This randomized clinical trial investigates if the provision of prognostic information regarding patients with heart failure to clinicians results in improved decision-making about treatment initiation and intensity, appropriate end-of-life care, and reduction in hospitalization or death rates.
Key Points
Question
Does providing clinicians with prognostic information about their patients with heart failure translate into improved decision-making about initiation and intensity of treatment, more appropriate end-of-life care, and a reduction in rates of hospitalization or death?
Findings
In this randomized clinical trial that included 3124 patients, the primary outcome—a composite of 30-day hospital readmissions and all-cause mortality at 1 year—occurred in 619 patients (38.9%) in the alert group and 603 patients (38.4%) in the usual-care group. No significant differences between study groups were noted in the prescription of heart failure medications at discharge, the placement of an implantable cardioverter-defibrillator, or referral to palliative care.
Meaning
Provision of mortality risk information did not affect clinical decision-making or improve outcomes in patients with heart failure.
Abstract
Importance
Heart failure is a major cause of morbidity and mortality worldwide. The use of risk scores has the potential to improve targeted use of interventions by clinicians that improve patient outcomes, but this hypothesis has not been tested in a randomized trial.
Objective
To evaluate whether prognostic information in heart failure translates into improved decisions about initiation and intensity of treatment, more appropriate end-of-life care, and a subsequent reduction in rates of hospitalization or death.
Design, Setting, and Participants
This was a pragmatic, multicenter, electronic health record–based, randomized clinical trial across the Yale New Haven Health System, comprising small community hospitals and large tertiary care centers. Patients hospitalized for heart failure who had N-terminal pro–brain natriuretic peptide (NT-proBNP) levels of greater than 500 pg/mL and received intravenous diuretics within 24 hours of admission were automatically randomly assigned to the alert (intervention) or usual-care groups.
Interventions
The alert group had their risk of 1-year mortality calculated using an algorithm that was derived and validated using similar historic patients in the electronic health record. This estimate, including a categorical risk assessment, was presented to clinicians while they were interacting with a patient’s electronic health record.
Main Outcomes and Measures
The primary outcome was a composite of 30-day hospital readmissions and all-cause mortality at 1 year.
Results
Between November 27, 2019, through March 7, 2021, 3124 patients were randomly assigned to the alert (1590 [50.9%]) or usual-care (1534 [49.1%]) group. The alert group had a median (IQR) age of 76.5 (65-86) years, and 796 were female patients (50.1%). Patients from the following race and ethnicity groups were included: 13 Asian (0.8%), 324 Black (20.4%), 136 Hispanic (8.6%), 1448 non-Hispanic (91.1%), 1126 White (70.8%), 6 other ethnicity (0.4%), and 127 other race (8.0%). The usual-care group had a median (IQR) age of 77 (65-86) years, and 788 were female patients (51.4%). Patients from the following race and ethnicity groups were included: 11 Asian (1.4%), 298 Black (19.4%), 162 Hispanic (10.6%), 1359 non-Hispanic (88.6%), 1077 White (70.2%), 13 other ethnicity (0.9%), and 137 other race (8.9%). Median (IQR) NT-proBNP levels were 3826 (1692-8241) pg/mL in the alert group and 3867 (1663-8917) pg/mL in the usual-care group. A total of 284 patients (17.9%) and 270 patients (17.6%) were admitted to the intensive care unit in the alert and usual-care groups, respectively. A total of 367 patients (23.1%) and 359 patients (23.4%) had a left ventricular ejection fraction of 40% or less in the alert and usual-care groups, respectively. The model achieved an area under the curve of 0.74 in the trial population. The primary outcome occurred in 619 patients (38.9%) in the alert group and 603 patients (39.3%) in the usual-care group (P = .89). There were no significant differences between study groups in the prescription of heart failure medications at discharge, the placement of an implantable cardioverter-defibrillator, or referral to palliative care.
Conclusions and Relevance
Provision of 1-year mortality estimates during heart failure hospitalization did not affect hospitalization or mortality, nor did it affect clinical decision-making.
Trial Registration
ClinicalTrials.gov Identifier NCT03845660
Introduction
Heart failure is a complex syndrome with substantial variability in the risk profile of individual patients.1 Clinical decision-making can be difficult, and more accurate prognostication has the potential to enhance targeted use of interventions that improve patient symptoms and reduce rates of adverse outcomes.2 Several risk-prediction tools have been derived from cohort studies or randomized trials, but their clinical effect has never been tested in a randomized fashion.3,4,5,6,7,8 Therefore, contemporary societal guidelines do not anchor recommendations for clinical management of heart failure in qualification of individual patient risk, leaving the onus for response to any risk assessment on the individual clinician.9,10,11
The widespread use of the electronic health record (EHR) has enabled embedded algorithms that use patient-level data fields to calculate risk scores and communicate these to clinicians during appropriate times in their workflow.12 This process can bypass many of the traditional barriers to integrating risk profiling of heart failure into usual care. We implemented this across an integrated health care system in a randomized fashion to evaluate the independent clinical effect of this information at the bedside of patients with heart failure. The Risk Evaluation and its Impact on Clinical Decision-Making and Outcomes in Heart Failure (REVEAL-HF) trial was an embedded pragmatic, multicenter, randomized clinical trial that informed clinicians about the 1-year predicted mortality of their hospitalized patients with heart failure using an internally validated algorithm from the EHR. We hypothesized that this information would translate into improved decisions about initiation and intensity of treatment, more appropriate end-of-life care, and a subsequent reduction in rates of hospitalization or death.
Methods
Trial Oversight and Study Population
The trial protocol was approved by the institutional review board at Yale School of Medicine and is accessible online (Supplement 1).13 A description of the study design was previously published.14 An independent data and safety monitoring board regularly monitored enrollment and safety data. Participants were automatically recruited when electronically identified from 4 teaching hospitals within the integrated Yale New Haven Health System with a shared EHR (Epic Systems). Asian, Black, White, and other race and Hispanic, non-Hispanic, and other ethnicity were identified via the EHR given prior information on differences in prognosis by race and ethnicity. The inclusion criteria were all adults 18 years or older who had an N-terminal pro–brain natriuretic peptide (NT-proBNP) level of 500 pg/mL or more (to convert to nanograms per liter, multiply by 1) and received intravenous loop diuretics within 24 hours of admission (eFigure 1 in Supplement 2). Individuals who had opted out of EHR research participation (<1% of the hospitalized population) were excluded, as were those receiving hospice care or under observation status. The requirement for informed consent was waived, as the alert was deemed unlikely to affect patient welfare, and informing patients of their prognosis would contaminate the usual-care group.
Trial Procedures
Once enrolled, patients were randomly assigned to either the alert (intervention) group or the usual-care group using an internal random-number rule. Randomization was performed at the patient rather than the clinician level, as inpatients at participating hospitals are often cared for by many clinicians who change throughout their hospital stay. In the intervention group, an alert displaying the predicted 1-year mortality rate, as well as other relevant information, was displayed to clinicians when they opened the order-entry portion of the medical record (Figure 1). Timing of the alert was chosen to maximize a potential effect on downstream clinician ordering decisions. The risk score was created based on retrospective data from patients from our health system to maximize validity, as previously described, and the variables included were as follows: age, weight, systolic blood pressure, red cell distribution width, blood urea nitrogen level, monocyte count, lymphocyte percentage, blood urea nitrogen/creatinine ratio, troponin level, NT-proBNP level, mean corpuscular volume, intensive care unit admission, and measurement of arterial pH level. The model achieved an area under the curve of 0.74 in internal validation before rollout in the prospective study (eMethods in Supplement 2).
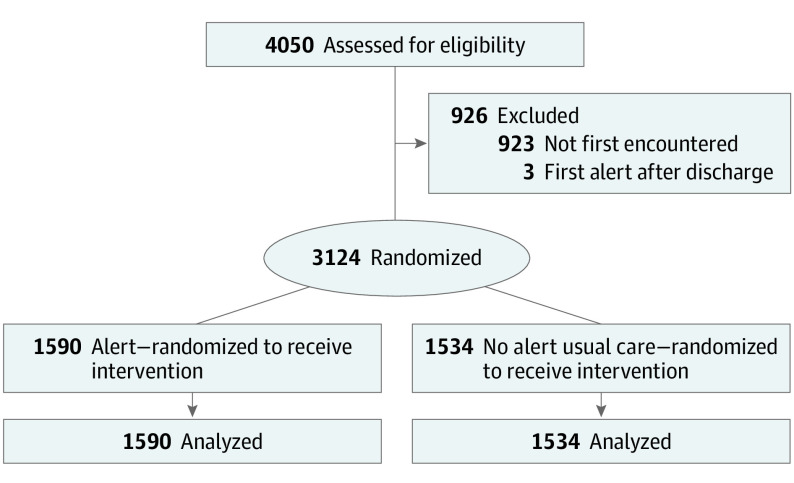
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
CONSORT diagram for the Risk Evaluation and its Impact on Clinical Decision-making and Outcomes in Heart Failure (REVEAL-HF) trial.
The alert was developed using recommended methodologies for clinical decision support: the correct information, person, format, channel, and workflow.15 Before initiation of the clinical trial, we conducted focus groups with clinicians who care for patients with heart failure to get feedback on the EHR alert design, user friendliness, and hindrance to workflow. We also sought input from experts in behavioral economics from the Yale School of Management. The alert was subsequently modified to reflect the suggestions made by the focus groups. Specifically, we created risk categories that corresponded with calculated risk of 1-year mortality: very low (<5%), low (5%-15%), medium (15%-30%), high (30%-50%), and very high (>50%) and color coded these to represent level of risk. Finally, we allowed clinicians to provide input of their personal assessment of the risk assessment by allowing them to select from 1 of the following options within the alert: (1) risk assessment seems appropriate, (2) risk assessment seems too high, (3) risk assessment seems too low, and (4) not sure. There were no mortality estimates available for patients elsewhere in the EHR.
Outcome Measures
The primary outcome was composite of 30-day hospital readmission and 1-year mortality. Secondary outcomes included length of hospital stay, discharge doses of heart failure therapies in patients who qualified, palliative care referrals during index hospitalization, placement of an implantable cardioverter-defibrillator, and cardiac transplant or implantation of a left ventricular–assist device. We also evaluated the relationship between the clinician assessment of the risk prediction and the primary outcome.
Statistical Analysis
Based on prior research, we estimated that the composite outcome of 30-day hospitalization and 1-year mortality would occur in approximately 30% of hospitalized patients with heart failure. A reduction in this proportion to 25% in the alert group would be considered clinically meaningful. To that end, a sample size of 1562 in each group would achieve a 90% power to detect a difference this large at a 2-sided α level of .05 as calculated using the Cochran-Mantel-Haenszel test, yielding a total population of 3124 patients needed to test our primary hypothesis. The primary analysis used the intention-to-treat principle. The proportion of patients experiencing the primary outcome in the alert and usual-care groups was compared using the Cochran-Mantel-Haenszel test, accounting for stratification by study hospital. Statistical significance was based on a P value of < .05. Data analyses were conducted using Stata software, version 15 (StataCorp) and R, version 3.5.1 (R Foundation for Statistical Computing).
Results
Patient Characteristics
The trial enrolled 3124 patients from November 27, 2019, through March 7, 2021, across 4 hospitals within the integrated Yale New Haven Health System. Patients were randomly assigned to the alert (1590 [50.9%]) or usual-care (1534 [49.1%]) group. Baseline characteristics for the study groups were well balanced and similar in all respects (Table). The alert group had a median (IQR) age of 76.5 (65-86) years; 796 were female patients (50.1%), and 794 were male patients (49.9%). Participants from the following race and ethnicity groups were included in the alert group: 13 Asian (0.8%), 324 Black (20.4%), 136 Hispanic (8.6%), 1448 non-Hispanic (91.1%), 1126 White (70.8%), 6 other ethnicity (0.4%), and 127 other race (8.0%). The usual-care group had a median (IQR) age of 77 (65-86) years; 788 were female patients (51.4%), and 746 were male patients (48.6%). Participants from the following race and ethnicity groups were included in the usual-care group: 22 Asian (1.4%), 298 Black (19.4%), 162 Hispanic (10.6%), 1359 non-Hispanic (88.6%), 1077 White (70.2%), 13 other ethnicity (0.9%), and 137 other race (8.9%). A total of 367 patients (23.1%) and 359 patients (23.4%) had a left ventricular ejection fraction of 40% or less in the alert and usual-care groups, respectively. Median (IQR) NT-proBNP levels were 3826 (1692-8241) pg/mL in the alert group and 3867 (1663-8917) pg/mL in the usual-care group. In the alert and usual-care groups, the most common comorbid conditions were atrial fibrillation (773 [48.6%] vs 778 [50.7%]), diabetes (694 [43.6%] vs 670 [43.7%]), chronic kidney disease (659 [41.4%] vs 623 [40.6%]), chronic obstructive pulmonary disease (478 [30.1%] vs 475 [31.0%]), and depression (375 [23.6%] vs 374 [24.4%]).
Table. Baseline Characteristics of Study Population.
| Characteristic | No. (%) | |
|---|---|---|
| Alert group (n = 1590) | Usual care (n = 1534) | |
| Age, median (IQR), y | 76.5 (65-86) | 77 (65-86) |
| Sex | ||
| Male | 794 (49.9) | 746 (48.6) |
| Female | 796 (50.1) | 788 (51.4) |
| Race and ethnicity | ||
| Asian | 13 (0.8) | 11 (1.4) |
| Black | 324 (20.4) | 298 (19.4) |
| Hispanic | 136 (8.6) | 162 (10.6) |
| Non-Hispanic | 1448 (91.1) | 1359 (88.6) |
| White | 1126 (70.8) | 1077 (70.2) |
| Other ethnicity | 6 (0.4) | 13 (0.9) |
| Other race | 127 (8.0) | 137 (8.9) |
| LVEF, median (IQR), % | 55 (37.5-62.5) | 55 (37.5-62.5) |
| LVEF ≤40% | 367 (23.1) | 359 (23.4) |
| NT-proBNP, median (IQR), pg/mL | 3826 (1692-8241) | 3867 (1663-8917) |
| Creatinine, median (IQR), mg/dL | 1.3 (0.9-2.0) | 1.3 (0.9-1.9) |
| Serum sodium, median (IQR), mmol/L | 139 (136-141) | 139 (136-141) |
| COVID+ | 199 (12.5) | 189 (12.3) |
| Medical history and admission location | ||
| Atrial fibrillation | 773 (48.6) | 778 (50.7) |
| Chronic kidney disease | 659 (41.4) | 623 (40.6) |
| Chronic obstructive pulmonary disease | 478 (30.1) | 475 (31.0) |
| Type 2 diabetes | 694 (43.6) | 670 (43.7) |
| Depression | 375 (23.6) | 374 (24.4) |
| ICU admission | 284 (17.9) | 270 (17.6) |
| Medications | ||
| ACE-I/ARB/ARNI | 766 (48.2) | 738 (48.1) |
| β-Blocker | 1307 (82.2) | 1264 (82.4) |
| Aldosterone blocker | 376 (23.6) | 385 (25.1) |
| SGLT2i | 138 (8.7) | 134 (8.7) |
| Loop diuretic | 1588 (99.9) | 1532 (99.9) |
| ICD/CRT | 235 (14.8) | 235 (15.3) |
| Risk categories at time of randomization | ||
| Very low risk (<5%) | 425 (26.7) | 360 (23.5) |
| Low risk (5%-15%) | 752 (47.3) | 770 (50.2) |
| Medium risk (15%-30%) | 307 (19.3) | 319 (20.8) |
| High risk (30%-50%) | 96 (6.0) | 77 (5.0) |
| Very high risk (>50%) | 10 (0.6) | 8 (0.5) |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CRT, cardiac resynchronization therapy; ICD; implantable cardioverter-defibrillator; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–brain natriuretic peptide; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
At enrollment, 82.2% of patients (2571 of 3124) were receiving β-blockers; 48.1% (1504 of 3124) were receiving an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or an angiotensin receptor neprilysin inhibitor; and 24.4% (761 of 3124) were receiving a mineralocorticoid receptor antagonist. A total of 272 of 3124 patients (8.7%) were receiving a sodium-glucose cotransporter 2 inhibitor, and 554 of 3124 patients (17.7%) were admitted to an intensive care unit.
Performance of the Risk Model
Predicted risk categories at the time of admission were equivalent between study groups. Approximately one-half of the patients (1522 of 3124 [48.7%]) were predicted to have a 1-year mortality between 5% and 15%; approximately 20.0% (626 of 3124) were in the medium risk group (15%-30%), and 25.1% (785 of 3124) were in the very low risk group (<5%). The model achieved a mean (SD) area under the curve of 0.74 (0.02) in this prospective trial, which was similar to its discrimination performance in the derivation and validation data set (eMethods in Supplement 2). During the study period, which occurred largely during the COVID-19 pandemic, we observed persistently higher rates of mortality than predicted by the algorithm, resulting in worse calibration compared with the derivation data set.
Treatment Decisions and Clinical Outcomes
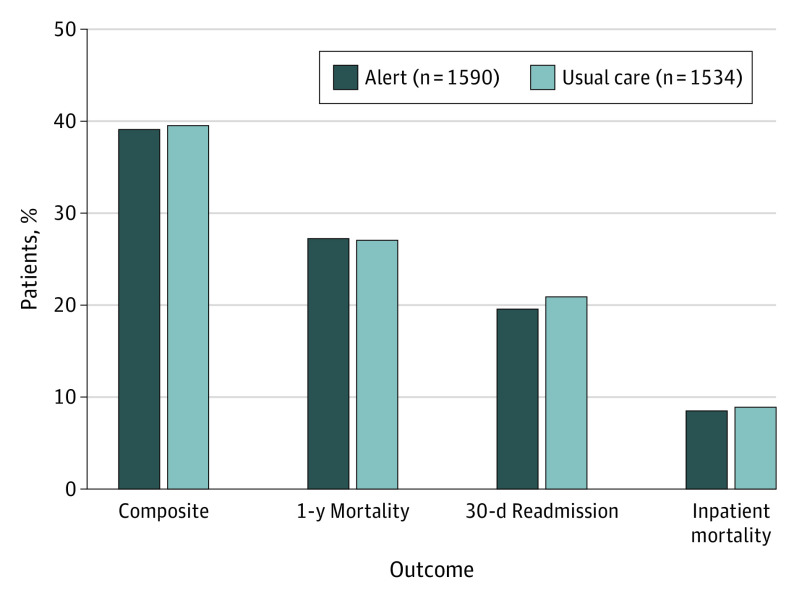
Patients were followed up for a median (IQR) time of 384 (286-473) days. The median (IQR) number of alerts per patient was (IQR) 9 (5-16) alerts. The median (IQR) number of alerts per clinician was 5 (2-12) alerts. There were no significant differences between the 2 groups in terms of the primary end point that occurred in 619 patients (38.9%) in the alert group compared with 603 patients (39.3%) in the usual-care group (Figure 2). There was no statistically significant evidence of differences between components of the primary end point, with an observed 1-year mortality of 27.1% in the alert group and 26.1% in the usual-care group (P = .89); rates of 30-day readmission were 19.4% and 20.7%, respectively (P = .39). Finally, there were no differences in length of hospital stay after randomization between study groups (alert vs usual care: median [IQR], 4.4 [2.2-9.4] days vs 4.3 [2.1-9.0] days; P = .28). There was no evidence of heterogeneity in effect of the alert according to site (P = .25) (eTable 1 in Supplement 2).
Figure 2. Primary Outcome and Components According to Study Group.
There were no significant differences between the 2 groups in terms of the primary end point that occurred in 619 patients (38.9%) in the alert group and 603 patients (39.3%) in the usual care group.
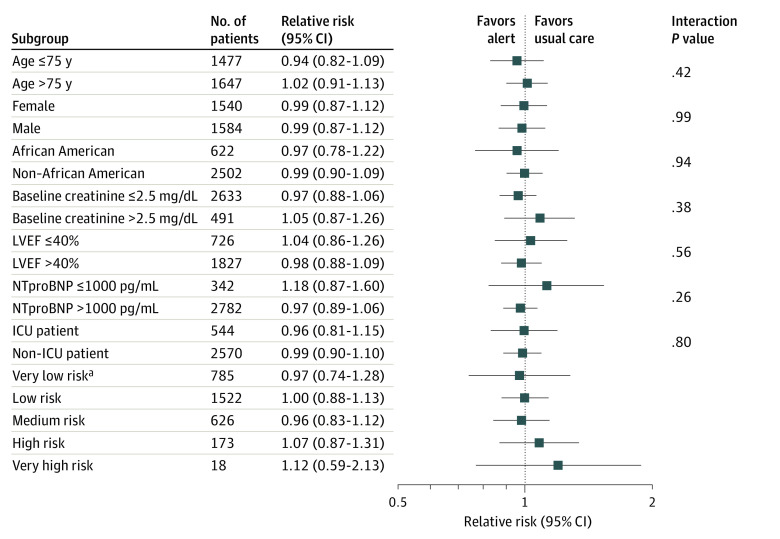
Prespecified subgroup analyses (Figure 3) showed that none of the baseline characteristics of the patients, including age (≤75 years vs >75 years), sex, race, baseline creatinine level (≤2.5 mg/dL vs >2.5 mg/dL; to convert to micromoles per liter, multiply by 88.4), left ventricular ejection fraction (≤40% vs >40%), NT-proBNP level (≤1000 pg/mL vs >1000 pg/mL), admission to the intensive care unit, or predicted risk category identified characteristics in which knowledge of risk might lead to improved outcomes.
Figure 3. Primary Outcome According to Prespecified Subgroups.
Prespecified subgroup analyses showed that none of the baseline characteristics of the patients including age (≤75 y vs >75 y), sex, race, baseline creatinine level (≤2.5 mg/dL vs >2.5 mg/dL), left ventricular ejection fraction (LVEF; ≤40% vs >40%), N-terminal pro–brain natriuretic peptide level (NT-proBNP; ≤1000 pg/mL vs >1000 pg/mL), admission to the intensive care unit (ICU), or predicted risk category identified characteristics in which knowledge of risk might lead to improved outcomes.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4; to convert NT-proBNP to nanograms per liter, multiply by 1.
aComparisons of relative risk (95% CI) between risk groups: very low risk (VLR) vs LR, 0.98; 95% CI, 0.74-1.28 vs 1.00; 95% CI, 0.88-1.13; P = .94; VLR vs medium risk (MR), 0.98; 95% CI, 0.74-1.28 vs 0.96; 95% CI, 0.83-1.12; P = .93; VLR vs high risk (HR), 0.98; 95% CI, 0.74-1.28 vs 95% CI, 1.07; 95% 0.87-1.31; P = .53; VLR vs VHR, 0.98; 95% CI, 0.74-1.28 vs 1.12; 95% CI, 0.59-2.13; P = .48.
There was no evidence that information about risk affected key treatment decisions in patients with heart failure (eTable 2 in Supplement 2; Figure 4). Specifically, we found no evidence of differences in discharge prescription of heart failure medical therapies. Patients with higher predicted risk tended to be less likely to be receiving these therapies, but this was equally balanced between study groups. Rates of new placement of an implantable cardioverter-defibrillator, implantation of left ventricular–assist device, and cardiac transplant were low during the study period and did not differ between groups. The percentage of patients referred to palliative care increased according to risk but also did not differ between groups.
Figure 4. Clinical Decision-making in Regard to Medical Therapy and Palliative Care Referral According to Alert Group.
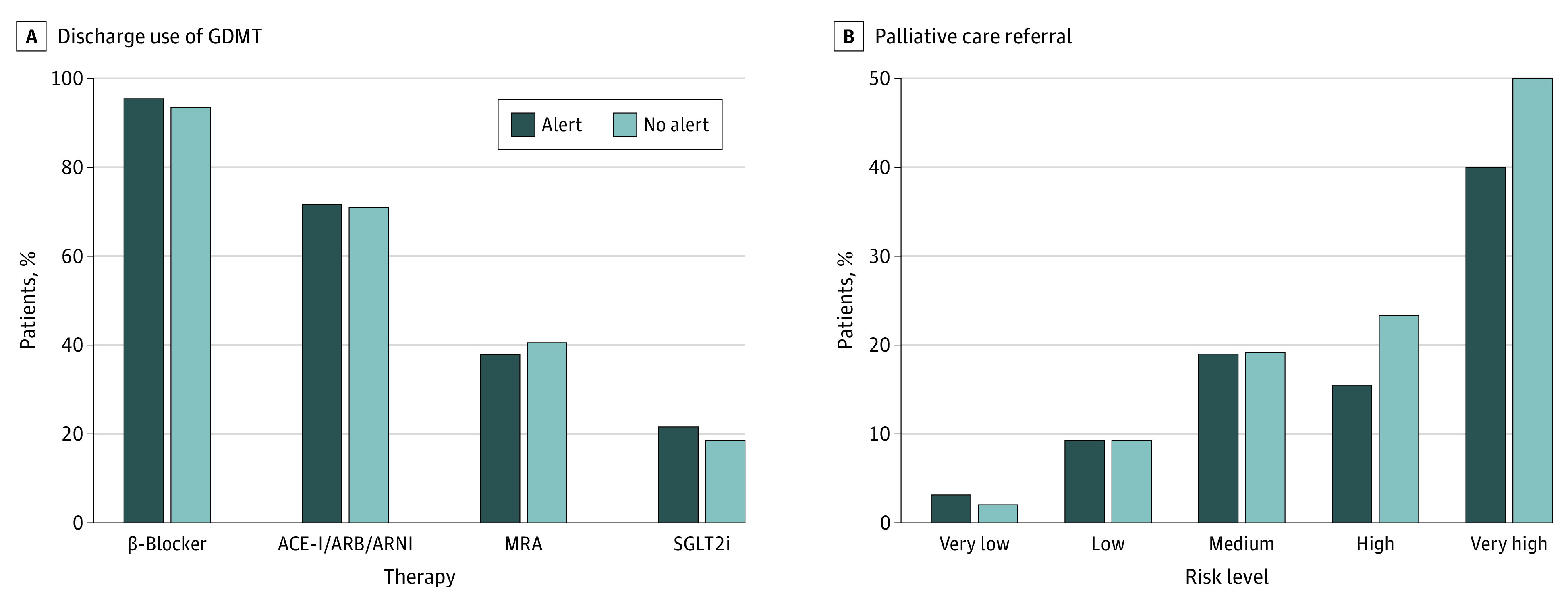
Discharge use of guideline-directed medical therapy (GDMT) in patients with left ventricular ejection fraction (LVEF) of 40% or less (A) and palliative care referral (B). There was no evidence of differences in discharge prescription of heart failure medical therapies in patients with LVEF of 40% or less or referral to palliative care. ACE-I indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; MRA, magnetic resonance angiography; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Clinician Interaction With Risk Prediction
Of the possible acknowledgments for the alert, a majority of clinicians (11 286 of 17 752 [63.6%]) clicked on not sure, 5207 of 17 752 [29.3%] clicked on risk assessment seems appropriate, and a minority 938 of 17 752 [5.3%] clicked on risk assessment seems too low or 299 of 17 752 [1.7%] clicked on risk assessment seems too high. Observed mortality rates according to predicted risk categories and clinician input are shown in eFigure 2 in Supplement 2. In patients with high predicted risk of 1-year mortality, there was a significant association between clinician opinion of estimated risk and observed mortality rate (P = .05), with a trend among those with very high predicted risk (P = .07) (eTable 3 in Supplement 2).
Discussion
In this embedded and pragmatic randomized clinical trial of hospitalized patients with heart failure, we found no evidence that risk estimates of 1-year mortality provided to clinicians during standard workflow led to reductions in rates of rehospitalization or mortality. There was no indication that this information affected the use of medical or surgical therapy, or even referral to palliative care. Bedside clinicians may require more prescriptive decision support, as these results call into question the hypothesis that prognostic information alone prompts changes in clinical decision-making that improves outcomes in patients with heart failure.
Implicit in the proliferation of risk scores to predict adverse events in patients with heart failure is the assumption that their use would allow clinicians to tailor interventions that benefit patients; our findings suggest a limited utility of an information-alone approach to heart failure prognostication.6,16 There are a number of potential reasons for these findings. First, the lack of specific recommendations tethered to risk assessment may have limited their clinical utility. However, societal guidelines currently do not have risk-based recommendations for medical and device therapy in patients with heart failure.9,10,11,17,18 Furthermore, we recently demonstrated in the Pragmatic Trial of Messaging to Providers About Treatment of Heart Failure (PROMPT-HF) that giving clinicians actionable information about patients with heart failure does lead to improvements in care.19 Not only do these results raise fundamental questions about the role of risk prediction in heart failure but also question our ongoing efforts to improve phenotyping and personalized care in heart failure against a backdrop that does not make well-defined distinctions in medical or device interventions across patients with widely different risk profiles.20 This is in contrast to other areas of cardiovascular medicine, such as primary prevention or acute coronary syndromes, as well as other areas of medicine (oncology, in particular), where risk assessments are directly related to clinical recommendations.21,22
Another explanation for our findings might be that the risk estimates did not add substantially to clinician intuition, thus mitigating any independent benefit from this information. Indeed, there was evidence that clinician judgment improved calibration among patients in the highest risk categories. Although there is a dearth of definitive studies on the topic of the accuracy of clinician risk prediction in heart failure, prior work negates this justification by consistently demonstrating worse performance by clinicians when compared with validated algorithms.23 A more likely explanation might be the concept of algorithm aversion, where forecasters—in this case, clinicians—tend to prefer prediction on the basis of intuition vs statistical algorithms, even if the latter are objectively superior.24,25 The high rates of adverse outcomes seen in our cohort along with wide-ranging underutilization of heart failure therapies and palliative care may suggest that accurate assessments of risk are not routinely taken into account during clinical decision-making; whether these were considered and ignored, or were inaccurate, will need further study.
Limitations
Our study had several limitations. First, REVEAL-HF was based out of a single health care system. However, we included a mix of small community hospitals and large tertiary care centers whose patient demographics aligned closely with those of hospitalized patients with heart failure across the US.26 In fact, our study had higher representation of Asian, Black, and Hispanic patients, women, and older adult patients than previously published acute heart failure trials. Our entirely pragmatic design also allowed for study enrollment based on objective criteria rather than curation by study personnel, increasing the generalizability of our patient population. Second, the study risk score was developed from a historic cohort of patients with heart failure seen at Yale (January 1, 2014, and April 14, 2018), using the inclusion and exclusion criteria of the trial, in order to maximize internal validity and most appropriately address the study hypothesis. Within this framework, our model’s performance in the trial population was equivalent in discrimination when compared with the derivation or validation data set, but its calibration was inferior. We hypothesize that this may be related to changes in cohort characteristics during the COVID-19 pandemic and an associated excess burden of heart failure deaths. Third, the results of the study may have simply demonstrated the phenomenon of alert fatigue, a tendency to disregard alerts owing to their abundance, even if they provide important information.27 Finally, clinical decision-making in a complex syndrome like heart failure is multifaceted, and prognostic information is but one of the many tools used to care for patients; it is entirely possible that this information was only helpful in subset of patients which our study was not sufficiently powered to detect.
Conclusions
In conclusion, the REVEAL-HF randomized clinical trial was a pragmatic study in a high-risk and representative population of hospitalized patients with heart failure that examined, for the first time (to our knowledge), whether knowledge about prognosis at 1 year affects clinical decision-making and patient outcomes. We found no evidence in support of this hypothesis.
Trial Protocol
eMethods. Creation and Performance of Mortality Risk Score
eTable 1. Composite Outcome by Hospital
eTable 2. Treatment Decisions According to Alert and Risk Levels
eTable 3. Clinical Opinion of Estimated Risk and Observed Mortality
eFigure 1. The REVEAL-HF Clinical Trial
eFigure 2. Interaction Between Predicted Risk Categories, Observed Rates of Mortality, and Clinician Assessment of Risk
Data Sharing Statement
References
- 1.Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 2.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2(5):440-446. doi: 10.1016/j.jchf.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators . Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. doi: 10.1001/jama.293.5.572 [DOI] [PubMed] [Google Scholar]
- 4.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581-2587. doi: 10.1001/jama.290.19.2581 [DOI] [PubMed] [Google Scholar]
- 5.Abraham WT, Fonarow GC, Albert NM, et al. ; OPTIMIZE-HF Investigators and Coordinators . Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008;52(5):347-356. doi: 10.1016/j.jacc.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 6.Peterson PN, Rumsfeld JS, Liang L, et al. ; American Heart Association Get With the Guidelines-Heart Failure Program . A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25-32. doi: 10.1161/CIRCOUTCOMES.109.854877 [DOI] [PubMed] [Google Scholar]
- 7.Khazanie P, Heizer GM, Hasselblad V, et al. Predictors of clinical outcomes in acute decompensated heart failure: Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure outcome models. Am Heart J. 2015;170(2):290-297. doi: 10.1016/j.ahj.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 8.Ahmad T, O’Brien EC, Schulte PJ, et al. Evaluation of the incremental prognostic utility of increasingly complex testing in chronic heart failure. Circ Heart Fail. 2015;8(4):709-716. doi: 10.1161/CIRCHEARTFAILURE.114.001996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonagh TA, Metra M, Adamo M, et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(48):4901. doi: 10.1093/eurheartj/ehab670 [DOI] [PubMed] [Google Scholar]
- 10.Maddox TM, Januzzi JL Jr, Allen LA, et al. ; Writing Committee . 2021 update to the 2017 ACC Expert Consensus Decision Pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772-810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e876-e894. doi: 10.1161/CIR.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 12.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385(9981):1966-1974. doi: 10.1016/S0140-6736(15)60266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risk Evaluation and Its Impact on Clinical Decision-making and Outcomes in Heart Failure (REVEAL-HF). ClinicalTrials.gov Identifier: NCT03845660. Updated April 22, 2022. Accessed Month Day, Year. https://clinicaltrials.gov/ct2/show/NCT03845660
- 14.Ahmad T, Yamamoto Y, Biswas A, et al. REVeAL-HF: design and rationale of a pragmatic randomized controlled trial embedded within routine clinical practice. JACC Heart Fail. 2021;9(6):409-419. doi: 10.1016/j.jchf.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 15.Tang PC, Young CY. ActiveGuidelines: integrating web-based guidelines with computer-based patient records. Proc AMIA Symp. 2000;843-847. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DS, Lee JS, Schull MJ, et al. Prospective validation of the emergency heart failure mortality risk grade for acute heart failure. Circulation. 2019;139(9):1146-1156. doi: 10.1161/CIRCULATIONAHA.118.035509 [DOI] [PubMed] [Google Scholar]
- 17.Ahmad T, Lund LH, Rao P, et al. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc. 2018;7(8):7. doi: 10.1161/JAHA.117.008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene SJ, Butler J, Spertus JA, et al. Comparison of New York Heart Association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(5):522-531. doi: 10.1001/jamacardio.2021.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol. 2022;79(22):2203-2213. doi: 10.1016/j.jacc.2022.03.338 [DOI] [PubMed] [Google Scholar]
- 20.Bayes-Genis A, Liu PP, Lanfear DE, et al. Omics phenotyping in heart failure: the next frontier. Eur Heart J. 2020;41(36):3477-3484. doi: 10.1093/eurheartj/ehaa270 [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139(25):e1162-e1177. doi: 10.1161/CIR.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 22.Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122(9):1302-1315. doi: 10.1161/CIRCRESAHA.117.310782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamokoski LM, Hasselblad V, Moser DK, et al. Prediction of rehospitalization and death in severe heart failure by physicians and nurses of the ESCAPE trial. J Card Fail. 2007;13(1):8-13. doi: 10.1016/j.cardfail.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 24.Dietvorst BJ, Simmons JP, Massey C. Algorithm aversion: people erroneously avoid algorithms after seeing them err. J Exp Psychol Gen. 2015;144(1):114-126. doi: 10.1037/xge0000033 [DOI] [PubMed] [Google Scholar]
- 25.Cervellin G, Borghi L, Lippi G. Do clinicians decide relying primarily on bayesians principles or on Gestalt perception? some pearls and pitfalls of Gestalt perception in medicine. Intern Emerg Med. 2014;9(5):513-519. doi: 10.1007/s11739-014-1049-8 [DOI] [PubMed] [Google Scholar]
- 26.Ghazi L, Desai NR, Simonov M, et al. Rationale and design of a cluster-randomized pragmatic trial aimed at improving use of guideline directed medical therapy in outpatients with heart failure: Pragmatic Trial of Messaging to Providers About Treatment of Heart Failure (PROMPT-HF). Am Heart J. 2022;244:107-115. doi: 10.1016/j.ahj.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 27.Payne TH. EHR-related alert fatigue: minimal progress to date, but much more can be done. BMJ Qual Saf. 2019;28(1):1-2. doi: 10.1136/bmjqs-2017-007737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Creation and Performance of Mortality Risk Score
eTable 1. Composite Outcome by Hospital
eTable 2. Treatment Decisions According to Alert and Risk Levels
eTable 3. Clinical Opinion of Estimated Risk and Observed Mortality
eFigure 1. The REVEAL-HF Clinical Trial
eFigure 2. Interaction Between Predicted Risk Categories, Observed Rates of Mortality, and Clinician Assessment of Risk
Data Sharing Statement