Abstract
An efficient and environmentally friendly method was established for designing novel 3-amino-1,4-dihydroquinoxaline-2-carbonitrile (1) via the reaction of bromomalononitrile and benzene-1,2-diamine under microwave irradiation in an excellent yield (93%). This targeted amino derivative was utilized for the construction of a series of Schiff bases (8–13). A new series of thiazolidinone derivatives (15–20) were synthesized in high yields (89–96%) via treatment of thioglycolic acid with Schiff bases (8–13) under microwave irradiation in high yields (89–96%). Moreover, new pyrimidine derivatives (26–30 and 35–38) were prepared by treatment of compound 1 with arylidenes (21–25) and/or alkylidenemalononitriles (31–34) using piperidine as a basic catalyst under microwave conditions. Based on elemental analyses and spectral data, the structures of the new assembled compounds were determined. The newly synthesized quinoxaline derivatives were screened and studied as an insecticidal agent against Aphis craccivora. The obtained results indicate that compound 16 is the most toxicological agent against nymphs of cowpea aphids (Aphis craccivora) compared to the other synthesized pyrimidine and thiazolidinone derivatives. The molecular docking study of the new quinoxaline derivatives registered that compound 16 had the highest binding score (−10.54 kcal/mol) and the thiazolidinone moiety formed hydrogen bonds with Trp143.
Introduction
Aphis craccivora, also identified as the cowpea aphid, is among the most threatening crop pests causing direct damage to plants by distorting and delaying plant growth.1,2 The molasses produced by the vector are applied on the plant and encourage mold growth with soot limiting photosynthesis.3 It hosts many plants such as Rosaceae, Malvaceae, Asteraceae, Caryophyllaceae, Solanaceae, Chenopodiaceae, and Ranunculaceae families, but it appears to prefer groups of the bean family.4−6 Aphids are vectors for a number of plant viruses such as mosaic virus, mottle virus, alfalfa mosaic virus, and peanut virus.7,8
Nicotinic acetylcholine receptors (nAChRs) are agonist-gated ion channels that belong to the Cys loop superfamily.9−11 They are widely dispersed throughout the nervous system and take part in the regulation of the main physiological functions and pathophysiological processes.12−14 Many therapeutic agents, toxicants, and insecticides target these nAChRs that mediate excitatory neurotransmission.15−17
There are many strategies for aphid control relying on eco-friendly agrochemicals.18−20 For example, (E)-β-farnesene is released from aphid cornicles to alarm others nearby, and it is the major component of warning pheromones for most aphid species.21 In addition to the alarm feature of (E)-β-farnesene, it exhibited aphicidal potential at a dose of 100 mg and revealed a synergistic effect when combined with imidacloprid to control aphid breeding.22 The disadvantage of its use is its instability attributed to its conjugated double bond.23 Another strategy for aphid control is the use of insect kinins, which are a group of neuropeptides with many biological functions and highly present in arthropods and invertebrates.24 Insect kinins exhibited aphicidal and antifeedant potential that can control and interfere with biological processes of insects such as muscle contraction, release of digestive enzymes, and water–sodium balance that lead to insect death.25 Unfortunately, there are many limitations of kinins for application in peptidase inactivation and easy degradation.26
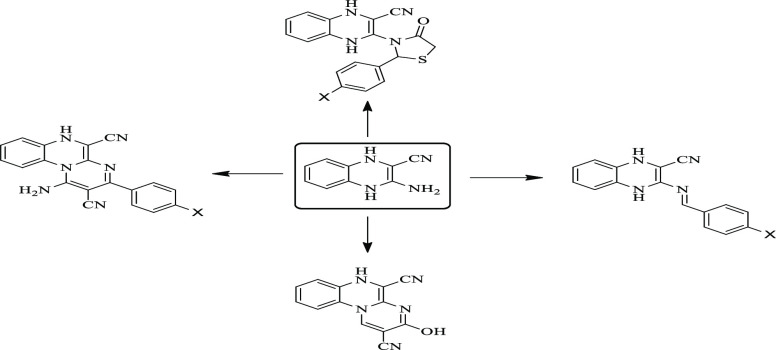
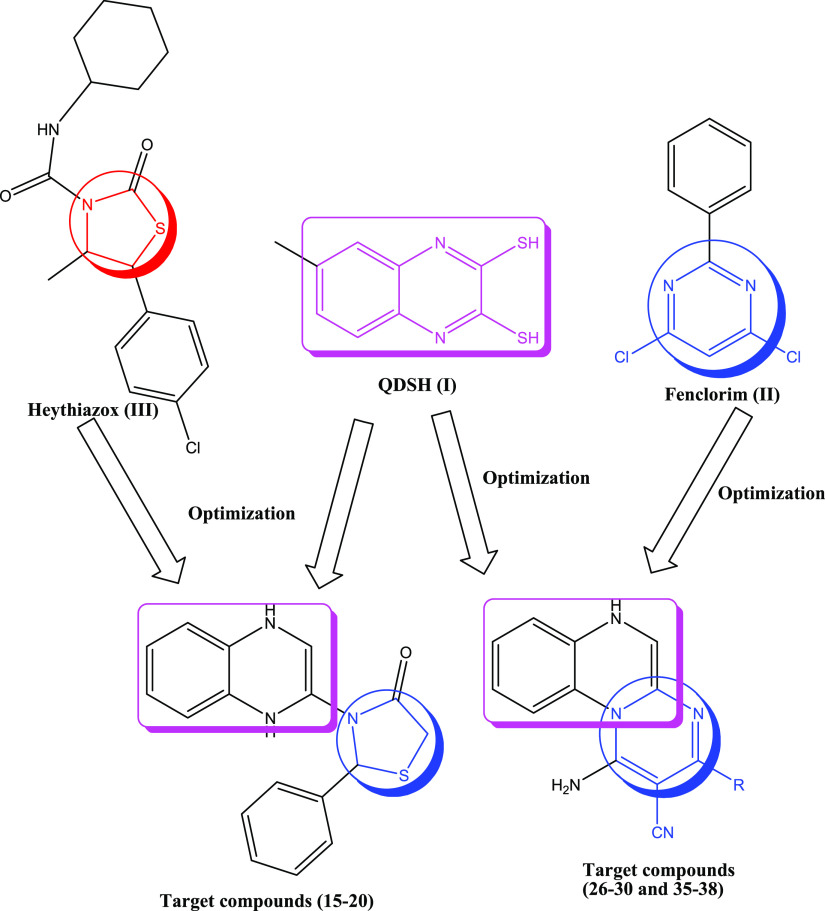
Quinoxaline-based compounds are among some molecular structures that have been recorded to be used for many different purposes in agrochemistry and medicine because of their biological potential as anti-inflammatory,27−29 antimicrobial,30−32 antifungal,33,34 antibiotic,35,36 and insecticidal agents.37−39 Moreover, quinoxalines were reported in the literature to be active inhibitors of nicotinic acetylcholine.40 QDSH (I) was reported in the literature to exhibit insecticidal potential utilized for controlling ticks and phytophagous mites41 (Figure 1).
Figure 1.
Design strategy of the novel target compounds 15–20, 26–30, and 35–38.
On the other hand, pyrimidine and thiazolidinone heterocycles attracted chemists’ attention due to their biological potential as well as their agrochemical effects. Both heterocycles exhibited potential as anticancer,42−45 anti-inflammatory,46−49 hypoglycemic,50,51 antifungal,52 antioxidant,53−55 antibacterial,31,56,57 and insecticidal agents58−61 and nicotinic acetylcholine inhibitors.62−65
For example, Fenclorim (II) is a herbicide used for controlling annual grasses, broadleaf weeds, and some edges66−68 (Figure 1). On the other hand, Heythiazox (III) belongs to a thiazolidinone derivative having nymphicidal and larvicidal potential toward mites and leafhoppers that could be administrated at any plant growth stage from budding till fruiting69 (Figure 1).
The abovementioned findings and our previous studies related to the discovery of novel bioactive agents70−78 encouraged us to construct novel quinoxaline derivatives and assess these novel candidates for their insecticidal potential against Aphis craccivora. Our design based upon combining the quinoxaline ring with the widely documented insecticidal pyrimidine heterocycle and/or thiazolidinone in one hybrid to obtain pyrimido[1,2-a]quinoxaline derivatives (26–30), 35–38, and thiazolidin-3-yl-1,4-dihydroquinoxaline (15–20) aims to increase the insecticidal activity and capacity to destroy Aphis craccivora as explained in Figure 1. A molecular docking study was carried out to propose the binding mode of the novel target compounds as insecticidal agents.
Results and Discussion
Chemistry
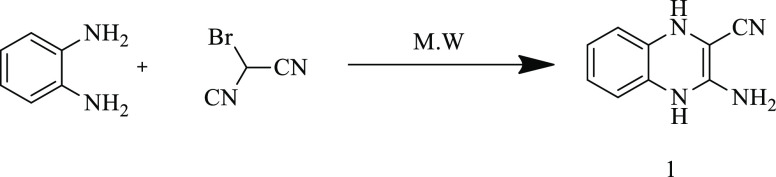
The parent compound 3-amino-1,4-dihydroquinoxaline-2-carbonitrile (1) was simply prepared via treating bromomalononitrile and benzene-1,2-diamine under microwave irradiation (Scheme 1). The IR spectrum of compound 1 revealed the appearance of NH2 and C≡N groups at 3436, 3344, and 2202 cm–1. The 1H NMR spectrum of compound 1 showed a new singlet signal at δ 10.93 ppm due to one NH group, and also, the aromatic signals corresponding to NH and NH2 groups appeared in the region δ 7.25–6.66 ppm (NH and NH2 disappeared on deuteration). The 13C NMR spectrum of compound 1 indicated the presence of (δ 150.11) C≡N, (130.89, 127.37, 123.43, 120.34, 116.08) CH=CH, and C=C.
Scheme 1. Synthesis of 3-Amino-1,4-dihydroquinoxaline-2-carbonitrile.
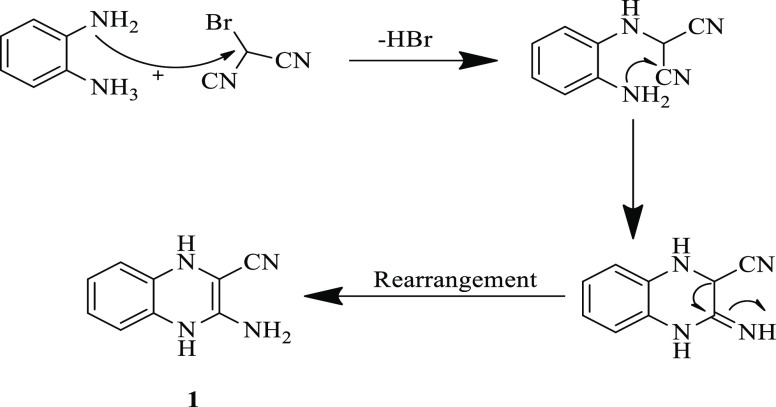
A possible mechanism of the formation of compound 1 is described in Scheme 2.
Scheme 2. Possible Mechanisms for the Synthesis of Compound 1.
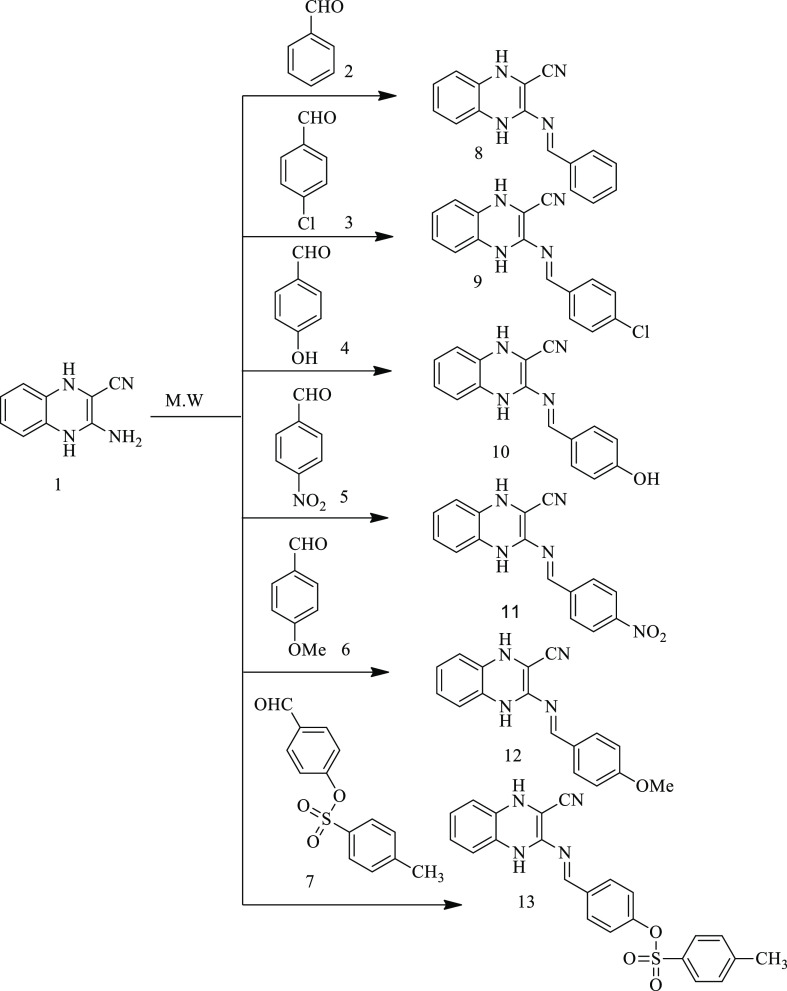
The starting material 1 was smoothly reacted with some aromatic aldehydes 2–7, namely, benzenecarbaldehyde (2), 4-chlorobenzenecarbaldehyde (3), 4-hydroxybenzenecarbaldehyde (4), 4-nitrobenzenecarbaldehyde (5), p-methoxybenzenecarbaldehyde (6), and 4-tosyloxybenzenecarbaldehyde (7) via microwave irradiation in ethanol for 8–12 min to afford the corresponding Schiff bases (8–13) (Scheme 3).
Scheme 3. Synthesis of Schiff Bases 8–13.
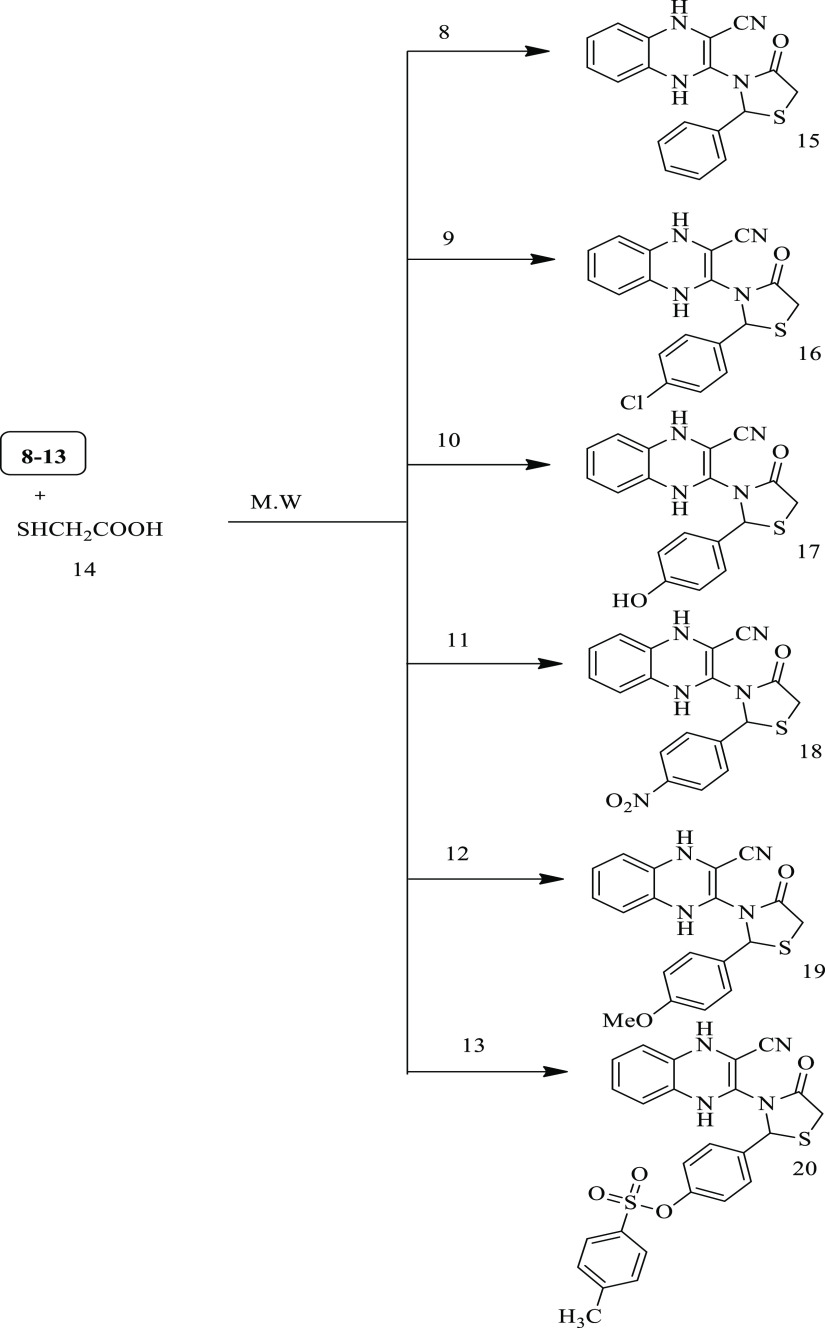
The structures of the obtained Schiff bases (8–13) were established by using IR, 1H NMR, 13C NMR, and elemental analyses. The IR spectra of these target compounds (8–13) displayed the absence of NH2 and CO bands of absorption and the appearance of a new band in the region 1630–1645 cm–1 due to CH=N groups. The 1H NMR (DMSO-d6) spectra exhibited, in addition to the expected aromatic protons signals, new singlet signals located in δ 8.60–8.25 ppm attributed to N=CH, at δ 10.89 ppm for the proton of the OH group (in compound 10), at δ 3.89 ppm for the OCH3 group (compound 12), and at δ 2.43 ppm for the CH3 group in compound 13. Moreover, their 13C NMR spectra indicated new signals that appeared at δ 155.88–152.12 ppm due to the CH=N group. Additionally, elemental analyses and mass spectra of compounds 8–13 confirmed the proposed structures. Further, 1,4-dihydroquinoxaline-2-carbonitrile derivatives (15–20) were synthesized via the treatment of thioglycolic acid (14) with Schiff bases (8–13) under microwave irradiation conditions (Scheme 4). The following are some of the benefits of this process: high yields (89–96%), shorter reaction time (10–15 min), easy workup, lower cost, and safety; pollution issues associated with toxic solvent use were avoided. The optimized results are summarized in Table 1.
Scheme 4. Synthesis of 3-(4-Oxo-2-phenyl-1,3-thiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile Derivatives (15–20).
Table 1. The Yields and Required Time for Thiazolidinone and Pyrimidine Formation Using Two Methods.
| microwave technique |
conventional method |
|||
|---|---|---|---|---|
| comp. no. | yield (%) | time (min) | yield (%) | time (h) |
| 15 | 95 | 10 | 52 | 6 |
| 16 | 89 | 15 | 61 | 7 |
| 17 | 94 | 11 | 55 | 7 |
| 18 | 90 | 14 | 62 | 7 |
| 19 | 96 | 12 | 60 | 6 |
| 20 | 93 | 12 | 57 | 6 |
| 26 | 85 | 8 | 56 | 6 |
| 27 | 88 | 10 | 57 | 5 |
| 28 | 86 | 9 | 62 | 5 |
| 29 | 89 | 12 | 60 | 6 |
| 30 | 87 | 9 | 58 | 7 |
| 35 | 95 | 11 | 55 | 6 |
| 36 | 90 | 13 | 59 | 7 |
| 37 | 94 | 12 | 65 | 6 |
| 38 | 91 | 10 | 57 | 7 |
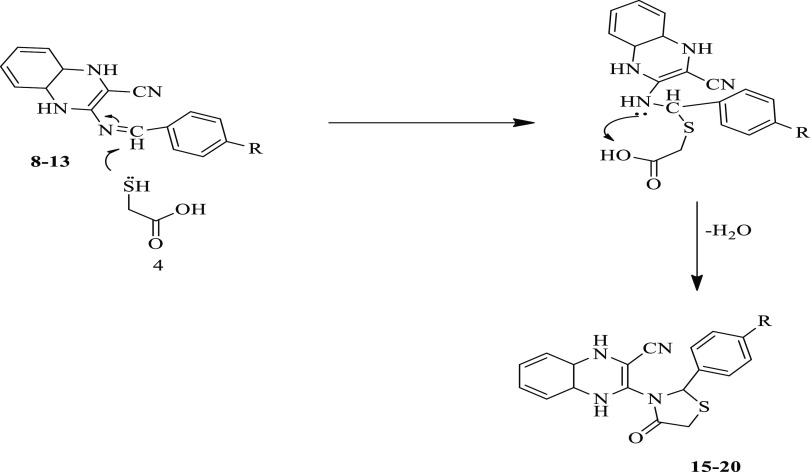
The IR spectra of compounds 15–20 showed the appearance of new carbonyl groups within the region 1641–1649 cm–1. Moreover, the 1H NMR (DMSO-d6) spectra showed, signals other than aromatic protons that are also present, new signals within the region δ 4.03–3.47 ppm consistent with the CH2 groups and singlet signals related to SCH– groups in the region δ 6.31–5.73 ppm. Furthermore, 13C NMR spectra and elemental analysis results of these compounds (15–20) confirmed the proposed structures of the thiazolidinone ring. For example, the spectrum of 13C NMR for compound 20 presented in addition to the expected aromatic signals the appearance of a new signal at δ 21.85 ppm due to the CH3 group, a new signal at δ 62.15 ppm for the CH2 group, and at δ 170.84 ppm because of the C–O group. Moreover, its DEPT-135 spectrum revealed no sign of carbonyl groups as well as the appearance of an opposite phase signal at δ 62.83 ppm for the CH2 group. The possible mechanism for the formation of compounds 15–20 is presented in Scheme 5.
Scheme 5. The Possible Mechanism for the Formation of Compounds 15–20.
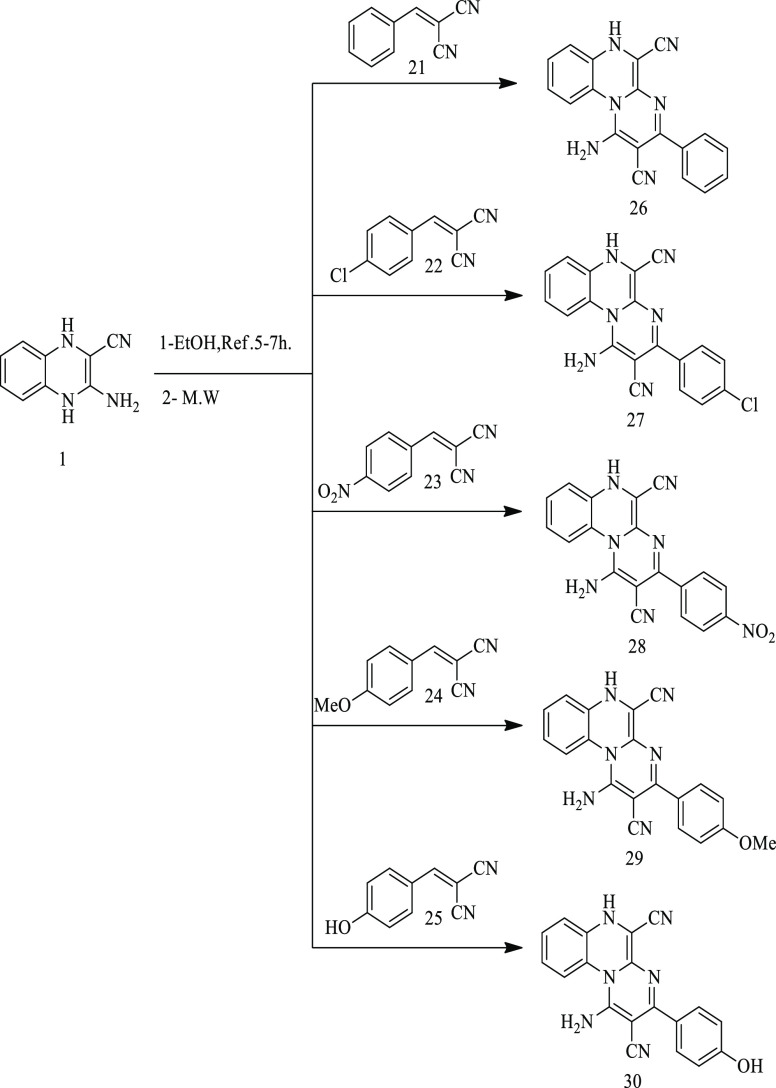
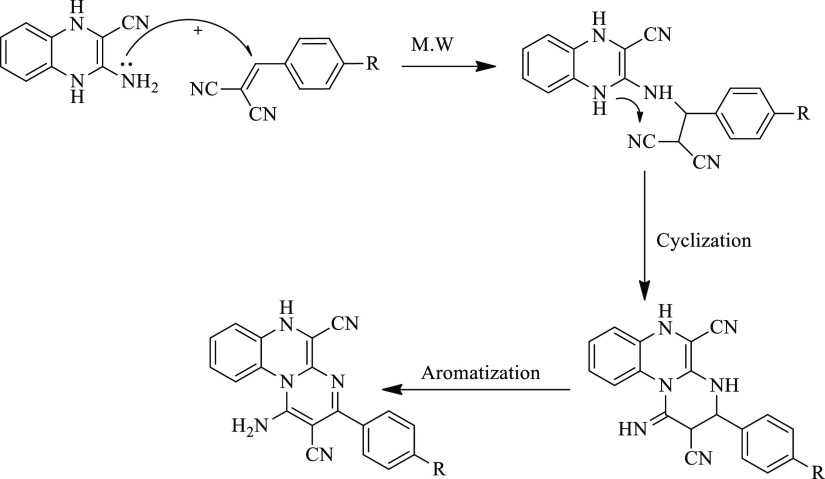
1-Amino-3-phenyl-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile derivatives (26–30) were synthesized via two methods. The first method was the treatment of compound 1 with some selected aromatic arylidenes (21–25) under refluxing in ethanol for 5–7 h. In this traditional method, the reaction took a lot of time with moderate yields (56–65%). The second method was the treatment of the previous mixture under microwave irradiation conditions in ethanol as displayed in Scheme 6 and Table 1. In this simple protocol, the targeted products (26–30) were obtained in high yields (85–89%) within a short reaction time (8–13 min). The optimized results are summarized in Table 1.
Scheme 6. Synthesis of Pyrimidine Derivatives 26–30.
The IR spectra of compounds 26–30 displayed the appearance of new NH2 and C≡N groups within the regions 3387–3194 and 2209–2222 cm–1, respectively, which occurred in regions different from those of the starting material. The 1H NMR spectrum in DMSO-d6 displayed the aromatic protons signals and singlet signals corresponding to NH and NH2 groups in the region δ 10.24–6.03 ppm. Furthermore, 13C NMR spectra and elemental analyses of compounds 26–30 confirmed the expected structures of the pyrimidine ring. The 13C NMR spectrum of compound 27, for example, displayed the following signals: δ 155.91, 153.53, 136.26, 134.94, 132.31, 131.63, 131.04, 129.88, 129.56, 129.38, 129.30, 128.58, 121.36, 117.69, 113.14 ppm. The formation of the pyrimidines 26–30 might be started through the nucleophilic attack of NH2 groups to activated carbon double bonds and subsequent cyclization via another nucleophilic attack of NH groups to C≡N groups. Finally, the obtained intermediate underwent aromatization via losing a hydrogen atom to afford the pyrimidine derivatives 26–30 (Scheme 7).
Scheme 7. The Plausible Mechanism for the Formation of Pyrimidine Derivatives 26–30.
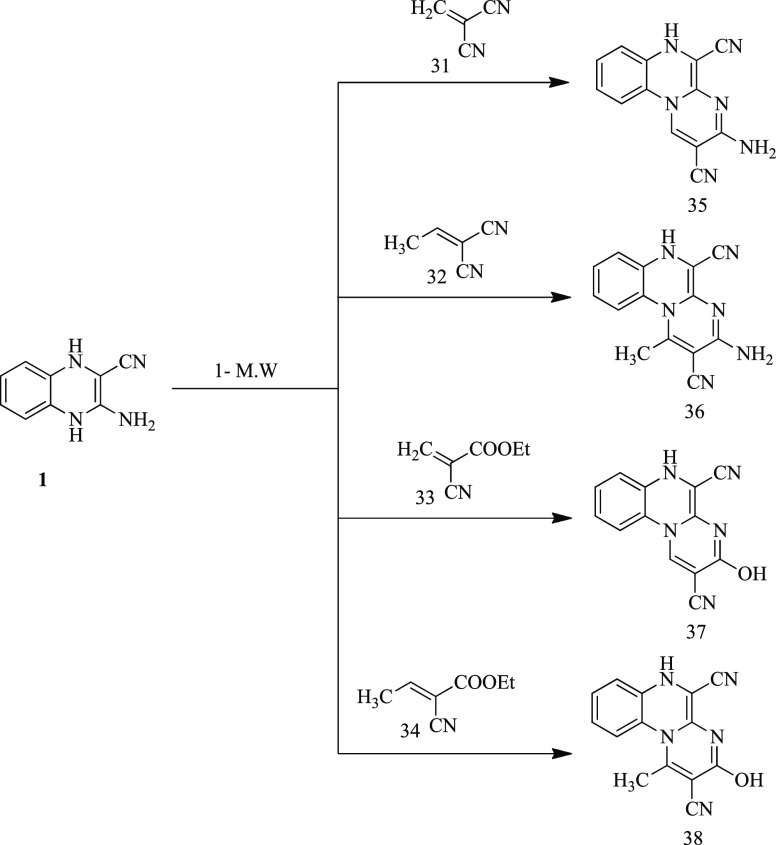
The treatment of a compound 1, with alkylidenemalononitriles (31–34)79,80 in ethanol containing few drops of piperidine (2–5 drops), afforded the corresponding pyrimidine derivatives (35–38) (Scheme 8) in low yields (55–65%). Meanwhile, irradiation of the aforesaid reaction mixture by microwaves for 10–13 min afforded the corresponding pyrimidine derivatives (35–38) in excellent yields (90–95%) (Table 1). New structures for these products (35–38) were derived from IR, 1H NMR, 13C NMR, and elemental analyses. The IR spectra of compounds 35–38 showed the disappearance of aldehydic C=O groups and the appearance of NH groups at 3222 and at 3204 cm–1 (derivatives 35 and 36, respectively) and OH groups at 3420 and 3446 (in compounds 37 and 38, respectively), in addition to C≡N groups at 2192–2223 cm–1. 1H NMR spectra of compounds 36–38 showed, in addition to the signals from the aromatic proton, new singlet signals related to NH protons at δ 10.21–9.73 ppm (disappeared on deuteration in compounds 35 and 36). In addition to the appearance of CH3 protons in compounds 36 and 38 at δ 3.79 and 3.26 ppm, respectively, their 13C NMR spectra showed new signals at δ 28.07 and 21.07 ppm due to CH3 groups, respectively.
Scheme 8. Synthesis of Pyrimidine Derivatives 35–38.
Insecticidal Bioefficacy Screening
Toxicological Activity Test for Nymphs of Cowpea Aphid (Aphis craccivora) Insects Following Treatment for 24 h
The insecticidal effectiveness of all synthesized compounds had been evaluated; results of compounds 1, 9, 15, 16, 18, 20, 26, 27, 28, 30, 37, and 38 against nymphs of Aphis craccivora are illustrated in Table 2. In the 24 h period after the treatment, bioefficacy results of the synthesized pyrimidine and thiazolidinone derivatives exhibited a high to low level of toxicological activity toward nymphs of cowpea aphids after 24 h of the testing with LC50. The values range from 0.021 to 1.023, that is, LC50 values of compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30, and 37 were 1.023, 0.101, 0.227, 0.021, 0.551, 0.053, 0.444, 0.284, 0.039, 0.334, 0.787, and 0.944 ppm, respectively. According to this result, compound 16 was the most toxic agent against nymphs of cowpea aphids (Aphis craccivora) after the treatment has been completed for 24 h compared to the other synthesized pyrimidine and thiazolidinone derivatives.
Table 2. Insecticidal Activity of Compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30, and 37 toward the Nymphs and Adults of Cowpea Aphid (Aphis craccivora) Insects after 24 h of Treatment.
| nymphs |
adults |
|||||
|---|---|---|---|---|---|---|
| comp. | LC50 (ppm) | slope | toxic ratio | LC50 (ppm) | slope | toxic ratio |
| 1 | 1.023 | 0.312 ± 0.031 | 0.020 | 2.101 | 0.362 ± 0.024 | 0.048 |
| 9 | 0.101 | 0.382 ± 0.031 | 0.207 | 0.570 | 0.316 ± 0.077 | 0.178 |
| 15 | 0.227 | 0.340 ± 0.033 | 0.092 | 0.620 | 0.231 ± 0.023 | 0.164 |
| 16 | 0.021 | 0.311 ± 0.032 | 1 | 0.102 | 0.132 ± 0.076 | 1 |
| 18 | 0.551 | 0.350 ± 0.035 | 0.381 | 1.226 | 0.233 ± 0.023 | 0.083 |
| 20 | 0.053 | 0.303 ± 0.030 | 0.396 | 0.267 | 0.270 ± 0.024 | 0.382 |
| 26 | 0.444 | 0.291 ± 0.038 | 0.047 | 1.485 | 0.193 ± 0.023 | 0.068 |
| 38 | 0.284 | 0.304 ± 0.032 | 0.073 | 1.470 | 0.247 ± 0.023 | 0.068 |
| 27 | 0.039 | 0.301 ± 0.0302 | 0.538 | 0.130 | 0.130 ± 0.077 | 0.784 |
| 28 | 0.334 | 0.391 ± 0.0303 | 0.062 | 1.660 | 0.371 ± 0.032 | 0.061 |
| 30 | 0.787 | 0.321 ± 0.0301 | 0.026 | 2.236 | 0.362 ± 0.209 | 0.045 |
Toxicological Activity Test for Adults of Cowpea Aphids (Aphis craccivora) after 24 h of Treatment
Results of compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30, and 37 were tested against cowpea aphids (Aphis craccivora). These can be seen in Table 2. The bioefficacy of synthesized compounds was measured 24 h after treatment, and the compounds exhibited a high to low level of toxicological activity against the adults of cowpea aphids (Aphis craccivora) because they were nearly as active as others after 24 h of testing with LC50. The values range from 0.101 to 2.236 for compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30 and 37 with LC50 values of 2.101, 0.570, 0.620, 0.102, 1.226, 0.267, 1.485, 1.470, 0.130, 1.660, 2.236, and 1.382 in ppm. This result indicated that compound toxicity was high for compound 16 against adults of Aphis craccivora with an LC50 value equal to 0.102 ppm.
Structure–Activity Relationship
Based on the toxicity value, the structure–activity relationship is shown in Table 2 and Figure 2. From the synthetic pyrimidine and thiazolidinone derivatives, compound 16 was more active in combating nymphs and adults of Aphis craccivora than the other pyrimidine and thiazolidinone derivatives. A high level of activity is associated with the compounds 16, 20, and 27, and there is a possibility that it is due to chlorophenyl, CN groups, and benzene sulfanyl moieties in their structure. Based on the toxicity results in Table 2, some structure–activity relationship could be concluded. Adding the thiazolidinone ring to the quinoxaline moiety markedly increased the insecticidal potential of the target compounds against Aphis craccivora. This is clear when comparing 3-amino-1,4-dihydroquinoxaline-2-carbonitrile (1) (LC50 = 2.101 ppm) with thiazolidin-3-ylquinoxaline derivatives 15–20, which exhibited a higher insecticidal potential within the LC50 range = 0.102–1.226 ppm. Regarding the substituents on the thiazolidinone moiety, attaching electron-withdrawing groups as p-chlorophenyl (compound 16, LC50 = 0.1 ppm) and benzenesulfonate (compound 20, LC50 = 0.26 ppm) to the thiazolidinone ring displayed a higher insecticidal potential than the phenyl ring (compound 15, LC50 = 0.6 ppm). On the other hand, hybridizing the pyrimidine ring with the quinoxaline moiety increased the insecticidal effect of the target compounds 26 (LC50 = 1.485 ppm), 27 (LC50 = 0.130 ppm), 28 (LC50 = 1.660 ppm), 37 (LC50 = 1.382 ppm), and 38 (LC50 = 1.470 ppm) except compound 30 (LC50 = 2.236 ppm), which recorded a comparable insecticidal effect against Aphis craccivora to that registered by the quinoxaline compound 1 (LC50 = 2.101 ppm).
Figure 2.
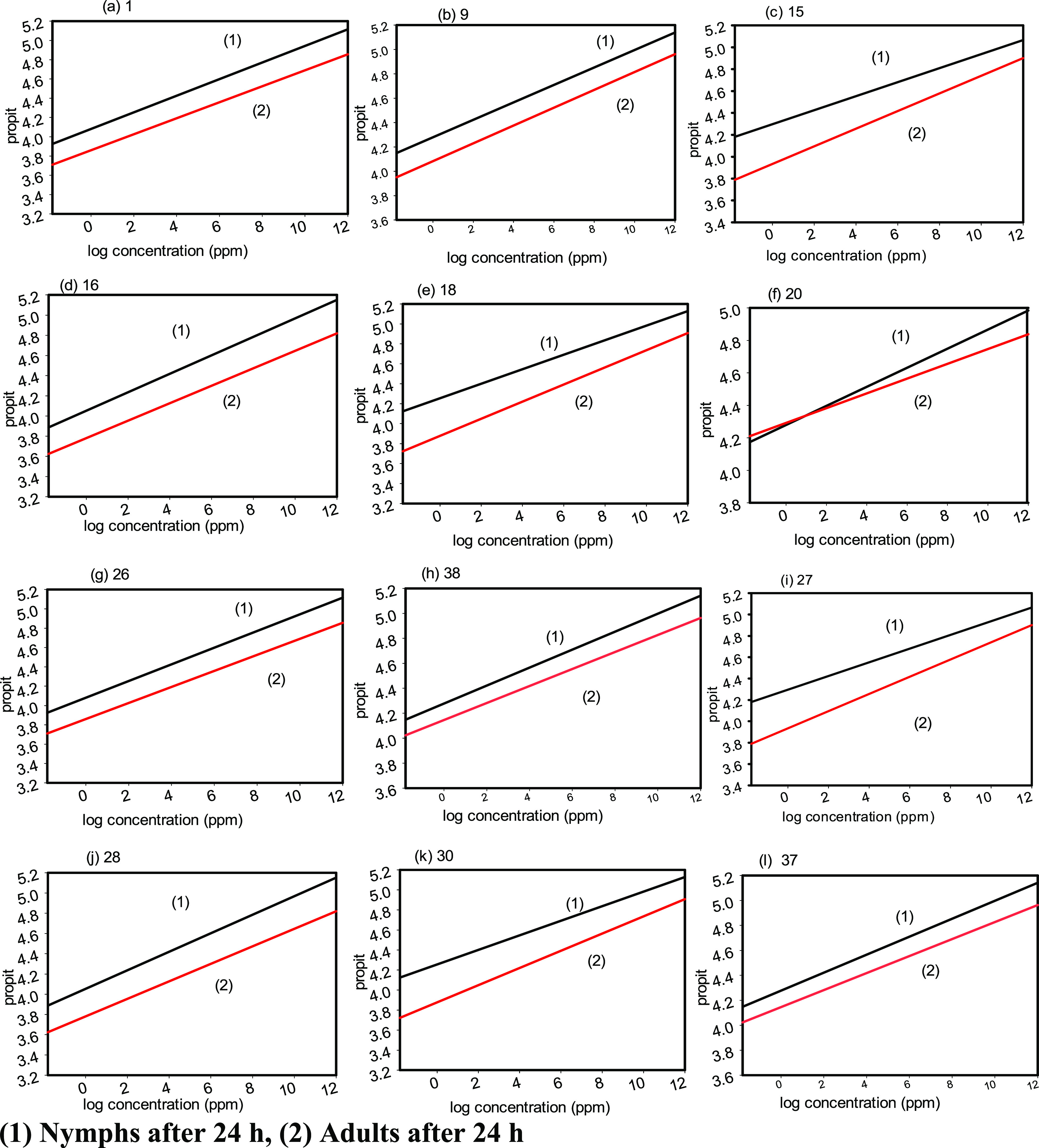
(a–l) Insecticidal activities of compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30, and 37 for the nymphs and adults of cowpea aphid (Aphis craccivora) insects following a 24 h treatment period.
From the results recorded in Table 2, we conclude that the most toxic compounds to cowpea aphids are 16, 20, and 27.
Docking Study
Using X-ray crystal structures, a docking study was conducted for acetylcholine (protein AChBP) from Lymnaea stagnalis (PDB 2ZJU) due to the unavailability of Aphis craccivora crystal structures. The cocrystallized ligand imidaclorid was redocked within the binding region of AChBP with RMSD = 1.3201 and binding energy score = −8.17 kcal/mol. The target compounds 1, 9, 15, 16, 18, 20, 26, 38, 27, 28, 30, and 37 were docked within the AChBP and H-bonding amino acid residues, and H-bond lengths and binding energy scores are recorded in Table 3.
Table 3. The Binding Interactions and Affinity (kcal/mol) of 1, 9, 15, 16, 18, 20, 26, 27, 28, 30, 38, and 37 within AChBP Binding Regions.
| compound | affinity (kcal/mol) | no. of hydrogen bonds | distance (Å) from the main residue | functional group | |
|---|---|---|---|---|---|
| 1 | –5.34 | 1 | Trp143 | 2.58 | NH2 |
| 9 | –8.51 | 1 | Trp143 | 2.69 | C=N |
| 15 | –8.45 | 3 | Trp143 | 3.15 | C≡N |
| Thr144 | 3.09 | NH | |||
| Cys187 | 2.94 | thiazolidinone S | |||
| 16 | –10.54 | 1 | Trp143 | 3.17 | thiazolidinone C=O |
| 18 | –7.42 | 2 | Cys147 | 3.07 | NH |
| Trp143 | 2.94 | thiazolidinone S | |||
| 20 | –8.42 | 3 | Glu190 | 2.86 | NH |
| Tyr192 | 3.08 | C≡N | |||
| Trp143 | 3.21 | S=O | |||
| 26 | –7.39 | 1 | Trp143 | 2.78 | NH2 |
| 27 | –9.46 | 2 | Cys188 | 2.56 | NH |
| Cys187 | 3.05 | NH | |||
| 28 | –8.27 | 3 | Trp143 | 2.89 | C≡N |
| Cys188 | 3.09 | NH | |||
| Trp143 | 2.62 | C≡N | |||
| 30 | –7.07 | 2 | Cys188 | 3.03 | NH |
| 38 | –7.11 | 1 | Trp143 | 2.88 | NH2 |
| 37 | –7.08 | 1 | Trp143 | 2.60 | NH2 |
| imidacloprid | –8.17 | 1 | Trp143 | 2.83 | pyridyl N |
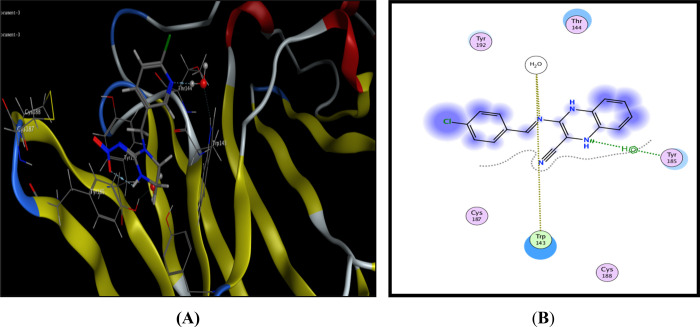
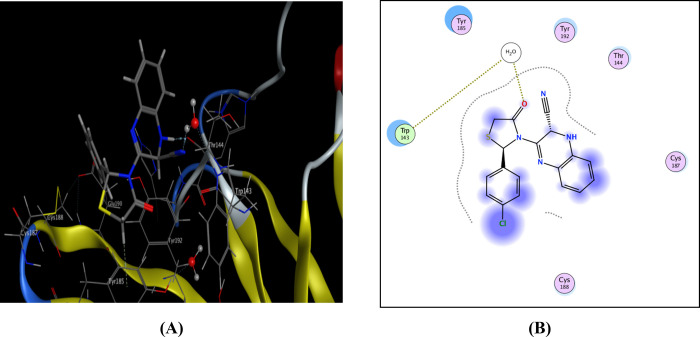
Compound 9 displayed similar fitting with the AChBP binding site as imidaclorid with binding score = −8.51 kcal/mol. Furthermore, this compound 9 formed two binding interactions; azomethine N bonded with Trp143 via H-bonds, and the quinoxaline moiety interacted with Tyr385 via arene-H interactions (Figure 3).
Figure 3.
Possible binding interaction of compound 9 within AChBP. (A) 3D binding with Trp143 and (B) 2D binding of compound 9 inside active sites.
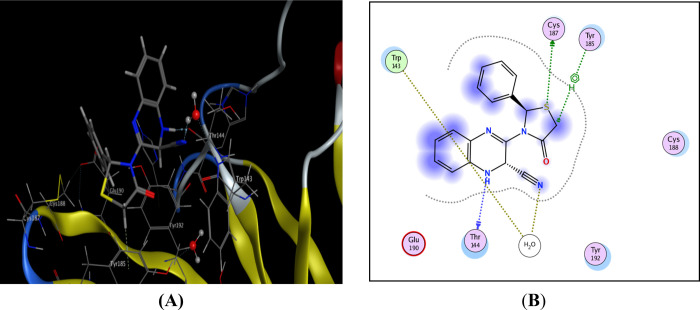
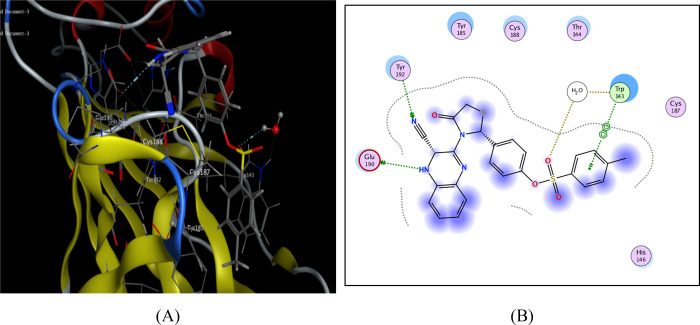
On the other hand, the thiazolidinoquinoxaline derivative 15 produced arene-H interactions with the thiazolidino moiety in addition to 3 hydrogen bonds as follows: (i) Trp143 with CN, (ii) Thr144 with quinoxaline NH, and (iii) Cys187 with thiazolidino S (Figure 4).
Figure 4.
Possible binding interaction of compound 15 throughout AChBP. (A) 3D binding with Trp143, Thr144, and Cys187 and (B) 2D binding of compound 15 inside active sites.
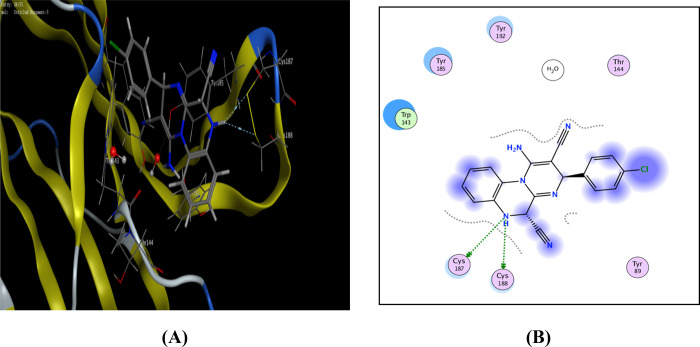
Regarding the most active compound toward nymph of cowpea aphids (16), it registered the highest binding score (−10.54 kcal/mol), and the thiazolidinone C=O formed a hydrogen bond with Trp143 (Figure 5).
Figure 5.
Possible binding interaction of compound 16 throughout AChBP. (A) 3D binding with Trp143 and (B) 2D binding of compound 16 inside active sites.
In addition, the phenyl ring of compound 20 made hydrophobic interactions with Trp143 with the binding score equal to −8.42 kcal/mol. Additionally, this compound revealed hydrogen bonding with Glu190, Tyr192, and Trp143 as depicted in Figure 6.
Figure 6.
Possible binding interaction of compound 20 within AChBP. (A) 3D binding with Glu199, Tyr192, and Trp143 and (B) 2D binding of compound 20 inside active sites.
Finally, compound 27 formed two hydrogen bonds through interactions of the NH group of quinoxaline with Cys188 and Cys187 amino acid residues (Figure 7).
Figure 7.
Possible binding interaction of compound 27 throughout AChBP. (A) 3D binding with Cys187 and Cys188 and (B) 2D binding of compound 27 to active sites.
Experimental Section
Chemistry
We purchased all commercially available reagents from Merck, Aldrich, and Fluka and did not purify them further. The reactions were monitored via thin-layer chromatography (TLC), using precoated silica gel G/UV-254 plates (Merck 60F254) and UV light (254/365 nm). Using a Kofler melting point apparatus, melting points were detected and uncorrected. As a result of the attenuated total reflection (ATR) method, FT-IR spectra were recorded with an FT-IR ALPHA Bruker platinum ATR spectrometer and are given in cm–1. 1H NMR and 13C NMR (DMSO-d6) spectra were recorded at 400 and 100 MHz, respectively, on a Bruker BioSpin AG spectrometer and DEPT-135 (ppm). Mass spectra were obtained at 70 eV using a GCMS-QP 1000EX spectrometer from Shimadzu. PerkinElmer CHN analyzer models were used to obtain the elemental analyses. Kenstar OM9925E (2450 MHz, 800 W) microwave ovens were used to carry out microwave irradiation.
Synthesis of 3-Amino-1,4-dihydroquinoxaline-2-carbonitrile (1)
A mixture of bromomalononitrile (2.0 mmol) and benzene-1,2-diamine (2.0 mmol) was added into 5.0 mL of absolute ethanol; then, the mixture was irradiated in a microwave oven for 5 min. Once the product was cooled to room temperature, the formed precipitate was filtered off, washed with cold ethanol (2 × 10 mL), dried, and crystallized from ethanol.
Yield: 93%; color: brown solid; m.p. 186–188 °C; IR (ATR): 3436, 3344 (NH2), 3235, 3176 (2NH), 2202 (C≡N) cm–1; 1H NMR: δ 10.93 (s, 1H, NH), 7.25–6.66 (m, 7H, 4-CHarom. + NH2, +NH); 13C NMR: δ 150.11, 130.89, 127.37, 123.43, 120.34, 116.08; MS, m/z (%): 172 (M+, 15); anal. calcd. for C9H8N4 (172.19): C, 62.78%; H, 4.68%; N, 32.54%. Found: C, 62.84%; H, 4.62%; N, 32.59%.
Schiff Bases (8–13): General Procedure for Their Synthesis
Mixtures of quinoxaline 1 (2.0 mmol) and (2.0 mmol) aromatic aldehydes, namely, benzenecarbaldehyde (2), 4-chlorobenzenecarbaldehyde (3), 4-hydroxybenzenecarbaldehyde (4), 4-nitrobenzenecarbaldehyde (5), p-methoxybenzenecarbaldehyde (6), and 4-tosyloxybenzenecarbaldehyde (7) were prepared via microwave irradiation in ethanol for 8–12 min to afford the corresponding Schiff bases 8–13. After cooling to room temperature, the solid product was filtered off and washed in water (3 × 5 mL). After drying, ethanol was used for crystallization.
3-(Benzylideneamino)-1,4-dihydroquinoxaline-2-carbonitrile (8)
Yield: 77%; color: yellow solid; m.p. 148–150 °C; IR (ATR): 3367, 3175 (NH), 2219 (C≡N), 1630 (CH=N) cm–1; 1H NMR: δ 9.93 (s, 1H, NH), 8.65 (s, 1H, CH=N), 8.60–6.66 (m, 10H, 9-CHarom. and NH); 13C NMR: δ 155.23, 131.68, 128.13, 123.83, 120.53, 116.16, 104.32; MS, m/z (%): 260 (M+, 30); anal. calcd. for C16H12N4 (260.29): C, 73.83%; H, 4.65%; N, 21.46%. Found: C, 73.76%; H, 4.71%; N, 21.58%.
3-((4-Chlorobenzylidene)amino)-1,4-dihydroquinoxaline-2-carbonitrile (9)
Yield: 70%; color: light yellow solid; m.p. 118–120 °C; IR (ATR): 3368, 3168 (2NH), 2192 (C≡N), 1645 (CH=N) cm–1; 1H NMR: δ 10.23 (s, 1H, NH), 8.25 (s, 1H, CH=N), 8.04–6.66 (m, 9H, 8-CHarom. and NH); 13C NMR: δ 155.88, 150.61, 148.61, 131.40, 130.52, 126.20, 120.14, 108.89, 106.70, 102.17 ppm; MS, m/z (%): 294 (M+, 25); anal. calcd. for C16H11ClN4 (294.74): C, 65.20%; H, 3.76%; N, 19.01%. Found: C, 65.25%; H, 3.71%; N, 19.12%.
3-((4-Hydroxybenzylidene)amino)-1,4-dihydroquinoxaline-2-carbonitrile (10)
Yield: 75%; color: pale yellow solid; m.p. 126–128 °C; IR (ATR): 3172–3400 (2NH, OH), 2206 (C≡N), 1635 (CH=N) cm–1; 1H NMR: δ 10.23 (s, 1H, NH), 8.60 (s, 1H, CH=N), 8.51–8.31 (m, 9H, 8-CHarom. and NH), 10.89 (s, 1H, OH); 13C NMR: δ 153.45, 131.68, 128.13, 123.83, 120.53, 116.16, 103.21; MS, m/z (%): 276 (M+, 15); anal. calcd. for C16H12N4O (276.29): C, 69.55%; H, 3.76%; N, 19.01%. Found: C, 65.25%; H, 4.67%; N, 20.14%.
3-((4-Nitrobenzylidene)amino)-1,4-dihydroquinoxaline-2-carbonitrile (11)
Yield: 78%; color: orange solid; m.p. 140–142 °C; IR (ATR): 3375 (NH), 3169 (NH), 2217 (C≡N), 1643 (CH=N) cm–1; 1H NMR: δ 10.25 (s, 1H, NH), 8.28 (s, 1H, CH=N), 8.03–6.66 (m, 9H, 8-CHarom. + NH); 13C NMR: δ 152.12, 131.79, 128.23, 123.91, 120.64, 116.31, 103.41; MS, m/z (%): 305 (M+, 30); anal. calcd. for C16H11N5O2 (305.29): C, 62.95%; H, 3.63%; N, 22.94%. Found: C, 62.98%; H, 3.67%; N, 22.98%.
3-((4-Methoxybenzylidene)amino)-1,4-dihydroquinoxaline-2-carbonitrile (12)
Yield: 74%; color: brown solid; m.p. 155–157 °C; IR (ATR): 3374, 3168 (2NH), 2219 (C≡N), 1636 (CH=N) cm–1; 1H NMR: δ 10.25 (s, 1H, NH), 8.29 (s, 1H, CH=N), 8.01–6.66 (m, 9H, 8-CHarom. + NH), 3.89 (s, 3H, OCH3); 13C NMR: δ 152.10, 131.79, 128.23, 123.91, 120.64, 116.31, 103.41; MS, m/z (%): 290 (M+, 20); anal. calcd. for C17H14N4O (290.32): C, 70.33%; H, 4.86%; N, 19.30%. Found: C, 70.35%; H, 4.89%; N, 19.34%.
4-((3-Cyano-1,4-dihydroquinoxalin-2-yl)imino)methylphenyl-4-methylbenzenesulfonate (13)
Yield: 76%; color: reddish brown solid; m.p. 145–147 °C; IR (ATR): 3374, 168 (2NH), 2298 (CH3), 2206 (C≡N), 1634 (CH=N) cm–1; 1H NMR: δ 10.25 (s, 1H, NH), 8.24 (s, 1H, CH=N), 8.14–6.66 (m, 13H, 12-CHarom. + NH), 2.43 (s, 3H, CH3); 13C NMR: δ 152.12, 131.79, 128.23, 123.91, 120.64, 116.31, 103.41; MS, m/z (%): 430 (M+, 45); anal. calcd. for C23H18N4O3S (430.48): C, 64.17%; H, 4.21%; N, 13.01%; S, 7.45%. Found: C, 64.22%; H, 4.27%; N, 13.04%; S, 7.48%.
Compound Synthesis of 15–20: General Procedures
Method A (Microwave Method)
A mixture of Schiff bases 15–20 (2.0 mmol) and thioglycolic acid (2.2 mmol) in 5.0 mL of dry toluene was irradiated in an MW oven for an approved time as shown in Table 1. Upon completion of the reaction (TLC was used to check the progress), the reaction mixture was cooled, and to remove the unreacted acid, the reaction mixture was cooled and washed with dilute sodium bicarbonate solution. The organic layer was separated (toluene layer) and removed by a rotary evaporator, yielding the solid product that was purified through crystallization using ethanol.
Method B (Traditional Method)
A mixture of appropriate compound Schiff bases 15–20 (2.0 mmol) and thioglycolic acid (2.2 mmol) was refluxed in ethanol (20.0 mL) in dry toluene (5.0 mL) for 14–17 h (TLC monitoring). Cooling was applied to the reaction mixture that was washed with a dilute solution of sodium bicarbonate to remove the unreacted acid. The organic layer was separated (toluene layer) and removed by a rotary evaporator, yielding the solid product that was purified through crystallization using ethanol.
3-(4-Oxo-2-phenylthiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile (15)
Yield: 95%; color: brown solid; m.p. 164–166 °C; IR (ATR) cm–1: 3426, 3324 (2NH), 3094 (C–Harom.), 2976 (C–Haliph.), 2201 (CN), 1649 (C=O); 1H NMR: δ 7.93–6.75 (m, 11H, CHarom. + 2NH), 6.30 (s, 1H, N–CH–S), 4.30 (s, 2H, CH2); 13C NMR δ (ppm): 159.58, 130.63, 130.53, 122.92, 121.31, 120.74, 120.68, 120.53, 118.23, 116.08, 111.56, 65.65; MS, m/z (%): 334 (M+, 25); anal. calcd. for C18H14N4OS (334.39): C, 64.65%; H, 4.22%; N, 16.75%; S, 9.59%. Found: C, 64.69%; H, 4.24%; N, 16.78%; S, 9.63%.
3-(2-(4-Chlorophenyl)-4-oxothiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile (16)
Yield: 89%; color: deep green solid; m.p. 179–181 °C; IR (ATR) cm–1: 3296, 3176 (2NH), 3056 (C–Harom.), 2923 (C–Haliph.), 2192 (CN), 1648 (C=O); 1H NMR: δ 12.82 (s, 1H, NH), 8.06–7.03 (m, 10H, CHarom. + NH), 6.31 (s, 1H, CH), 4.25 (s, 2H, CH2); 13C NMR δ (ppm): 162.22, 155.66, 147.32, 146.36, 136.06, 132.70, 130.84, 130.51, 128.77, 128.48, 128.08, 121.51, 120.42, 96.89, 56.67; MS, m/z (%): 368 (M+, 45); anal. calcd. for C18H13ClN4OS (368.84): C, 58.61%; H, 3.55%; N, 15.19%; S, 8.69%. Found: C, 58.65%; H, 3.48%; N, 15.26%; S, 8.58%.
3-(2-(4-Hydroxyphenyl)-4-oxothiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile (17)
Yield: 94%; color: gray solid; m.p. 192–194 °C; IR (ATR) cm–1: 3314, 3267 (2NH), 3090 (C–Harom.), 2981 (C–Haliph.), 2193 (CN), 1648 (C=O); 1H NMR: δ 11.43 (br, 1H, OH), 8.017–7.14 (m, 11H, CHarom. + NH), 5.97 (s, 1H, N–CH–S), 4.15 (s, 2H, CH2); 13C NMR δ (ppm): 173.05, 156.90, 150.87, 146.65, 138.19, 136.46, 133.54, 130.08, 128.35, 128.05, 127.95, 122.76, 120.12, 98.63, 60.96; MS, m/z (%): 350 (M+, 16); anal. calcd for C18H14N4O2S (350.39): C, 61.70%; H, 4.03%; N, 15.99%; S, 9.15%. Found: C, 61.62%; H, 4.12%; N, 16.07%; S, 9.22%.
3-(2-(4-Nitrophenyl)-4-oxothiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile (18)
Yield: 90%; color: reddish brown solid; m.p. 178–180 °C; IR (ATR) cm–1: 3321, 3298 (2NH), 3097 (C–Harom.), 2996 (C–Haliph.), 2192 (CN), 1647 (C=O); 1H NMR: δ 9.36 (s, 1H, NH), 8.54–7.32 (m, 9H, CHarom. + NH), 5.73 (s, 1H, N–CH–S), 4.08 (s, 2H, CH2); 13C NMR δ (ppm): 170.54, 152.54, 148.09, 146.24, 137.87, 135.12, 131.04, 130.0, 127.90, 126.25, 125.61, 121.99, 120.10, 96.11, 59.15; MS, m/z (%): 379 (M+, 25); anal. calcd. for C18H13N5O3S (379.39): C, 56.98%; H, 3.45%; N, 18.46%; S, 8.45%. Found: C, 57.07%; H, 3.51%; N, 18.37%; S, 8.54%.
3-(2-(4-Methoxyphenyl)-4-oxothiazolidin-3-yl)-1,4-dihydroquinoxaline-2-carbonitrile (19)
Yield: 96%; color: green solid; m.p. 196–198 °C; IR (ATR) cm–1: 3376, 3265 (2NH), 3091 (C–Harom.), 2987 (C–Haliph.), 2194 (CN), 1645 (C=O); 1H NMR: δ 12.45 (s, 1H, NH), 8.65–6.81 (m, 9H, CHarom. + NH), 6.21 (s, 1H, CH), 3.82 (s, 3H, OMe), 3.47 (s, 2H, CH2); 13C NMR δ (ppm): 171.03, 150.83, 150.46, 148.87, 131.12, 129.79, 112.92, 110.47, 109.23, 106.69, 102.85, 62.30, 58.44; MS, m/z (%): 364 (M+, 10); anal. calcd for C19H16N4O2S (364.10): C, 62.62%; H, 4.43%; N, 15.37%; S, 8.80%. Found: C, 62.71%; H, 4.49%; N, 15.42%; S, 8.73%.
4-(3-(3-Cyano-1,4-dihydroquinoxalin-2-yl)-4-oxothiazolidin-2-yl)phenyl-4-methylbenzenesulfonate (20)
Yield: 93%; color: deep green solid; m.p. 183–185 °C; IR (ATR) cm–1: 3311, 3204 (2NH), 3081 (C–Harom.), 2183 (CN), 1671 (C=O), 1641 (C=O); 1H NMR: δ 10.19 (s, 1H, NH), 7.95–6.90 (m, 13H, CHarom. + NH), 5.59 (s, 1H, N–CH–S), 4.25 (s, 2H, CH2), 2.44 (s, 3H, CH3); 13C NMR δ (ppm): 170.84, 148.82, 146.02, 131.50, 130.65, 130.17, 129.29, 127.31, 126.68, 126.01, 124.68, 123.71, 123.44, 122.52, 98.39, 62.15, 21.58; DEPT-135: 130.65, 130.61, 129.29, 128.51, 126.67, 125.76, 125.17, 122.83, 122.78, 97.73, 62.83, 21.86; MS, m/z (%): 504 (M+, 35); anal. calcd. for C25H20N4O4S2 (504.58): C, 59.51%; H, 4.00%; N, 11.10%; S, 12.71%. Found: C, 59.45%; H, 3.95%; N, 11.17%; S, 12.68%.
Synthesis of Pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile Derivatives 26–38
Method A (Microwave Method)
Compound 1 (2 mmol), in a mixture of aliphatic aldehydes (2 mmol), namely, formaldehyde or acetaldehyde, with malononitrile or ethyl cyanoacetate (2 mmol) or arylidine malononitrile (21–25) (2 mmol) in ethanol (2–5 drops), was irradiated in an MW oven for an approved time as shown in Table 1; upon completion of the reaction (TLC was used to check the progress), using filtration and drying, the solid precipitate was collected from the reaction mixture after it was cooled to room temperature, using dioxane to crystallize.
Method B (Traditional Method)
For 5–7 h, the previous mixture was refluxed into a solution of ethanol (2–5 drops) (TLC). After allowing the reaction mixture to cool to room temperature, by pouring the mixture into ice water that has been acidified with hydrochloric acid, filtration was used to collect the solid precipitate, which was dried and crystallized from dioxane.
1-Amino-3-phenyl-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (26)
Yield: 85%; color: yellow solid; m.p. 214–216 °C; IR (ATR) cm–1: 3314–3278 (NH2), 3198 (NH), 3082 (C–Harom.), 2221 (CN); 1H NMR: δ 10.82 (s, 1H, NH), 8.14–6.94 (m, 11H, CHarom. + NH2); 13C NMR δ (ppm): 156.03, 154.35, 141.67, 139.09, 136.56, 135.83, 130.87, 129.27, 129.06, 128.24, 127.71, 127.35, 124.50, 116.57, 112.15; MS, m/z (%): 324 (M+, 15); anal. calcd. for C19H12N6 (324.34): C, 70.36; H, 3.73; N, 25.91. Found: C, 70.42; H, 3.78; N, 25.96.
1-Amino-3-(4-chlorophenyl)-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (27)
Yield: 88%; color: red solid; m.p. 227–229 °C; IR (ATR) cm–1: 3387, 3316 (NH2), 3187 (NH), 3080 (C–Harom.), 2222 (CN); 1H NMR: δ 9.80 (s, 1H, NH), 8.48–6.55 (m, 10H, CHarom. + NH2); 13C NMR δ (ppm): 155.91, 153.53, 136.26, 134.93, 132.31, 131.04, 129.88, 129.56, 129.38, 129.30, 128.58, 121.36, 117.69, 113.14; MS, m/z (%): 358 (M+, 10); anal. calcd. for C19H11ClN6 (358.78): C, 63.60; H, 3.09; N, 23.42. Found: C, 63.53; H, 3.16; N, 23.37.
1-Amino-3-(4-nitrophenyl)-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (28)
Yield: 86%; color: yellow solid; m.p. 204–205 °C; IR (ATR) cm–1: 3365, 3214 (NH2), 3184 (NH), 3088 (C–Harom.), 2216 (CN); 1H NMR: δ 10.03 (s, 1H, NH), 8.64–6.57 (m, 10H, CHarom. + NH2); 13C NMR δ (ppm): 137.40, 135.38, 134.08, 131.97, 129.97, 129.91, 129.52, 129.22, 128.78, 127.56, 124.06, 116.24; MS, m/z (%): 369 (M+, 30); anal. calcd. for C19H11N7O2 (369.34): C, 61.79; H, 3.00; N, 26.55. Found: C, 61.84; H, 3.13; N, 26.43.
1-Amino-3-(4-methoxyphenyl)-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (29)
Yield: 89%; color: brown solid; m.p. 208–210 °C; IR (ATR) cm–1: 3328, 3207 (NH2), 3188 (NH), 3088 (C–Harom.), 2981 (C–Haliph.), 2188 (CN); 1H NMR: δ 9.19 (s, 1H, NH), 7.41–6.99 (m, 10H, CHarom. + NH2), 3.92 (s, 3H, OCH3); MS, m/z (%): 354 (M+, 16); anal. calcd. for C20H14N6O (354.36): C, 67.79; H, 3.98; N, 23.72. Found: C, 67.83; H, 4.01; N, 3.75.
1-Amino-3-(4-hydroxyphenyl)-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (30)
Yield: 87%; color: red solid; m.p. 208–210 °C; IR (ATR) cm–1: 3452 (OH), 3339, 3194 (NH2), 3102 (NH), 3079 (C–Harom.), 2209 (CN); 1H NMR: δ 12.95 (s, 1H, OH), 8.99 (s, 1H, NH), 8.04–6.70 (m, 10H, CHarom. + NH2); 13C NMR δ (ppm): 156.56, 154.28, 150.32, 149.81, 146.97, 146.40, 137.19, 132.37, 132.02, 130.81, 130.34, 128.65, 122.49, 121.62, 119.25; MS, m/z (%): 340 (M+, 25); anal. calcd. for C19H12N6O (340.34): C, 67.05; H, 3.55; N, 24.69. Found: C, 67.08; H, 3.59; N, 24.71.
3-Amino-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (35)
Yield: 95%; color: green solid; m.p. 146–148 °C; IR (ATR) cm–1: 3435, 3348 (NH2), 3222 (NH), 3070 (C–Harom.), 2205 (CN); 1H NMR: δ 10.21 (s, 1H, NH), 8.12 (s, 2H, NH2), 7.73–7.18 (m, 5H, Ar); 13C NMR δ (ppm): 151.71, 150.74, 146.43, 137.84, 136.17, 131.66, 130.84, 129.29, 128.10, 122.78, 119.58, 116.91; MS, m/z (%): 248 (M+, 35); anal. calcd. for C13H8N6 (248.24): C, 62.90%; H, 3.25%; N, 33.85%. Found: C, 62.84%; H, 3.31%; N, 33.93%.
3-Amino-1-methyl-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (36)
Yield: 90%; color: orange solid; m.p. 162–168 °C; IR (ATR) cm–1: 3362, 3310 (NH2), 3204 (NH), 3082 (C–Harom.), 2217 (CN); 1H NMR: δ 9.73 (s, 1H, NH), 7.37–6.34 (m, 6H, Ar + NH2), 3.79 (3H, CH3); 13C NMR δ (ppm): 149.87, 146.65, 137.44, 134.71, 132.41, 131.99, 130.75, 128.65, 122.89, 122.17, 28.07; MS, m/z (%): 262 (M+, 11); anal. calcd. for C14H10N6 (262.26): C, 64.11%; H, 3.84%; N, 32.04%. Found: C, 64.18%; H, 3.78%; N, 31.97%.
3-Hydroxy-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (37)
Yield: 94%; color: reddish brown solid; m.p. 145–147 °C; IR (ATR) cm–1: 3420 (OH), 3216 (NH), 3074 (C–Harom.), 2223 (CN); 1H NMR: δ 10.65 (s, 1H, OH), 10.21 (s, 1H, NH), 7.73–7.18 (m, 5H, Ar + CH); 13C NMR δ (ppm): 157.24, 153.51, 148.09, 140.17, 138.97, 132.56, 130.94, 129.20, 128.83, 124.05, 118.34, 116.47; MS, m/z (%): 249 (M+, 16); anal. calcd. for C13H7N5O (249.22): C, 62.65%; H, 2.83%; N, 28.10%. Found: C, 62.68%; H, 2.76%; N, 28.16%.
3-Hydroxy-1-methyl-6H-pyrimido[1,2-a]quinoxaline-2,5-dicarbonitrile (38)
Yield: 94%; color: reddish brown solid; m.p. 168–170 °C; IR (ATR) cm–1: 3446 (OH), 3204 (NH), 3063 (C–Harom.), 2924 (C–Haliph.), 2192 (CN); 1H NMR: δ 9.78 (s, 1H, OH), 7.87–7.38 (m, 5H, Ar + NH), 3.26 (3H, CH3); 13C NMR δ (ppm): 146.89, 146.36, 137.76, 136.53, 132.11, 131.99, 130.80, 130.13, 129.79, 129.00, 128.04, 126.61, 124.97, 123.71, 21.88; MS, m/z (%): 263 (M+,25); anal. calcd. for C14H9N5O (263.25): C, 63.87%; H, 3.45%; N, 26.60%. Found: C, 63.94%; H, 3.52%; N, 26.56%.
Insecticidal Bioefficacy Screening
The insecticidal activity was evaluated with the leaf dip bioassay on some synthesized pyrimidine and thiazolidinone derivatives.81−84 We report here the results of lab testing for the target compounds so that we can determine the concentrations required to kill 50% (LC50) of nymphs and adults of cowpea aphid (Aphis craccivora) insects. In this study, five concentrations of pyrimidine and thiazolidinone derivatives were prepared, and a surfactant, 0.1% Tween 80, was used. Similar sizes of 50 nymphs and 50 adults of cowpea aphid (Aphis craccivora) insects were dipped for 10 s in every concentration of synthesized target compounds; this was repeated three times. The testing of insects was performed by leaving them to dry at room temperature for about half an hour in which the control samples (the samples were soaked in distilled water and Tween 80) of insects were also utilized. After the used insects had dried, they were transferred to disks (9 cm size) and then left for a 24 h period at 22 ± 2 °C and 60 ± 5% relative humidity. Using a new binocular microscope, the aphid mortality was measured 24 h after the test. Aphids that were unable to move were considered dead. The insecticide bioactivity test of every target compound was repeated twice, and Abbott’s formula was used to correct the given data.85 The measured mortality relapse line was dissected by probit analysis.86 Sun’s equations were used to determine the harmfulness index.87 The batches of cowpea aphid (Aphis craccivora) insects were gathered from bean fields of an agricultural research center farm during the 2021/2022 season.
Docking Study
The crystal structure of acetylcholine cocrystallized with imidacloprid was obtained from the Protein Data Bank (code: 2ZJU).88 MOE2015.06 was utilized to perform this study for the novel constructed quinoxalines inside acetylcholine-active regions. Imidacloprid was redocked within acetylcholine to validate the docking study, and the RMSD was equal to 1.2785. The constructed quinoxalines’ 3D structures were built using the MOE molecular builder; then, these quinoxalines were protonated, energy-minimized, and saved as mdb files followed by docking within acetylcholine applying the previously reported procedures.89−91
Conclusions
In this study, novel thiazolidinones 15–20 and pyrimidine derivatives 26–30 and 35–38 possessing quinoxaline moieties were synthesized, and different spectral techniques were used to identify and confirm their chemical structures. The synthesized compounds were evaluated in vitro for their insecticidal potential against Aphis craccivora. The synthesized compounds 16, 20, and 27 displayed the highest toxicity activity against the tested strains. The 4-chlorophenylthiazolidino derivative 16 was the most toxicological agent against nymphs of cowpea aphids (Aphis craccivora) with LC50 = 0.021 ppm compared to the other synthesized pyrimidine and thiazolidinone derivatives. The molecular docking study of the new quinoxaline derivatives registered that compound 16 had the highest binding score (−10.54 kcal/mol) and the thiazolidinone moiety formed hydrogen bonds with Trp143.
Acknowledgments
Financial support from the Deanship of Graduate Studies at Jouf University for funding and supporting this research, through the initiative of DGS, Graduate Students Research support (GSR) at Jouf University, Saudi Arabia, is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03332.
Data that support the findings of this study including all IR, 1H NMR, and 13C NMR spectral data for synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Parankusam S.; Katamreddy S.; Bommineni P. R.; Bhatnagar-Mathur P.; Sharma K. K.. Insights into insect resistance in pulse crops: Problems and preventions. Pulse Improvement: Springer; 2018. p. 137–173. [Google Scholar]
- Arogundade O.; Ajose T.; Matthew J. O.; Osijo I. A. Current and Emerging Pests and Diseases of Cucumber (Cucumis sativus L.) in Africa. Cucumber Econ. Values Cultiv. Breed. 2021, 179. [Google Scholar]
- Kroschel J.; Mujica N.; Okonya J.; Alyokhin A.. Insect pests affecting potatoes in tropical, subtropical, and temperate regions. The Potato Crop; Springer: Cham; 2020. p. 251–306. [Google Scholar]
- Pawar M. M.; Shivanna B.; Prasannakumar M.; Parivallal P. B.; Suresh K.; Meenakshi N. Spatial distribution and community structure of microbiota associated with cowpea aphid (Aphis craccivora Koch). 3 Biotech. 2022, 12, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. Aphids. Pests and Their Management; Springer; 2018. p. 871–908. [Google Scholar]
- Ebert T.; Cartwright B. Biology and ecology of Aphis gossypii Glover (Homoptera: aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Thottappilly G. Plant virus diseases of importance to African agriculture. J. Phytopathol. 1992, 134, 265–288. 10.1111/j.1439-0434.1992.tb01236.x. [DOI] [Google Scholar]
- Li J.; Gu H.; Liu Y.; Wei S.; Hu G.; Wang X. RNA-seq reveals plant virus composition and diversity in alfalfa, thrips, and aphids in Beijing, China. Arch. Virol. 2021, 166, 1711–1722. 10.1007/s00705-021-05067-1. [DOI] [PubMed] [Google Scholar]
- Bouzat C.; Sine S. M. Nicotinic acetylcholine receptors at the single-channel level. Br. J. Pharmacol. 2018, 175, 1789–1804. 10.1111/bph.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke P.; Nauen R.; Beck M. E. Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew. Chem., Int. Ed. 2013, 52, 9464–9485. 10.1002/anie.201302550. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Wang Y.; Zhang Z.; Zhao Z.; Cymes G. D.; Tajkhorshid E.; et al. Cryo-EM structures of a lipid-sensitive pentameric ligand-gated ion channel embedded in a phosphatidylcholine-only bilayer. Proc. Natl. Acad. Sci. 2020, 117, 1788–1798. 10.1073/pnas.1906823117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M.; Pistillo F.; Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96, 302–311. 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Fritschy J. M.; Panzanelli P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014, 39, 1845–1865. 10.1111/ejn.12534. [DOI] [PubMed] [Google Scholar]
- Anderson G.; Seo M.; Berk M.; Carvalho A.; Maes M. Gut permeability and microbiota in Parkinson’s disease: role of depression, tryptophan catabolites, oxidative and nitrosative stress and melatonergic pathways. Curr. Pharm. Des. 2016, 22, 6142–6151. 10.2174/1381612822666160906161513. [DOI] [PubMed] [Google Scholar]
- Marrs T. C.; Maynard R. L. Neurotranmission systems as targets for toxicants: a review. Cell Biol. Toxicol. 2013, 29, 381–396. 10.1007/s10565-013-9259-9. [DOI] [PubMed] [Google Scholar]
- Soreq H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015, 38, 448–458. 10.1016/j.tins.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Ofek K.; Soreq H. Cholinergic involvement and manipulation approaches in multiple system disorders. Chem.-Biol. Interact. 2013, 203, 113–119. 10.1016/j.cbi.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Zhang J.; Song D.; Duan H.; Li W.; Yang X. Novel (E)-β-farnesene analogues containing 2-nitroiminohexahydro-1, 3, 5-triazine: synthesis and biological activity evaluation. Molecules 2016, 21, 825. 10.3390/molecules21070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. Employing eco-friendly potato disease management allows organic tropical Indian production systems to prosper. Asian J. Food Agro-Ind. 2009, 5, 80–S87. [Google Scholar]
- a Hussein B. R. M.; Ali A. M. Multicomponent Reaction for Synthesis of Novel 2-Tosyloxyphenylpyridines. J. Heterocycl. Chem. 2019, 56, 1420–1425. 10.1002/jhet.3521. [DOI] [Google Scholar]; b Khodairy A.; Ali A. M.; El-Wassimy M. T. 4-Toluenesulfonamide as a Building Block for Synthesis of Novel Triazepines, Pyrimidines, and Azoles. J. Heterocycl. Chem. 2016, 53, 1544–1553. 10.1002/jhet.2461. [DOI] [Google Scholar]; c Khodairy A.; Ali A. M.; Aboelez M. O.; El-Wassimy M. T. One-Pot Multicomponent Synthesis of Novel 2-Tosyloxyphenylpyrans under Green and Conventional Condition with Anti-inflammatory Activity. J. Heterocycl. Chem. 2017, 54, 1442–1449. 10.1002/jhet.2730. [DOI] [Google Scholar]; d Mourad A. F. E.; Amer A. A.; El-Shaieb K. M.; Ali A. M.; Aly A. A. 4-Hydroxy-1-phenylquinolin-2 (1H)-one in One pot Synthesis of Pyrimidoquinolines and Related Compounds under Microwave Irradiation and Conventional Conditions. J. Heterocycl. Chem. 2016, 53, 383–388. 10.1002/jhet.2286. [DOI] [Google Scholar]; e Khodairy A.; Shaaban K. M.; Ali M. A.; El-Wassimy M. T.; Nagwa S. A. Eco-friendly and efficiently synthesis, anti-inflammatory activity of 4-tosyloxyphenylpyrans via multi-component reaction under ultrasonic irradiation and room temperature conditions. J. Chem. Pharm. Res. 2015, 7, 332–340. [Google Scholar]; f Khodairy A.; Ali A. M.; El-Wassimy M. T. Synthesis of Novel Chromene, Pyridine, Pyrazole, Pyrimidine, and Imidazole Derivatives via One-pot Multicomponent Reaction. J. Heterocycl. Chem. 2017, 54, 3342–3349. 10.1002/jhet.2954. [DOI] [Google Scholar]; g Khodairy A.; Ali A. M.; El-Wassimy M. T. Synthesis and Reactions of New Thiazoles and Pyrimidines Containing Sulfonate Moiety. J. Heterocycl. Chem. 2018, 55, 964–970. 10.1002/jhet.3126. [DOI] [Google Scholar]; h Ahmed E. A.; Soliman A. M. M.; Ali M. A.; El-Remaily M. A. A. Boosting the catalytic performance of zinc linked amino acid complex as an eco-friendly for synthesis of novel pyrimidines in aqueous medium. Appl. Organomet. Chem. 2021, 35, e6197 10.1002/aoc.6197. [DOI] [Google Scholar]
- Zhang R.; Wang B.; Grossi G.; Falabella P.; Liu Y.; Yan S.; et al. Molecular basis of alarm pheromone detection in aphids. Curr. Biol. 2017, 27, 55–61. 10.1016/j.cub.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Dryburgh J. L.Effects of Virus Infection and Volatiles on Aphid Virus Vector Behavior on Sweetpotato; Louisiana State University and Agricultural & Mechanical College, 2019.
- Qin Y. G.; Yang Z. K.; Song D. L.; Wang Q.; Gu S. H.; Li W. H.; et al. Bioactivities of synthetic salicylate-substituted carboxyl (E)-β-Farnesene derivatives as ecofriendly agrochemicals and their binding mechanism with potential targets in aphid olfactory system. Pest Manage. Sci. 2020, 76, 2465–2472. 10.1002/ps.5787. [DOI] [PubMed] [Google Scholar]
- Smagghe G.; Mahdian K.; Zubrzak P.; Nachman R. J. Antifeedant activity and high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae) induced by biostable insect kinin analogs. Peptides. 2010, 31, 498–505. 10.1016/j.peptides.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Li X.; Song D.; Ling Y.; Zhou Y.; Yang X. Synthesis, aphicidal activity and conformation of novel insect kinin analogues as potential eco-friendly insecticides. Pest Manage. Sci. 2020, 76, 3432–3439. 10.1002/ps.5721. [DOI] [PubMed] [Google Scholar]
- Zhou Y. l.; Li X. l.; Zhang Y. m.; Shi Y.; Li H. h.; Zhang Z. A novel bee-friendly peptidomimetic insecticide: Synthesis, aphicidal activity and 3D-QSAR study of insect kinin analogs at Phe2 modification. Pest Manage. Sci. 2022, 2952. 10.1002/ps.6920. [DOI] [PubMed] [Google Scholar]
- Tariq S.; Somakala K.; Amir M. Quinoxaline: An insight into the recent pharmacological advances. Eur. J. Med. Chem. 2018, 143, 542–557. 10.1016/j.ejmech.2017.11.064. [DOI] [PubMed] [Google Scholar]
- Abu-Hashem A. A.; Gouda M. A.; Badria F. A. Synthesis of some new pyrimido [2′, 1′: 2, 3] thiazolo [4, 5-b] quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1976–1981. 10.1016/j.ejmech.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Tariq S.; Alam O.; Amir M. Synthesis, anti-inflammatory, p38α MAP kinase inhibitory activities and molecular docking studies of quinoxaline derivatives containing triazole moiety. Bioorg. Chem. 2018, 76, 343–358. 10.1016/j.bioorg.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Ghoneim A. A.; Elkanzi N. A. A.; Bakr R. B. Synthesis and studies molecular docking of some new thioxobenzo [g] pteridine derivatives and 1, 4-dihydroquinoxaline derivatives with glycosidic moiety. J. Taibah Univ. Sci. 2018, 12, 774–782. 10.1080/16583655.2018.1510163. [DOI] [Google Scholar]
- Hrichi H.; Ahmed E. N. A.; Badawy B. R. Novel β-lactams and thiazolidinone derivatives from 1, 4-dihydroquinoxaline Schiff’s base: Synthesis, antimicrobial activity and molecular docking studies. Chem. J. Mold. 2020, 15, 86–94. 10.19261/cjm.2019.647. [DOI] [Google Scholar]
- Bakr R. B.; Elkanzi N. A. A.; Ghoneim A. A.; Moustafa S. Synthesis,Molecular Docking Studies and in Vitro Antimicrobial Evaluation of Novel Pyrimido[1, 2-a] Quinoxaline Derivatives. Heterocycles 2018, 96, 1941–1957. 10.3987/COM-18-13955. [DOI] [Google Scholar]
- Xu H.; Fan L. Synthesis and antifungal activities of novel 5, 6-dihydro-indolo [1, 2-a] quinoxaline derivatives. Eur. J. Med. Chem. 2011, 46, 1919–1925. 10.1016/j.ejmech.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Tang X.; Zhou Q.; Zhan W.; Hu D.; Zhou R.; Sun N.; et al. Synthesis of novel antibacterial and antifungal quinoxaline derivatives. RSC Adv. 2022, 12, 2399–2407. 10.1039/D1RA07559D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. B.; Kim Y. H.; Park J. Y.; Kim S. K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. 10.1016/j.bmcl.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Bailly C.; Echepare S.; Gago F.; Waring M. J. Recognition elements that determine affinity and sequence-specific binding to DNA of 2QN, a biosynthetic bis-quinoline analogue of echinomycin. Anti-Cancer Drug Des. 1999, 14, 291–303. [PubMed] [Google Scholar]
- Liu X.-H.; Yu W.; Min L.-J.; Wedge D. E.; Tan C.-X.; Weng J.-Q.; et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020, 68, 7324–7332. 10.1021/acs.jafc.0c01042. [DOI] [PubMed] [Google Scholar]
- Baashen M. Quinoxaline-2, 3 (1 H, 4 H)-dithione: Synthesis and reactions. Phosphorus, Sulfur Silicon Relat. Elem. 2018, 193, 350–357. 10.1080/10426507.2018.1424166. [DOI] [Google Scholar]
- Islami M. R.; Hassani Z. One-pot and efficient protocol for synthesis of quinoxaline derivatives. ARKIVOC 2008, 15, 280–287. [Google Scholar]
- Cohen E. D.; Miller R. F. Quinoxalines block the mechanism of directional selectivity in ganglion cells of the rabbit retina. Proc. Natl. Acad. Sci. 1995, 92, 1127–1131. 10.1073/pnas.92.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojakovick A. S.; March R. B. Insecticide cyclic nucleotide interactions: I. quinoxalinedithiol derivatives: a new group of potent phosphodiesterase inhibitors. Pestic. Biochem. Physiol. 1976, 6, 10–19. 10.1016/0048-3575(76)90003-1. [DOI] [Google Scholar]
- Abdelgawad M. A.; Bakr R. B.; Alkhoja O. A.; Mohamed W. R. Design, synthesis and antitumor activity of novel pyrazolo [3, 4-d] pyrimidine derivatives as EGFR-TK inhibitors. Bioorg. Chem. 2016, 66, 88–96. 10.1016/j.bioorg.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Abdellatif K. R. A.; Bakr R. B. New advances in synthesis and clinical aspects of pyrazolo [3, 4-d] pyrimidine scaffolds. Bioorg. Chem. 2018, 78, 341–357. 10.1016/j.bioorg.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Bakr R. B.; Mehany A. (3, 5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo [3, 4-d] pyrimidin-4-ylamino) phenyl] methanone. Mol. Ther. 2016, 2016, M915. 10.3390/M915. [DOI] [Google Scholar]
- Buzun K.; Gornowicz A.; Lesyk R.; Kryshchyshyn-Dylevych A.; Gzella A.; Czarnomysy R. 2-{5-[(Z, 2 Z)-2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-oxo-2-thioxothiazolidin-3-yl}-3-methylbutanoic Acid as a Potential Anti-Breast Cancer Molecule. Int. J. Mol. Sci. 2022, 23, 4091. 10.3390/ijms23084091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelgawad M. A.; Bakr R. B.; Azouz A. A. Novel pyrimidine-pyridine hybrids: synthesis, cyclooxygenase inhibition, anti-inflammatory activity and ulcerogenic liability. Bioorg. Chem. 2018, 77, 339–348. 10.1016/j.bioorg.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Bakr R. B.; Ghoneim A. A.; Azouz A. A. Selective cyclooxygenase inhibition and ulcerogenic liability of some newly prepared anti-inflammatory agents having thiazolo [4, 5-d] pyrimidine scaffold. Bioorg. Chem. 2019, 88, 102964 10.1016/j.bioorg.2019.102964. [DOI] [PubMed] [Google Scholar]
- Abdelgawad M. A.; Al-Sanea M. M.; Musa A.; Elmowafy M.; El-Damasy A. K.; Azouz A. A.; Ghoneim M. M.; Bakr R. B. Docking Study, Synthesis, and Anti-Inflammatory Potential of Some New Pyridopyrimidine-Derived Compounds. J. Inflammation Res. 2022, 15, 451–463. 10.2147/JIR.S343263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelgawad M. A.; Elkanzi N. A. A.; Musa A.; Ghoneim M. M.; Ahmad W.; Elmowafy M.; et al. Optimization of pyrazolo [1, 5-a] pyrimidine based compounds with pyridine scaffold: Synthesis, biological evaluation and molecular modeling study. Arabian J. Chem. 2022, 15, 104015 10.1016/j.arabjc.2022.104015. [DOI] [Google Scholar]
- Morja M. I.; Chikhalia K. H. Iron-catalyzed intermolecular cross-dehydrogenative C (sp3)–H/C (sp)–H coupling of pyrimidine bearing 4-thiazolidinones with terminal alkynes. Mol. Diversity 2022, 1–9. 10.1007/s11030-021-10363-8. [DOI] [PubMed] [Google Scholar]
- Baboo P.; Gautam G.; Gupta S. K. Strategies for the Synthesis and Biological Screening of Thiazolidinone Derivatives. Asian J. Res. Chem. 2017, 10, 240–248. 10.5958/0974-4150.2017.00039.6. [DOI] [Google Scholar]
- Elkanzi N. A. A.; Bakr R. B. Microwave Assisted, Antimicrobial Activity and Molecular Modeling of Some Synthesized Newly Pyrimidine Derivatives Using 1, 4-diazabicyclo [2.2. 2] octane as a Catalyst. Lett. Drug Des. Discovery 2020, 17, 1538–1551. 10.2174/1570180817999200802033351. [DOI] [Google Scholar]
- Abdelgawad M. A.; Bakr R. B.; Ahmad W.; Al-Sanea M. M.; Elshemy H. A. New pyrimidine-benzoxazole/benzimidazole hybrids: Synthesis, antioxidant, cytotoxic activity, in vitro cyclooxygenase and phospholipase A2-V inhibition. Bioorg. Chem. 2019, 92, 103218 10.1016/j.bioorg.2019.103218. [DOI] [PubMed] [Google Scholar]
- Mohammed H. A.; Attia S. K.; Nessim M. I.; Shaaban M. E.-B.; El-Bassoussi A. A. Studies on some thiazolidinones as antioxidants for local base oil. Egypt. J. Chem. 2019, 62, 1219–1234. 10.21608/EJCHEM.2019.6662.1560. [DOI] [Google Scholar]
- Djukic M.; Fesatidou M.; Xenikakis I.; Geronikaki A.; Angelova V. T.; Savic V.; et al. In vitro antioxidant activity of thiazolidinone derivatives of 1, 3-thiazole and 1, 3, 4-thiadiazole. Chem.-Biol. Interact. 2018, 286, 119–131. 10.1016/j.cbi.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Adki N.; Rana N.; Palthya R. N. Synthesis and biological evaluation of pyrazole analogues linked with 1, 2, 3-triazole and 4-thiazolidinone as antimicrobial agents. Curr. Chem. Lett. 2022, 11, 139–146. 10.5267/j.ccl.2021.8.001. [DOI] [Google Scholar]
- Bakr R. B.; Elkanzi N. A. A. Preparation of some novel thiazolidinones, imidazolinones, and azetidinone bearing pyridine and pyrimidine moieties with antimicrobial activity. J. Heterocyclic Chem. 2020, 57, 2977–2989. 10.1002/jhet.4009. [DOI] [Google Scholar]
- Rezaei M.; Mohammadi H. T.; Mahdavi A.; Shourian M.; Ghafouri H. Evaluation of thiazolidinone derivatives as a new class of mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2018, 108, 205–213. 10.1016/j.ijbiomac.2017.11.147. [DOI] [PubMed] [Google Scholar]
- Satpathy S.; Gotyal B.; Babu V. R.; Meena P.. New insecticide molecules in IPM; ENVIS, 2022, 18. [Google Scholar]
- Liu X. H.; Wang Q.; Sun Z. H.; Wedge D. E.; Becnel J. J.; Estep A. S.; Tan C. X.; Weng J. Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manage. Sci. 2017, 73, 953–959. 10.1002/ps.4370. [DOI] [PubMed] [Google Scholar]
- Ismail M. F.; Madkour H. M. F.; Salem M. S.; Mohamed A. M. M.; Aly A. F. Design, synthesis and insecticidal activity of new 1, 3, 4-thiadiazole and 1, 3, 4-thiadiazolo [3, 2-a] pyrimidine derivatives under solvent-free conditions. Synth. Commun. 2021, 51, 2644–2660. 10.1080/00397911.2021.1945106. [DOI] [Google Scholar]
- Fang T.; Sun C.; Xu Y.; Yuan J.; Wang Y.; Xing J. Synthesis, insecticidal activities and molecular docking studies on cis-nitenpyram analogues with a flexible amido segment anchored on tetrahydropyrimidine ring. Chem. Res. Chin. Univ. 2014, 30, 931–936. 10.1007/s40242-014-4191-y. [DOI] [Google Scholar]
- Liu Z.; Li Q. X.; Song B. Recent research progress in and perspectives of mesoionic insecticides: Nicotinic acetylcholine receptor inhibitors. J. Agric. Food Chem. 2020, 68, 11039–11053. 10.1021/acs.jafc.0c02376. [DOI] [PubMed] [Google Scholar]
- Cordova D.; Benner E. A.; Schroeder M. E.; Holyoke C. W. Jr.; Zhang W.; Pahutski T. F.; et al. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 2016, 74, 32–41. 10.1016/j.ibmb.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Rauter A. P.; Padilha M.; Figueiredo J. A.; Ismael M. I.; Justino J.; Ferreira H.; et al. Bioactive Pseudo-C-nucleosides Containing Thiazole, Thiazolidinone, and Tetrazole Rings. J. Carbohydr. Chem. 2005, 24, 275–296. 10.1081/CAR-200060396. [DOI] [Google Scholar]
- Deng X.; Zheng W.; Jin C.; Zhan Q.; Bai L. Novel phenylpyrimidine derivatives containing a hydrazone moiety protect rice seedlings from injury by metolachlor. Bioorg. Chem. 2021, 108, 104645 10.1016/j.bioorg.2021.104645. [DOI] [PubMed] [Google Scholar]
- Guan A.; Liu C.; Yang X.; Dekeyser M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. 10.1021/cr4005605. [DOI] [PubMed] [Google Scholar]
- Wang L.; Yang Z.; Pan S.; Zhu M.; Guan A.; Sun X.; et al. A new potential aphicide against Myzus persicae: Design, synthesis and 3D-QSAR of novel phenoxypyridine derivatives containing 4-aminopyrimidine. J. Mol. Struct. 2022, 1262, 132949 10.1016/j.molstruc.2022.132949. [DOI] [Google Scholar]
- Kodandaram M.; Rai A.; Halder J. Novel insecticides for management of insect pests in vegetable crops: A review. Veg. Science. 2010, 37, 109–123. [Google Scholar]
- Elkanzi N. A. A.; El Azab I. H.; Bakr R. B. Design, Synthesis, and In Silico Molecular Docking Study of Some Novel Thiochromene Derivatives with Antimicrobial Potential. Polycyclic Aromat. Compd. 2022, 1–20. 10.1080/10406638.2022.2041052. [DOI] [Google Scholar]
- AL-Shammri K. N.; Elkanzi N. A. A.; Arafa W. A.; Althobaiti I. O.; Bakr R. B.; Moustafa S. M. N. Novel indan-1, 3-dione derivatives: Design, green synthesis, effect against tomato damping-off disease caused by Fusarium oxysporum and in silico molecular docking study. Arabian J. Chem. 2022, 15, 103731 10.1016/j.arabjc.2022.103731. [DOI] [Google Scholar]
- Bakr R. B.; Azab I. H. E.; Elkanzi N. A. A. Thiochromene candidates: design, synthesis, antimicrobial potential and in silico docking study. J. Iranian Chem. Soc. 2022, 19, 1413–1423. 10.1007/s13738-021-02391-w. [DOI] [Google Scholar]
- El Azab I. H.; Bakr R. B.; Elkanzi N. A. A. Facile one-pot multicomponent synthesis of pyrazolo-thiazole substituted pyridines with potential anti-proliferative activity: synthesis, in vitro and in silico studies. Molecules 2021, 26, 3103. 10.3390/molecules26113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelgawad M. A.; Musa A.; Almalki A. H.; Alzarea S. I.; Mostafa E. M.; Hegazy M. M.; Mostafa-Hedeab G.; Ghoneim M. M.; Parambi D. G. T.; Bakr R. B.; al-Muaikel N. S.; Alanazi A. S.; Alharbi M.; Ahmad W.; Bukhari S. N. A.; al-Sanea M. M. Novel Phenolic Compounds as Potential Dual EGFR and COX-2 Inhibitors: Design, Semisynthesis, in vitro Biological Evaluation and in silico Insights. Drug Des., Dev. Ther. 2021, 15, 2325. 10.2147/DDDT.S310820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkanzi N. A. A.; Hrichi H.; Bakr R. B.; Hendawy O.; Alruwaili M. M.; Alruwaili E. D.; et al. Synthesis, in vitro evaluation and molecular docking of new pyrazole derivatives bearing 1, 5, 10, 10a-tetrahydrobenzo [g] quinoline-3-carbonitrile moiety as potent antibacterial agents. J. Iran. Chem. Soc. 2021, 18, 977–991. 10.1007/s13738-020-02086-8. [DOI] [Google Scholar]
- Elkanzi N. A. A.; Ghoneim A. A.; Bakr R. B. Design, efficient synthesis and antimicrobial evaluation of some novel pyrano [2, 3-b][1, 8] naphthyridine and pyrrolo [2, 3-f][1, 8] naphth-yridine derivatives. Pharma Chem. 2019, 11, 6–13. [Google Scholar]
- Abdelgawad M. A.; Elkanzi N. A. A.; Nayl A.; Musa A.; Alotaibi N. H.; Arafa W.; et al. Targeting tumor cells with pyrazolo [3, 4-d] pyrimidine scaffold: A literature review on synthetic approaches, structure activity relationship, structural and target-based mechanisms. Arabian J. Chem. 2022, 103781. [Google Scholar]
- Mathew B.; Parambi D. G.; Singh M.; Hendawy O. M.; Al-Sanea M. M.; Bakr R.; Protopine B.. Naturally Occurring Chemicals Against Alzheimer’s Disease: Elsevier; 2021. p. 167–174, 10.1016/B978-0-12-819212-2.00014-1. [DOI] [Google Scholar]
- Khalafallah A.; Abd Elal R.; Elkanzi N. A. A. Heterocyclic fused rings with bridgehead nitrogen atoms: Single step synthesis of several polyfunctionally substituted fused pyridines. Heterocycl. Commun. 2002, 8, 397–406. 10.1515/HC.2002.8.4.397. [DOI] [Google Scholar]
- Raslan M. A.; El Aal R. A.; Hassan M.; Ahamed N. A.; Sadek K. Studies on fused azoles: Synthesis of several polyfunctionally substituted fused azoles. J. Chin. Chem. Soc. 2001, 48, 91–99. 10.1002/jccs.200100017. [DOI] [Google Scholar]
- Gad M. A.; Aref S. A.; Abdelhamid A. A.; Elwassimy M. M.; Abdel-Raheem S. A. A. Biologically active organic compounds as insect growth regulators (IGRs): introduction, mode of action, and some synthetic methods. Curr. Chem. Lett. 2021, 10, 393–412. 10.5267/j.ccl.2021.5.004. [DOI] [Google Scholar]
- Abdelhamid A. A.; Elsaghiera A. M. M.; Aref S. A.; Gad M. A.; Ahmed N. A.; Abdel-Raheem S. A. A. Preparation and biological activity evaluation of some benzoylthiourea and benzoylurea compounds. Curr. Chem. Lett. 2021, 10, 371–376. 10.5267/j.ccl.2021.6.001. [DOI] [Google Scholar]
- Abdelhamid A. A.; Salama K. S.; Elsayed A. M.; Gad M. A.; Ali Ali El-Remaily M. A. Synthesis and Toxicological Effect of Some New Pyrrole Derivatives as Prospective Insecticidal Agents against the Cotton Leafworm, Spodoptera littoralis (Boisduval). ACS Omega 2022, 3990. 10.1021/acsomega.1c05049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid A. A.; Elwassimy M. M.; Aref S. A.; Gad M. A. Chemical design and bioefficacy screening of new insect growth regulators as potential insecticidal agents against Spodoptera littoralis (Boisd.). Biotechnol. Rep. 2019, 24, e00394 10.1016/j.btre.2019.e00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Finney D. J.Probit analysis: a statistical treatment of the sigmoid response curve: Cambridge university press: Cambridge; 1952. [Google Scholar]
- Sun Y.-P. Toxicity Index-an Improved Method of comparing the relative Toxicity of Insecticides. J. Econ. Entomol. 1950, 43, 45–53. 10.1093/jee/43.1.45. [DOI] [Google Scholar]
- Ihara M.; Okajima T.; Yamashita A.; Oda T.; Hirata K.; Nishiwaki H.; et al. Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoid insecticides imidacloprid and clothianidin. Invertebr. Neurosci. 2008, 8, 71–81. 10.1007/s10158-008-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkanzi N. A. A.; Hrichi H.; Bakr R. B. Antioxidant, Antimicrobial, and Molecular Docking Studies of Novel 1, 4-naphthoquinone Derivatives. Lett. Drug Des. Discov. 2022, 19, 654. 10.2174/1570180819666211228091055. [DOI] [Google Scholar]
- B Bakr R.; BM Mehany A.; RA Abdellatif K. Synthesis, EGFR Inhibition and Anti-cancer Activity of New 3, 6-dimethyl-1-phenyl-4-(substituted-methoxy) pyrazolo [3, 4-d] pyrimidine Derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 1389–1400. 10.2174/1872211311666170213105004. [DOI] [PubMed] [Google Scholar]
- Abdellatif K.; Abdelall E.; Abdelgawad M. A.; Ahmed R. R.; Bakr R. B. Synthesis, docking study and antitumor evaluation of certain newly synthesized pyrazolo [3, 4-d] pyrimidine derivatives. J. Organ Chem Indian. 2014, 10, 157–167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.