
Arrival chest x-ray with arrow pointing projectile in neck zone 1.
Central Message.
We introduce a novel, multidisciplinary, and minimally invasive approach to managing penetrating esophageal trauma with concomitant mediastinal abscess formation and vascular injury using thoracoscopy, transluminal endoscopic drainage placement (Houdini), and endovascular and endoluminal stenting.
Although the incidence of esophageal perforations caused by trauma remains low, the morbidity and mortality associated with them remain high.1, 2, 3 Traditional management of these types of injuries involves surgical exposure of the esophagus and a 2-layered repair in stable patients.3, 4, 5 In hemodynamically unstable patients or patients with extreme esophageal destruction, this type of injury may warrant a damage-control operation with esophageal diversion.1,6 Over the past decade, as technology and techniques have advanced, our ability to manage these injuries in a less-invasive manner has become more prevalent.7, 8, 9 Techniques in flexible endoscopy, advanced fluoroscopy, and esophageal stenting and clipping have introduced new methods for minimally invasive management of these complex injuries.7,8,10, 11, 12, 13 These injuries may be associated with mediastinal abscess and fluid collections that mandate drainage.8,10,12 These new techniques provide a safe approach to appropriate candidates and avoid the morbidity of major open surgery,1,13 in multiple body cavities, and in the setting of infected fields submitted to blast injury. This case report demonstrates the minimally invasive approach to an esophageal gunshot wound (GSW) with associated mediastinal abscess and vascular injury integrating these new evolving techniques. Patient consent for procedure, perioperative care, and publication was obtained.
Clinical Summary
The patient is a 26-year-old man who presented with a GSW to the right neck. There was concern for tracheal or esophageal injury because the bullet was lodged near the left first rib on chest x-ray (Figure 1), suggesting transcervical and mediastinal pathway. A computed tomography angiography of the neck (Figure 2) was performed that revealed a posterior mediastinal hematoma as well as a right vertebral artery injury resulting in occlusion of the artery. A bronchoscopy and upper endoscopy were performed by the trauma team with only the findings of esophageal mucosal bruising. Clinical suspicion remained high for an injury, so a barium swallow was performed that demonstrated contrast leakage into the neck and mediastinum from the upper esophagus.
Figure 1.
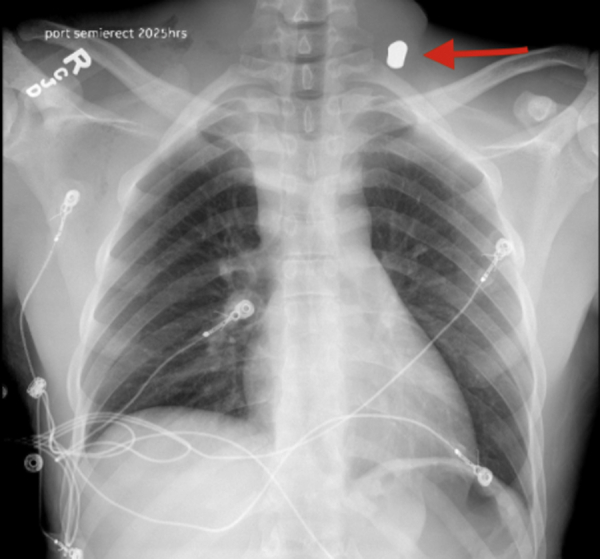
Arrival chest x-ray with arrow pointing the projectile in neck zone 1.
Figure 2.
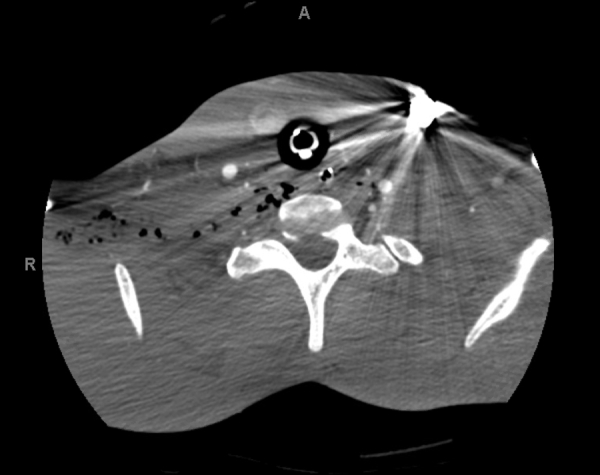
Computed tomography angiography of the neck demonstrating trans-mediastinal trajectory of the projectile located in the left lower neck.
At this time, the patient had started developing fevers, tachycardia, and oliguria. The thoracic surgery team was consulted, and the patient was brought to the operating 2 days from the injury. Bronchoscopy ruled out airway injury, but upper endoscopy revealed a GSW immediately below the cricopharyngeus muscle at the right-anterior esophagus. With the use of transluminal endoscopy with a 5.4-mm diameter pediatric scope and antibiotic irrigation (cefazolin and fluconazole), the tract from the esophagus to the skin was identified. On the table, fluoroscopic contrast study was performed and antibiotic solution used extensively for irrigation and estimation of cavitary and possible mediastinal drainage pathways. Extension into posterior thoracic inlet parallel to esophagus raised concerns for more extensive blast trauma. Then using a fluoroscopic and endoscopic technique, a Jagwire High Performance Guidewire (Boston Scientific) was threaded through the bullet tract from the skin entrance site and into the lumen of the esophagus gaining transluminal control of the trajectory of injury (Video 1). A drain was then backed out transluminally in a retrograde fashion from the injury site in the esophagus and into the posterior mediastinum. A #10 flat Jackson-Pratt drain tubing (Cardinal Health) was pulled through the bullet's skin entrance site and secured in place. The flat portion was marked with silk tips to aid in endoscopic manipulation. A 100 × 23-mm fully covered esophageal stent (Alimaxx-ES; Merit Endotek) was placed over the injury to provide further esophageal protection, reduce surrounding tissues spaces, stabilize transluminal drain, and minimize further contamination of the mediastinum with anticipation of possible progressive blast injury. An immediate sub-cricopharyngeal placement of the proximal end is ensured, with attention to keeping struts below the sphincter to avoid globus sensation. In the absence of a refluxogenic hiatal hernia and with a stable tubular esophagus, a percutaneous endoscopic gastrostomy (PEG) was then placed for the protracted oral intake period to come without risk of feeding formula wash-back into the stented area. Post–stent placement of a PEG was preferred because frail wound tissues are protected and direct endoscopic guidance of the gastrostomy bumper with an endoscope was done to avoid displacement or damage to exposed stent edges. The patient's straight entrance, nontortuous tubular anatomy of his esophagus, and our center's extensive experience with transluminal endoscopic approaches are taken into account for this maneuver. An endoscopic “push” PEG or gastrojejunostomy, unavailable to the authors on the night in question, is a safer option for centers less familiar with this approach (Video 2).
The patient was then placed in the left lateral decubitus position, and a right video-assisted thoracoscopic surgery was performed to proceed with mediastinal drainage. Posterior and anterior mediastinal pleura were opened, identifying a purulent cavity communicating with the cervical space in the retroesophageal prevertebral space. Two Jackson-Pratt drains placed in parallel across the cervical space and mediastinum and a chest tube were secured for wide drainage of the injured area, anticipating possible further breakdown in associated blast injury tissues (Video 3). The patient tolerated the procedure well, was extubated immediately postoperatively, and had an uneventful recovery. He underwent stenting of his right vertebral artery injury by vascular surgery (5 mm × 5 cm Gore Viahbahn) on postoperative day 2. Antibiotics and an antifungal (piperacillin/tazobactam eventually converted to enteral amoxicillin and clavulanate and fluconazole) were continued throughout the patient's hospital course. His chest tube and one of his thoracic drains were removed before discharge. He was discharged home on postoperative day 8.
The first stent exchange was performed approximately 2 weeks later. His drains were both “cracked” backed out approximately 1 cm at this time to avoid suction injury and commence “controlled fistula” management. His bullet wound entrance healed within 2 weeks. As anticipated, further breakdown occurred lower on the esophagus and was well and directly controlled per mediastinal drains and lower extension of stent. This was in the form of ischemic-like ulceration. He underwent serial endoscopic stent exchanges and drain manipulations by sequential retraction over the following 2 months every 2 to 3 weeks. This totaled 3 stent exchanges and 4 endoscopies. Patient tolerated clear per os intake during this time without globus sensation. His fistula closed, and the final stent was removed 3 months after the injury. The bullet was surgically removed at this time because it had migrated to skin level and was causing patient discomfort. He was monitored radiographically with computed tomography scans after his final stent removal to ensure no fluid collections or infections were developing, as well as integrity of the vascular structures. The patient was noncompliant with dietary restrictions throughout his course and used his gastrostomy tube only intermittently, so this was removed approximately 4 months after injury. Clinical and contrast imaging follow-up up to 3 years show no sequalae.
Discussion
This case report demonstrates successful, minimally invasive management of a penetrating esophageal trauma across the neck and mediastinum with associated vascular lesion and blast effect. Given the complex injuries that may be associated with trauma patients, this type of innovation is paramount in improving outcomes and reducing morbidity and surgical trauma that escalate with multiple cavity interventions and wide debridement in these situations. This patient also had a proximal vertebral artery injury that was managed in a minimally invasive endovascular fashion by the vascular surgery team. The coordination of care and communication among trauma, vascular, and thoracic surgery in this case is an example of interdisciplinary communication and planning that helped a patient with a potentially devastating injury walk out of the hospital with minimal morbidity.
Esophageal stenting is not without risks, technical failures, or need for reintervention. A revision of 201 esophageal stenting articles comparing 785 surgical repair strategies brings attention to the limitations and variances on each approach.14 Factors such as delay from onset of injury to intervention from 24 to 48 hours, compounding injuries, hemodynamic instability, sepsis, and need for other extensive resection or surgical procedure, all present in our patient, were recognized as conditioning failure of primary surgical approach. Stent and “go home” is a fallacy. It is well established that resuscitation, antibiotics, adequate drainage, reliable enteral access, and monitoring for stent migration are also key components of successful management of these patients.15 Other comparative studies have reported propensity-matched multicenter comparison of treatments with an esophageal stent or an operative repair for an acute esophageal perforation.16 In these well-matched groups, the esophageal stent cohort presents significant favorable differences in morbidity (4% vs 43%; P = .02), mean length of stay (6 vs 11 days; P = .0007), time to oral intake (3 vs 8 days; P = .0004), and cost ($91,000 vs $142,000; P < .0001) when compared with patients receiving surgical repair. When specifically looking at the trauma population and mechanisms, blunt injuries do worse than penetrating irrespective of approach. Granularity of results is murky when attempts are made to differentiate thoracic versus cervical esophageal injury.17 Thus, our patient's close-range GSW combining a penetrating and blunt blast component presented a complex choice. It is our group's approach to closely monitor high esophageal stents with endoscopy and imaging. Crossing of the cricopharyngeal plane with struts carries significant risks further than discomfort, that is, aspiration, recurrent and laryngeal nerves palsy, pharyngeal erosions, and vascular and laryngotracheal fistulation. Compulsive attention to landing of the stent below this level and regular stent exchanges to vary pressure points is important in mitigating these. Endoluminal stitching is an adjunct to prevent migration in experienced hands when available. It is all these complexities, risks, patient's mechanism of injury, and condition to our choice of interventions, which although minimally invasive, are extensive and incorporating directed drainage, soilage control, nutrition, and attention to other injuries.
Although the patient did undergo numerous episodes of general anesthesia for outpatient endoscopies and stent exchanges, his esophageal injury healed well without neurovascular deficits or need for open incisions in a blast-impacted and contaminated neck and chest. This technique is not appropriate for all patients, such as those with hemodynamic instability or a low tolerance for repeated episodes of general anesthesia. This patient required close clinical follow-up, screening endoscopies, and prolonged antibiotics. The multispecialty pathway presented is not meant as a surgical shortcut. These approaches do not substitute sound surgical open principles of infection source drainage, hemostasis control, nutrition, viable tissue interposition, preservation, and repair. They methodically preserve them with the use of emerging techniques in situations where a traditional open approach may be a source of greater surgical trauma, physiologic insult, and increased failure and morbidity. A tertiary center with not only access to them but also large case numbers and expertise in these techniques is also necessary, as well as an open and clear communication strategy with the patient and family of the risks, surgical approaches and alternatives, and follow-up. Despite some noncompliance issues, this patient followed up appropriately and participated in his care, which led to a good outcome. This approach would not be successful in patients lost to follow-up.
Conclusions
As shown in this case report, when the appropriate patient is chosen, the Houdini drains and minimally invasive techniques can be effective in managing complex penetrating esophageal and vascular injuries with minimal short- or long-term morbidity, while respecting the surgical principles of traditional open surgery as a mandate.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Preoperative imaging, drain preparation, endoscopic transluminal drain (Houdini) and stent placement, and thoracoscopic mediastinal abscess drainage. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.
Houdini drain preparation, placement, and stent deployment. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.
Thoracoscopic mediastinal abscess drainage and drain placement. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.
References
- 1.Petrone P., Kassimi K., Jimenez-Gomez M., Betancourt A., Axelrad A., Marini C.P. Management of esophageal injuries secondary to trauma. Injury. 2017;48:1735–1742. doi: 10.1016/j.injury.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Asensio J.A., Chahwan S., Forno W., MacKersie R., Wall M., Lake J., et al. Penetrating esophageal injuries: multicenter study of the American Association for the Surgery of Trauma. J Trauma. 2001;50:289–296. doi: 10.1097/00005373-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Patel M.S., Malinoski D.J., Zhou L., Neal M.L., Hoyt D.B. Penetrating oesophageal injury: a contemporary analysis of the national trauma data bank. Injury. 2013;44:48–55. doi: 10.1016/j.injury.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Brinster C.J., Singhal S., Lee L., Marshall M.B., Kaiser L.R., Kucharczuk J.C. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475–1483. doi: 10.1016/j.athoracsur.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Madiba T.E., Muckart D.J. Penetrating injuries to the cervical oesophagus: is routine exploration mandatory? Ann R Coll Surg Engl. 2003;85:162–166. doi: 10.1308/003588403321661307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi S., Schraufnagel D.P., Siddiqui H.U., Javorski M.J., Mace A., Elnaggar A.S., et al. Recent advancements in the minimally invasive management of esophageal perforation, leaks, and fistulae. Expert Rev Med Devices. 2019;16:197–209. doi: 10.1080/17434440.2019.1582329. [DOI] [PubMed] [Google Scholar]
- 7.Gurwara S., Clayton S. Esophageal perforations: an endoscopic approach to management. Curr Gastroenterol Rep. 2019;21:57. doi: 10.1007/s11894-019-0730-5. [DOI] [PubMed] [Google Scholar]
- 8.Watkins J.R., Farivar A.S. Endoluminal therapies for esophageal perforations and leaks. Thorac Surg Clin. 2018;28:541–554. doi: 10.1016/j.thorsurg.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Sepesi B., Raymond D.P., Peters J.H. Esophageal perforation: surgical, endoscopic and medical management strategies. Curr Opin Gastroenterol. 2010;26:379–383. doi: 10.1097/MOG.0b013e32833ae2d7. [DOI] [PubMed] [Google Scholar]
- 10.Saxena P., Khashab M.A. Endoscopic management of esophageal perforations: who, when, and how? Curr Treat Options Gastroenterol. 2017;15:35–45. doi: 10.1007/s11938-017-0117-3. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Esquivel R., Raju G.S. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep. 2013;15:321–329. doi: 10.1007/s11894-013-0321-9. [DOI] [PubMed] [Google Scholar]
- 12.Dasari B.V., Neely D., Kennedy A., Spence G., Rice P., Mackle E., et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 13.Ben-David K., Behrns K., Hochwald S., Rossidis G., Caban A., Crippen C., et al. Esophageal perforation management using a multidisciplinary minimally invasive treatment algorithm. J Am Coll Surg. 2014;218:768–774. doi: 10.1016/j.jamcollsurg.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Persson S., Rouvelas I., Irino T., Lundell L. Outcomes following the main treatment options in patients with a leaking esophagus: a systematic literature review. Dis Esophagus. 2017;30:1–10. doi: 10.1093/dote/dox108. [DOI] [PubMed] [Google Scholar]
- 15.Herrera A., Freeman R.K. The evolution and current utility of esophageal stent placement for the treatment of acute esophageal perforation. Thorac Surg Clin. 2016;26:305–314. doi: 10.1016/j.thorsurg.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Freeman R.K., Herrera A., Ascioti A.J., Dake M., Mahidhara R.S. A propensity-matched comparison of cost and outcomes after esophageal stent placement or primary surgical repair for iatrogenic esophageal perforation. J Thorac Cardiovasc Surg. 2015;149:1550–1555. doi: 10.1016/j.jtcvs.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 17.Raff L.A., Schinnerer E.A., Maine R.G., Jansen J., Noorbakhsh M.R., Spigel Z., et al. Contemporary management of traumatic cervical and thoracic esophageal perforation: the results of an Eastern Association for the Surgery of Trauma multi-institutional study. J Trauma Acute Care Surg. 2020;89:691–697. doi: 10.1097/TA.0000000000002841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative imaging, drain preparation, endoscopic transluminal drain (Houdini) and stent placement, and thoracoscopic mediastinal abscess drainage. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.
Houdini drain preparation, placement, and stent deployment. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.
Thoracoscopic mediastinal abscess drainage and drain placement. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00306-6/fulltext.


