Abstract
Twelve Cu-based ternary (Cu–Me1–S, Me1 = Fe, Sn, or Sb) and quaternary (Cu–Me2–Sn–S, Me2 = Fe, Zn, or V) nanocrystalline sulfides are shown as perspective antibacterial materials here. They were prepared from elemental precursors by a one-step solvent-free mechanochemical synthesis in a 100 g batch using scalable eccentric vibratory ball milling. Most of the products have shown strong antibacterial activity against Escherichia coli and Staphylococcus aureus bacteria. For instance, stannite Cu2FeSnS4 and mohite Cu2SnS3 were the most active against E. coli, whereas kesterite Cu2ZnSnS4 and rhodostannite Cu2FeSn3S8 exhibited the highest antibacterial activity against S. aureus. In general, stannite has shown the best antibacterial properties out of all the studied samples. Five out of twelve products have been prepared using mechanochemical synthesis for the first time in a scalable fashion here. The presented synthetic approach is a promising alternative to traditional syntheses of nanomaterials suitable for biological applications and shows ternary and quaternary sulfides as potential candidates for the next-generation antibacterial agents.
1. Introduction
Contemporary human society is confronted with several critical concerns, and infection diseases are among the most important ones.1 Antibiotics worked very well against bacteria in the past. However, nowadays, antimicrobial resistance has been reaching a critical level.2 Insight into new antibacterially active materials, not yet known to the microbes, is therefore a reasonable approach. Finding a chemically stable, non-toxic, and low-cost antibacterial material is of utmost importance. Metal sulphides mimicking safe natural minerals seem to be rising stars in this area.1,3 More binary metal sulphides perfectly serve this role. The antibacterial potential of Ag2S,4−8 CdS,9−17 CuS18−27 MoS2,28−38 and ZnS39−44 has been studied many times. There are few publications on the antimicrobial action of more exotic binary sulfides like SnS2,45 CoS2,46 NiS,47 and In2S3.48 Binary sulfides are most often complemented by another compounds like oxides to get the composite with good antibacterial activity. The works on the antibacterial activity of ternary and quaternary sulfides are even more scarce, namely just the antibacterial potential of Cu2SnS3,49 Cu2ZnSnS4,50,51 and Cu12Sb4S1352 has been discovered so far.
There are many synthetic pathways for sulfide nanoscale production,3,53,54 among which a scalable, solvent-free one-step methodology called mechanochemical synthesis has found an inevitable place.55,56 One of the scalable alternatives to the lab-scale mechanochemical synthesis is eccentric vibratory milling57 and it has been sufficiently applied to prepare both sulfides58−65 and selenides.66
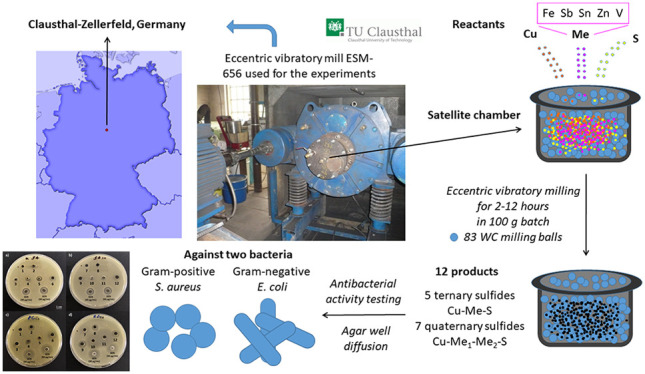
This article provides a comprehensive viewpoint on the antibacterial potential of twelve Cu-based ternary and quaternary sulfides mechanochemically synthesized in a scalable fashion. For the most of the products, the antibacterial activity has not been reported yet. Moreover, five of them have not even been prepared mechanochemically so far. As the eccentric vibratory mill used for the experiments in this study was located at Technical University of Clausthal, Germany, a birthplace of famous microbiologist Robert Koch,67 the samples in this study are labeled as KOCHx (x being a sample number), as a tribute to him.
2. Materials and Methods
2.1. Materials
For mechanochemical synthesis of chalcopyrite CuFeS2, chatkalite Cu6FeSn2S8, stannoidite Cu8Fe3Sn2S12, skinnerite Cu3SbS3, and stannite Cu2FeSnS4, the following precursors were used: copper (Merck, Germany, 99,7% purity), antimony (Merck, Germany, 99.8% purity), tin (Nihon Seiko, Japan, 99% purity), iron (WINLAB, Germany, 99% purity), and sulfur (CG-Chemikalien, Germany, 99% purity).
2.2. Mechanochemical Synthesis
The details on the mechanochemical syntheses of the majority of the studied compounds can be found in the following publications: mawsonite Cu6Fe2SnS8,58 rhodostannite Cu2FeSn3S8,59 kesterite Cu2ZnSnS4,60,65 tetrahedrite Cu12Sb4S13,61 colusite Cu13VSn3S16,62 mohite Cu2SnS3,63 and famatinite Cu3SbS4.64 The weights of precursors for all the compounds (including those newly prepared) and the corresponding milling time are provided in Table 1.
Table 1. Weights of the Precursors and Milling Time for the Mechanochemical Syntheses of Ternary and Quaternary Cu-Based Sulfides Synthesized in this Studya.
| weight
(g) |
milling time (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| sample | desired phase | phase name | Cu | Fe | Sb | Sn | S | |
| KOCH 1 | CuFeS2 | chalcopyrite | 34.6 | 30.4 | 35.0 | 720 | ||
| KOCH 258 | Cu6Fe2SnS8 | mawsonite | 53.9 | 12.9 | 13.7 | 29.5 | 240 | |
| KOCH 3 | Cu6FeSn2S8 | chatkalite | 41 | 6 | 25.5 | 27.5 | 120 | |
| KOCH 4 | Cu8Fe3Sn2S12 | stannoidite | 39.2 | 12.9 | 18.3 | 29.6 | 120 | |
| KOCH 559 | Cu2FeSn3S8 | rhodostannite | 16 | 7 | 32.2 | 44.8 | 600 | |
| KOCH 660 | Cu2ZnSnS4 | kesterite | 28.9 | 14.9 | 27.0 | 29.2 | 360 | |
| KOCH 761 | Cu12Sb4S13 | tetrahedrite | 45.8 | 29.2 | 25.0 | 240 | ||
| KOCH 862 | Cu13VSn3S16 | colusite | 47.3 | 20.4 | 29.4 | 720 | ||
| KOCH 963 | Cu2SnS3 | mohite | 37.2 | 34.7 | 28.1 | 180 | ||
| KOCH 1064 | Cu3SbS4 | famatinite | 43.3 | 27.6 | 29.1 | 240 | ||
| KOCH 11 | Cu3SbS3 | skinnerite | 46.7 | 29.8 | 23.5 | 120 | ||
| KOCH 12 | Cu2FeSnS4 | stannite | 29.6 | 13.0 | 27.6 | 29.8 | 120 | |
For colusite (KOCH8), also 2.9 g of vanadium was used for the synthesis.
The rest of the milling conditions was similar for all the samples. The mechanochemical syntheses were carried out in an industrial eccentric vibratory ball mill ESM 656-0.5ks (Siebtechnik, Germany) working under the following conditions: A 5 L steel satellite milling chamber attached to the main corpus of the mill, 83 tungsten carbide balls with a diameter of 35 mm and a total mass of 30 kg, 80% ball filling in the milling chamber, amplitude of inhomogeneous vibrations 20 mm, rotational speed of the eccenter 960 min–1, and an argon atmosphere. The total feed of reaction precursors was 100 g per batch. The milling was performed for different times which are mentioned in the appropriate sections.
2.3. Characterization
2.3.1. X-ray Diffraction
The phase composition of all mechanochemically prepared products was analyzed by X-ray diffraction (XRD, Rigaku SmartLab 3 kW) in a Bragg–Brentano geometry in a 2θ-angle range of 10–90° using Cu Kα radiation with 30 kV and 40 mA and a scan speed of 4° min–1. The phase identification in the acquired XRD patterns was performed using the HighScore Plus software (PANalytical B.V., The Netherlands, version 3.0e) in the 2θ-angle range of 15–65°.
2.3.2. ζ-Potential Measurements
ζ-potential was measured in the diluted water solution of sodium chloride (10 mM) using Zetasizer Nano ZS (Malvern, Malvern, U.K.) setup, the electrophoretic mobility of the particles being converted to zeta potential using the Smoluchowski equation built in the Malvern Zetasizer software. The measurements were performed in triplicate with at least 12 sub-runs for each sample.
2.4. Antibacterial Activity
The antibacterial properties of the samples were evaluated by the agar well diffusion method by a slight modification in the process reported in.68 The tested bacteria (Staphylococcus aureus CCM 4223 and Escherichia coli CCM 3988) were obtained from the Czech collection of microorganisms (CCM, Brno, Czech Republic). The procedure used was as follows:
Bacteria were cultured overnight, aerobically at 37 °C in Luria–Bertani (LB) medium (Sigma-Aldrich, Saint-Louis, MO) with agitation. After this, bacteria were mixed with 50% glycerol (Mikrochem, Pezinok, Slovakia) and frozen glycerol stock cultures were maintained at −20 °C. Before the experimental use, cultures were transferred to LB medium and incubated for 24 h, and used as the source of inoculum for each experiment.
Plate count agar (HIMEDIA, Mumbai, India) medium was cooled to 42 °C after autoclaving, inoculated overnight with liquid bacterial culture to a cell density of 5 × 105 colony-forming units per mililiter (cfu/mL).
20 mL of this inoculated agar was pipetted into a 90 mm diameter Petri dish.
Once the agar was solidified, five mm diameter wells were punched in the agar and filled with 50 μL of samples prepared in the form of suspensions (prepared by dispersing 20 mg of KOCHx samples in 1 mL of distilled water). Gentamicin sulfate (Biosera, Nuaille, France) with the concentration of 30 mM was used as a positive control.
The plates were incubated for 24 h at 37 °C.
Afterward, the plates were photographed and the inhibition zones were measured by the ImageJ 1.53e software (National Institutes of Health, Bethesda, MD). The values used for the calculation are mean values obtained from 3 replicate tests.
The antibacterial activity was calculated by applying the formula reported in:68 %Relative inhibition zone diameter (RIZD = [(IZD sample – IZD negative control)/IZD gentamicin] × 100, where RIZD is the relative inhibition zone diameter (%) and IZD is the inhibition zone diameter (mm). As a negative control, the inhibition zones of distilled water equal to 0 were taken. The inhibition zone diameter (IZD) was obtained by measuring the diameter of the transparent zone and subtracting the size of the wells (5 mm).
3. Results and Discussion
3.1. X-ray Diffraction
The formation of nanoparticles by mechanochemistry is well-known.55,69 In general, two phenomena are observed during high-energy milling, mechanical activation and mechanochemical reaction.70,71 While the first one can be considered a top-down approach (by diminishing the crystallite size down to the nanoscale), the second one might also be considered as a bottom-up strategy, as the size of the produced nanoparticles can be increased with prolonged milling. The particle size of the precursors is being reduced down to the nanoscale and simultaneously, the reaction between them occurs on the grain boundaries. The produced nanoparticles are most often present in the form of microcrystalline agglomerates; however, the X-ray diffraction usually reveals nanoscale dimensions of the individual crystallites.
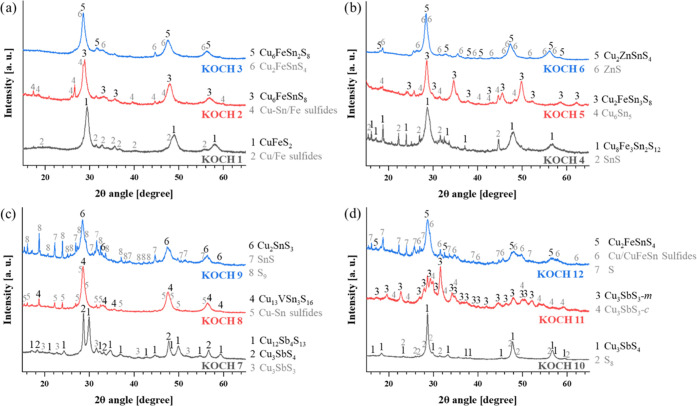
The XRD patterns of all the samples synthesized in the present study and their principal identified phases are provided in Figure 1.
Figure 1.
XRD patterns of all powders under study containing mostly ternary and quaternary sulfides: (a) KOCH 1-3, (b) KOCH 4-6, (c) KOCH 7–9, (d) KOCH 10–12. High-intensity diffraction peaks are marked in black for the main present phase, additional secondary phases are marked in grey for each system.
The mechanochemical syntheses of ternary and quaternary Cu sulfides represent an excellent scalable method to obtain a predetermined Cu–sulfide phase.63,65 In general, all samples were composed of the desired ternary or quaternary copper sulfide as a major phase. However, secondary phases were also present either as a result of an incomplete reaction between the initial reactants or because of the fact that they represent intermediate phases formed on the reaction pathway to the desired compound. A significant peak broadening clearly shows the nanocrystalline character of the products, and this has been also confirmed by calculations from XRD data and transmission electron microscopy (TEM) analysis for seven products that have been synthesized before.58−65
It can be deduced that the secondary phases are present in small amounts by comparing the peak intensities of the identified phases in each processed system. Only in the synthesis of tetrahedrite (Cu12Sb4S13, KOCH7 sample), similar peak intensity of famatinite (Cu3SbS4) was registered. The presence of secondary phases is something that must be followed for scalable intentions in potential industrial applications, especially if the presence of a specific secondary phase compromises the antibacterial activity of the final product. This can be exemplified in the KOCH7 system, where neither of the two ternary Cu-based sulfides did contribute to the antibacterial response, as will be shown later.
An overview of the identified phases in each processed sample, together with their crystallographic information references or synthesis methodologies is provided in the supplementary material (Table S1).
3.2. Antibacterial Activity
3.2.1. Relative Inhibition Zone Diameter (RIZD) determination using the agar well diffusion method
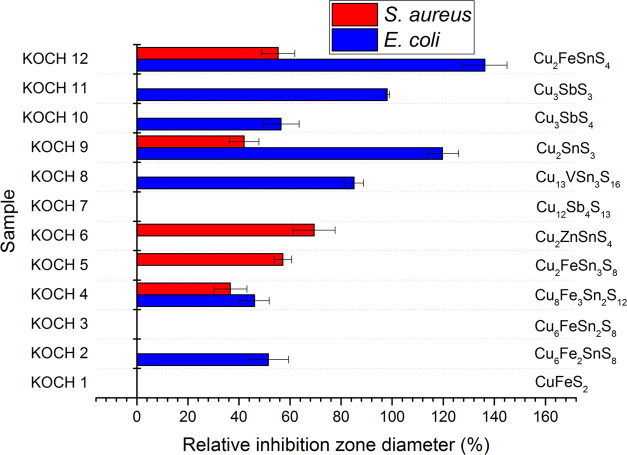
Twelve Cu-based sulfides were subjected to antibacterial tests using the agar well diffusion method. The relative inhibition zone diameters (RIZDs) received for both bacterial strains are summarized in Figure 2 and representative images from the experiments can be seen in the supporting file (Figure S1).
Figure 2.
Relative inhibition zone diameter (RIZD) for all twelve studied samples for both S. aureus and E. coli. The positive control was antibiotic gentamicin with a concentration of 30 μg/mL and its RIZD was taken as 100%.
It can be seen that only three samples did not exhibit any antibacterial activity, namely chalcopyrite CuFeS2, chatkalite Cu6FeSn2S8, and tetrahedrite Cu12Sb4S13. Out of these three compounds, only the antibacterial potential of tetrahedrite has been revealed so far.52 In the mentioned study, the E. coli bacteria could be almost completely destroyed by the Cu12Sb4S13 film in 10 min. The fact that tetrahedrite was not active in our case might be connected with the difference in the methodology used to prepare the material, which results in dissimilar properties (e.g., in nonidentical particle size).
For all other nine samples, at least some activity could be observed. In general, the activity was better against gram-negative E. coli (7 out of 12 investigated samples were active and RIZD values were slightly larger for the other bacteria). In the case of gram-positive S. aureus, the activity was registered in 5 out of 12 samples. Interestingly, different compounds were active against different types of bacteria, e.g., kesterite Cu2ZnSnS4 and rhodostannite Cu2FeSn3S8 were active only against gram-positive S. aureus, whereas skinnerite Cu3SbS3, famatinite Cu3SbS4, collusite Cu13VSn3S16, and mawsonite Cu6Fe2SnS8 were active only against gram-negative E. coli. The activity against both types of bacteria was detected only scarcely (in 3 out of 12 samples), namely for stannite Cu2FeSnS4, mohite Cu2SnS3, and stannoidite Cu8Fe3Sn2S12. The highest RIZD was evidenced in the case of stannite (136.25 ± 8.6%) against E. coli. Against S. aureus, the best activity out of 12 studied samples was evidenced for kesterite Cu2ZnSnS4 (69.41 ± 8.21%).
The antibacterial potential of kesterite has already been studied earlier.50,51 The zone of inhibition (ZOI) reported in50 was 3 and 5 mm for E. coli and S. aureus, respectively, being in accordance with better activity of this compound against S. aureus observed also in our case. In the mentioned study, 1 mg of the powder was introduced into the well, which is the same as in our case (accepting the premise that the powder was homogeneously distributed in the distilled water before testing). The mechanism of antibacterial action of kesterite is most probably connected with the electrostatic interaction due to the opposite surface charges of the compound and the bacterial cell wall.72
The antibacterial effect of mohite Cu2SnS3 has been revealed by Lokhande et al. upon testing it against the same bacteria as in our case (S. aureus and E. coli). The antibacterial action was stronger against E. coli, similar to our study. The authors ascribed the difference to the thickness of the walls of the two bacteria, being 80 nm thick for S. aureus and only 10 nm thick for E. coli, respectively,73 making the former one more resistant. The authors have demonstrated the damage caused to the E. coli and S. aureus cells by providing the scanning electron microscopy (SEM) images of the bacteria after being subjected to the effect of CTS nanocrystals. While E. coli bacterial cells’ length increased as a result of the exposure to the Cu2SnS3 nanoparticles, the shrinkage of cellular texture and extrusion of intracellular fluids were observed in the case of S. aureus. Similar to kesterite, the authors also ascribe the mechanism of action to the electrostatic interaction between the oppositely charged species (positive charge of the nanoparticles and negative charge of the cell wall).74,75
3.2.2. Relationship between Antibacterial Action and ζ-Potential Measurements
The surface charge of the compounds, which is expressed by their ζ-potential, can play a decisive role in the antibacterial action. ζ-potential reflects the stability of the reaction systems in the form of colloidal dispersions.76
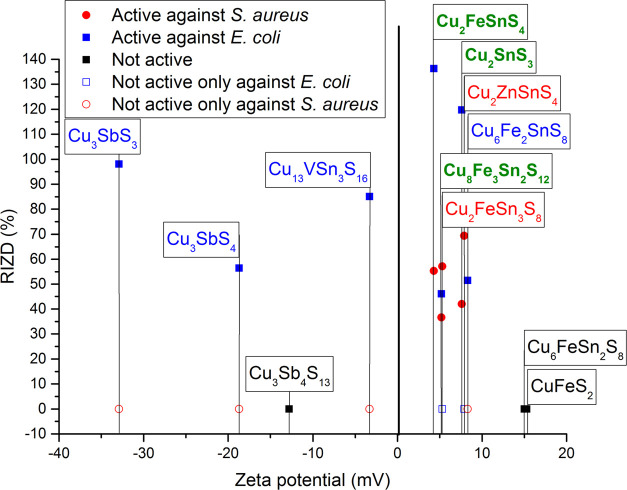
Therefore, ζ-potential measurements for all samples were performed. The measured values are summarized in Figure 3 and the Electronic Supporting Information (Table S2). It can be seen that the whole range of ζ-potential spanning from very negative to very positive values was evidenced in our case, the most negative being in the case of Cu3SbS3 (−32.9 mV) and the most positive in the case of chalcopyrite CuFeS2 (15.3 mV). Similar results have been observed in the literature.77−79 The presence of iron seems to lead to the positive ζ-potential values (KOCH 1–5, 12). This is a result of the dissolution of Fe2+ ions originating from iron particles and their transfer into the solution.77 This is also the case of Zn2+ ions (KOCH 9).80 On the other hand, the presence of antimony yields negative ζ-potential values (samples KOCH 7, 10 and 11), as evidenced for chalcostibite CuSbS2.78 The direct correlation between the ζ-potential values and the antibacterial action can be seen in Figure 3.
Figure 3.
Dependence of antibacterial activity expressed as RIZD on ζ-potential. The formulas of the main phases in the samples are in different color: green- active against both bacteria, red—active only against S. aureus, blue—active only against E. coli, black—not active against either bacteria.
The correlation of ζ-potential values and the RIZD detected for all samples provides us with some clues. First, it seems that a very positive ζ-potential value is not beneficial, as when it was higher than 10 mV, no antibacterial action was observed. Second, there is a markable difference in the fact that ZP values are beneficial for the antibacterial action against the given bacterial species. While quite a wide range of ZP values (from almost −35 to +8 mV) are efficient against E. coli, there is quite a narrow window for the action against S. aureus (values between +4 and +10 mV). The products with the negative ZP values are not active against S. aureus, but are only active against E. coli, so there might be some correlation between the negative surface charge of the given sulfides and the properties of the gram-negative E. coli bacteria.
In three out of four studies reporting the antibacterial activity of the multinary sulfides published so far, the authors also determined the minimum inhibitory concentration (MIC). It is defined as the lowest concentration of a drug that will inhibit the visible growth of an organism after overnight incubation. The MIC value for CZTS nanocrystals prepared in50 was 500 μg/mL against all tested bacteria, including E. coli and S. aureus. The antibacterial action of kesterite was tested against these bacterial cell lines (among others) also in51 and the MIC value was in the range of 128–512 μg/mL, thus the results are in accordance. Lokhande et al.49 observed a significant reduction in the growth of bacterial colonies (reporting this to be MIC) after the introduction of 1 and 3 mL of mohite Cu2SnS3 solution (solvent unspecified) for S. aureus and E. coli, respectively; thus the activity was better against S. aureus in this case. However, as mentioned earlier, the ZOI obtained by the agar well diffusion method in that study was larger for E. coli than for S. aureus, so the results from the two complementary methods are contradictory. In the present research, we also tried to determine the MIC value, however, as our powders are black, their dispersion in the solution with bacteria caused the solution to become opaque, which hampered the proper determination of MIC, as this is done spectrophotometrically.
Conclusions
Twelve ternary and quaternary nano-sulfides were successfully prepared by a mechanochemical one-step solvent-free synthesis in a 100 g batch using an eccentric vibratory ball mill. The synthesis is perfectly feasible just by solid-state milling of the elemental precursors, as in the majority of experiments, the desired sulfides were prepared as main phases. The agar well diffusion method has shown that at the concentration of 20 mg/mL, most of the products are efficient antibacterial agents, throwing some light on the influence of the chemical composition on the antibacterial action. In general, better activity of the studied sulfides was evidenced against gram-negative E. coli; however, in two cases (kesterite Cu2ZnSnS4 and rhodostannite Cu3FeSnS8), only the activity against S. aureus was observed. The most potent agents were found to be stannite Cu2FeSnS4 and mohite Cu2SnS3. The former one exhibited a significant antibacterial action against both types of bacteria. Only three out of twelve products (namely, chalcopyrite CuFeS2, chatkalite Cu6FeSn2S8, and tetrahedrite Cu12Sb4S13) did not show any activity at the studied concentration. The investigation on the relationship between ζ-potential values and antibacterial activity has revealed that the products with the negative ζ-potential values were efficient only against E. coli bacteria, thus there might be some relationship between their negative charge and the properties of the E. coli cell wall. The present research has shown nanocrystalline sulfides as interesting alternatives to traditional antibacterial agents and also the robustness of the mechanochemical synthesis performed on a larger scale.
Acknowledgments
The present study was financially supported by the Slovak Research and Development Agency under the Contract No. APVV-18-0357 and that of The Ministry of Education, Science, Research and Sport of the Slovak Republic Grant Agency (projects 2/0112/22 and 2/0103/20). This work was realized within the frame of the project, Research Centre of Advanced Materials and Technologies for Recent and Future Applications, PROMATECH, “ITMS 26220220186, supported by the Operational Program “Research and Development” financed through the European Regional Development Fund. The support of COST Action CA18112 MechSustInd (www.mechsustind.eu), supported by the COST Association (European Cooperation in Science and Technology, www.cost.eu) is also acknowledged. The authors also acknowledge very valuable pieces of advice and discussions with Dr. Adrian Augustyniak from West Pomeranian University of Technology in Szczecin (Poland).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01657.
Description of all identified crystallographic phases in KOCH 1–12 samples; including the crystallographic identification card numbers from Crystallography Open Database (COD); representative photographs from agar well diffusion tests showing transparent zones of the active samples; table listing the exact ζ-potential values detected for KOCH 1–12 samples (PDF)
The authors declare no competing financial interest.
Notes
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
Supplementary Material
References
- Han H. C.; Yang J. J.; Li X. Y.; Qi Y.; Yang Z. Y.; Han Z. J.; Jiang Y. Y.; Stenzel M.; Li H.; Yin Y. X.; Du Y.; Liu J. R.; Wang F. L. Shining light on transition metal sulfides: New choices as highly efficient antibacterial agents. Nano Res. 2021, 14, 2512–2534. 10.1007/s12274-021-3293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- Coughlan C.; Ibáñez M.; Dobrozhan O.; Singh A.; Cabot A.; Ryan K. M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. 10.1021/acs.chemrev.6b00376. [DOI] [PubMed] [Google Scholar]
- Levard C.; Hotze E. M.; Colman B. P.; Dale A. L.; Truong L.; Yang X. Y.; Bone A. J.; Brown G. E.; Tanguay R. L.; Di Giulio R. T.; Bernhardt E. S.; Meyer J. N.; Wiesner M. R.; Lowry G. V. Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity. Environ. Sci. Technol. 2013, 47, 13440–13448. 10.1021/es403527n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibiya P. N.; Moloto M. J. Synthesis, characterisation and antimicrobial effect of starch capped silver sulphide nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Nanotechnol. 2017, 14, 385–398. 10.1504/IJNT.2017.082437. [DOI] [Google Scholar]
- Fakhri A.; Pourmand M.; Khakpour R.; Behrouz S. Structural, optical, photoluminescence and antibacterial properties of copper-doped silver sulfide nanoparticles. J. Photochem. Photobiol., B 2015, 149, 78–83. 10.1016/j.jphotobiol.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Delgado-Beleño Y.; Martinez-Nuñez C. E.; Cortez-Valadez M.; Flores-Lopez N. S.; Flores-Acosta M. Optical properties of silver, silver sulfide and silver selenide nanoparticles and antibacterial applications. Mater. Res. Bull. 2018, 99, 385–392. 10.1016/j.materresbull.2017.11.015. [DOI] [Google Scholar]
- Wang J.; Sun J. Y.; Huang J.; Fakhri A.; Gupta V. K. Synthesis and its characterization of silver sulfide/nickel titanate/chitosan nanocomposites for photocatalysis and water splitting under visible light, and antibacterial studies. Mater. Chem. Phys. 2021, 272, 124990 10.1016/j.matchemphys.2021.124990. [DOI] [Google Scholar]
- Gupta V. K.; Pathania D.; Asif M.; Sharma G. Liquid phase synthesis of pectin-cadmium sulfide nanocomposite and its photocatalytic and antibacterial activity. J. Mol. Liq. 2014, 196, 107–112. 10.1016/j.molliq.2014.03.021. [DOI] [Google Scholar]
- Barman J.; Sultana F. Optoelectronic and antimicrobial activity of composite zinc oxide and cadmium sulphide quantum dots and application in water treatment. Indian J. Pure Appl. Phys. 2017, 55, 231–236. [Google Scholar]
- Shivashankarappa A.; Sanjay K. R. Escherichia coli- based synthesis of cadmium sulfide nanoparticles, characterization, antimicrobial and cytotoxicity studies. Braz. J. Microbiol. 2020, 51, 939–948. 10.1007/s42770-020-00238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana T. K.; Maji S. K.; Pal A.; Maiti R. P.; Dolai T. K.; Chatterjee K. Photocatalytic and antibacterial activity of cadmium sulphide/zinc oxide nanocomposite with varied morphology. J. Colloid Interface Sci. 2016, 480, 9–16. 10.1016/j.jcis.2016.06.073. [DOI] [PubMed] [Google Scholar]
- Dorraji M. S. S.; Ashjari H. R.; Rasoulifard M. H.; Rastgouy-Houjaghan M. Polyurethane foam-cadmium sulfide nanocomposite with open cell structure: Dye removal and antibacterial applications. Korean J. Chem. Eng. 2017, 34, 547–554. 10.1007/s11814-016-0261-9. [DOI] [Google Scholar]
- Alsaggaf M. S.; Elbaz A. F.; El Badawy S.; Moussa S. H. Anticancer and Antibacterial Activity of Cadmium Sulfide Nanoparticles by Aspergillus niger. Adv. Polym. Technol. 2020, 2020, 1–13. 10.1155/2020/4909054. [DOI] [Google Scholar]
- Ashengroph M.; Khaledi A.; Bolbanabad E. M. Extracellular biosynthesis of cadmium sulphide quantum dot using cell-free extract of Pseudomonas chlororaphis CHR05 and its antibacterial activity. Process Biochem. 2020, 89, 63–70. 10.1016/j.procbio.2019.10.028. [DOI] [Google Scholar]
- Liu L.; Sun M. Q.; Zhang H. J.; Yu Q. L.; Li M. C.; Qi Y.; Zhang C. D.; Gao G. D.; Yuan Y. J.; Zhai H. H.; Chen W.; Alvarez P. J. J. Facet Energy and Reactivity versus Cytotoxicity: The Surprising Behavior of CdS Nanorods. Nano Lett. 2016, 16, 688–694. 10.1021/acs.nanolett.5b04487. [DOI] [PubMed] [Google Scholar]
- Shalabayev Z.; Baláž M.; Khan N.; Nurlan Y.; Augustyniak A.; Daneu N.; Tatykaev B. B.; Dutková E.; Burashev G.; Casas Luna M.; Džunda R.; Bureš R.; Čelko L.; Ilin A.; Burkitbayev M. Sustainable synthesis of cadmium sulfide applicable in photocatalysis, hydrogen production, and as an antibacterial agent using two mechanochemical protocols. Nanomaterials 2022, 12, 1250 10.3390/nano12081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addae E.; Dong X. L.; McCoy E.; Yang C.; Chen W.; Yang L. J. Investigation of antimicrobial activity of photothermal therapeutic gold/copper sulfide core/shell nanoparticles to bacterial spores and cells. J. Biol. Eng. 2014, 8, 11 10.1186/1754-1611-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Y.; Hua X. W.; Wu F. G.; Li B. L.; Liu P. D.; Gu N.; Wang Z. F.; Chen Z. Synthesis of Ultrastable Copper Sulfide Nanoclusters via Trapping the Reaction Intermediate: Potential Anticancer and Antibacterial Applications. ACS Appl. Mater. Interfaces 2015, 7, 7082–7092. 10.1021/acsami.5b01214. [DOI] [PubMed] [Google Scholar]
- Ahmed K. B. A.; Anbazhagan V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. 10.1039/C7RA05636B. [DOI] [Google Scholar]
- Ahmed K. B. A.; Subramaniyan S. B.; Banu S. F.; Nithyanand P.; Anbazhagan V. Jacalin-copper sulfide nanoparticles complex enhance the antibacterial activity against drug resistant bacteria via cell surface glycan recognition. Colloids Surf., B 2018, 163, 209–217. 10.1016/j.colsurfb.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Shalabayev Z.; Baláž M.; Daneu N.; Dutková E.; Bujňáková Z.; Kaňuchová M.; Danková Z.; Balážová L’.; Urakaev F.; Tkáčiková L.; Burkitbayev M. Sulfur-Mediated Mechanochemical Synthesis of Spherical and Needle-Like Copper Sulfide Nanocrystals with Antibacterial Activity. ACS Sustainable Chem. Eng. 2019, 7, 12897–12909. 10.1021/acssuschemeng.9b01849. [DOI] [Google Scholar]
- Wang Y.; Wang W.; Liu B. J.; Yu D. Preparation of durable antibacterial and electrically conductive polyacrylonitrile fibers by copper sulfide coating. J. Appl. Polym. Sci. 2017, 134, 45496 10.1002/app.45496. [DOI] [Google Scholar]
- Subramaniyan S. B.; Vijayakumar S.; Megarajan S.; Kamlekar R. K.; Anbazhagan V. Remarkable Effect of Jacalin in Diminishing the Protein Corona Interference in the Antibacterial Activity of Pectin-Capped Copper Sulfide Nanoparticles. ACS Omega 2019, 4, 14049–14056. 10.1021/acsomega.9b01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioni C.; Borzenkov M.; D’Alfonso L.; Sperandeo P.; Polissi A.; Cucca L.; Dacarro G.; Grisoli P.; Pallavicini P.; D’Agostino A.; Taglietti A. Self-Assembled Monolayers of Copper Sulfide Nanoparticles on Glass as Antibacterial Coatings. Nanomaterials 2020, 10, 352 10.3390/nano10020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S.; Rhim J. W. Fabrication of Copper Sulfide Nanoparticles and Limonene Incorporated Pullulan/Carrageenan-Based Film with Improved Mechanical and Antibacterial Properties. Polymers 2020, 12, 2665 10.3390/polym12112665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhit M.; El Naga A. O. A.; El Saied M.; Maksoud M. I. A. A. Radiation-induced synthesis of copper sulfide nanotubes with improved catalytic and antibacterial activities. Environ. Sci. Pollut. Res. 2021, 28, 44467–44478. 10.1007/s11356-021-13482-9. [DOI] [PubMed] [Google Scholar]
- Liu C.; Kong D. S.; Hsu P. C.; Yuan H. T.; Lee H. W.; Liu Y. Y.; Wang H. T.; Wang S.; Yan K.; Lin D. C.; Maraccini P. A.; Parker K. M.; Boehm A. B.; Cui Y. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 2016, 11, 1098–1104. 10.1038/nnano.2016.138. [DOI] [PubMed] [Google Scholar]
- Yin W. Y.; Yu J.; Lv F. T.; Yan L.; Zheng L. R.; Gu Z. J.; Zhao Y. L. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. 10.1021/acsnano.6b05810. [DOI] [PubMed] [Google Scholar]
- Yang X.; Li J.; Liang T.; Ma C. Y.; Zhang Y. Y.; Chen H. Z.; Hanagata N.; Su H. X.; Xu M. S. Antibacterial activity of two-dimensional MoS2 sheets. Nanoscale 2014, 6, 10126–10133. 10.1039/C4NR01965B. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Zhang X.; Yin W. Y.; Ma D. Q.; Xie C. J.; Zheng L. R.; Dong X. H.; Mei L. Q.; Yu J.; Wang C. Z.; Gu Z. J.; Zhao Y. L. Functionalized MoS2 Nanovehicle with Near-Infrared Laser-Mediated Nitric Oxide Release and Photothermal Activities for Advanced Bacteria-Infected Wound Therapy. Small 2018, 14, 1802290 10.1002/smll.201802290. [DOI] [PubMed] [Google Scholar]
- Cao F. F.; Ju E. G.; Zhang Y.; Wang Z. Z.; Liu C. Q.; Li W.; Huang Y. Y.; Dong K.; Ren J. S.; Qu X. G. An Efficient and Benign Antimicrobial Depot Based on Silver -Infused MoS2. ACS Nano 2017, 11, 4651–4659. 10.1021/acsnano.7b00343. [DOI] [PubMed] [Google Scholar]
- Zhang W. T.; Shi S.; Wang Y. R.; Yu S. X.; Zhu W. X.; Zhang X.; Zhang D. H.; Yang B. W.; Wang X.; Wang J. L. Versatile molybdenum disulfide based antibacterial composites for in vitro enhanced sterilization and in vivo focal infection therapy. Nanoscale 2016, 8, 11642–11648. 10.1039/C6NR01243D. [DOI] [PubMed] [Google Scholar]
- Mutalik C.; Krisnawati D. I.; Patil S. B.; Khafid M.; Atmojo D. S.; Santoso P.; Lu S. C.; Wang D. Y.; Kuo S. R. Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application. ACS Sustainable Chem. Eng. 2021, 9, 7904–7912. 10.1021/acssuschemeng.1c01868. [DOI] [Google Scholar]
- Xu J. G.; Cai R.; Zhang Y. G.; Mu X. Y. Molybdenum disulfide-based materials with enzyme-like characteristics for biological applications. Colloids Surf., B 2021, 200, 111575 10.1016/j.colsurfb.2021.111575. [DOI] [PubMed] [Google Scholar]
- Zhao Y. C.; Jia Y. X.; Xu J. Y.; Han L.; He F.; Jiang X. Y. The antibacterial activities of MoS2 nanosheets towards multi-drug resistant bacteria. Chem. Commun. 2021, 57, 2998–3001. 10.1039/D1CC00327E. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Roy S.; Sarkar A.; Jaiswal A. Reusable MoS2-Modified Antibacterial Fabrics with Photothermal Disinfection Properties for Repurposing of Personal Protective Masks. ACS Appl. Mater. Interfaces 2021, 13, 12912–12927. 10.1021/acsami.1c00083. [DOI] [PubMed] [Google Scholar]
- Shi J. P.; Li J.; Wang Y.; Cheng J. J.; Zhang C. Y. Recent advances in MoS2-based photothermal therapy for cancer and infectious disease treatment. J. Mater. Chem. B 2020, 8, 5793–5807. 10.1039/D0TB01018A. [DOI] [PubMed] [Google Scholar]
- Mani S. K.; Manickam S.; Muthusamy V.; Thangaraj R. Antimicrobial Activity and Photocatalytic Degradation Properties of Zinc Sulfide Nanoparticles Synthesized by Using Plant Extracts. J. Nanostruct. 2018, 8, 107–118. [Google Scholar]
- Sathishkumar M.; Rajamanickam A. T.; Saroja M. Characterization, antimicrobial activity and photocatalytic degradation properties of pure and biosynthesized zinc sulfide nanoparticles using plant extracts. J. Mater. Sci.: Mater. Electron. 2018, 29, 14200–14209. 10.1007/s10854-018-9553-7. [DOI] [Google Scholar]
- Kannan S.; Subiramaniyam N. P.; Sathishkumar M. A novel green synthesis approach for improved photocatalytic activity and antibacterial properties of zinc sulfide nanoparticles using plant extract of Acalypha indica and Tridax procumbens. J. Mater. Sci.: Mater. Electron. 2020, 31, 9846–9859. 10.1007/s10854-020-03529-x. [DOI] [Google Scholar]
- Morshedtalab Z.; Rahimi G.; Emami-Nejad A.; Farasat A.; Mohammadbeygi A.; Ghaedamini N.; Negahdary M. Antibacterial Assessment of Zinc Sulfide Nanoparticles against Streptococcus pyogenes and Aeinetobacter baumannii. Curr. Top. Med. Chem. 2020, 20, 1042–1055. 10.2174/1381612826666200406095246. [DOI] [PubMed] [Google Scholar]
- Giridhar M.; Naik H. S. B.; Prabhakar M. C.; Naik M. M.; Ballesh N.; Mahesh M. C. Synthesis, characterization and antibacterial activity of water-soluble dye-capped zinc sulphide nanoparticles from waste Zn-C battery. Bull. Mater. Sci. 2021, 44, 6 10.1007/s12034-020-02287-0. [DOI] [Google Scholar]
- Li G. P.; Zhai J. F.; Li D.; Fang X. N.; Jiang H.; Dong Q. Z.; Wang E. K. One-pot synthesis of monodispersed ZnS nanospheres with high antibacterial activity. J. Mater. Chem. 2010, 20, 9215–9219. 10.1039/c0jm01776k. [DOI] [Google Scholar]
- Fakhri A.; Behrouz S.; Pourmand M. Synthesis, photocatalytic and antimicrobial properties of SnO2, SnS2 and SnO2/SnS2 nanostructure. J. Photochem. Photobiol., B 2015, 149, 45–50. 10.1016/j.jphotobiol.2015.05.017. [DOI] [PubMed] [Google Scholar]
- He X. Y.; Gan J. G.; Fakhri A.; Dizaji B. F.; Azarbaijan M. H.; Hosseini M. Preparation of ceric oxide and cobalt sulfide-ceric oxide/cellulose-chitosan nanocomposites as a novel catalyst for efficient photocatalysis and antimicrobial study. Int. J. Biol. Macromol. 2020, 143, 952–957. 10.1016/j.ijbiomac.2019.09.155. [DOI] [PubMed] [Google Scholar]
- Ashraf M. A.; Li C.; Zhang D. Q.; Fakhri A. Graphene oxides as support for the synthesis of nickel sulfide-indium oxide nanocomposites for photocatalytic, antibacterial and antioxidant performances. Appl. Organomet. Chem. 2020, 34, e5354 10.1002/aoc.5354. [DOI] [Google Scholar]
- Tiss B.; Moualhi Y.; Bouguila N.; Kraini M.; Alaya S.; Croitoru C.; Ghiuta I.; Cristea D.; Patroi D.; Moura C.; Cunha L. Influence of the Physical Properties on the Antibacterial and Photocatalytic Behavior of Ag-Doped Indium Sulfide Film Deposited by Spray Pyrolysis. Coatings 2021, 11, 370 10.3390/coatings11040370. [DOI] [Google Scholar]
- Lokhande A. C.; Shelke A.; Babar P. T.; Kim J.; Lee D. J.; Kim I. C.; Lokhandee C. D.; Kim J. H. Novel antibacterial application of photovoltaic Cu2SnS3 (CTS) nanoparticles. RSC Adv. 2017, 7, 33737–33744. 10.1039/C7RA05194H. [DOI] [Google Scholar]
- Kumar R. S.; Maddirevula S.; Easwaran M.; Dananjaya S. H. S.; Kim M. D. Antibacterial activity of novel Cu2ZnSnS4 nanoparticles against pathogenic strains. RSC Adv. 2015, 5, 106400–106405. 10.1039/C5RA15027B. [DOI] [Google Scholar]
- Ocakoglu K.; Dizge N.; Colak S. G.; Ozay Y.; Bilici Z.; Yalcin M. S.; Ozdemir S.; Yatmaz H. C. Polyethersulfone membranes modified with CZTS nanoparticles for protein and dye separation: Improvement of antifouling and self-cleaning performance. Colloids Surf., A 2021, 616, 126230 10.1016/j.colsurfa.2021.126230. [DOI] [Google Scholar]
- Song C. Q.; Li T. C.; Guo W.; Gao Y.; Yang C. Y.; Zhang Q.; An D.; Huang W. C.; Yan M.; Guo C. S. Hydrophobic Cu12Sb4S13-deposited photothermal film for interfacial water evaporation and thermal antibacterial activity. New J. Chem. 2018, 42, 3175–3179. 10.1039/C7NJ04545J. [DOI] [Google Scholar]
- Kulkarni P.; Nataraj S. K.; Balakrishna R. G.; Nagaraju D. H.; Reddy M. V. Nanostructured binary and ternary metal sulfides: synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. 10.1039/C7TA07329A. [DOI] [Google Scholar]
- Liu Y. C.; Li Y.; Kang H. Y.; Jin T.; Jiao L. F. Design, synthesis, and energy-related applications of metal sulfides. Mater. Horiz. 2016, 3, 402–421. 10.1039/C6MH00075D. [DOI] [Google Scholar]
- Baláž P.; Achimovičová M.; Baláž M.; Billik P.; Cherkezova-Zheleva Z.; Criado J. M.; Delogu F.; Dutková E.; Gaffet E.; Gotor F. J.; Kumar R.; Mitov I.; Rojac T.; Senna M.; Streletskii A.; Wieczorek-Ciurowa K. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]
- Baláž P.; Baláž M.; Achimovičová M.; Bujňáková Z.; Dutková E. Chalcogenide mechanochemistry in materials science: insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. 10.1007/s10853-017-1174-7. [DOI] [Google Scholar]
- Gock E.; Kurrer K. E. Eccentric vibratory mills-a new energy efficient way for pulverization. Erzmetall 1996, 49, 434–442. [Google Scholar]
- Baláž P.; Hegedüs M.; Reece M.; Zhang R.; Su T.; Škorvánek I.; Briančin J.; Baláž M.; Mihálik M.; Tešinský M.; Achimovičová M. Mechanochemistry for thermoelectrics: Nanobulk Cu6Fe2SnS8/Cu2FeSnS4 composite synthesized in an industrial mill. J. Electron. Mater. 2019, 48, 1846–1856. 10.1007/s11664-019-06972-7. [DOI] [Google Scholar]
- Baláž M.; Dobrozhan O.; Tešinský M.; Zhang R.-Z.; Džunda R.; Dutková E.; Rajňák M.; Chen K.; Reece M. J.; Baláž P. Scalable and environmentally friendly mechanochemical synthesis of nanocrystalline rhodostannite (Cu2FeSn3S8). Powder Technol. 2021, 388, 192–200. 10.1016/j.powtec.2021.04.047. [DOI] [Google Scholar]
- Baláž P.; Hegedüs M.; Baláž M.; Daneu N.; Šiffalovič P.; Bujňáková Z.; Tóthová E.; Tešinský M.; Achimovičová M.; Briančin J.; Dutková E.; Kaňuchová M.; Fabián M.; Kitazono S.; Dobrozhan O. Photovoltaic Materials: Cu2ZnSnS4 (CZTS) Nanocrystals Synthesized via Industrially Scalable, Green, One-step Mechanochemical Process. Prog. Photovoltaics 2019, 27, 798–811. 10.1002/pip.3152. [DOI] [Google Scholar]
- Baláž P.; Guilmeau E.; Daneu N.; Dobrozhan O.; Baláž M.; Hegedus M.; Barbier T.; Achimovičová M.; Kaňuchová M.; Briančin J. Tetrahedrites synthesized via scalable mechanochemical process and spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 1922–1930. 10.1016/j.jeurceramsoc.2020.01.023. [DOI] [Google Scholar]
- Hegedüs M.; Achimovičová M.; Hui H. J.; Guelou G.; Lemoine P.; Fourati I.; Juraszek J.; Malaman B.; Baláž P.; Guilmeau E. Promoted crystallisation and cationic ordering in thermoelectric Cu26V2Sn6S32 colusite by eccentric vibratory ball milling. Dalton Trans. 2020, 49, 15828–15836. 10.1039/D0DT03368E. [DOI] [PubMed] [Google Scholar]
- Baláž P.; Achimovičová M.; Baláž M.; Chen K.; Dobrozhan O.; Guilmeau E.; Hejtmánek J.; Knížek K.; Kubíčková L.; Levinský P.; Puchý V.; Reece M. J.; Varga P.; Zhang R.-Z. Thermoelectric CuS-Based Materials Synthesized via a Scalable Mechanochemical Process. ACS Sustainable Chem. Eng. 2021, 9, 2003–2016. 10.1021/acssuschemeng.0c05555. [DOI] [Google Scholar]
- Dutková E.; Sayagués M. J.; Fabián M.; Baláž M.; Achimovičová M. Mechanochemically synthesized ternary chalcogenide Cu3SbS4 powders in a laboratory and an industrial mill. Mater. Lett. 2021, 291, 129566 10.1016/j.matlet.2021.129566. [DOI] [Google Scholar]
- Baláž P.; Hegedüs M.; Achimovičová M.; Baláž M.; Tešinský M.; Dutková E.; Kaňuchová M.; Briančin J. Semi-industrial green mechanochemical syntheses of solar cell absorbers based on quaternary sulfides. ACS Sustainable Chem. Eng. 2018, 6, 2132–2141. 10.1021/acssuschemeng.7b03563. [DOI] [Google Scholar]
- Achimovičová M.; Baláž M.; Girman V.; Kurimský J.; Briančin J.; Dutková E.; Gaborová K. Comparative Study of Nanostructured CuSe Semiconductor Synthesized in a Planetary and Vibratory Mill. Nanomaterials 2020, 10, 2038 10.3390/nano10102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metchnikoff E.The Founders of Modern Medicine: Pasteur, Koch, Lister; Walden Publications, 1939; p 387. [Google Scholar]
- Rojas J. J.; Ochoa V. J.; Ocampo S. A.; Muñoz J. F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complementary Altern. Med. 2006, 6, 2 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T.; McCormick P. G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146. 10.1023/b:jmsc.0000039199.56155.f9. [DOI] [Google Scholar]
- Boldyrev V. V.; Tkacova K. Mechanochemistry of solids: Past, present, and prospects. J. Mater. Synth. Process. 2000, 8, 121–132. 10.1023/A:1011347706721. [DOI] [Google Scholar]
- Baláž M.Environmental Mechanochemistry: Recycling Waste into Materials Using High-Energy Ball Milling; Springer, 2021; p 619. [Google Scholar]
- Li J. J.; Shen J.; Li Z. Q.; Li X. D.; Sun Z.; Hu Z. G.; Huang S. M. Wet chemical route to the synthesis of kesterite Cu2ZnSnS4 nanocrystals and their applications in lithium ion batteries. Mater. Lett. 2013, 92, 330–333. 10.1016/j.matlet.2012.10.125. [DOI] [Google Scholar]
- Salton M. R. J.; Kim K. S.; Baron S.. Structure. In Medical Microbiology, 4th ed.; University of Texas Medical Branch: Galveston (TX), 1996. [PubMed] [Google Scholar]
- Valodkar M.; Modi S.; Pal A.; Thakore S. Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. 10.1016/j.materresbull.2010.12.001. [DOI] [Google Scholar]
- Hou X.; Li Y.; Yan J. J.; Wang C. W. Highly efficient photocatalysis of p-type Cu2ZnSnS4 under visible-light illumination. Mater. Res. Bull. 2014, 60, 628–633. 10.1016/j.materresbull.2014.09.041. [DOI] [Google Scholar]
- Chirayil C. J.; Abraham J.; Mishra R. K.; George S. C.; Thomas S.. Instrumental Techniques for the Characterization of Nanoparticles. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier, 2017; Vol. 3, pp 1–36. [Google Scholar]
- Dutková E.; Bujňáková Z.; Kováč J.; Škorvánek I.; Sayagues M. J.; Zorkovská A.; Kováč J. Jr.; Baláž P. Mechanochemical synthesis, structural, magnetic, optical and electrooptical properties of CuFeS2 nanoparticles. Adv. Powder Technol. 2018, 29, 1820–1826. 10.1016/j.apt.2018.04.018. [DOI] [Google Scholar]
- Dutková E.; Sayagués M. J.; Fabián M.; Kováč J.; Baláž M.; Stahorský M. Mechanochemical synthesis of ternary chalcogenide chalcostibite CuSbS2 and its characterization. J. Mater. Sci.: Mater. Electron. 2021, 32, 22898–22909. 10.1007/s10854-021-06767-9. [DOI] [Google Scholar]
- Mitchell T. K.; Nguyen A. V.; Evans G. M. Heterocoagulation of chalcopyrite and pyrite minerals in flotation separation. Adv. Colloid Interface Sci. 2005, 114–115, 227–237. 10.1016/j.cis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Wang X.; Forssberg E.; Bolin N. J. The aqueous and surface chemistry of activation in the flotation of sulphide minerals—A review. Part II: A surface precipitation model. Miner. Process. Extr. Metall. Rev. 1989, 4, 167–199. 10.1080/08827508908952636. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.