Abstract
Epithelioid hemangioendothelioma is considered an uncommon tumor originating from vascular tissues. Although this disease is an extremely rare malignant cancer, its pleural subtype is even less common. We discuss a 68‐year‐old man with isolated pleural epithelioid hemangioendothelioma, along with a literature review of all similar cases.
Keywords: case report, epithelioid hemangioendothelioma, pleura, review
Primary malignantepithelioid hemangioendothelioma of the pleura in a 68‐year‐old male. A hypermetabolic nodule in the right lower lobe, adjacent to oblique right fissure with SUV of 4.39 at maximum and hypermetabolic primary tumoral involvement in the right parietal and mediastinal pleura, along with malignant right effusion were noted. Diffuse irregular pleural thickening is noted on the Right side, showing increased FDG activity (SUVmax: up to 7.98).

1. INTRODUCTION
Epithelioid hemangioendothelioma (EH) is considered an uncommon tumor originating from vascular tissue with intermediate behavior of malignancy originating from various sites such as the brain, meninges, bone, gastrointestinal tract, liver, skin, breast, testis, and mediastinum. 1 , 2 , 3 Although this tumor can affect various tissues, EH originating from the pleural is less described and believed to act more aggressively in its clinical course. 1 , 4 Here, we report a case of primary malignant EH of the pleura and a literature review.
2. CASE PRESENTATION
A 68‐year‐old man was referred to our clinic for pleuritic chest pain and weight loss (about 3 kg in 4 months). He was referred to our hematology clinic, located in Shiraz, Iran, and affiliated with Shiraz University of Medical Sciences. The patient had no past medical history of smoking or exposure to asbestos. Physical examination, laboratory findings, and abdominal pelvic sonography were not decisive for diagnosis. Chest x‐ray (CXR) revealed a thick wall cavity on the right side.
A chest computerized tomography (CT) revealed two calcified nodules on the left side of the chest, including a 5.5 mm‐sized and a 4 mm‐sized in the left lung apex. There was an enhanced irregular thickening of the right parietal pleura with severe pleural effusion and the passive collapse of the right lower lobe and parts of the right upper and middle lobe (Figure 1).
FIGURE 1.
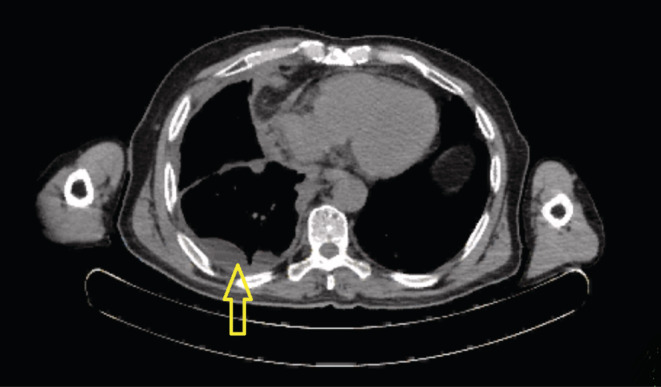
Chest computerized tomography in favor of left‐sided pleural effusion and mass (demonstrated with yellow arrow)
The patient underwent diagnostic thoracentesis that showed a total count: of 540/μl, Red blood cell (RBC): 380/μl, Neutrophil 2/μl, Lymphocyte 158/μl, Sugar fluid: 125 mg/dl, Protein fluid 4.2 gr/dl, Lactate dehydrogenase (LDH) 323 IU/L Adenosine deaminase (ADA) fluid: 5.0 U/L (all in the normal range) and negative bacteriology. Also, right pleural fluid tap cytology was negative for malignant cells.
The patient underwent thoracoscopy, and a biopsy of the pleura was taken. Grossly, the specimen consisted of a resected lung tissue measuring 4.0 × 3.0 × 2.0 cm in maximal dimension. Microscopic description showed numerous ill‐defined and coalescing nodules composed of hypercellular epithelioid cell proliferation with uniform nuclei and prominent nuclei, showing frequent intracytoplasmic vacuoles. The tumors were infiltrative and showed desmoplastic fibromyxoid stroma. The cytological atypia was overall mild with very focal pleomorphism, but the hypercellularity was prominent, necrosis was abundant, and the mitotic rate was highly elevated (>30/10 hpd). The tumor cells were variably positive for AE1.3, CAM 5.2, CD31, and ERG, while negative for CD34, MUC4, MOC31, BerEp4, TTF1, calretinin, P40, desmin, S100, and Melan‐A. KI69 index was elevated (70%). According to the above findings and the immunohistology evaluation, the diagnosis of pleural EHE was made. (Figure 2).
FIGURE 2.
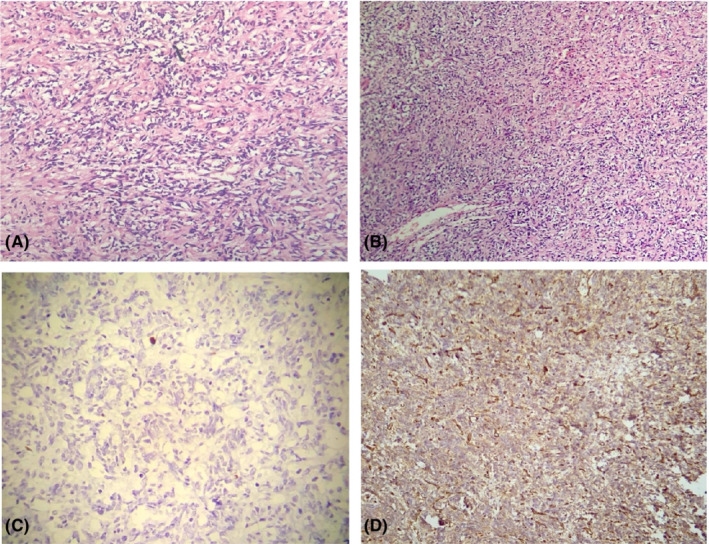
Pathological evaluation of primary malignant epithelioid hemangioendothelioma of the pleura; (A) section of tumor which shows a spindle cell proliferation without nuclear atypia (H&E, ×200); (B) spindle cell proliferation with hemangiopericytoma like vessels (H&E, ×100); (C) Ki67 staining which shows a low proliferative index; (D) positive CD34 immunostaining
Analyte‐specific reagents (ASR) disclaimer on the positive and negative controls stain appropriately. The patient was diagnosed with pleural EHE following the examination and laboratory results. So, a fluorodeoxyglucose positron emission tomography (FDG‐PET) scan was performed to evaluate the staging and metastasis of epithelioid hemangioendothelioma. The PET scan revealed two non‐FDG‐avid calcified nodules in the left upper lobe, a diffuse irregular pleural thickening on the right side that showed increased FDG activity with a standardized uptake value 5 7.98 at maximum, evidence of right pleural effusion with increased FDG uptake. No significant uptakes in other known nodules were noted, and no metastatic lesions were found (Figure 3).
FIGURE 3.
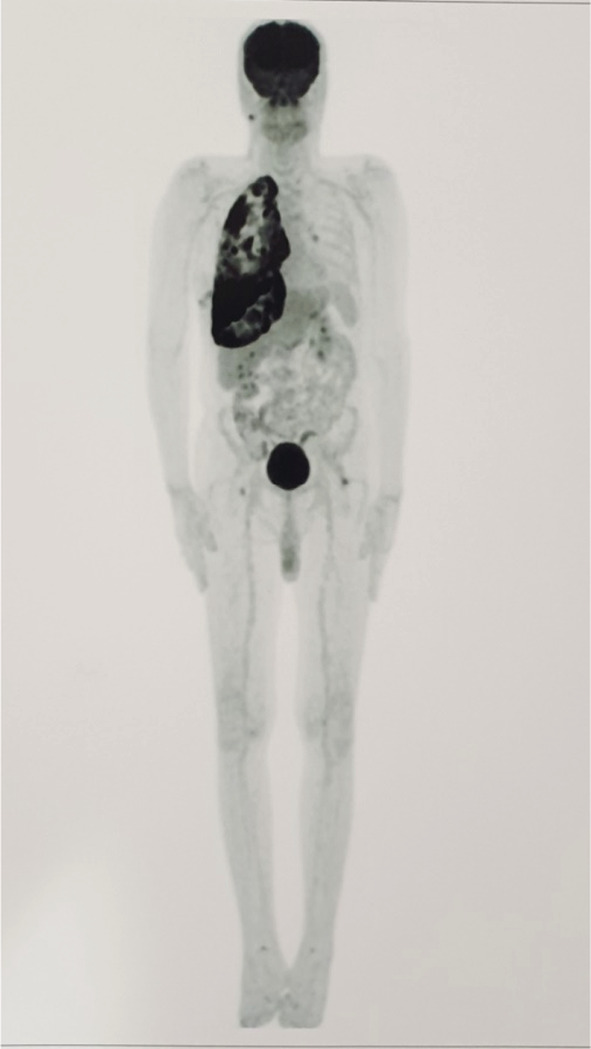
A hypermetabolic nodule in the right lower lobe, adjacent to oblique right fissure with SUV of 4.39 at maximum and hypermetabolic primary tumoral involvement in the right parietal and mediastinal pleura, along with malignant right effusion were noted. Diffuse irregular pleural thickening is noted on the right side, showing increased FDG activity (SUVmax: up to 7.98)
Due to no response to Pazopanib (800 mg once daily) for 4 months, paclitaxel (175 mg/m2) was started, and the patient was scheduled for pleurectomy; however, he did not consent and was lost to follow‐up.
3. DISCUSSION AND CONCLUSION
Although EHE is considered an extremely rare malignant cancer, and its pleural subtype is even less common, further characterizing its clinical course and treatment outcomes is necessary. Therefore, a thorough literature review was conducted. We recorded data of 49 patients from reports published in PubMed, Scholar, Medline, and Scopus using the terms “intravascular bronchoalveolar tumor” or “pleural epithelioid hemangioendothelioma” or a combination of both. Cases of pulmonary EHE with metastasis to the pleura and cases in which the histologic diagnosis was epithelioid angiosarcoma were excluded; however, distinguishing the tumors' primary site in pleural and lung involvement was not possible. Therefore, extracting the exact number of cases in the literature review is not possible. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 Since there was no available national or international database regarding EHE cases, we created a database based on all available reports in the literature. Additionally, we added our case of pleural EHE to this database, resulting in a total of 50 primary cases of pleural EHE. Figure 4 demonstrates the frequency of reported primary pleural malignant epithelioid hemangioendothelioma until now based on published research. Also, Table 1 demonstrates the clinical, demographical, and histological features of primary pleural hemangioendothelioma based on a review of published literature.
FIGURE 4.
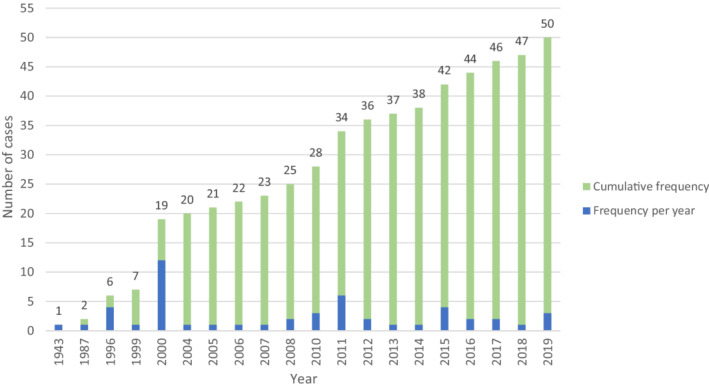
Frequency of primary pleural malignant epithelioid hemangioendothelioma from 1943 to 2019 based on published research
TABLE 1.
Clinical, demographical, and histological features of primary pleural hemangioendothelioma based on a review of published literature
| No of patients | Authors/reference | Age/sex | Symptoms (no.) | Asbestos exposure/cigarette | Chest radiography (no.) | Factors | Other involved sites | Treatment | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Stout et al. 6 | 61/M | Left lower chest pain, night sweats, weakness, hemoptysis | NA | NA (thickening of pleura in autopsy) | NA | Hilar lymph node, left lung, diaphragm | Thoracentesis | 8 |
| 2 | Yousem et al. 18 | 34/M | Dyspnea | NA | Bilateral pleural effusion | NA | None | None | 3 |
| 3 | Lin et al. 19 | 51/M | NA | Asbestos (+), cigarette (+) | Pleural thickening and effusion | Vimentin (+), Keratin (−), CD31(+), CD34 (+), UEA‐1 (+), VWF (+), Collagen IV (+) | None | Pleurectomy | NA |
| 4 | 58/M | NA | NA | Pleural thickening | Vimentin (+), CD34 (−), UEA‐1 (+), VWF (−), Collagen IV (+) | None | Pleurectomy | 6 | |
| 5 | 56/M | NA | NA | Pleural thickening | Vimentin (+), CD31 (+), CD34 (−), UEA‐1 (+), VWF (+), Collagen IV (+) | None | Pleurectomy | NA | |
| 6 | 42/M | NA | NA | Pleural thickening and effusion | Vimentin (+), Keratin (+), CD34 (+), UEA‐1 (+), Collagen IV (+) | None | Pleurectomy | NA | |
| 7 | Pinet et al. 2 | 50/F | None | NA | Right pleural effusion | Factor VIII (+), BNH9 (−) | Peritoneum | Carboplatin, Etoposide | 18 + (CR) |
| 8–11 | Crotty et al. 3 | 55‐71/M | Chest pain (3), dyspnea (3), cough (1), fever (1), weight loss (1) | Asbestos, 3/4 (+), cigarette, 3/4 (+) | Right pleural effusion (4), pleural thickening or mass (1) | CD31 (+), CD34 (+), Factor VIII (+) | Lung (2) mediastinum lymph node (1), liver (1) retroperitoneal lymph node (1) | Decortication surgery | 1–19 (mean = 10) |
| 12 | Zhang et al. 15 | 66/M | NA | NA | Pleural effusion | Cytokeratin, 5/5 (+), CEA, 1/4 (+), CD31, 5/5 (+), CD34, 4/5 (+), factor VIII, 4/5 (+) | None | NA | 6 |
| 13 | 45/M | NA | NA | Pleural effusion | None | NA | 6 | ||
| 14 | 60/M | NA | NA | Bloody pleural effusion | None | NA | 2 | ||
| 15 | 62/F | NA | NA | Pleural effusion, ascites | None | NA | 1 | ||
| 16 | 53/M | NA | NA | Pleural effusion | None | NA | 6 | ||
| 17 | Attanoos et al. 20 | 59/M | Pleuritic chest pain, dyspnea | Asbestos (+), cigarette (+) | Pleural effusion, diffuse pleural thickening | NA | None | NA | 7 |
| 18 | 73/M | Chest infection, dyspnea | Asbestos (+), cigarette (+) | Pleural effusion, pleural fibrosis, and mass | NA | None | NA | 9 | |
| 19 | 33/M | Cough, pleuritic chest pain, dyspnea | Asbestos (+), cigarette (+) | Pleural effusion, pleural thickening | NA | Mediastinum, diaphragm | NA | 8 | |
| 20 | Vitório et al. 21 | 61/M | Chest pain, weight loss, | Asbestos (+), cigarette (+) | Pleural effusion, pleural thickening, small pleural nodule | CD31 (+). CD34 (+), Factor VIII (+) | None | Cisplatin, Etoposide | 3 |
| 21 | Al‐Shraim et al. 22 | 51/M | Cough, dyspnea | NA | Left pleural effusion | CD31 (+), CD34 (+), Factor VIII (+) | Skin | INF‐α, decortication | 24 + |
| 22 | Knuuttila et al. 23 | F/72 | Dyspnea | NA | negative |
CAM5.2 (−), vimentin (+), Calretinin (−), Muscle Actin (−), CD34 (−), S‐100 (−), CK56 (−) EMA (−) |
None | NA | (113+) |
| 23 | Saqi et al. 24 | 37/M | Dyspnea | Asbestos (−), cigarette (−) | Right‐sided pleural effusion, Pleural thickening | NA | None | Carboplatin, Etoposide, Avastin, decortication, thoracentesis | 2 |
| 24 | Lee YJ et al. 25 | 31/F | Upper back and shoulder pain, chest pain | Asbestos (−), cigarette (−) | Nodular pleural thickening (in CT scan) | CD31 (+), CD34 (+), Factor VIII (+) | Lung, Bone | Radiation, Mesna‐Doxorubicin‐Ifosfamide‐Dacarbazine (MAID), Adriamycin | 10 |
| 25 | Martinez et al. 14 | 37/F | Cough, dyspnea, back pain | NA | Pleural effusion | Vimentin (+), CD31 (+), CD34 (−) | None | Interferon, Thalidomide | NA |
| 26 | Liu et al. 26 | 80/M | dyspnea, Right side chest pain | Asbestos (−), cigarette (−) | Right‐sided pleural effusion | CD31 (+), FLI1 (+), Pancytokeratin focal (+) | None | Chemotherapeutic, surgery | 6 |
| 27 | Andre et al. 27 | 65/F | Chest pain | Asbestos (−), cigarette (−) | Pleural effusion | Ca125 (+) | None | Carboplatin, etoposide | 6 |
| 28 | Bocchino et al. 1 | 58/F | Cough, dyspnea, chest pain | Asbestos (−), cigarette (−) | Pleural mass | CD31 (+), CD34 (+), factor VIII (+), ulex (+), Europeaus (+) | None | None | 3 |
| 29 | Kim et al. 28 | 46/F | Right‐sided chest pain, cough | NA | Right‐sided pleural effusion | CD31 (+), CD34 (+) | Chest wall, Esophagus | Carboplatin, etoposide, Resection | 23+ |
| 30 | Márquez‐Medina et al. 16 | 85/M | negative | NA | Right‐sided pleural effusion | CD31 (+), calretinin, (−) TTF‐1 (−), B72.3 (−), pankeratin (−), WT1 (−), CD34 (−), CD68 (−), p63 (−), 34betaE12 (−), CAM5.2 (−), S‐100 (−) | None | No treatment | 7 |
| 31 | Lazarus et al. 29 | 42/M | Cough dyspnea chest pain | NA | Right pleural effusion | NA | Skin |
Paclitaxel, bevacizumab |
8 |
| 32 | 42/M | Fever, cough | Asbestos (−), cigarette (+) | Left pleural effusion | CD31 (+), CD34 (+) | None | Carboplatin, etoposide, bevacizumab | 6 | |
| 33 | Chou et al. 30 | 42/M | Chest pain, cough | NA | Pleural effusion, pleural thickness | CD31 (+), CD34 (+), Vimentin (+), AE1/AE3 (−), Calretinin (−) | Bone (thoracic spine) | Partial pleurectomy, radiotherapy, chemotherapy | 14‐in progress |
| 34 | 27/M | Cough, hoarseness, chest tenderness | NA | Pleural mass, left hemidiaphragm paralysis, pleural effusion | CD31 (+), CD34 (−), FLI‐1 (+), Vimentin (+), AE1/AE3 (−), Calretinin (−), S‐100 (−) | None | Radiotherapy, pleurectomy, Doxorubicin, cisplatin | 7 ‐ CR | |
| 35 | Bansal et al. 31 | 51/F | left‐sided, non‐pleuritic chest pain, weight loss | NA | large loculated effusion with pleural thickening | CD31 (+), CD34 (+) | None | Doxorubicin | 4 |
| 36 | Photowala et al. 32 | NA | Hypoxemia, bilateral pleuretic chest pain, dyspnea | Asbestos (−), cigarette (−) | Small to moderate bilateral pleural effusion, Hemothorax | AE1/3 (+), CAM 5.2 (+), CD31 (+), calretinin (−), Cytokeratin‐5/6 (−), cytokeratin‐7 (−), cytokeratin‐20 (−), CD30 (−), CD117 (−), CD34 (−) | Pericardium | Thoracotomy | 5, |
| 37 | Yu et al. 33 | 39/F | NA | Asbestos (−), cigarette (−) | Left thoracic mass | NA | None | Carboplatin, etoposide | 14‐ CR |
| 38 | Ha et al. 17 | 71/M | Cough, dyspnea, fatigue, weight loss | Asbestos (−), cigarette (−) | Bilateral Pleural effusion, pleural and lung nodule, | CD31 (+), CD34 (−), CAMTA1 rearrangement (+) | None | NA | NA |
| 39 | Wethasinghe et al. 34 | 41/M | Right lower chest pain, weight loss | Asbestos (−), cigarette (−) | pleural thickening | NA | None | radiotherapy | 6 – In progress |
| 40 | Sanxi et al. 35 | 40/M | Chest pain, | Asbestos (−), cigarette (+) | Left pleural effusion, Thickening of pleura | CD31 (+), CD34 (+), vimentin (+), AE1 / AE3 (+), Calretinin (−), Ki‐67 (<1% low), CK5 / 6 (−), FVIIRAg (−) | None | Gemcitabine, cisplatin | NA |
| 41 | Salijevska et al. 36 | 36/F | Chest pain, dyspnea | Asbestos (−), cigarette (+) | complete opacification of the right hemothorax, right‐sided pleural effusion, pleural thickening | MNF116 (+), CK7 (+), D2‐40 (+), CD31 (+), CD34 (+) | lungs | Paclitaxel | 6 |
| 42 | Jamy et al. 9 | 24/M | Abdominal pain, chest pain, dyspnea, nausea and vomiting, hemoptysis | NA | Pleural effusion, pleural thickening | CD31 (+); MOC‐31 (−), Ber‐Ep4 (−), p63 (−), OCT‐4 (−), alpha‐fetoprotein (−) | None | Carboplatin, paclitaxel | 2 |
| 43 | Kanemura et al. 15 | 31/F | Dull back pain | NA | Pleural thickening, pleural effusion | AE1/AE3 (−), CAM5.2 (−), calretinin (−), WT‐1, CEA (−), TTF‐1 (−), D2‐40 (+), vimentin (+), CD34 (+), CD31 (+), factor VIII (+) | None | carboplatin, pemetrexed, Bevacizumab | 6 ‐ CR |
| 44 | Fan et al. 37 | 68/F | Back pain | Asbestos (−), cigarette (−) | Left pleural effusion and thickening | CD31 (+), M2a (+), carcinoembryonic antigen (D2‐40) (+), ERG (+), Ki67 (+), CD34 (−), CK7 (−), TTF‐1 (−), Napsin A (−), PCK (−), CK5/6 (−), WT‐1 (−), ALK D5f2 (−), E‐cadherin (−), Factor VIII (−) | None | NA | NA |
| 45 | Ferreiro et al 38 | 85/M | Left pleuritic pain, progressive dyspnea, dry cough. | Asbestos (−), cigarette (+) | bilateral pleural effusion | Vimentin (+), cytokeratin (AE1–AE3) (+), CD31 (+), calretinin. (−) Ki‐67 (high) | None | Symptomatic | 2 |
| 46 | Albano et al. 39 | 76/F | Chest pain, dyspnea, cough | NA | Pleural thickening, Right side pleural effusion | CD31(+), CD34 (+), panCK (−), AE1/AE3 (−), CK5/6 (−), EMA (−), calretinin (−), WT1 (−), TTF1 (−), Napsin (−) | None | Chest drainage | 6 |
| 47 | Rejeb et al. 37 | 79/M | Chest pain, dyspnea, weight loss | NA | Pleural thickening, right side pleural effusion | CD34 (+) | None | Etoposide, Cisplatin | 1 |
| 48 | Takenaka et al. 40 | 62/M | Right chest pain, dyspnea | Asbestos (−), cigarette (+) | Right side pleural effusion | Calretinin (−), CD31 (+), CD34 (+), CAMTA1 (+) | None | Extrapleural pneumonectomy, pazopanib | 3.5 |
| 49 | AlGhunaim et al. 41 | 49/M | Cough, dyspnea, weight loss, chest pain | NA | Hemothorax | CD34 (+), CD31 (+), CK7 (−), CK20 (−), calretinin (−), TTF1 (−), napsin (−) | None | Chemotherapy | NA |
| 50 | Our case | 68/M | Pleuritic chest pain, weight loss | Asbestos (−), cigarette (−) | Right side pleural effusion and mass | AE1.3 (+), CAM 5.2 (+), CD31 (+), ERG (+), CD34 (−), MUC4 (−), MOC31 (−), BerEp4 (−), TTF1 (−), calretinin (−), P40 (−), desmin (−), S100 (−), Melan‐A (−). KI69 elevated (70%). | None | Paclitaxel | 8 ‐ In progress |
Mallory initially described hemangioendothelioma (EH) in 1908, and later in 1943, Stout published a series of cases reporting a tumor of vascular origin from various sites that could be misdiagnosed as angiosarcoma. 6 , 36 In 1975, Dail and Leibow reported a tumor of intravascular bronchioloalveolar (IVBATT) which then in 1982 was named Epithelioid hemangioendothelioma (EHE) by Weiss and Enzinger, who reported twenty cases with gradually growing nodules of the lung with variant histology from malignant sarcomatous to benign granulomatous tumors. 4 , 9 , 15 , 37
A literature review of all EHE cases has demonstrated heterogeneity in its management and presentation. To our knowledge, this review summarizes by far the largest number of patients with pleural EHE. The average age of patients diagnosed with PEH was 53.72 (SD = 15.98) years, and 70% were males, which aligns with previous reports that pleural EHE commonly affects older men presenting with symptoms of the disease. In contrast, lung EHE affects middle‐aged and young women without symptoms 7 , 8 , 9 , 37 , 38 , 39 and cases in children are extremely rare. 40 Among the cases, 8 (16%) had a history of asbestos exposure, while 6 (12%) had a history of cigarette smoking. Common clinical symptoms of pleural EHE include pleuritic chest pain (54%), dyspnea (46%), sputum production and cough (30%), and weight loss (16%). In comparison, less frequent symptoms included shoulder or back pain (8%), hemoptysis (6%), fever (4%), fatigue (4%), nausea vomiting (2%), night sweating (2%), hypoxemia (2%), and hoarseness (2%). Multiple uncommon symptoms have been reported as the presentation of pleural EHE, such as the rise of pigmented lesions on the patients back representing Leser–Trélat sign which happens to be a paraneoplastic syndrome from an unknown etiology, compression effect of the tumor on the myocardium in a middle‐age woman and spontaneous bilateral hemothorax indicating the vascular nature of this tumor. 20 , 23 , 24 Amin et al. even reported a case of an accidental finding of EHE following a routine CXR due to gastroenteritis with pleural metastasis. 41
Although the exact etiology of EHE has not been established, several theories have been reported to play a role in its pathogenesis, such as the use of contraceptives, exposure to radiation or asbestosis, occupation contaminants such as heavy industry, vinyl chloride inhalation, smoking, the role of biological substances in stimulation endothelial proliferation and angiogenesis and translocations in chromosomes (e.g., genetic disorders, t (1; 3) (p36.23; q25), WWTR1–CAMTA1 gene fusion, YAP1–TFE3 fusion). 2 , 3 , 9 , 10 , 25 , 42 , 43 , 44 , 45 , 46 It is worth mentioning that the patient in our report had no predisposing factors.
Even though EHE has an indolent nature, progression, metastasis, and recurrences can occur. 47 , 48 , 49 The diagnosis of EHE is made upon histopathological evaluation and is characterized by solid nests of cells in a myxoid or hyaline stroma and tubulopapillary growth of short strands showing frequent intracytoplasmic vacuoles. 12 , 50 , 51 In addition, vascular immunohistochemistry markers may be helpful in the confirmation of the diagnosis. CD31 had the highest positivity based on reviewed literature (32/32), followed by CD34 (23/31), Vimentin (11/11), Factor VIII (8/9), VWF (5/6), UEA1 (4/4), collagen IV (4/4), Ki67 (3/4), FLI1 (2/2), CAM52 (2/4), Keratin (7/11), and AE1/AE3 (4/7). Factors that contribute to the tumor's aggressiveness include necrosis, an increase in cellularity, and a high mitotic rate which, in this case, can be labeled as malignant EHE. 47 , 50
Various malignancies can involve pleura; thus, the differential diagnosis of angiosarcomas, mesothelioma, adenocarcinoma, and metastatic neoplasms is essential to distinguish the exact pathology from another. 3 , 5 , 12 , 15 , 49 Mesothelial immunohistochemical markers such as WT1 and calretinin (0/11 in our study) can help segregate pleural EHE from mesothelioma. Lastly, distinguishing pleural EHE from angiosarcomas is vital since most angiosarcomas are fatal. 52
Based on our review, pleural effusion and pleural thickening (nodular or smooth) have been the typical manifestation of pleural EHE in radiological examination (76% and 32%, respectively). However, these findings should have been considered nonspecific due to their similarity to radiological findings in mesothelioma and diffuse pleural carcinomatosis. 7 , 9 , 39 , 49 Fibrosis, mediastinal lymphadenopathy, hemidiaphragm paralysis, hemothorax, and invasion of the diaphragm, ribs, and vertebrae were uncommon findings among pleural EH cases. 9 , 10 , 11 As our case had an increased 18‐fluorine‐fluorodeoxyglucose (18F‐FDG) activity, two reports also showed an increase in uptake of FDG in their cases, indicating the aggressiveness of the tumor and its rapidly growing feature. It can also detect other tumor foci in other parts of the body and as a marker for decision‐making in therapeutic approaches. 53 , 54 Malignant tumor cells often show higher FDG uptake, and SUV ≥2.5 is considered to be a criterion for malignancy (specificity, sensitivity, and accuracy of 82%, 97%, and 92%, respectively). 54
In literature, no standard treatment has been established for pleural EHE, yet, some studies used surgery (11: 22%), chemotherapy and radiotherapy (18: 36%), combination therapy (6: 12%), and some only symptomatic therapy (5: 10%). Surgical approaches are mostly considered when the tumor is local; nevertheless, microscopic tumors may remain in the chest wall when done without chemotherapy. 15 , 24 , 34 A combination of chemotherapy with surgical treatment, especially administering etoposide and carboplatin, has been effective in reports that led to a disease‐free period of more than 14 months. 19 , 24 Also, the use of six cycles of carboplatin and etoposide had resulted in the complete remission of the disease. In contrast, in another case, the combination use of etoposide and cisplatin resulted in only limited response in another case. 2 , 4 In 2008, Lee et al. reported a 31‐year‐old woman diagnosed with pleural EHE with extension to the bone and lung, which was treated with Doxorubicin, ifosfamide, mesna, and dacarbazine (MAID) and palliative radiotherapy on the spine which led to 10 months survival of the patient. 15 Also, a report demonstrated no benefit in administering Bevacizumab, an anti‐vascular endothelial growth factor monoclonal antibody, plus the use of etoposide and carboplatin.
After a period of observation, surgical resection is the treatment of choice in cases with proven unifocal disease or with technically resectable locoregional metastases. Pleural stripping or pneumonectomy may be considered individually, taking into account the severity of the disease and the expected benefit. Local ablative techniques may be considered when EHE involvement is limited to the lungs. 55
Despite the lack of a standard medical approach, patients with metastatic disease and unequivocal evidence of disease progression, worsening symptoms, or organ dysfunction are candidates for systemic treatment. Conventional soft tissue sarcomas chemotherapy appears to have very limited activity and should be reserved for cases that are more aggressive or rapidly progressing, such as high‐grade soft tissue sarcomas. 56 Therefore, further data regarding patients in clinical trials are required to draw a clear conclusion.
Interferon, 57 , 58 thalidomide, 59 multi‐tyrosine kinase inhibitors (usually with a strong vascular endothelial growth factor receptor [VEGFR] inhibitory property), 60 , 61 , 62 , 63 , 64 , 65 , 66 and mechanistic target of rapamycin (mTOR) inhibitors 59 , 67 , 68 , 69 , 70 have all shown antitumor activity retrospectively. In our report, the patient was initially treated with pazopanib, which inhibits tumors growth by inhibiting angiogenesis via inhibiting cell surface VEGFR (VEGFR‐1, VEGFR‐2, VEGFR‐3), platelet‐derived growth factor receptors (PDGFR‐alpha and ‐beta), fibroblast growth factor receptor (FGFR‐1 and ‐3), cytokine receptor (cKIT), interleukin‐2 receptor inducible T‐cell kinase, lymphocyte‐specific protein tyrosine kinase (Lck), and transmembrane glycoprotein receptor tyrosine kinase (c‐Fms). 71 Based on previous reports, mTOR inhibitors have shown the most clinical activity, with progression‐free survival and overall survival in 1 to 2 years, respectively, and approximately 10% of patients have even longer progression‐free survival. 55
In reviewed pleural EHE cases, out of 50 cases, 40 (80%) patients died, 4 had complete remission, and six are still pending, which demonstrated the disease's poor prognosis. The results of our review demonstrate an average of 9.98 months of disease course. In literature, the prognosis of EHE is variable depending on the origin site. Pulmonary EHE has been reported to have a better prognosis than pleural EHE despite the extensive involvement of parenchymal tissue with a long life expectancy. 37 , 38 According to reports, an indicator for poor prognosis includes being symptomatic at presentation, metastasis, peripheral lymphadenopathy, and lymphangitic tumor spread, extensive interstitial or intravascular tumor spread, fibrous pleuritis with an extrapleural proliferation of tumor cells, pleural effusion, and spindle tumor cells. 37 , 38 , 39 , 41 , 48
In conclusion, based on our review, although rare, taking into consideration the diagnosis of pleural EHE is essential due to the disease's high mortality. Further research and reporting of cases are necessary to add to the information about the clinical progression and the natural history of this rare malignancy to provide a therapeutic approach to the disease.
AUTHOR CONTRIBUTIONS
AR designed the study. RS collected the data and performed a review of the literature. AE and KR drafted the manuscript. All authors proofread and accepted the final version of the manuscript.
FUNDING INFORMATION
No financial support was received for this report.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICAL APPROVAL
Written informed consent was obtained from the patient in our study. The purpose of this research was thoroughly explained to the patient, and he was assured that their information would be kept confidential by the researcher. The present study was approved by the Medical Ethics Committee of the academy.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor‐in‐chief of this journal.
ACKNOWLEDGMENT
None to declare.
Rezvani A, Shahriarirad R, Erfani A, Ranjbar K. Primary malignant epithelioid hemangioendothelioma of the pleura: A review and report of a novel case. Clin Case Rep. 2022;10:e06211. doi: 10.1002/ccr3.6211
DATA AVAILABILITY STATEMENT
SPSS data of the participant can be requested from the authors. Please write to the corresponding author if you are interested in such data.
REFERENCES
- 1. Salijevska J, Watson R, Clifford A, Ritchie AI, Mauri F, Adeboyeku D. Pleural epithelioid hemangioendothelioma: literature summary and novel case report. J Clin Med Res. 2015;7(7):566‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Márquez‐Medina D, Samamé‐Pérezvargas JC, Tuset‐DerAbrain N, Montero‐Fernández A, Taberner‐Bonastre T, Porcel JM. Pleural epithelioid hemangioendothelioma in an elderly patient. A case report and review of the literature. Lung Cancer. 2011;73(1):116‐119. [DOI] [PubMed] [Google Scholar]
- 3. Martínez D, Iturbe J, Buzeki R, Caffaratti E. Hemangioendotelioma epitelioide de pleura. Presentacion de un caso con hemotorax. Rev Am Med Respir. 2008;8(2):73‐76. [Google Scholar]
- 4. Pinet C, Magnan A, Garbe L, Payan MJ, Vervloet D. Aggressive form of pleural epithelioid haemangioendothelioma: complete response after chemotherapy. Eur Respir J. 1999;14(1):237‐238. [DOI] [PubMed] [Google Scholar]
- 5. Attanoos R, Suvarna S, Rhead E, et al. Malignant vascular tumours of the pleura in “asbestos” workers and endothelial differentiation in malignant mesothelioma. Thorax. 2000;55(10):860‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stout AP. Hemangio‐endothelioma: a tumor of blood vessels featuring vascular endothelial cells. Ann Surg. 1943;118(3):445‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yousem SA, Hochholzer L. Unusual thoracic manifestations of epithelioid hemangioendothelioma. Arch Pathol Lab Med. 1987;111(5):459‐463. [PubMed] [Google Scholar]
- 8. Lin BT‐Y, Colby T, Gown AM, et al. Malignant vascular tumors of the serous membranes mimicking mesothelioma: a report of 14 cases. Am J Surg Pathol. 1996;20(12):1431‐1439. [DOI] [PubMed] [Google Scholar]
- 9. Crotty EJ, McAdams HP, Erasmus JJ, Sporn TA, Roggli VL. Epithelioid hemangioendothelioma of the pleura: clinical and radiologic features. Am J Roentgenol. 2000;175(6):1545‐1549. [DOI] [PubMed] [Google Scholar]
- 10. Zhang PJ, Livolsi VA, Brooks JJ. Malignant epithelioid vascular tumors of the pleura: report of a series and literature review. Hum Pathol. 2000;31(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 11. Vitório PK, Coletta EM, Morrone N, et al. Hemangioendotelioma epitelióide de pleura. J Bras Pneumol. 2004;30(1):60‐65. [Google Scholar]
- 12. Al‐Shraim M. Primary pleural epithelioid haemangioendothelioma with metastases to the skin. A case report and literature review. J Clin Pathol. 2005;58(1):107‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knuuttila A, Jee KJ, Taskinen E, Wolff H, Knuutila S, Anttila S. Spindle cell tumours of the pleura: a clinical, histological and comparative genomic hybridization analysis of 14 cases. Virchows Arch. 2006;448(2):135‐141. [DOI] [PubMed] [Google Scholar]
- 14. Saqi A, Nisbet L, Gagneja P, Leslie KO. Primary pleural epithelioid hemangioendothelioma with rhabdoid phenotype: report and review of the literature. Diagn Cytopathol. 2007;35(4):203‐208. [DOI] [PubMed] [Google Scholar]
- 15. Lee YJ, Chung MJ, Jeong KC, et al. Pleural epithelioid hemangioendothelioma. Yonsei Med J. 2008;49(6):1036‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JX, Shiau MC, Nonaka D. An 80‐year‐old man with shortness of breath and large right‐sided pleural effusion. Chest. 2010;138(5):1247‐1252. [DOI] [PubMed] [Google Scholar]
- 17. André ST, Valente C, Paiva B, Pêgo A, Carvalho L, Luís AS. Epithelioid hemangioendothelioma of the pleura ‐ a rare presentation of a clinical case. Rev Port Pneumol. 2010;16(3):477‐482. [DOI] [PubMed] [Google Scholar]
- 18. Bocchino M, Barra E, Lassandro F, Ranieri F, Muto R, Rea G. Primary pleural haemangioendothelioma in an Italian female patient: a case report and review of the literature. Monaldi Arch Chest Dis. 2010;73(3):135‐139. [DOI] [PubMed] [Google Scholar]
- 19. Kim EA, Lele SM, Lackner RP. Primary pleural epithelioid hemangioendothelioma. Ann Thorac Surg. 2011;91(1):301‐302. [DOI] [PubMed] [Google Scholar]
- 20. Lazarus A, Fuhrer G, Malekiani C, McKay S, Thurber J. Primary pleural epithelioid hemangioendothelioma (EHE)–two cases and review of the literature. Clin Respir J. 2011;5(1):e1‐e5. [DOI] [PubMed] [Google Scholar]
- 21. Chou Y‐Y, Hsieh Y‐S, Wu C‐C, How S‐W. Epithelioid hemangioendotheliomas of the lung and pleura: report of three cases. Formos J Surg. 2011;44(4):163‐167. [Google Scholar]
- 22. Bansal A, Chawla M, Cohen PJ, Kwon JS. Pleural epithelioid hemangioendothelioma. Lung. 2012;190(4):469‐470. [DOI] [PubMed] [Google Scholar]
- 23. Photowala F, Hatipoglu US, Garcia C. An older patient with bilateral non‐traumatic haemothoraces. BMJ Case Rep. 2012;2012:bcr0120125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu L, Gu T, Xiu Z, Shi E, Zhao X. Primary pleural epithelioid Hemangioendothelioma compressing the myocardium. J Card Surg. 2013;28(3):266‐268. [DOI] [PubMed] [Google Scholar]
- 25. Ha SY, Choi IH, Han J, et al. Pleural epithelioid hemangioendothelioma harboring CAMTA1 rearrangement. Lung Cancer. 2014;83(3):411‐415. [DOI] [PubMed] [Google Scholar]
- 26. Wethasinghe J, Sood J, Walmsley R, Milne D, Jafer A, Gordon‐Glassford N. Primary pleural epithelioid hemangioendothelioma mimicking as a posterior mediastinal tumor. Respirol Case Rep. 2015;3(2):75‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanxi A, Yalan B, Qinfeng Z, et al. Pleural epithelioid hemangioendothelioma: a case report and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38(3):174‐178. [PubMed] [Google Scholar]
- 28. Jamy OH, Huber B, Giri S. Pleural epitheliod hemangioendothelioma: what started as a liver fluke and ended up being almost mistaken for malignant mesothelioma. Ann Thorac Med. 2015;10(4):289‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanemura S, Kuribayashi K, Moriya Y, Shimizu S, Tsujimura T, Nakano T. Pemetrexed for epithelioid haemangioendothelioma of the pleura. Respirol Case Rep. 2016;4(6):e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan Y, Wang F, Li S, Ye C, Ying Y, Mao H. Pleural epithelioid hemangioendothelioma: a case report and literature review. J Natl Med Assoc. 2016;108(2):124‐129. [DOI] [PubMed] [Google Scholar]
- 31. Ferreiro L, San‐Jose E, Suarez‐Antelo J, et al. Spontaneous bilateral haemothorax as presentation of primary pleural epithelioid haemangioendothelioma. Clin Respir J. 2017;11(6):1079‐1085. [DOI] [PubMed] [Google Scholar]
- 32. Albano D, Bosio G, Tironi A, Bertagna F. 18F‐FDG PET/CT in pleural epithelioid hemangioendothelioma. Asia Ocean J Nucl Med Biol. 2017;5(1):70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rejeb SB, Ghachem DB, Dhaoui A, Marzouk SB, Bellil K. Primary pleural epithelioid hemangioendothelioma. Chest Dis Rep. 2018;6(1). [Google Scholar]
- 34. Takenaka M, Ichiki Y, Nabe Y, et al. Difficulty of treatment for pleural epithelioid hemangioendothelioma: a report of a case. Gen Thorac Cardiovasc Surg. 2019;68:1‐4. [DOI] [PubMed] [Google Scholar]
- 35. AlGhunaim EM, AlNajem H, AlShehab DS. Spontaneous bilateral Hemothorax as a case of epithelioid Hemangioendothelioma (EHE). Case Rep Oncol Med. 2019;2019:1854361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mallory FB. The results of the application of special histological methods to the study of tumors. J Exp Med. 1908;10(5):575‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dail DH, Liebow AA, Gmelich JT, et al. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983;51(3):452‐464. [DOI] [PubMed] [Google Scholar]
- 38. Kitaichi M, Nagai S, Nishimura K, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998;12(1):89‐96. [DOI] [PubMed] [Google Scholar]
- 39. Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986;3(4):259‐287. [PubMed] [Google Scholar]
- 40. Ellis GL, Kratochvil FJ 3rd. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol. 1986;61(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 41. Amin RM, Hiroshima K, Kokubo T, et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology. 2006;11(6):818‐825. [DOI] [PubMed] [Google Scholar]
- 42. Ledson M, Convery R, Carty A, Evans C. Epithelioid haemangioendothelioma. Thorax. 1999;54(6):560‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woelfel C, Liehr T, Weise A, et al. Molecular cytogenetic characterization of epithelioid hemangioendothelioma. Cancer Genet. 2011;204(12):671‐676. [DOI] [PubMed] [Google Scholar]
- 44. Mendlick MR, Nelson M, Pickering D, et al. Translocation t(1;3)(p36.3;q25) is a nonrandom aberration in epithelioid hemangioendothelioma. Am J Surg Pathol. 2001;25(5):684‐687. [DOI] [PubMed] [Google Scholar]
- 45. Tanas MR, Sboner A, Oliveira AM, et al. Identification of a disease‐defining gene fusion in epithelioid Hemangioendothelioma. Sci Transl Med. 2011;3(98):98ra82. [DOI] [PubMed] [Google Scholar]
- 46. Errani C, Zhang L, Sung YS, et al. A novel WWTR1‐CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50(8):644‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32(6):924‐927. [DOI] [PubMed] [Google Scholar]
- 48. Bahrami A, Allen TC, Cagle PT. Pulmonary epithelioid hemangioendothelioma mimicking mesothelioma. Pathol Int. 2008;58(11):730‐734. [DOI] [PubMed] [Google Scholar]
- 49. Lin BTY, Colby T, Gown AM, et al. Malignant vascular tumors of the serous membranes mimicking mesothelioma. Am J Surg Pathol. 1996;20(12):1431‐1439. [DOI] [PubMed] [Google Scholar]
- 50. Anderson T, Zhang L, Hameed M, Rusch V, Travis WD, Antonescu CR. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1‐CAMTA1 fusions. Am J Surg Pathol. 2015;39(1):132‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lau K, Massad M, Pollak C, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140(5):1312‐1318. [DOI] [PubMed] [Google Scholar]
- 52. Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50(5):970‐981. [DOI] [PubMed] [Google Scholar]
- 53. Watanabe S, Yano F, Kita T, et al. 18 F‐FDG‐PET/CT as an indicator for resection of pulmonary epithelioid hemangioendothelioma. Ann Nucl Med. 2008;22(6):521‐524. [DOI] [PubMed] [Google Scholar]
- 54. Patz EF Jr, Lowe VJ, Hoffman JM, et al. Focal pulmonary abnormalities: evaluation with F‐18 fluorodeoxyglucose PET scanning. Radiology. 1993;188(2):487‐490. [DOI] [PubMed] [Google Scholar]
- 55. Stacchiotti S, Miah AB, Frezza AM, et al. Epithelioid hemangioendothelioma, an ultra‐rare cancer: a consensus paper from the community of experts. ESMO Open. 2021;6(3):100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frezza AM, Ravi V, Lo Vullo S, et al. Systemic therapies in advanced epithelioid haemangioendothelioma: a retrospective international case series from the world sarcoma network and a review of literature. Cancer Med. 2021;10(8):2645‐2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moon SB, Kwon HJ, Park KW, Yun WJ, Jung SE. Clinical experience with infantile hepatic hemangioendothelioma. World J Surg. 2009;33:597‐602. [DOI] [PubMed] [Google Scholar]
- 58. Radzikowska E, Szczepulska‐Wojcik E, Chabowski M, Oniszh K, Langfort R, Roszkowski K. Pulmonary epithelioid haemangioendothelioma, interferon 2‐alpha treatment, case report. Pneumonol Alergol Pol. 2008;76:281‐285. [PubMed] [Google Scholar]
- 59. Soape MP, Verma R, Payne JD, Wachtel M, Hardwicke F, Cobos E. Treatment of hepatic epithelioid hemangioendothelioma: finding uses for thalidomide in a new era of medicine. Case Rep Gastrointest Med. 2015;2015:326795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saada E, Saint Paul MC, Gugenheim J, Follana P, François E. Metastatic hepatic epithelioid hemangio‐endothelioma: long‐term response to sunitinib malate. Oncol Res Treat. 2014;37:124‐126. [DOI] [PubMed] [Google Scholar]
- 61. Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC soft tissue and bone sarcoma group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88‐92. [DOI] [PubMed] [Google Scholar]
- 62. Chevreau C, Le Cesne A, Ray‐Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French sarcoma group (GSF/GETO). Cancer. 2013;119:2639‐2644. [DOI] [PubMed] [Google Scholar]
- 63. Agulnik M, Yarber JL, Okuno SH, et al. An open‐label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257‐263. [DOI] [PubMed] [Google Scholar]
- 64. Yousaf N, Maruzzo M, Judson I, Judson FC, Benson C. Systemic treatment options for epithelioid haemangioendothelioma: the Royal Marsden Hospital experience. Anticancer Res. 2015;35:473‐480. [PubMed] [Google Scholar]
- 65. Zheng Z, Wang H, Jiang H, Chen E, Zhang J, Xie X. Apatinib for the treatment of pulmonary epithelioid hemangioendothelioma: a case report and literature review. Medicine (Baltimore). 2017;96:e8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Semenisty V, Naroditsky I, Keidar Z, Bar‐Sela G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma, a suitable treatment option: case report and review of anti‐angiogenic treatment options. BMC Cancer. 2015;15:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg. 2006;82:2010‐2013. [DOI] [PubMed] [Google Scholar]
- 68. Engel ER, Cournoyer E, Adams DM, Stapleton S. A retrospective review of the use of sirolimus for pediatric patients with epithelioid hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42:e826‐e829. [DOI] [PubMed] [Google Scholar]
- 69. Riou S, Morelon E, Guibaud L, Chotel F, Chotel F, Marec‐Berard P. Efficacy of rapamycin for refractory hemangioendotheliomas in Maffucci's syndrome. J Clin Oncol. 2012;30:e213‐e215. [DOI] [PubMed] [Google Scholar]
- 70. Cohen EE, Wu K, Hartford C, et al. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin Cancer Res. 2012;18:4785‐4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hamberg P, Verweij J, Sleijfer S. (pre‐)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist. 2010;15(6):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
SPSS data of the participant can be requested from the authors. Please write to the corresponding author if you are interested in such data.


