Abstract
The safety of ultraviolet B (UVB) phototherapy with respect to cutaneous carcinogenesis has not been established for patients with chronic kidney disease. To investigate this issue, a nationwide cohort study of 10,805 patients with advanced chronic kidney disease was conducted using data from the National Health Insurance of Taiwan, the Taiwan Cancer Registry, and the national death registry. After a median follow-up of 75 months, 16 of 2,161 patients in the UVB group and 63 of 8,644 patients in the non-UVB group developed skin cancers. Compared with the non-UVB group, patients in the UVB group did not show an increased risk of skin cancer (hazard ratio 1.066; 95% confidence interval 0.584–1.944), non-melanoma skin cancer (hazard ratio 1.067; 95% confidence interval 0.571–1.996), or cutaneous melanoma (hazard ratio 1.009; 95% confidence interval 0.115–8.879). In addition, patients who received more UVB phototherapy did not show an increased risk of skin cancer. UVB phototherapy appears to be a safe treatment for uraemic pruritus in patients with chronic kidney disease.
Key words: UVB phototherapy, skin cancer, uraemic pruritus, chronic kidney disease
Patients with chronic kidney disease (CKD) have a high prevalence rate of pruritus, which has major influences on mortality and quality of life (1–3). Ultraviolet (UV) phototherapy is one of the principal treatments for uraemic pruritus (1). The therapeutic effects of UV phototherapy result from complex immunomodulation mechanisms, which involve apoptosis of lymphocytes and dendritic cells within the skin, as well as alterations in cytokine signalling (4–7).
UV radiation has been reported to be cutaneous carcinogenic (8), and observational studies of patients with psoriasis showed that psoralen and ultraviolet A (PUVA) phototherapy is associated with an increased risk of skin cancer (9–11). Studies on ultraviolet B (UVB) photo-therapy have not found an increased risk of skin cancer in patients with psoriasis, regardless of UVB type or skin phototype (11–15). However, the estimated number of patients needed to determine the carcinogenic risk associated with UVB phototherapy is in the thousands, with a follow-up of 10 years or more (16).
SIGNIFICANCE
Ultraviolet B phototherapy is widely used to relieve uraemic pruritus, but its long-term safety regarding cutaneous carcinogenic risk has not been established for patients with chronic kidney disease. In this nationwide cohort study of 10,805 patients with advanced chronic kidney disease and a median follow-up of 75 months, ultraviolet B phototherapy did not increase the risk of skin cancer, non-melanoma skin cancer, or cutaneous melanoma. Ultraviolet B phototherapy appears to be a safe treatment for uraemic pruritus in patients with advanced chronic kidney disease.
Patients with CKD or on long-term dialysis are subject to a higher incidence of skin cancer (17, 18), hence the risk of photocarcinogenesis should be evaluated carefully. However, evidence regarding the risk of skin cancer in patients with CKD treated with UVB phototherapy is scarce. The aim of this study was to assess the cutaneous carcinogenic risk of UVB phototherapy in a nationwide cohort of patients with CKD.
MATERIALS AND METHODS
Data sources
A cohort study was conducted using data from the National Health Insurance (NHI) of Taiwan, the Taiwan Cancer Registry (TCR), and the national death registry. These are de-identified data for the entire population of Taiwan and can only be analysed in the data centres run by the Health and Welfare Data Science Center, Ministry of Health and Welfare. The NHI system provides single-payer compulsory health insurance covering > 99% of Taiwan’s population since 1995 (19, 20). The NHI database comprises information including demographic data, outpatient records, inpatient records, and registries for patients with catastrophic illness (co-payments are waived for patients receiving medical treatments related to the registered diseases), as described in our previous studies (20, 21). Registration as a catastrophic-illness patient must meet strict criteria with supporting medical records, and the application should be submitted by a relevant specialist and must be verified by other senior specialists. Therefore, the diagnosis of the registered disease has been ascertained accurately. The NHI system applied the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) for disease coding and replaced the ICD-9-CM with the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) in 2016. Hospitals with ≥ 50 beds are obliged to report all newly diagnosed cancers to the TCR central office since 1979, using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) for cancer coding (22, 23). The death registry data provide the date and the cause of death. The Institutional Review Board of the Far Eastern Memorial Hospital approved this study and waived the requirement for informed consent because the database provided only de-identified information.
Study participants
Taiwan’s NHI reimburses patients with CKD for UVB phototherapy (treatment code: 51019B) for uraemic pruritus.
Inclusion criteria for this study were: patients with advanced CKD who received their first UVB phototherapy for uraemic pruritus from 2001 to 2005. A patient who began UVB phototherapy with any of the following criteria was defined as having advanced CKD: (i) required long-term dialysis therapy when receiving the first UVB phototherapy; or (ii) had initiated long-term dialysis therapy within one year after the first UVB phototherapy.
The earliest calendar year available for the NHI claims data is 1998; therefore patients who received the first UVB phototherapy earlier than 2001 were excluded, to ensure a washout period of 3 years. Other exclusion criteria were: patients younger than 20 years or who had a diagnosis of skin cancer at baseline; patients who had received PUVA phototherapy or who did not receive a prescription for phototherapy from a dermatologist.
Patients with advanced CKD who did not receive UVB phototherapy were randomly selected and matched by age, sex, and calendar year of dialysis initiation with those who received UVB phototherapy at a ratio of 1:4. To ensure that the patients were prescribed UVB phototherapy for uraemic pruritus, patients with a diagnosis of vitiligo, psoriasis, parapsoriasis, or cutaneous lymphoma (ICD-9-CM code: 374.53, 709.01, 696.0–696.2, 202; ICD-10-CM code: L80, H02.73, L40–L41, L94.5, C84.A, C82.6) were also excluded. Individuals requiring long-term dialysis therapy were registered as patients with catastrophic illness, with a specific diagnosis code (ICD-9-CM code: 585; ICD-10-CM code: N18.6) (20, 21). The date on which long-term dialysis therapy initiated was defined as the registration date for catastrophic illness status.
The participants who received UVB phototherapy were categorized as the UVB group and the matched participants who did not receive UVB phototherapy were categorized as the non-UVB group. The index date of a patient in the UVB group was defined as the date when the patient received the first UVB phototherapy. The time period was calculated from the date of first UVB phototherapy to the date of initiation of dialysis for each patient in the UVB group, and the index date of their matched patients in the non-UVB group was defined as the date with the same time period to the date of initiation of dialysis.
Exposure assessment
Patient data were obtained, including age, sex, occupation, and location of residence at the index date. A patient was defined as having an outdoor occupation if the registered occupation in the NHI was farmer, fisherman, or seaman (24). The total number of UVB phototherapy treatments received by each patient during the observation period was recorded. The effects of UVB phototherapy were evaluated by means of: (i) the binary variable: treatment with UVB phototherapy (UVB group vs non-UVB group); and (ii) the continuous variable: the total number of UVB phototherapy treatments received by each patient. Organ transplant recipients would be registered as patients with catastrophic illness. Patients who had received organ transplantation were identified using the registries of patients with catastrophic illness by specific diagnosis codes (Table SI1) because organ transplant recipients have a higher risk of skin cancer (25, 26). Patients receiving prescriptions of hydrochlorothiazide were also identified, since hydrochlorothiazide has been reported to be associated with an increased risk of skin cancer due to its photosensitizing properties (27). The daily record of the UV index at each administrative area in 2010 was obtained, which is provided by the Central Weather Bureau and the Environmental Protection Administration in Taiwan (24). The mean UV index at the location of residence was calculated according to the administrative area in which each patient resided (24, 28). To assess the influence of environmental UV exposure, the mean UV index at the location of residence was categorized as high (≥ 6) or low (< 6) (24).
Outcome measures
The outcomes of interest were the incidence of skin cancer, non-melanoma skin cancer (NMSC) and cutaneous melanoma. The incidence of skin cancer included the incidence of NMSC and cutaneous melanoma. A patient was defined as having NMSC by any one of the following criteria: (i) the inpatient or outpatient diagnosis included the code for NMSC (ICD-9-CM code: 173, 232; ICD-10-CM code: C44, C4A, D04) and a procedure code for lesion removal, or (ii) the TCR included a topography code for skin (C44) and a morphology code for histological type other than melanoma. A patient was defined as having cutaneous melanoma by any one of the following criteria: (i) the inpatient or outpatient diagnosis included the code for cutaneous melanoma (ICD-9-CM code: 172; ICD-10-CM code: C43, D03) and a procedure code for lesion removal, or (ii) the TCR included a topography code for skin (C44) and a morphology code of histological type for melanoma. The incidence date of skin cancer was defined as the date of the presence of a diagnosis code for skin cancer in the NHI or the date of diagnosis for skin cancer in the TCR, whichever had occurred earlier. The follow-up period started on the index date and ended on the incidence date of the outcome, the date of death, or 31 December 2017, whichever had occurred earlier.
Statistical analyses
Categorical variables are presented as frequencies with percentages, and continuous variables are presented as the mean values with standard deviations. A standardized difference < 0.1 indicated adequate balance between the UVB and the non-UVB groups (29). Kaplan–Meier cumulative incidence plots were constructed to show the time to event of the outcome, and the log-rank test was used to compare the incidence between the UVB and the non-UVB groups. For each outcome, univariate Cox proportional hazards models were used to estimate the crude hazard ratio (HR) and 95% confidence interval (95% CI) of: (i) the treatment of UVB phototherapy; or (ii) the number of UVB phototherapy treatments. In the multivariate Cox proportional hazards models evaluating UVB phototherapy, the HR and 95% CI were estimated by further adjusting the models with: (i) age and sex or (ii) age, sex, occupation, mean UV index, organ transplantation, and hydrochlorothiazide. The proportional hazards assumption was assessed using the test of weighted Schoenfeld residuals (30). To assess the robustness of the results, sensitivity analyses were performed by limiting the follow-up time of the Cox models to 3, 6, 9, 12, and 15 years. Fine and Gray’s subdistribution hazard model was used to consider the competing risks between death and skin cancer. A 2-sided p-value ≤ 0.05 was considered statistically significant. Statistical analyses were performed with SAS (version 9.4, SAS Institute, Cary, NC, USA) and Stata software (version 14, StataCorp LP, College Station, TX, USA).
RESULTS
Patient selection
The patient-selection process is shown in Fig. 1. The total number of individuals assessed was 26,777,346. Of these, 31,718 patients who received their first phototherapy in the period 2001 to 2005 were identified. After exclusion and matching, a total of 2,161 patients in the UVB group and 8,644 patients in the non-UVB group were enrolled and followed up until December 31, 2017.
Fig. 1.
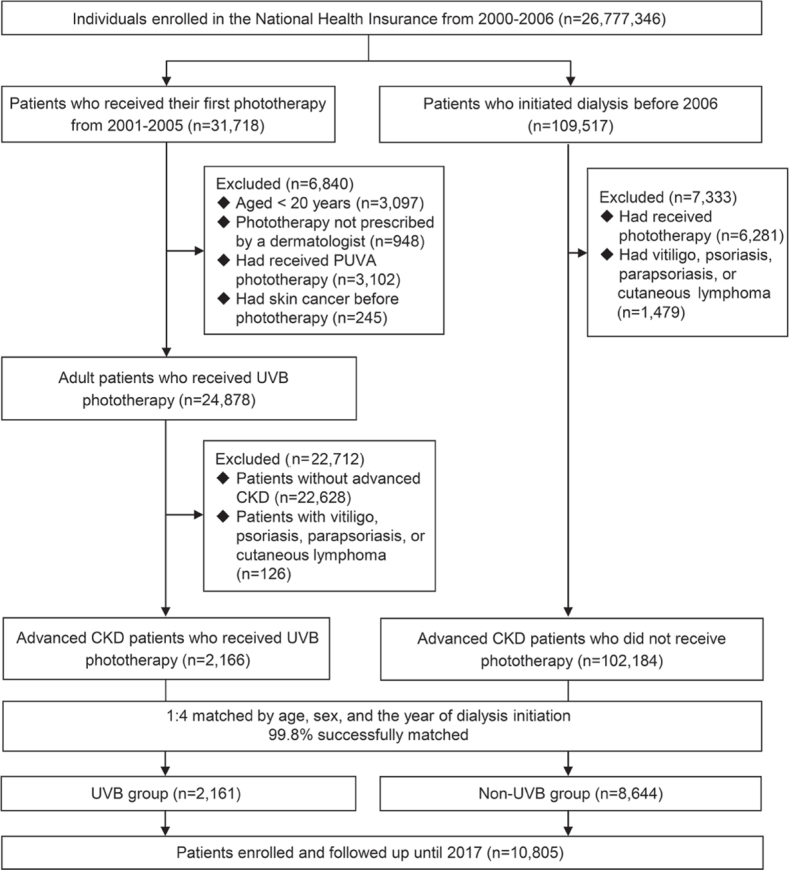
Summary of patient selection. CKD: chronic kidney disease; PUVA: psoralen and ultraviolet A; UVB: ultraviolet B.
Patient characteristics
Table I shows the baseline characteristics of the study participants. The mean age was 61.5 years, and 54% were men. Among the patients, 20% had outdoor occupations, 41.5% lived in locations with a high UV index, 7% received organ transplantation, and 32.9% used hydrochlorothiazide. The baseline characteristics were well balanced between the UVB and the non-UVB groups (all standardized differences <0.1). Patients in the UVB group received UVB phototherapy for a mean ± standard deviation of 7.4 ± 19.4 times; 29.3% received UVB phototherapy at least 6 times; and 8.7% at least 18 times.
Table I.
Baseline characteristics of the study participants
| Characteristics | UVB group | Non-UVB group | Standardized differencea |
|---|---|---|---|
| Patients, n | 2,161 | 8,644 | – |
| Age, years, mean (SD) | 61.5 (12.4) | 61.5 (12.4) | – |
| Male sex, n (%) | 1,168 (54.0) | 4,672 (54.0) | – |
| Year of dialysis initiation, n (%) | |||
| 1995–1998 | 613 (28.4) | 2,452 (28.4) | – |
| 1999–2001 | 711 (32.9) | 2,844 (32.9) | – |
| 2002–2006 | 837 (38.7) | 3,348 (38.7) | – |
| Outdoor occupation, n (%) | 416 (19.3) | 1,745 (20.2) | 0.08 |
| High UV indexb, n (%) | 903 (41.8) | 3,586 (41.5) | 0.02 |
| Organ transplantation, n (%) | 117 (5.4) | 640 (7.4) | 0.08 |
| Immunosuppressant drugs, n (%) | 130 (6.0) | 676 (7.8) | 0.07 |
| Hydrochlorothiazide, n (%) | 757 (35.0) | 2,798 (32.4) | 0.06 |
A standardized difference < 0.1 indicated adequate balance between the groups.
Mean UV index ≤ 6 at location of residence.
SD: standard deviation; UV: ultraviolet; UVB utraviolet B.
Association between UVB phototherapy and skin cancer
During a median follow-up time of 75 months, 16 patients (15 with NSMC and 1 with cutaneous melanoma) in the UVB group (0.74%) and 63 patients (58 with NMSC and 5 with cutaneous melanoma) in the non-UVB group (0.73%) developed skin cancers. The incidence rate of skin cancer was 1.23 cases per 1,000 patient-years for the UVB group and 0.98 cases per 1,000 person-years for the non-UVB group. The Kaplan–Meier cumulative incidence plot (Fig. 2) did not show a significant difference in the risk of skin cancers between the UVB and non-UVB groups (p = 0.45). Tables II and III show the results of Cox proportional hazards models for the influences of UVB phototherapy on each study outcome. In general, the univariate and multivariate Cox proportional hazards models had similar results. Compared with patients who did not receive UVB phototherapy, patients who received UVB phototherapy did not show a higher risk of skin cancer (adjusted HR 1.066; 95% CI 0.584–1.944), NMSC (adjusted HR 1.067; 95% CI 0.571–1.996), or cutaneous melanoma (adjusted HR 1.009; 95% CI 0.115–8.879) (Table II). Patients who received more UVB phototherapy treatments were not associated with an increased risk of skin cancer (adjusted HR 1.001; 95% CI 0.984–1.018), NMSC (adjusted HR 0.998; 95% CI 0.974–1.022), or cutaneous melanoma (adjusted HR 1.010; 95% CI 0.987–1.033) (Table III).
Fig. 2.
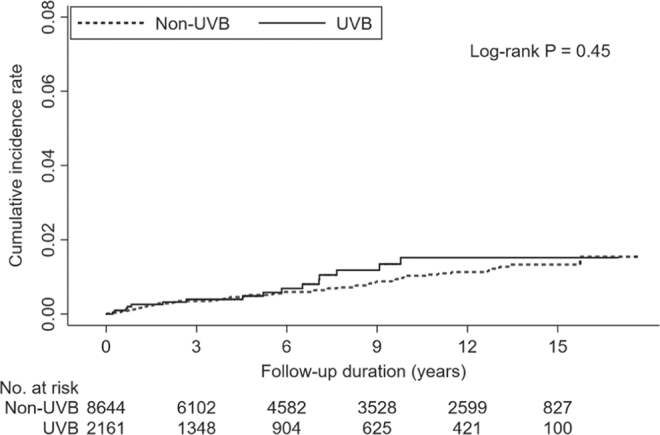
Kaplan–Meier cumulative incidence of time to event of skin cancer in the ultraviolet B (UVB) and non-UVB groups.
Table III.
Cox proportional hazards models for the influences of the number of ultraviolet B (UVB) phototherapy treatments on study outcomes
| Skin cancer | Non-melanoma skin cancer | Cutaneous melanoma | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Model 1a Number of UVB phototherapy treatments |
1.002 | 0.985–1.019 | 0.85 | 0.998 | 0.974–1.023 | 0.90 | 1.010 | 0.989–1.031 | 0.35 |
| Model 2b Number of UVB phototherapy treatments |
1.001 | 0.984–1.017 | 0.95 | 0.998 | 0.974–1.022 | 0.84 | 1.009 | 0.988–1.031 | 0.40 |
| Age, years | 1.048 | 1.028–1.069 | < 0.001 | 1.046 | 1.026–1.067 | <0.001 | 1.071 | 0.995–1.153 | 0.07 |
| Male sex | 1.676 | 1.055–2.662 | 0.03 | 1.571 | 0.976–2.531 | 0.06 | 4.525 | 0.519–39.416 | 0.17 |
| Model 3c Number of UVB phototherapy treatments |
1.001 | 0.984–1.018 | 0.90 | 0.998 | 0.974–1.022 | 0.87 | 1.010 | 0.987–1.033 | 0.42 |
| Age, years | 1.065 | 1.041–1.089 | < 0.001 | 1.061 | 1.037–1.086 | <0.001 | 1.115 | 1.017–1.222 | 0.02 |
| Male sex | 1.739 | 1.076–2.810 | 0.02 | 1.629 | 0.993–2.672 | 0.05 | 4.345 | 0.491–38.451 | 0.19 |
| Outdoor occupation | 2.102 | 1.292–3.422 | 0.003 | 1.964 | 1.180–3.269 | 0.01 | 4.674 | 0.874–24.991 | 0.07 |
| High UV indexd | 1.111 | 0.696–1.775 | 0.66 | 1.257 | 0.772–2.047 | 0.36 | 0.209 | 0.023–1.865 | 0.16 |
| Organ transplantation | 5.896 | 3.171–10.962 | < 0.001 | 5.379 | 2.811–10.293 | <0.001 | 17.139 | 1.961–149.772 | 0.01 |
| Hydrochlorothiazide | 1.373 | 0.865–2.181 | 0.18 | 1.328 | 0.819–2.154 | 0.25 | 2.060 | 0.407–10.411 | 0.38 |
Model 1: Univariate Cox proportional hazards model adjusted for the number of UVB phototherapy treatments received by each patient.
Model 2: Multivariate Cox proportional hazards model adjusted for the variable in model 1 and for age and sex.
Model 3: Multivariate Cox proportional hazards model adjusted for all variables in model 2 and for occupation, high UV index, organ transplantation, and the prescription of hydrochlorothiazide.
Mean UV index > 6 at location of residence.
The number of UVB phototherapy treatments was analysed as a continuous variable, and the HR indicates the effect of every 1 additional UVB phototherapy treatment received by the patient.
CI: confidence interval; HR: hazard ratio.
Table II.
Cox proportional hazards models for the treatment with ultraviolet B (UVB) phototherapy on study outcomes
| Skin cancer | Non-melanoma skin cancer | Cutaneous melanoma | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Model 1a | |||||||||
| Treatment with UVB phototherapy | 1.236 | 0.713–2.141 | 0.45 | 1.260 | 0.713–2.225 | 0.43 | 0.956 | 0.112–8.199 | 0.97 |
| Model 2b | |||||||||
| Treatment with UVB phototherapy | 1.255 | 0.724–2.176 | 0.42 | 1.279 | 0.724–2.261 | 0.40 | 0.969 | 0.113–8.337 | 0.98 |
| Age, years | 1.048 | 1.028–1.069 | < 0.001 | 1.046 | 1.026–1.067 | < 0.001 | 1.072 | 0.995–1.154 | 0.07 |
| Male sex | 1.679 | 1.057–2.666 | 0.03 | 1.574 | 0.977–2.535 | 0.06 | 4.398 | 0.511–37.869 | 0.18 |
| Model 3c | |||||||||
| Treatment with UVB phototherapy | 1.066 | 0.584–1.944 | 0.84 | 1.067 | 0.571–1.996 | 0.84 | 1.009 | 0.115–8.879 | 0.99 |
| Age, years | 1.065 | 1.041–1.089 | < 0.001 | 1.061 | 1.037–1.086 | < 0.001 | 1.117 | 1.018–1.224 | 0.02 |
| Male sex | 1.738 | 1.076–2.808 | 0.02 | 1.627 | 0.992–2.669 | 0.05 | 4.208 | 0.482–36.717 | 0.19 |
| Outdoor occupation | 2.104 | 1.293–3.423 | 0.003 | 1.974 | 1.186–3.285 | 0.01 | 4.451 | 0.846–23.417 | 0.08 |
| High UV indexd | 1.110 | 0.695–1.773 | 0.66 | 1.257 | 0.772–2.048 | 0.36 | 0.208 | 0.023–1.846 | 0.16 |
| Organ transplantation | 5.911 | 3.178–10.995 | < 0.001 | 5.389 | 2.815–10.314 | < 0.001 | 17.769 | 2.021–156.189 | 0.01 |
| Hydrochlorothiazide | 1.368 | 0.861–2.175 | 0.19 | 1.324 | 0.816–2.150 | 0.26 | 2.006 | 0.396–10.160 | 0.40 |
Model 1: Univariate Cox proportional hazards model adjusted for treatment with UVB phototherapy.
Model 2: Multivariate Cox for the variable in model 1 and for age and sex.
Model 3: Multivariate Cox proportional hazards model adjusted for all variables in index, organ transplantation, and the prescription of hydrochlorothiazide.
Mean UV index >6 at location of residence.
CI: confidence interval; HR: hazard ratio.
Sensitivity analyses and competing risk analyses
In the sensitivity analyses, different follow-up times were examined in the Cox proportional hazards models evaluating the treatment with UVB phototherapy and the number of UVB phototherapy treatments. Overall, the risk of skin cancer was not significantly associated with UVB phototherapy at follow-up times of 3, 6, 9, 12, and 15 years (Table IV). Considering the competing risks between death and skin cancer, the results of the univariate and multivariate models were similar to those of the main analyses. The risk of skin cancer was not increased among patients in the UVB group or among those who received more UVB phototherapy treatments (Table V).
Table IV.
Multivariate Cox proportional hazards models for the influences of ultraviolet B (UVB) phototherapy on skin cancer at different follow-up times
| Follow-up time | Treatment with UVB phototherapy | Number of UVB phototherapy treatments | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| 3 years | 0.832 | 0.319–2.172 | 0.71 | 1.004 | 0.984–1.025 | 0.68 |
| 6 years | 0.844 | 0.376–1.894 | 0.68 | 1.003 | 0.982–1.023 | 0.81 |
| 9 years | 1.074 | 0.557–2.071 | 0.83 | 1.003 | 0.987–1.018 | 0.74 |
| 12 years | 1.129 | 0.616–2.068 | 0.70 | 1.002 | 0.986–1.018 | 0.82 |
| 15 years | 1.068 | 0.585–1.950 | 0.83 | 1.001 | 0.984–1.018 | 0.89 |
Multivariate Cox proportional hazards models adjusted for the exposure variable of UVB phototherapy, age, sex, occupation, high Uv index, organ transplantation, and the prescription of hydrochlorothiazide at different follow-up times.
CI: confidence interval; HR: hazard ratio.
Table V.
Competing risk models for the influences of ultraviolet B (UVB) phototherapy on skin cancer
| Exposure variables | Treatment with UVB phototherapy | Number of UVB phototherapy treatments | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Model 1a | 1.017 | 0.587–1.760 | 0.95 | 1.003 | 0.995–1.012 | 0.49 |
| Model 2b | 1.016 | 0.587–1.760 | 0.95 | 1.003 | 0.995–1.011 | 0.50 |
| Model 3c | 0.916 | 0.504–1.664 | 0.77 | 1.004 | 0.995–1.012 | 0.39 |
Model 1: Univariate model adjusted for the exposure variable of UVB phototherapy, using the Fine and Gray's subdistribution hazard model to consider the competing risk between death and skin cancer.
Model 2: Multivariate model adjusted for the variable in model 1 and for age and sex.
Model 3: Multivariate model adjusted for all variables in model 2 and for occupation, high UV index, organ transplantation, and the prescription of hydrochlorothiazide.
CI: confidence interval; HR: hazard ratio.
DISCUSSION
This is the first nationwide cohort study to assess the safety of UVB phototherapy for uraemic pruritus in patients with advanced CKD. Among 2,161 patients who received UVB phototherapy and 8,644 patients who did not receive UVB phototherapy, this study found that treatment of uraemic pruritus with UVB phototherapy did not increase the risk of skin cancer. Neither the risk of NMSC nor the risk of cutaneous melanoma was increased in patients treated with UVB phototherapy. These results were not influenced by the follow-up time or the competing risk of death in the cohort.
In this study, the risk of skin cancer was significantly higher among patients who were male, older, had an outdoor occupation, or had undergone organ transplantation. Due to the low incidence of cutaneous melanoma in Taiwan (23), NMSC contributed to most of the skin cancer events in this study. The strong associations between organ transplantation and skin cancer could be explained by the use of immunosuppressants and decreased host immune surveillance among organ transplant recipients (25, 26). The associations between outdoor occupations and skin cancer could be due to more exposure to UV radiation among outdoor workers (31).
The current study shows that hydrochlorothiazide was not associated with the risk of skin cancer, NMSC, and cutaneous melanoma. The finding was consistent with a nationwide case-control study using Taiwan’s NHI claims data, which had reported that hydrochlorothiazide was not associated with an increased risk of lip cancer, non-lip NMSC, or melanoma (32). Similarly, a nationwide cohort study of South Korean population had not shown an increased risk of NMSC or melanoma among hydrochlorothiazide users (33). Studies reporting strong associations between hydrochlorothiazide and skin cancer were carried out in Caucasian populations (27, 34). Additional studies are needed to determine whether the difference in skin phototypes or lifestyles regarding sun exposure in Asian populations influence the cutaneous carcinogenic risk from hydrochlorothiazide. The current analyses did not show an association between skin cancer and the mean UV index at the location of residence. The latitudes of administrative areas in Taiwan range within 5°; therefore, the differences in environmental UV exposure might not be large enough to influence the risk of skin cancer in the current study cohort.
Retrospective cohort studies did not show an association between UVB phototherapy and cutaneous carcinogenesis in Asian and Caucasian patients (11–14). These studies mainly enrolled patients with psoriasis who were younger than 50 years and used the general population as their comparison group, therefore the results could not be extrapolated to patients with CKD. The current study enrolled a larger population with a longer follow-up period, focusing on patients with advanced CKD with a mean age above 60 years, which could provide sufficient power to detect the risk of skin cancer after receiving UVB phototherapy.
The strength of this study is its large sample size and long follow-up time. The NHI database was linked to the cancer and death registries to ensure that the outcomes were ascertained accurately. To meticulously assess the influences of UVB phototherapy, the treatment with UVB phototherapy and the number of UVB phototherapy treatments were examined. Both exposure variables showed similar results in the univariate and multivariable models. A further strength of this study was the rigorous statistical methods applied to minimize confounding factors in the analyses. In the multivariate models, most of the potential risk factors for skin cancer were taken into consideration. The sensitivity analyses and competing risk analyses all showed similar results, which supports the robustness of our analyses.
There are several limitations to the current study. First, the treatment code is the same for narrowband and broadband UVB phototherapy in Taiwan’s NHI; therefore it was not possible to assess different types of UVB phototherapy in the analyses. Secondly, the cohort was based on claims data and did not contain information about health-related behaviours or laboratory examination results. However, most of the risk factors were adjusted for in the multivariate analyses and sensitivity analyses were performed in different time periods, which supports the results of the main analyses. Thirdly, most of the patients in the UVB group received a relatively lower number of UVB phototherapy treatments. The effect of UVB phototherapy with large number of treatments would require further studies. Finally, the Taiwanese population is mainly Asian, with Fitzpatrick’s skin phototypes II to V (35). Additional studies are required in order determine whether the findings can be generalized to CKD populations of other races or skin phototypes.
In conclusion, treatment of uraemic pruritus with UVB phototherapy does not increase the cutaneous carcinogenic risk among patients with advanced CKD. The risk of NMSC or cutaneous melanoma is not influenced by UVB phototherapy. These analyses indicate that UVB phototherapy appears to be a safe treatment for uraemic pruritus in patients with advanced CKD.
ACKNOWLEDGEMENTS
This study was supported by research grants to Dr Hon-Yen Wu from the National Health Research Institutes, Taiwan (NHRI-EX108-10510PC, NHRI-EX110-11026PI) and the Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH-EX108-10510PC, FEMH-2020-C-025), as well as by research grants to Dr Mei-Ju Ko from the Department of Health, Taipei City Government (10701-62-005, 10801-62-007) and the Taipei City Hospital, Taipei City, Taiwan (TPCH-109-17, TPCH-108-21). The funders had no role in study design, data collection, data analysis, manuscript preparation or publication decisions.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Mettang T, Kremer AE. Uremic pruritus. Kidney Int 2015; 87: 685–691. [DOI] [PubMed] [Google Scholar]
- 2.Wu HY, Huang JW, Tsai WC, Peng YS, Chen HY, Yang JY, et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: a prospective cohort study. PLoS One 2018; 13: e0203474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HY, Peng YS, Chen HY, Tsai WC, Yang JY, Hsu SP, et al. A comparison of uremic pruritus in patients receiving peritoneal dialysis and hemodialysis. Medicine 2016; 95: e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tartar D, Bhutani T, Huynh M, Berger T, Koo J. Update on the immunological mechanism of action behind phototherapy. J Drugs Dermatol 2014; 13: 564–568. [PubMed] [Google Scholar]
- 5.Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant 2011; 26: 3338–3344. [DOI] [PubMed] [Google Scholar]
- 6.Ko MJ, Peng YS, Chen HY, Hsu SP, Pai MF, Yang JY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol 2014; 71: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 7.Ko MJ, Yang JY, Wu HY, Hu FC, Chen SI, Tsai PJ, et al. Narrow-band ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 2011; 165: 633–639. [DOI] [PubMed] [Google Scholar]
- 8.Christensen L, Suggs A, Baron E. Ultraviolet photobiology in dermatology. Adv Exp Med Biol 2017; 996: 89–104. [DOI] [PubMed] [Google Scholar]
- 9.Stern RS, Liebman EJ, Vakeva L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA follow-up study. J Natl Cancer Inst 1998; 90: 1278–1284. [DOI] [PubMed] [Google Scholar]
- 10.Stern RS. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol 2001; 44: 755–761. [DOI] [PubMed] [Google Scholar]
- 11.Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maitre M, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26: 22–31. [DOI] [PubMed] [Google Scholar]
- 12.Jo SJ, Kwon HH, Choi MR, Youn JI. No evidence for increased skin cancer risk in Koreans with skin phototypes III–V treated with narrowband UVB phototherapy. Acta Derm Venereol 2011; 91: 40–43. [DOI] [PubMed] [Google Scholar]
- 13.Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Derm Venereol 2004; 84: 370–374. [DOI] [PubMed] [Google Scholar]
- 14.Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol 2008; 159: 931–935. [DOI] [PubMed] [Google Scholar]
- 15.Black RJ, Gavin AT. Photocarcinogenic risk of narrowband ultraviolet B (TL-01) phototherapy: early follow-up data. Br J Dermatol 2006; 154: 566–567. [DOI] [PubMed] [Google Scholar]
- 16.Diffey BL, Farr PM. The challenge of follow-up in narrow-band ultraviolet B phototherapy. Br J Dermatol 2007; 157: 344–349. [DOI] [PubMed] [Google Scholar]
- 17.Wang CC, Tang CH, Huang SY, Huang KC, Sue YM. Risk of non-melanoma skin cancer in patients with chronic kidney disease and its relationship to uraemic pruritus. Acta Derm Venereol 2017; 97: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 18.Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet 2000; 355: 1886–1887. [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015; 175: 1527–1529. [DOI] [PubMed] [Google Scholar]
- 20.Wu HY, Fukuma S, Shimizu S, Norton EC, Tu YK, Hung KY, et al. Effects of higher quality of care on initiation of long-term dialysis in patients with CKD and diabetes. Am J Kidney Dis 2017; 70: 666–674. [DOI] [PubMed] [Google Scholar]
- 21.Wu HY, Peng CL, Chen PC, Chiang CK, Chang CJ, Huang JW, et al. Comparative effectiveness of angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers for major renal outcomes in patients with diabetes: a 15-year cohort study. PLoS One 2017; 12: e0177654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol 2015; 45: 291–296. [DOI] [PubMed] [Google Scholar]
- 23.Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc 2016; 115: 1076–1088. [DOI] [PubMed] [Google Scholar]
- 24.Yu HC, Lin CL, Chen ZT, Hu FR, Sung FC, Wang IJ. Risk of skin cancer in patients with pterygium: a nationwide population-based cohort study in Taiwan. Ocul Surf 2014; 12: 69–76. [DOI] [PubMed] [Google Scholar]
- 25.Lee KF, Tsai YT, Lin CY, Hsieh CB, Wu ST, Ke HY, et al. Cancer incidence among heart, kidney, and liver transplant recipients in Taiwan. PLoS One 2016; 11: e0155602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard MD, Su JC, Chong AH. Skin cancer following solid organ transplantation: a review of risk factors and models of care. Am J Clin Dermatol 2018; 19: 585–597. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen SA, Gaist D, Schmidt SAJ, Holmich LR, Friis S, Pottegard A. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J Am Acad Dermatol 2018; 78: 673–681. [DOI] [PubMed] [Google Scholar]
- 28.Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations – US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol 2005; 141: 477–481. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat-Simul C 2009; 38: 1228–1234. [Google Scholar]
- 30.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526. [Google Scholar]
- 31.Taylor HR. Aetiology of climatic droplet keratopathy and pterygium. Br J Ophthalmol 1980; 64: 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pottegard A, Pedersen SA, Schmidt SAJ, Lee CN, Hsu CK, Liao TC, et al. Use of hydrochlorothiazide and risk of skin cancer: a nationwide Taiwanese case-control study. Br J Cancer 2019; 121: 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park E, Lee Y, Jue MS. Hydrochlorothiazide use and the risk of skin cancer in patients with hypertensive disorder: a nationwide retrospective cohort study from Korea. Korean J Intern Med 2020; 35: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman GD, Asgari MM, Warton EM, Chan J, Habel LA. Antihypertensive drugs and lip cancer in non-Hispanic whites. Arch Intern Med 2012; 172: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YW, Chu CY. The minimal erythema dose of broadband ultraviolet B in Taiwanese. J Formos Med Assoc 2007; 106: 975–978. [DOI] [PubMed] [Google Scholar]


