Abstract
Aromatase is the requisite and limiting enzyme in the production of estrogens from androgens. Estrogens synthesized centrally have also emerged as a potent neuroprotectant in the vertebrate brain. Studies in rodents and songbirds have identified key mechanisms that underlie both; the injury-dependent induction of central aromatization, and the protective effects of centrally synthesized estrogens. Injury-induced aromatase expression in astrocytes occurs following a broad range of traumatic brain damage including excitotoxic, penetrating, and concussive injury. Responses to neural insult such as edema and inflammation involve signaling pathways the components of which are excellent candidates as inducers of this astrocytic response. Finally, estradiol from astrocytes exerts a paracrine neuroprotective influence via the potent inhibition of inflammatory pathways. Taken together, these data suggest a novel role for neural aromatization as a protective mechanism against the threat of inflammation and suggests that central estrogen provision is a wide-ranging neuroprotectant in the vertebrate brain.
Introduction
Estrogens have long been appreciated for their potent organizational and activational effects on sex-specific neural circuits. Recent evidence however, has also implicated 17β-estradiol (E2) in neuroprotection (Maggi et al., 2004; McEwen et al., 2001; Wise, 2003). In several species, elevations in peripheral E2 are associated with dramatic decreases in markers of cell death and the numbers of necrotic or apoptotic cells in the CNS (Belcredito et al., 2001; Bryant et al., 2006; Roselli, 2007; Simpkins et al., 2005; Simpkins and Dykens, 2008). Elevations in circulating E2 are likely the consequence of increases in the expression and activity of aromatase (estrogen-synthase), the rate-limiting enzyme in the synthesis of estrogens (Simpson et al., 1994). Indeed, aromatase expression has been documented in multiple peripheral tissues including the ovary, testes, placenta, bone, adipose, and adrenals (Vanselow et al., 1999).
The vertebrate brain is also a site of estrogen synthesis (Balthazart et al., 1990; Naftolin et al., 1996; Negri-Cesi et al., 2001; Peterson et al., 2005; Roselli et al., 2004; Roselli and Resko, 2001; Saldanha et al., 2011, 2000). In mammals, we have known about the critical role of hypothalamic aromatization in the development and activation of male-specific neural circuits and behaviors for some time; a phenomenon generalizable to multiple rodent species (MacLusky and Naftolin, 1981). While aromatase has been documented at extra-hypothalamic, forebrain sites of some other mammals, its physiological role remains somewhat equivocal (Naftolin et al., 1996; Roselli et al., 2004; Yague et al., 2008b; Zhong et al., 2017). Across species however, the constitutive expression of aromatase in the mammalian brain appears to be neuronal, suggested and documented with studies that have used in-situ hybridization and immunocytochemistry (Gottfried-Blackmore et al., 2008; Roselli, 2007; Yague et al., 2008a).
In birds, the role of neural aromatase in development and adulthood is perhaps better understood, due in part to pioneering work in Passeriformes (Gurney and Konishi, 1980), Columbiformes (Steimer and Hutchison, 1981, 1980), and Galliformes (Schumacher and Balthazart, 1984). Passerines (songbirds) distinguish themselves from other bird species since the expression of aromatase is particularly widespread and abundant (Metzdorf et al., 1999; Saldanha et al., 2000; Schlinger and Arnold, 1991). Among songbirds, the zebra finch (Taeniopygia guttata), appears particularly interesting because aromatase appears to be among the highest measured in the CNS of any homeotherm, and occurs at levels sufficient to enrich plasma levels of E2 in both sexes, but especially in males (Adkins-Regan and Ascenzi, 1990; Schlinger and Arnold, 1993, 1991). Indeed, the neural estrogen synthetic capability in this species has been confirmed via multiple techniques including enzyme activity (Schlinger and Arnold, 1992, 1991), in situ hybridization (Saldanha et al., 1998; Schlinger et al., 1994; Shen et al., 1995; Soma et al., 2003) and immunocytochemistry (Peterson et al., 2005; Saldanha et al., 2000). While more abundant in the songbird relative to mammals, the constitutive expression of aromatase nonetheless appears to be restricted to neurons in every avian species studied (Peterson et al., 2005; Saldanha et al., 2011, 2000; Shen et al., 1995).
Despite the assertions above, aromatization is neither exclusively constitutive nor neuronal in the mammalian and passerine brain. Schlinger et al., first presented intriguing data suggesting that primary dissociated cell cultures of hatchling songbird telencephalon, expressed aromatase in glial cells (Schlinger, 1994). This was later confirmed in vivo by several studies which documented the expression of aromatase in astrocytes following excitotoxicity or mechanical injury in rodent and songbirds (Azcoitia et al., 2010; Garcia-Segura et al., 1999; Peterson et al., 2001; Saldanha et al., 2005). Interestingly, in some cases the expression of injury-induced aromatase is also seen in radial glia suggesting that both types of astroglia are capable of induced estrogen synthesis in songbirds (Peterson et al., 2004).
Here we review literature on the various types of challenges that appear to reliably induce aromatase in multiple species and introduce the suggestion that neural, astrocytic aromatization appears to be a potent neuroprotectant regardless of the type of insult in some vertebrates. We then present what is known about the induction of aromatase, go on to discuss the regulation and consequences of injury-induced aromatase following various forms of central perturbation in songbirds and mammals. In closing we present data supporting a novel role for neural aromatase in neuroprotection from the consequences of peripheral inflammation, and end with the hypothesis that induced aromatase appears to have evolved in multiple species as a neuroprotectant against a broad range of threat to the CNS, with intimate ties to the innate immune system.
A broad range of neural damage induces aromatase expression in astroglia
Primary evidence suggesting an association between neural degeneration and aromatase expression in astrocytes was presented by Garcia-Segura (Garcia-Segura et al., 1999). In these studies, neurodegeneration was induced in rats and mice, either with an excitotoxic dose of kainic acid or a penetrating mechanical injury. Eight or ten days later respectively, subjects showed increased aromatase activity and expression in the hippocampus, the brain area where degeneration was most apparent. Notably, immunoreactive aromatase colocalized with glial fibrillary acidic protein (GFAP) revealing the astrocytic nature of the cells with induced aromatase expression. Importantly, no degeneration or astrocytic aromatase was detected in control animals, establishing a correlation between neurodegeneration and the induction of aromatase in astrocytes. Peterson et al., echoed these findings using adult zebra finches, and added that the induction of aromatase following penetrating damage was detectable as soon as 24 hours post-injury in the songbird (later work showed increases even more rapidly) (Peterson et al., 2001). Together, these studies opened a new area of investigation on the spatial and temporal specificity of E2 provision in the vertebrate brain, and accomplished two significant ends. First, they provided a context for earlier studies that reported astrocytic aromatase and an increase in aromatase activity in primary dissociated cell cultures of hatchling songbirds following the death of neurons in vitro (Schlinger et al., 1994). Perhaps more importantly, they strongly suggested an obligatory association between damage to the neuropil and central aromatization. We have learned that this association spans a wide range of neurotrauma including experimental models of stroke and concussive brain injury.
Ischemia and central aromatization.
Circulating E2 may be neuroprotective in experimental stroke models using rodents. Ischemic damage caused by medial carotid artery occlusion (MCAO) is lowest when it is performed on female rats in proestrus relative to the identical manipulation in diestrus or metestrus animals (Carswell et al., 2000). Correspondingly, experimentally induced decreases of circulating estrogens by ovariectomy, pharmacological inhibition, or normal reproductive senescence result in higher lesion volumes following MCAO, suggesting a role for aromatization from peripheral sources on neural injury and damage (Alkayed et al., 1998; Rusa et al., 1999; Sawada et al., 2000).
More recent work points to an extragonadal, and likely neural source for neuroprotective E2 following ischemia. McCullough et al., compared infarct volume following MCAO in female aromatase knock-out (ARKO) mice with ovariectomized wild-type (OVXWT) littermates and documented less damage in the latter, suggesting the possibility of an extragonadal neuroprotectant (McCullough et al., 2003). E2 via aromatization is the likely neuroprotectant since infarct size in OVXWT mice treated chronically with an aromatase inhibitor had comparable damage to that of ARKO mice and E2 treatment of ARKO mice completely ameliorated the increased susceptibility to MCAO-induced neural damage (McCullough et al., 2003). Indeed, the key site of aromatization in this neuroprotective effect may be the area around the lesion itself. In spontaneously hypertensive rats, MCAO increases the expression of aromatase in astrocytic processes in the penumbra of the neural infarct (Carswell et al., 2005). This upregulation is detectable at 24 hours and 8 days following treatment but is not 2 hours or 30 days after MCAO. Taken together these data strongly suggest that disruption of the neuropil due to experimental stroke can alter E2 provision in the rodent brain via the induction of aromatase expression in a manner seemingly similar to that of other types of neurotrauma.
Concussion and central aromatization.
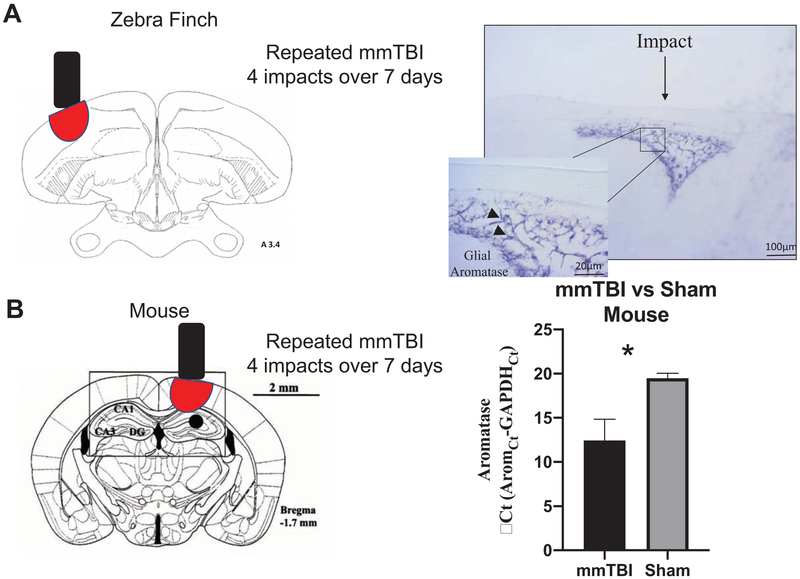
Our recent unpublished work has further extended the types of neural insult capable up altering the neural expression of aromatase. In the zebra finch and in the mouse, multiple mild concussive treatments using the controlled weight-drop technique reliably result in the upregulation or aromatase around the focus of concussive damage (see Figure 1). This upregulation is detectable at both the transcript and protein levels (in different animal models) and represents yet another type of neurotraumatic event that can upregulate aromatase in the homeotherm brain.
Figure 1.
Multiple mild concussive traumatic brain injury (mmTBI) were created by briefly anesthetizing subjects with isofluorane. Subjects were then placed on a modified weight drop apparatus (Laboratory weight drop device, Northeast Biomedical) where a 293.19 g rod was raised 1 cm above the head of the subject and dropped. The above procedure was repeated 4 times over 7 days and subjects were collected 10 days after the first injury. In zebra finches (A) mmTBI upregulates glial aromatase in astrocytes (purple) around the site of injury. Following mmTBI in mice (N=3/group), aromatase transcript was significantly upregulated in injured brains when compared to sham tissue following one-way ANOVA analysis. * denotes a significant difference at P <0.05 (one-way ANOVA)
The obvious question to be asked is: what signals, perhaps shared by these multiple types of neurotraumatic events, could be candidates as regulators of aromatase transcription in astrocytes? Several possibilities come immediately to mind, including cell death, changes in pressure and perhaps inflammatory signals.
Possible regulators of astrocytic aromatase expression
Cell Death.
Damage to the brain can be broadly separated into two phases, the initial primary physical trauma that results from the physical displacements of brain tissue, and the second injury that is the brain’s response to the trauma. The secondary injury includes the long-term effects of the initial trauma (necrosis or apoptosis), as well as the production of free radicals, increased excitotoxicity, and increased inflammation and edema (Bramlett and Dietrich, 2007; Lenzlinger et al., 2001; Maas et al., 2008; Marciano et al., 2002; Stein, 2008). Changes in signals associated with cell death are less compelling as a regulator of glial aromatase as multiple cells undergo pyknosis for various reasons at all times in the adult CNS. Furthermore, aromatase expression in the constitutive rodent and songbird brain appears to be neuronal, lessening the likelihood that signals associated with cell death could serve as triggers of astrocytic aromatase transcription. While one could argue that low levels of pyknosis may not be enough to reach a hypothetical threshold for the upregulation of aromatase in astrocytes, it is noteworthy that massive amounts of cell death occur every autumn within the nuclei of the song circuit, an interconnected set of brain areas necessary for singing behavior, in temperate-zone songbirds in the absence of observed glial aromatase expression, further lessening the likelihood of these signals in the regulation of astrocytic aromatase. Furthermore, in some songbirds like zebra finches, both male and females undergo massive neuronal proliferation during development, however females also undergo a period of neuronal pruning and regression that results in the sex differences observed in song nuclei number and size (Burek et al., 1997; Kirn and DeVoogd, 1989; Nordeen and Nordeen, 1988). Interestingly, this neuronal pruning or naturally occurring cell death does not result in glial aromatase expression either.
Edema.
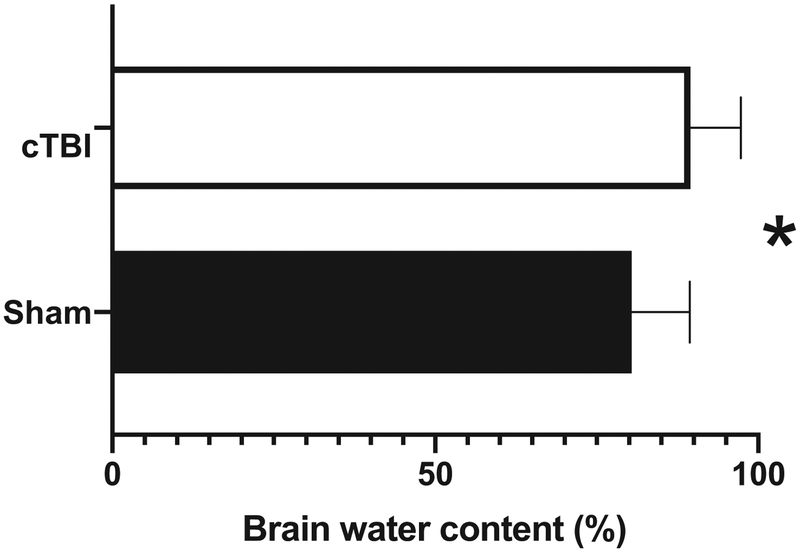
Cerebral edema has a crucial impact on morbidity following TBI as it increases cranial pressure, impairs cerebral perfusion and oxygenation, and contributes to the overall degeneration of the brain (Sorby-Adams et al., 2017; Stokum et al., 2015). Astrocytes are key participants in cerebral edema by virtue of their relationship with the cerebral vasculature, their unique compliment of solute and water transport proteins, and their general role in brain volume homeostasis (Stokum et al., 2015). Specifically, one of the effects of even a single concussive event is increased edema over controls (unpublished Figure 2). Estradiol reduces edema formation and infarct volume in vivo (O’Donnell et al., 2006). Changes in pressure are fairly ubiquitous in multiple forms of neurotrauma including stroke, penetrating, and concussive injuries, however to the best of our knowledge these data have not been tested in vivo (Sorby-Adams et al., 2017). Intriguing evidence suggests that astrocytes may be capable of sensing changes in pressure. Gatson et al., exposed astrocytic cultures to various pressures and reported increases in aromatase message relative to GAPDH relative to unmanipulated controls (Gatson et al., 2011). Furthermore, perihematomal cerebral edema specifically up-regulates thrombin that further activates secretion of the cytokines: tumor necrosis factor (TNF) and IL-1β from glial cells (Stokum et al., 2015). These data again point to inflammatory cascades as the likely candidate for the potent induction of injury-associated aromatase expression, however the specific mechanism(s) of this influence have only recently been revealed.
Figure 2.
A single concussive injury was directed to the brain of an anesthetized adult zebra finches as described in Figure 1. 6 hours following injury, a 4mm diameter brain sample was collected and immediately weighed for the wet weight (WW). Brain samples were then placed at 110C for 24 hours and weighed again for the dry weight (DW). Brain water content or edema was calculated as %H2O = (WW - DW) × 100/WW. Edema (as measured by brain water content) is significantly higher in birds that underwent concussive traumatic brain injury (cTBI) relative to shams as measured using a one tailed Mann-Whitney U. * denotes a significant difference at P <0.05
Inflammation.
Since increased inflammation is an important component of the secondary response to damage, we sought to examine the role of inflammation alone in the regulation of astrocytic aromatase expression. In adult zebra finches, we unilaterally exposed the neuropil to either the inflammagen phytohaemagglutinin (PHA) or vehicle without causing cell death or tissue damage (Duncan and Saldanha, 2011), thus we were able to determine the role of inflammation on the brain independent of the cell death that is generally associated with brain damage. Treatment with PHA increased inflammation as confirmed by the upregulation of the expression of two cytokines (interleukin 1b and 6 (IL-1b and IL-6)). Importantly, tissue exposed to PHA had significantly higher expression of aromatase and this upregulation was in astrocytes as confirmed by colocalization of aromatase with the astrocytic marker vimentin. Furthermore, this was done without causing cell damage or death as we were unable to detect apoptosis or pyknosis in the tissue. Thus, we were able to determine that neuroinflammation and not cell death is the necessary initiation factor for the induction of glial aromatase (Duncan and Saldanha, 2011). This hypothesis is consistent with previous data. Injury induced inflammation is mediated by a number of factors, specifically cytokines (Duncan and Saldanha, 2011). Cytokines dramatically regulate aromatase gene expression in several mammalian cells, including normal and malignant breast tissue (Honma et al., 2002; Morioka et al., 2000; Purohit et al., 2002). Taken together, the data strongly suggest that inflammatory signals, such as those resulting from cell damage, may be a potent regulator of astrocytic aromatase expression. A description of the direct tests of this hypothesis and the mechanism of this influence is discussed below.
Inflammation initiates astrocytic aromatase
Inflammation is part of the body’s immune system response to an irritant (pathogen, injury, chemical, or radiation). For many years, it was viewed mainly as a necessary, even beneficial, response to illness or injury. But now research has indicated that inflammation can be more harmful than helpful. While inflammation is necessary for the activation of a number of downstream pathways involved in neuronal repair, if this inflammation becomes chronic or goes unchecked secondary damage can and will occur (“A current view on inflammation,” 2017). Below we will highlight the genes that regulate both the induction and progress of the inflammatory process, specifically those genes and regulators that also mediate glial aromatase.
Prostaglandin E2 (PGE2).
In rats, administration of PGE2 during a restricted neonatal period induces E2 synthesis via neuronal aromatization (Amateau and McCarthy, 2004; MacLusky and Naftolin, 1981). PGE2 is a proinflammatory signal and is also induced following injury via the enzyme cyclooxygenase (COX)-2 (Chen, 2010; Davidson et al., 2001; Kalinski, 2012; Ricciotti and FitzGerald, 2011). Administration of the COX-1/2 inhibitor indomethacin during development reduced cerebellar aromatase and E2 and had dramatic effects on dendritic morphology and neurophysiology (Dean et al., 2012a, 2012b). Thus, local COX activity and consequent PGE2 synthesis regulate aromatase activity in the mammalian brain. Blocking PGE2 synthesis with indomethacin in the zebra finch brain, was the first identified method for blocking the well-known increase in aromatase and E2 in the adult zebra finch brain following injury (Pedersen et al., 2018; Pedersen and Saldanha, 2017). Interestingly, indomethacin reduced aromatase expression and E2 content at 6 hours, but not 24 hours following injury in females. However, in males, the inhibitory effect of indomethacin on aromatase and E2 was apparent at 24, but not 6 hours after treatment. These data suggest that COX activity, perhaps via consequent prostaglandin secretion, may induce aromatase expression and central E2, an effect that is detectable in temporally distinct patterns between sexes (Pedersen and Saldanha, 2017).
PGE2 has a high affinity for four known E-prostanoid (EP) receptors: EP 1-4 (Singh et al., 1997). Binding of PGE2 to these receptors can regulate aromatase and E2 via modulation of downstream signaling pathways in other systems (Duncan and Saldanha, 2011; Phillips et al., 1978; Reed et al., 1993). Surprisingly, EP-1 and EP-2 receptors were not represented in the zebra finch genome, however they may play a greater role in non-avian species for the purpose of this review we are going to focus on EP-3 and EP-4 (Warren et al., 2010). The expression of both EP-3 and EP-4 changed in a temporally distinct manner following brain injury, with expression of EP-4 being higher in both sexes, but EP-3 only being significantly higher in females at earlier time-points (Pedersen and Saldanha, 2017). Antagonism of prostanoid receptor(s) did prevent injury-induced E2 but in a sex-specific manner. Antagonism of EP-3 in males prevented E2 induction at 24 h, and antagonism of EP-4 prevented the induction in females at 6 h post-injury. Given this data, we believe that PGE2 may bind to EP-3 in males and EP-4 in females to achieve the robust induction of E2 that has been well-documented following penetrating brain injury (Pedersen et al., 2018; Pedersen and Saldanha, 2017).
Nuclear factor Kappa B (NFκB).
NFκB is a family of inducible transcription factors and gene regulators that play a variety of evolutionarily conserved roles in the immune system, specifically in the control of inflammation (Hayden and Ghosh, 2004). In its inactive form, NFκB is located in the cytoplasm and is natively bound to its inhibitor, IκB (inhibitor of κB) (Yamamoto and Gaynor, 2004). In response to TBI and concomitant TNF-α release (Hayden and Ghosh, 2004; Xu et al., 1998), the IκB kinase complex activates, which leads to the phosphorylation of IκB-α and IκB-β (DiDonato et al., 1997; Mercurio et al., 1997; Regnier et al., 1997; Shih et al., 2015; Zandi et al., 1997). Once activated, NFκB binds to the κB site of the promoter, which allows transcription of genes involved in the inflammatory response. NFκB-mediated neuroinflammation is the driving force behind the secondary wave of degeneration, and thus inhibition of this pathway has been a target for decreasing damage to the brain. In the avian brain, expression of NFκB is upregulated when compared to non-injury controls at 2 and 24 hours following injury. Thus, NFκB expression following injury occurs prior to glial aromatase expression and thus is in the right time and place to be involved in the activation of glial aromatase (Cook et al., 2018). Future research is ongoing to determine if NFκB is both necessary and sufficient to upregulate glial aromatase alone.
Cytokines.
Ironically, there appears to be a chicken and egg approach to cytokines following TBI. Cytokines such as LL-1β and TNF-α are inductive cues for many of the inflammatory agents that later induce further production of neuroinflammatory products. Thus it is possible that the inflammatory process is driven at least partly by cells crossing the impaired blood–brain barrier in TBI and spinal cord injury alone (Chodobski et al., 2011; Sundholm-Peters et al., 2004; Thelin et al., 2018). However, the process is further modified by local cytokine generation by glial cells, CNS macrophages, and neuronal cells (Freidin et al., 1992; Helmy et al., 2011; Sébire et al., 1993; Thelin et al., 2018). As previously reviewed, following injury both astrocytes and microglial are both upregulated and they are steroid sensitive. Furthermore, cytokines, such as IL-6 and TNF-α and PGE2, can all stimulate aromatase activity (Macdiarmid et al., 1994; Purohit et al., 1999; Zhao et al., 1996). Thus, we sought to investigate the consequences of specific cytokines that are upregulated following gliosis —namely, IL-1β and TNF-α on induced glial aromatase expression following TBI.
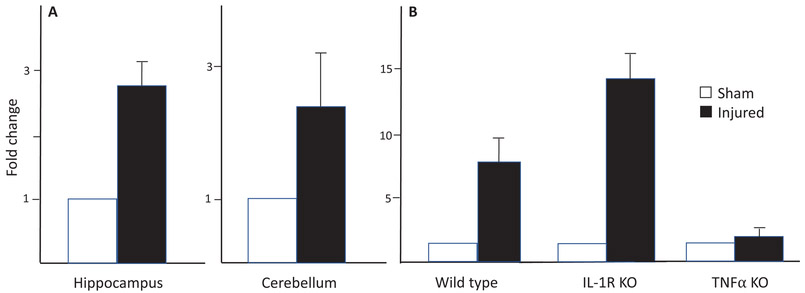
To determine if IL-1β and TNF-α signaling are necessary for the induction of aromatase, we tested the influence of a chronic penetrating injury on the expression of aromatase in wild type (WT) mice and knockout mice deficient in IL-1β receptors (IL1R KO) or TNF-α (TNF-α KO; unpublished Fig. 3). Aromatase mRNA was significantly higher in injured cortical samples compared to sham cortical samples for both WT and IL1R-KO mice, this effect was not apparent in the injured cortex of TNF-α KO animals, despite not differing in initial aromatase expression. These data suggest that TNF-α, but not IL-Ιβ signaling is necessary for the induction of aromatase following brain injury in mice (Figure 3). The mechanisms directly underlying the upregulation of aromatase by TNF-α are still understudied, however TNF-α is a key driver of aromatase promoter I.4-mediated expression in adipose tissue and this could be a possible site for regulation in the brain (Martínez-Chacón et al., 2018; Zhao et al., 1996). Future studies are necessary and ongoing to determine the molecular mechanisms underlying TNF-α induced glial aromatase expression and subsequent biosynthesis of estradiol following injury.
Figure 3.
Adult wild type C57BL/6 (WT) mice and either IL-1 receptor null (IL-1R KO) or TNFα (TNFα KO) were implanted with in-dwelling cannula directed towards the hippocampus. 10 days post-surgery, animals were sacrificed and total RNA was extracted from microdissections immediately adjacent to the cortical needle and the corresponding location in sham animals. Expression of aromatase relative to GAPDH was measured using quantitative PCR using primers specific for the aromatase mRNA sequence. Fold change in aromatase expression 10 days after (A) a unilateral penetrating injury to the hippocampus of male wild type adult mice, and (B) aromatase expression following the same injury in the hippocampus of wild-type, interleukin 1 receptor knockouts (IL-1RKO) and TNFαKO knockouts. Aromatase expression is increased in the hippocampus and also in the distal cerebellum (A). In the hippocampus itself (B) aromatase is upregulated in WT and IL-1RKO, but not in TNFαKO mice. * denotes a significant difference at P <0.05
Given the obvious differences in circulating hormone levels between males and females, and also the changes in hormone levels during menopause, it is not surprising that there are sex differences in the inflammatory response following injury. In fact, the neuroprotective effects of estrogen likely contribute to sex differences in neurodegenerative disease, ischemic stroke and traumatic brain injury. Various groups have shown sex differences in the induction of glial aromatase following injury. In songbirds and in mammals, inhibition of aromatase using fadrozole increases damage after traumatic brain injury in males, an effect that can be prevented with estrogen replacement (Azcoitia et al., 2001; Wynne et al., 2008). In the zebra finch, there is a greater upregulation of aromatase mRNA expression that is more rapid in female than in males after damage to the cerebellum (Mirzatoni et al., 2010). A similar female-biased sex difference in glial aromatase induction was also observed after injury to the zebra finch entopallium (Saldanha et al., 2013). There are also sex differences in aromatase induction specifically in astrocytes. Astrocytes isolated from female neonatal rat cortex have greater aromatase activity and expression than astrocytes from males. Female astrocytes are protected from cell death in response to oxygen glucose deprivation compared to males, and this sex difference is abolished by aromatase inhibition (Liu et al., 2007).
The neuroprotection in females is conferred in part by estrogens modulation of the immune response (Czlonkowska et al., 2006). In astrocytes specifically, estrogen and selective estrogen receptor modulators influence cytokine levels (Cerciat et al., 2010; Dodel et al., 1999). While not many studies have looked at sex differences in the timeline of cytokine induction after injury, in songbirds females have a greater up upregulation of IL-1β, but not IL-6, that occurs prior to the robust upregulation of aromatase that also has a female bias (Saldanha et al., 2013). Together paired with what we know about COX, PGE2, and EPs, these data suggest that males and females differ in their overall response to injury.
Aromatization-dependent effects on inflammation
There is still an unresolved paradox with respect to the immunomodulating role of estrogens. On one side, we recognize inhibition and suppression of inflammation in several animal models of chronic inflammatory diseases. On the other hand, we realize the immunosupportive role of estrogens in trauma/sepsis and the proinflammatory effects in some chronic autoimmune diseases in humans (Straub, 2007).
Estrogen Receptors.
In animals subjected to trauma and hemorrhage, a general inflammatory condition, Estrogen Receptor beta (ERβ) mRNA expression was increased, whereas Estrogen Receptor (ERα) expression was decreased (Schneider et al., 2000). These studies suggest inflammation-dependent up-regulation of ERβ relative to ERα in mammals (Straub, 2007). In zebra finches, both males and females had elevated ERα and ERβ at multiple time points following injury, but no change in G protein-coupled estrogen receptor (GPER1) was detected at any time point (Pedersen and Saldanha, 2017). Using pharmacological antagonists, the role for ERα vs ERβ was examined on neuroinflammation and response to injury. Antagonism of ERα, but not ERβ resulted in exaggerated neural PGE2 levels, suggesting that ERα mediates the anti-inflammatory action of estradiol (Pedersen and Saldanha, 2017).
Nuclear factor κB.
As stated previously, NF-κB is an important factor in proinflammatory signaling, however it can also interact with ER pathways (McKay and Cidlowski, 1999; Straub, 2007). In rodents, high levels of E2 block LPS-induced DNA binding and transcriptional activity of the p65 subunit of NF-κB by preventing its nuclear translocation (Ghisletti et al., 2005). Furthermore, ERα impairs TNF-induction of IL-6 by preventing binding of c-rel and, to a lesser extent, RelA proteins to the NF-κB site on the IL-6 promoter (Galien and Garcia, 1997; Kurebayashi et al., 1997; Straub, 2007). Following damage to the brain, transient middle cerebral artery occlusion in vivo, substantial apoptosis and inflammatory responses were observed, including IκB phosphorylation, NF-κB activation, and iNOS overexpression (Wen et al., 2004). In this model, E2 treatment (short-term pregnancy levels) produced strong protective effects by reducing infarct volume and neuronal apoptosis by inhibiting NF-κB activation and iNOS overexpression (Straub, 2007; Wen et al., 2004). Thus, high levels of E2 produced following injury inhibit NF-κB activation, reducing, therefore, the proinflammatory signaling that is activated immediately after injury.
Cytokines.
Aromatase decreases expression of TNF-α and IL-1β following injury in an E2 dependent manner (Pedersen et al., 2016). Most effects were apparent in both sexes except that E2 replacement decreased PGE2 levels in females, but its effect in males was not significant (Pedersen et al., 2016). Taken together, the data strongly suggest that injury-induced E2 synthesis is a potent modulator of neuroinflammation associated with brain damage from a penetrating brain injury in the finch brain. Interestingly, although similar in general, we found some intriguing differences in the anti-inflammatory effects of E2 across sexes. When aromatase is inhibited, females have a prolonged elevation of TNF-α and IL-1β, whereas only TNF-α remains high in males. In partial agreement, E2 administration lowers TNF-α in males and IL-1β in females but not vice versa (Pedersen et al., 2018).
Aromatization-dependent effects on degeneration.
It is very likely that injury induced astrocytic aromatization increases local levels of E2 around the injury site (Pedersen et al., 2016; 2018). We have excellent evidence supporting multiple modes of neuroprotection associated with peripheral changes in E2 including, but not limited to: apoptotic genes (D’Astous et al., 2006; Lisztwan et al., 2008; Singer et al., 1998), second messenger cascades (Mannella and Brinton, 2006; Quesada et al., 2008), neurotrophic factors (Buchanan et al., 2000; Fernandez–Galaz et al., 1997; McCarthy et al., 2002; Quesada and Micevych, 2004), mitochondrial function (Green et al., 2000; Prokai and Simpkins, 2007; Simpkins et al., 2005; Simpkins and Dykens, 2008), and inflammatory pathways (Arevalo et al., 2012; Cerciat et al., 2010). While specific evidence implicating these pathways in the role of injury-induced astrocytic provision of E2 in neuroprotection is less complete, we have good reason to believe the multiple aspects of cell-turnover are affected as consequences of astrocytic aromatization.
The majority of this work has been accomplished in songbirds and rats. In zebra finches, inhibition of injury-induced aromatase increases apoptosis relative to controls (Wynne and Saldanha, 2004). This increase in apoptosis is correlated with increased gliosis (Wynne et al., 2008) and collectively contributes to larger infarct volumes following aromatase inhibition (Saldanha et al., 2005; Wynne et al., 2008; Wynne and Saldanha, 2004). This pattern is matched well by more recent studies using global cerebral ischemia in rats where inhibition of injury induced aromatase via the administration of antisense oligonucleotides increased neuronal death relative to mis-sense probes (Zhang et al., 2014).
These exacerbations in neural damage are effectively ameliorated by concomitant E2 provision. Correspondingly aromatase inhibition with co-administered E2 decreases apoptosis, gliosis and infarct volume in zebra finches (Saldanha et al., 2005; Wynne et al., 2008). In association, E2 also increases indices of regeneration including the number of new cells around neural injury in songbirds (Walters et al., 2011). Collectively, it appears that injury-induced E2 provision promotes various indices of cellular preservation in songbirds and rodents and songbirds and contribute to the preservation and perhaps repair of damaged tissue.
A novel role for brain aromatase as a central protectant from peripheral threat
The last two decades have redirected interest in E2-dependent physiology into areas of neuroprotection and neural plasticity. As described above, multiple investigations using different species and several different models of neurotrauma have consistently reported an upregulation of aromatase, perhaps fueled by inflammatory cascades, whereby locally derived E2 is then available to modulate cellular turnover and promote neuroprotective mechanisms. We end however, with some very recent observations that perhaps even further widen our appreciation of neural aromatization as a watchful and rapidly responsive system that protects fragile neural tissue from potential damage due to signals from the periphery.
Given the potent inductive role of inflammatory cascades on neural aromatization (Duncan and Saldanha, 2011; Pedersen and Saldanha, 2017), we tested the possibility that peripheral increases in inflammation may likely modulate neural aromatase expression. Indeed, two hours post intra-peritoneal LPS administration, subjects showed decreased locomotion and elevations in the neural expression of TNFα and IL-1β, and had lost more weight as measured 24hr later. At the latter time point however, neural cytokine expression had returned to baseline, LPS-subjects were indistinguishable from controls in their locomotor activity, and peripheral injections of LPS increased aromatase enzyme activity in the brain relative to controls (Pedersen et al., 2018). These data suggest the possibility that the upregulation of aromatase observed 24hr post-LPS may be responsible for the return of neural cytokine expression to baseline and the observed decrease in sickness-like behavior, and this hypothesis is currently under investigation in our laboratory.
Of particular interest was the observation that this increase was not due to glial aromatase, but rather an increase in the number of aromatase-expressing neurons. This observation suggests the possibility that neurotrauma and peripheral infections may have cell-specific effects on aromatase expression. The nature of this difference remains to be understood. Remarkably, similar increases in neural aromatase have also been reported following inflammation during human development. Children between the years of 1 and 9 years and who suffered an inflammatory event prior to death have higher expression of aromatase in the cerebellum relative to those who did not experience an inflammatory event (Wright et al., 2019). Also in keeping with the data presented on brain injury in songbirds, the authors report a positive correlation between PGE2 and aromatase expression in this postmortem tissue. While the cellular source of these findings remains mysterious, the results point towards a tight association between inflammatory signals, both peripheral and central, in the regulation of neural aromatization. This neural aromatization appears to have evolved as a strong protectant across vertebrates.
Future Directions
The pluripotent role for central estrogens appears to now include an obligatory interaction with the innate immune system. This interaction seems to involve the normal CNS during development (Amateau and McCarthy, 2004; Garcia-Segura and McCarthy, 2004; McCarthy et al., 2002; Wright et al., 2019), but also following a range of physical insult to the brain. More broadly however, inflammatory signals can upregulate the expression of aromatase with little or no trauma to the CNS. Specifically, increases in inflammation caused by the systemic or central administration of inflammagens results in a dramatic upregulation in aromatase expression in the songbird brain (Duncan & Saldanha, 2011; Pedersen et al., 2018). Interestingly, this upregulation apparently occurs in astrocytes following central administration of PHA (Duncan & Saldanha, 2011) but is neuronal following peripheral LPS administration (Pedersen et al., 2018). This differential pattern suggests at least two novel aspects of the interaction between neuroinflammation and neurosteroidogenesis. Firstly, the cell-specific signals that result in neuronal and glial aromatase expression appear to be different. Secondly, the observed increase in neural aromatization following a peripheral threat suggests a novel sentinel and rapidly responsive aspect of neurosteroidogenesis. We hypothesize that neural aromatization may function to protect vulnerable neural circuits from impending, in addition to actual threat. In other words, the upregulation of neural aromatase following peripheral inflammation may reflect a protective mechanism where the vulnerability of the CNS is preemptively mitigated via estrogen synthesis upon detection of the peripheral threat. Neuroinflammation following peripheral activation of the immune system may provide one mechanism whereby the anti-inflammatory functions of central E2 synthesis can protect against the potential compromise of neural circuits following peripheral infection (Pedersen et al., 2018; Pedersen & Saldanha, 2018). While much work remains to be done toward understanding this complex interaction more fully, there remains little doubt that neurosteroidogenesis appears to be a powerful mechanism of neuroprotection in the vertebrate brain.
Highlights.
Estrogens are established effectors of sex-specific neural circuits.
Astrocytic aromatization is upregulated in response to a broad range of brain damage.
Estrogens synthesized by astrocytic aromatization potently inhibit neuroinflammation.
Neural aromatization may also protect the brain against peripheral inflammation and infection.
Acknowledgements
The authors thank the numerous undergraduate, graduate, postdoctoral and technical associates who have helped conduct the experiments upon which this review is framed. This work was supported by NIH NS 024767 and 085585 to CJS and the Vassar College Salmon Fund to KAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- A current view on inflammation, 2017. . Nat. Immunol 18, 825–825. 10.1038/ni.3798 [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Ascenzi M, 1990. Sexual differentiation of behavior in the zebra finch: effect of early gonadectomy or androgen treatment. Horm Behav 24, 114–127. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, 1998. Gender-linked brain injury in experimental stroke. Stroke 29, 159–65; discussion 166. 10.1161/01.str.29.1.159 [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM, 2004. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci 7, 643–650. 10.1038/nn1254 [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM, 2012. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J. Neuroendocrinol 24, 183–190. 10.1111/j.1365-2826.2011.02156.x; 10.1111/j.1365-2826.2011.02156.x [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM, 2010. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur. J. Neurosci 32, 1995–2002. 10.1111/j.1460-9568.2010.07516.x; 10.1111/j.1460-9568.2010.07516.x [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM, 2001. Brain aromatase is neuroprotective. J. Neurobiol 47, 318–329. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N, 1990. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: An immunocytochemical study. J. Comp. Neurol 301, 276–288. 10.1002/cne.903010210 [DOI] [PubMed] [Google Scholar]
- Belcredito S, Vegeto E, Brusadelli A, Ghisletti S, Mussi P, Ciana P, Maggi A, 2001. Estrogen neuroprotection: the involvement of the Bcl-2 binding protein BNIP2. Brain Res Brain Res Rev 37, 335–342. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD, 2007. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res 161, 125–141. https://doi.org/S0079-6123(06)61009-1 [pii] [DOI] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM, 2006. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine 29, 199–207. [DOI] [PubMed] [Google Scholar]
- Buchanan CD, Mahesh VB, Brann DW, 2000. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1). Biol. Reprod 62, 1710–21. 10.1095/biolreprod62.6.1710 [DOI] [PubMed] [Google Scholar]
- Burek MJ, Nordeen KW, Nordeen EJ, 1997. Sexually dimorphic neuron addition to an avian song-control region is not accounted for by sex differences in cell death. J Neurobiol 33, 61–71. [PubMed] [Google Scholar]
- Carswell HVO, Anderson NH, Morton JJ, McCulloch J, Dominiczak AF, Macrae IM, 2000. Investigation of Estrogen Status and Increased Stroke Sensitivity on Cerebral Blood Flow after a Focal Ischemic Insult. J. Cereb. Blood Flow Metab 20, 931–936. 10.1097/00004647-200006000-00005 [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM, 2005. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol 96, 89–91. [DOI] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA, 2010. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 58, 93–102. 10.1002/glia.20904; 10.1002/glia.20904 [DOI] [PubMed] [Google Scholar]
- Chen C, 2010. COX-2’s new role in inflammation. Nat. Chem. Biol 6, 401–402. 10.1038/nchembio.375 [DOI] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J, 2011. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res 2, 492–516. 10.1007/s12975-011-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Hung V, Duncan KA, 2018. Crosstalk between Estrogen Withdrawal and NFκB Signaling following Penetrating Brain Injury. Neuroimmunomodulation 25, 193–200. 10.1159/000493506 [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I, 2006. Gender Differences in Neurological Disease: Role of Estrogens and Cytokines. Endocrine 29, 243–256. 10.1385/ENDO:29:2:243 [DOI] [PubMed] [Google Scholar]
- D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T, 2006. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol 69, 1492–1498. [DOI] [PubMed] [Google Scholar]
- Davidson J, Abul H, Milton A, Rotondo D, 2001. Cytokines and cytokine inducers stimulate prostaglandin E 2 entry into the brain. Pflugers Archg. Arch. Eur. J. Physiol 442, 526–533. 10.1007/s004240100572 [DOI] [PubMed] [Google Scholar]
- Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM, 2012a. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur. J. Neurosci 35, 1218–1229. 10.1111/j.1460-9568.2012.08032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Wright CL, Hoffman JF, Wang M, Alger BE, McCarthy MM, 2012b. Prostaglandin E2 Stimulates Estradiol Synthesis in the Cerebellum Postnatally with Associated Effects on Purkinje Neuron Dendritic Arbor and Electrophysiological Properties. Endocrinology 153, 5415–5427. 10.1210/en.2012-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M, 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388, 548–554. 10.1038/41493 [DOI] [PubMed] [Google Scholar]
- Dodel RC, Du Y, Bales KR, Gao F, Paul SM, 1999. Sodium salicylate and 17beta-estradiol attenuate nuclear transcription factor NF-kappaB translocation in cultured rat astroglial cultures following exposure to amyloid A beta(1-40) and lipopolysaccharides. J Neurochem 73, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Saldanha CJ, 2011. Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J. Neuroinflammation 8, 81. 10.1186/1742-2094-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez–Galaz M., Morschl E, Chowen J., Torres–Aleman I, Naftolin F, Garcia–Segura L., 1997. Role of Astroglia and Insulin-Like Growth Factor-I in Gonadal Hormone-Dependent Synaptic Plasticity. Brain Res. Bull 44, 525–531. 10.1016/S0361-9230(97)00238-4 [DOI] [PubMed] [Google Scholar]
- Freidin M, Bennett MV, Kessler JA, 1992. Cultured sympathetic neurons synthesize and release the cytokine interleukin 1 beta. Proc. Natl. Acad. Sci. U. S. A 89, 10440–3. 10.1073/pnas.89.21.10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galien R, Garcia T, 1997. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF- B site. Nucleic Acids Res. 25, 2424–2429. 10.1093/nar/25.12.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, McCarthy MM, 2004. Minireview: Role of glia in neuroendocrine function. Endocrinology 145, 1082–1086. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB, 1999. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89, 567–578. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Simpkins JW, Yi KD, Idris AH, Minei JP, Wigginton JG, 2011. Aromatase Is Increased in Astrocytes in the Presence of Elevated Pressure. Endocrinology 152, 207–213. 10.1210/en.2010-0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E, 2005. 17 -Estradiol Inhibits Inflammatory Gene Expression by Controlling NF- B Intracellular Localization. Mol. Cell. Biol 25, 2957–2968. 10.1128/MCB.25.8.2957-2968.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K, 2008. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol 109, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Yang SH, Simpkins JW, 2000. Neuroprotective effects of phenolic A ring oestrogens. Novartis Found Symp 230, 202–220. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Konishi M, 1980. Hormone induced sexual differentiation of brain and behavior in zebra finches. Science (80-. ). 208, 1380–1382. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S, 2004. Signaling to NF-kappaB. Genes Dev 18, 2195–2224. 10.1101/gad.1228704 [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KLH, Menon DK, Pickard JD, Hutchinson PJA, 2011. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab 31, 658–70. 10.1038/jcbfm.2010.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, Okai T, 2002. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 49, 371–377. [DOI] [PubMed] [Google Scholar]
- Kalinski P, 2012. Regulation of Immune Responses by Prostaglandin E 2. J. Immunol 188, 21–28. 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, DeVoogd TJ, 1989. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J Neurosci 9, 3176–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi S, Miyashita Y, Hirose T, Kasayama S, Akira S, Kishimoto T, 1997. Characterization of mechanisms of interleukin-6 gene repression by estrogen receptor. J. Steroid Biochem. Mol. Biol 60, 11–7. [DOI] [PubMed] [Google Scholar]
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK, 2001. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol 24, 169–181. [DOI] [PubMed] [Google Scholar]
- Lisztwan J, Pornon A, Chen B, Chen S, Evans DB, 2008. The aromatase inhibitor letrozole and inhibitors of insulin-like growth factor I receptor synergistically induce apoptosis in in vitro models of estrogen-dependent breast cancer. Breast Cancer Res 10, R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ, 2007. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab 27, 135–141. [DOI] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R, 2008. Moderate and severe traumatic brain injury in adults. The Lancet.Neurology 7, 728–741. 10.1016/S1474-4422(08)70164-9 [doi] [DOI] [PubMed] [Google Scholar]
- Macdiarmid F, Wang D, Duncan LJ, Purohit A, Ghilchik MW, Reed MJ, 1994. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor. Mol. Cell. Endocrinol 106, 17–21. 10.1016/0303-7207(94)90181-3 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, 1981. Sexual differentiation of the central nervous system. Science (80-. ). 211, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E, 2004. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol 66, 291–313. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD, 2006. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci 26, 9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano PG, Eberwine JH, Ragupathi R, Saatman KE, Meaney DF, McIntosh TK, 2002. Expression profiling following traumatic brain injury: a review. Neurochem Res 27, 1147–1155. [DOI] [PubMed] [Google Scholar]
- Martínez-Chacón G, Brown KA, Docanto MM, Kumar H, Salminen S, Saarinen N, Mäkelä S, 2018. IL-10 suppresses TNF-α-induced expression of human aromatase gene in mammary adipose tissue. FASEB J. 32, 3361–3370. 10.1096/fj.201700938RRR [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Amateau SK, Mong JA, 2002. Steroid Modulation of Astrocytes in the Neonatal Brain: Implications for Adult Reproductive Function. Biol. Reprod 67, 691–698. 10.1095/biolreprod.102.003251 [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD, 2003. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci 23, 8701–8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA, 2001. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A 98, 7093–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA, 1999. Molecular Control of Immune/Inflammatory Responses: Interactions Between Nuclear Factor-κB and Steroid Receptor-Signaling Pathways. Endocr. Rev 20, 435–459. 10.1210/edrv.20.4.0375 [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A, 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science (80-. ). 278, 860–866. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L, 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol 407, 115–129. [PubMed] [Google Scholar]
- Mirzatoni A, Spence RD, Naranjo KC, Saldanha CJ, Schlinger BA, 2010. Injury-induced regulation of steroidogenic gene expression in the cerebellum. J. Neurotrauma 27, 1875–1882. 10.1089/neu.2010.1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka M, Shimodaira K, Kuwano Y, Fujikawa H, Saito H, Yanaihara T, 2000. Effect of interleukin-1beta on aromatase activity and cell proliferation in human osteoblast-like cells (HOS). Biochem Biophys Res Commun 268, 60–64. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J, 1996. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63, 149–155. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Motta M, Martini L, Celotti F, 2001. Aromatase expression and activity in male and female cultured rat hypothalamic neurons: effect of androgens. Mol. Cell. Endocrinol 178, 1–10. 10.1016/S0303-7207(01)00442-7 [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, 1988. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J Neurosci 8, 2869–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE, 2006. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26, 1234–1249. 10.1038/sj.jcbfm.9600278 [DOI] [PubMed] [Google Scholar]
- Pedersen AL, Brownrout JL, Saldanha CJ, 2018. Neuroinflammation and neurosteroidogenesis: Reciprocal modulation during injury to the adult zebra finch brain. Physiol Behav 187, 51–56. 10.1016/j.physbeh.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Pedersen AL, Nelson LH, Saldanha CJ, 2016. Centrally Synthesized Estradiol Is a Potent Anti-Inflammatory in the Injured Zebra Finch Brain. Endocrinology 157, 2041–2051. 10.1210/en.2015-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AL, Saldanha CJ, 2017. Reciprocal interactions between prostaglandin E2- and estradiol-dependent signaling pathways in the injured zebra finch brain. J Neuroinflammation 14, 262. 10.1186/s12974-017-1040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA, 2004. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol 475, 261–269. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA, 2001. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata). J. Neuroendocrinol 13, 317–323. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ, 2005. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings.Biological Sci. / R. Soc 272, 2089–2096. 10.1098/rspb.2005.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CA, Girit EZ, Kay JE, 1978. Changes in intracellular prostaglandin content during activation of lymphocytes by phytohaemagglutinin. FEBS Lett. 94, 115–9. 10.1016/0014-5793(78)80919-3 [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW, 2007. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther 114, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Newman SP, Reed MJ, 2002. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res 4, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Singh A, Ghilchik MW, Reed MJ, 1999. Inhibition of tumor necrosis factor alpha-stimulated aromatase activity by microtubule-stabilizing agents, paclitaxel and 2-methoxyestradiol. Biochem Biophys Res Commun 261, 214–217. [DOI] [PubMed] [Google Scholar]
- Quesada A, Lee BY, Micevych PE, 2008. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol 68, 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE, 2004. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res 75, 107–116. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Topping L, Coldham NG, Purohit A, Ghilchik MW, James VH, 1993. Control of aromatase activity in breast cancer cells: the role of cytokines and growth factors. J Steroid Biochem Mol Biol 44, 589–596. [DOI] [PubMed] [Google Scholar]
- Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M, 1997. Identification and characterization of an IkappaB kinase. Cell 90, 373–383. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA, 2011. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol 31, 986–1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Schrunk JM, Stormshak F, 2004. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol. Behav 83, 233–245. 10.1016/j.physbeh.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 2001. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J. Steroid Biochem. Mol. Biol 79, 247–53. [DOI] [PubMed] [Google Scholar]
- Roselli CF, 2007. Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol 106, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD, 1999. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke 30, 1665–70. 10.1161/01.str.30.8.1665 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Burstein SR, Duncan KA, 2013. Induced synthesis of estrogens by glia in the songbird brain. J. Neuroendocrinol 10.1111/jne.12067; 10.1111/jne.12067 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych PE, Schlinger BA, 1998. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm. Behav 34, 85–97. 10.1006/hbeh.1998.1447 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA, 2011. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32, 532–549. 10.1210/er.2011-0004. Epub 2011 May 26.; ID: 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD, 2005. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata). J. Neurobiol 64, 192–201. 10.1002/neu.20147 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA, 2000. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol 423, 619–630. [DOI] [PubMed] [Google Scholar]
- Sawada H, Udaka F, Izumi Y, Nishinaka K, Kawakami H, Nakamura S, Kameyama M, 2000. Cerebral white matter lesions are not associated with apoE genotype but with age and female sex in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 68, 653–656. 10.1136/jnnp.68.5.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, 1994. Frank A. Beach Award. Estrogens to song: picograms to sonograms. Horm Behav 28, 191–198. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP, 1994. Neuronal and non-neuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci 14, 7541–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP, 1993. Estrogen synthesis in vivo in the adult zebra finch: additional evidence that circulating estrogens can originate in brain. Endocrinology 133, 2610–2616. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP, 1992. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci U S A 89, 7650–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP, 1991. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci U S A 88, 4191–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH, 2000. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock 14, 347–53. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J, 1984. The postnatal demasculinization of sexual behavior in the Japanese quail (Coturnix coturnix japonica). Horm. Behav 18, 298–312. [DOI] [PubMed] [Google Scholar]
- Sébire G, Emilie D, Wallon C, Héry C, Devergne O, Delfraissy JF, Galanaud P, Tardieu M, 1993. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J. Immunol 150, 1517–23. [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP, 1995. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol 360, 172–184. [DOI] [PubMed] [Google Scholar]
- Shih RH, Wang CY, Yang CM, 2015. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci 8, 77. 10.3389/fnmol.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA, 2008. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev 57, 421–430. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA, 2005. Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord 4, 69–83. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, 1994. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15, 342–355. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM, 1998. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport 9, 2565–2568. [DOI] [PubMed] [Google Scholar]
- Singh A, Purohit A, Duncan LJ, Mokbel K, Ghilchik MW, Reed MJ, 1997. Control of aromatase activity in breast tumours: the role of the immune system. J. Steroid Biochem. Mol. Biol 61, 185–192. [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ, 2003. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol 56, 209–221. [DOI] [PubMed] [Google Scholar]
- Sorby-Adams AJ, Marcoionni AM, Dempsey ER, Woenig JA, Turner RJ, 2017. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int. J. Mol. Sci 18, 1788. 10.3390/ijms18081788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB, 1981. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature 292, 345–347. 10.1038/292345a0 [DOI] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB, 1980. Aromatization of testosterone within a discrete hypothalamic area associated with the behavioral action of androgen in the male dove. Brain Res. 192, 586–91. 10.1016/0006-8993(80)90912-9 [DOI] [PubMed] [Google Scholar]
- Stein DG, 2008. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev 57, 386–397. https://doi.org/S0165-0173(07)00112-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Kurland DB, Gerzanich V, Simard JM, 2015. Mechanisms of Astrocyte-Mediated Cerebral Edema. Neurochem. Res 40, 317–328. 10.1007/s11064-014-1374-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, 2007. The complex role of estrogens in inflammation. Endocr Rev 28, 521–574. 10.1210/er.2007-0001 [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG, 2004. Radial glia-like cells at the base of the lateral ventricles in adult mice. J Neurocytol 33, 153–164. [DOI] [PubMed] [Google Scholar]
- Thelin EP, Hall CE, Gupta K, Carpenter KLH, Chandran S, Hutchinson PJ, Patani R, Helmy A, 2018. Elucidating Pro-Inflammatory Cytokine Responses after Traumatic Brain Injury in a Human Stem Cell Model. J. Neurotrauma 35, 341–352. 10.1089/neu.2017.5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanselow J, Zsolnai A, Fesus L, Furbass R, Schwerin M, 1999. Placenta-specific transcripts of the aromatase encoding gene include different untranslated first exons in sheep and cattle. Eur. J. Biochem 265, 318–324. 10.1046/j.1432-1327.1999.00734.x [DOI] [PubMed] [Google Scholar]
- Walters BJ, Alexiades NG, Saldanha CJ, 2011. Intracerebral estrogen provision increases cytogenesis and neurogenesis in the injured zebra finch brain. Dev. Neurobiol 71, 170–181. 10.1002/dneu.20839;10.1002/dneu.20839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Kunstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong L, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TAF, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin Y-C, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backstrom N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Volker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AFA, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu W, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang S-P, Li X, Graves T, Fulton L, Nelson J, Chinwalla A, Hou S, Mardis ER, Wilson RK, 2010. The genome of a songbird. Nature 464, 757–762. 10.1038/nature08819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW, 2004. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 1008, 147–154. 10.1016/j.brainres.2004.02.019 [DOI] [PubMed] [Google Scholar]
- Wise PM, 2003. Estrogens: protective or risk factors in brain function? Prog. Neurobiol 69, 181–191. [DOI] [PubMed] [Google Scholar]
- Wright CL, Hoffman JH, McCarthy MM, 2019. Evidence that inflammation promotes estradiol synthesis in human cerebellum during early childhood. Transl. Psychiatry 9, 58. 10.1038/s41398-018-0363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne RD, Saldanha CJ, 2004. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J. Neuroendocrinol 16, 676–683. 10.1111/j.1365-2826.2004.01217.x [DOI] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ, 2008. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia 56, 97–105. 10.1002/glia.20594 [DOI] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY, 1998. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res 59, 135–142. [DOI] [PubMed] [Google Scholar]
- Yague JG, Garcia-Segura LM, Azcoitia I, 2008a. Selective transcriptional regulation of aromatase gene by vitamin D, dexamethasone, and mifepristone in human glioma cells. Endocrine. [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH, 2008b. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB, 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci 29, 72–79. 10.1016/j.tibs.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M, 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91, 243–252. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, Vadlamudi RK, Brann DW, 2014. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol Cell Endocrinol 389, 84–91. 10.1016/j.mce.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER, 1996. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol 10, 1350–1357. 10.1210/mend.10.11.8923461 [DOI] [PubMed] [Google Scholar]
- Zhong YH, Dhawan J, Kovoor JA, Sullivan J, Zhang WX, Choi D, Biegon A, 2017. Aromatase and neuroinflammation in rat focal brain ischemia. J. Steroid Biochem. Mol. Biol 174, 225–233. 10.1016/j.jsbmb.2017.09.019 [DOI] [PubMed] [Google Scholar]