Summary
Stroke is a leading cause of disability. While neurotechnology has shown promise for improving upper-limb recovery after stroke, efficacy in clinical trials has been variable. Our central thesis is that to improve clinical translation, we need to develop a common neurophysiological framework for understanding how neurotechnology alters network activity. Our perspective discusses principles for how motor networks, both healthy and those recovering from stroke, subserve reach-to-grasp movements. We focus on neural processing at the resolution of single movements, the timescale at which neurotechnologies are applied, and discuss how this activity might drive long-term plasticity. We propose that future studies should focus on cross-area communication and bridging our understanding of timescales ranging from single trials within a session to across multiple sessions. We hope that this perspective establishes a combined path forward for preclinical and clinical research with the goal of more robust clinical translation of neurotechnology.
A. Introduction
Of the ~800,000 people who have a stroke each year in the United States, a majority are left with long-term disability due to upper limb dysfunction and an inability to perform activities of daily living. This is despite current advances in upper-extremity rehabilitation (Lin et al., 2018). There is an urgent need for effective treatments.
Over the past decade, there has been substantial investment into “neurotechnology” to augment recovery of arm and hand function after stroke. This includes a diverse array of approaches such as non-invasive and invasive brain stimulation (Harvey et al., 2018; Levy et al., 2016), somatosensory nerve stimulation (Carrico et al., 2016; Conforto et al., 2010), brain-computer interface (BCI) based rehabilitation (Buch et al., 2008), and vagus nerve stimulation (Dawson et al., 2021). These various approaches have shown promise, but there is still large variability in their overall therapeutic efficacy. For example, while some clinical trials were negative, others have found evidence for overall significant benefits, but with variable effects across the population of enrolled patients (Dawson et al., 2021; Harvey et al., 2018; Kwakkel and Dobkin, 2021; Levy et al., 2016) (Table 1).
Table 1.
Selected Clinical Studies of Neurotechnology to Improve Upper Extremity Motor Function after Stroke.
| Study | N | Study design; Time after stroke; Severity levels included | Improvement | Primary Outcome Achieved# |
|---|---|---|---|---|
| Therapy | ||||
| EXCITE (Wolf et al., 2006) | 222 | RCT, constraint-induced movement (CIMT) therapy versus usual care; chronic stroke; range of severities | Wolf motor function test performance time decreased from 19.3 to 9.3 in CIMT versus 24 to 17.7 in usual care groups. Motor Activity Log (MAL) also increased more in the CIMT vs usual care. | + |
| CPASS (Dromerick et al., 2021) | 72 | RCT, 20 hours of additional (beyond usual care) task-specific motor therapy applied at either <= 30 day (acute) versus 2-3 months (subacute) versus >= 6 months (chronic) versus control (usual care); range of severities | Acute and subacute groups showed more ARAT change (~+6 points) as compared to controls while chronic group showed no difference (compared to control change). | + |
| Brain Stimulation | ||||
| NICHE (Harvey et al., 2018) | 167 | RCT, 1 Hz (low-frequency) TMS to contralesional (non-injured) motor cortex vs sham stimulation prior to therapy sessions; chronic stroke; range of severities | UE-FM improved significantly in both experimental and sham stimulation groups (~8 points). Also no differences in improvement in ARAT or Wolf motor function between groups. | − |
| (Allman et al., 2016) | 24 | 24, anodal tDCS versus sham paired with daily motor training for 9 days; chronic stroke; range of severities | UE-FM improved in both groups (~8 points, no differences between groups). ARAT and WMFT tests improved more in tDCS group compared to sham (10 versus 5 and 10 versus 3 for ARAT and WMFT respectively). | − |
| EVEREST (Levy et al., 2016) | 164 | RCT, Electrical epidural motor cortex stimulation (EECS) versus control prior to therapy; chronic stroke; moderate-severe UL deficits | Primary efficacy endpoint, defined as achieving minimum improvement of 4.5 points on UE-FM or 0.21 points of AMAT, was met by 32% in EECS vs 29% in control (no difference). | − |
| Brain Computer Interfaces | ||||
| (Ramos-Murguialday et al., 2013) | 32 | RCT, BCI- (SMR-) driven hand/arm orthosis vs random, non-contingent, movements of orthosis; chronic stroke; severe UL deficits | UE-FM scores improved 3.41 points in the BCI-contingent group and no improvement in the non-contingent group, a significant difference. | + |
| (Bhagat et al., 2020) | 10 | Single-arm study of 12 therapy sessions of BCI enabled exoskeleton for elbow training; chronic stroke, range of severities | UE-FM and ARAT scores improved by 3.92 and 5.35 points respectively. 80% of participants achieved MCID on either UE-FM or ARAT. | + |
| Nerve Stimulation | ||||
| (Conforto et al., 2010) | 22 | RCT, peripheral (median) nerve sensory stimulation at two different intensities (subsensory or suprasensory) immediately preceding motor training; subacute stroke; range of severities | Jebsen-Taylor hand scores improved in the subsensory intensity group but not in the suprasensory intensity group. | na |
| (Carrico et al., 2016) | 36 | RCT, peripheral nerve stimulation (2 hours) vs sham-stimulation followed by intensive task-oriented training (4 hours) in patients with severe; chronic stroke; severe UL deficits | There were statistically significant effects on UE-FM, WMFT, and ARAT post-intervention and at 1 month follow-up | + |
| (Dawson et al., 2021) | 108 | RCT, rehabilitation paired with VNS versus with sham stimulation; chronic stroke; moderate-severe UL deficits | UE-FM increased by 5 points in VNS group vs 2.4 points in control group. UE-FM MCID achieved in 47% (VNS) vs 24% (sham), a significant difference. | + |
UE-FM = upper extremity Fugl-Meyer, ARAT = action arm research test, AMAT = arm motor ability test, WMFT = wolf motor function test, RCT = randomized controlled trial, VNS = vagus nerve stimulation, MCID = minimally clinically important difference, BCI = brain computer interface, SMR = sensorimotor rhythm,
ongoing trial-targeted enrollment
The goal of this perspective is to propose a unified neurophysiological framework that accounts for basic and clinical research into these various approaches. We hope that a unified framework can also identify approaches to optimize neurotechnology and to significantly improve therapeutic efficacy. Such a framework is also critical for consideration of combined interventions (Coscia et al., 2019). We specifically propose that we need to understand how interventions modify neural activity patterns during task performance (i.e., the resolution at which interventions are applied) and also trigger long-term plasticity (Joy and Carmichael, 2021; Nudo et al., 1996). Moreover, because stroke is viewed as a “disconnection” syndrome (Catani and ffytche, 2005) – where cortical, subcortical and spinal networks are functionally disconnected because of loss of connectivity – we propose that characterizing and modulating the neurophysiological basis for network transmission is particularly important. We discuss how neural co-firing, especially at the level of neural ensembles, may be essential for network transmission and can be a target for modulation to improve motor function after stroke (Khanna et al., 2021; Ramanathan et al., 2018).
Anatomical and functional imaging studies have long proposed that loss of network connectivity explains clinical stroke syndromes and the potential for recovery (Catani and ffytche, 2005; Grefkes and Fink, 2014). Stroke can be anatomically classified as ‘cortical’, ‘subcortical’, or ‘mixed’ (Figure 1A,B). For example, ‘large-vessel’ stroke typically involves the anterior circulation arteries and leads to both cortical and subcortical damage. In contrast, ‘small-vessel’ subcortical strokes, which are particularly common (Figure 1C), result from occlusion of small perforator arteries and often lead to white matter damage. Observational studies have led to principles about the potential for recovery from these lesions. For instance, a cortical stroke (“lesion 1”) in the hand region of primary motor cortex (M1) leads to localized deficits in strength and dexterity of the hand with generally good functional recovery, though persistent deficits of fine dexterity may occur (Ostergard and Miller, 2019). In contrast, subcortical strokes, particularly those involving the white matter connecting multiple regions, can result in more complex phenotypes and longer lasting deficits (“lesion 2”) (Shelton and Reding, 2001). Such subcortical lesions can damage many inter-area connections, e.g., cortex-spinal cord, cortex-basal ganglia, and cortico-cortical connections. Subcortical lesions can also result in atrophy of previously connected subcortical nuclei; the degree of atrophy can also predict levels of impairment (Liew et al., 2021).
Figure 1. Vascular and structural anatomy of stroke syndromes.
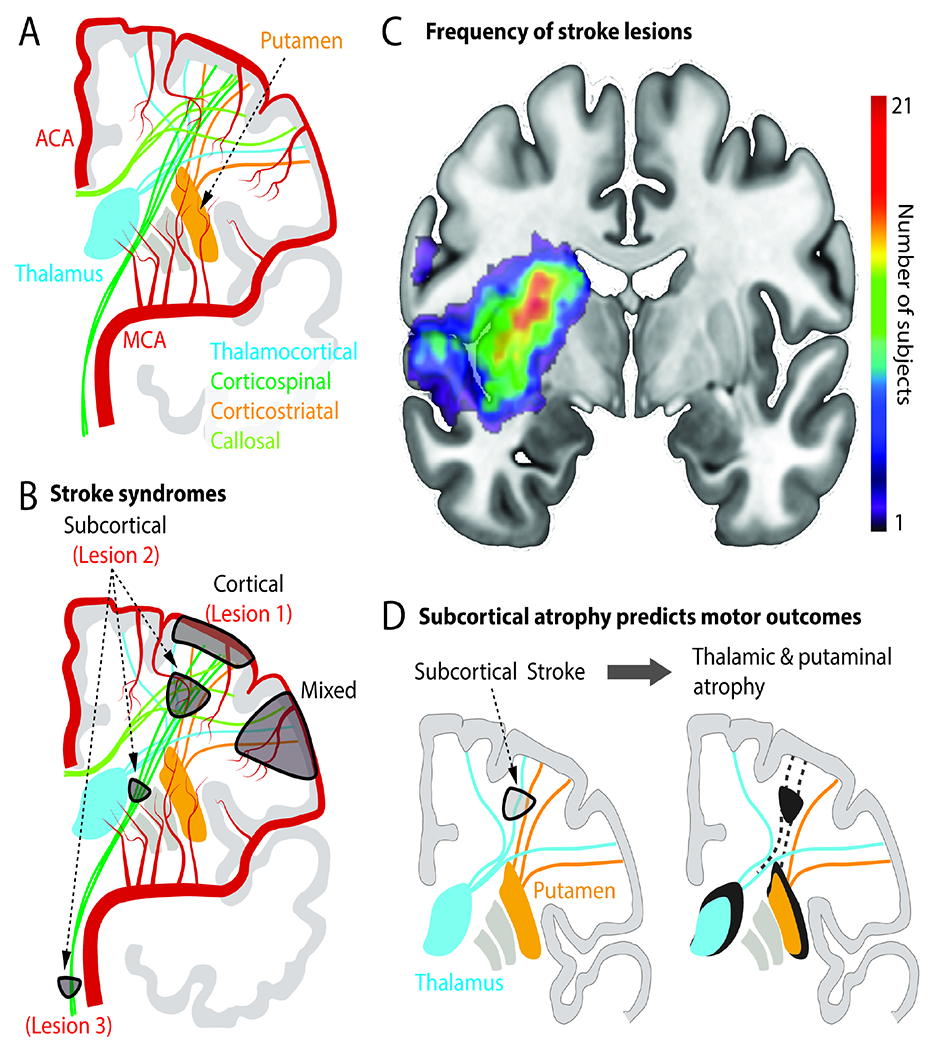
A. Illustration of the anterior vascular circulation, cortical and deep grey nuclei and connecting fibers. ACA=anterior cerebral artery; MCA=middle cerebral artery.
B. Common symptomatic stroke lesion types. Lesion 1 is pure cortical. Lesion 2 is in corona radiata (“subcortical”). Lesion 3 is the brainstem. A mixed cortical and subcortical lesion is also marked. These lesions are also referred to in Figure 2.
C. Human brain MRI with superimposed stroke “lesion density” map. Lesions previously published in (Lin et al., 2019) and (Lin et al., 2021). Colorbar illustrates the number of subjects with a lesion in that region in a cohort of n=65 patients with upper extremity deficits.
D. Atrophy in subcortical nuclei (thalamus, putamen) can emerge due to disconnection of these nuclei from cortex. Atrophy is associated with persistent motor deficits.
How may we develop a common neurophysiological and behavioral approach to understanding network disconnection and motor recovery? Given that the motor network is implicated in the transformation of the visual properties of an object to a plan for grasping it (Rizzolatti and Luppino, 2001; Schaffelhofer and Scherberger, 2016), we take the view that focusing on reach-to-grasp (RTG) of a set of objects provides a sensitive behavioral measure of network integrity. While it is certainly possible to conduct studies of isolated movements, we suggest that this would further expand the dichotomy between measures of function that assess ability to perform activities of daily living and measures of impairment, e.g., isolated assessments of single joints. While RTG actions are typically considered ‘functional measures’, they can also be used to inform levels of impairment through detailed kinematic measurements of single joints. It is important to note that apraxia, or the inability to make a skilled action despite preserved rudimentary movement control and strength, can be a confound in a functional task. However, the use of a set of simple geometric and complex objects can help with assessments of apraxia. Further, RTG measurements allow for the development of a comparative framework between animal models and human stroke patients. Thus, we take the view that rehabilitation of arm and hand function may be distilled down to a set of tasks (particularly RTG of a set of objects) that rely on activity in injured networks.
With respect to neurophysiology, we focus on local and cross-area neural activity at the resolution of single RTG actions. Reinforcement of these neural activity patterns are likely to drive the widely recognized benefits of task training during rehabilitation, such as driving activity-dependent plasticity (Cramer, 2008; Ganguly and Poo, 2013; Joy and Carmichael, 2021; Nudo et al., 1996). Importantly, neurotechnology is applied at this temporal resolution. For example, a single session of a stimulation therapy – transcranial direct current stimulation (tDCS) or vagal nerve stimulation (Allman et al., 2016; Dawson et al., 2021) – is applied concurrent with single movements. Complete characterization of the neurophysiology of single RTG movements will require multiscale measurements across species. Measures of spiking and field potentials (local field potentials/LFP or electrocorticography/ECoG) in non-human primates can be compared to ECoG and noninvasive EEG and magnetoencephalography (MEG) recordings in humans (Buzsaki et al., 2012).
We specifically focus on the consequences of damage to connectivity between cortical regions and subcortical nuclei (Guo et al., 2021; Liew et al., 2021). Although most clinical strokes involve damage to such pathways (Figure 1), their contribution has been relatively ignored in comparison to canonical descending pathways to brainstem and spinal cord such as the corticospinal tract (Darling et al., 2011; Stinear et al., 2012; Zaaimi et al., 2012). We thus discuss principles for local and cross-area neural processing in cortical, basal ganglia and thalamic networks, particularly how they might be perturbed by stroke and modulated by neurotechnology. We then discuss how this approach can also be applied to descending brainstem and spinal pathways.
B. Clinical stroke and outcome measures
Upper limb dysfunction after stroke is not simply one phenomenon, but rather a complex syndrome: loss of strength and dexterity, increases in movement variability, increased resting tone, and intrusion of ”abnormal synergies” (Krakauer and Carmichael, 2017). An abnormal flexor synergy occurs when a severely impaired stroke patient attempts to move the affected arm: the shoulder rises, the elbow contracts, and the palm faces up. In the intact nervous system, agonist and antagonist muscles are activated in a temporally precise manner during a movement (Mustard and Lee, 2004). Abnormal synergies may result from a loss of the precise regulation of the phasic timing between flexors and extensors.
No single outcome measure captures all aspects of upper limb impairment.
For example, the Fugl-Meyer, a commonly used motor outcome, largely quantifies the extent of movements that are abnormal (“in synergy”) versus normal (“out of synergy”) (Fugl-Meyer et al., 1975), but does not specifically quantify deficits in strength or dexterity. It is also clear that improvements in upper limb impairments (e.g., greater Fugl-Meyer score) do not generalize to improvements in performing daily tasks (Gordon, 1987). This has emphasized the need for measures of upper limb function that reflect activities of daily living, particularly as rehabilitation aims to improve function in daily life.
Functional measures include the Action Research Arm Test and the Wolf Motor Function Test. It is commonly believed that unlike the Fugl-Meyer, functional measures can show improvements in scores due to compensatory behaviors, e.g., compensatory changes in posture. However, it is also possible that improvements in a functional task result from restitution of lost function. For example, there may be improved joint range of motion, better regulation of across trial reliability or enhanced multi-joint coordination. Importantly, isolated measures of impairment may not capture such changes in dynamic movement control.
Kinematic measurements of impairments during a functional task – using measures of smoothness, joint range of motion, and muscle activations (Levin et al., 2019; Schwarz et al., 2019) – are important steps towards developing quantitative models of dynamic movement control. Notably, during clinical assessments, movements are usually only done once per session, ignoring trial-to-trial variability of the movement. Capturing kinematic variability across trials may be important as recovery in animal models is partially driven by regulation of neural variability (Balbinot et al., 2018; Guo et al., 2021; Ramanathan et al., 2018). Assessment of these parameters of impairment may also eventually allow links to specific damaged pathways.
C. Anatomy and physiology of recovery
C1. Anatomy
Classic lesion-function analysis built the foundation of neurology and the anatomical link between a brain “area” and symptoms. For example, damage to left-sided areas resulted in the localization of some aspects of language to Broca’s area (Dronkers et al., 2007). Broadly speaking, through neuroanatomic and neuroradiographic lesion-deficit correlation, the cortical, subcortical, and brainstem areas associated with upper limb impairments are known (Rondina et al., 2016). These are the same areas linked to RTG in non-human primates (Figure 2).
Figure 2. Distributed motor network for reach to grasp control and stroke syndromes.
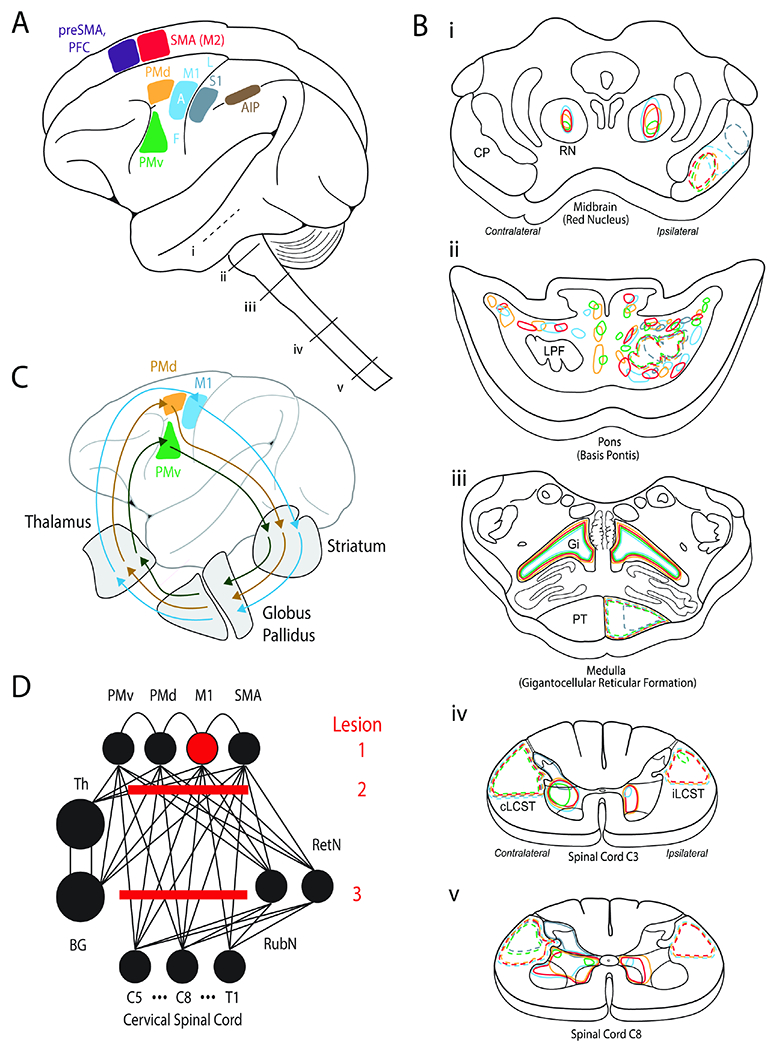
A. Cortical areas implicated in motor control. SMA=supplementary motor area or secondary motor area (M2). PFC=prefrontal cortex. PMd=dorsal premotor cortex. PMv=ventral premotor cortex.
M1=primary motor cortex. S1=primary somatosensory cortex. AIP=anterior inferior parietal. F=face. A=arm. L=leg. i-v show segments of the brainstem and spinal cord that are then illustrated in (B).
B. Expanded view of i-v segments from A. The color coding illustrates projections from cortical motor areas to each of the segments as delineated by tracer studies.
C. Parallel recurrent pathways between premotor and motor cortices to the basal ganglia and thalamus.
D. Highly connected neural network for motor control. Lesion 1 represents an isolated ‘hand knob’ cortical M1 stroke. Lesion 2 is in the corona radiata (“subcortical”), which is more common. Lesion 3 is also a common lesion in the brainstem.
Studies of the neuroanatomic basis of hemiparesis and recovery have largely focused on the corticospinal tract (CST), the major descending pathway from contralateral motor areas to spinal cord. CST anatomic integrity has been shown to: (i) relate to upper limb motor impairments (Maraka et al., 2014) (ii) predict recovery in the first three months (Lin et al., 2019) and (iii) predict motor gains with treatment (Cassidy et al., 2018). While the CST originating from M1 has been used to predict outcomes, the CST also receives contributions from premotor (both dorsal and ventral areas), and supplementary motor area (Figure 2A-B). Consistent with this, measuring loss of the CST from both M1 and ventral premotor areas can improve predictions of recovery (Ito et al.).
Alternative descending motor tracts such as the reticulospinal and rubrospinal pathways likely also play a role in recovery of upper limb function (Esposito et al., 2014; Ishida et al., 2019; Zaaimi et al., 2012) (Figure 2B, D). The reticulospinal pathways may contribute to maladaptive processes such as abnormal muscle synergies (Krakauer and Carmichael, 2017). It is worth noting that there are changes in the anatomy of brainstem rubrospinal and reticulospinal pathways when comparing rodents to non-human primates to humans (Olivares-Moreno et al., 2021). However, there may be general principles regarding the importance of brainstem nuclei in supporting motor recovery across species (Ishida et al., 2019; Zaaimi et al., 2012).
More recent attention has turned to mapping the effects of stroke on large-scale brain networks (Fox, 2018). It is well-established that stroke lesions damage both within and across hemisphere connections (Grefkes and Fink, 2014). Indeed, structural and functional connectivity approaches may serve as biomarkers to predict treatment response (Guggisberg et al., 2019). Stroke also disrupts connectivity between cortex and subcortical nuclei (Figure 1D, 2D). This is less well-appreciated but likely critical for recovery (Guo et al., 2021; Rimmele et al., 2018). For example, nigropallidal tract integrity predicts recovery of fine motor skills (Rimmele et al., 2018). Moreover, atrophy of ipsilesional thalamic and basal ganglia (putamen, nucleus accumbens) is associated with worse sensorimotor function (Liew et al., 2021). In subsequent sections we will discuss systems neuroscience studies that reveal the clinical consequences of damage to such pathways.
C2. Physiology
Early work proposed the importance of ‘cortical motor maps’ and descending pathways for recovery (Nudo et al., 1996) (Figure 3). In animal studies, a motor map is generated by intracortical microstimulation. Under anesthesia, microelectrodes deliver brief current pulses to focally stimulate cortical areas, resulting in activation of descending pathways and eliciting limb movements (Fig. 3A). The site of stimulation is then moved, and changes in evoked limb movements are noted, yielding a “motor map”. A change in motor maps thus reflects alterations in how descending inputs activate muscles. Analogously in humans, a motor map can be generated using transcranial magnetic stimulation (TMS) to focally stimulate cortical areas. The effects of stimulation can be measured using motor-evoked potentials (MEPs). A motor map can then be measured by changing sites of stimulation (Fig. 3B). MEPs are considered measures of CST integrity (Byblow et al., 2015). In both preclinical and clinical populations, successful rehabilitation has been associated with changes in the motor map, inferred to reflect an increased “cortical representation” of the rehabilitated limb (Fig. 3E–F, (Nudo et al., 1996; Wittenberg et al., 2003)). Similar changes are also observed with novel skill training (Fig. 3C-D, (Pascual-Leone et al., 1995; Plautz et al., 2000)). Importantly, the presence or absence of MEPs in distal muscles has been incorporated into predictive algorithms for recovery (Stinear et al., 2012). Together, this research highlights a clear principle: lack of descending connectivity from cortical motor areas to muscle implies poor recovery potential.
Figure 3: Motor maps and motor evoked potential.
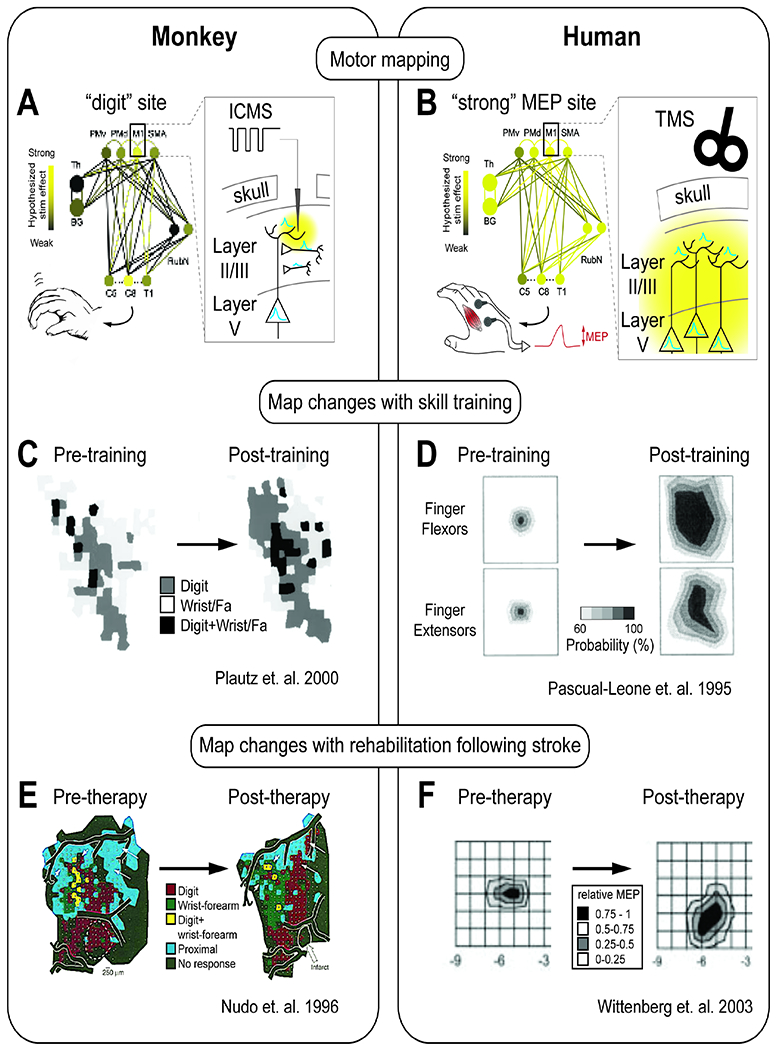
A. Intracortical microstimulation (ICMS) derived motor maps performed in preclinical studies. Microelectrodes are introduced into different cortical sites. Stimulation pulses are delivered, and observations of which limbs move are noted.
B. Transcranial magnetic stimulation (TMS) derived motor maps performed in humans. TMS coils are positioned to activate specific cortical sites. Stimulation is delivered, and muscle activity at one site is monitored to capture “motor-evoked potentials” (MEPs).
C. Training of a skilled RTG movement increases the digit, wrist, and forearm representation in motor maps (Plautz 2000).
D. Training of a skilled hand movement over 5 days increases the probability of evoking a motor potential (left = day 1, right = day 5) (Pascual-Leone 1995).
E. Following rehabilitation therapy after an infarct, an expansion of the evoked digit representation in the cortical map (Nudo, 1996)
F. Following rehabilitation therapy, an expansion of areas of cortex that can evoke an MEP (Wittenberg, 2003)
While descending connections are essential, it is also increasingly clear that motor network physiology is important. For example, in animal models, electrical stimulation can lead to rapid changes in RTG-related neural co-firing and rapid behavioral improvements (Khanna et al., 2021; Ramanathan et al., 2018). This indicates that for patients with a certain degree of intact descending connectivity, restoring neural activity patterns has a potential role in improving movement control. Fast timescale neurophysiology can be monitored at many spatial scales including EEG (~10 cm), ECoG (~1cm), or multi electrode arrays (~1 mm). There appears to be analogous signals in human subjects and animals, particularly in non-human primates (Figure 4).
Figure 4. Multiscale neural population activity during preparation and execution of movement.
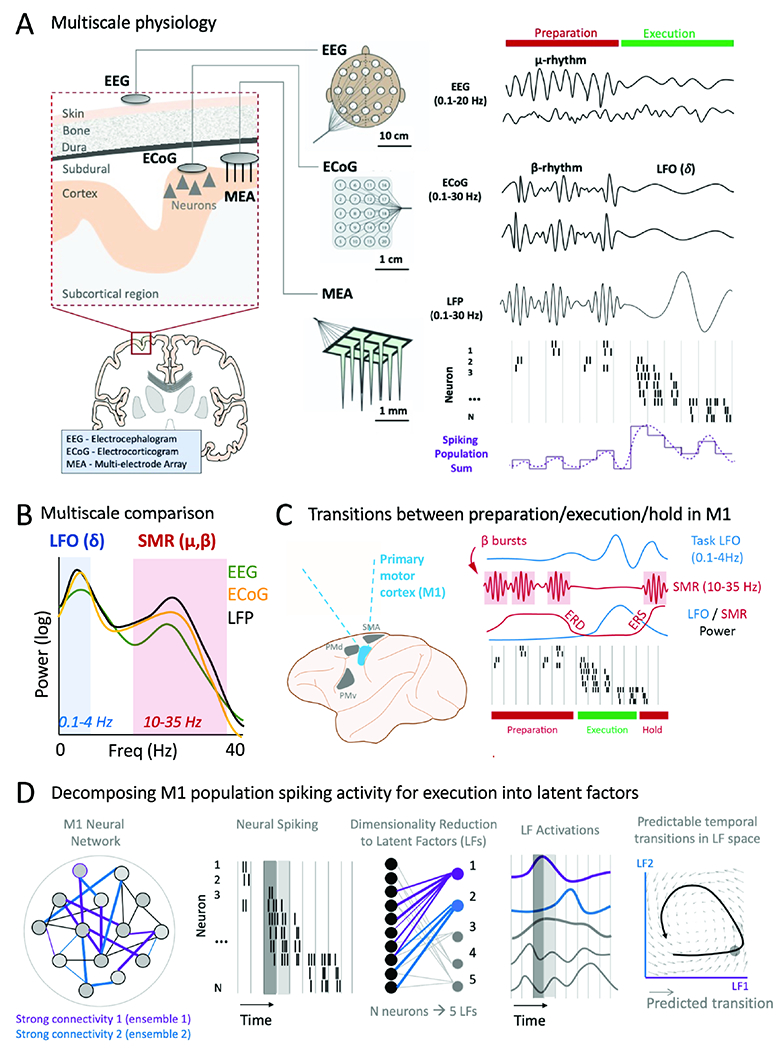
A. Multiscale recording of neural signals using EEG, ECoG and MEA during movement preparation and execution. ‘Population sum’ is simply the sum of all recording spiking activity for each time bin.
B. Power spectral density for combined movement and preparation, δ (delta) is a general reference to 0.1-4 Hz bands (synonymous with “LFO” in this review). SMR=sensorimotor rhythm and captures both the mu and beta rhythms.
C. Recordings in non-human primates during preparation, movement execution, and post-movement hold. There are sharp transitions from high β power to high LFO power; ERD (event-related desynchronization) and ERS (even-related synchronization) are associated with β power transitions.
D. Spiking activity associated with movement execution demonstrates complex spatiotemporal spiking patterns. These can be decomposed using dimensionality reduction methods into latent factors (LF). LF can be viewed as estimates of the functional connectivity (i.e., stable co-firing of patterns) within the recorded neural population. LF activation representation the activation of a given LF over time. Dark gray and light gray boxes illustrate different representations of neural population activity at two consecutive time points.
Early clinical studies focused on the sensorimotor rhythm, a neural “oscillation” in the 10-35 Hz range (β and μ, Figure 4A, B). The sensorimotor rhythm exhibits power decreases around movement onset termed ‘event-related desynchronization’. At movement end, there is increased amplitude, termed “event related synchronization” (Figure 4C). Prior studies have found links between changes in event related synchronization and desynchronization to levels of motor impairment after stroke. For example, there is a negative correlation between the extent of desynchronization and the level of upper limb motor impairment (Rossiter et al., 2014). Another study found longitudinal associations between the sensorimotor rhythm properties and longer-term motor recovery (Tang et al., 2020).
Recent studies have also found that low frequency oscillations (LFOs), a correlate of neural population spiking, can track upper limb recovery (Figure 5). LFOs represent the 0.1-4 Hz range in field potentials (Figure 4B) and are prominent during movement execution (Figure 5A, C). LFOs are closely related to the normal patterning of neural population spiking during movement and recovery (Hall et al., 2014; Lemke et al., 2019; Ramanathan et al., 2018). Interestingly, RTG-aligned LFOs in rodents, measured using field potentials and neural spiking activity, were diminished after stroke and recovery was closely correlated with their restoration (Ramanathan et al., 2018) (Figure 5A). Reaching-related LFOs, measured using ECoG, were also diminished in a human subject with chronic impairments after stroke, in contrast to two non-stroke subjects (Ramanathan et al., 2018), (Figure 5B). A subsequent report in a cohort of human subjects also found that movement-related LFOs could be measured using EEG and were a significant predictor of motor recovery in the first 3 months (Bönstrup et al., 2019) (Figure 5C).
Figure 5. Changes in task LFOs and neural variability with recovery.
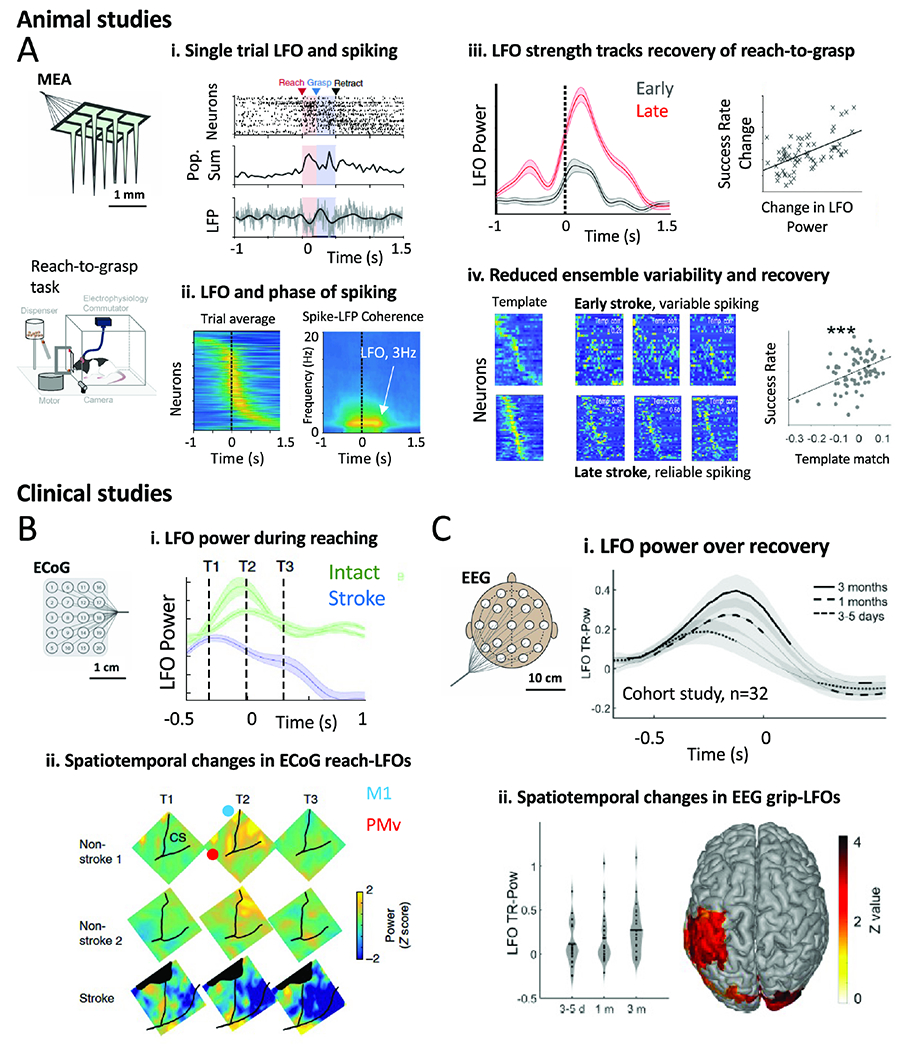
A. Rodent studies examining cortical correlates of recovery. MEAs were implanted in premotor cortex and a RTG task was used. i) Single neuron, population, and LFO neural features during a single trial of an intact animal executing a RTG movement. ii) Spike-phase coherence between neural population activity and field potential activity. iii) Change in LFO power and its relationship to behavioral recovery. (iv) Single trial neural pattern consistency (measured with a “template match” analysis) is predictive of recovery. In late, each single trial closely resembles the template; in early, there is marked variability from trial-to-trial.
B. A single stroke patient’s task-related LFO power measured with ECoG in comparison to two non-stroke subjects’ ECoG. There is reduced LFO power for movement in the stroke subject at all task timepoints. CS=central sulcus.
C. EEG based study in 32 subjects measured task-LFO strength during recovery. (i) 3 time points of measurement of LFOs (ii) increased grip-LFO power with time that correlated with recovery.
In summary, these observed changes in descending connectivity (motor maps) and neurophysiology have provided some basis for current neurotechnological approaches. However, neither changes in functional connectivity and physiology nor neurotechnological approaches are usually considered in a unified, multiscale, mechanistic framework
D. Current neurotechnology
D1. Brain stimulation
Several forms of non-invasive brain stimulation have been investigated to enhance recovery. They aim to alter short term excitability (measured using MEPs) and then facilitate activity-dependent plasticity when paired with rehabilitation. Specific effects can be dependent on stimulation parameters. For example, while low frequency repetitive TMS leads to attenuated MEPs, high frequency repetitive TMS enhances MEPs (Hummel and Cohen, 2006). Grounded in work done in smaller clinical trials (Hsu et al., 2012), the NICHE trial examined whether low frequency repetitive TMS to the non-injured M1 (when paired with rehabilitation over 6 weeks) can result in long term benefits in chronic stroke (Harvey et al., 2018). This type of stimulation is presumed to reduce excitability and thus normalize cross-hemispheric imbalances in inhibition. Unfortunately, there was no net benefit of stimulation seen in this trial.
Stimulation efficacy is usually assessed by measuring changes in TMS-evoked MEP amplitude (Figure 3). It is currently poorly understood how stimulation leads to short-term MEP changes. Moreover, there can be notable dissociations between changes in motor maps and long-term changes in performance (Nishibe et al., 2015). It is also important to note that TMS protocols were not designed to tap into particular activity patterns related to RTG; instead, they were developed to address reduced excitability around the stroke site or cross-hemispheric imbalances of excitation-inhibition (Clarkson and Carmichael, 2009). While repetitive stimulation is also widely thought to induce plasticity similar to long-term potentiation (Ziemann et al., 2008), it is unclear where in the motor network synaptic changes might occur (Figure 3 A,B). A ripe area for investigation is how and where plasticity might manifest and how this alters task activity, which is directly observable and causes movements.
Transcranial direct current stimulation (tDCS) is another form of noninvasive brain stimulation (Bolognini et al., 2011). Electrodes soaked in isotonic solution are applied and secured to the scalp over desired areas. In the case of the anode, excitability of the underlying brain tissue is believed to be increased. The advantage of tDCS is its ease of use and portability. There are multiple studies suggesting that tDCS might be beneficial as an adjunct to rehabilitation (Allman et al., 2016; Elsner et al., 2013). An ongoing clinical study is examining the effects of tDCS combined with constraint induced movement therapy (clinicaltrials.gov NCT03826030).
While tDCS is believed to cause subthreshold depolarization of neurons that also triggers plasticity (Fritsch et al., 2010), there is controversy as to whether this effect is plausible using typical current amplitudes (associated with low electrical field strengths of < 1 V/m) (Liu et al., 2018). Thus, while tDCS is promising, its mechanism of action is unclear. Optimization of intracranial electrical fields generated by tDCS could provide a path forward (Voroslakos et al., 2018). Invasive studies, where the effects of electrical fields on neural activity can be directly monitored, can also guide rational optimization of tDCS parameters (Khanna et al., 2021; Liu et al., 2018).
Invasive stimulation approaches have also been tested. Building off the success of prior pre-clinical studies (Plautz et al., 2003), the EVEREST trial tested the effects of epidural electrical stimulation (Levy et al., 2016). Epidural stimulation has the benefit of greater consistency in delivering currents. Here, 50% motor threshold stimulation at 50 Hz was delivered over a 6-week period. Unfortunately, the trial did not find a significant difference between the control and investigational groups 4 weeks after the study period. The neurophysiological target for stimulation, like TMS trials, was MEPs.
Finally, a pilot trial of cerebellar stimulation in chronic stroke patients is currently ongoing (Cooperrider et al., 2020). The study aims to modulate cortical areas using deep brain stimulation that targets the lateral cerebellar nucleus and the dentato-thalamo-cortical pathway. This approach is grounded in animal studies which have shown that such stimulation can boost cortical excitability and trigger structural plasticity (Park et al., 2015).
In summary, both noninvasive and invasive brain stimulation show promise. While clinical trials have been largely negative, there is still active research into optimization of stimulation parameters and better understanding of responders versus non-responders. A common target for current stimulation approaches is boosting cortical excitability, measured using MEP. However, the neural mechanisms underlying excitability are poorly understood, particularly how changes in excitability might impact the network activity patterns that drive RTG.
D2. Brain-computer interfaces (BCI)
Numerous studies have tested EEG based BCI neurofeedback training in stroke; patients learn strategies for volitional control of EEG signals using biofeedback. The feedback can range from disembodied signals – an abstracted relationship between EEG and cursor movements – to direct feedback using a wearable robotic system. BCI protocols have generally targeted the ipsilesional sensorimotor rhythm (Figure 4). For example, BCI therapies might instruct patients to desynchronize the sensorimotor rhythm and link that to the movement of an orthosis. Multiple pilot studies have now shown that the combined use of EEG BCI feedback and physical therapy can result in sustained improvements (Ramos-Murguialday et al., 2013). Interestingly, a recent small non-randomized study of neurofeedback using EEG low-frequency oscillations also found evidence for improvements with rehabilitation (Bhagat et al., 2020). Together, these studies suggest that BCI neurofeedback of the sensorimotor rhythm and low-frequency oscillations may be promising.
D3. Nerve Stimulation
Most post-stroke nerve stimulation techniques to enhance motor function have focused on peripheral sensory nerve stimulation or vagus nerve stimulation (VNS). Early on, it was recognized that prospects for motor recovery are reduced when there is impaired sensory function. This observation coupled with the known key role of sensory input for motor function led to efforts to modulate cortical sensory input from the paretic limb via repetitive peripheral stimulation. Small trials have shown some evidence of efficacy (Conforto et al., 2018; Conforto et al., 2010; Tu-Chan et al., 2017), especially in patients with severe hemiparesis (Carrico et al., 2016). Larger trials are needed.
Animal studies first provided evidence that VNS is safe and can enhance plasticity (Engineer et al., 2019). During rehabilitation, stimulation of the vagus nerve is timed to the end of movement repetitions, putatively reinforcing the practiced movements. Vagal nerve stimulation results in rapid activation of cholinergic (Ach) and noradrenergic (NE) systems (Engineer et al., 2019). In general, Ach neurons show phasic discharge in context of novelty or reinforcement feedback(Parikh et al., 2007). Similarly, correlated phasic discharge from neurons in the locus coeruleus (NE) predicts correct responses (Engineer et al., 2019). Together, it appears that brief bursts of ACh or NE may enhance attentional effects and improve “signal to noise”, thus facilitating the encoding of relevant task features (Sato et al., 1987). Furthermore, depletion of cortical ACh or NE can block cortical plasticity and impair learning (Engineer et al., 2019; Sato et al., 1987).
In a recent trial, after six weeks of therapy paired with VNS, participants randomized to the VNS group (n=53) had a significant increase in FMA-upper limb (5 points) compared to the control group (3.8 points) (Dawson et al., 2021). In addition, 90 days after the study was completed, a higher percentage of patients in the VNS group maintained clinically meaningful responses. It will be important to continue to define mechanisms of action and to specifically identify those patients who may benefit most (Kwakkel and Dobkin, 2021).
D4. Spinal stimulation
A growing body of literature has also suggested that spinal stimulation, which aims to boost descending inputs from supraspinal centers, is promising (Kaneshige et al., 2022; Powell et al., 2022; Zimmermann and Jackson, 2014). For example, a past study showed that after temporary inactivation of M1, closed loop stimulation of the cervical ventral spinal cord using ventral premotor cortex activity could boost EMG activity and upper limb function (Zimmermann and Jackson, 2014). More recently, epidural spinal cord stimulation has demonstrated great promise in restoring walking function in spinal cord injury patients (Wagner et al., 2018) by increasing excitability in spinal networks and making them more responsive to descending supraspinal commands. A recent pilot study presented promising initial results on spinal cord stimulation of afferent inputs for immediately improving upper limb impairment and function (Powell et al., 2022). It remains unclear precisely which descending inputs to the spine are modulated by these different approaches.
D5. Summary
Overall, various approaches have been tested to improve motor recovery after stroke. At present, it remains unclear which approach is best. While some approaches have been tested in larger phase clinical trials (Table 1), others are in early phase clinical testing. Much more research is required to fully elucidate the overall efficacy of approaches, particularly when considering the heterogeneity of stroke patients and their patterns of injury.
E. Interconnected cortical and subcortical areas underlying RTG
We now turn to studies that have characterized the local and cross-area activity patterns that drive RTG actions in intact animals. We primarily focus on anatomy and physiology in non-human primates. However, we will extensively refer to work done in rodents to establish a multispecies framework and to highlight causal manipulations.
We note that when using studies in animals to inform clinical stroke, it is important to consider the difference between short-term (e.g., optogenetics) versus permanent disruptions (e.g., lesion) (Otchy et al., 2015). Comparison of the network and behavioral consequences of temporary versus permanent lesioning can lead to different conclusions. For example, temporary disruptions might indicate that an area is key for a certain type of behavior but reveal little about the potential for recovery. Behavioral gains after lesions imply that the motor network can compensate over time (Cramer, 2008; Joy and Carmichael, 2021). Together, this suggests that the motor network has partially localized specialization (e.g., caudal bank of M1 is important for fine finger movements) but is also a highly distributed network with some degree of capacity for recovery through parallel pathways (Figure 2D).
While we will focus on descending pathways to subcortical regions, the brainstem, the spine, cortical areas and corticocortical connectivity are also important. M1 receives inputs from other areas (e.g., anterior intraparietal cortex, ventral premotor cortex, and supplementary motor area/SMA, Figure 2A). Together, this network is hypothesized to be responsible for the transformation of visual properties of an object to a plan for the appropriate grasp to execution of the grasp (Rizzolatti and Luppino, 2001; Schaffelhofer and Scherberger, 2016). RTG is often part of a larger sequence of movements. For example, in the kitchen, one will often pick up an object and then dry it with a towel. Inputs carrying knowledge of the “next action” from SMA (Mushiake et al., 1991) are likely needed to smoothly string the discrete actions together and keep track of progress throughout. Moreover, the ventral premotor area itself constitutes a motor control center with outputs to the reticular formation and cervical spinal cord (Morecraft et al., 2019). The ventral premotor area also strongly projects to M1 (Dum and Strick, 1991). Based on where it projects to the cervical spinal cord, distal control such as grasping possibly occurs through indirect connections such as corticocortical, subcortical, or intrinsic spinal circuits. We refer interested readers to excellent reviews on the contribution of the distributed cortical network for RTG (Davare et al., 2011; Rizzolatti and Luppino, 2001).
E1. Descending pathways
RTG control relies on interconnected cortical, brainstem and spinal motor areas with a somatotopic organization (Figure 2). Cortical motor areas contribute predominantly to motor control of the contralateral upper limb (Lemon, 2008), though there are ipsilateral contributions as well (~10-20% of CST fibers are uncrossed and may subserve proximal control). Premotor predominant pathways such as the corticoreticular tract provide bihemispheric input to the brainstem (Boyne et al., 2021b) (Figure 2B). Also, motor areas exhibit somatotopy, where specific sub-areas of M1, premotor cortex, and SMA control distinct parts of the body (Riehle and Vaadia, 2005). Together, this implies that the primate network is a mosaic of distinct areas, each with its own body representations and maps.
These areas give rise to parallel descending pathways, each having access to the brainstem and spinal cord (Figure 2B) (Darling et al., 2018; Kennedy et al., 1986; Lemon, 2008; McNeal et al., 2010; Morecraft et al., 2013; Morecraft et al., 2019). CST projections mostly terminate in the intermediate zone of the spinal cord, and M1 is a source of monosynaptic projections to spinal motor neurons (Porter, 1995). The observation that recovery of precise grip after training is still possible after experimental M1 lesions is likely because descending projections from multiple premotor regions can also contribute to recovery of dexterous prehension (Darling et al., 2018; Tohyama et al., 2017). Plasticity in spinal propriospinal networks has been implicated in such recovery.
Multiple studies in non-human primates have dissected the specific contributions of descending tracts. After extensive CST lesions, an initial upper limb flaccid paralysis is followed by recovery to a point where gross motor movements are possible (Lawrence and Kuypers, 1968). After a unilateral CST lesion, assessments of residual connectivity of the ipsilateral CST versus reticulospinal tract (using intra-brainstem stimulation paired with intracellular recordings) found imbalanced strengthening of reticulospinal tract to flexor but not extensor motor neurons, which mirrors the flexor posture and extensor weakness seen after stroke (Zaaimi et al., 2012). There was little apparent contribution from the ipsilateral CST. This suggests a dissociation between the cortical contributions to dexterous control, perhaps through CST strengthening, versus abnormal synergies perhaps through reticulospinal tract strengthening.
More work is required to reconcile this work highlighting parallel bihemispheric contributions to motor control with clinical studies in humans investigating hemispheric activation after stroke. For example, multiple clinical studies have found evidence that “activations” (defined by BOLD signals using fMRI) and derived patterns of connectivity between ipsilesional and contralesional cortical areas are associated with severity of deficits and recovery. One relatively robust finding is that contralesional motor areas are “overactive” after stroke and that the extent of contralesional hemisphere activation varies directly with motor deficits and injury to CST (Grefkes and Fink, 2011; Marshall et al., 2000). These findings can be interpreted as partly resulting from increased reticulospinal tract contributions from the contralesional hemisphere: cortical contributions to reticulospinal tract originate from bilateral and distributed regions of the motor network (particularly anterior) and descend to the brainstem to synapse in the pontomedullary reticular formation (Boyne et al., 2021a). Indeed, there is a line of work in humans specifically investigating contributions of the reticulospinal tract to stroke phenotypes; a residual and uninhibited reticulospinal tract may be responsible for abnormal synergistic movements (McPherson et al., 2018). Given that abnormal synergies after stroke impede functional RTG (Zackowski et al., 2004), increased cortico-cortical connectivity that enables control via the reticulospinal tract may, in fact, be detrimental. On the other hand, there is also evidence implying that the contralesional hemisphere could have a faciliatory role for recovery of motor function, particularly in patients with relatively mild deficits (Gerloff et al., 2006) and that contralesional CST strengthens after recovery from premotor/motor cortical lesions but not after the more severe frontoparietal lesions (Darling et al., 2018).
Based on our current understanding, the following model seems plausible: dexterous grasp control is mostly dependent on integrity of the ipsilesional motor network (particularly M1 and CST), while abnormal synergies, may manifest due to activity in contralesional, predominantly premotor contributions to the reticulospinal tract. Future work should consider how both hemispheres drive recovery of proximal movements versus dexterous prehension.
E2. Corticostriatal and corticothalamic pathways
Often under-appreciated in clinical stroke, the basal ganglia play an important role in RTG. Corticostriatal projections appear to be important for the generation of fast and coordinated RTG movements (Desmurget and Turner, 2010; Jin and Costa, 2015; Lemke et al., 2019). They are particularly important for automatic and vigorous transitions between submovements in a complex sequence (Jin and Costa, 2015; Park et al., 2020). Short-term inhibition of putamen disrupts the performance of a sequence of actions (Desmurget and Turner, 2010). In addition to its role in the execution of well-rehearsed movements, it also has a role in the learning of new skills, particularly for the automatization of movements (Yin et al., 2009).
Cortico-thalamic interactions (including reciprocal connections) also appear to be important for regulating cortical states prior to and during movement control (Nashef et al., 2021; Strick and Sterling, 1974). A recent study showed that silencing thalamic inputs to M1 severely disrupted cortical pattern generation and RTG behavior in mice (Sauerbrei et al., 2020). Further, theoretic work highlights that cortico-thalamo-cortical connections can quickly modify cortical dynamics, enabling the flexible production of distinct movement elements (Logiaco et al., 2021). Thus, temporally patterned inputs from thalamus appear to be essential for flexibly producing movement sequences, including RTG.
F. Physiological basis of normal RTG control
F1. Oscillations
As described above, the sensorimotor rhythm (SMR) and LFOs exhibit stereotyped responses across multiple spatial scales in the motor network during skilled execution (Figure 3A-C). Specifically, during preparation, mu (μ, 7-11 Hz) and beta (β, 10-35 Hz) frequencies, collectively called SMR, exhibit strong power that drops with movement. Higher pre-movement β power has been associated with a slower movement initiation (Khanna and Carmena, 2017), indicating that suppression of β is needed for execution. However, the power of β oscillations is also modulated by task uncertainty, cues distinct from movement periods and somatosensory feedback (Baker, 2007).
More recently, low-frequency oscillatory dynamics have emerged as a marker of active movement control following preparation. Studies in rodents, non-human primates and humans have found evidence for transient low-frequency oscillatory (LFO, 0.1-4 Hz) activity in the healthy motor cortex during skilled upper limb tasks (Hall et al., 2014; Ramanathan et al., 2018; Schalk et al., 2007). These bouts of oscillatory activity may establish the timing or sequencing of motor actions. Indeed when LFOs are recorded concurrently with population spiking activity or signals reflecting spiking, spiking exhibits bursts at the excitable phases of the LFO across rodents, non-human primates and human subjects (Hall et al., 2014; Natraj et al., 2022; Ramanathan et al., 2018), Figure 3C. As we will also discuss below, these signals may be particularly important for robust cross-area communication and be ‘condition invariant’, or independent of the specific movement being executed.
F2. Spiking in premotor and motor areas
There is a trend for longer timescale pre-movement activity in premotor and SMA areas versus a more dynamic and phasic movement-modulated activity in M1. The caudal bank of M1, with the highest density of corticomotoneuronal cells, exhibits both dynamic and tonic signals that covary with muscles during movement (Crammond and Kalaska, 2000; Shalit et al., 2012). In contrast, dorsal and ventral premotor show strong pre-movement or movement onset activity that is phasic and does not persist through the movement (Crammond and Kalaska, 2000). Given the connectivity of both premotor cortex, SMA and M1 to downstream targets, it is perhaps not surprising that “preparatory” activity is also found in reticular networks (Buford and Davidson, 2004) and the spinal cord (Prut and Fetz, 1999). One possibility is that long timescale activity in premotor areas, with their projections to red nucleus and reticular networks, is involved in predictive modulation of postural tone/reflex; for example, prior to reaching, there is consistent evidence of bilateral proximal/truncal muscle activity (Cisek et al., 2003). In contrast, caudal premotor cortex and M1 are likely to be more involved in prehension. Overall, a promising future direction would be to understand how activity patterns in bilateral networks (cortical, brainstem, cervical spine) regulate truncal versus proximal versus hand muscles. This might also reveal how activity patterns manifest in abnormal synergies after stroke.
F3. Neural population coding
Well-learned and highly stereotyped movements are associated with predictable neural ensemble responses (Georgopoulos et al., 1986; Shenoy et al., 2013). Initial studies focused on how neural responses covaried with kinematics and thus ‘represented’ movement. However, correlations with movement parameters may not reveal how each region contributes to movement control (Fetz, 1992; Shenoy et al., 2013). This is made clear by theoretical work simulating connected neural networks with multiple ‘hidden’ layers between control centers and muscle (Fetz, 1992). Such a hidden layer might resemble M1, which receives inputs and projects to brainstem and spinal cord. These studies suggest that a neuron’s response pattern reflects its input patterns and connectivity; activity patterns may or may not covary with kinematics. Together, this suggests that understanding how inputs and connectivity give rise to time-varying population patterns that drive downstream regions and eventually muscle is most important.
Without making claims about how a given area directly controls movements, we can explore how the activity of ensembles in an area vary with RTG. For stereotyped movements, M1 activity demonstrates highly stable sequential activity of neurons (Figure 3C–D, Figure 4A) (Lebedev et al., 2019; Peters et al., 2014). Such spatiotemporal activity can be represented by a few “latent factors” that define a low dimensional space (‘manifold’) (Figure 3D) (Gallego et al., 2017; Rouse and Schieber, 2018). The time varying activation of the latent factor is the latent factor activation (Fig 3D). For stereotyped behaviors, latent factor activity is temporally predictable: given a certain population neural pattern at time point t, the pattern at timepoint t + 1 is highly predictable (Churchland et al., 2012; Shenoy et al., 2013). Recent analysis has suggested that the sequential activation of neural ensembles could underlie these predictable dynamics (Lebedev et al., 2019). Thus, the reliable sequential activation of neural populations is likely to be an important motif for skilled performance.
A surprising finding is that sequential neural activity is similar across different reaching movements: i.e., “condition invariant’ (Kaufman et al., 2016). This suggests that the low-dimensional manifold and temporal patterns of population activity supporting movement control may consist of common elements used to prepare and execute movements, with small modifications to these general signals for selecting and driving each specific movement. Common elements may include drops in beta power at movement onset, LFO power increases during execution. Movement-specific elements may include the specific neural ensembles entrained to preparatory beta oscillations, the spatiotemporal details of LFOs, and the “condition-variant” component of latent factor activity.
Perhaps the common elements are part of a functional neural infrastructure needed to successfully communicate neural patterns across brain regions, as we will consider next. In the context of stroke, this offers hope that neurotechnology may not need to restore the details of each movement’s commanding neural signals, but instead could focus on approaches that restore the generalizable spatial and temporal properties of population signals.
F4. Cross-area population dynamics
Mammalian cortex has both strong local and cross-area connections. Such connectivity suggests an important role for interactions across areas. Neural oscillations such as β and LFOs could be a substrate for communication. β-oscillations are evident in the basal ganglia and thalamus as well as in cortex during preparation for movement (Figure 6A) (Cagnan et al., 2019); β-coupling across premotor-motor-parietal areas are also present during RTG (Dann et al., 2016). β oscillations are thought to be generated by rhythmic interactions between excitatory and inhibitory neurons and are hypothesized to be particularly well suited for “binding” neural ensembles across spatially distant regions. Given the role of β oscillations in maintaining working memory, and evidence of increased preparatory β power with an increased number of possible upcoming actions (Tzagarakis et al., 2010), one possible role of β oscillations is to bind together neural ensembles across the cortico-basal ganglia-thalamocortical network that encodes a particular action plan. β-rhythms may be best characterized as sparse non-synchronous spatial “bursts”. Therefore, spatiotemporally distinct ‘βs’ may simultaneously encode many possible upcoming actions (Figure 6A,B).
Figure 6. Proposed model of cross-area dynamics during preparation and movement control for daily tasks.
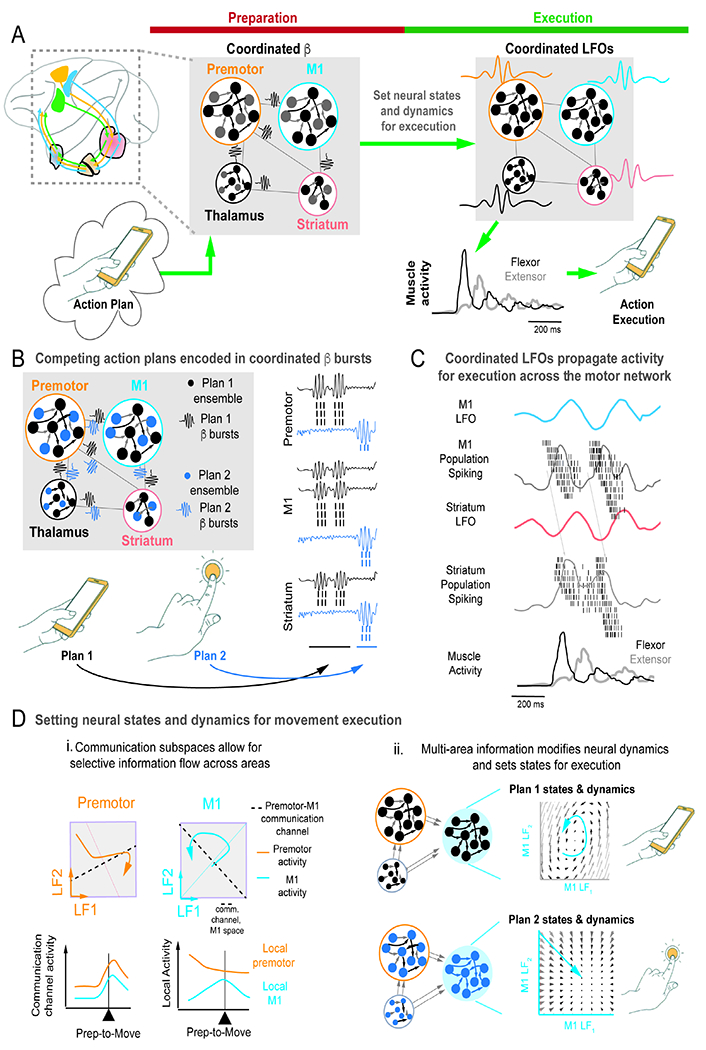
A. Proposed network-wide model of coordinated β bursts and LFOs underlying preparation and movement execution in RTG control.
B. Depicted is a proposed model of multiple potential movement plans that for actions involving different objects, and simultaneously maintained movement plans coordinated by network-wide β bursts.
C. Specific example of M1 to striatum communication facilitated by LFOs and population spiking.
D. Examples of communication channels across network nodes. (i) Communication space between premotor and M1 can coordinate components of activity. (ii) Example of premotor/thalamus setting states in M1 that allow execution of two motor plans. Each movement plan is associated with distinct temporal transitions (illustrated with distinct flow fields).
For movement execution, there is growing evidence that low-frequency activity can drive cross-area communication (Dann et al., 2016; Natraj et al., 2022)(Figure 6A,C). In rodent RTG, coherent LFOs were evident across M1, striatum and EMG (Lemke et al., 2019); pharmacological disruption of the striatum resulted in variable control and loss of LFOs in M1. There is also evidence of similar low-frequency coupling between thalamus and M1 (Gaidica et al., 2020). In humans, distributed LFOs with task-related phase-locking is evident across the human grasp network during prehension (Natraj et al., 2022). Finally, from a theoretical perspective, LFOs may create pockets of phasic excitability that synchronize ensembles, enabling more effective transmission (Hahn et al., 2019). Consistent with this idea is the finding that artificially recreating phasic excitability after stroke can improve RTG after stroke (Khanna et al., 2021). Together, this suggests that LFO coupling in cortical-BG-thalamus networks may drive reliable propagation of information during movement control (Figure 6C).
Mechanisms for inter-area communication in spiking ensembles have also been identified. Population spiking activity in two areas can influence each other via ‘subspaces’ that are defined by the axes in each area’s high dimensional space that drive activity in the second space (Figure 6D, i). Understanding what is transmitted between cortical-subcortical areas during movement control has only begun to being characterized (Semedo et al., 2019; Veuthey et al., 2020). Interestingly, recent theoretical work suggests that activity from one area can also profoundly influence the structure of the temporal predictions of population spiking activity (“neural dynamics”) in a downstream connected region (Figure 6D, ii) (Logiaco et al., 2021). This ability to change neural dynamics might be essential for the ability of the brain to flexibly execute many types of RTG behaviors in daily living.
F5. Variability, learning and population dynamics
The work reviewed above has been conducted in animals performing stereotyped and, thus likely, “feedforward” automatic movements. How do neural dynamics evolve with learning? There is growing evidence that reliable and stable ensemble patterns only emerge after achievement of skilled performance (Ganguly and Carmena, 2009; Peters et al., 2014). In contrast, there is marked variability in neural co-firing during early learning (Athalye et al., 2017). Such variability is likely a marker of neural and/or behavioral exploration to support learning and adaptation (Kondapavulur et al., 2022; Tumer and Brainard, 2007).
There is also evidence that the emergence of population dynamics requires delayed offline processing; this also highlights how task activity can drive long-term plasticity, both structural and in population dynamics. For example, stabilization of emergent population dynamics has been shown to require sleep-dependent processing (Kim et al., 2019); motor learning can also trigger synapse formation during sleep (Yang et al., 2014). Sleep-dependent reactivation of task-related awake population responses is essential for subsequent performance gains (Gulati et al., 2017; Kim et al., 2019); such reactivations appear to consolidate population activity patterns. Importantly, reactivation events are detected using latent factor templates created by using awake task activity during successful trials (Gulati et al., 2017). This strongly argues that the nervous system actively uses neural exploration to discover rewarding population patterns, and, with consolidation, an emergent neural population response emerges.
Finally, for grasping of objects (without reaching), population dynamics may not be as clearly discernable (Suresh et al., 2020). This suggests that for more complex motor actions that require feedback to execute, there may be less predictable low dimensional population responses. This may be due to a combination of greater trial-to-trial variability in a feedback-dependent task and variable somatosensory inputs driving motor networks. The presence of variability and lack of temporal predictability for both exploratory and feedback dependent behaviors further suggests that we need to consider network wide activity patterns, and not just M1, as a driver of movement control. The implications of exploratory behaviors and neural variability for recovery are discussed below.
G. Neuromodulation and RTG recovery
G1. Modulating low-frequency oscillations and movement control
As reviewed above, LFOs and associated spiking activity are linked to reliable movement control. What might be the physiological role of LFOs? Studies suggest that the presence of LFOs during execution periods are associated with stronger neural co-firing and reduced variability of spiking (Figure 5A).
Electrical stimulation that mimics the frequency content and phasic excitability of LFOs can significantly improve skilled RTG after stroke (Figure 7A–B) (Khanna et al., 2021). This study found that low-frequency electrical stimulation could significantly entrain the population of recorded neurons in the premotor perilesional cortex. The phase of entrainment was a significant predictor of behavior, such that performance was best when the RTG started at certain phases of stimulation. When examining how stimulation modified the neural patterns, it was found that the stimulation phases that improved behavior were the same that entrained neural activity and increased ensemble co-firing during RTG. Network modeling demonstrated a plausible mechanism of how population entrainment to a phase of stimulation may improve RTG. Trials that began at stimulation phases of entrainment, experienced a strengthening of the initial neural ensemble activation at the start of the RTG pattern (Figure 7A-B). This increase in strength and synchrony then enabled activity to propagate through the stroke-damaged network, possibly resulting in a more robust RTG. Based on the knowledge that LFOs are synchronized across the network, we expect that the normalization of RTG patterns extends beyond perilesional cortex and is important for observed effects (Figure 7D), i.e., phasic excitability can improve co-firing and “packet” propagation across the network.
Figure 7. Modulation of neural co-firing and neural activity propagation with low-frequency alternating current stimulation (ACS).
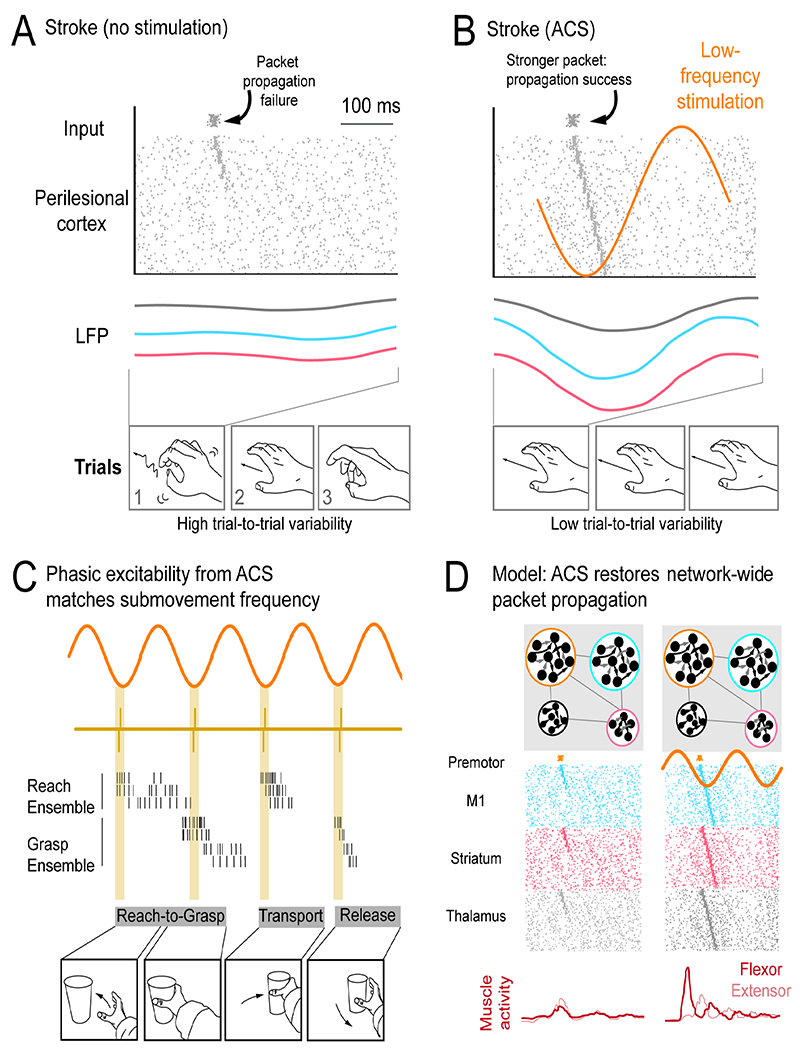
A. Failure of neural activity propagation in perilesional cortex (PLC). Poor propagation is associated with variable neural patterns and variable task performance.
B. ACS at 3Hz can boost propagation through boosting neural co-firing at phases of stimulation. Increased co-firing at the start of a RTG neural pattern can boost propagation through the weakened network.
C. ACS at ~3Hz may match sub movement rhythmicity, enabling distinct subcomponents of a longer movement to benefit from improved neural co-firing associated with ACS phase.
D. General proposal that ACS can restore phasic network excitability and allow improved patterning of muscle activity.
It is also notable that while movements initiated in alignment with the stimulation phase were associated with better performance, the grasp (which is delayed from movement start by 250-300 ms) was the specific component improved by the stimulation. Since stimulation was a 3Hz sine wave, for trials that began at the entraining phase, grasp onset was often also aligned to the stimulation phase. Thus, it may be that movement sub-components after initiation can also benefit from alignment to the entraining phase. Based on the notion that “sub-movements” of complex actions tend to occur at low frequencies (Hall et al., 2014; Lemke et al., 2019), low frequency stimulation may help organize activity patterns for multiple sub-movements during longer duration movements (Figure 7C).
Other invasive and non-invasive stimulation studies that boost motor cortical excitability may also be increasing synchronous firing and enhancing neural propagation to downstream targets. Given that changes in MEP are typically evident using these stimulation methods, presumed changes in excitation-inhibition balance could also modulate task related neural co-firing. This network-wide activation of neural co-firing might be consolidated with practice (including structural changes), yielding improvements in movement-control even after stimulation has ended.
Notably, neural populations are sensitive to the details of stimulation waveforms and frequency. Neural populations tend to fire precisely in response to “edges” (e.g., onset and offset) but lose precision with sustained non-varying inputs (Mainen and Sejnowski, 1995), e.g., direct current stimulation. Further, populations of cortical neurons have a refractory period. After a strong stimulation burst (e.g., after TMS), excitatory neurons reduce their firing rate for a period of ~50-150ms, likely due to recruitment of inhibitory networks (Li et al., 2017). Thus, stimulation delivered as direct current may not always effectively drive synchronous activity due to imprecision in spiking, nor may stimulation that is delivered at too high frequencies. Indeed, high frequency stimulation is commonly believed to interrupt pathologically synchronized activity in Parkinson’s Disease. Stimulation at low frequencies may then offer a particularly effective approach at increasing synchronous co-firing to improve cross-area propagation following stroke. Overall, this raises the importance of understanding how various waveforms and approaches interact with the complex spatiotemporal dynamics of neural populations, especially during RTG.
G2. Resting state delta and stroke
Because a growing body of literature has examined changes in resting state ‘delta’ (0.1-4 Hz) after stroke (Carmichael and Chesselet, 2002; Cassidy et al., 2020) and there are alterations in sleep architecture after stroke (NREM, slow oscillations and sleep delta, 0.1-4Hz) (Duss et al., 2017; Facchin et al., 2020; Kim et al., 2022), it is important to place task LFOs (also 0.1-4 Hz) in the context of other states. In the intact brain, delta power dominates all states, albeit at different amplitudes or power (Figure 8A). Interestingly, during both task LFOs and NREM, there is evidence of preserved ensemble activation between awake RTG and during sleep (Hall et al., 2014; Kim et al., 2019)(Figure 8B).
Figure 8. Evolution of resting, task and NREM sleep activity after stroke.
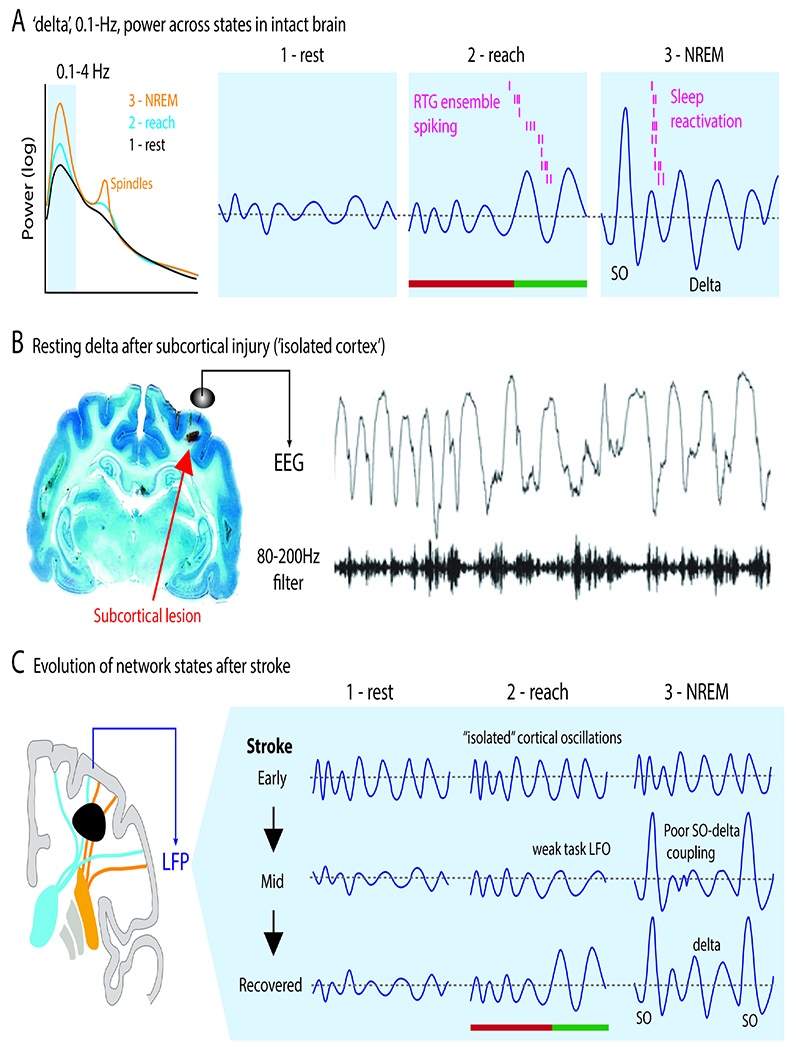
A. Activity patterns in the delta band across three network states. There is growing evidence that spiking activity linked to task LFOs is also reactivated during delta band slow-wave activity during sleep. Arrowhead indicates movement onset. Color for preparation vs execution as prior.
B. Reactivations in NREM sleep are prominent during slow oscillations (SO). Reactivations are detected using templates created – using dimensionality reduction methods, i.e., latent factors or LFs – from task related activity.
C. Increased resting state delta after a subcortical lesion that isolates cortex. This can be seen in both hemispheres.
D. Model of changes in delta patterns with recovery. Early states are associated with ‘autonomous’ delta waves that largely do not vary across states, including sleep. With late recovery, it possible for normal cortico-thalamic interactions and normal transitions between states. SO=slow oscillation.
How does resting state delta change after stroke? In the acute phase following stroke, abnormal increased resting delta is evident in patients (van Dellen et al., 2013) and in animal models (Nita et al., 2007). Longitudinal studies suggest that reductions of resting delta are associated with recovery (Cassidy et al., 2020). Peripheral neuromodulation that reduces resting delta amplitude is also associated with improved function (Tu-Chan et al., 2017). Animal studies have further shown that isolated injury to subcortical white matter is sufficient to trigger increased resting delta (Figure 8C) (Nita et al., 2007). Together, this suggests a model in which, particularly early after stroke, cortex is disconnected from subcortical structures and enters an autonomous oscillatory mode characterized by increased resting delta (Figure 8D).
Because abnormal delta persists into sleep, disordered sleep-dependent processing may account for poor functional gains with rehabilitation. Indeed, a recent study showed that “abnormal delta waves” after stroke may disrupt sleep processing (Kim et al., 2022). Abnormal delta waves appeared to reduce spindles coupling to slow oscillations; the coupling of spindles to slow oscillations is critical reactivations of emergent LFs and for memory consolidation (Kim et al., 2019). Strikingly, a recent study showed that modulation of neural firing during sleep can improve motor recovery after stroke, indicating a causal link between sleep processing and recovery (Facchin et al., 2020).
Together, this suggests that increased resting delta after stroke may be a consequence of disconnection of the cortex from subcortical areas. Persistence of delta into sleep likely disrupts sleep-dependent mechanisms of neural plasticity. Thus, neuromodulation to normalize 0.1-4 Hz patterning across states could allow a potentially unified approach for restoration of movement control, motor memory consolidation and regulation of plasticity (Figure 8D).
G3. Neuromodulators and recovery
We discussed above how VNS results in rapid activation of cholinergic and noradrenergic systems, perhaps enhancing attentional effects. Dopamine, a neuromodulator that strongly modulates behavior (Hikosaka et al., 2018), has also been studied. The dopamine system consists of midbrain synthesizing neurons that enable brain wide neuromodulation; major targets include the striatum and cortex. Studies in both animals and humans have used dopamine agonists (Gower and Tiberi, 2018). For example, an early study used a rodent RTG task to demonstrate faster recovery when a dopamine agonist was paired with training (Adkins and Jones, 2005). A study in non-human primates found that the nucleus accumbens, a major component of the striatum, is important for motor recovery after spinal cord injury (Sawada et al., 2015). Strikingly, they found that there was loss of a correlate of local population spiking in cortex with loss of the nucleus accumbens. This provides direct evidence that cross area dynamics associated with reward processing, and perhaps motivation, might directly modulate M1 and/or perilesional cortex.
There has also been clinical testing of treatment with dopamine agonists. In 2001, a single-center randomized, placebo-controlled study of 53 stroke patients found that L-DOPA could improve recovery (Scheidtmann et al., 2001). More recently, however, the DARS trial, a multicenter controlled trial, found no differences between groups for walking independence (Ford et al., 2019). There may be promise in designing future studies optimized for delivery of dopamine during single RTG trials.
H. Future Directions
In this perspective, we took a systems-neuroscience approach to recovery after stroke. We primarily focused on cortical-subcortical interactions and discussed principles for neuromodulation targeted to activity patterns during RTG. These principles can likely be extended to cortical-brainstem-spinal interactions. Below we discuss potential new research directions (Figure 9).
Figure 9. Framework of therapies.
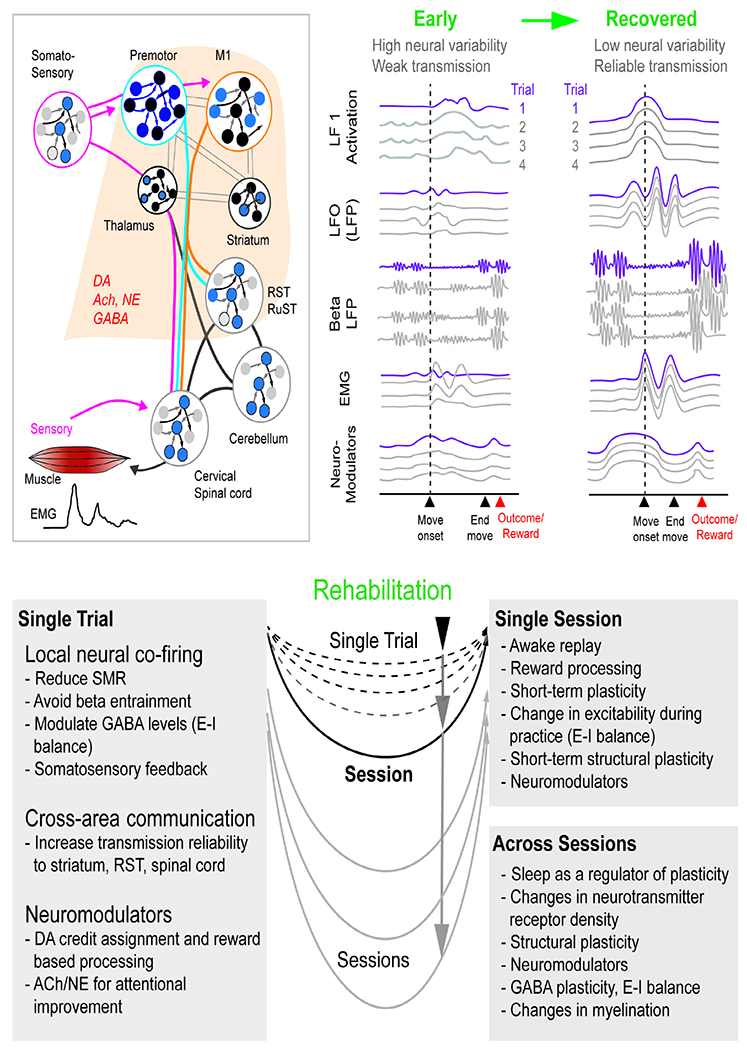
Top row illustrates circuits involved in movement control along with neuromodulators that can change circuit properties. Also shown is a model for early patterning of activity as well as a late (recovered) activity patterns. The traces highlight single-trial examples. The DA trace represent both tonic and phasic patterns. Rehabilitation is represented as a phenomenon that occurs at the level of single trials, single sessions and across sessions.
DA=dopamine, NE=norepinephrine, Ach=acetylcholine. E-I=Excitatory-Inhibitory
A common RTG framework.
We propose that focusing on RTG across species can allow the development of circuit-based outcome measures and allow a more direct mapping of circuit phenomena in non-human primates (spikes, LFP, ECoG) and human patients (ECoG, EEG). These approaches can answer fundamental questions. Which components of the distributed motor network are most critical for recovery? For a ‘functional task’ such as RTG, is there evidence of kinematic changes that imply true reinstitution of multi-joint movements (e.g., greater ability to generate smoother and coordinated RTG, changes in joint range of motion, changes in movement variability)? Does rehabilitation occur at the single trial and/or at the session level? What are the long-term effects of offline processing?
Excitability and task patterns.
Understanding how task activity relates to static measures of excitability - motor maps and MEP – will be valuable. Because stimulation induced changes in short-term excitability can be evident at a single session level (Nitsche and Paulus, 2000), it may be possible to directly link task activity and behavior. For example, it is possible that the extent of event related desynchronization or LFO peak is related to excitability. This model could also reveal insights into cell-type specific inhibition, which may then allow direct links to patterning of population activity.
Somatosensory feedback.
During RTG, proprioceptive and tactile sensory systems predict and monitor limb movements and grip forces (Johansson and Flanagan, 2009; Sobinov and Bensmaia, 2021). The anterior parietal lobe consists of somatosensory cortex, specifically Broadman areas 3a, 3b, 1, and 2, with area 2 having the strongest projections to M1 (Darian-Smith et al., 1993). Lesions that include parietal cortex may be particularly detrimental for recovery (Darling et al., 2016). The clinical parallel of this is the observation that patients with primary sensory deficits tend to have worse motor recovery (Meyer et al., 2016). Notably, repetitive peripheral stimulation can improve excitability and improve function after stroke (Conforto et al., 2010). Relatively little is known about mechanisms of action other than changes in motor maps. More recent work has suggested that sensory modulation can change resting delta as well as coupling of spiking to low frequency oscillations.
Physiological targets for neuromodulation.
Task-related ensemble co-firing (and its relation to LFOs) appear to be promising targets for brain stimulation. Understanding how sensorimotor desynchronization interacts with LFOs and neural co-firing is likely to be important for addressing deficits in preparation and execution. With respect to tDCS and transcranial alternating current stimulation, a detailed understanding of the achieved electrical field is essential to inform stimulation intensity and predict its neurophysiological effects (Liu et al., 2018). With respect to invasive stimulation, a diversity of waveforms and frequencies have been used. Understanding how specific stimulation parameters alter task activations can build a common framework.
Flexible motor control.
The neural basis of flexible motor control, particularly related to object interactions requiring feedback, is not well understood (Figure 6). We ultimately need to understand how the nervous system allows dexterous control of RTG, especially as it relates to activities of daily living where an individual has to a select among a large repertoire of actions and objects (Schaffelhofer and Scherberger, 2016; Suresh et al., 2020). Movement control related to this involves not just feedforward control, but also requires feedback from somatosensory modalities (Johansson and Flanagan, 2009; Sobinov and Bensmaia, 2021). These approaches require a departure from a majority of past and current studies in animals that predominantly focus on the neural basis of well-learned gross arm movement control and clinical trials that feature outcome measures with limited neurophysiological basis.
Linking activity to structural plasticity.
We have primarily focused on activity associated with RTG. We have also highlighted work causally linking such activity to task performance improvements and structural plasticity (Facchin et al., 2020; Joy and Carmichael, 2021; Kim et al., 2019; Yang et al., 2014). We thus take the view that rehabilitation can be partially modeled as a set of RTG tasks that rely on activity in injured networks, particularly across task and sleep states. Network activity is also known to reactivate during rest periods (Buch et al., 2021). While it is widely accepted that task activity can drive synaptic plasticity (Ganguly and Poo, 2013; Joy and Carmichael, 2021; Yin et al., 2009), determining how RTG and offline activity may precisely drive long-term structural plasticity is an important avenue for further research, particularly determining whether cortical, subcortical, brainstem, or spinal plasticity is most important.
Cross-area communication in cortex-brainstem-spine pathways.
Understanding cortical and subcortical communication with brainstem areas (red nucleus, reticular networks), spinal cord and muscles is essential. As reviewed above, spinal modulation may be effective in the presence of weak residual cortical inputs. However, it remains unclear how descending inputs to brainstem reticular networks (Esposito et al., 2014) and the spinal cord might result in abnormal synergies. It is also unknown if the goal should be to decouple inputs or to strengthen inputs in cortical and reticulospinal pathways.
Pathway specific measures and rehabilitation.
While it is well known that larger strokes and greater injury to specific motor pathways, i.e., the CST, leads to more severe deficits, upper extremity dysfunction after stroke is multifaceted and the links between aspects of hemiparesis and anatomy of particular pathways remain to be further investigated. It is possible that loss of dexterity and weakness are linked to loss of cortical inputs. The intrusion of synergies appears to be a separate phenomenon, perhaps from abnormal bihemispheric activation of reticulospinal pathways. Beyond this, it is currently difficult to map deficits to specific pathways. Mapping of structural MRI and fMRI findings from populations of stroke patients to specific deficits can suggest contributions. Alternatively, it is possible that deficits represent a disconnection of a complex network that cannot be more precisely parsed.
Computational models.
Developing sufficiently complex models of motor network function can be an important test bed to optimize neuromodulation approaches. While this is a challenging feat, monitoring activity from multiple areas simultaneously with known anatomical connectivity can allow neurophysiologically matched neural network modelling (Michaels et al., 2020). The models can also be used to explore the effects of lesioning (Michaels et al., 2020) and for discovering novel neuromodulation parameters (Khanna et al., 2021).
Neurotechnology has the great potential to improve upper limb function after stroke, but clinical outcomes are variable. Ganguly and colleagues propose a neurophysiological framework that establishes a path forward for basic and clinical research with a goal of improving the robustness of clinical translation.
Acknowledgements.
We wish to thank Richard Ivry, Harman Ghuman, Steven Massa, and Raymond Swanson for their comments and helpful discussion. This publication was supported by the National Institute of Health under Award Number R01NS117406 (K.G.), R01NS112424 (K.G.), 5K99 NS 124748 (P.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests. K.G. was previously a consultant to Cala Health. K.G. holds a patent that was filed by the UC Regents (Patent no: US 11,147,971 for closed-loop brain stimulation in stroke). A second patent application on nerve stimulation for stroke is pending.
References cited
- Adkins DL, and Jones TA (2005). D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Lett 380, 214–218. [DOI] [PubMed] [Google Scholar]
- Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, Stagg CJ, and Johansen-Berg H (2016). Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science translational medicine 8, 330re331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athalye VR, Ganguly K, Costa RM, and Carmena JM (2017). Emergence of Coordinated Neural Dynamics Underlies Neuroprosthetic Learning and Skillful Control. Neuron 93, 955–970 e955. [DOI] [PubMed] [Google Scholar]
- Baker SN (2007). Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 17, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinot G, Schuch CP, Jeffers MS, McDonald MW, Livingston-Thomas JM, and Corbett D (2018). Post-stroke kinematic analysis in rats reveals similar reaching abnormalities as humans. Scientific reports 8, 8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat NA, Yozbatiran N, Sullivan JL, Paranjape R, Losey C, Hernandez Z, Keser Z, Grossman R, Francisco GE, O’Malley MK, et al. (2020). Neural activity modulations and motor recovery following brain-exoskeleton interface mediated stroke rehabilitation. Neuroimage Clin 28, 102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, Banco E, Macea DD, Tesio L, Chessa C, et al. (2011). Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair 25, 819–829. [DOI] [PubMed] [Google Scholar]
- Bönstrup M, Krawinkel L, Schulz R, Cheng B, Feldheim J, Thomalla G, Cohen LG, and Gerloff C (2019). Low-Frequency Brain Oscillations Track Motor Recovery in Human Stroke. Ann Neurol 86, 853–865. [DOI] [PubMed] [Google Scholar]
- Boyne P, Awosika OO, and Luo Y (2021a). Mapping the corticoreticular pathway from cortex-wide anterograde axonal tracing in the mouse. J Neurosci Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne P, Difrancesco M, Awosika W, Williamson B, and Vannest J (2021b). Mapping the human corticoreticular pathway with multimodal delineation of the gigantocellular reticular nucleus and high-resolution diffusion tractography. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, Braun C, Dimyan MA, Ard T, Mellinger J, Caria A, Soekadar S, Fourkas A, et al. (2008). Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke 39, 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Claudino L, Quentin R, Bönstrup M, and Cohen LG (2021). Consolidation of human skill linked to waking hippocampo-neocortical replay. Cell reports 35, 109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford JA, and Davidson AG (2004). Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159, 284–300. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, and Koch C (2012). The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byblow WD, Stinear CM, Barber PA, Petoe MA, and Ackerley SJ (2015). Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 78, 848–859. [DOI] [PubMed] [Google Scholar]
- Cagnan H, Mallet N, Moll CKE, Gulberti A, Holt AB, Westphal M, Gerloff C, Engel AK, Hamel W, Magill PJ, et al. (2019). Temporal evolution of beta bursts in the parkinsonian cortical and basal ganglia network. Proc Natl Acad Sci U S A 116, 16095–16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, and Chesselet MF (2002). Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci 22, 6062–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Chelette KC 2nd, Westgate PM, Powell E, Nichols L, Fleischer A, and Sawaki L (2016). Nerve Stimulation Enhances Task-Oriented Training in Chronic, Severe Motor Deficit After Stroke: A Randomized Trial. Stroke 47, 1879–1884. [DOI] [PubMed] [Google Scholar]
- Cassidy JM, Tran G, Quinlan EB, and Cramer SC (2018). Neuroimaging Identifies Patients Most Likely to Respond to a Restorative Stroke Therapy. Stroke 49, 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JM, Wodeyar A, Wu J, Kaur K, Masuda AK, Srinivasan R, and Cramer SC (2020). Low-Frequency Oscillations Are a Biomarker of Injury and Recovery After Stroke. Stroke 51, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, and ffytche DH (2005). The rises and falls of disconnection syndromes. Brain 128, 2224–2239. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, and Shenoy KV (2012). Neural population dynamics during reaching. Nature 487, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, and Kalaska JF (2003). Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89, 922–942. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, and Carmichael ST (2009). Cortical excitability and post-stroke recovery. Biochem Soc Trans 37, 1412–1414. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Dos Anjos SM, Bernardo WM, Silva AAD, Conti J, Machado AG, and Cohen LG (2018). Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair 32, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SK, Baltieri SC, Scaff M, and Cohen LG (2010). Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair 24, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperrider J, Momin A, Baker KB, and Machado AG (2020). Cerebellar Neuromodulation for Stroke. Curr Phys Med Rehabil Rep 8, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscia M, Wessel MJ, Chaudary U, Millan JDR, Micera S, Guggisberg A, Vuadens P, Donoghue J, Birbaumer N, and Hummel FC (2019). Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 142, 2182–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC (2008). Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol 63, 549–560. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, and Kalaska JF (2000). Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84, 986–1005. [DOI] [PubMed] [Google Scholar]
- Dann B, Michaels JA, Schaffelhofer S, and Scherberger H (2016). Uniting functional network topology and oscillations in the fronto-parietal single unit network of behaving primates. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Darian-Smith I, Burman K, and Ratcliffe N (1993). Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J Comp Neurol 335, 200–213. [DOI] [PubMed] [Google Scholar]
- Darling WG, Ge J, Stilwell-Morecraft KS, Rotella DL, Pizzimenti MA, and Morecraft RJ (2018). Hand Motor Recovery Following Extensive Frontoparietal Cortical Injury Is Accompanied by Upregulated Corticoreticular Projections in Monkey. J Neurosci 38, 6323–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, and Morecraft RJ (2011). Functional recovery following motor cortex lesions in non-human primates: experimental implications for human stroke patients. J Integr Neurosci 10, 353–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Rotella DL, Hynes SM, Ge J, Stilwell-Morecraft K, and Morecraft RJ (2016). Sensorimotor cortex injury effects on recovery of contralesional dexterous movements in Macaca mulatta. Exp Neurol 281, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Kraskov A, Rothwell JC, and Lemon RN (2011). Interactions between areas of the cortical grasping network. Curr Opin Neurobiol 21, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, Alexander J, Ali R, Brown BL, Feng W, et al. (2021). Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 397, 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, and Turner RS (2010). Motor sequences and the basal ganglia: kinematics, not habits. J Neurosci 30, 7685–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick AW, Geed S, Barth J, Brady K, Giannetti ML, Mitchell A, Edwardson MA, Tan MT, Zhou Y, Newport EL, et al. (2021). Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, and Cabanis EA (2007). Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain 130, 1432–1441. [DOI] [PubMed] [Google Scholar]
- Dum RP, and Strick PL (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11, 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duss SB, Seiler A, Schmidt MH, Pace M, Adamantidis A, Müri RM, and Bassetti CL (2017). The role of sleep in recovery following ischemic stroke: A review of human and animal data. Neurobiol Sleep Circadian Rhythms 2, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, and Mehrholz J (2013). Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. The Cochrane database of systematic reviews 11, CD009645. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, and Hays SA (2019). Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke. Frontiers in Neuroscience 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, and Arber S (2014). Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508, 351–356. [DOI] [PubMed] [Google Scholar]
- Facchin L, Schone C, Mensen A, Bandarabadi M, Pilotto F, Saxena S, Libourel PA, Bassetti CLA, and Adamantidis AR (2020). Slow Waves Promote Sleep-Dependent Plasticity and Functional Recovery after Stroke. J Neurosci 40, 8637–8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE (1992). Are movement parameters recognizably coded in the activity of single neurons? Behavioral and Brain Sciences 15, 679–690. [Google Scholar]
- Ford GA, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, Pearn J, Ruddock S, Sackley CM, Saloniki EC, et al. (2019). Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 18, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD (2018). Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med 379, 2237–2245. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, and Lu B (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, and Steglind S (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7, 13–31. [PubMed] [Google Scholar]
- Gaidica M, Hurst A, Cyr C, and Leventhal DK (2020). Interactions Between Motor Thalamic Field Potentials and Single-Unit Spiking Are Correlated With Behavior in Rats. Frontiers in neural circuits 14, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego JA, Perich MG, Miller LE, and Solla SA (2017). Neural Manifolds for the Control of Movement. Neuron 94, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, and Carmena JM (2009). Emergence of a stable cortical map for neuroprosthetic control. Plos Biol 7, e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, and Poo MM (2013). Activity-dependent neural plasticity from bench to bedside. Neuron 80, 729–741. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, and Kettner RE (1986). Neuronal population coding of movement direction. Science 233, 1416–1419. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, et al. (2006). Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 129, 791–808. [DOI] [PubMed] [Google Scholar]
- Gordon J. (1987). Assumptions underlying physical therapy intervention: Theoretical and historical perspectives. Movement science: Foundations for physical therapy in rehabilitation, ed Carr J & Sheppard R.
- Gower A, and Tiberi M (2018). The Intersection of Central Dopamine System and Stroke: Potential Avenues Aiming at Enhancement of Motor Recovery. Frontiers in synaptic neuroscience 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, and Fink GR (2011). Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134, 1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, and Fink GR (2014). Connectivity-based approaches in stroke and recovery of function. Lancet Neurol 13, 206–216. [DOI] [PubMed] [Google Scholar]
- Guggisberg AG, Koch PJ, Hummel FC, and Buetefisch CM (2019). Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol 130, 1098–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati T, Guo L, Ramanathan DS, Bodepudi A, and Ganguly K (2017). Neural reactivations during sleep determine network credit assignment. Nat Neurosci 20, 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Kondapavulur S, Lemke SM, Won SJ, and Ganguly K (2021). Coordinated increase of reliable cortical and striatal ensemble activations during recovery after stroke. Cell reports 36, 109370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Ponce-Alvarez A, Deco G, Aertsen A, and Kumar A (2019). Portraits of communication in neuronal networks. Nat Rev Neurosci 20, 117–127. [DOI] [PubMed] [Google Scholar]
- Hall TM, de Carvalho F, and Jackson A (2014). A common structure underlies low-frequency cortical dynamics in movement, sleep, and sedation. Neuron 83, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RL, Edwards D, Dunning K, Fregni F, Stein J, Laine J, Rogers LM, Vox F, Durand-Sanchez A, Bockbrader M, et al. (2018). Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 49, 2138–2146. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Ghazizadeh A, Griggs W, and Amita H (2018). Parallel basal ganglia circuits for decision making. J Neural Transm (Vienna) 125, 515–529. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, and Lin YY (2012). Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke 43, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Hummel FC, and Cohen LG (2006). Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5, 708–712. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kobayashi K, Ueda Y, Shimizu T, Tajiri N, Isa T, and Hida H (2019). Dynamic Interaction between Cortico-Brainstem Pathways during Training-Induced Recovery in Stroke Model Rats. J Neurosci 39, 7306–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito KL, Kim B, Liu J, Soekadar SR, Winstein C, Yu C, Cramer SC, Schweighofer N, and Liew S-L Corticospinal Tract Lesion Load Originating From Both Ventral Premotor and Primary Motor Cortices Are Associated With Post-stroke Motor Severity. Neurorehabilitation and Neural Repair 0, 15459683211068441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, and Costa RM (2015). Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol 33, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, and Flanagan JR (2009). Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10, 345–359. [DOI] [PubMed] [Google Scholar]
- Joy MT, and Carmichael ST (2021). Encouraging an excitable brain state: mechanisms of brain repair in stroke. Nat Rev Neurosci 22, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M, Obara K, Suzuki M, Tazoe T, and Nishimura Y (2022). Tuning of motor outputs produced by spinal stimulation during voluntary control of torque directions in monkeys. bioRxiv, 2022.2003.2016.484577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Seely JS, Sussillo D, Ryu SI, Shenoy KV, and Churchland MM (2016). The Largest Response Component in the Motor Cortex Reflects Movement Timing but Not Movement Type. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PR, Gibson AR, and Houk JC (1986). Functional and anatomic differentiation between parvicellular and magnocellular regions of red nucleus in the monkey. Brain Res 364, 124–136. [DOI] [PubMed] [Google Scholar]
- Khanna P, and Carmena JM (2017). Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Totten D, Novik L, Roberts J, Morecraft RJ, and Ganguly K (2021). Low-frequency stimulation enhances ensemble co-firing and dexterity after stroke. Cell 184, 912–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gulati T, and Ganguly K (2019). Competing Roles of Slow Oscillations and Delta Waves in Memory Consolidation versus Forgetting. Cell 179, 514–526.e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guo L, Hishinuma A, Lemke S, Ramanathan DS, Won SJ, and Ganguly K (2022). Recovery of consolidation after sleep following stroke—interaction of slow waves, spindles, and GABA. Cell reports 38,110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapavulur S, Lemke SM, Darevsky D, Guo L, Khanna P, and Ganguly K (2022). Transition from predictable to variable motor cortex and striatal ensemble patterning during behavioral exploration. Nature communications 13, 2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, and Carmichael ST (2017). Broken Movement: The Neurobiology of Motor Recovery after Stroke (The MIT Press; ). [Google Scholar]
- Kwakkel G, and Dobkin BH (2021). Vagus Nerve Stimulation for Upper Limb Function: Significant Difference, but Clinically Important? Stroke 52, 3407–3409. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, and Kuypers HG (1968). The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91, 1–14. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Ossadtchi A, Mill NA, Urpí NA, Cervera MR, and Nicolelis MAL (2019). Analysis of neuronal ensemble activity reveals the pitfalls and shortcomings of rotation dynamics. Scientific reports 9, 18978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke SM, Ramanathan DS, Guo L, Won SJ, and Ganguly K (2019). Emergent modular neural control drives coordinated motor actions. Nat Neurosci 22, 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN (2008). Descending pathways in motor control. Annu Rev Neurosci 31, 195–218. [DOI] [PubMed] [Google Scholar]
- Levin MF, Hiengkaew V, Nilanont Y, Cheung D, Dai D, Shaw J, Bayley M, and Saposnik G (2019). Relationship Between Clinical Measures of Upper Limb Movement Quality and Activity Poststroke. Neurorehabil Neural Repair 33, 432–441. [DOI] [PubMed] [Google Scholar]
- Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, Cramer SC, and Venkatesan L (2016). Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil Neural Repair 30, 107–119. [DOI] [PubMed] [Google Scholar]
- Li B, Virtanen JP, Oeltermann A, Schwarz C, Giese MA, Ziemann U, and Benali A (2017). Lifting the veil on the dynamics of neuronal activities evoked by transcranial magnetic stimulation. eLife 6, e30552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew SL, Zavaliangos-Petropulu A, Schweighofer N, Jahanshad N, Lang CE, Lohse KR, Banaj N, Barisano G, Baugh LA, Bhattacharya AK, et al. (2021). Smaller spared subcortical nuclei are associated with worse post-stroke sensorimotor outcomes in 28 cohorts worldwide. Brain Commun 3, fcab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DJ, Cloutier AM, Erler KS, Cassidy JM, Snider SB, Ranford J, Parlman K, Giatsidis F, Burke JF, Schwamm LH, et al. (2019). Corticospinal Tract Injury Estimated From Acute Stroke Imaging Predicts Upper Extremity Motor Recovery After Stroke. Stroke, STROKEAHA119025898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DJ, Erler KS, Snider SB, Bonkhoff AK, DiCarlo JA, Lam N, Ranford J, Parlman K, Cohen A, Freeburn J, et al. (2021). Cognitive Demands Influence Upper Extremity Motor Performance During Recovery From Acute Stroke. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DJ, Finklestein SP, and Cramer SC (2018). New Directions in Treatments Targeting Stroke Recovery. Stroke 49, 3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Voroslakos M, Kronberg G, Henin S, Krause MR, Huang Y, Opitz A, Mehta A, Pack CC, Krekelberg B, et al. (2018). Immediate neurophysiological effects of transcranial electrical stimulation. Nature communications 9, 5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logiaco L, Abbott LF, and Escola S (2021). Thalamic control of cortical dynamics in a model of flexible motor sequencing. Cell reports 35, 109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, and Sejnowski TJ (1995). Reliability of spike timing in neocortical neurons. Science 268, 1503–1506. [DOI] [PubMed] [Google Scholar]
- Maraka S, Jiang Q, Jafari-Khouzani K, Li L, Malik S, Hamidian H, Zhang T, Lu M, Soltanian-Zadeh H, Chopp M, et al. (2014). Degree of corticospinal tract damage correlates with motor function after stroke. Annals of clinical and translational neurology 1, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, and DeLaPaz RL (2000). Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 31, 656–661. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, and Morecraft RJ (2010). Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol 518, 586–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JG, Chen A, Ellis MD, Yao J, Heckman CJ, and Dewald JPA (2018). Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol 596, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, De Bruyn N, Lafosse C, Van Dijk M, Michielsen M, Thijs L, Truyens V, Oostra K, Krumlinde-Sundholm L, Peeters A, et al. (2016). Somatosensory Impairments in the Upper Limb Poststroke: Distribution and Association With Motor Function and Visuospatial Neglect. Neurorehabil Neural Repair 30, 731–742. [DOI] [PubMed] [Google Scholar]
- Michaels JA, Schaffelhofer S, Agudelo-Toro A, and Scherberger H (2020). A goal-driven modular neural network predicts parietofrontal neural dynamics during grasping. Proc Natl Acad Sci U S A 117, 32124–32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, and Darling WG (2013). Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol 521, 4205–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, Rotella DL, Pizzimenti MA, and Darling WG (2019). Terminal organization of the corticospinal projection from the lateral premotor cortex to the cervical enlargement (C5-T1) in rhesus monkey. J Comp Neurol 527, 2761–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, and Tanji J (1991). Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol 66, 705–718. [DOI] [PubMed] [Google Scholar]
- Mustard B, and Lee RG (2004). Relationship between EMG patterns and kinematic properties for flexion movements at the human wrist. Exp Brain Res 66, 247–256. [DOI] [PubMed] [Google Scholar]
- Nashef A, Mitelman R, Harel R, Joshua M, and Prut Y (2021). Area-specific thalamocortical synchronization underlies the transition from motor planning to execution. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natraj N, Silversmith DB, Chang EF, and Ganguly K (2022). Compartmentalized dynamics within a common multi-area mesoscale manifold represent a repertoire of human hand movements. Neuron 110, 154–174.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe M, Urban ET 3rd, Barbay S, and Nudo RJ (2015). Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. Neurorehabil Neural Repair 29, 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita DA, Cisse Y, Timofeev I, and Steriade M (2007). Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex 17, 272–283. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, and Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, and Milliken GW (1996). Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272, 1791–1794. [DOI] [PubMed] [Google Scholar]
- Olivares-Moreno R, Rodriguez-Moreno P, Lopez-Virgen V, Macías M, Altamira-Camacho M, and Rojas-Piloni G (2021). Corticospinal vs Rubrospinal Revisited: An Evolutionary Perspective for Sensorimotor Integration. Frontiers in Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergard TA, and Miller JP (2019). Surgery for epilepsy in the primary motor cortex: A critical review. Epilepsy Behav 91, 13–19. [DOI] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, and Ölveczky BP (2015). Acute off-target effects of neural circuit manipulations. Nature 528, 358–363. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, and Sarter M (2007). Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Furmaga H, Cooperrider J, Gale JT, Baker KB, and Machado AG (2015). Modulation of Cortical Motor Evoked Potential After Stroke During Electrical Stimulation of the Lateral Cerebellar Nucleus. Brain stimulation 8, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Coddington LT, and Dudman JT (2020). Basal Ganglia Circuits for Action Specification. Annu Rev Neurosci 43, 485–507. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, and Hallett M (1995). Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, and Komiyama T (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, and Nudo RJ (2003). Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res 25, 801–810. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, and Nudo RJ (2000). Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiology of learning and memory 74, 27–55. [DOI] [PubMed] [Google Scholar]
- Porter RLR (1995). Corticospinal function and voluntary movement (Oxford; Oxford; New York: Clarendon Press ; Oxford University Press; ). [Google Scholar]
- Powell MP, Verma N, Sorensen E, Carranza E, Boos A, Fields D, Roy S, Ensel S, Barra B, Balzer J, et al. (2022). Epidural stimulation of the cervical spinal cord improves voluntary motor control in post-stroke upper limb paresis. medRxiv, 2022.2004.2011.22273635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, and Fetz EE (1999). Primate spinal interneurons show pre-movement instructed delay activity. Nature 401, 590–594. [DOI] [PubMed] [Google Scholar]
- Ramanathan DS, Guo L, Gulati T, Davidson G, Hishinuma AK, Won SJ, Knight RT, Chang EF, Swanson RA, and Ganguly K (2018). Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nature medicine 24, 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Laer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Vyziotis A, et al. (2013). Brain-machine-interface in chronic stroke rehabilitation: A controlled study. Ann Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle A, and Vaadia E (2005). Motor cortex in voluntary movements : a distributed system for distributed functions (Boca Raton: CRC Press; ). [Google Scholar]
- Rimmele DL, Frey BM, Cheng B, Schulz R, Krawinkel LA, Bönstrup M, Braass H, Gerloff C, and Thomalla G (2018). Association of Extrapyramidal Tracts’ Integrity With Performance in Fine Motor Skills After Stroke. Stroke 49, 2928–2932. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, and Luppino G (2001). The Cortical Motor System. Neuron 31, 889–901. [DOI] [PubMed] [Google Scholar]
- Rondina JM, Filippone M, Girolami M, and Ward NS (2016). Decoding post-stroke motor function from structural brain imaging. Neuroimage Clin 12, 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Boudrias MH, and Ward NS (2014). Do movement-related beta oscillations change after stroke? J Neurophysiol 112, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse AG, and Schieber MH (2018). Condition-Dependent Neural Dimensions Progressively Shift during Reach to Grasp. Cell reports 25, 3158–3168.e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hata Y, Masui H, and Tsumoto T (1987). A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol 58, 765–780. [DOI] [PubMed] [Google Scholar]
- Sauerbrei BA, Guo JZ, Cohen JD, Mischiati M, Guo W, Kabra M, Verma N, Mensh B, Branson K, and Hantman AW (2020). Cortical pattern generation during dexterous movement is input-driven. Nature 577, 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, and Nishimura Y (2015). Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science 350, 98–101. [DOI] [PubMed] [Google Scholar]
- Schaffelhofer S, and Scherberger H (2016). Object vision to hand action in macaque parietal, premotor, and motor cortices. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk G, Kubanek J, Miller KJ, Anderson NR, Leuthardt EC, Ojemann JG, Limbrick D, Moran D, Gerhardt LA, and Wolpaw JR (2007). Decoding two-dimensional movement trajectories using electrocorticographic signals in humans. J Neural Eng 4, 264–275. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K, Fries W, Muller F, and Koenig E (2001). Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 358, 787–790. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Kanzler CM, Lambercy O, Luft AR, and Veerbeek JM (2019). Systematic Review on Kinematic Assessments of Upper Limb Movements After Stroke. Stroke 50, 718–727. [DOI] [PubMed] [Google Scholar]
- Semedo JD, Zandvakili A, Machens CK, Yu BM, and Kohn A (2019). Cortical Areas Interact through a Communication Subspace. Neuron 102, 249–259.e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit U, Zinger N, Joshua M, and Prut Y (2012). Descending systems translate transient cortical commands into a sustained muscle activation signal. Cereb Cortex 22, 1904–1914. [DOI] [PubMed] [Google Scholar]
- Shelton FN, and Reding MJ (2001). Effect of lesion location on upper limb motor recovery after stroke. Stroke 32, 107–112. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, and Churchland MM (2013). Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci 36, 337–359. [DOI] [PubMed] [Google Scholar]
- Sobinov AR, and Bensmaia SJ (2021). The neural mechanisms of manual dexterity. Nat Rev Neurosci 22, 741–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Petoe M, Anwar S, and Byblow WD (2012). The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 135, 2527–2535. [DOI] [PubMed] [Google Scholar]
- Strick PL, and Sterling P (1974). Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J Comp Neurol 153, 77–106. [DOI] [PubMed] [Google Scholar]
- Suresh AK, Goodman JM, Okorokova EV, Kaufman M, Hatsopoulos NG, and Bensmaia SJ (2020). Neural population dynamics in motor cortex are different for reach and grasp. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CW, Hsiao FJ, Lee PL, Tsai YA, Hsu YF, Chen WT, Lin YY, Stagg CJ, and Lee IH (2020). β-Oscillations Reflect Recovery of the Paretic Upper Limb in Subacute Stroke. Neurorehabil Neural Repair 34, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama T, Kinoshita M, Kobayashi K, Isa K, Watanabe D, Kobayashi K, Liu M, and Isa T (2017). Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proc Natl Acad Sci U S A 114, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu-Chan AP, Natraj N, Godlove J, Abrams G, and Ganguly K (2017). Effects of somatosensory electrical stimulation on motor function and cortical oscillations. J Neuroeng Rehabil 14, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer EC, and Brainard MS (2007). Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature 450, 1240–1244. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, and Pellizzer G (2010). Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30, 11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen E, Hillebrand A, Douw L, Heimans JJ, Reijneveld JC, and Stam CJ (2013). Local polymorphic delta activity in cortical lesions causes global decreases in functional connectivity. Neuroimage 83, 524–532. [DOI] [PubMed] [Google Scholar]
- Veuthey TL, Derosier K, Kondapavulur S, and Ganguly K (2020). Single-trial cross-area neural population dynamics during long-term skill learning. Nature communications 11, 4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voroslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernandez-Ruiz A, Kozak G, Kineses ZT, Ivanyi B, Buzsaki G, et al. (2018). Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature communications 9, 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FB, Mignardot J-B, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seáñez I, Caban M, Pirondini E, et al. (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Taub E, Gerber LH, Hallett M, and Cohen LG (2003). Constraint-Induced Therapy in Stroke: Magnetic-Stimulation Motor Maps and Cerebral Activation. Neurorehabilitation and Neural Repair 17, 48–57. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, and Nichols-Larsen D (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama 296, 2095–2104. [DOI] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, and Gan WB (2014). Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, and Costa RM (2009). Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, and Baker SN (2012). Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135, 2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, and Bastian AJ (2004). How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain 127, 1035–1046. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, and Rothwell JC (2008). Consensus: Motor cortex plasticity protocols. Brain stimulation 1, 164–182. [DOI] [PubMed] [Google Scholar]
- Zimmermann JB, and Jackson A (2014). Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Frontiers in neuroscience 8, 87–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


