Abstract
Background
Pulmonary infection is a frequent complication among stroke patients and adversely affects clinical outcomes, increases the length of hospitalization stay and costs, and aggravates the financial burden of the national medical health system. Early identification and management of high-risk patients are necessary and imperative to reduce the incidence of stroke-associated pneumonia (SAP).
Aim
The evidence-based practice project evaluated the effectiveness of a standard care bundle intervention in preventing the occurrence of SAP.
Methods
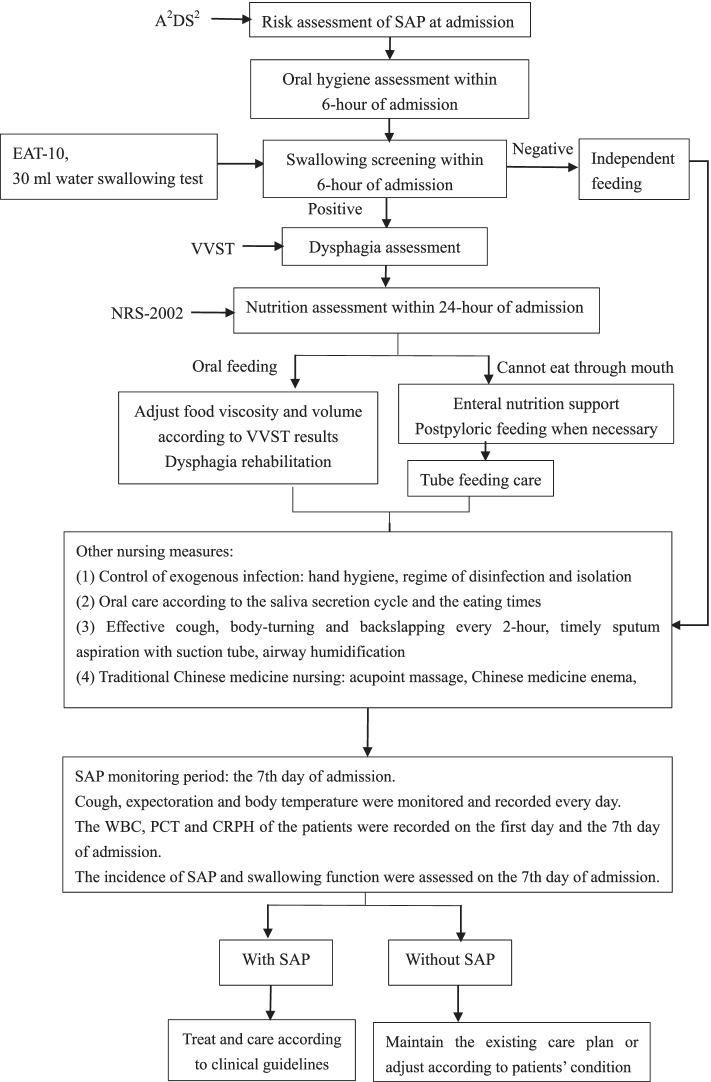
The project was conducted in a neurology department of a teaching hospital. Given the variation in assessment and management standards, evidence-based practice (EBP) methodology was used to establish a process for quality improvement. A thorough literature search was conducted to identify evidence-based interventions to manage and prevent SAP. Thorough critiques of the literature and synthesis of the evidence were completed. A systematic management flow and care bundle interventions were established. The care bundle included interventions, such as the utilization of tools for SAP risk screening; dysphagia screening and rehabilitation; feeding modification, oral care, airway management, position management, and the nursing techniques of traditional Chinese medicine.
Results
A significant improvement was observed in preventing SAP in patients in the postimplementation group compared with those in the preimplementation group (14.0% vs. 37.2%, p = 0.025). In addition, significantly lower duration of hospitalization, lower rate of aspiration, and improvements in albumin and oral hygiene were found after the implementation of the care bundle.
Conclusions
Evidence-based care bundles successfully empower nurses to reduce the incidence of SAP. The management flow of SAP prevention could be promoted to other units of the neurology department in the future. The results of the project reflect positively on the capacity to implement EBP in an acute care setting for stroke. The EBP methodology can be utilized to solve other clinical problems.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-022-02826-8.
Keywords: Stroke, Pneumonia, Evidence-based practice, Quality improvement, Prevention, Patient safety
Introduction
Stroke is a widely prevalent acute cerebrovascular disease with high morbidity and mortality [1]. Acute ischaemic stroke accounts for 60–80% of all strokes [1, 2]. Pulmonary infections frequently occur among stroke patients and adversely affect clinical outcomes, aggravating the financial burden on family and national medical health systems [3–5].
Stroke-associated pneumonia (SAP) refers to pulmonary infections that develop within the first 7 days of stroke onset among nonventilated patients, affecting 2.3 to 44% of stroke patients [6, 7]. Female sex, advanced age, dysphagia, the severity of acute stroke and disturbance of consciousness are the main risk factors for SAP [8–11]. Some of these factors have been used as scoring items in the scale to assess the risk of SAP. The combination of aspiration caused by these risk factors and acute stroke-induced immunosuppression leads to the increased incidence of SAP [12, 13].
Previous studies have shown that SAP can be prevented [14, 15]. In addition, 43–79% of SAP occurs within 72 h of acute stroke onset [14–16]. Therefore, early identification and management of high-risk patients are necessary to reduce the incidence of SAP [17].
Although many scholars have explored and evaluated the effects of different preventive interventions on SAP from different perspectives, systematic prevention recommendations or management flowcharts for SAP have not yet been developed. Effective nursing interventions include feeding management, respiratory tract management, dysphagia rehabilitation, cluster nursing interventions, and traditional Chinese medicine (TCM) nursing [9, 18, 19]. It is necessary to develop an evidence-based nursing scheme based on extensive evidence, including TCM nursing interventions.
Methods
Context
This project was implemented in the Neurology Department of The Second Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, which is a cerebrovascular disease medical center with more than 300 beds. A 28-bed internal medicine unit with more than 1200 annual cerebrovascular disease visits was designated for the project. The prevalence rate of SAP in the unit was 26.5% in 2018.
Purpose
This nurse-led project aimed to institute a best practice bundle and management process to reinforce systematic management of patients with acute stroke, resulting in a decrease in the incidence of SAP.
Population
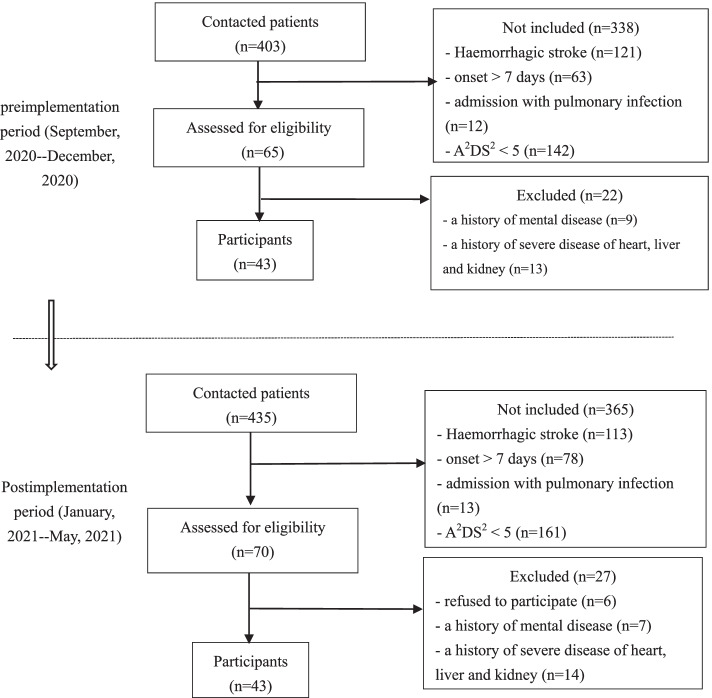
The population involved in the project was a convenience sample from September 2020 to May 2021. The inclusion criteria were as follows: (1) diagnosed with ischaemic stroke by computed tomography (CT) or magnetic resonance imaging (MRI); (2) between 18 and 80 years old; (3) time from symptom onset within 7 days; (4) admission without pulmonary infection; and (5) A2DS2 (Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity) score between 5 and 10. The exclusion criteria were: (1) a history of mental diseases; and (2) a history of severe diseases of the heart, liver and kidney (including malignant tumor of heart, liver, and kidney, acute myocardial infarction, allogeneic transplantation of the heart, liver, and kidney, end-stage renal disease or chronic renal failure and uremia, acute or subacute severe hepatitis, decompensated chronic liver failure, severe primary cardiomyopathy).
Sample size estimation was based on a similar study, which adopted an evidence-based care bundle to decrease the incidence of SAP from 43.3 to 16.7% [19]. With a power of 0.80, an alpha set at 0.05, and an effect size for the primary outcome (rate of SAP), each group required 43 participants.
Project design: the Iowa model
The Iowa Model of Evidence-Based Practice was utilized as a framework for project execution [20]. SAP in acute stroke patients was a problem-focused trigger, which was of high priority to the neurology unit. An interdisciplinary team was formed and responsible for the development and implementation of the evidence-based practice change. Relevant publications were gathered and evaluated for reliability, validity, and bias. The researchers found sufficient studies with consistent findings to support the following practice change.
The clinical nurse specialist conducted a systematic literature search. The goal was to identify what evidence-based interventions might exist to decrease the incidence of SAP. The Population, Intervention, Comparison, Outcome (PICO) framework was used to develop the search strategy: in patients with acute stroke, how do evidence-based nursing bundle interventions compared to conventional nursing interventions affect SAP rates? A Boolean search was completed using the following terms: (“acute stroke” OR “ischaemic acute stroke” OR “cerebral infraction” OR “cerebrovascular accident” OR “cerebrovascular apoplexy”) AND (“pneumonia” OR “stroke associated pneumonia” OR “SAP” OR “aspiration pneumonia” OR “inhalation pneumonia” OR “lung inflammation” OR “labor pneumonia”). The databases searched included the National Institute for Clinical Excellence (NICE), National Guideline Clearinghouse (NGC), Scottish Intercollegiate Guidelines Network (SING), MEDLINE, PubMed, Embase, Cochrane Library, Joanna Briggs Institute (JBI), EBSCO, Web of Science, Ovid, Chinese National Knowledge Infrastructure (CNKI), Wan Fang Data, VIP, and SinoMed. The inclusion criteria allowed articles about randomized controlled trials, quasi-experimental studies, case-control studies, and cohort studies published between 2009 and 2018. The search yielded 4766 articles and included 16 articles after critical appraisal with tools of Joanna Briggs Institute and AGREE II. These included 4 evidence-based clinical guidelines from America, England and Canada, 3 Chinese clinical guidelines and expert consensus, 3 evidence synthesis, 1 systematic review, 2 cohort trails and 3 random controlled trials. Evidence-based practice interventions were based on high quality clinical evidence and emphasized clinical expertise, patient values and expectations [21]. A gap analysis of current practice was completed to identify opportunities for improvement. Evidence-based knowledge was then applied to clinical practice through staff education and training, equipment availability, and environmental adjustments. The management scheme and flow were revised and detailed by consulting clinical medical, rehabilitation and nurse specialists and staff, who provided final feedback and adjustments based on FAME (feasibility, appropriateness, meaningfulness, effectiveness). Before implementing the final interventions, meetings were held to educate other providers and support staff (medical assistants and schedulers) about the project goals and to ask for their feedback. A 2-week period pilot study was conducted to assess feasibility and any improvement in outcomes.
Practice changes
The study was approved by the ethical committee of The Second Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine. Based on a literature review, SAP management recommendations included dysphagia screening and management, feeding management, oral hygiene management, position management, and TCM nursing techniques (Figs. 2 and Supplement 1).
Fig. 2.
The workflow of SAP prevention of patients with stroke
Nurses’ preparation before practice changes
Multiple staff meetings and face-to-face discussions were organized, and electronic materials were provided through the WeChat app to educate nursing staff about the bundle intervention protocol. Nursing education was done during a 2-week period before implementing the bundle nursing practice. The whole training was completed by the first author, and swallowing screening and rehabilitation were trained by senior speech and language therapists specializing in neurological rehabilitation. During the training process, the SAP prevention and management manual was produced based on the evidence, and sought for the support of the department head and rehabilitation therapists. At the end of the study, the nurses received a comprehensive real-situation examination by one senior speech therapist and one head nurse of the unit that included dysphagia screening, formulation and implementation of rehabilitation recommendations, and patient education. Those who passed the examination could implement the evidence-based care bundle in the stroke unit.
Data collection and analyses
The impact of the evidence-based care bundle on the rate of SAP and related patient outcomes was evaluated using data collected directly from patients and electronic medical records. In this study, SAP was diagnosed based on the medical documents and re-evaluated by the treating physician according to American CDC’s (Centers for Disease Control and Prevention) diagnostic criteria [22] for pneumonia (Supplement 2). This diagnostic standard is also recommended by the Consensus of Chinese experts on the diagnosis and treatment of stroke-associated pneumonia in 2019.
Patient-related data collected after implementation of the evidence-based care bundle (January 2021 to May 2021, prospective) were compared with data from the preimplementation period (September 2020 to December 2020, retrospective) (Fig. 1). Baseline data collected at admission included: age; sex; education; location of acute stroke; smoking; alcohol consumption; complications (diabetes, hypertension, heart disease); A2DS2 score; the results of 30 ml water swallowing test; aspiration (Supplement 3); National Institutes of Health Stroke Scale (NIHSS); oral hygiene [23]; nutrition risk screening-2002 (NRS-2002); serum albumin (ALB); white blood cell (WBC); procalcitonin (PCT); and hypersensitive C-reactive protein (CRPH). Data collected on the 7th day of admission were NIHSS, NRS-2002, ALB, WBC, PCT, CRPH, and incidence of SAP. The length of hospital stay and medical cost were collected at discharge. All data were collected and stored by the advanced practice nurse.
Fig. 1.
The flowchart of the study. A2DS2: Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity
Data analysis included descriptive statistics for all variables. The differences between the preimplementation and postimplementation groups were compared by the χ2 test, Fisher’s exact test, and Student’s t test or Mann–Whitney U test as appropriate. All data analysis was completed in SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
Table 1 presents the participants’ demographic, neurological and related data. There were no significant differences between the two groups in their baseline characteristics. There were also no significant differences between the two groups in infection-related indicators of SAP, which included WBC, PCT and CRPH (Table 2).
Table 1.
Baseline characteristics of the study population (n = 86)
| Variables | Preimplementation group (n = 43) |
Postimplementation group (n = 43) |
Z/F/U/χ2 | P |
|---|---|---|---|---|
| Age, years old | 69 (64, 76) | 68 (63, 74) | 846.0 | 0.497 |
| Sex, males | 24 (55.8) | 23 (53.5) | 0.047 | 0.829 |
| Employment | 2.708 | 0.305a | ||
| Employed | 1 (2.4) | 5 (11.6) | ||
| Retired | 35 (85.4) | 32 (74.4) | ||
| Unemployed | 5 (12.2) | 6 (14.0) | ||
| Risk factors | ||||
| Hypertension | 33 (76.7) | 31 (72.1) | 0.244 | 0.805 |
| Diabetes | 13 (30.2) | 15 (34.9) | 0.212 | 0.818 |
| Dyslipidemia | 10 (23.3) | 12 (27.9) | 0.244 | 0.805 |
| Atrial fibrillation | 14 (32.6) | 7 (16.3) | 3.087 | 0.131 |
| Smoking | 11 (25.6) | 14 (32.6) | 0.508 | 0.635 |
| Alcohol consumption | 4 (9.3) | 8 (18.6) | 1.550 | 0.351 |
| Stroke etiology | 2.437 | 0.543a | ||
| Cardioembolism | 12.3) | 0 (0.0) | ||
| Large-vessel disease | 24 (55.8) | 30 (69.8) | ||
| Small-vessel disease | 15 (34.9) | 11 (25.6) | ||
| Other causes | 3 (7.0) | 2 (4.7) | ||
| OCSP classification | 0.497 | 0.946 | ||
| Total anterior circulation infract | 7 (16.3) | 7 (16.3) | ||
| Partial anterior circulation infract | 24 (55.8) | 25 (58.1) | ||
| Posterior circulation | 6 (14.0) | 7 (16.3) | ||
| Lacunar infarct | 6 (14.0) | 4 (9.3) | ||
| Treatment protocols | 0.483a | |||
| Drugs | 37 (86.0) | 40 (93.0) | ||
| Non-drugs | 6 (14.0) | 3 (7.0) | ||
| Admission NIHSS score | 6 (3, 13) | 6 (4, 10) | 879.0 | 0.693 |
| Admission BP, mmHg | ||||
| Systolic BP | 160 (140, 175) | 153 (136, 175) | 842.0 | 0.476 |
| Diastolic BP | 87 (76, 95) | 91 (81, 96) | 788.5 | 0.240 |
| A2DS2 score | 6 (5, 6) | 6 (5, 7) | 802.5 | 0.262 |
| Oral hygiene score | 23 (17, 26) | 22 (18, 26) | 903.5 | 0.856 |
| NRS-2002 | 0.05 | 1.000 | ||
| ≥ 3 | 27 (62.8) | 28 (65.1) | ||
| < 3 | 16 (37.2) | 15 (34.9) | ||
| 30 ml water swallowing test | 2.828 | 0.243 | ||
| III | 22 (51.2) | 23 (53.5) | ||
| IV | 13 (30.2) | 17 (39.5) | ||
| V | 8 (18.6) | 3 (7.0) | ||
| The degree of dysphagia | 2.729 | 0.395a | ||
| 3 | 1 (2.3) | 0 (0.0) | ||
| 4 | 28 (65.1) | 23 (53.5) | ||
| 5 | 12 (27.9) | 18 (41.9) | ||
| 6 | 2 (4.7) | 2 (4.7) | ||
*The difference was statistically significant
aFisher’s exact test
Results are presented as n (%), mean ± standard deviation or median (interquartile range)
OCSP Oxfordshire Community Stroke Project; NIHSS National Institutes of Health Stroke Scale; BP blood pressure; A2DS2 Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity; NRS nutrition risk screening
Table 2.
Outcome indicators of the study population on the day of admission (n = 86)
| Variables | Preimplementation group (n = 43) |
Postimplementation group (n = 43) |
F/U /χ2 | P |
|---|---|---|---|---|
| Aspiration | 0 (0.0) | 0 (0.0) | / | |
| White blood cells (109/L) | 7.43 (5.89, 8.93) | 7.30 (5.60, 8.30) | 784.5 | 0.226 |
| Procalcitonin (ng/ml) | 0.045 (0.033, 0.056) | 0.048 (0.036, 0.072) | 737.5 | 0.106 |
| Hypersensitive C-reactive protein (mg/L) | 5.20 (4.02, 6.92) | 5.10 (3.24, 6.81) | 819.0 | 0.362 |
| Hemoglobin (g/L) | 116 (108, 136) | 120 (109, 133) | 865.0 | 0.607 |
| Serum albumin (g/L) | 36.93 ± 3.29 | 38.24 ± 3.31 | −1.838 | 0.070 |
aFisher’s exact test
Results are presented as n (%), mean ± standard deviation or median (interquartile range)
SAP
After the evidence-based process was implemented for 6 months, outcome evaluations were completed. Six of 43 (14.0%) patients in the postimplementation group were identified as diagnosed SAP, which was significantly lower than that in the preimplementation group (14.0% vs. 37.2%, p = 0.025). After the evidence-based care bundle implementation, the postimplementation group had lower levels of PCT (p = 0.007) and CRPH (p = 0.013) than the preimplementation group (Table 3).
Table 3.
All indicators of the study population (n = 86)
| Variables | Preimplementation group (n = 43) |
Postimplementation group (n = 43) |
t/U /χ2 | P |
|---|---|---|---|---|
| SAP | 16 (37.2) | 6 (14.0) | 6.108 | 0.025* |
| Indicators of infection | ||||
| White blood cells (109/L) | 7.49 (5.51, 9.30) | 7.20 (5.51, 8.40) | 863.5 | 0.598 |
| Procalcitonin (ng/ml) | 0.083 (0.054, 0.095) | 0.054 (0.044, 0.076) | 610.5 | 0.007* |
| Hypersensitive C-reactive protein (mg/L) | 8.74 (4.56, 13.60) | 5.20 (3.40, 6.70) | 636.5 | 0.013* |
| Length of stay, days | 13 (9, 18) | 11 (8, 14) | 687.0 | 0.040* |
| Total cost, USD | 20,351.62 (12,794.51, 32,790.98) | 18,139.21 (12,658.4, 32,666.31) | 843.0 | 0.481 |
| NIHSS score | 4 (2, 13) | 6 (2, 9) | 923.0 | 0.990 |
| Aspiration | 16 (37.2) | 4 (9.3) | 9.382 | 0.004* |
| NRS-2002 | 2.549 | 0.171 | ||
| ≥ 3 | 32 (74.4) | 25 (58.1) | ||
| < 3 | 11 (25.6) | 18 (41.9) | ||
| Serum albumin (g/L) | 33.92 ± 2.90 | 35.83 ± 4.20 | −2.459 | 0.016* |
| Oral hygiene score | 19 (15, 21) | 15 (13, 18) | 530.5 | 0.001* |
| 30 ml water swallowing test | 6.766 | 0.149 | ||
| I | 2 (4.7) | 10 (23.3) | ||
| II | 15 (34.9) | 12 (27.9) | ||
| III | 16 (37.2) | 15 (34.9) | ||
| IV | 6 (14.0) | 4 (9.3) | ||
| V | 4 (9.3) | 2 (4.7) | ||
| The degree of dysphagia | 7.097 | 0.131 | ||
| 0 | 2 (4.7) | 10 (23.3) | ||
| 2 | 15 (34.9) | 12 (27.9) | ||
| 4 | 17 (39.5) | 16 (37.2) | ||
| 5 | 6 (14.0) | 4 (9.3) | ||
| 6 | 3 (7.0) | 1 (2.3) | ||
*The difference was statistically significant
Results are presented as n (%), mean ± standard deviation or median (interquartile range)
SAP stroke-associated pneumonia; NIHSS National Institutes of Health Stroke Scale; NRS nutrition risk screening
Other findings
The mean length of hospital stay decreased from 14.47 ± 6.68 days before implementation to 11.74 ± 5.51 days after implementation. The cost of medical care of the postimplementation group was lower than that of the preimplementation group, but the difference was not statistically significant. The rate of aspiration of the postimplementation group was significantly lower than that of the preimplementation group. In addition, there were significant improvements in ALB, and oral hygiene (Table 3). The nurses’ compliance with using the entire care bundle was 77%.
Discussion
This project instituted an evidence-based practice bundle in the management of acute stroke patients to successfully reduce the incidence of SAP. The focus on dysphagia screening and rehabilitation; feeding modification, oral care, airway management, position management, and TCM nursing techniques were strategies introduced through the care bundle.
SAP can be prevented in a variety of ways. Many other important factors, such as head position, getting out of bed, continuous tube feeding, caregivers’ feeding experience, aspiration caused by vomiting or gastroesophageal reflux, oral hygiene, immunosuppression after acute stroke, and the lesion location of dysphagia, are also linked to the occurrence of SAP and must be considered in care plans to prevent respiratory infections in patients with acute stroke [24, 25]. Many previous studies have demonstrated kinds of prevention measures, but most of them are single or multiple preventive interventions and are not based on evidence. Evidence-based protocol initiated by nurses for syndrome management is scientific, effective, targeted, safe and systematic in stroke units [26]. The evidence-based practice model aimed to address the problems existing in current clinical practice by developing care bundle plans based on a thorough literature review and clinical scenarios, which made up for the conventional nursing mode based on experience and improved the effectiveness and safety of nursing practice.
Applying evidence-based practice methods to our project resulted in a decrease in the incidence of SAP. The infection indicators PCT and CRPH in the postimplementation group were significantly lower than those in the preimplementation group. The care bundle included dysphagia management, feeding management, oral hygiene management, position management, airway management and TCM nursing techniques. The above 6 aspects of the nursing interventions worked together to prevent SAP.
After the intervention, the nutrition indicators ALB and oral hygiene were significantly better in the postimplementation group than in the preimplementation group. Early nutritional risk screening, oral care and swallowing rehabilitation training could help patients increase food intake and improve malnutrition. In addition, the significant improvement in aspiration benefited from standard and systematic screening and management. The cost of medical care was lower than before but did not have statistical significance considering complex factors, such as treatment protocols, the severity of stroke and complications. All factors were combined to reduce the length of hospitalization stay.
Limitations
This was a single-center EBP project in an urban level 1 stroke center, and was limited by small sample size and restricted time for project implementation. Furthermore, some blood markers were not tested on the same day that the disease attacked, so bias may exist. Finally, the nurses’ compliance with using the entire care bundle needs to be improved in the future. The stroke center was a teaching hospital that had a frequent turnover of clinical nursing staff because of internship rotations, which required attention to who had been trained and who may have missed meetings or training sessions. Nonetheless, the clinically important findings in the prevention of SAP were promising and warrant further investigation with a larger sample.
Implications for nursing practice
Given the success of the initial project, the practice changes can be expanded to other neurology units in the clinical facility. This is in line with the guidance provided by the Iowa Model of Evidence-Based Practice for care quality improvement [27]. According to this model, the management plan and flow of SAP prevention can be promoted to other units of the neurology department in the future. Additionally, the implementation of evidence-based practice programs requires ongoing education for staff and changes in culture and behaviors. Future research should examine barriers to implementation and adherence to changes when changes are made.
Conclusion
SAP has presented challenges to nurses who care for patients with acute ischaemic stroke for a long time. An evidence-based practice project to empower nurses to reduce the incidence of SAP was successfully implemented. The results of the project reflect positively on the capacity to implement evidence-based practice in an acute care setting of stroke. These outcomes support the call to question traditional practice, which is necessary to advance the nursing profession in support of improved patient outcomes.
Supplementary Information
Acknowledgements
Thanks also to the concerned patients, nurses and doctors for their support.
Availability of data and material
The data analyzed during the current study is not publicly available because consent was not been obtained from the study participants for this. Data can be made available from the corresponding author on reasonable request after approved by the ethics committee.
Abbreviations
- A2DS2
Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity
- NICE
National Institute for Clinical Excellence
- NGC
National Guideline Clearinghouse
- SING
Scottish Intercollegiate Guidelines Network
- CT
Computerized Tomography
- MRI
Magnetic Resonance Imaging
- SAP
Stroke-associated pneumonia
- TCM
Traditional Chinese Medicine
- NIHSS
National Institutes of Health Stroke Scale
- NRS-2002
Nutrition risk screening-2002
- ALB
Serum albumin
- WBC
White blood cell
- PCT
Procalcitonin
- CRPH
Hypersensitive C-reactive protein
Authors’ contributions
ZYL, GZ and XPZ were responsible for the study design. RCY, JC and XPZ participated in the intervention training of research nurses. ZYL, DN, JC implemented the intervention. ZYL collected the data. ZYL and GZ was responsible for the statistical analysis. ZYL and LW wrote the first draft of the article. All authors read and approved the final manuscript.
Funding
This study was funded by Nursing Study Subjects of The Second Affiliated Hospital of Guangzhou University of Chinese Medicine [grant number YN2019HL08].
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. And all experimental protocols were approved by the Ethic Committee of the Guangdong Provincial Hospital of Chinese Medicine (B2020–298-01). All the information of the participants was confidential and informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhu-Yun Liu and Lin Wei contributed equally to this work.
Contributor Information
Zhu-Yun Liu, Email: lzhuyunsysu@163.com.
Lin Wei, Email: weilin22@126.com.
Ri-Chun Ye, Email: yerichun555@126.com.
Jiao Chen, Email: jasminechenjiao@163.com.
Dan Nie, Email: 380602759@qq.com.
Ge Zhang, Email: zhangge@gzucm.edu.cn.
Xiao-Pei Zhang, Email: xingyunxing021@163.com.
References
- 1.Benjamin EJ. Heart disease and stroke Statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146. doi: 10.1161/CIR.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LD, Liu JM, Yang Y, Peng B, Wang YL. Prevention and treatment of stroke in China still faces great challenges: summary of Chinese stroke prevention report 2018. Chin Circul J. 2019;34(2):105–119. doi: 10.3969/j.issn.1000-3614.2019.02.001. [DOI] [Google Scholar]
- 3.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Stroke outcome research Canada (SORCan) working group. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77(14):1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 4.Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214–3218. doi: 10.1161/STROKEAHA.110.610881. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc. 2012;21(1):61–67. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia. Stroke. 2015;46(8):2335–2340. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Cugy E, Sibon I. Stroke-associated pneumonia risk score: validity in a french stroke unit. J Stroke Cerebrovasc. 2017;26(1):225–229. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Hanchaiphiboolkul S. Risk factors for early infection after an acute cerebral infarction. J Med Assoc Thail. 2005;88(2):150–155. [PubMed] [Google Scholar]
- 9.Geng AX, Zhao K. Advances in nursing research on prediction and prevention of stroke-associated pneumonia. Chin J Nurs. 2017;S1:85–89. [Google Scholar]
- 10.Hannawi Y, Hannawi B, Rao C, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013;35(5):430–443. doi: 10.1159/000350199. [DOI] [PubMed] [Google Scholar]
- 11.Upadya A, Thorevska N, Sena KN, Manthous C, Amoateng-Adjepong Y. Predictors and consequences of pneumonia in critically ill patients with stroke. J Crit Care. 2004;19(1):16–22. doi: 10.1016/j.jcrc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 13.Teramoto S. Novel preventive and therapuetic strategy for post-stroke pneumonia. Expert Rev Neurother. 2009;9(8):1187–1200. doi: 10.1586/ern.09.72. [DOI] [PubMed] [Google Scholar]
- 14.Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10(4):357–371. doi: 10.1016/S1474-4422(10)70313-6. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 16.Neurology branch of Chinese Medical Association. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. Chin. J Neurol. 2015;48(4):246–25.(in Chinese). 10.3760/cma.j.issn.1006-7876.2015.04.002.
- 17.Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Pownall S, et al. Impact of dysphagia assessment and management on risk of stroke-associated pneumonia: a systematic review. Cerebrovasc Dis. 2018;46(3–4):99–107. doi: 10.1159/000492730. [DOI] [PubMed] [Google Scholar]
- 18.Qin HL. Observation on cluster nursing of traditional Chinese medicine in ICU patients with ischemic stroke associated pneumonia. SHANXI J OF TCM. 2016;12:52–54. [Google Scholar]
- 19.Zhou L. The intervention study of patients care bundles applied in the prevention of bacterial pneumonia in acute ischemic stroke associated pneumonia. 2019. Yun Nan University of Traditional Chinese Medicine. (in Chinese).
- 20.Brown CG. The Iowa model of evidence-based practice to promote quality care: an illustrated example in oncology nursing. Clin J Oncol Nurs. 2014;18(2):157–159. doi: 10.1188/14.cjon.157-159. [DOI] [PubMed] [Google Scholar]
- 21.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing & healthcare: a guide to best practice. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 22.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Chinese Expert Consensus Group on Rehabilitation Assessment and Treatment of Dysphagia 2017 expert consensus on assessment and treatment of dysphagia in China, part I assessment. Chin J Phys Med Rehabil. 2017;39(12):881–892. [Google Scholar]
- 24.Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Smith CJ, et al. Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: a systematic review. Dysphagia. 2020;35(5):735–744. doi: 10.1007/s00455-019-10061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZY, Zhang XP, Mo MM, Ye RC, Lin MQ. Impact of the systematic use of the volume-viscosity swallow test in patients with acute ischaemic stroke: a retrospective study. BMC Neurol. 2020;20(154):1. doi: 10.1186/s12883-020-01733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton S, McElduff P, Ward J, Grimshaw J, Dale S, D’Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia and swallowing dysfunction in acute stroke improves 90-day outcomes: QASC, a cluster randomised controlled trial. Lancet. 2011;378(9804):1699–1706. doi: 10.1016/S0140-6736(11)61485-2. [DOI] [PubMed] [Google Scholar]
- 27.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing and healthcare: a guide to best practice. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study is not publicly available because consent was not been obtained from the study participants for this. Data can be made available from the corresponding author on reasonable request after approved by the ethics committee.