Abstract
In songbirds and mammals, brain injury results in the upregulation of aromatase (estrogen synthase) expression in astroglia. The resulting, presumed synthesis of neural estradiol (E2) has neuroprotective effects including a decrease in neurodegeneration, neuroinflammation, and apoptosis. The development of therapeutic tools that exploit estrogenic neuroprotection in the treatment of neurotrauma require a precise quantification of the endogenous changes in neural aromatase and estradiol following brain injury. Surprisingly, the expected increase in neural estrogens following brain injury has not been demonstrated. Further, we are just beginning to unravel the mechanisms behind the protective effects of centrally synthesized estradiol. In the current study, levels of aromatase immunoprotein, neural estradiol, and steroid receptor mRNA were quantified in adult male and female zebra finches 48 hours following a unilateral penetrating brain injury. Both aromatase and estradiol were upregulated in the injured hemisphere of the brain compared to the uninjured hemisphere, demonstrating, for the first time, a robust increase in neural estradiol levels following injury. We did not detect an effect of injury on mRNA expression of the estrogen receptors (ER)-α, ER-β, or GPER-1, but observed a significant decrease in androgen receptor (AR) transcription in the injured lobe relative to the contralateral uninjured hemisphere. We conclude that mechanical damage causes a dramatic increase in local aromatization, and the resultant high levels of central estradiol are available to modulate steroid sensitive targets. Studies using alternate methods of receptor detection and/or time points may be necessary to understand the complete suite of mechanisms underlying the neuroprotective effects of induced estrogen synthesis in this animal model.
Keywords: Traumatic brain injury, aromatization, steroid receptor, neuroprotection
Introduction
The steroid hormone 17β-estradiol (E2) organizes and activates neural targets and critically modulates numerous physiological processes including sexual differentiation, reproduction and reproductive behavior (1–3). The synthesis of E2 is not limited to the periphery, but also occurs in the brain de novo, due to the expression of aromatase (estrogen synthase), the enzyme that converts testosterone to E2. In rodents, galliformes, and passerine songbirds, under normal conditions, the expression of aromatase is limited to neurons at diencephalic and telencephalic loci (4–8). This distribution of neuronal aromatase is believed to underlie the pluripotent role of E2 in the regulation of multiple behaviors and physiological endpoints in the vertebrate brain.
Neurotraumatic events such as traumatic brain injury (TBI), excitotoxic damage, and ischemia/anoxic conditions, however, are known to induce the expression of aromatase in glial cells. Specifically, in rodents, aromatase activity and astrocytic aromatase immunoreactivity are significantly increased at 8 days following administration of kainic acid and 10 days following a single penetrating brain injury (9). Similarly, a rapid upregulation of aromatase mRNA and aromatase immunoreactivity in astrocytes is seen in zebra finches (Taeniopygia guttata) within 24 hours after mechanical brain injury (10).
Glial aromatization following brain injury is believed to increase neural E2, and may play a neuroprotective role. Indeed, inhibition of aromatase activity by the injection of fadrozole into the site of a penetrating brain injury resulted in an increase in both the size of the injury itself and the density of apoptotic cells when compared to a penetrating brain injury injected with vehicle alone (11). Additionally, the inhibition of aromatase activity by fadrozole results in a significant increase in reactive gliosis 72 hours post-injury in the zebra finch brain and also increased neuronal damage in the mouse brain (12, 13). These results are consistent with additional studies suggesting increased neuronal damage following various types of brain injury in aromatase knock-out mice (13–15).
Importantly, estrogen provision in addition to fadrozole administration following a penetrating injury to the zebra finch brain decreases the number of apoptotic cells and cellular degeneration surrounding the lesion when compared to injured hemispheres injected with fadrozole alone (16). Similarly, administration of peripheral E2 attenuates the increased neuronal damage induced by aromatase inhibition following injury in mice (17). In addition to the mitigation of neuronal damage and secondary degeneration following brain injury, E2 has also been suggested to enhance neurogenesis after injury (18–20). The data strongly suggest that the neuroprotective effects of centrally synthesized E2 are a conserved characteristic across several vertebrates. This characteristic may be exploited in order to develop therapies that may mitigate the effects of TBI.
The development of therapies that exploit the neuroprotection afforded by estrogens require a better understanding of the endogenous changes in aromatization and E2 following TBI. However, while the phenomenology of glial aromatase induction and its neuroprotective consequences are well documented, we have very little quantitative information about injury dependent increases in neural aromatization and neural E2 (but see Garcia-Segura et al. and Lopez-Rodriguez et al.) (9, 21). Recently, Lopez-Rodriguez et al., (21) reported a decrease in neural E2 following TBI in rodents – a counterintuitive result given the robust increases in aromatase reported. Perhaps the rapid, reliable, and robust TBI-dependent increases of aromatase expression in the songbird could provide some resolution of this paradox? Indeed, to the best of our knowledge, endogenous neural alterations in E2 content following injury are completely unknown in the songbird, leaving a hole in our understanding of the downstream effects of neutrally synthesized E2.
The mechanism(s) whereby E2 is neuroprotective is well studied in the rodent, where the classical ERs, GPER-1 and even the androgen receptor (AR) have been implicated following neural injury (15, 22–27). However, in the finch, a lone study demonstrates a transient increase in AR following injury (28). The effect of injury on other steroid receptors, particularly the ERs is completely unknown in this species. Given the more rapid and dramatic induction of glial aromatase in this species, it may be beneficial to further characterize the mechanisms whereby glial estrogen-provision is neuroprotective in the finch.
In the present study we used a penetrating brain injury in adult male and female zebra finches to quantify changes in neural aromatase using semi-quantitative Western Blotting, neural E2 using EIA, and changes in expression of ER-α, ER-β, GPER-1 (GPR30), and AR using quantitative real-time PCR.
Materials and Methods
Animals
Adult zebra finches (>100 days post-hatch) were obtained from a commercial breeder and housed in same-sex aviaries at American University, under a 12/12-hour light/dark cycle, with food and water provided ad libitum. The American University Institutional Use and Animal Case Committee (IACUC) approved all animal procedures.
Stereotaxic Surgery
Adult male (n=5) and female (n=5) zebra finches were anesthetized with 60 mg/kg of ketamine and 10 mg/kg of xylazine in 0.9% saline (Henry Schein, Dublin, OH) and positioned in a stereotaxic apparatus with the head angled at 45°. The cranium was exposed and a unilateral craniotomy performed 6-mm anterior of the pineal gland and 2-mm lateral (left) of the midline. A 22 gauge Hamilton syringe (Hamilton Company, Reno, NV) was then slowly lowered 3-mm ventrally and held in place for 60 seconds before withdrawal. The scalp incision was sealed and animals were allowed to recover from surgery on a heating pad. 48 hours post-surgery, birds were decapitated and brains were rapidly extracted, dissected into quadrants (left anterior hemisphere (injured), left posterior hemisphere, right anterior hemisphere, and right posterior hemisphere and flash frozen on aluminum foil placed on dry ice. Tissue was then stored at −80 C until use.
Tissue Preparation
Tissue from the left (injured) anterior quadrant and right (uninjured) anterior quadrant was weighed and fully homogenized using disposable RNase-free pestle grinders with a battery-powered motor (Kimble Chase, Vineland, NJ) in ice-cold 0.1M phosphate buffer (PB) to a total volume of 1 mL. 300 μl of homogenate from each sample was aliquoted for protein extraction and use in immunoblotting, 300 μl was aliquoted for steroid extraction and enzyme immunoassay, and 300 μl was used for RNA extraction.
Experiment 1: Quantification of Aromatase Protein
Homogenate aliquoted for Western blotting was further lysed in 150 μl RIPA lysis buffer (Amresco, Solon, OH) supplemented with phosphatase and protease inhibitors (Bio-rad Laboratories, Hercules, CA), incubated for 35 minutes on ice, and finally centrifuged at 13,000 × g for 15 minutes at 4° C. Supernatant was immediately removed and protein quantified using a BCA assay (Thermo Scientific, Rockford, IL), then stored at −20° C until use. 25 μg of total protein from each sample was diluted with 4X Laemmli sample buffer (Bio-rad) and loaded into each well of a polyacrylamide gel (Mini-PROTEAN TGX; Bio-rad). Separated proteins were transferred electrophoretically to a PVDF membrane (Immun-Blot, Bio-rad) at 100 V for 90 minutes, followed by a 1 hour incubation of the membranes in a 5% blocking solution (Blotting grade blocker, Bio-rad in .1% TBS-T). The blots were then incubated in primary antibody against aromatase (anti-aromatase-zebra finch aromatase C-terminal (AZAC), 1:15,000 for 48 hours at 4°C). Following a series of washes in .1% TBS-T, the blot was incubated in anti-rabbit IgG, HRP-linked secondary antibody at 1:2,500 (Cell Signaling, Danvers, MA) for 1 hour at room temperature, washed in .1% TBS-T, and developed for 5 minutes with ECL (Clarity Western ECL Substrate; Bio-rad) for imaging. Following imaging, membranes were fully stripped with stripping buffer (Thermo Scientific), blocked in 5% blocking solution, and re-probed for GAPDH (Millipore, Billerica, MA) to normalize results. Band intensities were quantified using ImageJ software (NIH).
Experiment 2: Estradiol Enzyme Immunoassay
Tissue homogenate used for the measurement of E2 in females was obtained from the same animals utilized in Experiment 1. However, due to technical failures, we were unable to use homogenate from males in Experiment 1. We therefore utilized injured and uninjured male telencephalic homogenate from animals sacrificed 48hr after an injury using the identical penetrating tool (22 gauge needle) as that in Experiment 1. However, in these birds, the injury was created below a unilateral craniotomy 3-mm anterior of the pineal gland and 3-mm lateral (left) of the midline, and 48hr post-trauma brains were extracted and dissected into right and left hemispheres and flash frozen on dry ice. Thus, while location of the injury was slightly different, the nature of the injury and the time between trauma and tissue collection was identical. The methods utilized to analyze brain E2 levels have been validated in several recent songbird models including a demonstration that the combined liquid-liquid and solid-phase extraction provides sufficient purification for an accurate analysis of low tissue levels of E2 and, importantly, validation with results obtained with mass spectrometry (29–32).
Ether Extraction
Following homogenization of tissue in 1mL of .1M PB, 300 μl of each sample was ether extracted according to previously validated protocols (32, 33). 2 mL of diethyl ether (Sigma-Aldrich, St. Louis, MO) was added to each sample, and tubes were vortexed on a low setting for 30 seconds. Samples were next centrifuged at 2,100 rpm for 5 minutes at 4°C and tubes were submerged in a MeOH/dry ice bath for 1 minute to freeze the mixture. The organic phase of each sample was eluted into a clean, glass tube (Kimble Chase, glass 5 mL conical tubes) and set aside. The aqueous phase was thawed and the ether extraction process was repeated twice more on each sample. Following ether extraction, samples were dried under a gentle stream of air in a 50°C water bath until fully evaporated. Next, approximately 100 μl of a 1:1 MeOH/CH2Cl2 mixture (Sigma-Aldrich) was dripped down the sides of each tube, and samples were again evaporated under air in a 50°C water bath. Dried samples were stored at −20°C until re-suspension for solid-phase extraction.
Solid-Phase Extraction
Based on previous studies suggesting a greater recovery rate of E2 using combined ether and solid-phase extraction techniques (32), samples were resuspended in 500 μl of .1M PB and vortexed for 2 minutes at low speed before solid-phase extraction using a Visiprep SPE vacuum manifold (Sigma-Aldrich) and 1 mL/500 mg C18 cartridges (Agilent, Santa Clara, CA). Cartridges were conditioned by loading 500 μl of 100% MeOH and then equilibrating with 750 μl ultrapure H20 while under low vacuum pressure so as not to dry the cartridges. 500 μl of sample was then slowly loaded to each column and eluted into a waste collection container while under gentle vacuum pressure. Columns were next washed with 750 μl of ultrapure H20 and allowed to dry for 3 minutes under vacuum pressure. At this point, the waste collection container was replaced with the final collection tubes (Kimble Chase, glass 4 ml round-bottom tubes) and 250 μl of 100% MeOH was added to columns (2×) and allowed to soak for 2 minutes before elution by gravity. After both aliquots of MeOH passed through the columns, cartridges were allowed to dry under vacuum pressure for 5 minutes. Samples were fully evaporated under a gentle stream of air in a 50°C water bath and subsequently stored at −20°C until processing for EIA.
Estradiol Enzyme Immunoassay
Following steroid extraction using a combined liquid and solid-phase extraction protocol, samples were resuspended in 300 μl of EIA buffer (Cayman Chemical, Ann Arbor, MI) and vortexed on low speed for 1 minute. Samples were assayed in triplicates using an E2 Enzyme Immunoassay kit (Cayman Chemical) previously validated for use with zebra finch brain tissue according to manufacturer's instructions (30–32). Prior to ether and solid-phase extraction, an additional sample was spiked with radioinert E2 to the concentration of 256 pg/mL, processed in an identical manner, and assayed in triplicates alongside the experimental samples in order to estimate recovery. To ensure a lack of interference from our tissue matrix and reliability of measurement, we ran two-fold dilutions and validated recovery of the corrected concentration compared to the identical undiluted sample.
Experiment 3: Quantitative Polymerase-Chain Reaction (qPCR)
300 μl of Ribozol (Amresco) was added to 300 μl of aliquoted tissue homogenate, mixed thoroughly, and allowed to sit for 10 minutes at room temperature. RNA was subsequently extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer's instructions, and the purity of each extracted RNA sample was analyzed on an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). a260/280 ratios exceeded 1.8 for all samples. 250 ng of total RNA from each sample was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies, Frederick, MD), and 5 μl of the resulting cDNA was used to perform real-time quantitative PCR (RT-qPCR) for each reaction using Sybr Select Master Mix (Life Technologies) for a total reaction volume of 50 μl divided into 15 μl triplicates. Samples were run on 96-well optical plates using primers designed using zebra finch-specific sequences for ER-α, ER-β, Gper-1, AR, and GAPDH found in the Genbank database (Genbank accession numbers NM_001076701, XM_002200595, XM_004175666, NM_001076688, and NM_001198610, respectively). Relative quantitation was performed by comparison of the CT of each target gene between the experimental samples and calibrator/control (uninjured) samples in terms of fold-change (2−ΔΔCT). Expression of each target was normalized against the housekeeping gene GAPDH to control for any potential variations in amplification efficiency between samples.
Results
Statistical analyses
Relative optical densities for aromatase protein (Experiment 1), and delta CT values for ER-α, ER-β, GPER-1 and AR (Experiment 3) were analyzed using 2×2 mixed factorial ANOVAs with treatment (injured hemisphere vs. uninjured hemisphere) and sex as independent variables. For experiment 2, E2 levels were log transformed to correct for unequal variance across experimental groups. Due to the different experimental conditions between males and females for this portion of the experiment, males and females were analyzed separately using one-tailed paired t-tests with treatment as the within-subjects independent variable. All analyses were conducted using SPSS 17.0 statistical software (SPSS, Chicago, IL). Results were considered significant at a predetermined α = 0.05 level.
Experiment 1: Quantification of Aromatase Protein
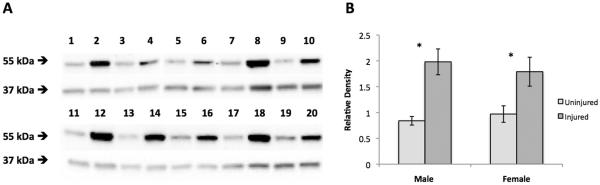
As previously reported (34), the antibody against aromatase recognizes a single band at approximately 55 kDa in zebra finch tissue (Figure 1a). The relative optical density of the band was measured relative to a band immunoreactive for GAPDH (37 kDa). A 2×2 factorial ANOVA revealed a significant main effect for injury, F (1, 8) = 43.32, p < .001, with the injured hemisphere showing higher levels of aromatase protein expression than the uninjured hemisphere (Figure 1b, 1.88 ± 0.19 vs. 0.91 ± 0.09, respectively). No significant main effect of sex (F (1, 8) = .013, p = .912) or interaction effect between injury and sex (F (1, 8) = 1.23, p = .30) was observed.
Figure 1.
Western blots (a) used for the analysis of aromatase (55 kDa) relative to GAPDH (37 kDa) protein expression 48 hr post surgery in uninjured (odd numbered lanes) and injured (even numbered lanes) brains of adult zebra finches. Lanes 1–4 and 15–20 represent male samples while lanes 5–14 represent female samples. Semi-quantitative analysis using the relative optical density of aromatase relative to GAPDH (b). Injured male and female birds showed higher aromatase expression relative to GAPDH (p< 0.001). * indicates a significant difference between injured and uninjured groups.
Experiment 2: Estradiol Enzyme Immunoassay
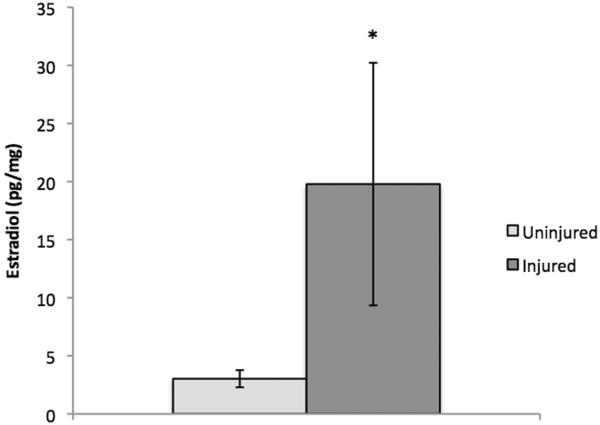
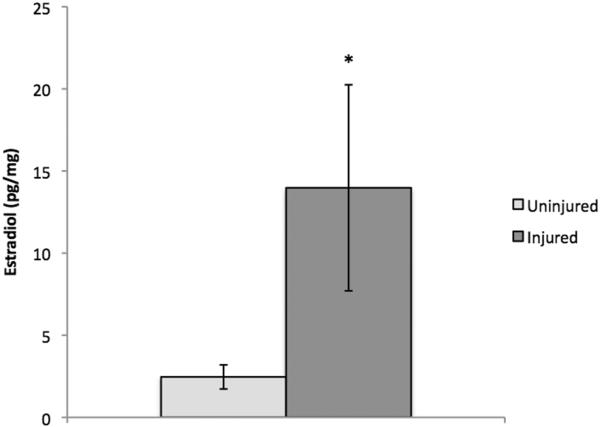
As described previously (32, 33), E2 is readily detectable in zebra finch brain. Statistical analyses using log-transformed data revealed that levels of E2 were significantly increased in injured brain tissue compared to uninjured brain tissue (Figure 2–3) of adult male (t (4) = −2.46, p = .035) and female (t (4) = 2.99, p = .02) zebra finches 48 hours after injury. Given the well-established and documented increase in aromatase expression and activity observed following injury in birds and mammals (15, 35), one-tailed t-tests were used in this analysis. Data from all samples fell within the detection range of 20–80% B/B0. A recovery rate of 42.87% for males and 99.2% for females was determined by spiking a sample with a known amount of radioinert E2 and comparing the sample concentration measured by the enzyme immunoassay against the expected concentration. We did not observe significant interference from our tissue matrix, as comparisons between undiluted samples and 2-fold diluted samples (corrected for concentration) showed less than a 10% difference, indicating sufficient pre-assay purification.
Figure 2.
E2 levels (pg/mg) in uninjured versus injured male zebra finch brain tissue 48 hours post-surgery. Neural E2 was significantly increased in the injured hemisphere compared to the contralateral uninjured hemisphere (p= 0.035). * indicates a significant difference between injured and uninjured groups.
Figure 3.
E2 levels (pg/mg) in uninjured versus injured female zebra finch brain tissue 48 hours post-surgery. Neural E2 was significantly increased in the injured hemisphere compared to the contralateral uninjured hemisphere (p= 0.020). * indicates a significant difference between injured and uninjured groups.
Experiment 3: Quantitative Real-time Polymerase Chain Reaction (qPCR)
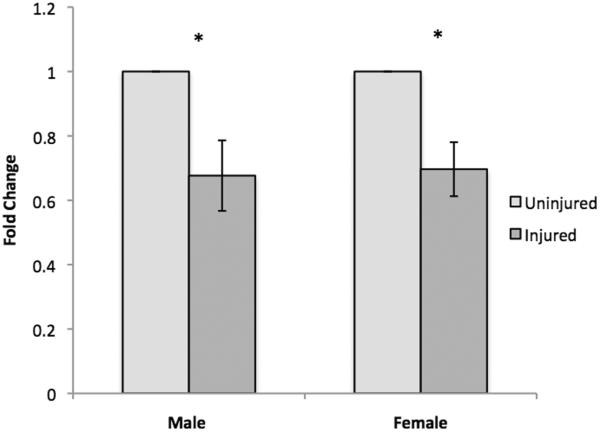
Delta Cts for ER-α, ER-β, GPER-1, and AR were compared between the injured and uninjured hemispheres of adult male and female (Table 1) zebra finches 48 hours after injury. 2×2 factorial ANOVAs for each receptor revealed no significant main effects of injury, sex, or an interaction effect for ER-α, ER-β, or GPER-1. Significant main effects of injury (F (1, 7) = 17.04, p= .004) and sex (F (1, 7) = 15.82, p= .005) were observed for AR, suggesting a significant down-regulation of AR expression in the injured hemisphere compared to the uninjured hemisphere (Figure 4, demonstrated in fold-change using the following equation: Fold change = 2ΔCT (Injured-Uninjured) assuming 100% efficiency).
Table 1.
Mean ΔCt values and standard errors for ER-α, ER-β, GPER-1 and AR in male and female uninjured and injured zebra finches (relative to GAPDH). P-values are shown for the main effects of sex, main effects of injury, and interaction effects. Significant results are indicated in bold.
| Gene | Mean ΔCT Uninjured Male | Mean ΔCT Injured Male | Mean ΔCT Uninjured Female | Mean ΔCT Injured Female | P-value Sex | P-value Injury | P-value Interaction |
|---|---|---|---|---|---|---|---|
| ER-α | 19.04 (1.34) | 18.43 (0.96) | 16.95 (0.10) | 17.38 (0.68) | .392 | .826 | .174 |
| ER-β | 12.10 (2.19) | 11.94 (1.48) | 18.04 (0.44) | 18.60 (0.39) | .032 | .460 | .276 |
| GPER-1 | 11.71 (0.74) | 12.28 (0.66) | 11.22 (0.36) | 11.26 (0.44) | .353 | .259 | .154 |
| AR | 8.75 (0.84) | 9.39 (0.65) | 11.91 (0.17) | 12.54 (0.24) | .005 | .004 | .812 |
Figure 4.
mRNA expression of androgen receptor (AR) in uninjured vs. injured hemispheres following brain injury. AR expression was significantly decreased in injured compared to uninjured hemispheres. * indicates a significant difference between injured and uninjured groups.
Discussion
While the upregulation of aromatase following penetrating brain injury has been well established in birds and mammals, the precise extent of this change has remained unclear. Additionally, while we expect that this observed increase in aromatase would result in a subsequent increase in neural E2, the neural content of this steroid following brain injury has not, to our knowledge, been previously quantified. In the current study, we used Western blotting to demonstrate a significant increase in aromatase immunoprotein in the injured compared to uninjured hemispheres of male and female zebra finch brains 48 hours after mechanical brain injury. Additionally, enzyme-immunoassays revealed a significant increase in central E2 at the same time point following TBI.
While injury significantly increased aromatase expression, there was no evidence of an influence of sex on this induction. The absence of a sex difference in aromatase upregulation at 48 hours after injury is consistent with previous research. While female zebra finches upregulate aromatase more rapidly following injury than males, both sexes appear to reach their maximal response 24 hours following injury (36). Several studies have shown an increase in aromatase transcription and translation following brain injury both in vitro and in vivo (8–11). A significant increase in aromatase mRNA and aromatase immunoreactivity can be observed around the site of injury at both 24 and 72 hours following a penetrating lesion of the zebra finch brain (10). Similarly, both mechanical and excitotoxic injury to the rodent brain results in an increase in aromatase enzyme activity and aromatase-immunoreactive cells (9). The current determination of an approximately 2.5 fold increase in aromatase immunoprotein 48 hours after brain injury not only supports previous research, but also provides the first semi-quantitative measure of the difference in endogenous protein levels between the injured and uninjured vertebrate brain.
The observed increase in aromatase immunoprotein may be an accurate indication of increases in aromatase activity. There is excellent agreement in the expression of the aromatase transcript, immunoprotein, and aromatase activity in the finch brain. Specifically, the distribution of aromatase mRNA, immunoreactive aromatase, and aromatase activity measured in punches or microdissected reveals high expression and activity at sites such as the hypothalamic preoptic area (HPOA), hippocampus (HP) and caudomedial nidopallium (NCM), and low expression in others such as the cerebellum (CB) and parolfactorium (PF) (5, 6, 37, 38). Given the concordance in patterns of expression and activity in the intact songbird brain, it is quite likely that the observed injury-induced increases in aromatase immunoprotein are good reflections of elevated aromatase activity following injury. This hypothesis is currently being directly tested in our laboratory.
In accordance with the noted increase in neural aromatase immunoprotein, E2 was increased approximately 4 fold in the injured hemisphere of adult zebra finches when compared to the uninjured contralateral hemisphere of the same animal. A large variability was observed with the measures of E2 on the injured side of the brain. We believe this may be a function of the fact that the injury can cause many changes in neurochemistry, anatomy, and physiology, all of which can contribute to variability in steroid concentrations. Despite this variability, however, the statistics suggest a significant and dramatic upregulation of E2 levels following injury. Of interest was the difference between the current results and a recent study measuring neural E2 levels in rodents following brain injury, which observed a decrease in levels at 24 and 72 hours, and 2 weeks following injury (21). The reason for the discrepancy in the pattern of results observed in rodents and songbirds is unknown, however, the increases in songbird aromatase and E2 following TBI are consistent.
Although males and females could not be directly compared in this portion of the experiment (see Methods) the extent of the increase in neural levels of E2 appears to be similar between sexes. Until the present studies, levels of neural E2 have not previously been quantified following TBI in avian models. The current study augments previous research by validating the idea that changes in aromatase can cause even larger alterations in local E2, and may additionally aid in elucidation of the mechanisms surrounding the neuroprotective effects of estradiol.
One limitation of the present study is that it is impossible to know if the levels of aromatase and E2 measured in the uninjured lobe reflect actual levels in an uninjured brain. While the data clearly demonstrate patterns of changes in aromatase and E2 following local injury, a comparison to uninjured animals is necessary before the present measures can be considered reflective of absolute measures. These studies are ongoing in our laboratory. Using the same extraction and analysis techniques utilized in our experiment, Chao et al. measured approximately 10pg/mg of E2 in the posterior and anterior portions of the telencephalon of uninjured male zebra finches. While this concentration is higher than our measurements for the male uninjured telencephalon of the brain, it is decreased compared to our concentrations from the male injured telencephalon (~19 pg/mg)(32).
Males and females had similar patterns of aromatase and E2 expression following injury. These findings were surprising as females have significantly lower levels of circulating testosterone, the substrate for the synthesis of E2 via aromatase. The substantial increase in injury-induced E2 in females suggests that this sex may either be increasing several steps of steroidogenesis prior to aromatization and/or utilizing an alternate androgenic substrate for aromatization. Indeed, a significant increase in translocator protein (TSPO), which initiates sex steroidogenesis via the transport of cholesterol into mitochondria, has been observed 2 days post-injury in the songbird cerebellum (39, 40). These findings suggest that not only aromatase, but also its substrate testosterone may be increased following injury to the brain in both males and females. Interestingly, TSPO was more highly expressed in females than males following injury, perhaps suggesting a compensation for lower levels of testosterone in the female brain. Further, the formation of E2 from the substrate dehydroepiandrosterone (DHEA), has been documented in the songbird brain (41–43). The precise mechanism of injury-induced changes in aromatase in the female finch are currently being intensely studied in our laboratory.
While some studies have described potential mechanisms for the neuroprotective actions of E2 in mammals, its mode of action remains far from clear. Injury to the mammalian brain has been shown to alter the expression of the classical estrogen receptors ER-α and ER-β, however it has been suggested that the neuroprotective effects of estradiol could also be mediated by the androgen receptor (AR) or alternate estrogen-binding proteins such as GPER-1 (15, 24–27). Administration of nonselective ER antagonists increased the extent of injury in mice following MCAO (22, 23), while administration of E2 failed to mitigate damage following injury in ER-α knockout mice. Similarly, administration of E2 failed to attenuate glutamate neurotoxicity in rodents treated with the GPER-1 antagonist G-15 (44). Astrocytic AR expression is increased 24 hours post-injury in the zebra finch brain, suggesting the involvement of androgen signaling following brain injury (28). The interaction of estradiol with these receptors or proteins could induce a multitude of neuroprotective cascades and mechanisms, including but not limited to the attenuation of proinflammatory mediators, promotion of protective neurotrophic/growth factors, increased clearance of excitotoxic glutamate through upregulation of glial glutamate transporters, and/or prevention of blood-brain barrier disruption (45). Numerous studies suggest E2 stimulates astrocytes to release neuroprotective growth factors including nerve growth factor (NFG), insulin-like growth factor (IGF-1), brain derived neurotrophic factor (BDNF), transforming growth factors alpha and beta (TGF-α and TGF-β), and glial cell line-derived neurotrophic factor (GDNF) (46–49).
Given previous studies establishing the involvement of the estrogen receptors ER-, ER-, and GPER-1 in neuroprotection following brain injury (15, 16, 18–20, 22–27), we were surprised to find no differences in the expression of these receptors in the present experiment. We did, however, observe a significant down-regulation of AR expression in the injured hemisphere compared to the uninjured hemisphere of both male and female zebra finches (Figure 4), which may reflect a decrease in central testosterone following aromatization to estradiol. These findings contrast with previous work showing an increase in AR protein expression 24 hours following brain injury in zebra finches (28). While the 48 hour time point was picked because in this species the peak of injury-induced aromatase expression occurs between 24 and 72hr post-surgery (12, 16), these differing results may indicate a time-dependent change in AR expression. Therefore, it may be necessary to investigate additional time points, and perhaps study protein expression in order to more completely understand the dynamics of steroid receptor alterations following mechanical brain injury in the zebra finch. Our data suggests a role of AR following traumatic brain injury, and may provide insight into the mediation of estradiol-induced neuroprotection. Since aromatization decreases local androgen in order to increase estrogens, it is likely that some neuroprotection could occur by the drop in local androgens in addition to that due to estrogens. The former may involve decreases in androgenic signaling. While this remains a hypothesis, it may be reflected in changes in AR.
In summary, we believe these data are among the first to better quantify injury-induced changes in central expression of aromatase protein and the consequent alterations in local E2 levels. These data may provide a framework for the development of viable therapies that seek to use the neuroprotective effects of local E2 as the basis for treatments for TBI.
Acknowledgements
We thank Benjamin Kussin-Shoptaw for excellent technical assistance. This work was supported by NIH 042767 and 080585 to CJS.
Footnotes
Author Disclosure Statement No competing financial interests exist.
References
- 1.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, Balthazart J. The postnatal demasculinization of sexual behavior in the Japanese quail (Coturnix coturnix japonica) Horm Behav. 1984;18(3):298–312. doi: 10.1016/0018-506x(84)90018-7. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208(4450):1380–3. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- 4.Schlinger BA. The activity and expression of aromatase in songbirds. Brain Res Bull. 1997;44(4):359–64. doi: 10.1016/s0361-9230(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 5.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360(1):172–84. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 6.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423(4):619–30. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31(2):129–48. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and non-neuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci. 1994;14(12):7541–52. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89(2):567–78. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 10.Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13(4):317–23. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- 11.Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16(8):676–83. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 12.Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- 13.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318–29. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 14.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003:1007298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 16.Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64(2):192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71(1):31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Walters BJ, Alexiades NG, Saldanha CJ. Intracerebral estrogen provision increases cytogenesis and neurogenesis in the injured zebra finch brain. Dev Neurobiol. 2011;71(2):170–81. doi: 10.1002/dneu.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters BJ, Saldanha CJ. Glial aromatization increases the expression of bone morphogenetic protein-2 in the injured zebra finch brain. J Neurochem. 2008;106(1):216–23. doi: 10.1111/j.1471-4159.2008.05352.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobiol. 2007;67(8):1107–17. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Rodriguez AB, Acaz-Fonseca E, Giatti S, Caruso D, Viveros MP, Melcangi RC, Garcia-Segura LM. Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology. 2015:561–11. doi: 10.1016/j.psyneuen.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schutz G, Schwaninger M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J Cereb Blood Flow Metab. 2010;30(5):935–42. doi: 10.1038/jcbfm.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20(1):112–8. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Arevalo MA, Santos-Galindo M, Acaz-Fonseca E, Azcoitia I, Garcia-Segura LM. Gonadal hormones and the control of reactive gliosis. Horm Behav. 2013;63(2):216–21. doi: 10.1016/j.yhbeh.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Ramirez VD. Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J Steroid Biochem Mol Biol. 1999;68(1–2):65–75. doi: 10.1016/s0960-0760(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 26.Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Reprod. 2002;67(5):1379–85. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450(3):256–71. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 28.Duncan KA, Moon J, Vartosis D, Zee I. Injury-induced expression of glial androgen receptor in the zebra finch brain. J Neurotrauma. 2013;30(22):1919–24. doi: 10.1089/neu.2013.2951. [DOI] [PubMed] [Google Scholar]
- 29.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21(3):191–9. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31(27):10034–8. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman AE, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155(3):503–10. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011:557. doi: 10.3389/fnana.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–34. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 35.Duncan KA, Walters BJ, Saldanha CJ. Traumatized and inflamed--but resilient: glial aromatization and the avian brain. Horm Behav. 2013;63(2):208–15. doi: 10.1016/j.yhbeh.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saldanha CJ, Burstein SR, Duncan KA. Induced synthesis of oestrogens by glia in the songbird brain. J Neuroendocrinol. 2013;25(11):1032–8. doi: 10.1111/jne.12067. [DOI] [PubMed] [Google Scholar]
- 37.Saldanha CJ, Schlinger BA. Estrogen synthesis and secretion in the brown-headed cowbird (Molothrus ater) Gen Comp Endocrinol. 1997;105(3):390–401. doi: 10.1006/gcen.1996.6841. [DOI] [PubMed] [Google Scholar]
- 38.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res Mol Brain Res. 1994;24(1–4):227–37. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 39.Mirzatoni A, Spence RD, Naranjo KC, Saldanha CJ, Schlinger BA. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J Neurotrauma. 2010;27(10):1875–82. doi: 10.1089/neu.2010.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol. 2000;161(1):102–14. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- 41.Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104(1):244–53. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlinger BA, Pradhan DS, Soma KK. 3beta-HSD activates DHEA in the songbird brain. Neurochem Int. 2008;52(4–5):611–20. doi: 10.1016/j.neuint.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145(4):1668–77. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 44.Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170(1):54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 45.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013:13771–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1) Biol Reprod. 2000;62(6):1710–21. doi: 10.1095/biolreprod62.6.1710. [DOI] [PubMed] [Google Scholar]
- 47.Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59(6):528–38. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- 48.Flores C, Salmaso N, Cain S, Rodaros D, Stewart J. Ovariectomy of adult rats leads to increased expression of astrocytic basic fibroblast growth factor in the ventral tegmental area and in dopaminergic projection regions of the entorhinal and prefrontal cortex. J Neurosci. 1999;19(19):8665–73. doi: 10.1523/JNEUROSCI.19-19-08665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karki P, Smith K, Johnson J, Jr., Lee E. Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-alpha in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol Cell Endocrinol. 2014;389(1–2):58–64. doi: 10.1016/j.mce.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]