Abstract
A three-step sequence for preparing remdesivir, an important anti-SARS-CoV-2 drug, is described. Employing N,N-dimethylformamide dimethyl acetal (DMF-DMA) as a protecting agent, this synthesis started from (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-furan-2-carbonitrile (GS-441524) and consisted of three reactions, including protection, phosphoramidation, and deprotection. The advantages of this approach are as follows: (1) the protecting group could be removed under a mild deprotection condition, which avoided the generation of the degraded impurity; (2) high stereoselectivity was achieved in the phosphorylated reaction; (3) this synthesis could be performed successively without purification of intermediates. Moreover, the overall yield of this approach on a gram scale could be up to 85% with an excellent purity of 99.4% analyzed by high-performance liquid chromatography (HPLC).
Introduction
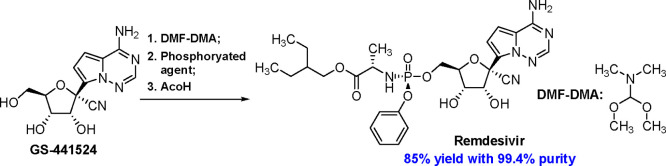
Remdesivir (1), an inhibitor of RNA-dependent RNA polymerase of the SARS-CoV-2 virus developed by Gilead Sciences,1−4 is the first drug to treat hospitalized patients with COVID-19.5−7 Recent study showed that the risk of hospitalization or death of COVID-19 outpatients with high risk could be reduced by 87% after receiving a three-day course of remdesivir.8 Moreover, the FDA authorized its emergency use in Covid-19 nonhospitalized patients based on the encouraging result of the clinical trial (NCT04501952).9 Therefore, enough supply of remdesivir is crucial to conquer the global health crisis caused by SARS-CoV-2.
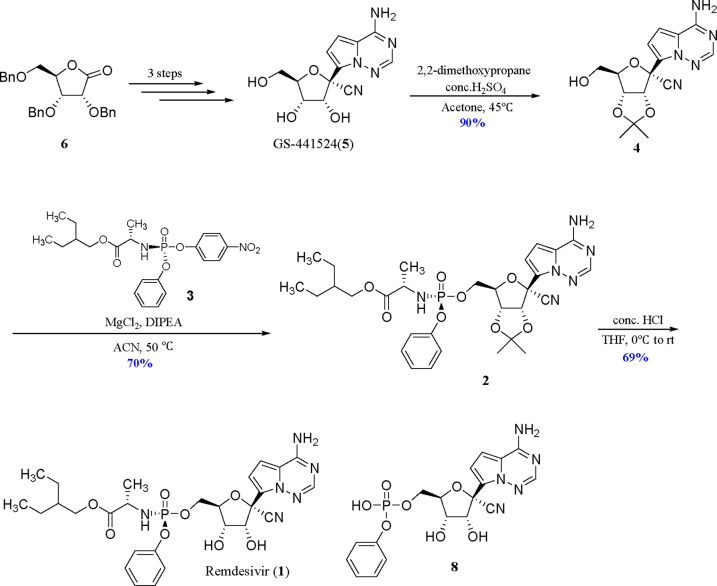
Structurally, remdesivir is a monophosphoramidate prodrug of a C-nucleoside analogue GS-441524. Given the great clinical demand, the synthesis of this antiviral prodrug has attracted chemists’ interest.10−15 The gram-scale synthesis of remdesivir from 2,3,5-tri-O-benzyl-d-ribonolactone (6) was reported by Gilead’s chemists (Scheme 1) and consisted of a six-step sequence.16 The low 48% overall yield of the last two reactions, including the phosphoramidation and the deprotection, might be the Achilles’ heel for the sufficient supply of remdesivir. Researchers have been studying the problem to address it. Recently, Zhang’s group reported the stereoselective synthesis of remdesivir by the coupling of phosphoramidoyl chloride (7) and acetonide-protected nucleoside 4 catalyzed by chiral imidazole derivatives with 73% overall yield (Scheme 2), which avoided the preparation of enantiomerically pure phosphorylated agent.17 Soon after, Hung’s group reported a similar approach to remdesivir with 70% yield by a one-pot method.18 Nonetheless, the instability of 7 posed a challenge to its purification and storage according to the study of phorsphoramidate derivatives reported previously,19 which might limit its application for commercial production.
Scheme 1. Gram-Scale Synthesis of Remdesivir Reported by Gilead’s Chemists and Acid-Induced Degraded Impurity (8).
Scheme 2. Imidazole-Derivative-Catalyzed Asymmetric Synthesis of Remdesivir.
As far as our group was concerned, intensive efforts were devoted to investigate those two reactions. In our study, methylmagnesium chloride (MeMgCl) could be used instead of MgCl2 and DIPEA in the phosphorylation reaction; moreover, the p-nitrophenylphosphoramidate 3 could be replaced with the pentafluorophenyl phosphoramidate 10 that had been used in the preparation of remdesivir in the literature.20,21 In the deprotection of acetonide, many reaction conditions, such as the concentration of hydrochloric acid, the solvents used, and the temperature, were screened, but no better result was obtained. Meanwhile, we found that the monophosphate impurity 8 was gradually produced before the compound 2 was consumed completely, which resulted from the hydrolysis of the phosphoramidate moiety of 1 in the presence of hydrochloric acid. Clearly, the side reaction caused by the harsh reaction condition resulted in the moderate 69% yield of this reaction. Therefore, a suitable protecting group at 2′,3′-dihydroxyls is key to achieve the high-efficient synthesis of remdesivir from GS-441524.
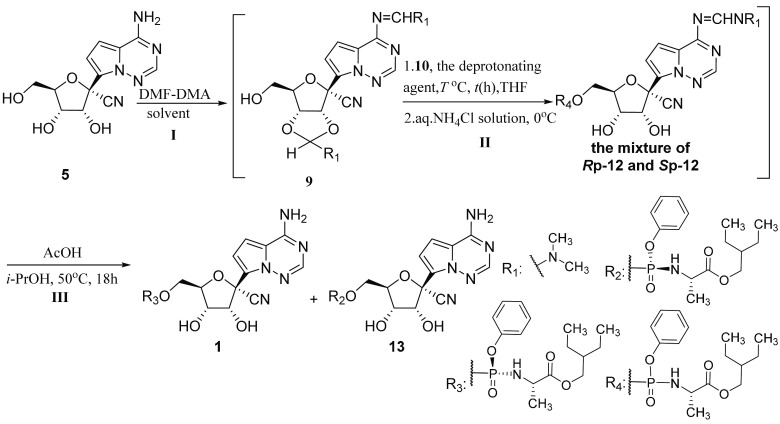
According to our previous study, N,N-dimethylformamide dimethyl acetal (DMF-DMA) is a good protecting agent that could mask 2′,3′-dihydroxyls and C4-amine of cytidine selectively to facilitate the esterification at 5′-hydroxyl group, furthermore, the formed dimethylaminomethylene (DMAM) group at the 2′,3′-dihydroxyls could be deprotected easily in the protic solvents, such as ethanol and isopropanol.22 Hence, employing DMF-DMA as a protecting agent, instead of 2,2-dimethoxypropane, might avoid the production of the byproduct 8. Herein, we developed a highly efficient approach for preparing remdesivir from GS-441524 (Scheme 3).
Scheme 3. Synthesis of Remdesivir from GS-441524 with DMF-DMA as the Protecting Agent.
Results and Discussion
The study started with the synthesis of the DMAM-protected nucleoside analogue 9. This intermediate was synthesized from the compound 5 with a quantitative yield in the presence of DMF-DMA (4.0 equiv.) in pyridine at 25 °C for 18 h. In view of the instability of the DMAM group at the 2′,3′-dihydroxyls, the progress of this reaction was indicated by the consumption of the starting material (monitored by thin layer chromatography (TLC); to prepare the TLC sample, the reaction mixture was added to methanol and the resulting solution was used as the sample). Meanwhile, compound 9 was used in the next step directly after concentration under reduced pressure. Subsequently, using methylmagnesium chloride (MeMgCl) (1.5 equiv.) as the deprotonating agent, the 9 was reacted with 10 (1.1 equiv.) to furnish the unstable intermediate 11. When the phosphorylation reaction was quenched with saturated NH4Cl solution, the DMAM group at 2′,3′-dihydroxyls of the compound 11 was concomitantly decomposed to provide the stable intermediate 12 with an 85% isolated yield, and the hydrolyzed impurity 8 was not observed by TLC analysis. With 12 in hand, the deprotection of the DMAM group at C6-amine was performed with AcOH (20 equiv.) in alcohol for 18 h at 50 °C and the product was afforded in 90% yield by chromatography purification. Overall, the yield of product 1 prepared from compound 5 was up to 76%, which was much higher than that of 43% reported by Gilead.16
Encouraged by the favorable outcome, we tried to optimize this new approach. Given the risk of the ester-transfer reaction in the amino acid ester moiety of compound 12 or 1 in ethanol, the deprotection reaction was conducted in isopropanol with lower reactivity, which proceeded smoothly as well and provided nearly the same result as using ethanol as the solvent. To simplify this synthesis, we quenched the phosphorylation reaction with a solution of AcOH (20 equiv.) in THF or isopropanol and the resulting mixture was warmed to 50 °C to remove the DMAM protection; however, this reaction progressed much slower than that using the pure compound 12 as the starting material. We speculated that the existence of magnesium ion (Mg2+) might affect the speed of the deprotection reaction. According to this hypothesis, the reaction mixture was extracted with ethyl acetate (EA) to remove Mg2+ after the phosphorylation reaction was quenched with saturated aq. NH4Cl solution. As expected, the reaction of crude 12 obtained from the workup of extraction and concentration with AcOH was continued for 18 h in isopropanol. Additionally, the protected nucleoside 9, because of its instability, was directly subjected to the next reaction after simple workup. Therefore, this synthesis could be conducted successively, which not only avoided the purification of intermediates but also greatly improved the synthetic efficiency.
Having the simplified synthesis in hand, the optimizations of reaction conditions were investigated. Because of the stability of product 1 and its isomer 13 in the conditions of the deprotection reaction, the ratio of two P-chiral isomers could be used to indicate the stereoselectivity of the phosphorylation reaction. To reduce the solvent type in the synthesis, we tried to replace pyridine with THF in the first step. The condensation reaction of compound 5 and DMF-DMA was performed in THF at 60 °C for 3 h. When the starting material 5 was consumed completely, the solvent was removed under reduced pressure and the resultant mixture was processed by solvent exchange with toluene once to remove the unreacted DMF-DMA and the methanol generated from the condensation reaction. The coupling of the obtained crude 9 and 10 (1.2 equiv.) in the presence of MeMgCl (1.5 equiv.) was conducted at −10 °C for 2 h. After simple posttreatment, the obtained 12 was used with AcOH in the deprotection reaction at 50 °C for 18 h. Disappointedly, this attempt provided the final product 1 with much lower d.r. (Table 1, entry 2, 36/1 d.r.) than that using pyridine as the solvent of the condensation reaction (entry 1, 161/1 d.r.). The obvious decrease in the stereoselectivity indicated that the remained pyridine in the crude 9 might exert a special effect on the phorsphorylation reaction of 10 and 9. Subsequently, 1 equiv. of pyridine was added to the phorsphorylation reaction but the ratio of 1 and 13 was only improved slightly (entry 3, LCAP of 92.7%., 42/1 d.r.). Nonetheless, the role of pyridine in the two reactions, including the condensation reaction and the phosphorylation reaction, needed to be studied further. Although pyridine was an infrequently used solvent, it was still chosen as the solvent of the condensation reaction in consideration of its benefit to the stereoselectivity in the synthesis.
Table 1. Condition Optimization of the Condensation Reaction (I) and the Phosphorylation Reaction (II)a.
|
I |
II |
III |
|||||
|---|---|---|---|---|---|---|---|
| entry | solvent | pyridine (equiv.) | deprotonating agent | T (°C) | T (h) | LCAP of 1 (%)d | d.r. ratioe (1/13) |
| 1 | pyridineb | MeMgCl | –10 | 2 | 96.7 | 161/1 | |
| 2 | THFc | MeMgCl | –10 | 2 | 93.0 | 36/1 | |
| 3 | THFc | 1.0 | MeMgCl | –10 | 2 | 92.7 | 42/1 |
| 4 | pyridineb | MeMgCl | –20 | 2 | 92.9 | 232/1 | |
| 5 | pyridineb | t-BuMgCl | –20 | 2 | 94.1 | 783/1 | |
General procedure: the condensation reaction of compound 5 (500 mg, 1.72 mmol, 1 equiv.) and DMF-DMA (820 mg, 6.88 mmol, 4.0 equiv.) afforded compound 9. The coupling of the obtained crude 9 and compound 10 (1.02 g, 2.06 mmol, 1.2 equiv.) was performed in the presence of the deprotonating agent (2.58 mmol, 1.5 equiv.). When crude 9 disappeared, the phosphorylation was quenched with aq. NH4Cl solution and provided compound 12. After simple aftertreatment, crude 12 was subjected to the solution of acetic acid (2.06 g, 34.4 mmol, 20.0 equiv.). Finally, product 1 was obtained.
The condensation was conducted at 25 °C for 3 h.
The condensation was conducted at 60 °C for 3 h.
LCAP: the area (%) of compound 1 determined by HPLC in the final reaction mixture.
The d.r. ratio was determined by HPLC.
Next, we studied the effects of the reaction temperature and the deprotonating agent on the phosphorylation reaction. When the reaction was conducted at −20 °C (entry 4), the LCAP of 1 slightly decreased to 92.9% and the value of d.r. was improved to 232/1, whereas at lower temperature (−40 °C), it took more than 8 h to finish the transformation of compound 12 to compound 1. Notably, the lower reaction temperature resulted in the lower reactivity. In addition, using t-butylmagnesium chloride (t-BuMgCl) as the deprotonating agent was studied (entry 5). Surprisingly, the stereoselectivity was significantly improved to 783/1 with comparable LCAP of 94.1%, and the reaction was finished in only 2 h.
On the basis of the optimized conditions, we investigated the feasibility for the synthesis of remdesivir on the gram-scale. In a 5 g scale synthesis, compound 5 was reacted with DMF-DMA to provide crude 9, and then 9 was phosphorylated by compound 10 in the presence of t-BuMgCl followed by quenching with saturated NH4Cl solution. After extraction and concentration, the obtained 12 was deprotected in the solution of AcOH in the isopropanol to furnish product 1 with 85% yield in 99.9:0.1 d.r. by chromatography purification.
Conclusions
In summary, facilitated by DMF-DMA as the protecting agent, a highly efficient synthesis of remdesivir from GS-441524 was developed. Compared to the 43% overall yield reported in the literature,16 this method provided remdesivir with 85% overall yield through a three-step sequence. Moreover, this synthesis could avoid the generation of the degraded impurity and be conducted successively without purification of intermediates. These advantages improved the synthetic efficiency of remdesivir greatly.
Experimental Section
General Information
(2R,3R,4S,5R)-2-(4-Aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile 5 and 2-ethylbutyl ((S)-(perfluorophenoxy)(phenoxy)phosphoryl)-l-alaninate 10 were obtained from Topharman Shanghai Co., Ltd. Other chemical reagents and solvents, such as DMF-DMA, 3.0 M methylmagnesium chloride solution in tetrahydrofuran (THF), 1.7 M t-butylmagnesium chloride solution in tetrahydrofuran, pyridine, THF, isopropanol, acetic acid, and ethyl acetate, were commercially available. 1H NMR, 13C{1H} NMR, and 31P NMR spectra were recorded on a Bruker 500 Hz instrument. Low-resolution mass spectra were measured on a Finnigan LTQ mass spectrometer (Thermo Fisher), and the corresponding high-resolution were measured on a Q-TOF mass spectrometer (Agilent G6520). HPLC spectra were obtained with an Agilent 1100 HPLC system.
((2R,3S,4R,5R)-5-(4-Aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl Phenyl Hydrogen Phosphate (8)
To a solution of 2 (0.50 g, 0.78 mmol), prepared from 5 according to the reported method, in tetrahydrofuran (10.0 mL) was slowly added 37% aq. hydrochloric acid (0.80 mL) at 0 °C. The reaction mixture was then warmed to 25 °C and stirred for 5 h. When TLC showed compound 2 had disappeared, the pH of the reaction mixture was adjusted to 8 by the addition of saturated aq. NaHCO3 solution. The resulting mixture was processed by reverse-phase HPLC using a YMC*GEL ODS column with gradient elution using acetonitrile and water. Finally, 8 was obtained with 99.6% purity as a white solid (54 mg, 15% yield). 1H NMR (500 MHz, DMSO) δ 8.12–7.71 (m, 3H), 7.18–7.14 (m, 2H), 7.10 (d, J = 7.9 Hz, 2H), 6.94–6.89 (m, 2H), 6.80 (d, J = 4.5 Hz, 1H), 6.29 (s, 1H), 5.60 (s, 1H), 4.62 (d, J = 5.0 Hz, 1H), 4.15 (dd, J = 9.5, 5.4 Hz, 1H), 3.98–3.92 (m, 2H), 3.85 (dt, J = 11.6, 5.8 Hz, 1H). 13C{1H} NMR (126 MHz, DMSO) δ 155.6, 153.9, 153.9, 147.8, 128.7, 124.0, 121.7, 120.0, 120.0, 117.2, 116.4, 110.4, 100.9, 83.3, 83.3, 78.3, 74.4, 70.3, 64.6, 64.6. 31P NMR (202 MHz, DMSO) δ −5.64. HRMS (ESI) m/z: [M + H]+ calcd for C18H19N5O7P+ 448.1017, found 448.1019.
2-Ethylbutyl((R)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyan-o-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)-l-alaninate (13)
Compound 4 (1.2 g, 3.6 mmol) and racemic 10 (Rp/Sp, 1/1) (2.0 g, 4.0 mmol) were dissolved in THF (20 mL). The resultant solution was cooled to −10 °C and 3.0 M methylmagnesium chloride solution in tetrahydrofuran (1.8 mL, 5.4 mmol) was added dropwise under a N2 atmosphere. After that, the reaction mixture was stirred for 1 h at −5 °C. When TLC indicated 10 was consumed completely, the reaction was quenched with acetic acid and then the solvent was removed under reduced pressure. The obtained mixture was redissolved in ethyl acetate. The resultant solution was washed with water, saturated aq. NaHCO3 solution and brine orderly, dried over Na2SO4, and concentrated. The crude 2, obtained from the above phosphorylated reaction, was added to the mixture of 37% aq. hydrochloric acid solution (3.6 mL) and tetrahydrofuran (25 mL) at 0 °C. After the reaction temperature rose to 25 °C, stirring was continued for 5 h. When TLC showed completion of this reaction, saturated aq. NaHCO3 solution was added until the pH of the reaction mixture was 8. The reaction mixture was concentrated to remove tetrahydrofuran and the resultant solution was extracted with ethyl acetate twice. The combined organic phase was washed with brine, dried over Na2SO4, and evaporated. The obtained concentrate was processed by a Daicel AD-H chiral column with isocratic elution by solution containing 70% hexane and 30% ethanol. Compound 13 was afforded with 92.9% purity as a white solid (585 mg, 27% yield). 1H NMR (500 MHz, DMSO) δ 7.97–7.80 (m, 3H), 7.32 (t, J = 7.9 Hz, 2H), 7.17–7.12 (m, 3H), 6.91 (d, J = 4.5 Hz, 1H), 6.84 (d, J = 4.5 Hz, 1H), 6.30 (d, J = 6.1 Hz, 1H), 6.02 (dd, J = 12.9, 10.1 Hz, 1H), 5.38 (d, J = 5.5 Hz, 1H), 4.65 (t, J = 5.5 Hz, 1H), 4.31–4.24 (m, 2H), 4.17–4.09 (m, 1H), 4.01–3.87 (m, 3H), 3.82–3.72 (m, 1H), 1.46–1.39 (m, 1H), 1.28–1.22 (m, 4H), 1.16 (d, J = 7.1 Hz, 3H), 0.80 (t, J = 7.5 Hz, 6H). 13C{1H} NMR (126 MHz, DMSO) δ 173.3, 173.3, 155.6, 150.7, 150.6, 147.9, 129.5, 124.5, 123.5, 120.2, 120.1, 116.9, 116.6, 110.4, 100.8, 82.2, 82.2, 78.8, 74.1, 69.9, 66.1, 65.2, 65.1, 49.9, 22.6, 22.5, 19.6, 19.6, 10.8, 10.8. 31P NMR (202 MHz, DMSO) δ 3.66. HRMS (ESI) m/z: [M + H]+ calcd for C27H36N6O8P+ 603.2327, found 603.2330.
2-Ethylbutyl ((S)-(((2R,3S,4R,5R)-5-cyano-5-(4-(((dimethylamino)methylene)amino-)pyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phen-oxy)phosphoryl)-l-alaninate (12)
DMF-DMA (1.64 g, 13.7 mmol) was added in one portion to the suspension of 5 (1.00 g, 3.42 mmol) in pyridine (5.0 mL) at 25 °C. The reaction mixture was stirred for 6 h at that temperature. When TLC indicated the full conversion of 5 (a sample was taken from the reaction mixture and quenched with methanol), the mixture was concentrated under reduced pressure. The obtained crude 9 and the phosphorylated agent 10 (1.86 g, 3.76 mmol) were dissolved in THF (25.0 mL). When the resultant solution was cooled to −10 °C, 3.0 M methylmagnesium chloride solution in tetrahydrofuran (1.7 mL, 5.13 mmol) was added dropwise under a N2 atmosphere and the reaction temperature was not higher than −5 °C during the process of dropping. When TLC indicated the phosphorylated reaction was completed, the reaction mixture was poured into saturated aq. NH4Cl solution and then concentrated to remove solvent. The obtained mixture was extracted with ethyl acetate twice. The organic phase was washed with water and brine orderly, dried over Na2SO4, and evaporated. The crude product was purified by chromatography purification with a gradient elution by solution containing CH2Cl2 and methanol (the ratio of CH2Cl2 and methanol was ranged from 80/1 to 30/1) to afford 12 with 97.5% purity as a foamy solid (1.91 g, 85% yield). 1H NMR (500 MHz, DMSO) δ 8.94 (s, 1H), 8.15 (s, 1H), 7.37–7.32 (m, 2H), 7.21–7.14 (m, 3H), 6.94 (d, J = 4.5 Hz, 1H), 6.80 (d, J = 4.5 Hz, 1H), 6.33 (d, J = 6.2 Hz, 1H), 6.02 (dd, J = 13.0, 10.1 Hz, 1H), 5.40 (d, J = 5.6 Hz, 1H), 4.72–4.66 (m, 1H), 4.29–4.21 (m, 2H), 4.10 (dt, J = 12.3, 6.2 Hz, 1H), 3.99 (dd, J = 10.9, 5.6 Hz, 1H), 3.95 (dd, J = 10.9, 5.9 Hz, 1H), 3.86 (dd, J = 10.9, 5.8 Hz, 1H), 3.84–3.77 (m, 1H), 3.24 (s, 3H), 3.18 (s, 3H), 1.45–1.37 (m, 1H), 1.28–1.18 (m, 7H), 0.79 (t, J = 7.5 Hz, 6H). 13C{1H} NMR (126 MHz, DMSO) δ 173.2, 173.2, 160.2, 158.1, 150.8, 150.7, 147.4, 129.6, 124.5, 123.4, 122.6, 120.1, 120.1, 116.8, 111.6, 101.8, 82.4, 82.4, 78.8, 74.0, 70.0, 66.1, 65.5, 65.4, 49.7, 41.1, 34.9, 22.6, 22.5, 19.8, 19.7, 10.8, 10.7. 31P NMR (202 MHz, DMSO) δ 3.68. HRMS (ESI) m/z: [M + H]+ calcd for C30H41N7O8P+ 658.2749, found 658.2739.
2-Ethylbutyl ((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cya-no-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)-l-alaninate (1)
AcOH (1.8 g, 30.0 mmol) was added to the solution of 12 (1.0 g, 1.5 mmol) in ethanol (15.0 mL) in one portion at 25 °C. After that, the reaction mixture was heated to 50 °C and stirred for 18 h under a N2 atmosphere. When TLC showed that 12 was consumed completely, the mixture was concentrated under reduced pressure. The residue was dissolved in ethyl acetate then washed with saturated aq. NaHCO3 solution, water and brine. The obtained organic phase was dried over Na2SO4 and evaporated. The crude product was subjected to the silica gel column with CH2Cl2 and methanol as the elution (CH2Cl2: methanol = 50:1 to 20:1) to provide 1 with 99.8% purity as a foamy solid (813 mg, 90% yield). 1H NMR (500 MHz, DMSO) δ 8.00–7.81 (m, 3H), 7.38–7.31 (m, 2H), 7.22–7.15 (m, 3H), 6.90 (d, J = 4.5 Hz, 1H), 6.83 (d, J = 4.5 Hz, 1H), 6.33 (d, J = 6.1 Hz, 1H), 6.03 (dd, J = 13.0, 10.1 Hz, 1H), 5.37 (d, J = 5.8 Hz, 1H), 4.68–4.62 (m, 1H), 4.29–4.22 (m, 2H), 4.13–4.07 (m, 1H), 3.95 (dd, J = 10.9, 5.8 Hz, 2H), 3.90–3.78 (m, 2H), 1.45–1.37 (m, 1H), 1.28–1.19 (m, 7H), 0.79 (t, J = 7.5 Hz, 6H). 13C{1H} (126 MHz, DMSO) δ 173.2, 173.2, 155.6, 150.8, 150.7, 147.9, 129.6, 124.5, 123.5, 120.1, 120.0, 116.9, 116.6, 110.4, 100.8, 82.1, 82.0, 79.0, 74.0, 69.9, 66.1, 65.4, 65.4, 49.7, 22.6, 22.5, 19.8, 19.7, 10.8, 10.7. 31P NMR (202 MHz, DMSO) δ 3.69. HRMS (ESI) m/z: [M + H]+ calcd for C27H36N6O8P+ 603.2327, found 603.2328.
Preparation of 1 from 5 without Purification of Intermediates on a 5 g Scale.
To a suspension of 5 (5.0 g, 17.2 mmol) in pyridine (20.0 mL) was added DMF-DMA (8.2 g, 68.8 mmol). The reaction mixture was stirred for 6 h at 25 °C. When the condensation reaction of 5 and DMF-DMA was finished, pyridine was removed under reduced pressure. The obtained 9 and 10 (10.2 g, 20.6 mmol) were dissolved in THF (50.0 mL). When the resultant solution was cooled to −20 °C, 1.7 M t-butylmagnesium chloride solution in tetrahydrofuran (15.2 mL, 25.8 mmol) was added dropwise under a N2 atmosphere and the temperature was kept under −15 °C. When the coupling reaction of 9 and 10 was completed, the reaction mixture was poured into saturated aq. NH4Cl solution. After that, the resultant solution was concentrated and then redissolved in ethyl acetate. The obtained solution was washed with water and brine orderly, dried over Na2SO4, and concentrated under reduced pressure. The residue was added to the mixture of AcOH (20.6 g, 0.34 mol) and isopropanol (100.0 mL). The temperature then rose to 50 °C and stirring was continued for 18 h. When the deprotection reaction was completed, the reaction mixture was evaporated. The obtained mixture was dissolved in ethyl acetate and neutralized with saturated aq. NaHCO3 solution. When the pH of the mixture was 8, the organic phase was separated, washed with water and brine orderly, dried over Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column using the mixture of CH2Cl2 and methanol (CH2Cl2: methanol = 50:1–20:1) to give 1 with 99.4% purity as a foamy solid (8.8 g, 85% yield).
Acknowledgments
This work was financed by the grant from Shanghai Science and Technology Committee in China (21S11903100).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02835.
1H, 13C{1H}, and 31P NMR spectra of compounds and HRMS spectrum (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yin W.; Mao C.; Luan X.; Shen D. D.; Shen Q.; Su H.; Wang X.; Zhou F.; Zhao W.; Gao M.; Chang S.; Xie Y. C.; Tian G.; Jiang H. W.; Tao S. C.; Shen J.; Jiang Y.; Jiang H.; Xu Y.; Zhang S.; Zhang Y.; Xu H. E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Woolner E.; Perry J. K.; Feng J. Y.; Porter D. P.; Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon A.; Le N. T.; Selisko B.; Eydoux C.; Alvarez K.; Guillemot J. C.; Decroly E.; Peersen O.; Ferron F.; Canard B. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020, 178, 104793. 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakchaure P. D.; Ghosh S.; Ganguly B. Revealing the Inhibition Mechanism of RNA-Dependent RNA Polymerase (RdRp) of SARS-CoV-2 by Remdesivir and Nucleotide Analogues: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2020, 124, 10641–10652. 10.1021/acs.jpcb.0c06747. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang D.; Du G.; Du R.; Zhao J.; Jin Y.; Fu S.; Gao L.; Cheng Z.; Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. D.; Lye D. C. B.; Hui D. S.; Marks K. M.; Bruno R.; Montejano R.; Spinner C. D.; Galli M.; Ahn M. Y.; Nahass R. G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19—the science of safety and effectiveness. U.S. Food and Drug Administration. https://www.fda.gov/drugs/news-events-human-drugs/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness.
- Gottlieb R. L.; Vaca C. E.; Paredes R.; Mera J.; Webb B. J.; Perez G.; Oguchi G.; Ryan P.; Nielsen B. U.; Brown M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUA 046 Gilead Remdesivir LOA Outpatients. U.S. Food and Drug Administration. https://www.fda.gov/media/137564/download.
- Paymode D. J.; Cardoso F. S. P.; Agrawal T.; Tomlin J. W.; Cook D. W.; Burns J. M.; Stringham R. W.; Sieber J. D.; Gupton B. F.; Snead D. R. Expanding Access to Remdesivir via an Improved Pyrrolotriazine Synthesis: Supply Centered Synthesis. Org. Lett. 2020, 22, 7656–7661. 10.1021/acs.orglett.0c02848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Hu T.; Zhang Y.; Wei D.; Zheng W.; Zhu F.; Tian G.; Aisa H. A.; Shen J. Weinreb Amide Approach to the Practical Synthesis of a Key Remdesivir Intermediate. J. Org. Chem. 2021, 86, 5065–5072. 10.1021/acs.joc.0c02986. [DOI] [PubMed] [Google Scholar]
- Von Keutz T.; Williams J. D.; Kappe C. O. Flash Chemistry Approach to Organometallic C-Glycosylation for the Synthesis of Remdesivir. Org. Process Res. Dev. 2021, 25, 1015–1021. 10.1021/acs.oprd.1c00024. [DOI] [Google Scholar]
- Vieira T.; Stevens A. C.; Chtchemelinine A.; Gao D.; Badalov P.; Heumann L. Development of a Large-Scale Cyanation Process Using Continuous Flow Chemistry En Route to the Synthesis of Remdesivir. Org. Process Res. Dev. 2020, 24, 2113–2121. 10.1021/acs.oprd.0c00172. [DOI] [PubMed] [Google Scholar]
- Xue F.; Zhou X.; Zhou R.; Zhou X.; Xiao D.; Gu E.; Guo X.; Xiang J.; Wang K.; Yang L.; et al. Improvement of the C-glycosylation Step for the Synthesis of Remdesivir. Org. Process Res. Dev. 2020, 24, 1772–1777. 10.1021/acs.oprd.0c00310. [DOI] [PubMed] [Google Scholar]
- Kumar Palli K.; Ghosh P.; Krishna Avula S.; Sridhara Shanmukha Rao B.; Patil A. D.; Ghosh S.; Sudhakar G.; Raji Reddy C.; Mainkar P. S.; Chandrasekhar S. Total synthesis of remdesivir. Tetrahedron Lett. 2022, 88, 153590. 10.1016/j.tetlet.2021.153590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T. K.; Jordan R.; Lo M. K.; Ray A. S.; Mackman R. L.; Soloveva V.; Siegel D.; Perron M.; Bannister R.; Hui H. C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Zhang L.; Huo X.; Zhang Z.; Yuan Q.; Li P.; Chen J.; Zou Y.; Wu Z.; Zhang W. Catalytic Asymmetric Synthesis of the anti-COVID-19 Drug Remdesivir. Angew. Chem., Int. Ed. Engl. 2020, 59, 20814–20819. 10.1002/anie.202011527. [DOI] [PubMed] [Google Scholar]
- Gannedi V.; Villuri B. K.; Reddy S. N.; Ku C. C.; Wong C. H.; Hung S. C. Practical Remdesivir Synthesis through One-Pot Organocatalyzed Asymmetric (S)-P-Phosphoramidation. J. Org. Chem. 2021, 86, 4977–4985. 10.1021/acs.joc.0c02888. [DOI] [PubMed] [Google Scholar]
- Ross B. S.; Ganapati Reddy P.; Zhang H.-R.; Rachakonda S.; Sofia M. J. Synthesis of diastereomerically pure nucleotide phosphoramidates. J. Org. Chem. 2011, 76, 8311–8319. 10.1021/jo201492m. [DOI] [PubMed] [Google Scholar]
- Chun B. K.; Clarke M. O.; Doerffler E.; Hui H. C.; Jordan R.; Mackman R. L.; Ray A. S.; Siegel D.. Methods for Treating Filoviridae Virus Infections. WO2016069826, 2016.
- Hu T.; Xie Y.; Zhu F.; Gong X.; Liu Y.; Xue H.; Aisa H. A.; Shen J. One-Pot” Synthesis of Molnupiravir from Cytidine. Org. Process Res. Dev. 2022, 26, 358–364. 10.1021/acs.oprd.1c00419. [DOI] [Google Scholar]
- Hu T.; Xie Y.; Liu Y.; Xue H.; Zhu F. A.; Aisa H.; Shen J.. A Convenient and Cost Efficient Route Suitable for “One-Pot” Synthesis of Molnupiravir.ChemRxiv, 2021. 10.26434/chemrxiv.14208206.v1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.