Abstract
Polymer networks crosslinked via non-covalent interactions afford interesting materials for a wide range of applications due to their self-healing capability, recyclability, and tunable material properties. However, when strong non-covalent binding motifs in combination with high crosslink density are used, processing of the materials becomes troublesome because of high viscosities and the formation of insoluble gels. Here, we present an approach to control the processability of grafted polymers containing strong non-covalent interactions by balancing the interplay of intra- and intermolecular hydrogen bonding. A library of copolymers with different degrees of polymerization and content of protected ureido-pyrimidinone-urea (UPy-urea) grafts was prepared. Photo-deprotection in a good solvent like tetrahydrofuran (THF) at low concentrations (≤1 mg mL–1) created intramolecularly assembled nanoparticles. Remarkably, the intrinsic viscosity of these nanoparticle solutions was an order of magnitude lower compared to solutions of the intermolecularly assembled analogues, highlighting the crucial role of intra- versus intermolecular interactions. Due to the strong hydrogen bonds between UPy dimers, the intramolecularly assembled structures were kinetically trapped. As a result, the polymer nanoparticles were readily processed into a bulk material, without causing major changes in the morphology as verified by atomic force microscopy. Subsequent intermolecular crosslinking of the nanoparticle film, by heating to temperatures where the hydrogen-bond exchange becomes fast, resulted in a crosslinked network. The reversibility of the hereby obtained polymer networks was shown by retrieving the intramolecularly assembled nanoparticles via redissolution and sonication of the intermolecularly crosslinked film in THF with a small amount of acid. Our results highlight that the stability and processability of highly supramolecularly crosslinked polymers can be controlled both in solution and in bulk by using the interplay of intra- and intermolecular non-covalent interactions in grafted polymers.
Introduction
In our current polymer-infused society, huge quantities of polymers are produced everyday and many of these end as waste, contributing to environmental pollution.1 This has urged scientists to develop sustainable “dynamic polymer systems”2 such as dynamic covalent3−7 and non-covalent polymers.8−12 The incorporated dynamicity enables polymers to be reprocessed multiple times in almost any shape via thermodynamic control.13 In dynamic covalent polymers, labile covalent bonds allow for reprocessing via dissociative and associative mechanisms, giving the polymer the ability to be reshaped.14 However, high temperatures and pressures are often required in order to induce sufficient mobility and to trigger the bond exchange.15−17 On the contrary, non-covalent polymers generally relax at shorter time scales (minutes compared to hours), enabling faster dynamics even at room temperature.18,19 In addition, the rate of association and dissociation of non-covalent interactions is highly dependent on temperature. This provides non-covalent polymers with excellent processing properties as shown by linear chain extension of low molecular weight polymers with hydrogen bonding motifs.20,21 Using a strong ureidopyrimidinone (UPy) motif, our group achieved material properties similar to conventional macromolecules at room temperature yet exhibiting superb processing properties at slightly elevated temperatures.22
Besides telechelic non-covalent polymers, various architectures and non-covalent motifs have been developed over the past decades, giving rise to a wide range of precisely tailorable material properties.23−27 However, processing becomes more troublesome when multifunctional polymers containing strong non-covalent bonds are used to achieve high performance functional, self-healing, and adaptable materials.28 Due to the high degree of connectivity, polymers exhibit extremely long relaxation times (up to days), leading to high viscosities or the formation of insoluble gels during processing.29 Besides supramolecular interactions, entanglements play a crucial role in processing as well since they significantly slow down the dynamics of the polymer.30 Logically, an effective approach to circumvent this problem is to restrain the polymer chains from forming entanglements, which was nicely shown by Kuhn and Balmer31 and later by Hawker et al.32 using covalently crosslinked single-chain polymeric nanoparticles. They prepared a copolymer solution containing isocyanate functionalities after which various diamines were added under dilute conditions to intramolecularly crosslink the polymers. Subsequent viscosity studies demonstrated the strength of this approach by achieving significantly lower viscosities for the nanoparticles compared to the random coil analogues. At the same time, our group showed the facile preparation of metastable polymeric nanoparticles based on hydrogen bonding potentially applicable for solution processable supramolecular materials.33,34 This concept has been used to study single-chain polymer nanoparticles (SCPNs);35,36 however, this has not been worked out with the idea to broaden the scope of processing supramolecular polymers into widely applied sustainable materials.
Herein, we report a strategy to enhance the processability and to control the stability of supramolecular grafted polymers by controlling intra- versus intermolecular interactions. In this study, ring-opening metathesis polymerization (ROMP) of norbornene monomers using a second generation Grubbs catalyst is applied due to easy functionalization and high resilience of the catalyst toward supramolecular moieties that can be obtained in high quantities. A library of norbornene polymers, grafted with protected UPy supramolecular motifs, is synthesized followed by intramolecular assembly into polymer nanoparticles in a controlled fashion by selective photo-deprotection using 365 nm light. Herein, the strongly dimerizing UPy groups complemented with lateral stacking of urea moieties37,38 and high glass transition temperatures (Tg) of polynorbornene ensured the formation of stable, intramolecularly crosslinked nanoparticles. Weak hydrogen bonding motifs,39−42 in contrast, exhibit fast exchange dynamics, resulting in higher probabilities of intermolecular crosslinking, and are therefore less suitable for this processing approach.35 The solvent generally plays a crucial role in this process as strong hydrogen bonding complexes should be formed while at the same time interparticle aggregation should be prevented. Previous studies on similar UPy grafted polymer systems showed that tetrahydrofuran (THF) works well43 and will therefore be predominantly used as a solvent. After intramolecular assembly, the particle processing (Figure 1, 1 → 2) and the intra-to-intermolecular rearrangement (Figure 1, 2 → 3) are studied. This work provides structural insights into the relation between intra- and intermolecular non-covalent interactions and reveals how this interplay can be used to regulate the solution viscosity and stability of supramolecular grafted polymers during processing. Moreover, polymer redissolution studies are presented as a proof of concept to address the reversibility of the system (Figure 1, 3 → 1 and 2 → 1) and therewith the potential of this sustainable and novel processing approach toward high-performance supramolecular materials.
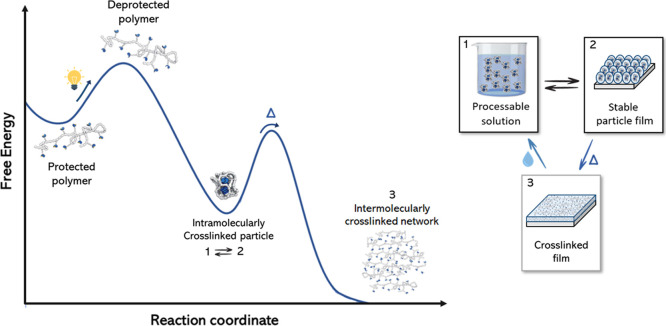
Figure 1.
Schematic representation of the energy diagram for sequential intramolecular assembly and intra-to-intermolecular assembly of UPy-urea grafted polymers into intramolecularly crosslinked nanoparticles (1) and an intermolecularly crosslinked network (3), respectively. In the intermediate kinetically trapped state, the intramolecularly crosslinked nanoparticles exhibit significantly lower viscosities allowing for easier processing into stable particle films (2).
Results and Discussion
Synthetic Design and Synthesis
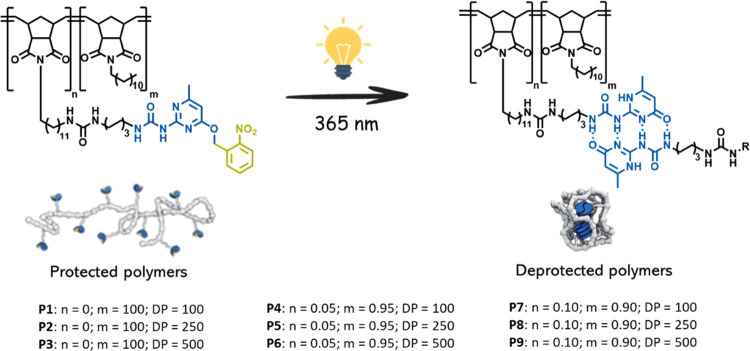
The synthetic approach of the monomers and polymers is similar to that described in previous work44 from our group (a complete reaction scheme can be seen in the Supporting Information). Here, we designed and synthesized a library of poly(norbornenes) grafted with hydrogen bonding building blocks using ring-opening metathesis polymerization (ROMP). The N-substituted cis-5-norbornene-2,3-dicarboxylic imides enable straightforward functionalization. Polymerization using the second generation Grubbs catalyst provided high monomer conversions as the first and third generation Grubbs catalysts resulted in significantly lower conversions. In order to intramolecularly assemble the polymers into nanoparticles, we use o-nitrobenzyl protected UPy as the precursor of the supramolecular motif with an adjacent urea moiety for lateral stacking.37,38 The comonomer is functionalized with an aliphatic dodecyl chain that provides solubility to the polymeric nanoparticles. With the aim of examining various structural effects on the nanoparticle stability, we deliberately prepared a library of polymers containing 0, 5, and 10 mol % UPy-urea as comonomers with varying degrees of polymerization (100, 250, and 500).
First, an amine-functionalized norbornene was acquired by a condensation reaction of endo/exo- cis-5-norbornene-2,3-dicarboxylic anhydride and an excess of 1,12-diaminododecane in the presence of triethylamine. Subsequent addition of 1-(6-isocyanatohexyl)-3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl)urea followed by protection of the UPy with o-nitrobenzyl chloride without intermediate purification yielded the supramolecular norbornene monomer. The dodecyl-functionalized norbornene monomer was obtained in high yields via a single condensation step of n-dodecylamine with the norbornene precursor (Scheme S1).
Using ROMP and the second generation Grubbs catalyst, the majority of (co)polymers (P1–P5, P7, and P8) was successfully prepared at room temperature in dichloromethane, achieving high monomer conversions (97–99%, Table 1) and a high degree of incorporation of functional monomer 5 (Scheme S2). Copolymers P6 and P9 (high molecular weight copolymers) were prepared at 50 °C in dichloroethane, obtaining slightly lower monomer conversions of 80 and 88%, respectively. A higher temperature was applied for these copolymerizations to prevent the complexation of protected UPy with small amounts of Grubbs catalyst.45 The conversion and incorporation of functional protected monomers was analyzed using 1H-NMR spectroscopy (Table 1). The full molecular characterization of the copolymers can be found in the SI (Figures S1–S7).
Table 1. Characterization of Polymers P1–P9 by NMR and SEC.
| polymera | UPy feedb [mol %] | conversionc [%] | observed UPyd [mol %] | DPe | Mnf [KDa] | Đg |
|---|---|---|---|---|---|---|
| P1 | 0 | 100 | 100 | 51 | 2.15 | |
| P2 | 0 | 99 | 247 | 76 | 2.16 | |
| P3 | 0 | 99 | 495 | 135 | 2.06 | |
| P4 | 5 | 100 | 4 | 100 | 55 | 2.05 |
| P5 | 5 | 99 | 5 | 248 | 84 | 1.81 |
| P6 | 5 | 80 | 5 | 400 | 187 | 1.70 |
| P7 | 10 | 99 | 9 | 99 | 66 | 1.85 |
| P8 | 10 | 97 | 8 | 242 | 89 | 1.92 |
| P9 | 10 | 88 | 8 | 440 | 161 | 1.45 |
Theoretical molar percentage of UPy-urea monomer 5 incorporated into the polymer.
Monomer conversion determined by 1H-NMR spectroscopy in chloroform-d.
Observed molar percentage of UPy-urea monomer 5 incorporated into the polymer.
Degree of polymerization (DP) calculated from the monomer conversion.
Number average molecular weight (Mn) measured by SEC at 1 mg mL–1 in THF.
Molar mass dispersity index obtained from SEC.
Intramolecular Assembly of the Supramolecularly Grafted Polymers
In our next step, we followed the assembly of the polymers into intramolecularly, non-covalently crosslinked polymeric nanoparticles. In contrast to the intermolecularly crosslinked polymer network analogue, the intramolecularly crosslinked particles refer to polymers adopting a collapsed particle morphology of one or a few polymer chains. First, the protected polymers P4–P9 were dissolved at 1 mg mL–1 in THF, which is a good solvent for the polymer backbone. THF also minimizes interparticle aggregation and allows the formation of strong hydrogen bonds after deprotection.43 Subsequently, the o-nitrobenzyl protecting group was cleaved using 365 nm light irradiation (Figure 2). The conversion of the deprotection step was monitored by 1H-NMR spectroscopy (Figure S10), and full deprotection was achieved after 2 h.
Figure 2.
Schematic representation of the collapse of o-nitrobenzyl protected UPy-urea grafted polynorbornene into deprotected intramolecularly crosslinked nanoparticles.
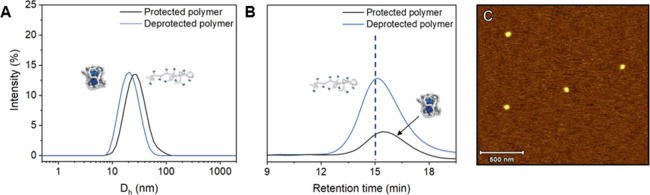
The intramolecular assembly was assessed by DLS and size exclusion chromatography (SEC) (Figure 3A,B). All deprotected polymers P4–P9 exhibited a significant increase in retention time in SEC, as illustrated for polymer P5 in Figure 3B; hence, a lower apparent molecular weight is observed, indicative of intramolecular crosslinking into (kinetically trapped) nanoparticles (Table S1). In accordance with the SEC data, DLS experiments also displayed a significant reduction of the hydrodynamic diameter Dh (13–29%), further confirming the intramolecularly crosslinked nature after UV light deprotection (polymer P5, Figure 3A). Furthermore, the narrow distribution suggests that the nanoparticles are uniform in size, and large multi-chain aggregates are nearly absent. To support this hypothesis, the deprotected intramolecularly assembled nanoparticle solution of P5 was dropcasted on freshly cleaved mica and visualized by AFM. Drying effects and agglomeration of the nanoparticles were prevented by dilution of the polymer solution to 10–8 mg mL–1 in THF before dropcasting. Also in this case, uniform nanoparticles were observed (Figure 3C), which is consistent with the results from earlier works.33,34,43
Figure 3.
Polymer P5 before and after deprotection by UV-light irradiation (365 nm) for 2 h at 1 mg mL–1 in THF demonstrating (A) a reduction in Dh (DLS) and (B) shortening of the retention time (SEC). (C) AFM height image of polymeric nanoparticles P5 dropcasted from a 10–8 mg mL–1 solution in THF on freshly cleaved mica.
Processability of UPy-Urea Grafted Polymers
Impact of Intra- versus Intermolecular Interactions on Viscosity
To examine the contributions of intra- and intermolecular interactions on the processability of the supramolecular grafted polymers, viscosity measurements were performed at different concentrations in THF. Assuming that our polymer solutions behave as Newtonian fluids, the dynamic viscosities were measured using a falling ball viscometer followed by consecutive calculation of the specific and reduced viscosity. In the next step, the intrinsic viscosity is acquired by extrapolation of the reduced viscosity to zero (η → 0). This provides us with structural knowledge on the contribution of the deprotected polymeric nanoparticles to the overall solution viscosity and therefore the processability.
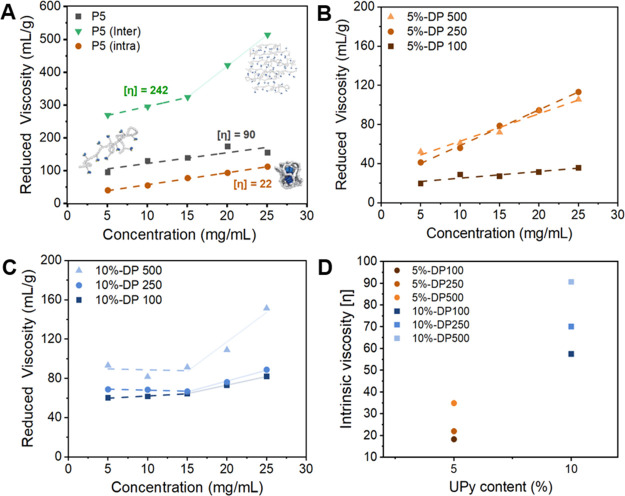
First, the effects of protective group and intra- versus intermolecular crosslinking were determined by comparing the viscosities of three differently prepared polymer solutions of P5 (Figure 4A). For clarity and clear distinction, the deprotected intramolecularly crosslinked nanoparticles and deprotected intermolecularly crosslinked network conformations will be described as P5 (intra) and P5 (inter), respectively, from this point onward. Samples of P5 (intra) are prepared by deprotection of P5 under dilute conditions (1 mg mL–1) promoting intramolecular collapse followed by removal of the solvent to the desired concentration for the measurements (between 5 and 25 mg mL–1). Samples of P5 (inter) were obtained by performing the deprotection at higher concentrations, 5–25 mg mL–1, which favors the formation of intermolecular non-covalent crosslinks. The protected polymer will be simply denoted as P5 and is used without any further preparation steps. Figure 4A shows the reduced viscosity plotted against the concentration for P5, P5 (intra), and P5 (inter). By subsequent extrapolation (dashed lines) of the data points, the intrinsic viscosities could be extracted. As expected, the intrinsic viscosities of P5 and P5 (inter) are significantly higher than those of P5 (intra). Likely, the high viscosity in P5 and P5 (inter) is a direct consequence of the large contribution of intermolecular interactions (such as hydrogen bonding) to viscosity. The difference in intrinsic viscosity between P5 and P5 (inter) can be explained by the stronger hydrogen bonding interactions between UPy groups compared to the protected UPy units. P5 (intra), in contrast, shows lower viscosities, which is rationalized by limited intermolecular interactions, a result of the intramolecular assembly of the polymer under dilute conditions. Interestingly, the intrinsic viscosity [η] (22 mL g–1) of the intramolecularly crosslinked P5 (intra) is about an order magnitude lower than that of P5 (inter) ([η] = 242 mL g–1), showcasing the potential of this approach for a better processability. Moreover, a sharp onset in viscosity was observed for concentrations higher than 15 mg mL–1, only occurring for P5 (inter). This rapid increase in viscosity is presumably caused by exceedance of the concentration at which intermolecular interactions become more dominant for P5 (inter), leading to local network formation. On the contrary, P5 and P5 (intra) do not show this sharp increase at higher concentrations, most likely due to the lower association constant and high stability of intramolecular crosslinks, respectively. Consequently, the critical concentration for these polymer conformations is probably significantly higher than the concentration range measured.
Figure 4.
(A) Reduced viscosity plots of protected polymer P5 (black), deprotected P5 (inter) (green), and deprotected P5 (intra) (brown). Reduced viscosity comparison of (B) deprotected P4–P6 (intra) containing 5 mol % UPy-urea and (C) deprotected P7–P9 (inter) containing 10 mol % UPy-urea. Viscosities were measured at room temperature. The corresponding extrapolations of the concentration to zero (c → 0) indicated by the dotted lines provide the intrinsic viscosities [η] of the polymer. (D) Intrinsic viscosity plotted as a function of grafting density.
Effect of UPy-Urea Content and Degree of Polymerization on the Nanoparticle Solution Viscosity
Besides the effect of polymer conformation and association strength, the content of UPy-urea and polymer length can also have a significant impact on the processability. Therefore, we compared the intrinsic viscosities of P4–P9 (intra) in their intramolecularly crosslinked state. For P4–P6 (intra), containing 5 mol % of UPy-urea grafts (Figure 4B), a decrease in intrinsic viscosity is observed for shorter chain lengths, which means that smaller nanoparticles tend to be more stable and form less intermolecular interactions. In turn, longer chain lengths generally tend to result in higher viscosities. Furthermore, the number of UPy moieties per chain is higher for longer chain lengths; hence, the probability of forming intermolecular interactions will also be higher, leading to increased viscosities.
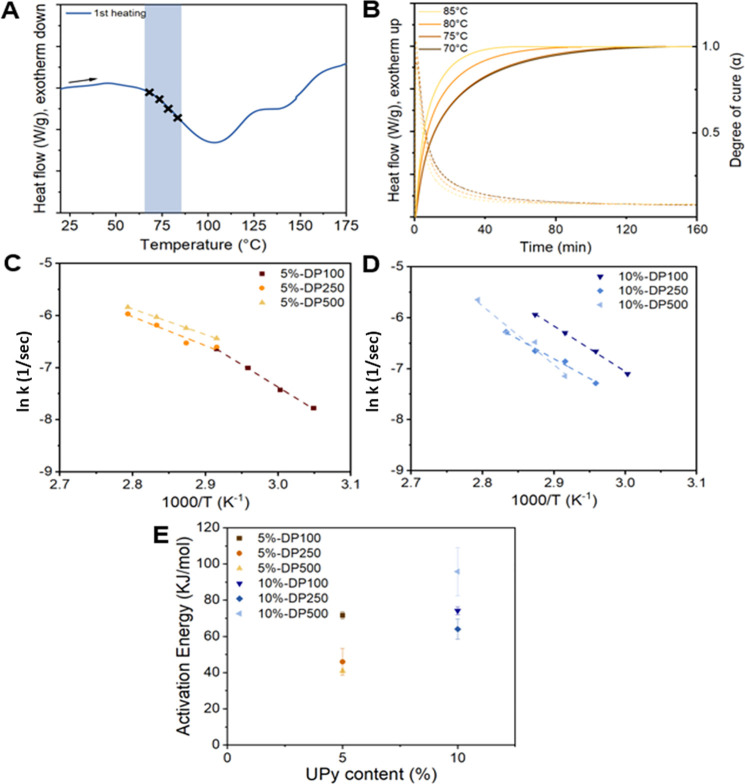
A similar trend was observed for P7–P9 (intra) (10 mol % UPy-urea), which supports our hypothesis on the effect of chain length on the viscosity (Figure 4C). Furthermore, as shown in Figure 4D, the absolute values for the intrinsic viscosity of P7–P9 (intra) ([η] = 50–90) are significantly higher than those for P4–P6 (intra) ([η] = 20–35). This can be attributed to the increased probability of forming intermolecular interactions during the intramolecular assembly for a higher content of UPy-urea grafts. Moreover, a sharp increase in viscosity similar to P5 (inter) was observed for polymers P7–P9 (intra) starting from 15 mg mL–1, most probably again due to crossing the concentration at which the intermolecular interactions become dominant.
Hence, the chain length and content of UPy-urea grafts play a crucial role in the ability to assemble into defined intramolecularly crosslinked nanoparticles and therefore the processability of the polymer solutions. Since intramolecular assembly seems to keep the viscosities of the solutions low, even after concentrating them, it is likely that this will significantly improve the processability of non-covalently grafted polymers.
Intra-to-Intermolecular Assembly of Polymeric Nanoparticles
Visualization of Nanoparticle Intermolecular Assembly
As shown in the previous section, the use of strong and long-lived quadruple hydrogen-bonded UPy dimers limits the probability of intermolecular crosslinking between nanoparticles (kinetic trapping)46,47 and provide nanoparticle stability. Hence, by using these stable UPy-urea moieties, we anticipate that it is possible to bridge the gap between solution and bulk without altering the nanoparticle morphology during processing. Since molecular characterization techniques do not provide a distinction between the intra- and intermolecularly crosslinked state, we selected AFM measurements to elucidate this change in morphology going from nanoparticles to a network-like structure.
The AFM samples were prepared by dropcasting a dilute 10–3 mg mL–1 nanoparticle solution of P5 (intra) on freshly cleaved mica from chloroform instead of THF. Chloroform is known to promote interparticle aggregation upon drying.43 As a result, we expect a morphology indicative of agglomerated nanoparticles held together by weak van der Waals interactions (Figure 5A–C), which in turn would allow us to visualize the morphology change. Next, the samples were heated to 90 °C in a stepwise fashion (10 °C/step) followed by equilibration for 30 min at each step for a temperature-induced change in morphology prior to measuring at room temperature. No significant variations in morphology were observed up till 70 °C (Figure 5C), which is still below the Tg of deprotected polymer P5 (Table 2). Upon further heating to 85 °C and exceeding the Tg of the polymer, the morphology changed from agglomerated nanoparticles to very flat round features with larger surface areas (Figure 5D). This change can most probably be attributed to the nanoparticle disassembly because of the increased dynamics followed by the formation of an intermolecularly crosslinked network. We postulated that this process is initiated by segmental relaxation of the polymer backbone (α-relaxation) above its Tg, which will be further studied in detail in the next section. It is proposed that the kinetically trapped intramolecular assembled polymers at high concentrations are transformed into the thermodynamically more stable crosslinked material. As the enthalpy of the interactions is more or less equal in both states, it is probably a delicate balance in entropy.
Figure 5.
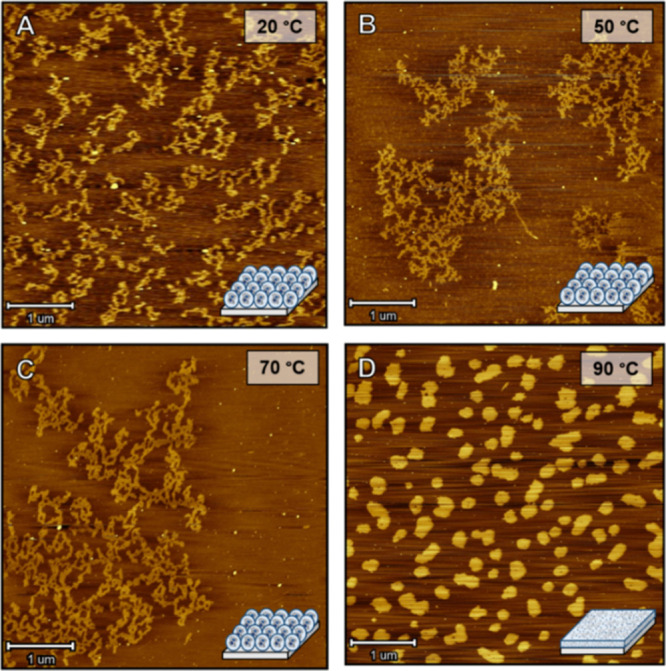
(A–D) Variable-temperature AFM images following the disassembly process of intramolecularly crosslinked polymeric nanoparticles to intermolecularly crosslinked networks occurring above the Tg. The sample was dropcasted from a 10–3 mg/mL solution of polymer P5 (intra) in CHCl3 on freshly cleaved mica and left for 30 min at the desired temperature before taking an image.
Table 2. Thermal Characterization of Polymers P1–P9.
| entrya | Tg,pc (°C) | Tg,dpd (°C) | To,1e (°C) | Tmax,1e (°C) | To,2f (°C) | Tmax,2f (°C) | ΔHdisg (J/g) | EAh (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| P1 | 87b | |||||||
| P2 | 87b | |||||||
| P3 | 85b | |||||||
| P4 | 79 | 62 | 52 | 85 | 100 | 120 | 91 | 71.6 ± 2.1 |
| P5 | 78 | 61 | 65 | 102 | 125 | 141 | 85 | 46.0 ± 7.4 |
| P6 | 78 | 60 | 61 | 93 | 110 | 130 | 71 | 40.9 ± 1.4 |
| P7 | 70 | 52 | 56 | 89 | 103 | 121 | 121 | 74.2 ± 2.2 |
| P8 | 68 | 55 | 66 | 99 | 121 | 130 | 98 | 64.0 ± 5.5 |
| P9 | 70 | 53 | 65 | 98 | 112 | 133 | 51 | 56.0 ± 12.5 |
Glass transition temperature of the non-functional control polymers (Tg,p).
Glass transition temperature of the o-nitrobenzyl protected UPy-urea grafted polymers (Tg,p) in their intermolecularly crosslinked conformation.
Glass transition temperature of the deprotected UPy-urea grafted polymers (Tg,dp) in their intermolecularly crosslinked conformation.
Onset temperatures (To,1 and To,2) for both exotherms observed in the first heating run of deprotected UPy-urea grafted polymers in their intramolecularly crosslinked conformation.
Corresponding maximum temperatures (Tmax,1 and Tmax,2) for both exotherms observed in the first heating run of deprotected UPy-urea grafted polymers in their intramolecularly crosslinked conformation.
Disassembly enthalpy of both exotherms observed in the first heating run of deprotected UPy-urea grafted polymers in their nanoparticle conformation.
Activation energy required for disassembly of the intramolecularly crosslinked nanoparticles into an intermolecularly assembled crosslinked network.
Thermal Properties and Dynamics of UPy-Urea Grafted Polymers
To corroborate that disassembly of the particles is initiated by the α-relaxation process, differential scanning calorimetry (DSC) measurements were conducted on non-functionalized polymers P1–P3 (Figure S11) and on both the protected polymers P4–P9 and deprotected intramolecularly crosslinked polymers P4–P9 (intra). The DSC samples for P4–P9 (intra) were prepared in a similar manner to those for the AFM measurements, wherein a 1 mg mL–1 nanoparticle solution in THF was concentrated to 10 mg mL–1. Subsequent dropcasting of aliquot amounts of solution on a glass slide and drying under reduced pressure at room temperature for at least 3 h provided us with nanoparticle films suitable for DSC measurements. A more detailed description of the sample preparation is reported in the SI.
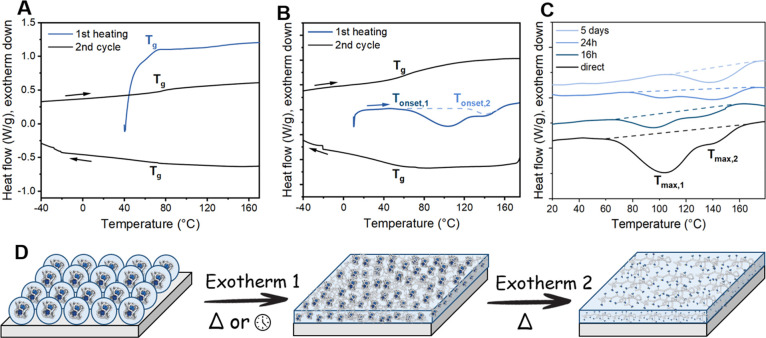
In Figure 6A (and Figure S12), the first heating run and the first cycle of protected polymers P4–P9 show one transition, a Tg that varies between 68 and 79 °C (Table 2) depending on the UPy-urea content. However, P4–P9 (intra) all exhibited two large overlapping exotherms, which we attribute to the disassembly of polymeric nanoparticles into an intermolecularly crosslinked network (Figure 6B). The first exotherm appears just above the Tg of the polymers and is assigned to the α-relaxation of the norbornene backbone. The second exotherm is generally observed between 120 and 150 °C, depending on the polymer length, as a shoulder of the first exotherm. This transition most probably originates from the subsequent α*-relaxation of the polymer backbone initiated by a dynamic UPy dissociation–association process, which is commonly observed in this temperature range.48 We assume that the nanoparticle disassembly predominantly occurs during the second exotherm since intermolecular crosslinking can only occur upon UPy dissociation.
Figure 6.
DSC traces of (A) protected polymer P5 and (B) deprotected P5 (intra) (first and second heating and second cooling runs). (C) Aging effect observed for P5 (intra) related to α-relaxation (exotherm 1, first heating run). A temperature ramp of 10 K min–1 was used. (D) Illustration of the two consecutive relaxation processes leading to stepwise nanoparticle disassembly. The first transition represents α-relaxation occurring both over time (days) or with temperature (50–70 °C), while the second transition representing α*-relaxation predominantly occurs with temperatures at 100–125 °C.
When comparing the onset and peak temperatures at which the two relaxation processes occur for polymers P4–P9 (intra) (To,1, Tmax,1, To,2, and Tmax,2), no significant differences were observed (Table 2). Moreover, by comparing the Tg of non-functional polymers P1–P3 and protected polymers P4–P9, a decreasing trend is observed upon increasing the UPy-urea content. This is presumably caused by the increased free volume created by the bulky UPy-urea grafts. A similar decreasing trend in Tg is observed for deprotected polymers P4–P9 (intra) in the second heating run (Table 2).
To confirm that the two aforementioned exotherms originate from two distinct relaxations, aging experiments were conducted on polymer P5 (intra). Hereto, samples of P5 (intra) were prepared as described earlier and left at room temperature for 0, 16, and 24 h as well as 5 days. As observed in the thermogram in Figure 6C, the first exotherm visible at about 110 °C starts to diminish after 16 h and has completely disappeared after 5 days. On the contrary, the second exotherm is barely affected by the aging process indicative of a thermally initiated process or a process with exceptionally high relaxation times (up to months) at room temperature. This supports that the second exotherm and relaxation process are predominantly dictated by UPy dissociation, while the first exotherm is dominated by polymer backbone relaxation as shown in the cartoon in Figure 6D.
Nanoparticle Disassembly Kinetics
To get a thorough understanding of the polymer relaxation and the associated energy release, we performed kinetic DSC studies on all intramolecularly crosslinked nanoparticle films for P4–P9 (intra). Herein, the method used is derived from the Borchardt and Daniels kinetic approach.49 We assumed that the nanoparticle relaxation occurs via first-order kinetics following the Arrhenius law. First, a thermogram was acquired to determine appropriate temperatures for the isothermal measurements. The selected temperatures generally range from Tonset to 50% of the height of Tpeak as indicated by the black crosses in Figure 7A. Subsequently, four isotherms at the selected temperatures for each polymer were measured (dashed lines) and converted into the degree of cure as a function of time (solid lines, Figure 7B). From these conversion curves, the specific reaction constant k(T) for every temperature was determined for α = 0.1. By plotting ln[k(T)] versus 1000/T (Figure 7C,D) and fitting the Arrhenius law, estimated values of the activation energy for nanoparticle relaxation were obtained for polymers P4–P9 (intra) (Table 2). The results show that polymers with higher DPs result in lower activation energies, while no significant trend was observed upon increasing the content of UPy-urea grafts (5 to 10 mol %) (Figure 7E). Interestingly, the absolute activation energy values seem to be comparable to averaged values for UPy dissociation50 and viscous flow of conventional polymers,51 arguably indicating the presence of a synergistic process. However, in-depth mechanistic studies would be required to support this hypothesis.
Figure 7.
(A) Temperature determination for isothermal experiments from the first heating run of polymer P5 (intra) in the nanoparticle conformation. A temperature ramp of 10 K min–1 was used. (B) Isothermal DSC traces between 70 and 85 °C (dotted lines) with corresponding degrees of cure (α) (solid lines). Arrhenius analysis of (C) polymers P4–P6 (intra) and (D) polymers P7–P9 (intra). (E) Activation energy for nanoparticle relaxation shown as a function of grafting density.
Redissolution of Intra- and Intermolecularly Crosslinked Polymer Films
The dynamic nature of the supramolecular networks enables recycling of the polymers, either thermally or by redissolution. This prompted us to test the reversibility of this process by reobtaining uniform nanoparticle solutions from both the intramolecularly crosslinked nanoparticle and the intermolecularly crosslinked network films. Herein, films of P5 (intra) as prepared for DSC analysis were sonicated for 2 min in an excess of THF. The quantity of solvent was then reduced to reach a concentration of 1 mg mL–1 after which they were subjected to DLS analysis and compared with the initial intramolecular assembly measurements (Figure 8).
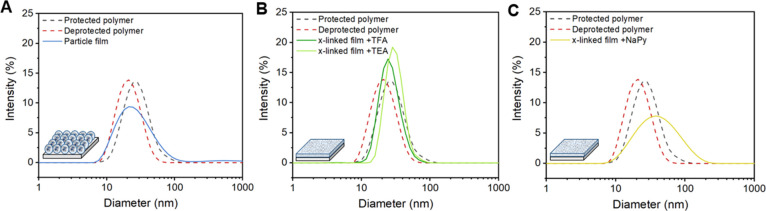
Figure 8.
DLS intensity distributions as a function of the hydrodynamic diameter (Dh) of redissolved intra- and intermolecularly crosslinked films of polymer P5 upon 2 min of sonication at 1 mg mL–1 in THF. (A) Upon redissolution of the nanoparticle film P5 (intra); (B) after redissolution of an intermolecular crosslinked film P5 (inter) sequentially followed by addition of 100 μL of TFA and by 100 μL of TEA; and (C) after redissolution of an intermolecular crosslinked film P5 (inter) upon addition of an excess of naphthyridine (NaPy).
After redissolution of the nanoparticle film of P5 (intra) in THF (Figure S14) and sonication for 2 min, the nanoparticles fully recover to their initial sizes and show a uniform yet slightly broader size distribution, possibly due to nanoparticle aggregation (Figure 8A, blue). Contrarily, the crosslinked polymer film of P5 (inter) could not be redissolved due to the highly crosslinked nature of the material (Figure S15A). However, upon addition of a small amount of trifluoroacetic acid (TFA), which disrupts the hydrogen bonding of the UPy dimers,34 the crosslinked film redissolved (Figure S15B). Subsequent DLS analysis revealed similarly sized polymeric nanoparticles with a narrow size distribution presumably in their random coil conformation due to UPy dimer inhibition by TFA (Figure 8B, dark green). Unfortunately, consecutive neutralization of TFA upon addition of an aliquot amount of triethylamine (TEA) as a base did not result in smaller nanoparticles but slightly larger-sized nanoparticles (Figure 8B, light green). An explanation could be that interparticle interactions are promoted by the presence of the TEA-TFA salt. Nevertheless, redissolution of the crosslinked polymer film P5 (inter) and thus reversibility of the system were possible. Furthermore, we tried to redissolve the intermolecularly crosslinked film of P5 (inter) by adding an excess of naphthyridine (NaPy), which forms a heterocomplex with UPy and would thus act as a dimerization inhibitor.52 However, only partial redissolution could be acquired (Figure S15C). Possibly, the equilibrium that is formed between the homo- and heterodimers could have resulted in a complex mixture, eventually leading to a broader size distribution (Figure 8C, yellow). Hence, the use of NaPy is a less suitable approach to achieve reversibility in this system.
Conclusions
In this work, we successfully presented a novel approach showing the enhanced processability and stability of supramolecular grafted polymers by controlling the intra- and intermolecular interactions. Hereby, we used the concentration as a tool for intramolecular polymer chain collapse, resulting in significantly lower viscosities than their intermolecularly crosslinked analogues. In general, increasing the chain length of the polymers and content of UPy-urea increased the propensity for intermolecular interactions and thus lead to higher viscosities. After processing the nanoparticles into the bulk, AFM analysis provided a first indication of nanoparticle disassembly indicated by a change in morphology upon exceedance of the Tg of the grafted polymers. Subsequent DSC analysis demonstrated the formation of stable nanoparticle films and revealed two exothermic relaxation processes during the first heating run in contrast to the processed random coil polymers. Herein, the two processes are related to the α- and α*-relaxation, in which the latter provides the intramolecularly trapped nanoparticles with sufficient energy to form an intermolecular crosslinked network. Further in-depth DSC analysis on the kinetics of nanoparticle relaxation in the bulk revealed a similar trend to that in solution, demonstrating the effect of chain length and UPy content on the activation barrier. Ultimately, by redissolution of intermolecularly crosslinked polymer films in the presence of small amounts of additive, we could show reversibility in this system. These fundamental and structural insights into the interplay of inter- and intramolecular crosslinking broaden the scope of applications for supramolecular polymers and increase their potential in the field of sustainability. We envision that this novel processing approach of non-covalently grafted polymers will lead to further development in the field of reprocessable plastics.
Acknowledgments
We would like to acknowledge the Dutch Ministry of Education, Culture and Science (Gravity program 024.001.035) and European Research Council for funding (H2020-EU.1.1., SYNMAT project, ID 788618).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.macromol.2c00976.
Experimental details, materials and methods, detailed synthetic procedures of all monomers, polymers, and intermediates, NMR spectra of final monomers and polymers, FTIR spectra of all polymers, NMR spectra of intramolecular folding of polymer P5, variable temperature and isothermal DSC traces of polymers, and photographs of redissolution studies (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gibb B. C. Plastics Are Forever. Nat. Chem. 2019, 11, 394–395. 10.1038/s41557-019-0260-7. [DOI] [PubMed] [Google Scholar]
- Roy N.; Bruchmann B.; Lehn J. M. DYNAMERS: Dynamic Polymers as Self-Healing Materials. Chem. Soc. Rev. 2015, 44, 3786–3807. 10.1039/c5cs00194c. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Shin S.; Kim T.; Lee D.; Seok C.; Lee M. Switchable Nanoporous Sheets by the Aqueous Self-Assembly of Aromatic Macrobicycles. Angew. Chem. 2013, 52, 6426–6429. 10.1002/anie.201210373. [DOI] [PubMed] [Google Scholar]
- Ying H.; Zhang Y.; Cheng J.. Dynamic Urea Bond for the Design of Reversible and Self-Healing Polymers. Nat. Commun. 2014, 5. 10.1038/ncomms4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarnal D.; Capelot M.; Tournilhac F.; Leibler L. Silica-like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. 10.1126/science.1212648. [DOI] [PubMed] [Google Scholar]
- Denissen W.; Rivero G.; Nicolaÿ R.; Leibler L.; Winne J. M.; Du Prez F. E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. 10.1002/adfm.201404553. [DOI] [Google Scholar]
- Mukherjee S.; Brooks W. L. A.; Dai Y.; Sumerlin B. S. Doubly-Dynamic-Covalent Polymers Composed of Oxime and Oxanorbornene Links. Polym. Chem. 2016, 7, 1971–1978. 10.1039/c5py02046h. [DOI] [Google Scholar]
- Sijbesma R. P.; Beijer F. H.; Brunsveld L.; Folmer B. J. B.; Hirschberg J. H. K. K. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- Fouquey C.; Lehn J.-M.; Levelut A.-M. Molecular Recognition Directed Self-Assembly of Supramolecular Liquid Crystalline Polymers from Complementary Chiral Components. Adv. Mater. 1990, 2, 254–257. 10.1002/adma.19900020506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavenor N. A.; Murnin M. J.; Horne W. S. Supramolecular Metal-Coordination Polymers, Nets, and Frameworks from Synthetic Coiled-Coil Peptides. J. Am. Chem. Soc. 2017, 139, 5–2215. 10.1021/jacs.7b00651. [DOI] [PubMed] [Google Scholar]
- Paper P. H.; Wu S.; Cai C.; Li F.; Tan Z.; Dong S. Deep Eutectic Supramolecular Polymers : Bulk Supramolecular Materials. Angew. Chem., Int. Ed. 2020, 59, 11871–11875. 10.1002/anie.202004104. [DOI] [PubMed] [Google Scholar]
- Ren X.; Wang X.; Sun Y.; Chi X.; Mangel D.; Wang H.; Sessler J. L. Amidinium–Carboxylate Salt Bridge Mediated Proton-Coupled Electron Transfer in a Donor–Acceptor Supramolecular System. Org. Chem. Front. 2019, 6, 584–590. 10.1039/c8qo01408f. [DOI] [Google Scholar]
- Rowan S. J.; Cantrill S. J.; Cousins G. R. L.; Sanders J. K. M.; Stoddart J. F. Dynamic covalent chemistry. Angew. Chem. Int Ed. 2002, 41, 898–952. . [DOI] [PubMed] [Google Scholar]
- Winne J. M.; Leibler L.; Du Prez F. Dynamic Covalent Chemistry in Polymer Networks: A Mechanistic Perspective. Polym. Chem. 2019, 6091–6108. 10.1039/C9PY01260E. [DOI] [Google Scholar]
- Liu W.; Zhang C.; Zhang H.; Zhao N.; Yu Z.; Xu J. Oxime-Based and Catalyst-Free Dynamic Covalent Polyurethanes. J. Am. Chem. Soc. 2017, 139, 8678–8684. 10.1021/jacs.7b03967. [DOI] [PubMed] [Google Scholar]
- Majumdar S.; Zhang H.; Soleimani M.; Benthem R. A. T. M. V.; Heuts J. P. A.; Sijbesma R. P. Phosphate Triester Dynamic Covalent Networks. ACS Macro Lett. 2020, 9, 1753–1758. 10.1021/acsmacrolett.0c00636. [DOI] [PubMed] [Google Scholar]
- Tretbar C. A.; Neal J. A.; Guan Z. Direct Silyl Ether Metathesis for Vitrimers with Exceptional Thermal Stability. J. Am. Chem. Soc. 2019, 141, 16595–16599. 10.1021/jacs.9b08876. [DOI] [PubMed] [Google Scholar]
- Oya N.; Ikezaki T.; Yoshie N. A Crystalline Supramolecular Polymer with Self-Healing Capability at Room Temperature. Polym. J. 2013, 45, 955–961. 10.1038/pj.2012.230. [DOI] [Google Scholar]
- Liu J.; Soo C.; Tan Y.; Yu Z.; Li N.; Abell C.; Scherman O. A. Tough Supramolecular Polymer Networks with Extreme Stretchability and Fast Room-Temperature Self-Healing. Adv. Mater. 2017, 29, 1–7. 10.1002/adma.201605325. [DOI] [PubMed] [Google Scholar]
- Rowan S. J.; Suwanmala P.; Sivakova S. Nucleobase-Induced Supramolecular Polymerization in the Solid State. J. Polym. Sci., Part A: Polym. Chem. 2003, 41, 3589–3596. 10.1002/pola.10967. [DOI] [Google Scholar]
- Zimmerman N.; Moore J. S.; Zimmerman S. C. Polymer Chemistry Comes Full Circle. Chem. & Ind. 1998, 604–610. [Google Scholar]
- Sijbesma R. P.; Meijer E. W. Supramolecular Polymers at Work. Mater. Today 2004, 7, 34–39. 10.1016/S1369-7021(04)00187-7. [DOI] [Google Scholar]
- Abbasi M.; Faust L.; Wilhelm M. Comb and Bottlebrush Polymers with Superior Rheological and Mechanical Properties. Adv. Mater. 2019, 31, 1–11. 10.1002/adma.201806484. [DOI] [PubMed] [Google Scholar]
- Wu W.; Wang W.; Li J. Star Polymers : Advances in Biomedical Applications. Prog. Polym. Sci. 2015, 46, 55–85. 10.1016/j.progpolymsci.2015.02.002. [DOI] [Google Scholar]
- Balkenende D. W. R.; Monnier C. A.; Fiore G. L.; Weder C. Optically Responsive Supramolecular Polymer Glasses. Nat. Commun. 2016, 7, 1–9. 10.1038/ncomms10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers B. A. G.; Sleczkowski M. L.; Wouters F.; Engels T. A. P.; Meijer E. W.; Palmans A. R. A. Polymer Chemistry Tuning Polymer Properties of Non-Covalent Crosslinked PDMS by Varying Supramolecular Interaction Strength. Polym. Chem. 2020, 11, 2847–2854. 10.1039/d0py00139b. [DOI] [Google Scholar]
- Galeazzi S.; Hermans T. M.; Paolino M.; Anzini M.; Mennuni L.; Giordani A.; Caselli G.; Makovec F.; Meijer E. W.; Vomero S.; Cappelli A. Multivalent Supramolecular Dendrimer-Based Drugs. Biomacromolecules 2010, 11, 182–186. 10.1021/bm901055a. [DOI] [PubMed] [Google Scholar]
- O’Donnell A. D.; Salimi S.; Hart L. R.; Babra T. S.; Greenland B. W.; Hayes W. Applications of Supramolecular Polymer Networks. React. Funct. Polym. 2022, 172, 105209 10.1016/j.reactfunctpolym.2022.105209. [DOI] [Google Scholar]
- Elkins C. L.; Park T.; Mckee M. G.; Long T. E. Synthesis and Characterization of Poly ( 2-Ethylhexyl Methacrylate ) Copolymers Containing Pendant , Self-Complementary Multiple-Hydrogen-Bonding Sites. J. Polym. Sci., Part A: Polym. Chem. 2005, 43, 4618–4631. 10.1002/pola.20961. [DOI] [Google Scholar]
- Feldman K. E.; Kade M. J.; Meijer E. W.; Hawker C. J.; Kramer E. J. Model Transient Networks from Strongly Hydrogen-Bonded Polymers. Macromolecules 2009, 42, 9072–9081. 10.1021/ma901668w. [DOI] [Google Scholar]
- Kuhn W.; Balmer G. Crosslinking of Single Linear Macromolecules. J. Polym. Sci. 1962, 57, 311–319. 10.1002/pol.1962.1205716524. [DOI] [Google Scholar]
- Beck J. B.; Killops K. L.; Kang T.; Sivanandan K.; Bayles A.; Mackay M. E.; Wooley K. L.; Hawker C. J. Facile Preparation of Nanoparticles by Intramolecular Cross-Linking of Isocyanate Functionalized Copolymers. Macromolecules 2009, 42, 5629–5635. 10.1021/ma900899v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berda E. B.; Foster E. J.; Meijer E. W. Toward Controlling Folding in Synthetic Polymers: Fabricating and Characterizing Supramolecular Single-Chain Nanoparticles. Macromolecules 2010, 43, 1430–1437. 10.1021/ma902393h. [DOI] [Google Scholar]
- Foster E. J.; Berda E. B.; Meijer E. W. Metastable Supramolecular Polymer Nanoparticles via Intramolecular Collapse of Single Polymer Chains. J. Am. Chem. Soc. 2009, 131, 6964–6966. 10.1021/ja901687d. [DOI] [PubMed] [Google Scholar]
- ter Huurne G. M.; Voets I. K.; Palmans A. R. A.; Meijer E. W. Effect of Intra- versus Intermolecular Cross-Linking on the Supramolecular Folding of a Polymer Chain. Macromolecules 2018, 51, 8853–8861. 10.1021/acs.macromol.8b01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposo J. A.; Rubio-Cervilla J.; Moreno A. J.; Lo Verso F.; Bacova P.; Arbe A.; Colmenero J. Folding Single Chains to Single-Chain Nanoparticles via Reversible Interactions: What Size Reduction Can One Expect?. Macromolecules 2017, 50, 1732–1739. 10.1021/acs.macromol.6b02427. [DOI] [Google Scholar]
- Etter M. C.; Urbañczyk-lipkowska Z.; Zia-ebrahimi M.; Panunto T. W. Hydrogen Bond Directed Cocrystallization and Molecular Recognition Properties of Diarylureas. J. Am. Chem. Soc. 1990, 16, 8415–8426. 10.1021/ja00179a028. [DOI] [Google Scholar]
- Kautz H.; van Beek D. J. M.; Sijbesma R. P.; Meijer E. W. Cooperative End-to-End and Lateral Hydrogen-Bonding Motifs in Supramolecular Thermoplastic Elastomers. Macromolecules 2006, 39, 4265–4267. 10.1021/ma060706z. [DOI] [Google Scholar]
- Greenland B. W.; Burattini S.; Hayes W.; Colquhoun H. M. Design, Synthesis and Computational Modelling of Aromatic Tweezer-Molecules as Models for Chain-Folding Polymer Blends. Tetrahedron 2008, 64, 8346–8354. 10.1016/j.tet.2008.05.077. [DOI] [Google Scholar]
- Ouhib F.; Raynal M.; Jouvelet B.; Isare B.; Bouteiller L. Hydrogen Bonded Supramolecular Polymers in Moderately Polar Solvents. Chem. Commun. 2011, 47, 10683–10685. 10.1039/c1cc14590h. [DOI] [PubMed] [Google Scholar]
- Coubrough H. M.; Reynolds M.; Goodchild J. A.; Connell S. D. A.; Mattsson J.; Wilson A. J. Assembly of Miscible Supramolecular Network Blends Using DDA·AAD Hydrogen-Bonding Interactions of Pendent Side-Chains. Polym. Chem. 2020, 11, 3593–3604. 10.1039/c9py01913h. [DOI] [Google Scholar]
- Herbst F.; Binder W. H. Comparing Solution and Melt-State Association of Hydrogen Bonds in Supramolecular Polymers. Polym. Chem. 2013, 4, 3602–3609. 10.1039/c3py00362k. [DOI] [Google Scholar]
- Stals P. J. M.; Gillissen M. A. J.; Nicolaÿ R.; Palmans A. R. A.; Meijer E. W. The Balance between Intramolecular Hydrogen Bonding, Polymer Solubility and Rigidity in Single-Chain Polymeric Nanoparticles. Polym. Chem. 2013, 4, 2584–2597. 10.1039/c3py00094j. [DOI] [Google Scholar]
- Foster E. J.; Berda E. B.; Meijer E. W. Tuning the Size of Supramolecular Single-Chain Polymer Nanoparticles. J. Polym. Sci., Part A: Polym. Chem. 2011, 49, 118–126. 10.1002/pola.24426. [DOI] [Google Scholar]
- Jawiczuk M.; Marczyk A.; Trzaskowski B.. Decomposition of Ruthenium Olefin Metathesis. Catalysts 2020, 10, 887, 10.3390/catal10080887. [DOI] [Google Scholar]
- Tang X.; Feula A.; Baker B. C.; Melia K.; Hermida Merino D.; Hamley I. W.; Buckley C. P.; Hayes W.; Siviour C. R. A Dynamic Supramolecular Polyurethane Network Whose Mechanical Properties Are Kinetically Controlled. Polymer 2017, 133, 143–150. 10.1016/j.polymer.2017.11.005. [DOI] [Google Scholar]
- Hohl D. K.; Ferahian A. C.; Montero De Espinosa L.; Weder C. Toughening of Glassy Supramolecular Polymer Networks. ACS Macro Lett. 2019, 8, 1484–1490. 10.1021/acsmacrolett.9b00710. [DOI] [PubMed] [Google Scholar]
- Wubbenhorst M.; Van Turnhout J.; Folmer B. J. B.; Sijbesma R. P.; Meijer E. W. Complex Dynamics of Hydrogen Bonded. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 365–372. 10.1109/94.933345. [DOI] [Google Scholar]
- Wang J.; Laborie M. G.; Wolcott M. P. Comparison of Model-Fitting Kinetics for Predicting the Cure Behavior of Commercial Phenol – Formaldehyde Resins. J. Appl. Polym. Sci. 2007, 105, 1289–1296. 10.1002/app. [DOI] [Google Scholar]
- Genderen M. H. P.; Wolfgang H. Quadruple Hydrogen Bonds of Ureido-Pyrimidinone Moieties Investigated in the Solid State by 1 H Double-Quantum MAS NMR Spectroscopy. Phys. Chem. 2002, 4, 3750–3758. 10.1039/b203333j. [DOI] [Google Scholar]
- Lou Y.; Lei Q.; Wu G. Research on Polymer Viscous Flow Activation Energy and Non-Newtonian Index Model Based on Feature Size. Adv. Polym. Technol. 2019, 1–11. 10.1155/2019/1070427. [DOI] [Google Scholar]
- Paffen T. F. E.; Ercolani G.; Greef T. F. A. D.; Meijer E. W. Supramolecular Buffering by Ring–Chain Competition. J. Am. Chem. Soc. 2015, 137, 1501–1509. 10.1021/ja5110377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.