Abstract
Background
Diabetic ketoacidosis (DKA) is a potentially life-threatening complication of type 1 diabetes mellitus (T1DM) that has increased during the COVID-19 pandemic. This study will not only shed light on such life-threatening complications but also be a step to increase the awareness of healthcare providers about such complications in the upcoming pandemic waves and increased dependence on telemedicine. Thus, we aimed to further investigate the increase of DKA in pediatrics.
Methods
PubMed, Web of Science, and Scopus were broadly searched for studies assessing the incidence of DKA in pediatrics during the COVID-19 pandemic.
Results
Our study included 24 papers with a total of 124,597 children with diabetes. A statistically significant increase occurred in the risk of DKA among newly diagnosed T1DM patients during the pandemic (RR 1.41; 95% CI 1.19, 1.67; p < 0.01; I2 = 86%), especially in the severe form of DKA (RR 1.66: 95% CI 1.3, 2.11) when compared to before.
Conclusion
DKA in newly diagnosed children with T1DM has increased during the pandemic and presented with a severe form. This may reflect that COVID-19 may have contributed not only to the development but also the severity of DKA.
Impact
Diabetic ketoacidosis (DKA) is a life-threatening complication of type 1 diabetes mellitus (T1DM) that has increased during the COVID-19 pandemic.
Our study included 25 papers with a total of 124,597 children with diabetes. A statistically significant increase occurred in the risk of DKA among newly diagnosed T1DM patients during the pandemic.
Our findings reflect that COVID-19 may have an altered presentation in T1DM and can be related to DKA severity.
Introduction
Announced by the World Health Organization in January 2020 as a global health emergency and in March 2020 as a pandemic, coronavirus disease 2019 (COVID-19) is a severe respiratory illness.1,2 COVID-19 is highly infective, presenting with a range of symptoms; however, up to 80% of symptomatic COVID-19 infections feature only flu-like symptoms with no complications;3 advanced complications such as renal or circulatory failure have been reported with severe cases or with other comorbidities or risk factors including old age, hypertension, cardiovascular diseases, or diabetes, especially type 1 diabetes mellitus (T1DM).4
One of the most common chronic illnesses in children, increasing over recent decades, T1DM is a metabolic disease characterized by a deficit in the production of insulin with various effects on the body’s metabolism. The rapid and early diagnosis of T1DM is crucial to prevent its progression to diabetic ketoacidosis (DKA).5 The frequency of DKA differs widely by region, ranging from 15% in Europe to 70% in North America.6 DKA is the main life-threatening acute complication associated with the onset of T1DM.7 Furthermore, it is an entirely preventable condition, yet it is a leading cause of T1DM morbidity and increased hospitalization and length of stay because it is frequently mismanaged.7 However, DKA mortality rates have significantly declined in the past 20 years to below 1%.8 In 2018, The National Diabetes Inpatient Audits recently found no significant reduction in the number of hospitalized people developing DKA, attributed to under-treatment and incorrect timing of insulin administration which have worsened since 2011 (4% in 2017).5
Several studies reported an increase of new T1DM cases in children among COVID-19 patients.9,10 Not only has the frequency of T1DM increased but also the frequency of DKA, which has been reported with high percentages in several studies.11–13 However, other studies have not reported any increase in the rates of DKA.14
Proving an increase in DKA during the COVID-19 pandemic will not only shed light on such life-threatening complications but also be a step to overcome the fear of approaching healthcare settings, increase awareness about such complications during the upcoming waves, and our increased dependence on telemedicine.15–18 Thus, our meta-analysis and systematic review aimed to further investigate the relationship between COVID-19 and pediatric DKA to fill the knowledge gap, settle the controversy between different studies,10–14,19,20 and answer the question of whether DKA has increased in the COVID-19 pandemic.
Methods
Ethical approval
All protocols of our study followed the regulations of the research ethics committee of Assiut University.
This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.21
Search strategy
We conducted our search of the following databases: PubMed, WOS, and Scopus, using broad terms and keywords for the concepts of DKA and COVID in children up to October 20, 2021. The full details of the systematic search as illustrated in Table 1. We imported initial search records into an excel sheet. After duplicates removal, three authors (A.E., H.H.E.-L., and M.A.) screened all included studies according to our eligibility criteria by title and abstract. Any relevant studies and conflict studies were shifted to full-text screening. Conflicts in full-text reviewing were resolved in a discussion. An additional manual search was performed by screening references of the included articles, literature reviews, and PubMed-related articles.
Table 1.
The search strategy.
| Databases | Restrictions | Term | Items found |
|---|---|---|---|
| PubMed | Restricted to title and abstract | ("diabetic ketoacidosis" OR "Diabetic Acidosis" OR "Diabetic Ketoacidosis" OR "Diabetic Acidosis" OR "Diabetic Ketosis" OR "Diabetic Ketoses") AND("COVID 19" OR "COVID-19" OR "2019-nCoV" OR "2019 nCoV" OR "Coronavirus Disease-19" OR "Coronavirus Disease 19" OR "2019 Novel Coronavirus Disease" OR "2019 Novel Coronavirus" OR "COVID19" OR "Coronavirus Disease 2019" OR "SARS Coronavirus 2" OR "SARS-CoV-2" OR "SARS CoV 2") AND (Children OR child* OR teen* OR preteen OR Adolescent OR baby OR infant OR kid OR youth OR toddler OR neonate) | 56 |
| Scopus | 45 | ||
| Web of Science | Restricted to topic | 50 | |
| Total | 151 |
Eligibility criteria
Our eligibility criteria are (a) studies that assessed the development of DKA in children with diabetes during the COVID-19 pandemic, (b) published in international peer-reviewed journals indexed in Scopus, WOS, PubMed and (c) no limits to language. We excluded animal studies, reviews, case reports, and commentary.
Data extraction
M.A. and H.H.E.-L. independently extracted data about baseline characteristics from the included studies using a standardized Excel sheet; first author name, year of publication, study design, country, sample size, characteristics of participants (sex and age), type of diabetes, new-onset or already diagnosed DM, aim and results. The same authors independently extracted data for the quantitative analysis; the number of DKA incidence in children with diabetes, degree of DKA (mild, moderate, and severe), months of measurement, type, and the onset of diabetes.
Data analysis
The Meta package of R software version 4.1.022 was used to analyze the pooled risk ratio with a 95% confidence interval. The random-effect model was employed in our meta-analyses. The I2 and χ2 tests were used to evaluate heterogeneity. A p value less than 0.05 was considered significant. The data extracted are categorical and presented as a percentage.
Our first analysis is calculating the risk ratio of DKA in prepandemic and post-pandemic stratified by the onset of diabetes (new-onset, pre-existing or mixed of both), and our second meta-analysis is comparing the degree of DKA (severe, moderate, or mild) in prepandemic and post-pandemic stratified by the onset of diabetes. The criteria used for grading the severity of DKA were according to the International Society for Pediatric and Adolescent Diabetes:6
Mild: venous pH <7.3 or serum bicarbonate <15 mmol/L.
Moderate: pH <7.2, serum bicarbonate <10 mmol/L.
Severe: pH <7.1, serum bicarbonate <5 mmol/L.
We also conducted leave-one-out meta-analyses on each subset of the studies by leaving one study out at each analysis and constructed the Funnel plots to evaluate the publication bias.
Results
Search results
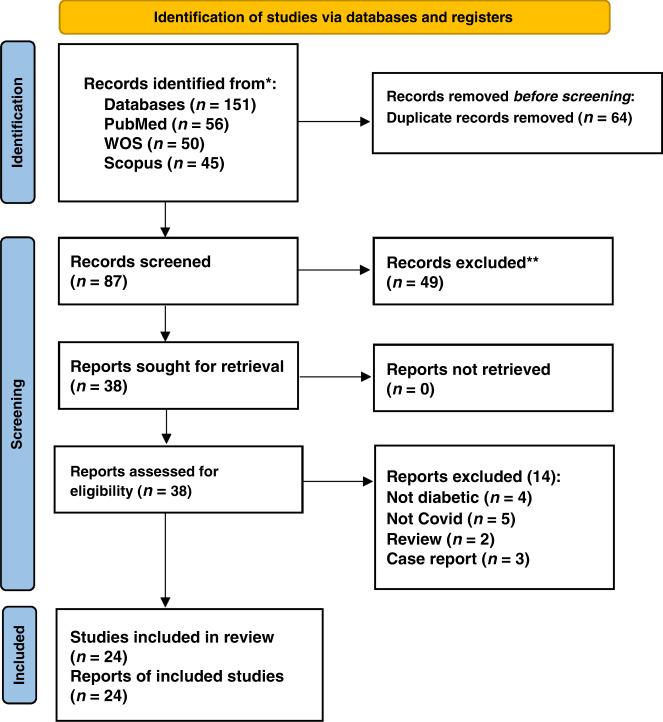
Our search strategy resulted in a total of 151 studies. After the title and abstract screening and removing the duplicates, 113 articles were eliminated, and 38 full-text articles were evaluated for eligibility. Following the full-text screening, 24 papers11–14,19,20,23–41 met our criteria. Finally, 17 studies were included in our meta-analysis, and 7 were included only in our systematic review as they did not provide sufficient data to be included in the meta-analysis, so we included them as qualitative analysis (Fig. 1).
Fig. 1. PRISMA flowchart.
The PRISMA flow diagram for the systematic review detailing the database searches, the number of Records screened, and the full texts retrieved.
Summary of included studies
The studies included 124,597 children with diabetes with a mean age of 8.8 years. Of these, 15 were retrospective cohort studies, 2 were multicenter observational studies, and 7 were multicenter cross-sectional studies. The studies included in our meta-analysis were 12 conducted in Europe (Poland, Turkey, the UK, Germany, and Romania), 3 in Asia (Saudi Arabia and China), and 2 in North America (the USA and Canada). The baseline characteristics are illustrated in Tables 2 and 3.
Table 2.
Studies included in the systematic review.
| Author, year | Country | Study design | Number of diabetic patients | Age | Gender (male) | DKA patients | DM type | New or pre-existing | HbA1C | Aim | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||||
| Kamrath, 2021 | Germany | Multicenter cohort study | 3238 | 9.8 (6.0–12.9)a | 1799 (55.6%) | 1094 | Type 1 | New-onset | NR | Estimation of the relative risk of DKA in DM patients associated with COVID | In 1st half of the year, there is a significant association between the incidence of COVID and DKA with RR of 1.4 in every 50 COVID cases per week but in the 2nd half of the year, this association was insignificant. | |||||
| Ramgopal, 2021 | USA | Cross-sectional study | Emergency patient 27,874,730 (51,708 DKA) | Emergency patient 1,913,085 (5985 DKA) | 4.8 (1.6–10.7)a | 5.7 (1.8–12.2)a | 987,805 (51.6%) | 987,805 (51.6%) | 51,708 | 5985 | NR | NR | NR | Identification changes in the presentations of pediatric emergencies during COVID compared to the last 20 years | Emergency utilization was low after the pandemic in most diagnoses and increased in DKA rate | |
| Kucharska, 2020 | Poland | Cross-sectional study | 1961 | NR | Less than 18 years | 1054 boys (53.72%) | NR | 31.75% (622) | 36.67% | Type 1 | New-onset | 11.79 ± 2.63% | 13.41 ± 2.50% | Determination if COVID-19 lockdown affected the incidence rate of type 1 DM in the pediatric | Incidence rate of DM decreases in pandemic with worse conditions than in previous years | |
| Elbarbary, 2020 | Italy | Cross-sectional electronic survey | 86 DM patients | Under 18 years | 20 (23%) | 44 | 61 type 125 type 2 | Pre-existing | 7.6 (SD 1.6) | Determination of the management practice of HCP caring for pediatric patients with DM during COVID-19 | 15% reported a higher incidence of DKA. The majority of centers did not have DM COVID-19 positive, and from those who had, were just mild/moderate disease course | |||||
| Fisler, 2020 | USA | Retrospective cohort study | 5 DM | Under 21 years | 37 (48%) | 2 | NR | Pre-existing | NR | Determination factors associated with PICU admission in COVID-19 patients | DKA is one of the most indications of PICU admission | |||||
| Alonso, 2020 | USA | Survey |
266 61 hospitalized 205 non-hospitalized (44 DKA) |
Less than 19 years | 133 (50%) | 44 | Type 1 | Pre-existing | 11 in hospitalized; 8.2 in non-hospitalized | Description of the outcomes of COVID-19 in children and adolescents with type 1 diabetes and which factor increased the risk of disease | DKA was the most common adverse event for hospitalization and high HBA1c was significantly associated with hospitalization | |||||
| Sherif, 2021 | Egypt | Retrospective observational study | 36 patients | Mean and SD 8.4 ± 3.8 | 19 (52%) | 34 | Type 1 | 29 new; 7 pre-existing | 11.6 ± 2.2 | Determination of the characteristics of pediatric patients with type 1 DM during the pandemic and the prevalence of new-onset DM among patients with DKA | The pandemic increases the prevalence and severity of DKA in diabetic patients | |||||
aMedian and IQR.
Table 3.
Studies included in meta-analysis.
| Author, year | Country | Total number | Study design | Subgroup | Gender, n (%) | Age (mean ± SD) | Number | HbA1C ± SD | Type of diabetes (%) | Type of DM (%) | COVID tests used for diagnosis (X-ray, PCR, antigen testing (–)) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Pre-existing | New-onset | Type I | Type II | |||||||||
| Alaqeel, 2021 | Saudi Arabia | 260 | Retrospective cohort study | Prepandemic | 69 (44.80) | 85 (55.20) | 9.7 ± 0.24 | 154 | 11.3 ± 0.2 | 97 (62.9) | 65 (61.3) | 154 (100) | 0 | – |
| Pandemic | 51 (48.10) | 55 (51.90) | 10.0 ± 0.3 | 106 | 12.1 ± 0.2 | 57 (37.0) | 41 (38.7) | 106 (100) | 0 | |||||
| Boboc, 2021 | Bucharest | 459 | Retrospective cohort study | Prepandemic | 170 (54.49) | 142 (45.51) | 7.59 | 312 | 11.32 ± 2.18 | 0 | 312 (100) | 312 (100) | 0 | PCR and antibody |
| Pandemic | 75 (51.02) | 72 (48.98) | 7.59 | 147 | 12.47 ± 2.19 | 0 | 147 (100) | 312 (100) | 0 | |||||
| Bogale, 2021 | USA | 412 | Retrospective | Prepandemic | 218 (58.90) | 152 (41.10) | 10.0 ± 4.29 | 370 | 12.0 ± 2.38 | 0 | 370 (100) | 370 (100) | 0 | – |
| Pandemic | 23 (54.80) | 19 (45.20) | 9.2 ± 4.55 | 42 | 12.2 ± 2.47 | 0 | 42 (100) | 370 (100) | 0 | |||||
| Danne, 2021 | Germany | 56,801 | Case–control | Prepandemic a | (51.7) | (48.3) | 13.4 (10.1, 16.2)a | 16,735 | 7.8 (7.0, 8.9)a | NR | NR | NR | NR | – |
| Pandemic b | (52.0) | (48) | 13.5 (10.2, 16.2)a | 12,157 | 7.6 (6.8, 8.6)a | NR | NR | NR | NR | |||||
| Prepandemic b | (51.6) | (48.4) | 13.4 (10.2, 16.2)a | 14,523 | 7.8 (7.0, 8.9)a | NR | NR | NR | NR | |||||
| Pandemic b | (51.9) | (48.1) | 13.6 (10.2, 16.4)a | 13,386 | 7.8 (6.9, 8.9)a | NRa | NR | NR | NR | |||||
| Dilek, 2021 | Turkey | 120 | Cross-sectional | Prepandemic | 21 (45.70) | 25 (54.30) | 10.5 | 46 | 10.7 | 0 | 46 (100) | 46 (100) | 0 | PCR and antibody |
| Pandemic | 35 (47.30) | 39 (52.70) | 10 | 74 | 11.7 | 0 | 74 (100) | 74 (100) | 0 | |||||
| Dzygalo, 2020 | Poland | 86 | Cohort | Prepandemic | 26 (50.00) | 26 (50.00) | 9.59 ± 4.7 | 52 | 11.5 ± 2.2 | 0 | 52 (100) | 52 (100) | 0 | – |
| Pandemic | 22 (64.70) | 12 (35.30) | 9.90 ± 4.9 | 34 | 12.9 ± 2.4 | 0 | 34 (100) | 34 (100) | 0 | |||||
| Han, 2021 | South Korea | 19 | Retrospective | Prepandemic 2017 | 2 (50.00) | 2 (50.00) | 11.50 ± 5.07 | 4 | 13.50 ± 0.84 | 1 (25.0) | 3 (75.0) | 3 (75.0) | 1 (25.0) | – |
| 2018 | 1 (20.00) | 4 (80.00) | 9.60 ± 4.62 | 5 | 13.08 ± 1.20 | 2 (40.0) | 3 (60.0) | 4 (80.0) | 1 (20.0) | |||||
| 2019 | 2 (66.70) | 1 (33.30) | 13.33 ± 2.08 | 3 | 12.23 ± 2.83 | 1 (33.3) | 2 (66.7) | 3 (100.0) | 0 | |||||
| Prepandemic total | 5 (41.67) | 7 (58.33) | 10.44 ± 4.62 | 12 | 13.27 ± 1.02 | 4 (33.33) | 8 (66.67) | 10 (83.33) | 2 (16.67) | |||||
| Pandemic | 1 (14.30) | 6 (85.70) | 12.57 ± 2.37 | 7 | 12.46 ± 1.91 | 0 | 7 (100.0) | 7 (100.0) | 0 | |||||
| Ho, 2021 | Canada | 221 | Retrospective | Prepandemic | 47 (41.20) | 67 (58.80) | 9.43 | 114 | NR | 0 | 114 (100) | 114 | 0 | – |
| Pandemic | 46 (43.00) | 61 (57.00) | 9.62 | 107 | NR | 0 | 107 (100) | 107 | 0 | |||||
| Jacob, 2021 | Israel | 304 | Retrospective cross-sectional | Prepandemic | NR | NR | 12.0 (8.7–15.0)a | 154 | NR | 74 (48.05) | 80 (51.94) | 154 (100) | 0 | – |
| Pandemic | NR | NR | 12.0 (8.7–14.1)a | 150 | NR | 64 (42.67) | 86 (57.33) | 150 (100) | 0 | |||||
| Lawrence, 2021 | UK | 53 | Case–control | Prepandemic 2015 | (33.00) | (67.00) | 8.4 ± 5.3 | 9 | 12.0 ± 2.8 | 0 | 9 (100) | 9 (100) | 0 | – |
| 2016 | (50.00) | (50.00) | 10.2 ± 5.4 | 6 | 10.5 ± 2.1 | 0 | 6 (100) | 6 (100) | 0 | |||||
| 2017 | (63.00) | (37.00) | 9.1 ± 4.2 | 8 | 10.6 ± 3.1 | 0 | 8 (100) | 8 (100) | 0 | |||||
| 2018 | (50.00) | (50.00) | 10.2 ± 4.9 | 10 | 11.4 ± 2.4 | 0 | 10 (100) | 10 (100) | 0 | |||||
| 2019 | (56.00) | (44.00) | 7.9 ± 4.0 | 9 | 12.1 ± 3.2 | 0 | 9 (100) | 9 (100) | 0 | |||||
| Prepandemic total | 252 (50.4) | 248 (49.6) | 9.08 ± 4.61 | 42 | 11.40 ± 2.72 | 0 | 42 (100) | 42 (100) | 0 | |||||
| Pandemic | (27.00) | (73.00) | 8.0 ± 4.3 | 11 | 12.3 ± 2.7 | 0 | 11 (100) | 11 (100) | 0 | |||||
| Lee, 2021 | China | 45 | Retrospective | Prepandemic | 23 (51.11) | 22 | 15.8 ± 6.13 | 45 | 7.70 ± 1.38 | NR | NR | 45 | 0 | – |
| Pandemic | 23 (51.11) | 22 | 15.8 ± 6.13 | 45 | 8.30 ± 2.05 | NR | NR | 45 | 0 | |||||
| Loh, 2021 | Germany | 125 | Case–control | Prepandemic | 36 (49.30) | 37 (50.70) | 10.64 ± 1.03 | 73 | 10.9 ± 0.65 | 55 (75.34) | 18 (24.65) | NR | NR | |
| Pandemic | 21 (40.40) | 31 (59.60) | 9.48 ± 1.36 | 52 | 10.27 ± 0.59 | 40 (76.9) | 12 (23.1) | 50 | 2 | |||||
| Mamelia, 2021 | Italy | 880 | Prospective cohort | Prepandemic 2017 | 111 (55.00) | 91 (45.00) | 8.7 ± 4.3 | 202 | NR | 0 | 202 (100) | 202 (100) | 0 | PCR |
| 2018 | 103 (53.90) | 88 (46.10) | 8.7 ± 3.9 | 191 | NR | 0 | 191 (100) | 191 (100) | 0 | |||||
| 2019 | 117 (50.60) | 114 (49.40) | 8.9 ± 4.1 | 231 | NR | 0 | 231 (100) | 231 (100) | 0 | |||||
| Prepandemic total | 331 (53.04) | 293 (46.96) | 8.77 ± 4.1 | 624 | NR | 0 | 624 (100) | 624 (100) | 0 | |||||
| Pandemic | 146 (57.00) | 110 (43.00) | 8.5 ± 4.2 | 256 | NR | 0 | 256 (100) | 256 (100) | 0 | |||||
| McGlacken-Byrne, 2021 | UK | 47 | Cross-sectional | Prepandemic | 15 (50.00) | 15 (50.00) | 11.4 (range 2.2–17.6)r | 30 | 10.4 ± 3.2 | 0 | 30 (100) | 30 (100) | 0 | PCR and antibody |
| Pandemic | 9 (52.90) | 8 (47.10) | 10.6 (range 3.2–16.3)r | 17 | 13.0 ± 1.7 | 0 | 17 (100) | 17 (100) | 0 | |||||
| Monkemoller, 2021 | Germany | 1491 | Prospective cohort | Prepandemic 2018 | 254 (55.7) | 202 (44.3) | 9.7 (5.8-13.2)a | 456 | NR | 0 | 456 (100) | 456 (100) | 0 | – |
| Prepandemic 2019 | 263 (52.3) | 240 (47.7) | 9.1 (5.5-12.6)a | 503 | NR | 0 | 503 (100) | 503 (100) | 0 | |||||
| Prepandemic total | 517 (53.91) | 442 (46.09) | –a | 959 | NR | 0 | 959 (100) | 959 (100) | 0 | |||||
| Pandemic | 327 (61.5) | 205 (38.5) | 9.9 (5.8-12.9)a | 532 | NR | 0 | 532 (100) | 532 (100) | 0 | |||||
| Rabbone, 2020 | Italy | 368 | Prospective cohort | Prepandemic | NR | NR | NR | 208 | NR | NR | NR | NR | NR | PCR and antibody |
| Pandemic | NR | NR | NR | 160 | NR | NR | NR | NR | NR | |||||
| Salmi, 2021 | Finland | 45 | Retrospective cohort | Prepandemic | 15 (60.00) | 10 (40.00) | 9.5 (6.2–11.4)a | 25 | 12.4 (11.0–14.0)a | NR | 25 (100) | 25 (100) | 0 | PCR and antibody |
| Pandemic | 11 (55.00) | 9 (45.00) | 10.0 (8.1–12.3)a | 20 | 12.8 (11.8–14.0)a | NR | 20 (100) | 20 (100) | 0 | |||||
aMedian and IQR.
r Median and range.
Meta-analysis
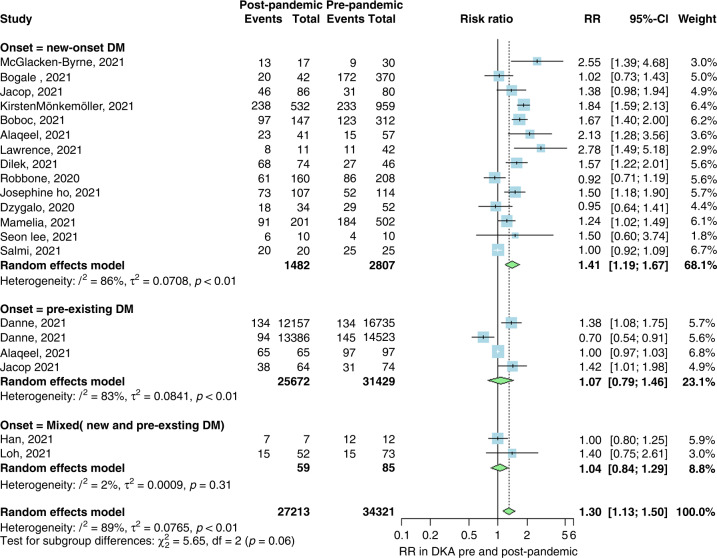
Our first analysis included 17 studies with 34,321 patients in the prepandemic group and 27,213 patients in the control or pandemic group. We performed three subgroup analyses for the incidence of DKA in newly diagnosed T1DM patients during the pandemic, pre-existing T1DM patients before the pandemic, and mixed between new and pre-existing T1DM patients. Our first subgroup analysis investigated the incidental risk of DKA in newly diagnosed T1DM patients during the pandemic, which showed a significantly increased risk (RR 1.41; 95% CI 1.19, 1.67; p < 0.01; I2 = 86%). There was no significant increase in the risk of DKA during the pandemic among pre-existing T1DM patients and mixed patients (RR 1.07; 95% CI 0.79, 1.46 and RR 1.04; 95% CI 0.84, 1.29, respectively; Fig. 2).
Fig. 2. Forest for DKA.
Forest plot summarizing the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the onset of diabetes (new-onset, pre-existing or mixed of both). SD standard deviation, CI confidence interval.
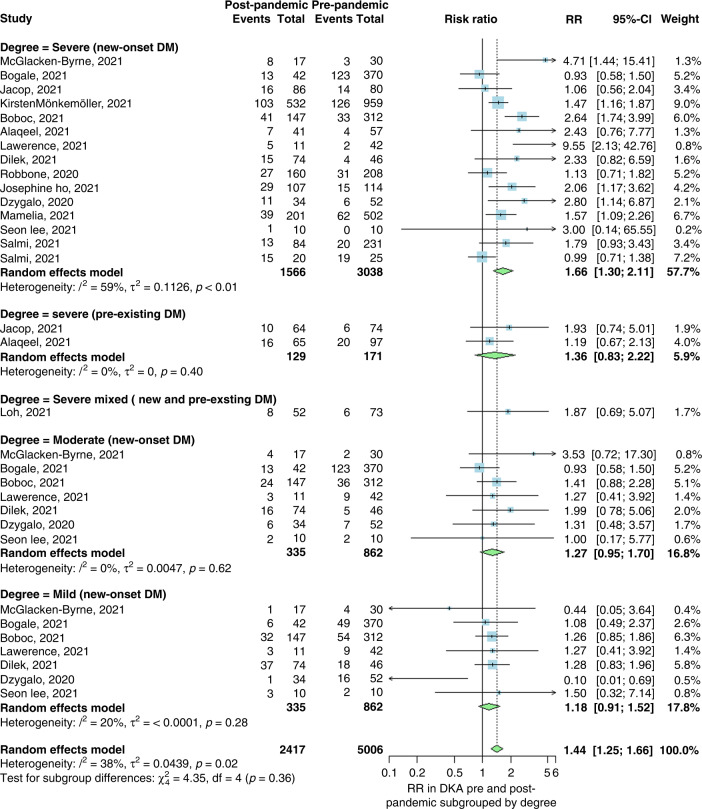
The second analysis of 15 studies included 5006 patients in the prepandemic group and 2417 in the pandemic group, with a cumulative significant RR of DKA of 1.44 (95% CI 1.25, 1.66; p < 0.01; I2 = 38%). This showed an increased risk of DKA during the pandemic, consistent with our first analysis. Furthermore, three subgroup analyses were performed for the severity (severe, moderate, and mild) of DKA in newly diagnosed T1DM patients during the pandemic. The first subgroup analysis included 3038 patients in the prepandemic group and 1566 in the pandemic group, investigating the risk of developing severe DKA. The analysis showed a significant increase in severe DKA during the pandemic (RR 1.66: 95% CI 1.3, 2.11) when compared to the prepandemic time. Our second and third subgroup analyses investigated the risk of moderate and mild DKA. They showed a statistically insignificant increase in the risk of developing both moderate and mild DKA during the pandemic time (RR 1.27; 95% CI 0.95, 1.7 and RR 1.18; 95% CI 0.91, 1.52), respectively. Moreover, severe DKA was analyzed in T1DM patients diagnosed prepandemic to show an increased risk of severity during the pandemic (RR 1.36; CI 95% 0.83, 2.22; p = 0.4), but it was statistically insignificant. Likewise, the severity of DKA in mixed new and prepandemic T1DM-diagnosed patients also showed an increase in risk during the pandemic (RR 1.87; 95% CI 0.69, 5.07; Fig. 3).
Fig. 3. Forest for degree.
Forest plot summarizing the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the degree of DKA (severe, moderate, or mild). SD standard deviation, CI confidence interval.
Visual inspection of the funnel plots of our meta-analyses revealed some asymmetrical distribution of the studies, as shown in Fig. 4.
Fig. 4. Funnel plots.
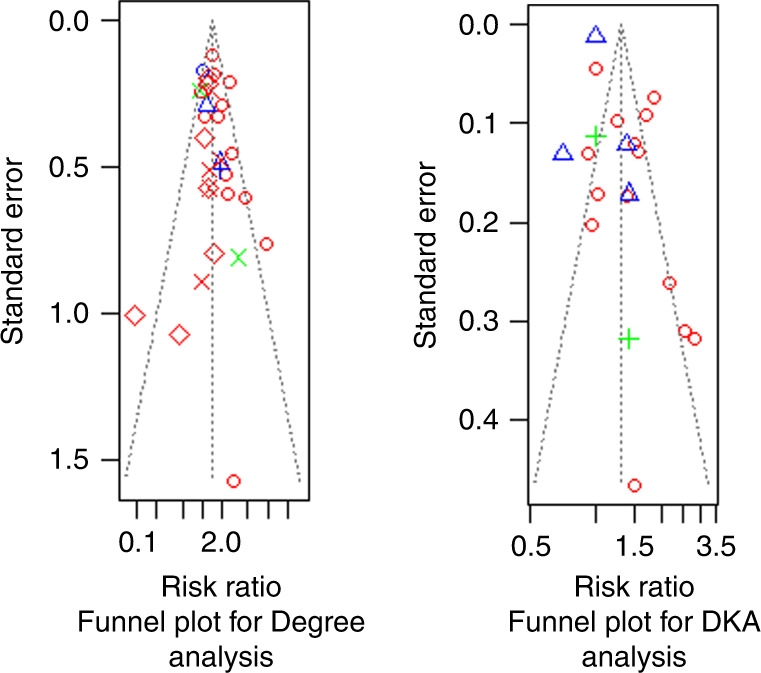
Funnel plots showing publication bias in studies included in the analysis calculating the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the onset of diabetes, and for the studies included in the analysis calculating the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the degree of DKA.
Sensitivity analysis
A leave-one-out analysis revealed that no single study affected the overall effect in either analysis (Figs. 5 and 6).
Fig. 5. Leave for DKA.
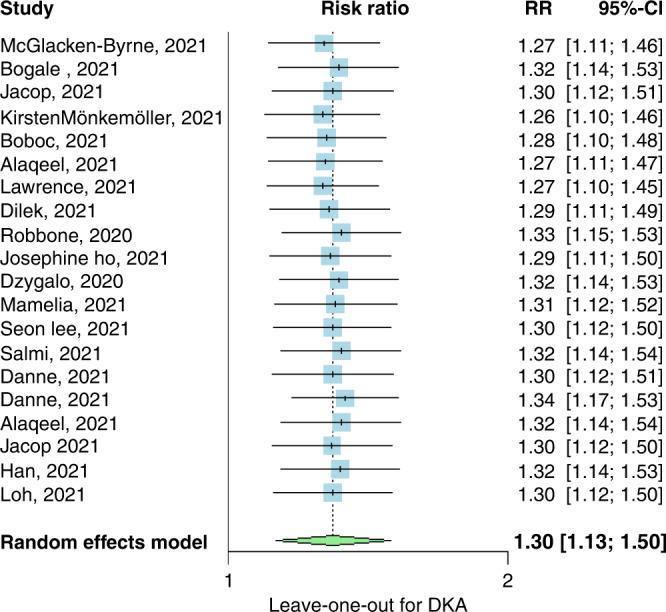
Leave-one-out meta-analysis of studies calculating the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the onset of diabetes, CI confidence interval.
Fig. 6. Leave for the degree.
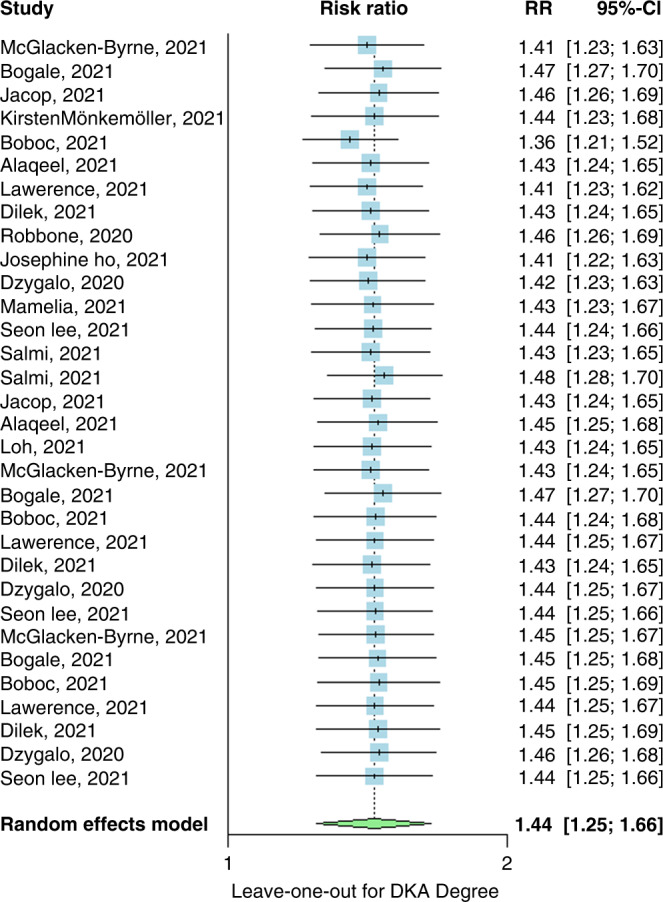
Leave-one-out meta-analysis of studies calculating the risk ratio of DKA in pre-pandemic and post-pandemic stratified by the degree of DKA, CI confidence interval.
Qualitative assessment
Kamrath et al.24 performed a multicenter cohort study on new-onset DKA in patients diagnosed with T1DM and COVID-19. Among 3238 patients with diabetes, DKA developed in 1094, with a significant relationship between the development of DKA and COVID-19 patients with diabetes in the first half of the year 2020; the relationship was insignificant in the second half.
Ramgopal et al.27 performed a cross-sectional study to identify changes in the presentations of pediatric emergencies during the pandemic compared to the last 20 years. Among the study population, 5985 (31%) DKA patients presented to the emergency room during the pandemic compared to 51,708 (18%) prepandemic, which emphasized that emergency utilization was low during the pandemic for most diagnoses, but with a noticeable increase in DKA presentations.
Kucharska et al.25 performed a cross-sectional study that included 1961 patients to determine whether COVID-19 lockdown was associated with an increasing incidence rate of T1DM in children. Out of 1961 patients, new-onset DKA was observed in 36.6% during the pandemic compared to 31.75% prepandemic.
Elbarbary et al.29 performed a multicenter cross-sectional study on 86 patients with diabetes under 18 years of age to determine whether management practice changed during the COVID-19 pandemic. Of the 86 patients, 44 developed DKA, and 15% reported a higher incidence of DKA during the pandemic. Most centers did not have COVID-19-positive patients with diabetes, and those who did showed a mild or moderate disease course.
Fisler et al.26 performed a retrospective cohort study to indicate the main causes of pediatric intensive care unit (PICU) admission during the pandemic. Of five patients with diabetes, two had DKA and were referred to the PICU, making DKA one of the main indications for PICU admission.
Alonso et al.30 performed a survey that included 266 patients with diabetes aged under 19 years to describe the outcomes of COVID-19 in children with T1DM and which factors increased the risk of disease. Out of 266 patients, 44 had DKA, making it one of the most common adverse events for hospitalization.
Sherif et al.23 performed a retrospective observational study including 36 patients with T1DM to determine the characteristics of pediatric patients with T1DM during the pandemic and the prevalence of new-onset T1DM and DKA. Of the 36 with T1DM, 29 developed DKA, proving that the pandemic increased the prevalence and severity of DKA in patients with diabetes.
Quality assessment
For cohort studies, judged by following the National Occupational Standards (NOS) guidelines, all studies were of good quality. However, the study of Elbarbary29 was of poor quality, primarily due to lacking comparability of cohorts based on the design. Based on the NOS scoring system, two studies, Danne38 and Loh,33 were of good quality, given scores of eight each. Two more studies, Kucharska25 and Lawrence,13 did not adjust the selection of cases as well as their comparability. Hence, they were of fair quality, scoring six each (Tables 4 and 5).
Table 4.
NOS quality assessment.
| Study ID | Newcastle-Ottawa Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Overall score (out of 9) | ||||||
| Representativeness of the exposed cohort (score: ★) | Selection of the non-exposed cohort (score: ★) | Ascertainment of exposure (score: ★) | Demonstration that outcome of interest was not present at the start of the study (score: ★) | Comparability of cohorts on the basis of the design or analysis (score: ★★) | Assessment of outcome (score: ★) | Was follow-up long enough for outcomes to occur (maximum: ★) | Adequacy of follow-up of cohorts (maximum: ★) | ||
| Danne, 2021 | ★ | ★ | ★ | – | ★★ | ★ | ★ | ★ | 8 |
| Kucharska, 2021 | ★ | ★ | ★ | – | – | ★ | ★ | ★ | 6 |
| Lawrence, 2021 | ★ | ★ | ★ | – | – | ★ | ★ | ★ | 6 |
| Loh, 2021 | ★ | ★ | ★ | – | ★★ | ★ | ★ | ★ | 8 |
Table 5.
NIH quality assessment.
| Title | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | N13 | N14 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alaqeel, 2021 | * | * | * | * | – | * | * | / | * | – | * | – | * | – | 9 |
| Boboc, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Bogale, 2021 | * | – | * | * | – | – | * | / | * | – | * | – | * | – | 7 |
| Danne, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Dilek, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Dzygalo, 2020 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Han, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | * | 9 |
| Ho, 2021 | * | * | * | * | – | * | * | / | * | – | * | – | * | – | 9 |
| Jacob, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Jama, 2020 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Kamrath, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Lee, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Mameli, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | * | 9 |
| McGlacken-Byrne, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | * | 9 |
| Rabbone, 2020 | * | * | * | * | – | – | – | / | * | – | * | – | * | – | 7 |
| Ramgopal, 2021 | * | * | * | * | – | – | * | / | * | – | – | – | * | – | 7 |
| Salmi, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Alonso, 2021 | * | * | * | * | – | – | * | / | * | – | * | – | * | – | 8 |
| Elbarbary, 2020 | – | * | * | – | – | – | – | / | – | – | – | – | * | – | 3 |
| Fisler, 2020 | * | * | * | * | – | – | – | / | * | – | – | – | * | – | 6 |
| Sherif, 2021 | * | * | * | * | – | – | / | / | / | – | * | – | * | – | 6 |
* = Yes; – = No; / = Not applicable.
1. Was the research paper question or goal stated clearly?
2. Was the study population specified clearly and defined?
3. Was the percentage of participation of eligible people at least 50%?
4. Were all the participants chosen from populations alike (including the same time period)? Were inclusion and exclusion criteria for being in the study stated and applied to all participants uniformly?
5. Was a sample size justification, power description, or variance and effect estimates given?
6. For the analyses in this paper, were the exposure(s) of interest measured before the outcome(s) were measured?
7. Was the timeframe enough so that one could reasonably expect to see an association between exposure and outcome if it was present?
8. For exposures that can be variable in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as a continuous variable)?
9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and applied uniformly to all study subjects?
10. Was the exposure(s) assessed many times (more than 1 time) over the timeline of the study?
11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and applied uniformly to all study subjects?
12. Were the outcome assessors blinded to the exposure status of subjects?
13. Was the loss to follow-up after baseline 20% or lower?
14. Were key potential confounding variables measured and modified statistically for their effect on the relationship between exposure(s) and outcome(s)?
Discussion
Auto-immune diseases such as T1DM can be caused by several environmental factors, such as viral, genetic, or immunological agents. Because COVID-19 covers both the viral causative agent and the immunological factor (exhausting the immune system), several studies have reported an increase in the bidirectional relationship between COVID-19 and diabetes.6,42
Our systematic review and meta-analyses include 24 studies of 124,597 children with diabetes that revealed that the incidental risk of DKA significantly increased during the pandemic in newly diagnosed T1DM patients, but with an insignificant increase in pre-existing T1DM pediatric patients. Furthermore, subgroup analyses of the DKA degree revealed a statistically significant risk of the severe form of DKA during the pandemic, and the mild and the moderate forms insignificantly increased, which reflects the impact of COVID-19 on other chronic diseases and its burden on healthcare systems.
As of January 5, 2022, COVID-19 has infected more than 290 million cases, with more than five million deaths.43,44 Having a chronic disease worsens the prognosis of COVID-19 infection and increases the mortality rate.45 With an increase in 2019 of 20 million cases per year,46,47 diabetes affected more than 537 million cases in 2020. Patients are expected to increase by 2030 to more than 643 million.46 The pre-existing pandemic of diabetes has been superimposed with the COVID-19 pandemic, resulting in a significantly vulnerable and huge COVID-19 patient population with diabetes, which is consistent with our data, which has proven to increase the risk of T1DM during pandemic time compared to prepandemic time. Diabetes-related immunodeficiency can predispose to COVID-19 infection, and cytokine storms caused by COVID-19 can further stimulate the immune response toward the pancreas cells, promoting the process of developing diabetes, especially type 1.48,49
Several studies revealed that patients with diabetes, when compared to non-diabetics, had more inflammatory cells and a higher risk of mortality due to COVID-19, ICU admission, and the need for mechanical ventilation.50 The angiotensin-converting enzyme-1 receptor of severe acute respiratory syndrome coronavirus (SARS-CoV) has been expressed in humans on the pancreatic beta cells and pancreatic microvasculature,51 and SARS-CoV has been proposed to replicate inside the pancreatic cells, precipitating T1DM and DKA.52,53
Including hyperosmolar hyperglycemia syndrome and the overlapping syndrome of hyperosmolar ketoacidosis, DKA is the most common hyperglycemic crisis.54 DKA happens in the setting of decreased glucose breakdown in cases of a relative or absolute deficiency of insulin, so the body metabolism shifts to lipolysis, producing excess ketone bodies,54 a state of severe metabolic acidosis. Reported DKA cases have been increasing during the pandemic, and several reasons have been proposed but need further research to determine the definitive pathophysiology. In a cohort study of 658 patients, Li et al.55 reported that COVID-19 infection not only induced DKA in patients with diabetes but also induced ketoacidosis in healthy COVID-19-infected patients. They also found a positive correlation between ketoacidosis and length of hospital stay, which is consistent with our data of increasing the risk of both DKA as the frequency of cases and the severity of DKA during the pandemic. This increase in pediatric DKA can be explained by the parents’ fear to access primary healthcare settings during the COVID-19 pandemic; thus, this delay contributes to increasing the DKA incidence in children.11,15
Limitations
The substantial heterogeneity reported in some subgroups is the main limitation. However, this challenge was overcome by using the DerSimonian and Laird random-effects model. This is based on an inverse variance approach, where the studies are weighted according to their level of heterogeneity by conducting leave-one-out meta-analyses. It showed that no study significantly affected the overall estimate or heterogeneity, which was minimized by conducting subgroup analyses. The heterogeneity present may be due to different populations, methods of diagnosis, and variants of COVID-19 that affected pediatric patients. Another limitation was the presence of some asymmetry in the funnel plots, which can be explained by the authors’ underreporting of studies without a proven hypothesis.
Conclusion
DKA in newly diagnosed T1DM children has increased during the pandemic and presented with a severe form. This may reflect that COVID-19 may have contributed not only to the development but also the severity of DKA. We introduce these insights to healthcare providers to educate patients about the importance of timely attendance to the emergency department for non-COVID symptoms.
Acknowledgements
The authors would like to thank Dr. Dina Khaled for her support.
Author contributions
K.S., A.E., H.H.E.-L., M.A., and A.E. designed the study and analyzed the data. E.M.H., A.M.A., F.-A.A., S.F.T., and A.A.O. drafted the manuscript. All authors were involved in the critical analysis of the final version of the manuscript. All authors approved the manuscript as submitted and agree to be accountable for all aspects of the work.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anas Elgenidy, Ahmed K. Awad.
References
- 1.Zahran AM, et al. Association of follicular helper T and follicular regulatory T cells with severity and hyperglycemia in hospitalized COVID-19 patients. Virulence. 2022;13:569–577. doi: 10.1080/21505594.2022.2047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed S, et al. Is COVID-19 a systemic disease? Coronaviruses. 2021;2:e060521189167. [Google Scholar]
- 3.El-Badawy O, et al. COVID-19 infection in patients with comorbidities: clinical and immunological insight. Clin. Appl Thromb. Hemost. 2022;28:10760296221107889. doi: 10.1177/10760296221107889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiga M, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamad IL, et al. Evaluation of pulmonary function changes in children with type 1 diabetes mellitus in Upper Egypt. Ther. Adv. Endocrinol. Metab. 2015;6:87–91. doi: 10.1177/2042018815580514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfsdorf JI, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes. 2018;19:155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 7.Evans K. Diabetic ketoacidosis: update on management. Clin. Med. J. R. Coll. Physicians Lond. 2019;19:396–398. doi: 10.7861/clinmed.2019-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. Declining death rates from hyperglycemic crisis among adults with diabetes, U.S., 1985-2002. Diabetes Care. 2006;29:2018–2022. doi: 10.2337/dc06-0311. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176:414–415. doi: 10.1001/jamapediatrics.2021.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unsworth R, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 11.Rabbone I, et al. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43:2870–2872. doi: 10.2337/dc20-1321. [DOI] [PubMed] [Google Scholar]
- 12.Kamrath C, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324:801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence C, et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet. Med. 2021;38:1–5. doi: 10.1111/dme.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogale KT, et al. The impact of COVID-19 pandemic on prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes: a single-centre study in central Pennsylvania. Endocrinol. Diabetes Metab. 2021;4:29–32. doi: 10.1002/edm2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wersäll JH, et al. Delayed referral is common even when new-onset diabetes is suspected in children. A Swedish prospective observational study of diabetic ketoacidosis at onset of Type 1 diabetes. Pediatr. Diabetes. 2021;22:900–908. doi: 10.1111/pedi.13229. [DOI] [PubMed] [Google Scholar]
- 16.March CA, et al. Paediatric diabetes care during the COVID-19 pandemic: lessons learned in scaling up telemedicine services. Endocrinol. Diabetes Metab. 2021;4:4–9. doi: 10.1002/edm2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Sofiani ME, et al. Rapid implementation of a diabetes telemedicine clinic during the Coronavirus Disease 2019 outbreak: our protocol, experience, and satisfaction reports in Saudi Arabia. J. Diabetes Sci. Technol. 2020;15:329–338. doi: 10.1177/1932296820947094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Predieri B, et al. Glycemic control improvement in italian children and adolescents with type 1 diabetes followed through telemedicine during lockdown due to the COVID-19 pandemic. Front. Endocrinol. (Lausanne). 2020;11:1–10. doi: 10.3389/fendo.2020.595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boboc, A. A. et al. The impact of SARS-COV-2 pandemic on the new cases of T1DM in children. A single-centre cohort study. J. Pers. Med.11, 551 (2021). [DOI] [PMC free article] [PubMed]
- 20.Alaqeel A, et al. The impact of COVID-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi Children. Front. Endocrinol. (Lausanne). 2021;12:8–12. doi: 10.3389/fendo.2021.669302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ372, n71 (2021). [DOI] [PMC free article] [PubMed]
- 22.R Foundation for Statistical Computing, Vienna, A. R Core Team, R: A Language and Environment for Statistical Computing. https://www.r-project.org/ (2020).
- 23.Sherif EM, et al. Clinical characteristics and outcome of hospitalized children and adolescent patients with type 1 diabetes during the COVID-19 pandemic: data from a single center surveillance study in Egypt. J. Pediatr. Endocrinol. Metab. 2021;34:925–936. doi: 10.1515/jpem-2021-0099. [DOI] [PubMed] [Google Scholar]
- 24.Kamrath, C. et al. Incidence of COVID-19 and risk of diabetic ketoacidosis in new-onset type 1 diabetes. Pediatrics148, e2021050856 (2021). [DOI] [PubMed]
- 25.Zubkiewicz-Kucharska A, et al. Diagnosis of type 1 diabetes during the SARS-CoV-2 pandemic: does lockdown affect the incidence and clinical status of patients? Adv. Clin. Exp. Med. 2021;30:127–134. doi: 10.17219/acem/130359. [DOI] [PubMed] [Google Scholar]
- 26.Fisler, G. et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann. Intensive Care10, 171 (2020). [DOI] [PMC free article] [PubMed]
- 27.Ramgopal S, et al. Forecast modeling to identify changes in pediatric emergency department utilization during the COVID-19 pandemic. Am. J. Emerg. Med. 2021;49:142–147. doi: 10.1016/j.ajem.2021.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, et al. Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic. Yeungnam Univ. J. Med. 2021 doi: 10.12701/yujm.2021.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbarbary NS, et al. COVID-19 outbreak and pediatric diabetes: perceptions of health care professionals worldwide. Pediatr. Diabetes. 2020;21:1083–1092. doi: 10.1111/pedi.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso GT, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J. Diabetes. 2021;13:681–687. doi: 10.1111/1753-0407.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGlacken-Byrne SM, et al. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: a multi-centre study of the first COVID-19 wave. Diabet. Med. 2021;38:1–7. doi: 10.1111/dme.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmi, H. et al. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch. Dis. Child.107, 180–185 (2022). [DOI] [PubMed]
- 33.Loh, C., et al. Diabetic ketoacidosis in pediatric patients with type 1- and type 2 diabetes during the COVID-19 pandemic. Metabolism122, 154842 (2021). [DOI] [PMC free article] [PubMed]
- 34.Mameli C, et al. Type 1 diabetes onset in Lombardy region, Italy, during the COVID-19 pandemic: the double-wave occurrence. EClinicalMedicine. 2021;39:101067. doi: 10.1016/j.eclinm.2021.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mönkemöller K, et al. Is it possible to prevent diabetic ketoacidosis at diagnosis of pediatric type 1 diabetes? Lessons from the COVID-19 pandemic. Monatsschr. Kinderheilkd. 2021;169:451–460. doi: 10.1007/s00112-020-01108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho J, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr. Diabetes. 2021;22:552–557. doi: 10.1111/pedi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob R, et al. Diabetic ketoacidosis at emergency department presentation during the first months of the SARS-CoV-2 pandemic in Israel: a multicenter cross-sectional study. Diabetes Ther. 2021;12:1569–1574. doi: 10.1007/s13300-021-01049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danne T, et al. A worldwide perspective on COVID-19 and diabetes management in 22,820 children from the SWEET Project: diabetic ketoacidosis rates increase and glycemic control is maintained. Diabetes Technol. Ther. 2021;23:632–641. doi: 10.1089/dia.2021.0110. [DOI] [PubMed] [Google Scholar]
- 39.Dżygało K, et al. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediatr. Endocrinol. Diabetes Metab. 2020;26:167–175. doi: 10.5114/pedm.2020.101003. [DOI] [PubMed] [Google Scholar]
- 40.Han MJ, Heo JH. Increased incidence of pediatric diabetic ketoacidosis after covid-19: a two-center retrospective study in Korea. Diabetes Metab. Syndr. Obes. Targets Ther. 2021;14:783–790. doi: 10.2147/DMSO.S294458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dilek SÖ, et al. Changes in the presentation of newly diagnosed type 1 diabetes in children during the COVID-19 pandemic in a tertiary center in Southern Turkey. J. Pediatr. Endocrinol. Metab. 2021;34:1303–1309. doi: 10.1515/jpem-2021-0287. [DOI] [PubMed] [Google Scholar]
- 42.DiMeglio LA, et al. Type 1 diabetes. Lancet. 2018;391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Timeline. “COVID-19. Coronavirus disease (COVID-19).” pandemic Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed: 2022-01-05.
- 44.Dong E, et al. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Lancet Inf. Dis. 2020;19:533–534. [Google Scholar]
- 45.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 46.International Diabetes Federation, IDF Diabetes Atlas, International Diabetes Federation. Diabetes Research and Clinical Practice (2020). [DOI] [PubMed]
- 47.Lima-Martínez MM, et al. COVID-19 and diabetes: a bidirectional relationship. Clin. Investig. Arterioscler. 2021;33:151–157. doi: 10.1016/j.arteri.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Accili D. Can COVID-19 cause diabetes? Nat. Metab. 2021;3:123–125. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang J, et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan, China. Am. J. Med. 2020;110:697–700. doi: 10.1016/j.amjmed.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fignani D, et al. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front. Endocrinol. (Lausanne). 2020;11:1–19. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller JA, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 53.Palermo NE, et al. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J. Clin. Endocrinol. Metab. 2020;105:2819–2829. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steenkamp DW, et al. Adult hyperglycemic crisis: a review and perspective. Curr. Diab. Rep. 2013;13:130–137. doi: 10.1007/s11892-012-0342-z. [DOI] [PubMed] [Google Scholar]
- 55.Li J, et al. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.