Abstract
Gas hydrate risks minimization in deepsea hydrocarbon flowlines, especially in high water to oil ratios, and is critical for the oil and gas flow assurance industry. Although there are several reviews on gas hydrate mitigation in gas-dominated systems, limited reviews have been dedicated to the understanding and mechanism of hydrate formation and mitigation in oil-dominated systems. Hence, this review article discusses and summarizes the prior studies on the hydrate formation behavior and mitigation in oil-dominated multiphase systems. The factors (such as oil volume or water cut, bubble point, and hydrate formers) that affect hydrate formation in oil systems are also discussed in detail. Furthermore, insight into the hydrate mitigation and mechanism in oil systems is also presented in this review. Also, a detailed table on the various studied hydrate tests in oil systems, including the experimental methods, inhibitor type, conventions, and testing conditions, is provided in this work. The findings presented in this work are relevant for developing the best solution to manage hydrate formation in oil-dominated systems for the oil and gas industry.
1. Introduction
Gas hydrates are solid crystalline ice-like materials of gas molecules (C2H6, CO2, CH4, and other lighter weights) trapped in water molecules at low temperatures and high pressures.1 Hammerschmidt et al.2 reported that hydrates in the production or transportation pipeline are among the most significant concerns in oil and gas flow assurance. Thus, the oil and gas sector constantly seeks ways to avoid hydrate formation in pipelines to avoid blockages and potential danger.3,4 The base of hydrate inhibition in thermodynamic hydrate inhibitors (THIs) keeps the operation system’s temperature and pressure out of the hydrate formation region. The system conditions can be shifted from a hydrate-stable zone to a hydrate-free zone by heating, employing insulated pipelines, and depressurization.5 However, long-distance pipelines are more expensive to heat and insulate.6,7 Water removal is a critical technique to avoid hydrate formation. It has been applied to many long-distance transportation lines.8 However, removing water from oil and gas is difficult or expensive, particularly in the production pipelines.9
An efficient technique to inhibit hydrate formation is to inject chemical additives (called hydrate inhibitors) into the pipeline. Hydrate inhibitors include kinetic hydrate inhibitors (KHIs), antiagglomerant inhibitors, and thermodynamic hydrate inhibitors (THIs).10 The first two methods are called low-dose inhibitors. In the water–oil–gas system, antiagglomerate inhibitors must be used, and water cut should be less than 50 wt %.11 Water cut could vary depending on the system used. In the oil-dominated system, oil volume is more than water and gas, while for a gas-dominated system, the gas phase is large compared to oil and water. The water-dominant system primarily refers to a water-dominant phase inside the pipelines/cell where the volume of water is higher than that of gas and oil.12 In gas-dominated pipelines, antiagglomerate inhibitors are not significantly impacted due to high water cuts. Compared to gas-based pipelines, the hydrate blockage in oil-based pipelines/systems is different due to different phases and hydrocarbons. The presence of oil in the system makes it complex, with phenomena such as water entrainment or dispersion of water droplets in the continuous oil phase due to fluid shear and surface-active components in the oil. Also, the hydrate formation and growth occur at the water–oil/water–gas interface, leading to additional complications compared to the gas-dominated system. Such complications affect the hydrate inhibition mechanism and formation behavior in the oil systems and thus require special or slightly different techniques to manage hydrate growth in the oil-dominated gas hydrate.13
There are generally two phases in a gas-dominated system/pipeline: gas and water exist simultaneously; however, the hydrate phase might be formed when the pipeline operates within the hydrate phase behavior. When the gas and water disperse in each other within the hydrate stability zone, they flow through the pipeline and form hydrate particles or hydrate slurry.13,14 During hydrate formation, the hydrogen-bonded water network opens its pores due to low-temperature and high-pressure conditions that encapsulate the small gas molecules, causing hydrate formation. The solid–liquid ratio of hydrate slurry grows as the hydrate concentration increases, causing hydrate deposition in the pipelines. The pipeline will be shut down due to the drop in liquid levels.15
Additionally, water and gas can form a hydrate layer inside the pipelines, which accumulates with time and reduces the pipe’s diameter, ultimately blocking the pipelines. On the other hand, the oil-dominated systems have extra layers of oil in between the water and gas phases. In forming hydrate in oil pipelines, the gas should be dissolved in the water system first. Then nucleation starts, and hydrate begins in the pipelines. Thus, preventing hydrate nuclei and growth is more critical in gas-dominated pipelines than oil-dominated ones. Consequently, the hydrate grows fast, and it is challenging to keep the pipes from being blocked. To avoid hydrate formation, thermodynamic hydrate inhibitors (THIs) and kinetic hydrate inhibitors (KHIs) are necessary in the natural gas pipeline, mainly when it operates under high-pressure and low-temperature conditions, which is usually the case in offshore scenarios. A drawback of THIs is that they require large volumes to mitigate hydrates effectively, particularly for oil and gas wells with a high water cut.16
Many factors affect the formation of hydrate plugging in a multiphase mixed rich liquid (gas–oil–water) system, resulting in different impacts on hydrate particle coalescence and deposition. As a result, gas hydrates plug pipelines in various ways, making it more challenging to prevent and manage hydrate formation in the oil- or multiphase-dominated system.17
This study reviews the main factors affecting gas hydrate as well as the hydrate inhibitors that contribute to inhibiting hydrates in oil-dominated pipelines, reviews research efforts over the past few years to increase KHI performance, and summarizes the applications of kinetic hydrate inhibitors (KHIs), antiagglomerate inhibitors, and thermal hydrate inhibitors (THIs).
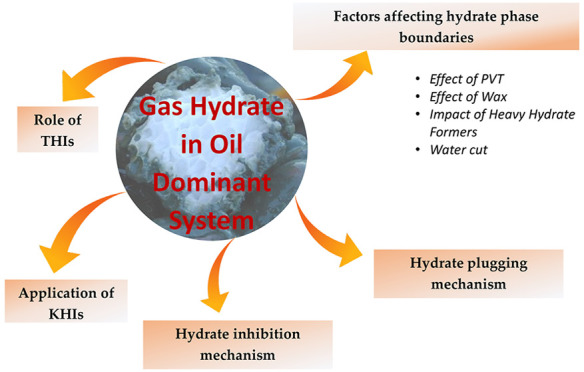
2. Factors Affecting Hydrate Phase Boundaries in Oil-Dominated Systems
In oil systems, several factors affect the hydrate phase boundaries. This section discusses the various factors that affect hydrate phase boundary conditions in oil-dominated systems.
2.1. Water Cut, Liquid Loadings, and Kihara Potential Parameters
Hydrate-formation components can be found in the vapor, oil, and aqueous phases, and producing a high velocity of the fluid’s vapor–liquid equilibrium is essential. As a result, a fine-tuned EoS fluid model can serve as the foundation for most dissociation hydrate curves. EoS models are often set to match the PVT data at reservoir temperatures over 65 °C.18−20 There is an argument made regarding an error during the crucial stage that could be developed; such fluid models might not indicate accurate fugacity predictions over the range of temperatures encountered, causing errors in hydrate predictions as well as the prediction of the temperature that depends on the fugacities in a typical production system.21 According to the previous research by Golsanami et al.,22 using bubble point data at low temperatures to tune the EoS fluid model has little effect on hydrate dissociation condition predictions.
The mutual solubility of hydrocarbons and water is very small, and in most cases, the behavior of the hydrocarbon phase is studied separately from the water phase. However,20 studies by Lv et al. have shown that at a given pressure above the bubble point of the system a high water cut could shift the hydrate phase boundary to lower temperatures.21 Chang-Sheng23 conducted experiments at five different water cuts in an oil system to study the effect of different water cuts (5, 10, 15, 20, and 25 vol %), and the natural gas solubilities in the emulsion systems were also examined. The results of the experiments indicated that the solubility of natural gas in emulsion systems increases practically linearly with pressure and decreases with water cut. For systems with lower water cut, a delayed hydrate formation stage exists, while rapid hydrate formation occurs. The gas–liquid dissolution equilibrium phase does not appear in the pressure curve for systems with higher water cut. The amount of gas consumed due to hydrate formation is much larger at a high water cut than at a low water cut. During the hydrate formation process, fractional distillation for natural gas components is also possible. Moreover, the experiments on hydrate dissociation also showed that the dissociation rate and the amount of dissociated gas increase with the increase of water cut.
Figure 1 shows the pump ΔP with two different liquid loadings (50 and 75 vol %) with two different velocities (1 and 1.75 m/s) as a function of hydrate for liquid loading. The results showed that there is not much effect on transition, implying that the hydrate concentration in water determines the pressure drop. It is important to notice that for a given hydrate the absolute hydrate volume would be higher at higher liquid loading, showing that more hydrates could be transported without a rise in the pump ΔP.24
Figure 1.
Impact of liquid loading on the pump during hydration of two different velocities (reproduced with permission from ref (24)).
According to previous studies by Avlonitis and Sergeeva et al.,24,25 most available hydrate dissociation studies are restricted to low- and moderate-pressure situations (e.g., less than 30 MPa). Typically, these data are used to tune the Kihara potential variables of hydrate formers. However, using these variables to predict the hydrate stability zones of oil systems at high pressures, significantly above the system’s bubble point, may result in a large deviation.25
2.2. Effect of Wax
Wax hydrate formation could be possible at certain pressures and temperatures in a reservoir fluid system containing water and heavy and light hydrocarbons. The thermodynamic behavior of one solid may be affected by the formation of another solid.26 The formation of gas hydrate can eliminate light components from fluid phases (e.g., C1, C2, C3, i-C4, n-C4, N2, CO2, and H2S), which can increase waxy component concentrations, affecting the wax phase behavior.27 On the other hand, the wax formation can eliminate heavy hydrocarbons, resulting in a higher concentration of light components in the remaining fluid. The temperature and pressure of hydrate dissociation may be affected by differences in hydrate-former composition.28−30
Furthermore, the wax formation can provide necessary nucleation sites, and in some cases, it may promote hydrate formation by lowering the required subcooling and vice versa. Although wax and hydration have been extensively studied, they were traditionally assumed to be unrelated issues.31 The estimated hydrate phase boundaries ignoring the impact of wax formation may be overly optimistic (i.e., showing lower hydrate formation temperatures). Similarly, if the hydrate formation is ignored, the wax problem could be underestimated. As a result, integrated wax and hydrate research is required.
2.3. Impact of Heavy Hydrate Formers
Components of reservoir fluids are usually categorized up to and including C5 and C6, and heavier components are grouped into single carbon number (SCN) categories; i.e., there is no additional information available on single heavy hydrate formers (HHFs). As a result, the effect of HHFs on the hydrate phase boundary cannot be taken into consideration.32 Cyclopentane has a significant effect on the location of the hydrate phase boundary33 being able to shift the hydrate dissociation temperature by almost 0.5 °C when 0.5 mol % of the non-hydrate-forming C6 fraction in a reservoir fluid is removed and replaced by 0.5 mol % of cyclopentane. Table 1 provides real case study data that show the distribution of components in three different reservoir fluids from the North Sea.34 As can be seen, the assumption of Rasmussen and Pedersen35 that 0.5 mol % of the C6 fraction in a reservoir fluid is cyclopentane is probably a conservative assumption for some reservoir fluids.29
Table 1. Component Distribution of Mole Fraction Reported by Three Different Reservoir Fluids from the Actual Case Study in the North Sea Reported by Mohammadi34.
| mole fraction in SCN |
||||
|---|---|---|---|---|
| SCN | components | North Sea oil | North Sea GC2 | |
| C6 | 2,2-DM-C4 | 0.02 | 0.01 | 0.01 |
| Cy-C5 | 0.09 | 0.08 | 0.05 | |
| 2,3-DM-C4 | 0.04 | 0.03 | 0.04 | |
| 2-M-C5 | 0.23 | 0.25 | 0.29 | |
| 3-M-C5 | 0.14 | 0.15 | 0.19 | |
| normal-C6 | 0.48 | 0.49 | 0.42 | |
| C7 | M-Cy-C5 | 0.12 | 0.14 | 0.15 |
| 2,2-DM-C5 | - | 0.00 | 0.01 | |
| 2,3-DM-C5 | - | 0.02 | 0.04 | |
| 2,4-DM-C5 | 0.01 | 0.02 | 0.01 | |
| 3,3-DM-C5 | - | 0.00 | 0.00 | |
| 3-E-C5 | - | 0.00 | 0.01 | |
| 2,2,3-TM-C4 | - | 0.00 | 0.00 | |
| benzene | 0.20 | 0.16 | 0.13 | |
| Cy-C6 | 0.26 | 0.24 | 0.10 | |
| 2-M-C6 | 0.00 | 0.06 | 0.08 | |
| 1,1-DM-Cy-C5 | 0.05 | 0.01 | 0.01 | |
| 3-M-C6 | 0.05 | 0.06 | 0.11 | |
| 1,cis-3-DM-Cy-C5 | 0.00 | 0.02 | 0.02 | |
| 1,trans-3-DM-Cy-C5 | 0.02 | 0.02 | 0.02 | |
| 1,trans-2-DM-Cy-C5 | 0.02 | 0.04 | 0.04 | |
| normal-C7 | 0.18 | 0.21 | 0.25 | |
| unspecified C7 | 0.08 | - | - | |
3. Hydrate-Plugging Mechanism in the Multiphase Systems
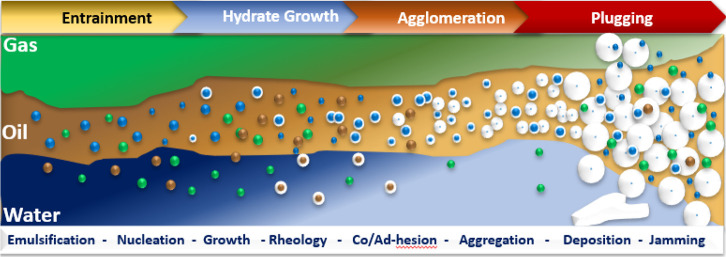
According to various media, the subsea mixed pipeline systems are divided into two different phases: oil-dominated and gas-dominated systems.23,36 Thus, due to the water cut difference, the term rich liquid phase is used for those systems that contain more liquid phase such as an oil-based system and a water-based system compared to the rich gas phase which has more gas phase than liquid. The hydrate formation and blocking caused by hydrates are different at each stage depending on the environmental circumstances.37 However, there are some surface-active substances present during the oil blasting stage such as asphaltenes.38 When the water cut is below 50%, flow shear produces a relatively stable brine/oil emulsion in which the aqueous phase is sufficiently disseminated in the oil phase. This stage is classified as an oil-based system. With increasing water cut, a portion of the free water splits under the emulsion system to produce a partially dispersing system. However, there are no clear criteria for water-based systems or PD systems.39 In combination with the hypothesis reported by Farhadian et al.,40 it has been recommended that an oil/water system is considered a PD system if the water cuts are below the oil emulsion phase transition point and considered a water-based system when the water cut is more than the oil emulsion. The hydrate plugging generally occurs in the rich liquid system, which mainly involves liquid hydrocarbons, dissolved gas, water, and bulk hydrates. This makes research and exploration of this flow assurance more challenging. As demonstrated in Figure 2, the formation of hydrates in the flow of the rich liquid phase is primarily driven by four different stages. First is the preparation stage, where the emulsification of the water and oil phases in the system produces a surface site for hydrate formation. When a large number of hydrate particles form at the oil–water interface, they are blocked in the form of deposits on the solid surface or slurry, causing a shift toward the flow rate to a lower rate.41 The particles are at the packing stage, whereby the hydrate particles constantly combine to form large hydrates that accumulate on top of the deposited layer found on the surface, reducing the flow rate due to the particles’ interfaces. The plugging stage, where the hydrates constantly form causing plugging, is when the flow of the liquid phase in the pipe is not moving/flowing.12 In the aqueous phase, hydrate particles are blocked in the system of the rich liquid phase; the binding force between them is negligible; and the hydrate particles have always been distributed. Due to the bridging force, the hydrate particles continuously aggregate, creating a bigger hydrate when dispersed into a continuous oil phase.42−44
Figure 2.
Hydrate process of the particle coalescing in a liquid phase (diagram reproduced with permission from ref (45)).
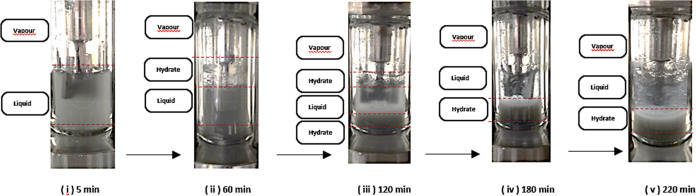
Furthermore, some researchers believe that the pressure changes and initial flow drop impact the hydrate formation and plugging.39 For example, Song et al. reported that based on different flow rates and pressure drops in the (drilling oil + NG + water) two forms of hydration can cause the pipeline plug. The hydrates are deposited on the pipe in the first type that is continually deposited (Figure 3). The process has four steps: in the steady-state stage, no hydrates are formed where the pressure drops, and the liquid phase does not change, as shown in Figure 3(i). A drastic reduction in pressure causes the initial formation of hydrate to occur, which would reduce the fluid flow in the pipelines. The fluid becomes more viscous as hydrate is formed, as does the frictional force, causing the pressure drop to increase as the flow rate drops. Hydrate formation results in the distribution of the hydrates in the liquid phase, resulting in silt-like hydrates (Figure. 3(ii)); sedimentation of hydrate particles results in the hydrate layer, which is a continuous process (Figure. 3(iii)); and the growth phase, which is time-dependent, slowly converts the liquid into the hydrate.46
Figure 3.
First type of hydrate-plugging process at low water cut (experiments conducted in Bhajan’s Lab).
The second type is when there is high water cut and flow. The oil–water solution is formed by gravity; in this form, the hydrate slurry accumulation results in a sudden rise in the fluid’s viscosity and a severe reduction in inflow (Figure 4).39 This kind of process takes place as follows: the stable flow where no occurrence of hydrate is found and where there is no change in flow rate and in the pressure drop on the liquid phase, the mixture of oil and water which flows smoothly, and last the formation of the hydrate stage, which is a rapid process.15 However, because of the significant volume of water present in the system, a viscous hydrate slurry forms in the boundaries of liquid gas space; this is the equilibrium stage, whereby the formation of hydrate is attained. The layering process starts at this point, as indicated in Figure 4(ii). Two layers are formed: the upper layer, drilling oil, has a mixture of oil–water with low density, and the bottom layer has a significant amount of hydrate. When the hydrate is rapidly formed in the mixed layer, the plugging stage occurs, which causes the mixed layer to be more viscous, leading a drop in the flow rate and an increase in the fractional resistance. With the increase of friction, the pressure drop first increases and then decreases as the flow rate decreases.24 The shear forces differ due to different water cuts for the two kinds of plugging processes. As a result, the liquid-phase stratification phenomena are not signifyingly witnessed in the initial stage of the plugging process, and the liquid-phase stratification phenomena are not observed.39
Figure 4.
Second type of plugging process under a high water cut (experiments conducted in Bhajan’s Lab).
Furthermore, the second hydrate plugging process is rapid since it has a high water cut. The mass fraction of produced hydrates is negligible. As a result, no stable hydrate is deposited in layer forms. There are no details discussed for the reasons behind the two hydration forms; however, the process of hydrate plugging is frequently a result of several mechanisms as well as the influence on hydrate particle coalescence which is not yet considered. Moreover, the two techniques of hydrate plugging and investigations of the mechanisms of pipeline plugging are classified into three groups depending on different water cuts: the oil-based system, the water-based system, and the PD system.
3.1.1. Hydrate-Plugging Mechanisms in the Oil-Dominated Systems
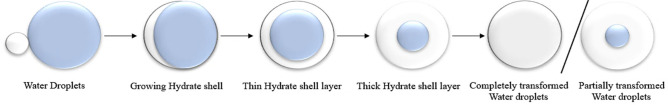
The water cut in early offshore oilfields was low. A previous study into the formation and plugging mechanisms in oil-based dominated systems has been conducted.39 As illustrated in Figure 5, the development of hydrate particles in oil systems is now separated into three different stages.47 On the water droplet’s outer surface, a hydrate shell layer is formed by the combination of water droplets with oil. The shell’s inside is slowly transformed to hydrate, which occurred because of the effects of mass and heat transfer, and some water droplets remain unconverted.48 Researchers Ning, Guanxing, and Gao et al.49,50 have developed numerous plugging hydrate mechanisms to describe the pipeline-plugging process to study hydrate plugging in oil-based systems. The plugging process of the oil-based system is currently divided into the coalescence particle’s adhesion of hydrate to the pipe wall and the concept of hydrate particle deposition, depending on various elements such as the temperature, pressure drop, water cut, flow rate, pipe material, subcooling degree, and conveying medium.37
Figure 5.
Schematic depicting the conversion of water droplets to hydrates in an oil-based system (diagram reproduced with permission from ref (39)).
In oil-dominated systems, hydrate particles tend to aggregate and/or deposit on the pipeline wall due to capillary cohesive forces, balanced against shear eddies that break aggregates or deposit these hydrate aggregates, and deposits may eventually lead to hydrate plugs.51,52 Turner53 proposed a simplified four-step conceptual mechanism on the process of hydrate plug formation in oil-dominated systems (Figure 1): (1) hydrate shell growth at the water–oil interface; (2) water entrainment in the oil phase; (3) aggregation via capillary attraction; and (4) catastrophic plugging of hydrate particles and aggregates.
3.1.2. Hydrate-Plugging Mechanisms in Water-Dominated Systems
Water-based systems have a water layer that is continuous and free. As the oil and gas industries develop, there is more production of the water content, and as a result the additional water cannot be removed any further or emulsified in the free layer of water and in oil. Shear forces will disperse some oil and gas in the free water layer. The higher water cut in these systems makes use of chemical inhibitor injection for hydrate prevention extremely expensive.54
The plugging hydrate formation mechanisms in water-based systems are different from those in oil-based systems. There is a study conducted by Joshi et al.55 on the cohesion forces in water via a micromechanical force apparatus. The study concludes that the magnitude of hydrate cohesion forces in water is comparatively low and cannot clarify the agglomeration particle as part of the plugging mechanism. Agglomeration of the particles is considered a key factor of the plugging mechanism hydrates in oil-based systems.
3.1.3. Hydrate-Plugging Mechanisms in Gas-Dominated Systems
In the gas-based systems, the system could be categorized by a predominant gaseous phase with slight amounts of the liquid phase of the hydrocarbon such as water and condensate gas. Hammerschmidt56 is the first who found that gas hydrates were causing blocking in the natural gas transposition/production pipelines above the hydrate formation temperatures. Mewes and Dorstewitz57 were the first to publish a flowloop investigation study on gas systems. The hydrates that were found formed first on the pipeline wall at the water–gas interaction. After that, the hydrate layer started to grow along the pipeline wall, until the whole diameter of the pipeline was blocked with hydrates. Kruka and Hatton58 did an experimental study using a flow loop to observe the hydrate buildup at the pipeline wall and the hydrate formation in the bulk liquid in a horizontal 3 in. pipe. They found that the plugging mechanism in the gas pipelines is due to the buildup on the pipe wall, which causes plugging from moderate blockage to full blockage.
4. Evaluation Methods for THIs and KHIs
The evaluation of both KHIs and THIs is mostly performed through experimental techniques. THI and KHI gas hydrate experiments were conducted using high-pressure differential scanning calorimetry (DSC), hydrate equilibrium cells, rocking cells, autoclaves, flow loops, and a sapphire hydrate reactor.59 The main parameters studied were temperature, pressure, time, and volume during the hydrate formation and dissociation experiments. However, further analyses were carried out using temperature, nuclear magnetic resonance (NMR), Raman spectroscopy magnetic resonance imaging, and X-ray diffraction.60,15 In addition, THIs required large amounts of chemicals since the range of concentrations is high, which will cause a higher OPEX/CAPEX cost, as stated by Creek et al.54 The hydrate risk management techniques recently have gained a lot of attention since they play a significant role in reducing the cost. The latest research has suggested using low-dosage hydrate inhibitors (LDHIs), which are separated into two types, antiagglomerates (AAs) and kinetic hydrate inhibitors (KHIs), and consume low concentration, and they have a lower cost compared to the THIs. Moreover, they provide flowline blockage control and contribute to shifting the flowline to the hydrate stability zone.
Of these mechanisms, film growth, which is the hydrate formation on the pipe surface from water droplets settling/condensing, will lead to an arterial stenosis of the pipeline. It was found to be one of the main contributors to the increase in pressure drops observed in a field trial test and industrial-scale flow loop tests.61,62 Moreover, current hydrate management methods are based on controlling the hydrate formation and agglomeration. These methods might not inhibit hydrate deposition on the pipe wall and may still lead to a hydrate plugging risk in the hydrate stability zone.61 These facts motivate the development of hydrate deposition modeling in gas- and oil-dominated systems. Quantitative risk modeling of hydrate bedding in an oil-continuous system was developed based on a physical force balance on a single water droplet, which defines the hydrate bedding rate and limits. A critical velocity model derived from an energy balance was also used to determine the critical agglomerate size for suspension.63 Additionally, hydrate film growth and deposition processes have been simulated, respectively, in gas-dominated systems. A combined heat and mass transfer model was used to describe hydrate film growth. Ding et al.64 proposed five controlling factors in the hydrate deposition process in water-in-oil emulsion systems, including the driving force of hydrate formation, the amount of “adhesive” water, the property of the surface, the mass transfer coefficient of the surface, and the flow shear rate.
4.1. Experimental Evaluation of Thermodynamic Hydrate Inhibitors (THIs)
The isochoric temperature search method or the T-cycle method is used in most research groups to obtain the hydrate dissociation/stability curve, i.e., the hydrate aqueous liquid–vapor equilibrium (HLVE).65 This method will first charge the reactor with an aqueous solution with or without a thermodynamic inhibitor. After injecting the gas at the experimental pressure, the system is left to saturate the solution with a gas.66 Once constant temperature and pressure values have been obtained, the system temperature is gradually reduced to allow hydrate formation, which is identified by a pressure reduction in the vessel or visual observation. In contrast to hydrate formation, hydrate dissociation is a slow process.67 Two heating methods are used to determine the equilibrium point during hydrate dissociation, continuous heating, and step heating. The temperature system continuously increases during a very slow heating rate of 0.00005 °C/h in the continuous heating approach.68 The temperature is raised stepwise with a 0.5 K increment for each step and a sufficient holding time of 48 h in the step heating technique to reach steady-state equilibrium.69 As a result, measuring gas hydrate dissociation equilibrium points takes 24–48 h. A pressure–temperature (P–T) diagram is generated for each experimental run.70 A sample P–T diagram generated during a T-cycle experiment is shown in Figure 6. The equilibrium point in a P–T diagram is the point where the heating and cooling lines intersect or the curve sharply changes (shown with a red circle). In the presence of thermodynamic inhibitors, the equilibrium points shift to the left side of the hydrate phase boundary. The average hydration depression temperature (DT) is calculated using the data collected during the tests.60 In comparison, the gas hydrate dissociation temperature with and without additives is the hydrate depression temperature. It is a critical indicator for evaluating the performance of any thermodynamic OHI.66
Figure 6.
Pressure vs temperature diagram generated by a T-cycle experiment (reproduced with permission from ref (71)).
4.2. Evaluation of Experimental Kinetic Hydrate Inhibitors (KHIs)
The induction time is typically used to evaluate potential KHIs in an experimental setting. The onset of hydrate nucleation begins when gas starts being entrapped inside the water/oil cells and is associated with the temperature spike due to the exothermic nature of hydrate formation, known as the induction time. The temperature and pressure conditions of the system are favorable for the generation of nuclei during the induction time.31 The nucleation and crystallization of hydrates are both probabilistic and stochastic. As a result, there are certain uncertainties in the experimental findings and screening.72 By repeating the experiments under similar conditions, the uncertainties can be minimized. The repeatability of the experiment verifies the performance of potential; many research groups use the performance of a potential KHI. The constant cooling method is used to determine induction time. Having an accurate understanding of the induction time can help to prevent hydrate formation in pipes.72
On the other hand, induction time is not an appropriate variable to use when evaluating the kinetic inhibition performance of various inhibitors investigated by various researchers. This is because the induction time can be affected by various factors such as sampling, the degree of subcooling, the driving force, and stirring. As a result, relative inhibitory power (RIP) is determined and used as criteria to compare the efficacy of various KHIs in this study.73 The following is a formula for calculating relative inhibitory power:
| 1 |
The memory effect is related to the history of the hydrate, indicating that the hydrate’s structure remains in the solution, another important parameter in gas hydrate kinetics.74 The solution with a memory effect is more likely to form hydrates than a fresh solution, reducing the induction time. For the oil and gas industry, the memory effect has significant implications. For example, hydrates form first if the pipeline is free of KHI or if KHI is not operating correctly.75 The hydrate dissociation should be properly escorted after its formation by removing the water phase. If the water phase is not completely inhibited, there is a great chance that the hydrate plug will rebuild shortly.76 In kinetic hydrate experiments, the sample for each run should be fresh to avoid the memory effect.
Furthermore, the driving force or subcooling temperature is a significant element in a hydrating system that can impact induction time at a given pressure. The difference between the hydrate equilibrium temperature and the experimental temperature is called subcooling temperature. A kinetic inhibitor’s induction time should be as long as (preferably greater than) the oil/gas stream’s residence time in the pipeline.77
5. Role of THIs in Oil-Based Systems
In general, researchers use the hydrate–liquid–vapor equilibrium (HLVE) curve to investigate the thermodynamic effect of gas hydrate inhibitors/promoters in oil-dominated systems by using the T-cycle method in which the equilibrium point is the main sign showing whether inhibitors/promoters with a inhibitor are helping to shift the hydrate equilibrium curve to high pressure and a low-temperature zone. The various inhibitors have been studied/tested in the oil system as THIs for CO2/CH4, such as MEG, PVP, Span 20, ionic liquid, etc.).66 As shown in Table 2, the experimental details of all reported measured THI data in gas hydrate in an oil-dominated system are presented in Table 2.
Table 2. Consolidated Thermodynamic Data Used in Oil-Dominated Systems.
| no. | type of equipment | gas | oil type | oil volume | water cut (%) | inhibitor type | conc. (wt %) | T (°C) | P (MPa) | remarks | ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | sapphire rocking cell RCS6 | CH4 | crude oil | 125 mL | 50 | decane | (3–10) | 25 to −5 | up to 10 | Some oil components (likely resins, and asphaltenes) contribute to the hydrate nucleation in water, oil, and gas systems. | (92) |
| surfynol 420 | |||||||||||
| span 80 | |||||||||||
| 2 | rocking cell | CO2 | crude oil | 100 mL | 20 | methanol | 12 | – 20 to 40 | 20 | The existence of crude oil in a multiphase system inhibits the production of gas hydrates, leading to a delay in hydrate form. | (79) |
| MEG | |||||||||||
| NaCl | |||||||||||
| KCl | |||||||||||
| 3 | rocking cell | CH4 | mineral oil 70T | 30 | NACL | 3.5 | 13 to −5 | 7 | Antiagglomerates aid in the formation of transportable hydrate slurry; however, at low concentrations of AA, temporary hydrate agglomeration was observed. | (80) | |
| MEG | 6.6 | ||||||||||
| 4 | stainless-steel chamber | CO2 | wax content and mineral oil | 125 mL | adsorption in water | ASP | 3 | 30 to 20 | 27 | The impact of asphaltenes on hydrate nucleation was more significant than waxes. | (93) |
| 5 | pressure cell | CH4 | conroe oil | 65% | 35 | 4 | 4.92 | The amount of hydrate formed over time increased as the water fraction increased; this increase is most likely extrinsic. | (45) | ||
| 6 | flow loop | CH4 | condensate gas + water + diesel oil–water | 5 vol % | 95 | antiagglomerant CAA | 2.0 | 4 and 1 | 10 | Despite the initial water cut, the adopted antiagglomerate may disperse hydrate particles in the fluid phase and the formed. | (82) |
| 10 vol % | 90 | ||||||||||
| 15 vol % | 85 | ||||||||||
| 20 vol % | 80 | ||||||||||
| 25 vol % | 75 | ||||||||||
| 7 | rocking cell system | CH4 | condensate liquid and crude oil | 2–7 mL of crude oil | 30–80 | antiagglomerate | 0.2–1 | 4 | 10 | Thermodynamic inhibition and reduction of AA adsorption on the hydrate surface are two possible effects of salt on AA performance. | (83) |
| 8 | HP-mDSC | CH4 | vegetable oil | 115 g | 55 | triethylamine | 30 | 20 to −20 | 25 | Some hydrate inhibitors, such as quaternary salts, contain chloride as a counterion, which can cause corrosion. | (40) |
| diisocyanate | |||||||||||
| PVP | |||||||||||
| polyethylene | |||||||||||
| glycol | |||||||||||
| 9 | rocking cell | CH4 | mineral oil 70T | - | 60 | MEG | 6.6 | 20 to 1 | 4.5–3.5 | Inhibited systems have a higher risk of hydrate agglomeration than noninhibited systems under certain conditions. | (81) |
| NACL | 3.5 | ||||||||||
| arquad | 0.5 | ||||||||||
| 10 | rocking cell | CH4 | crude oil | 0% | 100 | Luvicap-Bio | 20 to −60 | 10 | The percentage of the liquid hydrocarbon phase increases, and there is a clear inhibitory impact on hydrate formation. | (77) | |
| 20% | 80 | ||||||||||
| 30% | 70 | ||||||||||
| 40% | 60 |
Andrey S. Stoporev78 used the sapphire rocking cell RCS6 to conduct the thermodynamic study on CH4 gas in an oil system in the presence of crude oil with 50% water cut by using three different inhibitors (n-decane, Surfynol 420, and span 80) with a concentration range from 3 to 10 wt %. The experiment was tested under high pressure up to 10 MPa and temperature from 25 to −5 °C, and the results showed that n-decane and span 80 result in a significant decrease in the scatter of DT values at which nucleation of both structure I and structure II hydrate takes place, where the addition of a nonionic surfactant Surfynol 420 to the system does not much affect the methane hydrate.
Jai Krishna79 used four different inhibitors (methanol, MEG, NaCl, and KCl) with a concentration of 12 wt % under 20% water cut with a range of temperatures from −20 to 40 °C and pressure of 20 MPa, and the experiment was conducted using a rocking cell for CO2 hydrate on crude oil. The results show that MEG has the highest impact on shifting the equilibrium curve to higher temperature and pressure, where MEG helps to shift the equilibrium temperature around 1 point compared to the methanol and NaCl helps to shift the equilibrium temperature around 0.6 where KCl has the least impact among them.
Erland conducted two thermodynamics studies80,81 using NaCl and MEG for both studies but under different concentrations (3.5 and 6.6 wt %) on CH4 gas hydrate with the presence of mineral oil 70T, and both studies used different water cuts. The first study was conducted at a 30% water cut and the second study at 60% water cut at the same experimental conditions of 7 MPa pressure and temperature from 13 to −5 °C, where both studies show that the effect of those two different thermodynamics inhibitors has a significant impact on CH4 hydrate where it helps to shift the equilibrium temperature around 0.6–0.7 °C point. Moreover, the authors reported that inhibited systems have a higher risk of hydrate agglomeration than noninhibited systems under certain conditions. However, both authors Erland80,81 and Jai Krishna79 used the same thermodynamics inhibitors, but a different system clearly shows that the results of MEG and NaCl have a higher impact on CO2 systems compared to CH4 systems. Peng82 used flow loop on the CH4 gas hydrate system with the presence of condensate gas and diesel on different water cut ranges from 95 to 75%, and during this study antiagglomerate CAA played the role of the inhibitor with the concentration of 2 wt %; on the other hand, Zhao83 used 1 wt % antiagglomerate as well but on a brine system with the presence of condensate liquid and crude oil using a rocking cell with almost the same experimental conditions. It was found that the performance of AA on a brine water system has a more significant impact compared to the pure water system on hydrate inhibition.
6. Application of KHIs in Oil-Based Systems
Compared to thermodynamic studies, there are few studies on the kinetics of gas hydrate mitigation/enhancement in the oil system. Because gas hydrate formation kinetics is very probabilistic and dependent on factors such as apparatus design, experimental technique, reactor wall roughness, driving force, and impurity in a sample, the kinetic data gathered were evaluated differently (Bavoh et al.).9 Nucleation time, rate, and gas uptake during hydrate formation are the three main kinetic indicators used to evaluate gas hydrate’s inhibition/promotion performance in oil-dominated systems.65 Among the others, nucleation time is usually selected since it reflects the efficacy of oil/gas inhibitors in delaying hydrate formation. Based on kinetic experiments, some inhibitors are extremely poor gas hydrate kinetic inhibitors. They are kinetic promoters rather than inhibitors.31 On the other hand, their kinetic inhibition strength lies in their potential to delay the rate of hydrate formation and gas uptake. The most common commercial types of KHIs are water-soluble polymeric compounds such as polyvinylpyrrolidone (PVP) and poly(N-vinylcaprolactam) (PVCap),84 which can perturb the water structure and adsorb on the hydrate surface to prevent further growth of gas hydrate. Furthermore, combining the KHIs and appropriate synergists may improve the inhibition performance of these inhibitors. In fact, the synergists can help KHIs retard the nucleation or slow down the hydrate growth rate more effectively. Poly(ethylene oxide)s (PEOs), glycol ether compounds, quaternary ammonium ionic liquid salts, and hydroxyl ethyl cellulose (HEC) were found to be synergists to some KHIs.85,86 Authors typically determine the kinetic inhibition parameters of induction time and inhibition time. As shown in Table 3, the experimental details of all reported KHI data of gas hydrates in the oil-dominated system are presented in Table 3.
Table 3. Consolidated Kinetic Data Used in Oil-Dominated Systems.
| no. | type of equipment | gas | oil type | oil volume | water cut (%) | inhibitor type | conc. (wt %) | T (°C) | P (MPa) | remarks | ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | high-pressure cell | CH4 | diesel oil | 285 mL | 30 | antiagglomerant | 0 | 1.2 | 4 | Different water cuts were studied (30, 60, and 90%) with and without the AA. They found that AA helped to increase the induction time by 25 min. | (6) |
| 285 mL | 30 | 0 | 2 | 4.5 | |||||||
| 285 mL | 30 | 0.5 | 2 | 4 | |||||||
| 285 mL | 30 | 1 | 2 | 4 | |||||||
| 570 mL | 60 | 0 | 1.2 | 4 | |||||||
| 570 mL | 60 | 0 | 2 | 4 | |||||||
| 570 mL | 60 | 0.5 | 2 | 4.5 | |||||||
| 855 mL | 90 | 0 | 1.2 | 4 | |||||||
| 855 mL | 90 | 0 | 2 | 4.5 | |||||||
| 855 mL | 90 | 0.5 | 2 | 4 | |||||||
| 855 mL | 90 | 1 | 2 | 4 | |||||||
| 2 | rocking cell apparatus | CH4 | crude oil | 0 | 100 pure water | no KHI | 0 | 0.1 | 1 | Starch, chitosan, glycine, PVP, and mSA-RmAFP1 are the inhibitors in order. It has been discovered that mSA- RmAFP1 can reduce the rate of generation of SNG hydrates. | (89) |
| 0 | 100 brine water 3.5 wt % | no KHI | 0 | 0.1 | 1 | ||||||
| 0 | 100 pure water | mSA-RmAFP1 | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 pure water | chitosan | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 pure water | starch | 2.25 | 0.1 | |||||||
| 0 | 100 pure water | PVP | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 pure water | glycine | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 pure water | mSA-RmAFP1 | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 brine water 3.5 wt % | chitosan | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 brine water 3.5 wt % | starch | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 brine water 3.5 wt % | PVP | 2.25 | 0.1 | 1 | ||||||
| 0 | 100 brine water 3.5 wt % | glycine | 2.25 | 0.1 | 1 | ||||||
| 15% | 85 | no KHI | 0 | 0.1 | 1 | ||||||
| 15% | 85 | mSA-RmAFP1 | 2.25 | 0.1 | 1 | ||||||
| 15% | 85 | chitosan | 2.25 | 0.1 | 1 | ||||||
| 15% | 85 | starch | 2.25 | 0.1 | 1 | ||||||
| 15% | 85 | PVP | 2.25 | 0.1 | 1 | ||||||
| 15% | 85 | glycine | 2.25 | 0.1 | 1 | ||||||
| 3 | high-pressure sapphire autoclave | CH4 | paraffin oil | 89% | 11 | antiagglomerant | 2 | 3.17 | 5.78 | In the autoclave, hydrate particles formed a moving bed, which was followed by full dispersion of water and oil, rapid hydrate growth, and deposition on the wall. | (36) |
| 89% | 11 | 1.9 | 5.77 | ||||||||
| 89% | 11 | 5.9 | 5.88 | ||||||||
| 89% | 11 | 5.7 | 5.89 | ||||||||
| 78% | 22 | 6.3 | 5.99 | ||||||||
| 78% | 22 | 6.3 | 5.99 | ||||||||
| 78% | 22 | 4.9 | 5.94 | ||||||||
| 78% | 22 | 6.8 | 6.01 | ||||||||
| 70% | 30 | 1.9 | 5.83 | ||||||||
| 70% | 30 | 2.9 | 5.86 | ||||||||
| 70% | 30 | 2.7 | 5.86 | ||||||||
| 70% | 30 | 4.8 | 5.91 | ||||||||
| 50% | 50 | 1.2 | 5.73 | ||||||||
| 50% | 50 | 1.3 | 5.72 | ||||||||
| 50% | 50 | 6.4 | 5.91 | ||||||||
| 50% | 50 | 6.9 | 5.92 | ||||||||
| 30% | 70 | 1.2 | 5.66 | ||||||||
| 30% | 70 | 4.8 | 5.81 | ||||||||
| 30% | 70 | 3.3 | 5.76 | ||||||||
| 30% | 70 | 5.3 | 5.83 | ||||||||
| 4 | high-pressure sapphire autoclave | CH4 | conroe oil | 95% | 5 | NA | NA | 4 | 3.98 | The mass transfer of methane through hydrate shells appears to control hydrate formation in these water-in-oil dispersions. | (45) |
| 65% | 35 | NA | NA | ||||||||
| 65% | 35 | NA | NA | ||||||||
| 65% | 35 | NA | NA | ||||||||
| 65% | 35 | NA | NA | ||||||||
| 5 | stirring autoclave | CH4 | diesel oil | 209 mL | 5 | rhamnolipid | 3 | 6 | 5.50 | When Span 20, rhamnolipid, and compounding AAs were added in water/diesel oil systems, water cut affected not only the amount of dissociated methane hydrate but also the maximum dissociation rate of methane hydrate. | (23) |
| 198 mL | 10 | 3 | |||||||||
| 176 mL | 20 | 3 | |||||||||
| 198 mL | 10 | 1 | |||||||||
| 198 mL | 10 | 0.5 | |||||||||
| 198 mL | 10 | 0 | |||||||||
| 209 mL | 5 | Span 20 | 0.1 | ||||||||
| 198 mL | 10 | 0.1 | |||||||||
| 209 mL | 5 | 0.5 | |||||||||
| 198 mL | 10 | 0.5 | |||||||||
| 198 mL | 10 | 2.0 | |||||||||
| 209 mL | 5 | Span 20:esters polymer | 3.0 | ||||||||
| 198 mL | 10 | 3.0 | |||||||||
| 209 mL | 5 | 1:02 | |||||||||
| 198 mL | 10 | 1:02 | |||||||||
| 176 mL | 20 | 1:02 | |||||||||
| 154 mL | 30 | 1:02 | |||||||||
| 6 | high-pressure sapphire autoclave | CH4 | diesel oil | 95 mL | 5 | antiagglomerant | 3 | 4 | 0.2 | The solubility of natural gas in an emulsion system grows practically linearly with pressure, while it decreases with water cut. There is an initial slow hydrate formation stage for systems with water cuts of 5, 10, and 15% vol, whereas rapid hydrate formation occurs and the process of the gas–liquid dissolving equilibrium does not appear in the pressure curve at 20 and 25 vol %. | (87) |
| 0.4 | |||||||||||
| 0.8 | |||||||||||
| 1.2 | |||||||||||
| 90 mL | 10 | 0.2 | |||||||||
| 0.5 | |||||||||||
| 0.8 | |||||||||||
| 1.2 | |||||||||||
| 1.35 | |||||||||||
| 85 mL | 15 | 0.5 | |||||||||
| 0.8 | |||||||||||
| 1 | |||||||||||
| 1.2 | |||||||||||
| 1.35 | |||||||||||
| 80 mL | 20 | 0.3 | |||||||||
| 0.6 | |||||||||||
| 0.9 | |||||||||||
| 1.2 | |||||||||||
| 1.35 | |||||||||||
| 75 mL | 25 | 0.2 | |||||||||
| 0.4 | |||||||||||
| 0.8 | |||||||||||
| 7 | high-pressure sapphire autoclave | CH4 | diesel oil | 481 mL | 10 | lubrizol | 0 | 3 | 6.5 | High water cuts with or without surfactants had a lower dissociation ratio (25%) than lower water cuts, resulting in enhanced self-preservation effects. | (10) |
| 428 mL | 20 | 0 | |||||||||
| 374 mL | 30 | 0 | |||||||||
| 428 mL | 20 | 0.06 | |||||||||
| 428 mL | 20 | TBAB | 0.06 | ||||||||
| 428 mL | 20 | 0 | |||||||||
| 0 | 100 | 0 | |||||||||
| 530 mL | 99 | 0 | |||||||||
| 507 mL | 95 | 0 | |||||||||
| 530 mL | 99 | TBAB | 0.06 | ||||||||
| 530 mL | 99 | lubrizol | 0.06 | ||||||||
| 0 | 100 | TBAB | 0.06 | ||||||||
| 0 | 100 | lubrizol | 0.06 | ||||||||
| 8 | high-pressure sapphire autoclave | CH4 | diesel oil | 300 mL | up to 30 | Span20 with different promoters (SDS,L-1,TBAB) | 0 | 3 | 7 | SDS/L-l had a greater effect on increasing hydrate growth, which could significantly increase the kinetics of methane hydrate formation in the emulsion system, whereas Tween80 and TBAB prevented methane hydrate formation in the emulsion to some amount. | (94) |
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | |||||||||||
| 1 | |||||||||||
| 0.5 | |||||||||||
| 0.25 | |||||||||||
| 0.1 | |||||||||||
| 1 | |||||||||||
| 0.5 | |||||||||||
| 0.25 | |||||||||||
| 0.1 | |||||||||||
| 0.5 | |||||||||||
| 0.5 | 6.5 | ||||||||||
| 0.5 | 6 | ||||||||||
| 0.5 | 5.5 | ||||||||||
| 0.5 | 7 | ||||||||||
| 0.5 | 6.5 | ||||||||||
| 0.5 | 6 | ||||||||||
| 0.5 | 5.5 | ||||||||||
| 9 | stainless steel (SS-316) cell | CH4 | mineral oil | 40 | PVP | 2 | 4 | 8 | In comparison to PVP, MEG is a better choice. l-Tyrosine is not a good choice in terms of hydrate formation induction time. | (95) | |
| MEG | 20 | 7 | |||||||||
| 10 | high-pressure hydrate reaction system | CH4 | light oil and asphaltene | 4 mL | 90 | PVP (K-15) | 2000 ppm | 2 | 5 | Results demonstrated that light oil components mainly promote hydrate growth at the initial stage because of the enhanced methane solubility in the oil phase and the slight emulsification effect under mechanical stirring. | (91) |
| luvicap EG | |||||||||||
| inhibex 501 | |||||||||||
| 11 | autoclave | CH4 | seabed oil | 10 mL | (20–50) and (60–100) | antiagglomerant | 0.2 and 0.5 | 20 to 1 | 14 | AA is less toxic than QAs. The T surfactant used in this study has the ability to reduce the risk of hydrate obstruction in offshore gas flowlines as well as capture oil from deep water spills. | (88) |
Table 3 presented different experiments of KHIs on different systems. Song6 used a high-pressure cell reactor to test the performance of AA with 1 wt % concentration on CH4 hydrate in an oil system using diesel oil, to delay the induction time under three different water cuts (30%, 60%, and 90%) with a range of temperatures (1.2–2) and pressures (4–4.5 MPa), so the three different water cuts have been studied with and without the AA to check the performance of the AA. However, it has been found that the AA helps to increase the induction time to around 25 min for 30% and 90% water cuts and 9 min for 60% water cut on the other hand. Chang-Sheng87 used AA as well to study the solubility of natural gas in emulsion systems conducted using high-pressure sapphire autoclaves on CH4 hydrate in an oil system (diesel oil) with an AA concentration of 3 wt %. The result shows that the solubility of natural gas in emulsion systems increases almost linearly with the increase of pressure and decreases with the increase of water cut. Moreover, Sun88 studied the toxicology of AA compared to methanol and other chemicals using an autoclave on CH4 hydrate in an oil system using seabed oil with a low concentration of AA, around 0.5 wt %. This has proven that AA has lower toxicity compared to QAs.
Mu and Nicolas89 used a rocking cell apparatus to study the CH4 hydrate with crude oil on two different systems, pure and brine water, with a water cut of 85% on five different inhibitors (glycine, PVP, starch, chitosan, and mSA-RmAFP1). The comparison made in terms of inhibition strength (°C) when compared in terms of systems with brine water gave a higher inhibition strength for all inhibitors, which is around 0.4 °C; however, in terms of inhibitors, mSA-RmAFP1 gave the highest inhibition strength of around 5.5 °C. After that come PVP at 4 °C and then glycine at 3 °C, and Saberi90 studied the impact of PVP on the induction time with two different concentrations, 1 and 2 wt % of l-tyrosine, 1 wt % of MEG, and 10 wt % and a mix of MEG and PVP on different concentrations and 2 wt % of PVP + 10 wt % of MEG and 2 wt % of PVP + 20 wt % of MEG using Stainless Steel (SS-316) on CH4 hydrate with mineral oil with 40% water cut under 8 MPa pressure and 4.7 °C temperature. It has been reported that PVP gave the highest induction time around 85 min compared to MEG at 51 min, but after mixing PVP and MEG the induction time was increased. For 2 wt % of PVP + 10 wt % of MEG, the hydrate was formed after 90 min, and 2 wt % of PVP + 20 wt % of MEG showed the highest time where the hydrate was formed after 170 min; moreover, Zi91 simulated the CH4 hydrate with light oil and asphaltene with 90% water cut, 2 °C temperature, and pressure of 5 MPa on three different inhibitor PVPs (K-14, Luvicap EG, and Inhibex 501 with 2000 ppm concentration), where PVP also showed the most effective inhibitor.
Kele76 studied 20 spans with a different promoter (SDS, L-1, TBAB) with a low concentration of 0.5 wt % of the effect on the induction time and the gas consumption by using a high-pressure sapphire autoclave with a water cut up to 30% under 7 MPa pressure and 7 °C for temperature in terms of the induction time. Tween80 and TBAB prevented methane hydrate formation in the emulsion to some amount, which is around 10 min. Gas consumption of SDS gave the highest gas consumption around 0.06811 mol/min.
7. Hydrate Inhibition Mechanism in Oil-Dominated Systems
The hydrate inhibition mechanism in oil-dominated systems is similar but different to some extent for gas-dominated systems. Generally, the thermodynamic gas hydrate inhibition using conventional THIs like methanol, glycols, and salts is similar to the gas-dominated systems. The THI effect is via alteration of the water activity toward hydrate formation. This leads to the changes in the thermodynamic properties of the hydrate formation system in oil.
On the other hand, the kinetic and AA inhibition mechanism systems seems to differ in some cases when these inhibitors are used in oil-dominated systems. The use of KHIs such as AFP and PVP shows the adsorption-inhibition mechanism in oil-dominated systems. According to Mu and Solms,96 such KHIs reduce the transport rate and adsorption capacity of gas molecules to the hydrate surface, thus preventing hydrate growth and plugs. The reason the inhibition mechanism of both oil- and gas-dominated systems is similar might be due to the type of oil systems used by various authors. Most authors use oils which do not reflect the true composition of crude oil and thus exhibit different inhibition mechanisms. In the case of crude oil, two types of additional hydrate inhibition mechanisms are added to the inhibitor behavior. In a crude oil system, the burial of gas compositions which are promoters (propane, butane, and pentane) leads to the inhibition of hydrate formation (in the sense of thermodynamic behavior). Second, the hydrocarbon phase introduces an additional impediment for hydrate-forming guest molecules to move toward the aqueous phase. This is mainly controlled by the aromatic components in the crude oil since they are more soluble in water than paraffins. A molecular dynamics simulation further confirms that crude oil compositions (saturates, aromatics, resins, and asphaltenes), especially asphaltenes at a certain concentration, lead to hydrate inhibition or low hydrate inhibition. When asphaltenes are formed at the water–gas interface, they significantly inhibit hydrates.97 Lastly, AAs are mostly effective in oil systems; hence, they produce water-in-oil emulsions which are converted into hydrate particles. However, there are some AAs that can work without the need for the oil nor emulsion formation.98
8. Future Recommendations
Based on the findings of this review, the following future recommendations are needed for a better understanding of hydrate inhibition in oil-dominated systems.
The use of real or actual crude oil for experimental testing of inhibitors is recommended since the currently used synthetic oils do not properly represent the composition present in real crude oil.
There is a need to properly establish the hydrate inhibition and formation mechanism of inhibitors in oil systems, especially models that depend on the system’s driving force.
More research should be conducted to model the thermodynamics and kinetics of hydrate formation in crude oil systems. This should be done for both empirical and artificial intelligence approaches.
Also, it is recommended to test novel hydrate inhibitors for gas hydrate inhibition in oil systems at different water cuts and concentrations. Such novel inhibitors could be amino acids, ionic liquids, nanoparticles, new biosolvents, etc.
Current studies should focus on using flow loop apparatuses to mimic a real flow assurance environment.
Last but not least, more molecular level simulations are needed to support the experimental results and provide deep insights into the hydrate inhibition and formation mechanism in oil-dominated systems.
9. Conclusion
Based on the findings in this review, the conclusion drawn indicates that factors such as wax deposition do occur in oil-dominated systems that mix with the presence of hydrates. This results in critical challenges in preventing hydrate formation in such systems. Also, oil bubble point, water cut, and the presence of heavy hydrate formers are critical factors that also affect hydrate formation in oil-dominated systems. The preparation stage, particle packing stage, and plugging stage are the main steps involved in the hydrate formation mechanism in oil-dominated systems. The presence of both KHIs and AAs is able to prevent hydrate formation in oil-dominated systems, although very limited inhibitors have been tested in oil-dominated systems in the open literature. Also, more MD and modeling of hydrate formation and mitigation in oil-dominated systems are needed to provide useful knowledge for future breakthroughs in the oil and gas flow assurance sector.
Acknowledgments
The authors would like to express their gratitude to the Department of Chemical Engineering at Universiti Teknologi PETRONAS for the financial support under Joint Research Project (JRP) No. 015MD0-073. The authors would also like to acknowledge and appreciate the Research Centre for CO2 Capture for providing laboratory and technical services.
The authors declare no competing financial interest.
References
- Nashed O.; Sabil K. M.; Lal B.; Ismail L.; Jaafar A. J. Study of 1-(2-Hydroxyethyle) 3-Methylimidazolium Halide as Thermodynamic Inhibitors. Appl. Mech. Mater. 2014, 625, 337–340. 10.4028/www.scientific.net/AMM.625.337. [DOI] [Google Scholar]
- Hammerschmidt E. G. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26 (8), 851–855. 10.1021/ie50296a010. [DOI] [Google Scholar]
- Pavlenko A. M.; Koshlak H. Intensification of Gas Hydrate Formation Processes by Renewal of Interfacial Area between Phases. Energies 2021, 14, 5912. 10.3390/en14185912. [DOI] [Google Scholar]
- Chen J.; Zeng Y.; Liu C.; Kang M.; Chen G.; Deng B.; Zeng F. Methane Hydrate Dissociation from Anti-Agglomerants Containing Oil Dominated Dispersed Systems. Fuel 2021, 294, 120561. 10.1016/j.fuel.2021.120561. [DOI] [Google Scholar]
- Ul Haq I.; Qasim A.; Lal B.; Zaini D. B.; Foo K. S.; Mubashir M.; Khoo K. S.; Vo D. V. N.; Leroy E.; Show P. L. Ionic Liquids for the Inhibition of Gas Hydrates. A Review. Environ. Chem. Lett. 2022, 20, 2165. 10.1007/s10311-021-01359-9. [DOI] [Google Scholar]
- Song G.; Li Y.; Wang W.; Jiang K.; Shi Z.; Yao S. Hydrate Formation in Oil-Water Systems: Investigations of the Influences of Water Cut and Anti-Agglomerant. Chin. J. Chem. Eng. 2020, 28 (2), 369–377. 10.1016/j.cjche.2019.07.024. [DOI] [Google Scholar]
- Robinson D. B.; Ng H. J. Hydrate Formation and Inhibition in Gas or Gas Condensate Streams. J. Can. Pet. Technol. 1986, 25 (4), 26–30. 10.2118/86-04-01. [DOI] [Google Scholar]
- Johal K. S.Flow Assurance Technology Options For Deepwater & Long Distance Oil & Gas Transport. Offshore Mediterranean Conference and Exhibition, March 28, 2007; p OMC-2007-071.
- Bavoh C. B.; Lal B.; Osei H.; Sabil K. M.; Mukhtar H. A Review on the Role of Amino Acids in Gas Hydrate Inhibition, CO2 Capture and Sequestration, and Natural Gas Storage. J. Nat. Gas Sci. Eng. 2019, 64, 52. 10.1016/j.jngse.2019.01.020. [DOI] [Google Scholar]
- Lv Y. N.; Jia M. L.; Chen J.; Sun C. Y.; Gong J.; Chen G. J.; Liu B.; Ren N.; Guo S. Di; Li Q. P. Self-Preservation Effect for Hydrate Dissociation in Water + Diesel Oil Dispersion Systems. Energy Fuels 2015, 29 (9), 5563–5572. 10.1021/acs.energyfuels.5b00837. [DOI] [Google Scholar]
- Koh C. A.; Sloan E. D.; Sum A. K.; Wu D. T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. 10.1146/annurev-chembioeng-061010-114152. [DOI] [PubMed] [Google Scholar]
- Zerpa L. E.; Aman Z. M.; Joshi S.; Rao I.; Sloan E. D.; Koh C.; Sum A.. Predicting Hydrate Blockages in Oil, Gas and Water-Dominated Systems. Paper presented at the Offshore Technology Conference; Houston, Texas, USA, April 2012. 10.4043/23490-MS. [DOI] [Google Scholar]
- Farhang F.; Nguyen A. V.; Hampton M. A. Influence of Sodium Halides on the Kinetics of CO 2 Hydrate Formation. Energy Fuels 2014, 28 (2), 1220–1229. 10.1021/ef401549m. [DOI] [Google Scholar]
- Bavoh C. B.; Lal B.; Osei H.; Sabil K. M.; Mukhtar H. A Review on the Role of Amino Acids in Gas Hydrate Inhibition, CO2 Capture and Sequestration, and Natural Gas Storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. 10.1016/j.jngse.2019.01.020. [DOI] [Google Scholar]
- Wang Y.; Fan S.; Lang X. Reviews of Gas Hydrate Inhibitors in Gas-Dominant Pipelines and Application of Kinetic Hydrate Inhibitors in China. Chin. J. Chem. Eng. 2019, 27 (9), 2118–2132. 10.1016/j.cjche.2019.02.023. [DOI] [Google Scholar]
- Xiao C.; Adidharma H. Dual Function Inhibitors for Methane Hydrate. Chem. Eng. Sci. 2009, 64 (7), 1522–1527. 10.1016/j.ces.2008.12.031. [DOI] [Google Scholar]
- Makwashi N.; Ahmed T. Gas Hydrate Formation: Impact on Oil and Gas Production and Prevention Strategies. Nigerian Research Journal of Engineering and Environmental Sciences 2021, 6 (1), 61–75. 10.5281/zenodo.5047631. [DOI] [Google Scholar]
- Kelland M. A.; Pomicpic J.; Ghosh R.; Undheim C.; Hemmingsen T. H.; Zhang Q.; Varfolomeev M. A.; Pavelyev R. S.; Vinogradova S. S. Multi-Functional Oilfield Production Chemicals: Maleic-Based Polymers for Gas Hydrate and Corrosion Inhibition. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1201 (1), 012081. 10.1088/1757-899X/1201/1/012081. [DOI] [Google Scholar]
- Chen J.; Liu J.; Chen G. J.; Sun C. Y.; Jia M. L.; Liu B.; Si S.; Ren N. Insights into Methane Hydrate Formation, Agglomeration, and Dissociation in Water + Diesel Oil Dispersed System. Energy Convers. Manag. 2014, 86, 886–891. 10.1016/j.enconman.2014.06.056. [DOI] [Google Scholar]
- Yu L. C. Y.; Charlton T. B.; Aman Z. M.; Wu D. T.; Koh C. A. Hydrate Growth on Methane Gas Bubbles in the Presence of Salt. Langmuir 2020, 36, 84. 10.1021/acs.langmuir.9b03451. [DOI] [PubMed] [Google Scholar]
- Stoporev A. S.; Semenov A. P.; Medvedev V. I.; Mendgaziev R. I.; Istomin V. A.; Sergeeva D. V.; Manakov A. Y.; Vinokurov V. A. Formation and Agglomeration of Gas Hydrates in Gas - Organic Liquid - Water Systems in a Stirred Reactor: Role of Resins/Asphaltenes/Surfactants. J. Pet. Sci. Eng. 2019, 176, 952–961. 10.1016/j.petrol.2019.02.002. [DOI] [Google Scholar]
- Golsanami N. Evaluating the Effect of New Gas Solubility and Bubble Point Models on PVT Parameters and Optimizing Injected Gas Rate in Gas-Lift Dual Gradient Drilling. Energies 2021, 14, 1513. 10.3390/en14051513. [DOI] [Google Scholar]
- Xiang C. S.; Peng B. Z.; Liu H.; Sun C. Y.; Chen G. J.; Sun B. J. Hydrate Formation/Dissociation in (Natural Gas + Water + Diesel Oil) Emulsion Systems. Energies 2013, 6 (2), 1009–1022. 10.3390/en6021009. [DOI] [Google Scholar]
- Joshi S. V.; Grasso G. A.; Lafond P. G.; Rao I.; Webb E.; Zerpa L. E.; Sloan E. D.; Koh C. A.; Sum A. K. Experimental Flowloop Investigations of Gas Hydrate Formation in High Water Cut Systems. Chem. Eng. Sci. 2013, 97, 198–209. 10.1016/j.ces.2013.04.019. [DOI] [Google Scholar]
- Chen X.; Li H. New Pragmatic Strategies for Optimizing Kihara Potential Parameters Used in van Der Waals-Platteeuw Hydrate Model. Chem. Eng. Sci. 2022, 248, 117213. 10.1016/j.ces.2021.117213. [DOI] [Google Scholar]
- Liu H.; Zhan S.; Li R.; Liu Y.; Guo P.; Wang Z.; Du J.; Wen Y.; Dai P.; Liao H. High-Efficiency Natural-Gas Storage Method Involving Formation of Gas Hydrate in Water/Oil-Cyclopentane Emulsion. Chem. Eng. J. 2020, 400 (March), 125369. 10.1016/j.cej.2020.125369. [DOI] [Google Scholar]
- Zheng H.; Huang Q.; Wang W.; Long Z.; Kusalik P. G. Induction Time of Hydrate Formation in Water-in-Oil Emulsions. Ind. Eng. Chem. Res. 2017, 56 (29), 8330–8339. 10.1021/acs.iecr.7b01332. [DOI] [Google Scholar]
- Zhang D.; Huang Q.; Li R.; Wang W.; Zhu X.; Li H.; Wang Y. Effects of Waxes on Hydrate Behaviors in Water-in-Oil Emulsions Containing Asphaltenes. Chem. Eng. Sci. 2021, 244, 116831. 10.1016/j.ces.2021.116831. [DOI] [Google Scholar]
- Zhang D.; Huang Q.; Wang W.; Li H.; Zheng H.; Li R.; Li W.; Kong W. Effects of Waxes and Asphaltenes on CO2 Hydrate Nucleation and Decomposition in Oil-Dominated Systems. J. Nat. Gas Sci. Eng. 2021, 88, 103799. 10.1016/j.jngse.2021.103799. [DOI] [Google Scholar]
- Song G.; Ning Y.; Guo P.; Li Y.; Wang W. Investigation on Hydrate Growth at the Oil-Water Interface: In the Presence of Wax and Surfactant. Langmuir 2021, 37 (22), 6838–6845. 10.1021/acs.langmuir.1c01060. [DOI] [PubMed] [Google Scholar]
- Lim V. W. S.; Metaxas P. J.; Johns M. L.; Haandrikman G.; Crosby D.; Aman Z. M.; May E. F. The Delay of Gas Hydrate Formation by Kinetic Inhibitors. Chem. Eng. J. 2021, 411, 128478. 10.1016/j.cej.2021.128478. [DOI] [Google Scholar]
- Zi M.; Chen D.; Wu G. Molecular Dynamics Simulation of Methane Hydrate Formation on Metal Surface with Oil. Chem. Eng. Sci. 2018, 191, 253–261. 10.1016/j.ces.2018.06.070. [DOI] [Google Scholar]
- Brown E.; Khan M. N.; Salmin D.; Wells J.; Wang S.; Peters C. J.; Koh C. A. Cyclopentane Hydrate Cohesion Measurements and Phase Equilibrium Predictions. J. Nat. Gas Sci. Eng. 2016, 35, 1435–1440. 10.1016/j.jngse.2016.05.016. [DOI] [Google Scholar]
- Mohammadi A. H.; Ji H.; Burgass R. W.; Bashir A.; Tohidi B.. Gas Hydrates in Oil Systems. SPE Eur. Annu. Conf. Exhib. 2006, SPE 99437. [Google Scholar]
- Boissé Lomax L.; Bayly M. A.; Hjalgrim H.; Møller R. S.; Vlaar A. M.; Aaberg K. M.; Marquardt I.; Gandolfo L. C.; Willemsen M.; Kamsteeg E.-J.; et al. ‘North Sea’ Progressive Myoclonus Epilepsy: Phenotype of Subjects with GOSR2 Mutation. Brain 2013, 136 (4), 1146–1154. 10.1093/brain/awt021. [DOI] [PubMed] [Google Scholar]
- Akhfash M.; Aman Z. M.; Ahn S. Y.; Johns M. L.; May E. F. Gas Hydrate Plug Formation in Partially-Dispersed Water-Oil Systems. Chem. Eng. Sci. 2016, 140, 337–347. 10.1016/j.ces.2015.09.032. [DOI] [Google Scholar]
- Høiland S.; Askvik K. M.; Fotland P.; Alagic E.; Barth T.; Fadnes F. Wettability of Freon Hydrates in Crude Oil/Brine Emulsions. J. Colloid Interface Sci. 2005, 287 (1), 217–225. 10.1016/j.jcis.2005.01.080. [DOI] [PubMed] [Google Scholar]
- Biessikirski A.; Pytlik M.; Kuterasinski Łu.; Dworzak M.ł; Twardosz M.ł; Napruszewska B. D. Influence of the Ammonium Nitrate (V) Porous Prill Assortments and Absorption Index on Ammonium Nitrate Fuel Oil Blasting Properties. Energies 2020, 13, 3763. 10.3390/en13153763. [DOI] [Google Scholar]
- Song S.; Liu Z.; Zhou L.; Shang L.; Wang Y. Research Progress on Hydrate Plugging in Multiphase Mixed Rich-Liquid Transportation Pipelines. Front. Energy 2020, 10.1007/s11708-020-0688-x. [DOI] [Google Scholar]
- Farhadian A.; Varfolomeev M. A.; Rezaeisadat M.; Semenov A. P.; Stoporev A. S. Toward a Bio-Based Hybrid Inhibition of Gas Hydrate and Corrosion for Flow Assurance. Energy 2020, 210, 118549. 10.1016/j.energy.2020.118549. [DOI] [Google Scholar]
- Qin H.; Qu A.; Wang Y.; Zerpa L.; Koh C.; Bodnar S.; Daly S.; Palermo T.; Mateen K.. Predicting Hydrate Plugging Risk in Oil Dominated Systems Using a Transient Hydrate Film Growth Prediction Tool. Offshore Technology Conference, May 4, 2020; p D031S039R004. 10.4043/30545-MS. [DOI]
- Shi G.; Song S.; Shi B.; Gong J.; Chen D. A New Transient Model for Hydrate Slurry Flow in Oil-Dominated Flowlines. J. Pet. Sci. Eng. 2021, 196, 108003. 10.1016/j.petrol.2020.108003. [DOI] [Google Scholar]
- Liu Y.; Lv X.; Shi B.; Zhou S.; Lei Y.; Yu P.; Chen Y.; Song S.; Ma Q.; Gong J.; Yan K. Rheological Study of Low Wax Content Hydrate Slurries Considering Phase Interactions. J. Nat. Gas Sci. Eng. 2021, 94, 104106. 10.1016/j.jngse.2021.104106. [DOI] [Google Scholar]
- Cao X.; Yang K.; Li T.; Xiong N.; Yang W.; Bian J. Numerical Simulation of Hydrate Particle Behaviors in Gas-Liquid Flow for Horizontal and Inclined Pipeline. Case Stud. Therm. Eng. 2021, 27, 101294. 10.1016/j.csite.2021.101294. [DOI] [Google Scholar]
- Turner D. J.; Miller K. T.; Dendy Sloan E. Methane Hydrate Formation and an Inward Growing Shell Model in Water-in-Oil Dispersions. Chem. Eng. Sci. 2009, 64 (18), 3996–4004. 10.1016/j.ces.2009.05.051. [DOI] [Google Scholar]
- De Almeida V.; Serris E.; Cameirão A.; Herri J.-M.; Abadie E.; Glénat P. Monitoring Gas Hydrates under Multiphase Flow in a High Pressure Flow Loop by Means of an Acoustic Emission Technology. J. Nat. Gas Sci. Eng. 2022, 97, 104338. 10.1016/j.jngse.2021.104338. [DOI] [Google Scholar]
- Morrissy S. A.; McKenzie A. J.; Graham B. F.; Johns M. L.; May E. F.; Aman Z. M. Reduction of Clathrate Hydrate Film Growth Rate by Naturally Occurring Surface Active Components. Energy Fuels 2017, 31 (6), 5798–5805. 10.1021/acs.energyfuels.6b02942. [DOI] [Google Scholar]
- Yan K. Le; Sun C. Y.; Zou B.; Jiang S. X.; Zhang H. X.; Wu J. F. Application of Laser Measurement on the Evaluation of Gas Hydrate Anti-Agglomerant. Xiandai Huagong/Modern Chem. Ind. 2015, 35 (9), 176–179. 10.16606/j.cnki.issn0253-4320.2015.09.044. [DOI] [Google Scholar]
- Gao D.; Xie J.; Huang S.; Wu S.; Wu P.; Huang W. Research and Application of Evaluation Methods for Functional Characteristics of Oil-Based Drilling Fluid in Shale Gas Wells. Geofluids 2021, 2021, 1. 10.1155/2021/8814032. [DOI] [Google Scholar]
- Ning Y.; Li Y.; Song G.; Wang W.; Liu X.; Liu Z.; Zhang J. Investigation on Hydrate Formation and Growth Characteristics in Dissolved Asphaltene-Containing Water-In-Oil Emulsion. Langmuir 2021, 37 (37), 11072–11083. 10.1021/acs.langmuir.1c01698. [DOI] [PubMed] [Google Scholar]
- Sloan E. D.Natural Gas Hydrates in Flow Assurance; Gulf Professional Publishing, 2010. [Google Scholar]
- Sloan E. D.; Koh C. A.. Clathrate Hydrates of Natural Gases Third Edition. Chem. Ind.CRC Press. 2008, 119. [Google Scholar]
- Turner D. J.Clathrate Hydrate Formation in Water-in-Oil Dispersions. Colorado School of Mines; 2005. [Google Scholar]
- Creek J. L.; Subramanian S.; Estanga D. A.. New Method for Managing Hydrates in Deepwater Tiebacks. In Offshore Technology Conference; OnePetro, 2011. [Google Scholar]
- Dieker L. E.; Taylor C. J.; Koh C. A.; Sloan E. D.. Micromechanical Adhesion Force Measurements between Cyclopentane Hydrate Particles. In Proceedings of the 6th International Conference on Gas Hydrates; Citeseer, 2008; pp 12–16.
- Mech D.; Pandey G.; Sangwai J. S. Effect of Molecular Weight of Polyethylene Glycol on the Equilibrium Dissociation Pressures of Methane Hydrate System. J. Chem. Eng. Data 2015, 60 (6), 1878–1885. 10.1021/acs.jced.5b00088. [DOI] [Google Scholar]
- Dorstewitz F.; Mewes D. The Influence of Heat Transfer on the Formation of Hydrate Layers in Pipes. Int. J. Heat Mass Transfer 1994, 37 (14), 2131–2137. 10.1016/0017-9310(94)90314-X. [DOI] [Google Scholar]
- Hatton G. J.; Kruka V. R. Hydrate Blockage Formation-Analysis of Werner Bolley Field Test Data Deep. CTR 2002, 5201–5209. [Google Scholar]
- Sayed M. A.; Saini R. K.; AlAli E.; Kalgaonkar R.; Arnous A. Safer Dual-Functional Gas Hydrate Dissolver and Inhibitor to Replace Methanol. Energy Fuels 2021, 35 (17), 13731–13742. 10.1021/acs.energyfuels.1c01781. [DOI] [Google Scholar]
- Partoon B.; Javanmardi J. Effect of Mixed Thermodynamic and Kinetic Hydrate Promoters on Methane Hydrate Phase Boundary and Formation Kinetics. J. Chem. Eng. Data 2013, 58 (3), 501–509. 10.1021/je301153t. [DOI] [Google Scholar]
- Lachance J. W.; Talley L. D.; Shatto D. P.; Turner D. J.; Eaton M. W. Formation of Hydrate Slurries in a Once-through Operation. Energy Fuels 2012, 26 (7), 4059–4066. 10.1021/ef3002197. [DOI] [Google Scholar]
- Kobayashi I.; Nakajima M.; Chun K.; Kikuchi Y.; Fujita H. Silicon Array of Elongated Through-holes for Monodisperse Emulsion Droplets. AIChE J. 2002, 48 (8), 1639–1644. 10.1002/aic.690480807. [DOI] [Google Scholar]
- Turian R. M.; Hsu F.-L.; Ma T.-W. Estimation of the Critical Velocity in Pipeline Flow of Slurries. Powder Technol. 1987, 51 (1), 35–47. 10.1016/0032-5910(87)80038-4. [DOI] [Google Scholar]
- Ding L.; Shi B.; Wang J.; Liu Y.; Lv X.; Wu H.; Wang W.; Lou X.; Gong J. Hydrate Deposition on Cold Pipe Walls in Water-in-Oil (w/o) Emulsion Systems. Energy Fuels 2017, 31 (9), 8865–8876. 10.1021/acs.energyfuels.7b00559. [DOI] [Google Scholar]
- Bavoh C. B.; Nashed O.; Rehman A. N.; Othaman N. A. A. B.; Lal B.; Sabil K. M. Ionic Liquids as Gas Hydrate Thermodynamic Inhibitors. Ind. Eng. Chem. Res. 2021, 60 (44), 15835–15873. 10.1021/acs.iecr.1c01401. [DOI] [Google Scholar]
- Khan M. S.; Bavoh C. B.; Partoon B.; Lal B.; Bustam M. A.; Shariff A. M. Thermodynamic Effect of Ammonium Based Ionic Liquids on CO 2 Hydrates Phase Boundary. J. Mol. Liq. 2017, 238, 533–539. 10.1016/j.molliq.2017.05.045. [DOI] [Google Scholar]
- Paz P.; Netto T. A. On the Rheological Properties of Thermodynamic Hydrate Inhibitors Used in Offshore Oil and Gas Production. J. Mar. Sci. Eng. 2020, 8 (11), 1–15. 10.3390/jmse8110878. [DOI] [Google Scholar]
- Yaqub S.; lal B.; Kok Keong L. Thermodynamic and Kinetic Effect of Biodegradable Polymers on Carbondioxide Hydrates. J. Ind. Eng. Chem. 2019, 79, 131–145. 10.1016/j.jiec.2019.06.017. [DOI] [Google Scholar]
- Nashed O.; Partoon B.; Lal B.; Sabil K. M.; Shariff A. M. Review the Impact of Nanoparticles on the Thermodynamics and Kinetics of Gas Hydrate Formation. Journal of Natural Gas Science and Engineering 2018, 55 (May), 452–465. 10.1016/j.jngse.2018.05.022. [DOI] [Google Scholar]
- Sahith S. J. K.; Pedapati S. R.; Lal B. Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines. Appl. Sci. 2020, 10 (15), 5052. 10.3390/app10155052. [DOI] [Google Scholar]
- Sayani J. K. S.; Pedapati S. R.; Lal B. Phase Behavior Study on Gas Hydrates Formation in Gas Dominant Multiphase Pipelines with Crude Oil and High CO2Mixed Gas. Sci. Rep. 2020, 10 (1), 1–12. 10.1038/s41598-020-71509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoh C. B.; Nashed O.; Khan M. S.; Partoon B.; Lal B.; Sharif A. M. The Impact of Amino Acids on Methane Hydrate Phase Boundary and Formation Kinetics. J. Chem. Thermodyn. 2018, 117, 48–53. 10.1016/j.jct.2017.09.001. [DOI] [Google Scholar]
- Bavoh C. B.; Lal B.; Ben-Awuah J.; Khan M. S.; Ofori-Sarpong G. Kinetics of Mixed Amino Acid and Ionic Liquid on CO2 Hydrate Formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495 (1), 1–8. 10.1088/1757-899X/495/1/012073. [DOI] [Google Scholar]
- Khan M. S.; Bavoh C. B.; Foo K. S.; Shariff A. M.; Kassim Z.; Othman N. A. B.; Lal B.; Ahmed I.; Rahman M. A.; Gomari S. R. Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates. Molecules 2021, 26 (2), 1–15. 10.3390/molecules26020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqub S.; Lal B.; Mellon N. B.; Sufian S. B. Effect of the Natural Green Materials on Methane Hydrate Formation Kinetics. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458 (1), 012074. 10.1088/1757-899X/458/1/012074. [DOI] [Google Scholar]
- Kele Y.; Yuemeng R.; Cheng L.; Anshan X.; Xiaofang L. Methane Hydrate Formation Behaviors in High Water-Cut Oil-in-Water Systems with Hydrate Promoters. RSC Adv. 2021, 11 (49), 30597–30609. 10.1039/D1RA03501K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraboina N.; Pachitsas S.; Von Solms N. Natural Gas Hydrate Formation and Inhibition in Gas/Crude Oil/Aqueous Systems. Fuel 2015, 148, 186–190. 10.1016/j.fuel.2015.01.103. [DOI] [Google Scholar]
- Stoporev A. S.; Semenov A. P.; Medvedev V. I.; Kidyarov B. I.; Manakov A. Y.; Vinokurov V. A. Nucleation of Gas Hydrates in Multiphase Systems with Several Types of Interfaces. J. Therm. Anal. Calorim. 2018, 134 (1), 783–795. 10.1007/s10973-018-7352-2. [DOI] [Google Scholar]
- Sayani J. K. S.; Pedapati S. R.; Lal B. Phase Behavior Study on Gas Hydrates Formation in Gas Dominant Multiphase Pipelines with Crude Oil and High CO2Mixed Gas. Sci. Rep. 2020, 10 (1), 1–12. 10.1038/s41598-020-71509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume E. O.; Kakitani C.; Merino-Garcia D.; Morales R. E. M.; Sum A. K. Experimental Study of the Formation and Deposition of Gas Hydrates in Non-Emulsifying Oil and Condensate Systems. Chem. Eng. Sci. 2016, 155, 111–126. 10.1016/j.ces.2016.07.046. [DOI] [Google Scholar]
- Straume E. O.; Kakitani C.; Morales R.; Sum A. Study of Gas Hydrate Formation and Deposition Mechanisms in Hydrocarbon Systems. Geology 2018, 10.26678/abcm.encit2016.cit2016-0255. [DOI] [Google Scholar]
- Peng B. Z.; Chen J.; Sun C. Y.; Dandekar A.; Guo S. H.; Liu B.; Mu L.; Yang L. Y.; Li W. Z.; Chen G. J. Flow Characteristics and Morphology of Hydrate Slurry Formed from (Natural Gas+diesel Oil/Condensate Oil+water) System Containing Anti-Agglomerant. Chem. Eng. Sci. 2012, 84, 333–344. 10.1016/j.ces.2012.08.030. [DOI] [Google Scholar]
- Zhao H.; Sun M.; Firoozabadi A. Anti-Agglomeration of Natural Gas Hydrates in Liquid Condensate and Crude Oil at Constant Pressure Conditions. Fuel 2016, 180, 187–193. 10.1016/j.fuel.2016.03.029. [DOI] [Google Scholar]
- Xu S.; Fan S.; Fang S.; Wang Y.; Lang X. Excellent Synergy Effect on Preventing CH4 Hydrate Formation When Glycine Meets Polyvinylcaprolactam. Fuel 2017, 206, 19–26. 10.1016/j.fuel.2017.05.030. [DOI] [Google Scholar]
- Mottahedin M.; Zarenezhad B.; Varaminian F. Effects of Process Variables on the Initial Gas Hydrate Formation Rate: The Case of Ethane Hydrate Formation in the Absence or Presence of SDS Kinetic Promoter. J. Mol. Liq. 2014, 198, 57–62. 10.1016/j.molliq.2014.06.026. [DOI] [Google Scholar]
- Roosta H.; Dashti A.; Hossein Mazloumi S.; Varaminian F. Inhibition and Promotion Effects of Modified HECs and Modified Starches on the Growth Rate of Hydrate in Methane-Propane-Water System. J. Mol. Liq. 2017, 243, 553–563. 10.1016/j.molliq.2017.08.070. [DOI] [Google Scholar]
- Chen J.; Zeng Y.; Liu C.; Kang M.; Chen G.; Deng B.; Zeng F. Methane Hydrate Dissociation from Anti-Agglomerants Containing Oil Dominated Dispersed Systems. Fuel 2021, 294, 120561. 10.1016/j.fuel.2021.120561. [DOI] [Google Scholar]
- Sun M.; Firoozabadi A. New Surfactant for Hydrate Anti-Agglomeration in Hydrocarbon Flowlines and Seabed Oil Capture. J. Colloid Interface Sci. 2013, 402, 312–319. 10.1016/j.jcis.2013.02.053. [DOI] [PubMed] [Google Scholar]
- Mu L.; von Solms N. Inhibition of Natural Gas Hydrate in the System Containing Salts and Crude Oil. J. Pet. Sci. Eng. 2020, 188, 106940. 10.1016/j.petrol.2020.106940. [DOI] [Google Scholar]
- Saberi A.; Alamdari A.; Rasoolzadeh A.; Mohammadi A. H. Insights into Kinetic Inhibition Effects of MEG, PVP, and L-Tyrosine Aqueous Solutions on Natural Gas Hydrate Formation. Pet. Sci. 2021, 18 (2), 495–508. 10.1007/s12182-020-00515-0. [DOI] [Google Scholar]
- Zi M.; Chen D.; Wu G. Molecular Dynamics Simulation of Methane Hydrate Formation on Metal Surface with Oil. Chem. Eng. Sci. 2018, 191, 253–261. 10.1016/j.ces.2018.06.070. [DOI] [Google Scholar]
- Stoporev A. S.; Semenov A. P.; Medvedev V. I.; Kidyarov B. I.; Manakov A. Y.; Vinokurov V. A. Nucleation of Gas Hydrates in Multiphase Systems with Several Types of Interfaces. J. Therm. Anal. Calorim. 2018, 134 (1), 783–795. 10.1007/s10973-018-7352-2. [DOI] [Google Scholar]
- Wang B.; Dong H.; Liu Y.; Lv X.; Liu Y.; Zhao J.; Song Y. Evaluation of Thermal Stimulation on Gas Production from Depressurized Methane Hydrate Deposits☆. Appl. Energy 2018, 227, 710–718. 10.1016/j.apenergy.2017.08.005. [DOI] [Google Scholar]
- Kele Y.; Yuemeng R.; Cheng L.; Anshan X.; Xiaofang L. Methane Hydrate Formation Behaviors in High Water-Cut Oil-in-Water Systems with Hydrate Promoters. RSC Adv. 2021, 11 (49), 30597–30609. 10.1039/D1RA03501K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi A.; Alamdari A.; Rasoolzadeh A.; Mohammadi A. H. Insights into Kinetic Inhibition Effects of MEG, PVP, and L-Tyrosine Aqueous Solutions on Natural Gas Hydrate Formation. Pet. Sci. 2021, 18 (2), 495–508. 10.1007/s12182-020-00515-0. [DOI] [Google Scholar]
- Mu L.; von Solms N. Inhibition of Natural Gas Hydrate in the System Containing Salts and Crude Oil. J. Pet. Sci. Eng. 2020, 188, 106940. 10.1016/j.petrol.2020.106940. [DOI] [Google Scholar]
- Zi M.; Chen D.; Ji H.; Wu G. Effects of Asphaltenes on the Formation and Decomposition of Methane Hydrate: A Molecular Dynamics Study. Energy Fuels 2016, 30 (7), 5643–5650. 10.1021/acs.energyfuels.6b01040. [DOI] [Google Scholar]
- Delgado-Linares J. G.; Majid A. A. A.; Sloan E. D.; Koh C. A.; Sum A. K. Model Water-in-Oil Emulsions for Gas Hydrate Studies in Oil Continuous Systems. Energy Fuels 2013, 27 (8), 4564–4573. 10.1021/ef4004768. [DOI] [Google Scholar]