Abstract
In addition to their well-studied and crucial effects on brain development and aging, an increasing number of investigations across vertebrate species indicate that estrogens like 17β-estradiol (E2) have pronounced and rapid effects on cognitive function. The incidence and regulation of the E2-synthesizing enzyme aromatase at the synapse in regions of the brain responsible for learning, memory, social communication and other complex cognitive processes suggests that local E2 production and action affects the acute and chronic activity of individual neurons and circuits. Songbirds in particular are excellent models for the study of this “synaptocrine” hormone provision given that aromatase is abundantly expressed in neuronal soma, dendrites, and at the synapse across many brain regions in both sexes. Additionally, songbirds readily acquire and recall memories in laboratory settings, and their stereotyped behaviors may be manipulated and measured with relative ease. This leads to a rather unparalleled advantage in the use of these animals in studies of the role of neural aromatization in cognition. In this review we describe the results of a number of experiments in songbird species with a focus on the influence of synaptic E2 provision on two cognitive processes: auditory discrimination reliant on the caudomedial nidopallium (NCM), a telencephalic region likely homologous to the auditory cortex in mammals, and spatial memory dependent on the hippocampus. Data from these studies are providing evidence that the local and acute provision of E2 modulates the hormonal, electrical, and cognitive outputs of the vertebrate brain and aids in memory acquisition, retention, and perhaps the confluence of memory systems.
Keywords: aromatase, songbird, estrogen, synapse, hippocampus, perception, NCM
INTRODUCTION
The action of estrogens like 17β-estradiol (E2) on the brain during development and in adulthood results in a number of anatomical and physiological alterations. E2 masculinizes hypothalamic circuits in developing rodents (Arnold and Gorski, 1984), those in the telencephalon of songbirds (Gurney and Konishi, 1980), and feminizes the perinatal brain (Arnold and Gorski, 1984; Adkins-Regan and Ascenzi, 1990; Adkins-Regan et al., 1994). In adulthood, E2 activates masculine copulatory and aggressive behaviors and is necessary for the expression of feminine sexual behaviors in mammals and birds (Harding, 1986; Harding et al., 1988; Balthazart et al., 1996). This work establishes a pivotal and conserved role for E2 in the organization and activation of circuits necessary for sexual and aggressive behaviors in a number of vertebrates (MacLusky and Naftolin, 1981; Adkins-Regan and Watson, 1990; Balthazart et al., 1992).
Several of these effects are considered neural responses to chronic, peripheral changes in circulating E2, most likely synthesized in one or more peripheral tissues. Indeed, steroidogenic tissues express a variety of enzymes that metabolize cholesterol into several steroids including progestins, androgens, and estrogens. E2 production occurs when androgens are metabolized by P450 aromatase (CYP19, hereafter aromatase (see Simpson et al., 1994). While the ovary is one of the primary sources of circulating E2 in female vertebrates, males also synthesize E2 in testicular, adrenal, and adipose tissues (Simpson and Davis, 2001). In both sexes, E2 can access the general circulation where it can affect target tissues at distant loci, including the brain.
Independent of peripheral sources, a high level of E2 synthesis in the brain is achieved via the expression of aromatase in neurons that can convert circulating androgen to locally high, temporally-specific, and spatially-constrained levels of E2. Aromatase is expressed in diencephalic neurons in most species where its role in providing E2 to circuits underlying reproductive behaviors is well established (MacLusky and Naftolin, 1981; Balthazart, 1997). Songbirds express aromatase in large amounts at several brain regions, which provides an excellent model for studies on the role of local E2 synthesis in brain function (Schlinger and Arnold, 1991; Schlinger and Arnold, 1992). Specifically, there is abundant and widespread aromatase expression, measurable by biochemical activity (formation of E2 from radiolabeled androgen) and labeling of the aromatase transcript and protein, at several telencephalic loci in passerines (Vockel et al., 1990a, 1990b; Schlinger and Arnold, 1991; Schlinger and Arnold, 1992; Saldanha and Schlinger, 1997; Foidart et al., 1998; Saldanha et al., 1998; Saldanha et al., 1999; Saldanha et al., 2000; Silverin et al., 2000; Soma et al., 2003). In some of these regions, in addition to the long-lasting effects on brain and behavior mentioned above, E2 also modifies synaptic processes with temporal and spatial specificity, acutely regulating pre- and postsynaptic functionality and behavior. These acute effects are likely not responses to peripheral changes in E2, since plasma levels of this steroid do not appear to fluctuate rapidly. Perhaps there are additional modes of E2 provision more consistent with rapid alterations in steroid availability? Newer evidence from ultrastructural studies supports this contention as we now have a greater understanding of the acute and rapid alterations in synaptic E2 provision via the presynaptic expression and activity of aromatase (Peterson et al., 2005; Rohmann et al., 2007).
The passerine telencephalon appears to be the primary source of central E2 in zebra finches (Taeniopygia guttata), but brain areas vary in terms of the relative abundance of aromatase in soma and processes. In the caudomedial nidopallium (NCM; homologous to auditory cortex in mammals), somal and synaptic aromatase are abundant, and sex differences in expression have been described (Saldanha et al., 2000; Peterson et al., 2005; Remage-Healey et al., 2012). In contrast, in the hippocampus, a region central to spatial memory formation in vertebrates, despite aromatase activity that is higher than in any other brain nucleus (Saldanha et al., 1998), as well as the presence of aromatase mRNA (Shen et al., 1994; 1995; Saldanha et al., 1998), somal aromatase protein is low to undetectable (Figure 1; Saldanha et al., 2000; Peterson et al., 2005; Saldanha et al., 2012). Immunoelectron microscopy reveals an abundance of aromatase in presynaptic boutons and postsynaptic dendrites at this locus, the former being more abundant in males than in females (Peterson et al., 2005). Correspondingly, aromatase activity is higher in synaptosomal than microsomal fractions from tissue homogenates and in males relative to females (Rohmann et al., 2007).
Figure 1.
Reconstructions of coronal sections through the zebra finch brain at the levels of the hippocampus (HP; panel A) and caudomedial nidopallium (NCM; panel B). The mesopallium (M), cerebellum (Cb), and robust nucleus of the arcopallium (RA) are labeled for reference. Dashed boxes in each panel denote the areas in which aromatase mRNA expression is depicted in the HP (panel C) and NCM (panel D). The lateral ventricle (lv) and nidopallium (N) are labeled for reference in these panels, and the asterisks indicate areas of background hybridization. The scale bars in panels C and D = 100 µm. Figure adapted from Saldanha et al. (2008).
The localization and pattern of aromatase expression in the songbird brain allows for the testing of specific hypotheses about the regulation and role of aromatase at the synapse, and has directly fueled the articulation of the “synaptocrine hypothesis” (Saldanha et al., 2011). That presynaptic aromatase is spatially and readily dissociable from somal aromatase – and that “online” E2 levels in the brain can be reliably measured – affords an elegant scenario for the study of this hypothesis (Saldanha et al., 2003; Remage-Healey et al., 2011a; Saldanha et al., 2011; Saldanha et al., 2012). Perhaps not surprisingly, many of these hypotheses involve the role of synaptocrine E2 provision on cognitive processes like song discrimination and spatial memory behavior.
ROLE OF NEURAL AROMATIZATION IN SONG LEARNING AND SONG DISCRIMINATION
Zebra finches are part of a group of animals that learn their vocal communicative signals during a sensitive period in development (Immelmann, 1969). Song behavior is regulated by a network of interconnected nuclei and regions necessary for song learning, song production, and auditory perception. For example, lesions to the sensorimotor nucleus HVC (proper name) at any point in development or adulthood disrupt song output (Scharff and Nottebohm, 1991; Simpson and Vicario, 1990). Interconnections exist among HVC, other cortical nuclei, as well as striatal and thalamic regions (Bottjer et al., 1989), forming a closed loop that mirrors mammalian basal ganglia-thalamocortical pathways essential to vocal learning and control (see Brainard and Doupe, 2000).
Regions of the song control circuit contain abundant estrogen receptors (Gahr and Metzdorf, 1997; Metzdorf et al., 1999; Acharya and Veney, 2012) and all of the enzymes necessary for steroid hormone synthesis from cholesterol (Shen et al., 1994; Shen et al., 1995; Saldanha and Coomaralingam, 2005; Saldanha et al., 2000; London et al., 2006). HVC has long been described as responsive to E2 during development and adulthood (Gurney and Konishi, 1980; Gurney, 1981; Walters and Harding, 1988; Casto and Ball, 1996; Oberlander et al., 2004). While HVC punches reveal abundant aromatase activity in developing and adult zebra finches (Vockel et al., 1990a, 1990b), measures of message (Shen et al., 1994; 1995) and protein immunoproduct (Saldanha et al., 2000; Saldanha and Coomaralingam, 2005) reveal no aromatase-expressing somata within the adult HVC.
In the past two decades the function of regions involved in the auditory memories of song in birds has been intensely investigated, made possible primarily by the study of gene products whose expression can be selectively induced in response to specific auditory signals (Clayton, 1997; Mello, 2002). Male and female birds that perceive their own song or the songs of conspecifics show upregulation of both the protein and message of two of these immediate early genes, c-FOS and/or ZENK (an acronym for Zif268, Egr-1, NGF1-A, Krox-24), in areas outside of the traditional song control nuclei, most notably the caudomedial nidopallium (NCM; homologous to mammalian auditory cortex; Mello et al., 1992; Mello and Clayton, 1994; Mello et al., 1995; Jarvis and Nottebohm, 1997; Mello and Ribeiro, 1998; Duffy et al., 1999; Kruse et al., 2000; Bolhuis et al., 2001; Gentner et al., 2001; Stripling et al., 2001; Bailey et al., 2002; Eda-Fujiwara et al., 2003; Terpstra et al., 2004, 2006). Bilateral lesions of NCM in adult male zebra finches attenuated preferences for the song of a tutor (Gobes and Bolhuis, 2007), and inhibition of protein synthesis in the NCM of juvenile males during the song learning period led poor song production in adulthood (London and Clayton, 2008). Collectively, these studies indicate that neurons within NCM are integral to song recognition, including that which is not a male’s own per se (Bolhuis and Gahr, 2006).
In zebra finches, aromatase is abundant in somata, dendritic arbors, and terminals in NCM (Saldanha et al., 1999; Saldanha et al., 2000; Peterson et al., 2005), and estrogen receptors are expressed throughout the region (Balthazart et al., 1992; Bernard et al., 1999; Metzdorf et al., 1999; Acharya and Veney, 2012). A variety of studies have examined the role of E2 manipulation on song preference, immediate early gene activity, and/or the neurophysiological activity of NCM cells in male and female zebra finches as well as seasonally-breeding birds (reviewed in Cornil et al., 2012; Maney and Pinaud, 2012; De Groof et al., 2013). For example, E2 implants in female zebra finches result in a preference for songs with longer bouts (Clayton and Pröve, 1989), more complex songs (Vyas et al., 2009), increase extracellular multiunit activity in NCM in response to training with conspecific songs (Yoder et al., 2014), and in female white-throated sparrows (Zonotrichia albicollis) cause an increased ZENK expression in NCM, but primarily rostrally (Sanford et al., 2010). Male zebra finches implanted with testosterone –presumably aromatized by NCM neurons – learn to discriminate between their own song and the song of another conspecific in fewer trials than controls (Cynx and Nottebohm, 1992). Also, treatment of males with the aromatase inhibitor fadrozole, followed by song training through passive playback, resulted in a decreased neuronal memory in NCM for the training songs relative to saline treatment (Yoder et al., 2012). In male European starlings (Sturnus vulgaris), E2 implants result in higher recognition accuracy of conspecific songs depending on photoperiod (Calisi et al., 2014).
While there are clear effects of peripheral E2 manipulation on NCM activity and cognition, the expression of aromatase and estrogen receptors in the region, its size, and its direct and indirect connections with other regions of the song circuit (Vates et al., 1996) make possible the measurement of local E2 fluctuations and electrophysiological recordings following hormone manipulation and/or song presentations. Exposure to the songs of conspecifics increases levels of E2 in NCM while testosterone levels decrease (Remage-Healey et al., 2008). Interestingly, aromatase activity in singing males is elevated in synaptosomes in this region of the posterior telencephalon, a result not observed when birds are exposed to song playback (Remage-Healey et al., 2009). Locally-produced E2 significantly and rapidly enhanced the firing rates of NCM neurons in zebra finches (for up to 30 min), and inhibition of this local synthesis by ATD or antagonism of E2 action by tamoxifen rapidly attenuates electrical activity, an effect that lasts hours following manipulation (Tremere et al., 2009). In a separate study, fadrozole into the NCM of the left but not right hemisphere disrupts normal preference for a bird’s own song over conspecific song and decreases E2 levels, while E2 into the left increased the electrophysiological activity of neurons to the bird’s own song and forward/reverse conspecific songs (Remage-Healey et al., 2010). Finally, song exposure appears to recruit E2-producing neurons in NCM more so than those that are responsive to E2 (Jeong et al., 2011). Collectively, these studies indicate that neural aromatization in the NCM is important to song discrimination and therefore behavioral preferences.
Additional work has examined the responsiveness of neurons efferent to NCM, specifically those of HVC (Vates et al., 1996). Cells of HVC also show selectivity to a bird’s own song (Doupe and Solis, 1997). Acute treatment of NCM with E2 increases the activity of its cells to auditory stimulation as above, and concomitantly within neurons of HVC to the sound of the bird’s own song (Remage-Healey and Joshi, 2012). Moreover, following E2 administration in NCM, the baseline firing rates and those to all auditory stimuli increased in neurons in the nucleus interfacialis of the nidopallium (NIf), generally considered an interface between the auditory and motor systems (Lewandowski et al., 2013). Specifically, inhibition of E2 synthesis in NCM decreased these responses in NIf and HVC neurons (Pawlisch and Remage-Healey, 2014). These data indicate a role for local and rapid E2 provision in sensorimotor integration following auditory discrimination (see also Maney et al., 2006).
ROLE OF NEURAL AROMATIZATION IN SONGBIRD SPATIAL MEMORY FUNCTION
The focus of the role of E2 in cognition has been primarily on learning and memory behavior dependent on the hippocampus and associated structures of the medial temporal lobe in mammals. Most of these studies have focused on genomic processes that modify dendritic morphology, soma size, and the protection against cognitive decline and neurodegeneration (recently reviewed by Luine, 2014). In avian species, the hippocampus shows a number of structural and functional homologies with its mammalian counterpart (reviewed in Colombo and Broadbent, 2002; Macphail, 2002; Mayer et al., 2013). The examination of the role of the hippocampus in birds has generally centered on the acquisition and consolidation of spatial memories important for food caching and homing behaviors. The recovery of stored food is common in many avian species, specifically the parids and corvids (Kamil and Balda, 1990; Shettleworth, 1990), and homing behavior is conventionally associated with the pigeon, a columbiforme (Gagliardo et al., 1999). Remembering locations of food caches is dependent on the hippocampus. For example, a bilateral lesion to the structure in a black-capped chickadee reduces the accuracy of cache recovery but not the amount of caching or attempts to find the stored food (Sherry and Vaccarino, 1989; Hampton and Shettleworth, 1996). In homing pigeons, lesions to the hippocampus produce deficits in recognition of the home loft and of surrounding familiar landmarks; orientation back home is normal but slower (Bingman et al., 1988; Gagliardo et al., 1999; Strasser and Bingman, 1996). Elaborate studies in jays point to roles of the hippocampus that appear to integrate more than just memory for spatial location and are thought of as more “human-like,” the use of memories of past events in planning for the future (Clayton and Dickinson, 1998), despite current motivational states (Raby et al., 2007). These studies harken toward a role for the hippocampus in more “episodic-like” memories (Clayton and Dickinson, 1998).
A few experiments to date have examined spatial memory ability and the role of the hippocampus in zebra finches. In one (Patel et al., 1997), food-deprived adult zebra finches were trained to remember the location of a food source in a compartment covered by a cardboard flap. Other equally-sized compartments were also covered with flaps but contained no food. Male and female birds with bilateral hippocampal lesions were inhibited in their retrieval of the food source location as indicated by the number of flaps lifted over compartments that did not contain food, and there was no sex difference in performance. Another study (Watanabe and Bischof, 2004) examined the ability of hippocampal-lesioned adult males to find (via extra-maze cues) previously located seed in one of four identically colored feeders on the floor of an experimental chamber. Birds given bilateral hippocampal lesions prior to training were impaired in their ability to correctly choose a baited feeder, and lesions made following successful training did not maintain that learning (to the level of intact birds) nor allow the birds to relearn the task. Interestingly, birds with damage to the hippocampus eventually found the food in the same time as controls, although more visits to potentially baited feeders were made, suggesting use of an alternative, non-spatial strategy. These same hippocampal lesions do not affect the ability of birds to differentiate between different patterned (dot vs. stripe) feeders (Watanabe et al., 2008), a result that parallels work in another songbird, the chickadee (Hampton and Shettleworth, 1996), in that hippocampal lesions affect spatial orientation only, not discrimination of color or pattern. Additional studies examined the expression of the immediate early genes c-FOS and ZENK and found that these protein products were higher in the hippocampus during acquisition than recall of the task (Bischof et al., 2006; Mayer et al., 2010).
Zebra finches, however, do not cache food, and therefore tasks commonly used with food-storers such as those in the parid and corvid families (Capaldi et al., 1999; Krebs et al., 1989) may not be suitable or relevant. Although zebra finches in the wild search for seed primarily on the ground, they prefer to remain perched when not foraging (Zann, 1996). Bailey et al. (2009) also examined whether spatial memory could be disrupted following destruction of hippocampal tissue. Acquisition and performance were measured using a novel apparatus similar to a “T-” maze in which food-deprived birds flew up a central column and ate food from one of six baited cups that were mirrored in left and right arms (three cups per arm). Once the behavior of these birds reached a criterion level – signaling acquisition – birds were tested one hour later for memory of the food location. Birds lesioned as juveniles or adults showed an attenuation in spatial memory performance when tested in adulthood.
Given the high concentration of aromatase-containing synapses in the songbird hippocampus, it is tempting to speculate that the primary consequence of the destruction of hippocampal tissue is a loss of E2 production and action, compromising certain phases of learning and/or memory. While not directly testing the role of neural aromatization in the hippocampus on spatial memory function, a few studies have examined the short- and long-term effects of E2 treatment. Testosterone and E2 fed to great tits (Parus major) did not affect spatial memory performance shortly after ingestion but led to gradual improvements over the 5 days of treatment, an effect that subsided when hormone delivery ceased (Hodgson et al., 2008). Combinations of E2 implants and inhibition of aromatase synthesis with fadrozole in zebra finches suggests more of a role for E2 in the retrieval rather than the acquisition of a location in a four-arm maze (Rensel et al., 2013). Oberlander et al. (2004) castrated adult male zebra finches and implanted them subcutaneously with blanks, testosterone, dihydrotestosterone (DHT), or E2. Spatial memory acquisition and performance were measured using a task similar to that of Patel et al. (2007). Birds implanted with DHT did not learn the spatial task, and blank-implanted birds showed minimal learning over the course of the experiment. However, birds implanted with testosterone or E2 clearly demonstrated acquisition, with it being the most rapid in animals implanted with E2 relative to all other groups. While this study indicated that aromatization was a likely modulator of spatial memory performance in this species, this could not be more conclusively determined without direct manipulation of local hippocampal aromatization.
As indicated above, aromatase activity in the zebra finch hippocampus is higher than in any other brain region (Saldanha et al., 1998), in fact two to three times that in the diencephalon where the role of aromatase in copulatory and aggressive behaviors is well established (Saldanha and Schlinger, 1997; Schlinger, 1997; Saldanha et al., 1998). Aromatase mRNA is abundant in the hippocampus of zebra finches (Figure 1; Shen et al., 1995, Saldanha and Schlinger, 1997; Saldanha et al., 1998), but immunoproduct is found in only a small subset of hippocampal neurons (Saldanha et al., 2000) and somal aromatase immunoreactivity is low to undetectable (Saldanha et al., 2000; Peterson et al., 2005; Saldanha et al., 2012). Examination of aromatase immunoproduct at the electron microscopic level, however, reveals an abundance of aromatase in presynaptic boutons (Peterson et al., 2005). Regions with cells containing little or no somal aromatase, like the hippocampus, may be capable of E2 provision by aromatization in terminals. Thus, the songbird hippocampus and its associated spatial memory function appear ideal for understanding the role of presynaptic aromatization in learning and memory processes.
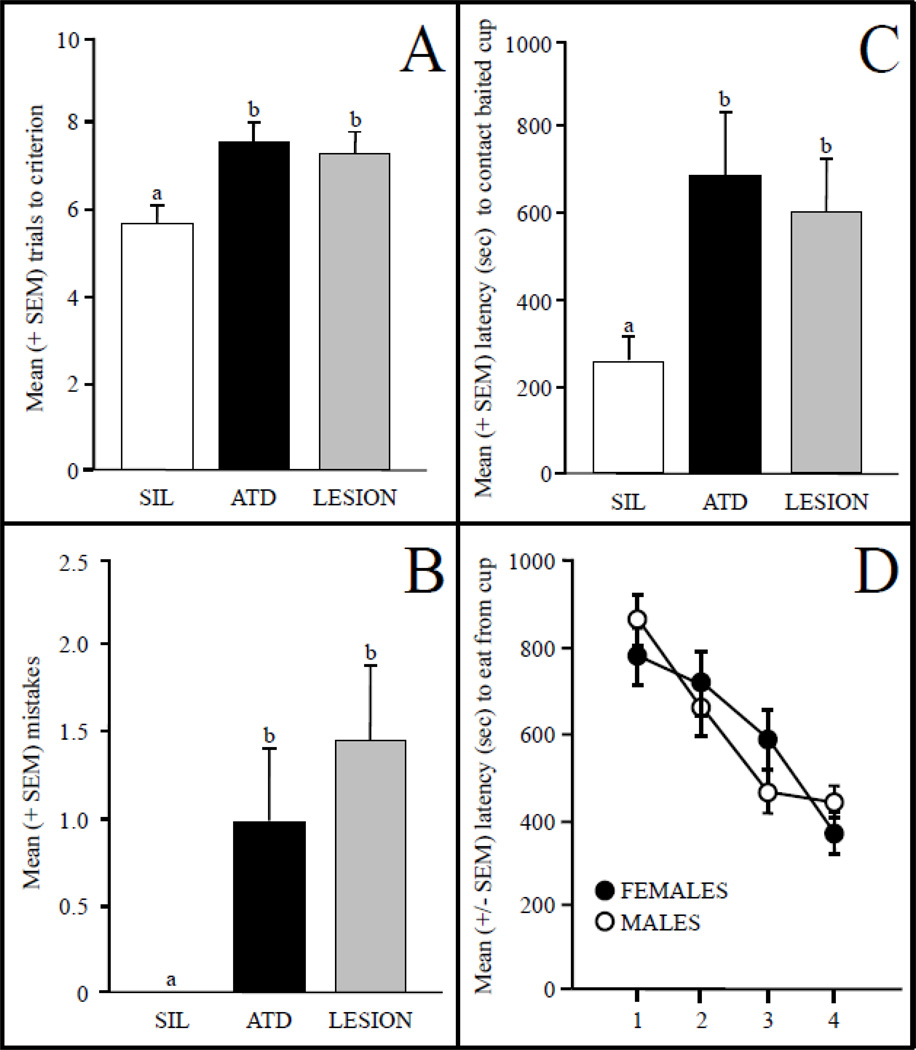
The dorsal and superficial location of the avian hippocampus, combined with its abundance of synaptic but not somal aromatase, allows for the local manipulation of neural aromatization toward understanding its role in cognitive function. Recently, we inhibited hippocampal aromatization in adult male zebra finches by placing a silicone pellet soaked with a lipophilic aromatase inhibitor, 1,4,6-androstatriene-3,17-dione (ATD), directly on the surface of the brain following bilateral craniotomies (Bailey et al., 2013). Control animals received only silicone into the openings, and excitotoxic lesions of the hippocampus were made in another group. Spatial memory function was determined as above (Bailey et al., 2009) 72 hours later, and hippocampal E2 levels and aromatase expression were also measured. Local application of ATD decreased acquisition of the spatial memory task relative to controls and, in probe trials, increased the time to contact the cup that contained seed as well as the number of mistakes (Figure 2). Notably, the spatial memory behavior of ATD-treated birds was indiscernible from that of birds with lesions of the structure. In addition, ATD treatment decreased hippocampal E2 levels by about 40% relative to silastic implants but had no effect on E2 levels in NCM, which sits just ventral to the hippocampus. Also, levels of E2 in brain were much higher than those in plasma, suggesting that the primarily synaptic aromatization in the hippocampus is crucial to cognitive function, a result in agreement with studies detailed above indicating context-dependent and behavioral selectivity of local E2 synthesis (Remage-Healey et al., 2008, 2009, 2013; Chao et al., 2014).
Figure 2.
Number of trials to reach criterion in birds whose hippocampus (HP) was treated with silicone (SIL) pellets as a control, the aromatase inhibitor ATD, or in which lesions were produced with an excitotoxin (panel A). In panel B, the average numbers of mistakes made by birds in the first probe trial are indicated, and their latencies to reach the baited cup in that trial are displayed in panel C. Inhibition of aromatase in the HP significantly increased the number of learning trials as well as mistakes prior to contacting and latencies to reach the learned cup relative to the control group. Interestingly, performance by ATD birds was statistically indistinguishable from birds with lesions of the HP. In a separate study, male and female zebra finches did not differ in acquisition rate in this task (data not shown), but the latencies of male birds decreased at a significantly faster rate than those of females over the probe trials (panel D). Data from Bailey et al. (2009, 2013).
Experiments regarding the role of local E2 synthesis in the hippocampus of songbirds have added to the growing literature on the utility of avian models in the study of intricate cognitive processes (Salwiczek et al., 2010). While there is a body of data indicating that aromatization in the hippocampus affects synaptic physiology, the mechanisms leading to the “spatiotemporal fidelity” are less well understood but are under examination (Balthazart and Ball, 2011; Remage-Healey et al., 2011a; Saldanha et al., 2011; Saldanha et al., 2012; Schlinger et al., 2014). Moreover, while modulation of local or global E2 production affects spatial memory in zebra finches (Oberlander et al., 2004; Bailey et al., 2013; Rensel et al., 2013), what remains unclear is whether failures of retrieval in these various tests are due to inabilities of hippocampal cells to acquire, consolidate, retain, or recall the spatial tasks. Measurement or more temporally-specific manipulation of locally-produced E2 during acquisition and retention phases may more precisely specify the role of neural aromatization.
Finally, the studies to date that have more directly examined the potential role of neural aromatization on passerine spatial memory function have used only zebra finches. These birds do not store food, migrate, or defend a large territory, and thus do not have the spatial memory demands as site-specific migrants or food-storing songbirds that rely upon spatial maps and memory for local environmental cues in order to survive seasons of inclement weather. Given the presence of aromatase in the hippocampus of songbird species, that spatial memory function provides scientists with a critical set of behaviors that are performed reliably in the wild, and that the structure undergoes dramatic seasonal changes in some songbird species (reviewed in Sherry and MacDougall-Shackleton, 2014), the vital role of neural aromatization can be expanded using others in the songbird order in which seasonal hippocampal plasticity is more imperative to heightened cognitive function and thus survival.
CONVERGENCE OF COGNITIVE SYSTEMS DEPENDENT ON NEURAL AROMATIZATION
Experiments on songbirds – who express abundant synaptic aromatase and who reliably perform perceptual and spatial memory behaviors in laboratory settings – have provided data indicating that neural aromatization is a powerful regulator of two cognitive processes: auditory discrimination reliant on the NCM and spatial memory dependent on the hippocampus, regions long known to be primary sites for aromatization in the brain (Saldanha et al., 1999). Given the behavioral and neuroendocrine overlaps, they may also be useful in elucidating interactions among the brain’s multiple memory systems. Additionally, some aspects of these behaviors subserved by the hippocampus and NCM are sexually dimorphic, permitting analysis of how these dimorphic regions develop, are maintained in adulthood, respond to challenge (e.g.., stress, damage, etc.), and are perhaps affected by aging (Saldanha and Schlinger, 2005), especially as we are beginning to understand some of the pre- and postsynaptic mechanisms regulating this synaptocrine communication.
Given the patterns of aromatase expression in the songbird brain, and the well-documented roles of local E2, one possible integration of the roles of the passerine hippocampus and structures of the song circuit (like NCM) in spatial memory function and song learning, respectively, may be to consider naturally occurring behaviors that critically rely upon both perceptual and spatial memories. For example, this might include a memory for the context within which songs are heard rather than the song itself. Specifically, the identity (individual bird), orientation (spatial location), and temporal context (time of day and/or year) of incident song may likely involve hippocampal pathways (Marler, 1997). In a certain sense, it is these very characteristics that may provide the “who/what”, “where”, and “when” that permits song discrimination and memory to be considered in context.
Those functions subserved by the NCM and hippocampus – and the importance of these memories to reproductive success and survival – make songbirds excellent models for the study of the convergence or overlap of these memory systems. In addition to the endocrine/hormonal data indicated above, work from several studies in songbirds also point toward this intersection. For example, activity of the immediate early genes c-FOS and/or ZENK are detected in the hippocampus during acquisition but not recall of a spatial memory task (Bischof et al., 2006; Mayer et al., 2010), following presentations of novel conspecific songs or tutor songs (Bolhuis et al., 2001; Bolhuis et al., 2000; Cheng and Clayton, 2004; Eda-Fujiwara et al., 2003; Kimpo and Doupe, 1997), conspecific as opposed to heterospecific song presentations (Bailey et al., 2002; Bailey and Wade, 2003, 2005), conspecific songs paired with a novel visual stimulus (Kruse et al., 2004), and mate versus non-mate calls (Vignal et al., 2008). Two characteristics of this expression are of extreme interest. Firstly, immediate early gene expression in the hippocampus occurs in a highly specific context that suggests a comparison or association between songs across species, physical context, or mate (Bailey and Wade, 2003, 2005). Secondly, this expression occurs almost exclusively in females and not males (Gobes et al., 2009). Interestingly, lesions to a subdivision of the hippocampus in adult female but not male zebra finches results in a loss of preference for tutor over novel conspecific songs, while the normal preference for conspecific over heterospecific songs remain intact in both sexes (Bailey et al., 2009). In detail, birds were given ibotenic acid lesions of the dorsolateral subdivision of the hippocampus (which appears, based on connectivity studies (Székely, 1999; Székely and Krebs, 1996), to be the primary input and output) just prior to song template formation (20 days post-hatch), sensorimotor integration (45 days post-hatch), or in adulthood (100 days post-hatch). As adults, in addition to spatial memory tests described above, tests of song production in males and song preference in males and females were given. Preferences for the song of a tutor versus that of a novel male conspecific, as well as a novel male conspecific versus a heterospecific song, were tested in an apparatus in which songs were played simultaneously. Difference scores for each measure (time spent in the side of the chamber broadcasting tutor’s song minus other conspecific, and zebra finch song minus heterospecific song) were then analyzed. Hippocampal lesions at any age did not affect song production or preference in males, nor was song preference affected in females lesioned at 20 or 45 days post-hatching. Intriguingly, only one group – females lesioned as adults – showed no preference for their tutor’s song compared to that of a novel conspecific male, based on the difference in times spent in each half of the chamber playing those songs.
While to our knowledge no data exist that indicate direct projections to or from the hippocampus and nuclei or regions important to song learning or perception like the NCM, several indirect projections could provide hippocampal neurons with information relevant to past or “on-line” song behavior. For instance, there are hippocampal projections with the dorsomedial portion of the thalamic nucleus (Székely and Krebs, 1996), perhaps providing the cells access to the thalamocortical circuit involved in song perception and sensorimotor integration (Vates et al., 1997) briefly noted above. Interestingly, these context-dependent effects in the hippocampus are also observed in the NCM. ZENK mRNA increases in NCM by the pairing of conspecific song with multi-colored lights (Kruse et al., 2004); a similar effect is seen in a neighboring auditory region (Avey et al., 2005). In male zebra finches, video playback of conspecific song stimuli significantly increases local E2 levels in NCM, an effect not observed in females (Remage-Healey et al., 2012).
These data collectively suggest a role for the hippocampus and NCM as interfaces between the widely investigated and described systems of the brain essential to perceptual (song learning and discrimination), spatial, and therefore perhaps episodic-like (contextual stimuli like time of day, place, identity of a specific bird, etc.) memories. The separation of pathways that underlie explicit and implicit memory function have been long studied in several mammalian model systems including humans, non-human primates, and rodents (Squire, 1992; Tulving, 2002). We have learned much from these studies, including the distinct and overlapping neural pathways that are activated during the learning and performance of procedural memory-reliant tasks (Graybiel, 1995), and those that are activated during the learning and performance of explicit memory-reliant ones (Squire, 1992; Tulving, 2002). The songbird may offer resolution to difficulties in determining the convergence of these systems – and the importance of neural aromatization – in that there are implicit and explicit memory tasks that are regularly performed in the wild, are highly selected for, and are relatively easy to study under laboratory conditions.
MECHANISMS OF AROMATASE ACTIVITY AND SYNAPTIC E2 PROVISION
The activity of aromatase and E2 provision are regulated by excitatory stimuli. In cells from quail hypothalamus, aromatase protein is rapidly phosphorylated by a Ca++-dependent mechanism in vitro (Balthazart and Ball, 2006); Ca++-dependent second messengers (Ca++/calmodulin protein kinase, as well as protein kinases A and C) control aromatase activity in somata (Balthazart et al., 2003a, 2003b, 2005, 2006) and are likely candidates for the control of synaptic aromatase. Calcium-dependent mechanisms also increase the production and release of E2 in cells of NCM (Remage-Healey et al., 2011b). Glutamate agonists increase and antagonists decrease aromatase activity and E2 production in hypothalamus (Balthazart et al., 2006) and auditory forebrain (Remage-Healey et al., 2008, Remage-Healey et al., 2011; Jeong et al., 2011). In the zebra finch hippocampus, aromatase and N-methyl-D-aspartate receptors are co-expressed in hippocampal neurons, and these glutamate receptors are detected pre- and postsynaptically (Saldanha et al., 2004). Moreover, E2 treatment increased the frequency of presynaptic receptors, indicating a role for E2 in autoreception and increases in synaptic strength. In rodent hippocampus, E2 modulates pre- and postsynaptic currents rapidly and is also strongly influenced by glutamate (Woolley 2007; Hojo et al., 2004). These findings propose a coupling of electrical and hormonal signaling at the vertebrate synapse. Rapid E2-dependent changes in intracellular signal transduction pathways in postsynaptic cells (McEwen and Alves, 1999; Akama and McEwen, 2003) support the idea that synaptic aromatase may be involved in use-dependent synaptic physiology and raises the possibility that synaptic E2 may function more like a neurotransmitter than a neuromodulator (Schlinger and Saldanha, 2005; Balthazart and Ball, 2006; Mukai et al., 2006; Saldanha et al., 2011). This mode of signal transduction is strongly supported by the localization of fixed-membrane estrogen receptors at extra-nuclear sites – including dendritic spines – in the songbird (Acharya and Veney, 2012) and rodent (Milner et al., 2005; Hammes and Levin, 2007; Woolley, 2007) brain, as well as modifications in intracellular pathways similar to those in rodents (Frick, 2015) like pERK, pCREB, and Egr-1/ZENK (Cheng and Clayton, 2004; Maney et al., 2006; Heimovics et al., 2012). Additional studies could agonize or antagonize these receptors, inhibit components of specific signaling cascades, or measure their cellular and subcellular levels during phases in which birds – as indicated above – acquire, consolidate, retain, or recall the spatial tasks, to obtain a more comprehensive understanding of the postsynaptic mechanisms, specifically, that are downstream from the action of E2.
SEX DIFFERENCES IN NEURAL AROMATAZATION AND BEHAVIOR
In regard to cognitive processes involving song discrimination learning and memory and spatial memory acquisition and performance, there are a number of sex differences in the ability of neurons to synthesize and respond to E2 as well as the behaviors that are subserved by the hippocampus and NCM in birds. For example, in the zebra finch hippocampus, males have more aromatase-positive presynaptic boutons and a higher proportion of synapses that express aromatase (Peterson et al., 2005), and in general there are more cells expressing membrane-bound G-protein coupled estrogen receptors in males than in females (Acharya and Veney, 2012). Interestingly, in tests of spatial memory, there are no sex differences in acquisition, but males perform better than females at retention trials over time as measured via latency to remember the location of a cup containing seed (Figure 2; Bailey et al., 2009).
In NCM, while there does not appear to be a sex difference in the amount of somal aromatase, adult males have more aromatase-positive fibers (Saldanha et al., 2000). In this region, increases in E2 levels occur during presentation of auditory and visual stimuli to males but only auditory stimuli in females (Remage-Healey et al., 2012). Also, a number of studies indicate differences in immediate early gene activity following presentations of conspecific and heterospecific song signals, as in birds 30 days post-hatching (Bailey and Wade, 2003) but not 45 days (Bailey and Wade, 2005). Interestingly, around the same developmental time period, baseline neural E2 levels in the caudal forebrain do not differ and increase in similar ways in response to passive song tutoring in juvenile males and females. However, differences do emerge as animals approach sexual maturity (Chao et al., 2014). These structural and functional sexual dimorphisms of the songbird NCM are providing insight into the mechanisms of local and acute E2 production and signaling (recently reviewed in Krentzel and Remage-Healey, 2015).
Studies concerning both the hippocampus and NCM advocate that the sex differences in E2-provision may lead to sexually dimorphic cognitive behavior. The questions remaining, however, are when these differences develop, and whether they lead to the sexually dimorphic cognitive function in adulthood.
SUMMARY
Over the past several years, experiments with members of the order Passeriformes have filled considerable gaps in our knowledge regarding the role of neural aromatization in cognition. While the vertebrate brain has long been studied as a target and source of E2, recent studies have documented the role of that which is neurally-derived and its effects on the acute and chronic activity of neurons, circuits, and the complex cognitive processes they underlie. Songbirds remain excellent models for the study of this “synaptocrine” hormone provision given abundant levels of aromatase in axon terminals in brain regions that underlie the acquisition and performance of congruent cognitive processes that can easily be manipulated and measured in laboratory settings. These studies have begun to elucidate the mechanisms involved in neural aromatization, E2 provision, as well as E2 action at the pre- and postsynaptic cells that leads to auditory discrimination and spatial memory function. Moreover, developmental and sex differences in neural aromatization may provide insight into sensitive period- and sexually dimorphic-dependent cognitive function.
Highlights.
Estrogens are synthesized at the synapse in the songbird brain.
Brain regions important for perception, learning and memory express synaptic aromatase.
Synaptic aromatase may be rapidly modulated.
Synaptic aromatase appears critical for complex social behaviors and memory function.
ACKNOWLEDGMENTS
Data from some of the experiments reviewed in this manuscript, as well as its preparation, were funded by National Institutes of Health grants NIH 042767 and 080585 to CJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David J. Bailey, Email: david.bailey@snc.edu.
Colin J. Saldanha, Email: saldanha@american.edu.
REFERENCES
- Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev. Neurobiol. 2012;72:1433–1446. doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Ascenzi M. Sexual differentiation of behavior in the zebra finch: effect of early gonadectomy or androgen treatment. Horm. Behav. 1990;24:114–127. doi: 10.1016/0018-506x(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Watson JT. Sexual dimorphism in the avian brain is not limited to the song system of songbirds: a morphometric analysis of the brain of the quail (Coturnix japonica) Brain Res. 1990;514:320–326. doi: 10.1016/0006-8993(90)91427-i. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual differentiation of brain and behavior in the zebra finch: critical periods for effects of early estrogen treatment. J. Neurobiol. 1994;25:865–877. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J. Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav. Brain Res. 2005;165:247–253. doi: 10.1016/j.bbr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J. Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Mol. Brain Res. 2003;116:147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. FOS and ZENK responses in 45 day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav. Brain Res. 2005;162:108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J, Saldanha CJ. Hippocampal lesions in zebra finches impair spatial memory performance, but not song - a developmental study of independent memory systems. Dev. Neurobiol. 2009;69:491–504. doi: 10.1002/dneu.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, De Clerck A, Foidart A. Behavioral demasculinization of female quail is induced by estrogens: studies with the new aromatase inhibitor, R76713. Horm. Behav. 1992;26:179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemcani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm. Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Balthazart J. Steroid control and sexual differentiation of brain aromatase. J. Steroid Biochem. Mol. Biol. 1997;61:323–339. [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur. J. Neurosci. 2003a;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Cornil CA, Ball GF. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. J. Steroid Biochem. Mol. Biol. 2003b;86:367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Bingman VP, Ioalé P, Casini G, Bagnoli P. Hippocampal ablated homing pigeons show a persistent impairment in the time taken to return home. J. Comp. Physiol. A. 1988;163:559–563. [Google Scholar]
- Bischof HJ, Lieshoff C, Watanabe S. Spatial memory and hippocampal function in a non-foodstoring songbird, the zebra finch (Taeniopygia guttata) Rev. Neurosci. 2006;17:43–52. doi: 10.1515/revneuro.2006.17.1-2.43. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Zijlstra GGO, den Boer-Visser AM, Van der Zee EA. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc. Natl. Acad. Sci., USA. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Hetebrij E, den Boer-Visser AM, De Groot JH, Zijlstra GGO. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur. J. Neurosci. 2001;13:2165–2170. doi: 10.1046/j.0953-816x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat. Rev. Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J. Comp. Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat. Rev. Neurosci. 2000;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Calisi RM, Knudsen DP, Krause JS, Wingfield JC, Gentner TQ. Estradiol differentially affects auditory recognition and learning according to photoperiodic state in the adult male songbird, European starling (Sturnus vulgaris) Peer. J. 2013;1:150. doi: 10.7717/peerj.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi EA, Robinson GE, Fahrbach SE. Neuroethology of spatial learning: the birds and the bees. Annu. Rev. Psychol. 1999;50:651–682. doi: 10.1146/annurev.psych.50.1.651. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ball GF. Early administration of 17beta-estradiol partially masculinizes song control regions and alpha2-adrenergic receptor distribution in European starlings (Sturnus vulgaris) Horm. Behav. 1996;30:387–406. doi: 10.1006/hbeh.1996.0044. [DOI] [PubMed] [Google Scholar]
- Chao A, Paon AP, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev. Neurobiol. 2014 doi: 10.1002/dneu.22228. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-Y, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosophorylation in zebra finch auditory forebrain during song presentation. J. Neurosci. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. Role of gene regulation in song circuit development and song learning. J. Neurobiol. 1997;33:549–571. [PubMed] [Google Scholar]
- Clayton NS, Pröve E. Song discrimination in female zebra finches and Bengalese finches. Anim. Behav. 1989;38:352–354. [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Colombo M, Broadbent N. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neurosci. Biobehav. Rev. 2000;24:465–484. doi: 10.1016/s0149-7634(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front. Neuroendocrinol. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Nottebohm F. Role of gender, season, and familiarity in discrimination of conspecific song by zebra finches (Taeniopygia guttata) Proc. Natl. Acad. Sci. USA. 1992;89:1368–1371. doi: 10.1073/pnas.89.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groof G, Poirier C, George I, Hausberger M, Van der Linden A. Functional changes between seasons in the male songbird auditory forebrain. Front. Behav. Neurosci. 2013;7:196. doi: 10.3389/fnbeh.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ. Song- and order-selective neurons in the songbird anterior forebrain and their emergence during vocal development. J. Neurosci. 1997;17:1147–1167. doi: 10.1523/JNEUROSCI.17-03-01147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DL, Bentley GE, Ball GF. Does sex or photoperiodic condition influence ZENK induction in response to song in European starlings? Brain Res. 1999;844:78–82. doi: 10.1016/s0006-8993(99)01915-0. [DOI] [PubMed] [Google Scholar]
- Eda-Fujiwara H, Satoh R, Bolhuis JJ, Kimura T. Neuronal activation in female budgerigars is localized and related to male song complexity. Eur. J. Neurosci. 2003;17:149–154. doi: 10.1046/j.1460-9568.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- Foidart A, Silverin B, Baillien M, Harada N, Balthazart J. Neuroanatomical distribution and variations across the reproductive cycle of aromatase activity and aromatase immunoreactive cells in the pied flycatcher (Ficedula hypoleuca) Horm. Behav. 1998;33:180–196. doi: 10.1006/hbeh.1998.1448. [DOI] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardo A, Ioalé P, Bingman VP. Homing in pigeons: The role of the hippocampal formation in the representation of landmarks used for navigation. J. Neurosci. 1999;19:311–315. doi: 10.1523/JNEUROSCI.19-01-00311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res. Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Gentner T, Hulse S, Duffy D, Ball G. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J. Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr. Biol. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Gobes SMH, Ter Haar SM, Vignal C, Vergne AL, Mathevon N, Bolhuis JJ. Differential responsiveness in brain and behavior to sexually dimorphic long calls in male and female zebra finches. J. Comp. Neurol. 2009;516:312–320. doi: 10.1002/cne.22113. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr. Opin. Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1382. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Gurney ME. Hormonal control of cell form and number in the zebra finch song system. J. Neurosci. 1981;1:658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endo. Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Shettleworth SJ. Hippocampal lesions impair memory for location but not color in passerine birds. Behav. Neurosci. 1996;110:831–835. doi: 10.1037//0735-7044.110.4.831. [DOI] [PubMed] [Google Scholar]
- Harding CF. The importance of androgen metabolism in the regulation of reproductive behavior in the avian male. Poult. Sci. 1986;65:2344–2351. doi: 10.3382/ps.0652344. [DOI] [PubMed] [Google Scholar]
- Harding CF, Walters MJ, Collado D, Sheridan K. Hormonal specificity and activation of social behavior in male red-winged blackbirds. Horm. Behav. 1988;22:402–418. doi: 10.1016/0018-506x(88)90011-6. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17β-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinol. 2012;153:1364–1376. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- Hodgson ZG, Meddle SL, Christians JK, Sperry TS, Healy SD. Influence of sex steroid hormones on spatial memory in a songbird. J. Comp. Physiol. A. 2008;194:963–969. doi: 10.1007/s00359-008-0369-4. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci, USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge: University Press; 1969. pp. 61–74. [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci, USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur. J. of Neurosci. 2011;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil AC, Balda RP. Spatial memory in seed-caching corvids. Psychol. Learn. Motiv. 1990;26:1–25. [Google Scholar]
- Kimpo RR, Doupe AJ. FOS is induced by singing in distinct neuronal populations in a motor network. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proc. Natl. Acad. Sci., USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Remage-Healey L. Sex differences and rapid estrogen signaling: A look at songbird audition. Front. Neuroendocrinol. 2015. doi: 10.1016/j.yfrne.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol. Learn. Mem. 2004;82:99–108. doi: 10.1016/j.nlm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lewandowski B, Vyssotski A, Hahnloser RH, Schmidt M. At the interface of the auditory and vocal motor systems: NIf and its role in vocal processing, production and learning. J. Physiol. Paris. 2013;107:178–192. doi: 10.1016/j.jphysparis.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat. Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function: Past, present and future. Horm. Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Macphail EM. The role of the avian hippocampus in spatial memory. Psicologica. 2002;23:93–108. [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur. J. of Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney D, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front. Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. J. Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- Mayer U, Watanabe S, Bischof HJ. Hippocampal activation of immediate early genes Zenk and c-Fos in zebra finches (Taeniopygia guttata) during learning and recall of a spatial memory task. Neurobiol. Learn. Mem. 2010;93:322–329. doi: 10.1016/j.nlm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Mayer U, Watanabe S, Bischof HJ. Spatial memory and the avian hippocampus: research in zebra finches. J. Physiol. Paris. 2013;107:2–12. doi: 10.1016/j.jphysparis.2012.05.002. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc. Natl. Acad. Sci., USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J. Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J. Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J. Comp. Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J. Comp. Physiol. A. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanakaa Y, Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochimica et Biophysica Acta (BBA) 2010;1800:1030–1044. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm. Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Patel SN, Clayton NS, Krebs JR. Hippocampal tissue transplants reverse lesion-induced spatial memory deficits in zebra finches (Taeniopygia guttata) J. Neurosci. 1997;17:3861–3869. doi: 10.1523/JNEUROSCI.17-10-03861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlisch BA, Remage-Healey L. Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an ‘interface’nucleus. Neuroscience. 2015;284:522–535. doi: 10.1016/j.neuroscience.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc. Biol. Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychol. 2003;1497:1–7. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western-scrub jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J. Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman ME, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Natl. Acad. Sci., USA. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: evidence for “synaptocrine” signaling. Front. Endocrinol. 2. 2011a doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci. 2011b;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J. of Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J. Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of estrogens during auditory processing in a songbird. J. Neuroendocinol. 2013;25:1024–1031. doi: 10.1111/jne.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol. Learn. Mem. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. J. Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA. Estrogen synthesis and secretion in the brown-headed cowbird (Molothrus ater) Gen. Comp. Endocrinol. 1997;105:390–401. doi: 10.1006/gcen.1996.6841. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm. Behav. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Clayton NS, Schlinger BA. Androgen metabolism in the juvenile oscine forebrain: a cross-species analysis at neural sites implicated in memory function. J. Neurobiol. 1999;40:397–406. doi: 10.1002/(sici)1097-4695(19990905)40:3<397::aid-neu11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Peterson RS, Yarram L, Schlinger BA. The synaptocrine hypothesis: a novel mechanism of estrogen delivery. Horm. Behav. 2003;44:74. [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic responsive neurons in the songbird brain--a doble label immunocytochemical study. Gen. Comp. Endocrinol. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endo. Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. In: Neuroanatomical Distribution of Aromatase in Birds: Cellular and Subcellular Analyses. Balthazart J, Ball GF, editors. New York, NY: Oxford University Press; 2012. pp. 100–114. [Google Scholar]
- Salwiczek LH, Watanabe A, Clayton NS. Ten years of research into avian models of episodic-like memory and its implications for developmental and comparative cognition. Behav. Brain Res. 2010;215:221–234. doi: 10.1016/j.bbr.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev. Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J. Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc. Natl. Acad. Sci. USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc. Natl. Acad. Sci. USA. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA. The activity and expression of aromatase in songbirds. Brain Res. Bull. 1997;44:359–364. doi: 10.1016/s0361-9230(97)00215-3. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Saldanha CJ. Songbirds: A novel perspective on estrogens and the aging brain. AGE. 2005;27:287–296. doi: 10.1007/s11357-005-4555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Remage-Healey L, Rensel M. Establishing regional specificity of neuroestrogen action. Gen. Comp. Endocrinol. 2014;205:235–241. doi: 10.1016/j.ygcen.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res. Mol. Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J. Comp. Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Vaccarino AL. Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 1989;103:308–318. [Google Scholar]
- Sherry DF, MacDougall-Shackleton SA. Seasonal change in the avian hippocampus. Front. Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Spatial memory in food-storing birds. Philos. Trans. R. Soc. Lond. B. 1990;329:143–151. [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen. Comp. Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J. Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J. of Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis of findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Strasser R, Bingman VP. The relative importance of location and feature cues for homing pigeon (Columba livia) goal recognition. J. Comp. Psychol. 1996;110:77–87. doi: 10.1037/0735-7036.110.1.77. [DOI] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: Role of genomic and electrophysiological activities. J. Neurobiol. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Székely AD. The avian hippocampal formation: subdivisions and connectivity. Behav. Brain Res. 1999;98:219–225. doi: 10.1016/s0166-4328(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Székely AD, Krebs JR. Efferent connectivity of the hippocampal formation of the zebra finch (Taenopygia guttata): An anterograde pathway tracing study using Phaseolus vulgaris leucoagglutinin . J. Comp. Neurol. 1996;368:198–214. doi: 10.1002/(SICI)1096-9861(19960429)368:2<198::AID-CNE3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J. Neurosci. 2004;24:4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, Van der Burg JMM, den Boer-Visser AM. Localised brain activation specific to auditory memory in a female songbird. J. Comp. Neurol. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J. Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata) J. Comp. Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vignal C, Bouchut C, Mathevon N. Sound-induced brain activity depends on stimulus subjective salience in female zebra finches. Curr. Rev. Biol. 2008;331:347–356. doi: 10.1016/j.crvi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990a;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J. Neurobiol. 1990b;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, Borg L, Bogdan D. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches’ behavioral responses to songs. Horm. Behav. 2009;55:50–59. doi: 10.1016/j.yhbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Harding CF. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Horm. Behav. 1988;22:207–218. doi: 10.1016/0018-506x(88)90067-0. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Bischof H-J. Effects of hippocampal lesions on acquisition and retention of spatial learning in zebra finches. Behav. Brain Res. 2004;155:147–152. doi: 10.1016/j.bbr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Maier U, Bischof H-J. Pattern discrimination is affected by entopallial but not by hippocampal lesions in zebra finches. Behav. Brain Res. 2008;190:201–205. doi: 10.1016/j.bbr.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;14:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Phan ML, Lu K, Vicario DS. He hears, she hears: Are there sex differences in auditory processing? Dev. Neurobiol. 2014 doi: 10.1002/dneu.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. London: Oxford University Press; 1996. [Google Scholar]