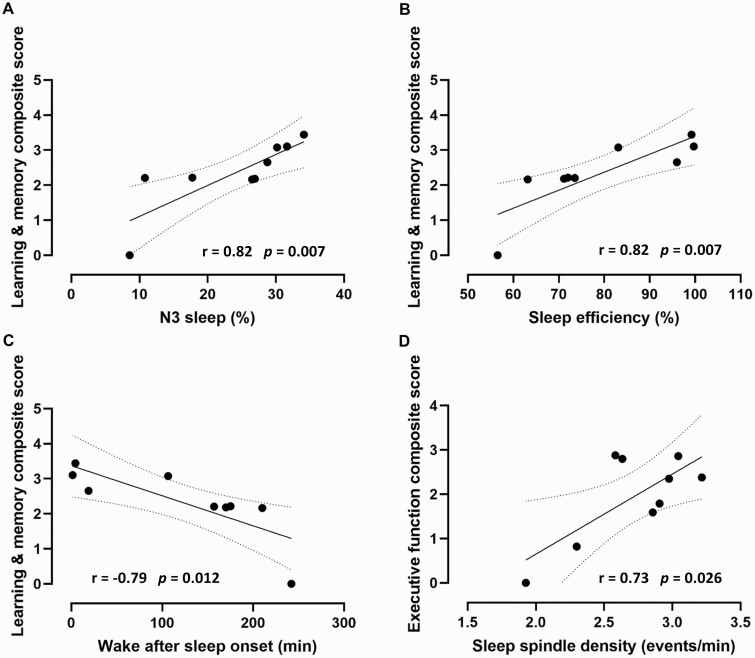
In the subset of TBI patients who were tested at both the hospitalized and follow-up stages, we explored the association between their acute sleep architecture and oscillations and their long-term cognitive outcomes, represented by the two cognitive composite scores created by principal component analyses. The learning and memory composite score was associated with percentage of time spent in N3 sleep (r = 0.82, p = 0.007), sleep efficiency (r = 0.82, p = 0.007), and wake after sleep onset (r = −0.79, p = 0.012). Indeed, higher percentage of time spent in N3 sleep, higher sleep efficiency, and shorter wake after sleep onset during hospitalization correlated with better learning and memory at follow-up (Figure 3, A–C). Conversely, the executive functioning composite score was only associated with acute spindle density (r = 0.73, p = 0.026). More precisely, higher spindle density during hospitalization correlated with better executive functioning at follow-up (Figure 3D). This association was not observed with spindle density measured in the chronic stage.
Figure 3.
Effect of sleep during TBI hospitalization on cognitive outcomes. Better cognitive outcomes 1–4 years following the initial injury were associated with better sleep during hospitalization in the subset of patients that were tested at both stages. Better learning and memory outcomes (composite score including the HVLT and BVMT immediate and delayed recalls) were associated with (A) more stage N3 deep sleep, (B) higher sleep efficiency, and (C) lower wake after sleep onset during hospitalization. (D) Better executive functioning outcomes (composite score including the TMT part B-minus-A and the Tower of London total moves, total correct, and total time) were associated with higher sleep spindle density during hospitalization. Composite scores were originally centered on zero and were translated upwards along the y axis to represent them without crossing the x-axis. Dotted lines represent the 95% confidence interval.
As sensitivity analyses to control for other variables that could affect the long-term cognitive functioning of TBI patients, we investigated if the cognitive composite scores were associated with age, injury severity (as measured by the GCS score at hospital admission and the duration of post-traumatic amnesia), ICU and total hospital lengths of stay, time since injury at moment of testing, and time since stopping continuous intravenous sedation at moment of testing. No significant associations were found. In addition, to control for the hospital-related variables that could affect our sleep measurements, we also investigated if the sleep of hospitalized TBI patients was associated with ICU and total hospital lengths of stay, time since injury at moment of testing, and time since stopping continuous intravenous sedation at moment of testing. No significant associations were found.
Discussion
We used a prospective longitudinal design and polysomnography to characterize the sleep of patients with moderate-to-severe TBI during early hospitalization and years after the injury. We found that patients hospitalized for severe traumatic injuries (TBI or OSCI) had a highly fragmented sleep, which was surprisingly accompanied by a high proportion of slow-wave sleep in TBI patients only. Furthermore, we confirmed our hypothesis that better sleep during hospitalization in the acute stage of the injury is associated with better long-term cognitive outcomes in TBI patients. Indeed, better learning, memory and executive functioning 1–4 years after injury were strongly associated with less fragmented and deeper sleep during hospitalization, but not with age or injury severity markers. These results highlight the importance of sleep in the acute stage of TBI, as it could be a strong predictor of neurological recovery, either as a promoter or as an early marker of cognitive outcomes. These results build on previous actigraphy studies which showed that the recovery of a consolidated sleep-wake cycle during the acute stage was associated with short-term outcomes, namely consciousness levels [7], post-traumatic amnesia resolution [12], and motor abilities [10]. Taken together, these results may suggest that better sleep during hospitalization predicts short- and long-term cognitive outcomes after TBI. They also call for further research on the early management of sleep disturbances as sleep could possibly promote optimal recovery after severe brain damage.
With no surprise, sleep in regular hospital units after ICU discharge was different from what is seen in laboratory conditions for healthy individuals. Both acute TBI and OSCI groups had less efficient and more fragmented sleep probably due, in part, to the hospital environment (e.g. noise, light, nursing interventions) and pain, two factors known to disrupt sleep [34–36]. However, while this was accompanied by lighter sleep in OSCI patients without moderate-to-severe TBI, TBI patients had a deeper sleep characterized by more slow-wave sleep. Slow-wave sleep is heavily involved in neuroplasticity, neurogenesis, and glymphatic clearance of metabolites [13–16, 19–21, 37]. In recent years, the study of glymphatic clearance during sleep has especially been a rapidly expanding field, as it is now becoming clear that slow-wave sleep drives this important process [19–21]. Given that these three processes underlying nervous system mending and upkeep should be massively solicited when the brain sustained important damage, they may explain the sleep patterns we observed in acute TBI patients. Results from the present study could point to the brain injury itself as an important factor in the brain’s heightened need for deeper sleep during critical care, which probably reflects the ongoing cerebral recuperation and reorganization following the especially severe TBI we studied. Previous studies in animal models support this hypothesis as they have observed increased sleep need shortly following a single fluid percussion or closed head TBI [38, 39]. We could hypothesize that the elevated sleep needs in our patients could also be due to previous sleep deprivation incurred during ICU stay or by prolonged periods of continuous sedation which may not fully replace sleep. However, neither ICU length of stay nor time since stopping continuous sedation was associated with sleep characteristics. In addition, studies in rats have advanced that prolonged sedation and anesthesia do not result in sleep deprivation [40, 41]. In terms of sleep oscillations, acute TBI patients had a decreased spindle density. Spindles are less prevalent towards the beginning of the night when sleep pressure is high and deeper sleep prevails [42, 43]. Although still involved in neuroplasticity, their reduction could be a result of the non-significant but present decrease in N2 sleep, which may be a trade-off incurred by the concomitant shift towards deeper sleep.
It is important to consider that sleep may be a marker of neurological recovery rather than a promoter, or may potentially be both a marker and a promoter. Our experimental design does not allow us to conclude whether cognitive outcomes are influenced directly by sleep or if both result from a common factor such as global neurological recovery. On one hand, a recent study advanced that the return of a consolidated sleep-wake cycle occurred in parallel with the return of consciousness, and not as a cause [7]. On the other hand, the previously discussed mechanisms could explain sleep’s role in promoting recovery. Long-term cognitive outcomes in our relatively small sample were associated with sleep but not with injury-related markers, and other studies have shown that sleep disorders exacerbate TBI cognitive impairment and other symptoms [44, 45]. We surmise that both sides may be intertwined in a feedback loop, as the brain needs healthy sleep the same way sleep needs a healthy brain.
Limitations
Almost all acute hospitalized patients were being administered one or more types of medication, with some of them known to influence sleep. The OSCI group was administered more analgesics and muscle relaxants than the TBI group, including opioids that are known to increase N2 sleep at the expense of N3 sleep [46, 47]. This is likely due to the severity of their orthopedic injury and/or to their greater awareness and ability to communicate their pain, even though most moderate-to-severe TBI patients also had significant orthopedic injuries. As such, resulting pain levels must have fluctuated and differed between groups and may have differently affected sleep. Conversely, a bigger proportion of the TBI group than the OSCI group was administered various psychoactive medications with very diverse effects on sleep [47]. Altogether, the mesh of fluctuating pain and medication makes it difficult to specifically characterize their effects on sleep in our limited samples, and even harder to compare with others as most TBI studies are comprised of very diverse samples and medication intake may not have been reported in detail. Other factors that may have affected our results include repeated nursing interventions and other hospital disruptions, which are partly accounted for in the experimental design by using a control group hospitalized in a similar environment. Overall, this highlights the importance of carefully detailing clinical factors when investigating this patient population.
It also has to be noted that the relatively small sample size of the hospitalized TBI group could be a limiting factor in the interpretation of the results, although we found very large effects due to the severe nature of the injury and its heavy neurological impact. In addition, it is expected that sleeping in the hospital environment could result in high inter-night variability, and therefore, a single night of polysomnography recording could limit the representativity of the whole hospital admission.
Clinical impact
Seeing as the hospital environment is typically not well suited for good sleep [34, 35], and that most sleep disorders following TBI stay undiagnosed and untreated for years [45], these findings and the recent literature suggest that the enactment of strategies to target sleep early on could possibly improve short- and long-term functional recovery, quality of life and return to work. In acute care units, the implementation of hospital protocols and pharmacological treatments that account for sleep may be necessary to allow for optimal recovery of TBI patients [35, 48]. In rehabilitation units and in the long-term, non-pharmacological interventions such as cognitive behavioral therapy and sleep hygiene practices have proven effective in improving sleep for individuals with TBI [44, 49]. Melatonin, melatonin agonists, and luminotherapy have also succeeded in improving sleep, fatigue, and cognitive functioning following TBI [50–53]. Novel strategies such as sleep enhancement may also prove useful. In animal models, sleep modulation to enhance slow-wave activity acutely after TBI markedly reduced axonal damage and cognitive decline 2 weeks later [54]. In healthy humans, acoustic and transcranial stimulation to enhance slow oscillations during sleep were successful in potentiating memory in the short-term [55, 56], although longer-term studies are necessary to evaluate their therapeutic effectiveness. Altogether, sleep appears to be an important modifiable factor to target early on and throughout recovery. In addition, sleep could prove to be a strong marker of neurological recovery, and thus, its monitoring during hospitalization following a TBI could allow targeting individuals that could benefit from other interventions to promote recovery.
Contributor Information
Erlan Sanchez, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Neuroscience, Université de Montréal, Montreal, Quebec, Canada.
Hélène Blais, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada.
Catherine Duclos, Montreal General Hospital, McGill University Health Centre, Montreal, Quebec, Canada; School of Physical and Occupational Therapy, McGill University, Montreal, Quebec, Canada.
Caroline Arbour, Centre Intégré de Traumatologie, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Faculty of Nursing, Université de Montréal, Montreal, Quebec, Canada.
Solenne Van Der Maren, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Psychology, Université de Montréal, Montreal, Quebec, Canada.
Héjar El-Khatib, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Psychology, Université de Montréal, Montreal, Quebec, Canada.
Andrée-Ann Baril, Douglas Mental Health University Institute, Montréal, Quebec, Canada; Department of Psychiatry, McGill University, Montréal, Quebec, Canada.
Francis Bernard, Centre Intégré de Traumatologie, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Medicine, Université de Montréal, Montreal, Quebec, Canada.
Julie Carrier, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Psychology, Université de Montréal, Montreal, Quebec, Canada.
Nadia Gosselin, Center for Advanced Research in Sleep Medicine, Centre Intégré Universitaire de Santé et Services Sociaux du Nord de l’Île-de-Montréal, Montreal, Quebec, Canada; Department of Psychology, Université de Montréal, Montreal, Quebec, Canada.
Funding
This study was funded by government granting agencies with grants to NG as a principal investigator: Canadian Institutes of Health Research (CIHR MOP 115172), Chaire de recherche du Canada, and Fonds de Recherche du Québec – Santé (FRQS #34851).
Disclosure Statement
None declared.
Data Availability
Relevant data that support the findings of this study are available from the corresponding author upon request.
References
- 1. Nguyen R, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(6):774–785. [DOI] [PubMed] [Google Scholar]
- 2. Kraus JF, Chu LD. Textbook of traumatic brain injury. In: Silver JM, McAllister TW, Yudofsky SC, eds. Arlington, VA: American Psychiatric Publishing; 2005:3–26. [Google Scholar]
- 3. Stocchetti N, et al. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathias J, et al. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 2012;13(7):898–905. [DOI] [PubMed] [Google Scholar]
- 5. Vaishnavi S, et al. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198–205. [DOI] [PubMed] [Google Scholar]
- 6. Duclos C, et al. The impact of poor sleep on cognition and activities of daily living after traumatic brain injury: a review. Aust Occup Ther J. 2015;62(1):2–12. [DOI] [PubMed] [Google Scholar]
- 7. Duclos C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2017;88(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duclos C, et al. Rest-activity cycle disturbances in the acute phase of moderate to severe traumatic brain injury. Neurorehabil Neural Repair. 2014;28(5):472–482. [DOI] [PubMed] [Google Scholar]
- 9. Duclos C, et al. Sleep–wake disturbances in hospitalized patients with traumatic brain injury: association with brain trauma but not with an abnormal melatonin circadian rhythm. Sleep. 2020;43(1). doi: 10.1093/sleep/zsz191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming MK, et al. Sleep disruption after brain injury is associated with worse motor outcomes and slower functional recovery. Neurorehabil Neural Repair. 2020; 34(7):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams ET, et al. Injury, sleep, and functional outcome in hospital patients with traumatic brain injury. J Neurosci Nurs. 2019;51(3):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makley MJ, et al. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabil Neural Repair. 2009;23(4):320–326. [DOI] [PubMed] [Google Scholar]
- 13. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kreutzmann JC, et al. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–190. [DOI] [PubMed] [Google Scholar]
- 16. Meerlo P, et al. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13(3):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schabus M, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23(7):1738–1746. [DOI] [PubMed] [Google Scholar]
- 18. Fogel SM, et al. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–1165. [DOI] [PubMed] [Google Scholar]
- 19. Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holth JK, et al. The sleep–wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363(6429):880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fultz NE, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiseman-Hakes C, et al. Sleep in the acute phase of severe traumatic brain injury: a snapshot of polysomnography. Neurorehabil Neural Repair. 2016;30(8):713–721. [DOI] [PubMed] [Google Scholar]
- 23. Menon DK, et al. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640. [DOI] [PubMed] [Google Scholar]
- 24. Teasdale G, et al. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 25. Nakase-Richardson R, et al. Classification schema of posttraumatic amnesia duration-based injury severity relative to 1-year outcome: analysis of individuals with moderate and severe traumatic brain injury. Arch Phys Med Rehabil. 2009;90(1):17–19. [DOI] [PubMed] [Google Scholar]
- 26. El-Khatib H, et al. Towards a better understanding of increased sleep duration in the chronic phase of moderate to severe traumatic brain injury: an actigraphy study. Sleep Med. 2018;59:67–75. doi: 10.1016/j.sleep.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 27. Sanchez E, et al. Brain white matter damage and its association with neuronal synchrony during sleep. Brain. 2019;142(3):674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez E, et al. Sleep spindles are resilient to extensive white matter deterioration. Brain Commun. 2020;2(2):fcaa071. doi: 10.1093/braincomms/fcaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El-Khatib H, et al. Slow wave activity moderates the association between new learning and traumatic brain injury severity. Sleep. 2021;44(4). doi: 10.1093/sleep/zsaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus C, Vaughn B.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 31. Carrier J, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33(4):758–766. [DOI] [PubMed] [Google Scholar]
- 32. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- 33. Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8(2):26. [Google Scholar]
- 34. Kulpatcharapong S, et al. Sleep quality of hospitalized patients, contributing factors, and prevalence of associated disorders. Sleep Disord. 2020;2020:8518396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wesselius HM, et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018;178(9):1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bergmann TO, et al. Acute changes in motor cortical excitability during slow oscillatory and constant anodal transcranial direct current stimulation. J Neurophysiol. 2009;102(4):2303–2311. [DOI] [PubMed] [Google Scholar]
- 38. Rowe RK, et al. Diffuse brain injury induces acute post-traumatic sleep. PLoS One. 2014;9(1):e82507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noain D, et al. Increased sleep need and reduction of tuberomammillary histamine neurons after rodent traumatic brain injury. J Neurotrauma. 2018;35(1):85–93. [DOI] [PubMed] [Google Scholar]
- 40. Nelson AB, et al. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33(12):1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tung A, et al. Anesthesia and sleep. Sleep Med Rev. 2004;8(3):213–225. [DOI] [PubMed] [Google Scholar]
- 42. Aeschbach D, et al. All-night dynamics of the human sleep EEG. J Sleep Res. 1993;2(2):70–81. [DOI] [PubMed] [Google Scholar]
- 43. Dijk DJ, et al. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiseman-Hakes C, et al. Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Inj. 2013;27(12):1364–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ouellet MC, et al. Sleep–wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14(7):746–757. [DOI] [PubMed] [Google Scholar]
- 46. Dimsdale JE, et al. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(1):33–36. [PubMed] [Google Scholar]
- 47. Mollayeva T, Shapiro C. Medication effects. In: Kushida C, ed. The Encyclopedia of Sleep. Vol 2. Waltham, MA: Academic Press; 2013: 330–337. [Google Scholar]
- 48. Driver S, et al. Pharmacological management of sleep after traumatic brain injury. NeuroRehabilitation. 2018;43(3):347–353. [DOI] [PubMed] [Google Scholar]
- 49. Ford ME, et al. Non-pharmacological treatment for insomnia following acquired brain injury: a systematic review. Sleep Med Rev. 2020;50:101255. [DOI] [PubMed] [Google Scholar]
- 50. Grima NA, et al. Efficacy of melatonin for sleep disturbance following traumatic brain injury: a randomised controlled trial. BMC Med. 2018;16(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lequerica A, et al. Pilot study on the effect of ramelteon on sleep disturbance after traumatic brain injury: preliminary evidence from a clinical trial. Arch Phys Med Rehabil. 2015;96(10):1802–1809. [DOI] [PubMed] [Google Scholar]
- 52. Killgore WDS, et al. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. 2020;134:104679. [DOI] [PubMed] [Google Scholar]
- 53. Connolly LJ, et al. Home-based light therapy for fatigue following acquired brain injury: a pilot randomized controlled trial. BMC Neurol. 2021;21(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morawska MM, et al. Sleep modulation alleviates axonal damage and cognitive decline after rodent traumatic brain injury. J Neurosci. 2016;36(12):3422–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 56. Papalambros NA, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci. 2017;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data that support the findings of this study are available from the corresponding author upon request.