Abstract
Extracellular RNAs (exRNAs) are novel circulating factors that can be used as biomarkers in various diseases. Their unique and diverse kinds, as well as their role as biomarkers, make them significant biomarkers. There has been immense work carried out since the discovery of exRNAs in circulation and other biological fluids to catalog and determine whether exRNAs may be utilized as indicators for health and illness. In this review, we aim to understand the current state of exRNAs in relation to various diseases and their potential as biomarkers. We will also review current issues and challenges faced in using exRNAs, with clinical and lab trials, that can be used as viable markers for different diseases.
Keywords: Extracellular RNAs (exRNAs), Extracellular vehicles (EVs), Cancer, Biomarkers, Exosomes
Background
Extracellular RNAs (exRNAs) are a heterogeneous population of different ribonucleic acids that are found in all biofluids (blood plasma, urine, saliva, etc.). exRNAs are involved in cell differentiation, apoptosis and cell-to-cell communication and regulate various physiological processes, thus modulating gene expression. Previously, exRNAs were thought to be readily degraded by RNases, but recent advances in liquid biopsy have revealed the stability and persistence of exRNAs with the aid of certain other molecules [1, 2]. A decade ago, two important studies revealed that exRNAs exist in conjugation with proteins or lipids, forming complexes and thus enabling the exRNAs to withstand degradation for a longer time [3, 4]. This aspect challenged the intrinsic tendency of exRNAs to escape degradation [5].
The diversity and abundance of exRNAs depend greatly on the sources, such as intracellular compartments, blood and tissues, which makes exRNAs a potential noninvasive biomarker of various diseases. On the diagnostic front, exRNA profiling provides early detection of tumors, hepatic and kidney complications, aging and various other diseases, while on the therapeutic front, exRNAs enclosed in vesicles may offer a safe and precise drug delivery system and well-engineered nanoparticles.
Exosome biology
Recent research on exRNAs has depicted their dynamic activities that occur and how they communicate information between the cells that play an influential role as signaling molecules. ExRNAs can be divided into two types: coding RNAs, which encode proteins, and noncoding RNAs (ncRNAs), which have various functions, such as assisting the translation of proteins and regulating gene expression. ExRNA protection is largely hidden by the incorporation of membrane-containing vesicles, which are released by cells and are known as potent vehicles of communication. These vesicles are mainly associated with proteins, nucleic acids, and lipids, which perform several biological functions between the parent and recipient cells. Extracellular vehicles (EVs) are mainly classified into microvesicles, exosomes, and apoptotic bodies, which play a crucial role in maintaining homeostasis and in the excretion of unwanted molecular substances [6, 7]. Some microRNAs (miRNAs) are nonexosomal, i.e., they are not associated with exosomes and can be released either by 1) passive release from injured cells or 2) active secretion by protein-mRNA complexes [8]. Messenger RNA (mRNA), which is rarely found among exRNA subpopulations, is usually isolated from certain biological fluids, such as saliva, blood, and urine. Another class of exRNA is miRNAs, owing to their abundance in the cytosol; they regulate various cellular activities, including growth, differentiation and cell death, thus considered as major drivers of cell fate [9–13]. Similarly, exosomal transfer RNAs (tRNA) have shown to be in greater amounts in patients of certain disease as compared to healthy individuals [14]. Nevertheless, the exact mechanism of tRNA fragments is uncertain, but they regulate some pathways and transfer components to other cells to be recognized [15, 16]. In addition, PIWI-interacting RNAs (piRNAs) are one of the largest classes of noncoding RNA (ncRNA) molecules found in the extracellular environment and reside in EVs. However, they are present in meaningful numbers in a few cells and are upregulated in red blood cells compared with plasma [17]. Small ncRNAs (sncRNAs) are more abundant than tRNAs but less abundant than exosomes. The ribonucleoproteins (RNPs), including helicases, polymerases and chaperones, control RNA quality and are involved in the degradation of defective RNAs through surveillance machinery [18–20].
What truly defines the characteristics of an exRNA is its origin. Biogenesis is the formation of a cell from another preexisting cell. However, exRNA biogenesis is not limited to a single mode or mechanism. Often, exRNAs are encapsulated in lipoprotein vesicles to evade degradation [1] (Fig. 1). As described previously, various cellular entities are responsible for exRNAs, and exosomes in particular have been linked to RNA as their carriers. One common biogenesis mechanism is endocytosis [21], where two different cells, secreting cells and recipient cells, are responsible for exRNA biogenesis. Endosomes and lysosomes secreted via endocytosis develop into late-stage endosomes and are excreted out of the cell as either exosomes, lipoproteins, or RNPs and then transported toward the recipient cell [1]. Exosomes are analogous to high-density lipoproteins (HDL) and low-density lipoproteins (LDL) in the transportation of exRNAs. For example, HDL transports a specific profile of exRNA from a variety of cell types to recipient cells [22]. Finally, the RNPs are stabilized to carry the exRNA [2], which suggests the possibility of exRNAs existing in more carrier-free forms rather than in association with vesicular bodies. These extracellular vesicular bodies soon enter the recipient cell via cell receptors and other complexes to alter the expression of various genes. These expression profiles drive differences in diseases among individuals, making them novel biomarkers of disease [21]. In 2010, researchers studied the release of HEK293 cell-derived exosomal miRNAs and discovered a dynamically controlled secretory ceramide-dependent mechanism. This mechanism could promote the sorting of endosomes into exocytic multivesicular bodies (MVBs) [23]. Later, another study confirmed the reliance of miRNA secretion into endosomes on ceramide and KRAS mutations [24]. However, there is still much uncertainty about the actual mechanisms involved. In more recent studies, after reaffirming the enigmatic nature of exRNAs, it was observed that many methods of identifying RNA were more related to extracellular complexes [2].
Fig. 1.
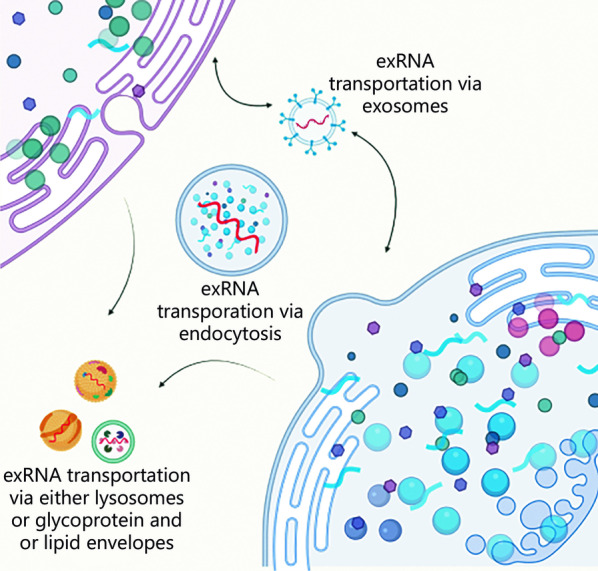
A general view of exRNA biogenesis and its primary function as a transportation cellular entity. exRNA extracellular RNA
Extracellular RNA as a diagnostic biomarker
The expeditious use of exRNA in clinical trials is due to its expression in various health conditions; therefore, it has been recognized as a potential biomarker. For example, miRNAs have been studied extensively in the clinical profiles of patients with a variety of diseases, suggesting that they are promising molecular markers for diagnosis and treatment. For instance, miR-21 has been reported in hepatitis, chronic kidney disease (CKD), and brain tumor, in which it has shown significant correlations with other parameters [25–27].
Other types of exRNA have also been reported as molecular markers, such as circulating RNAs (circRNAs), which are highly stable, conserved, and have tissue-specific expression patterns and have been suggested as potential diagnostic and prognostic biomarkers [28]. Multiple sclerosis (MS) currently does not have a single definite diagnostic test, and there are ongoing efforts to discover a diagnostic marker [29]. Fortunately, it is possible to utilize exRNAs as a diagnostic biomarker in MS, as many studies have revealed exRNA associations with diagnostic and therapeutic fields [30].
In 2017, Ebrahimkhani et al. [31] tried to fill the gap when they used a blood-based assay for serum exosomal miRNA detection in a cohort (n = 36, patients = 25, and control = 11) of MS patients who were experiencing relapse and remission. They found several miRNAs as biomarkers in the diagnosis of MS. Despite this, many types of cancers are also associated with clinical applications of exRNAs, and they have also been shown to acquire both beneficial and detrimental effects on specific conditions. For example, in a study, 5 exRNAs, including 3 mRNAs (SPINK7, PPL, and SEMA4B) and 2 microRNAs (MIR140-5p and MIR301a), showed downregulation in gastric cancer (GC). These exRNA candidates were isolated from the saliva of GC patients, which shows their ultimate potential utility for the use as noninvasive biomarkers in monitoring GC [32].
In another study, miR-21 showed significant elevations in the serum of glioblastoma patients, and these single exRNAs may target and regulate hundreds of genes [33]. Some particular retrotransposon RNAs originating from tumor cells have shown increased expression compared to normal cells, making them easier to isolate from biofluids. This characteristic makes them a unique and potential biomarker in cancers [34]. To some extent, exRNAs have been found to have a significant role in CKD, but the exact relevance remains unclear. Several changed expression patterns have also been detected in CKD during hemodialysis and after kidney transplantation [35].
A comparative study described a comparison between lupus nephritis (LN) patients and controls through miRNA profiles of kidney biopsy. miRNA microarray chip analysis of renal biopsies revealed differential expression of approximately 66 miRNAs, thus concluding that exRNAs may be an element in the pathogenesis of LN and a potential invasive diagnostic biomarker [36]. A detailed description of the biomarkers of exRNAs in various diseases is given in Table 1.
Table 1.
Overview of several exRNAs as diagnostic biomarkers in different diseases
| Biomarker | Type of disease | Method | Medium | Significance | References |
|---|---|---|---|---|---|
| miR-29 | Liver cirrhosis | qPCR | Serum | Lower levels show disease progression | [37] |
| miR-29a | Liver fibrosis | qPCR | Serum | Low levels show advanced liver fibrosis | [38] |
| miR-122/miR-155 | Acute and chronic liver injury | TaqMan MicroRNA assay | Serum and plasma | Upregulated in hepatocytes and a central regulator of inflammation | [39] |
| miR-30 | Muscle injury, muscle disuse atrophy | qRT-PCR | Skeletal muscle | An interesting biomarker of muscle homeostasis and muscle disease | [40] |
| miR-223 | Acute liver failure and liver cirrhosis | qPCR | Tissue and serum | Upregulation restricted to hepatocytes, also showed significantly higher levels in serum | [41] |
| miR-21 amplification | Glioblastoma | RT-PCR, microarray, Western blotting, immunohistochemical analysis | Cell lines, CSF | Regulator of EGFR expression, cell-cycle and signaling pathways | [26, 42] |
| miR-603/miR-181d ratio | RT-PCR, Western blotting | Tissue, cell line | Coregulators of MGMT expression | [26] | |
| EGFR amplification | RT-PCR, Western blotting | Tissue | Enhanced cell survival and proliferation via EGFR-PI3K pathway | [26] | |
| miR140-5p/miR301a | GC | RT-qPCR | Saliva | Downregulated in the GC | [32] |
| miR-26a/b | Oral squamous cell carcinoma | PCR | Epithelial tissue | Downregulation and function as a tumor suppressor | [43] |
| miR‐34a | Brain aging | qPCR | Blood and Plasma | Accessible biomarkers for age‐dependent changes in the brain | [44] |
| miR-24-3p | Aging | RT-qPCR array, RT-qPCR validation | Saliva | Nonspecific screening biomarker for aging | [45] |
| miR-29a/miR-29b | GDM | PCR | Serum | Downregulated in the GDM | [46] |
| miR-150/miR-192/miR-27a | Diabetes mellitus | Microarray profiling | Blood | Correlation between raised levels of fasting glucose and altered levels of miR-27a and miR-320a | [47] |
| miR-9/miR-29a | T2DM | qPCR | Serum | Deregulated in T2DM | [48] |
| miR-155/miR-181a | T1DM | Microarray profiling and qPCR | Serum | Deregulated in T1DM | [49] |
| miR-21 | Type 1 autoimmune hepatitis | Real-time qPCR | Serum | Correlation with the histological grades of inflammation | [27, 50] |
| miRNA-let-7a/miRNA-92a/miRNA-648a | Multiple sclerosis | qPCR | Plasma | Significantly lower expression | [51] |
| miR-15b/miR-34a/ miR-636 | Diabetic kidney disease | qRT-PCR | Urine | Upregulated in urine pellets | [52] |
| miR-21-5p | Diabetic kidney disease | qPCR | Urine | Upregulation and associated with pathogenesis of renal dysfunction | [53] |
| miR-21 | Renal fibrosis in diabetic nephropathy | qRT-PCR | Renal cortical tissue | Targets known fibrotic signaling proteins | [25] |
GC gastric cancer, GDM gestational diabetes mellitus, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, CSF cerebrospinal fluid, EGFR epidermal growth factor receptor, GFR glomerular filtration rate, PCR polymerase chain reaction, qPCR quantitative PCR, qRT-PCR quantitative reverse transcription polymerase chain reaction, RT-qPCR reverse transcription quantitative PCR, MGMT O6-methylguanine methyl transferase
Extracellular RNAs in cancer
Extracellular RNAs have been shown to be potential candidates in cancer diagnosis and prognosis because they are highly specific and highly sensitive indicators, reflecting the dynamics of the cells more accurately than DNA [54]. The noninvasive nature of exRNA biomarkers makes them more reliable candidates in the diagnostics of several cancers. For example, in the clinical diagnosis of GC, different expression levels of extracellular vesicle-derived exRNAs have been detected. Most of these exRNA biomarkers can even enhance the ability to distinguish a benign or malignant tumor [55]. Tumor cells release more vesicles than normal cells, which ultimately affects the tumor microenvironment, thus promoting tumor growth. For instance, long noncoding RNAs (lncRNAs) and miRNAs released by hepatocellular carcinoma cells into surrounding cells altered normal functioning and promoted the multifocal progression of tumors [56].
A number of exRNA biomarkers along with their expression abundance have been described. The high-throughput RNA sequencing and microarray analysis of these biomarkers can help researchers to collect extensive and valuable data about several cancers [57]. Previously, a number of studies have suggested that exRNAs loaded in vesicles are released into the circulation by tumor cells to communicate with cells in close proximity as well as with distant cells [58]. These vesicles play a promoting role in primary breast cancer development, invasion and metastasis. Prostate cancer is another prevalent malignancy that affects the male reproductive system. While examining cancerous prostate tissues, differential expression of reactive oxygen species, the p53 pathway, oncogenes and tumor suppressor genes in tumor vesicles was found [59]. Furthermore, urine contains biomarkers for malignancies of the reproductive system. PCA3, for example, is a lncRNA that is expressed and found in considerable amounts in patients with prostate cancer [60]. A comprehensive study found various exRNA signatures with significant potential for prostate cancer diagnosis [61]. Moreover, the study of some other exRNAs, such as miRNAs and piRNAs, has revealed complete extracellular ncRNA data in human saliva for advanced research on biomarkers [62]. Various oncogenic and oncosuppressor miRNAs, including miR-21, miR-223, miR-378e, miR-143 and miR-10b, have been reported to increase the invasion of tumors in a variety of cancers [63]. Last, a high correlation was observed among 7 differentially expressed genes and lung cancer through RNA sequencing technology [64]. Despite the fact that a number of exRNA biomarkers have been discovered for cancer diagnosis, a systematic identification of these novel biomarkers can provide accurate knowledge about the populations of EV-associated exRNAs and the analyses of their cargo.
Role of extracellular RNA in liver disease
Extracellular RNAs are vital molecular entities in the pathogenesis of various diseases, including liver cirrhosis, liver fibrosis, and chronic hepatitis.
Disease progression, as well as the healing process through regeneration, is regulated by various types of exRNAs (especially miRNAs), most of which have been distinguished as biomarkers for liver diseases. Hepatic stellate cells (HSCs) during liver fibrosis differentiate into transitional cells called myofibroblasts, which promote extracellular milieu release. These activated HSCs start to produce cellular communication network factor 2 (CCN2), which is related to the underexpression of miR-214, showing its regulatory effect on CCN2 [65]. Furthermore, miR-199a-5p targets the CCN2 3′-UTR and inhibits the production of CCN2; when they are transported to activated HSCs, they inhibit CCN2 3′-UTR activity [66]. This increases the extracellular material, thus causing liver fibrosis.
Hepatocellular carcinoma may also develop as a consequence of liver cirrhosis, making it difficult to diagnose the disease. An extensive investigation of proteomics through the serum of liver cancer patients, liver cirrhosis patients and control patients verified a hypothesis differentiating liver cancer and liver cirrhosis [67]. miRNA-451a was shown to be differentially expressed in the serum of 25 liver cirrhosis patients with early-stage liver cancer in comparison with 74 cirrhotic patients without liver cancer [68]. Nonalcoholic steatohepatitis (NASH) can lead to liver fibrosis; for example, miR-122 is an early biomarker for liver injury due to NASH. The upregulation of miR-122 was compared with serum alanine aminotransferase (ALT) [69]. A previous study revealed that the downregulation of miR-122 promotes alterations in lipid metabolism [70], as exRNAs usually form lipoprotein-RNA complexes [71]. However, the mechanism of lipid homeostasis under the effect of miR-122 has yet to be defined further.
Nonalcoholic fatty liver disease (NAFLD) can progressively lead to NASH. A combined analysis of four serum exRNAs (miR-21-5p, miR-151a-3p, miR-192-5p, and miR-4449) showed satisfactory diagnostic potential for NASH in NAFLD [72]. The plasma levels of novel tRNA-derived fragments predicted liver fibrogenesis risk in NAFLD [73]. miR-20a and miR-27a are useful biomarkers, as they have shown enhanced downregulation upon the downregulation of miR-126. The two previously mentioned exRNAs are significantly associated with the severity of NAFLD. On the other hand, miR-122 aids in the amplification of viral translation in hepatitis C by changing the structure of the internal ribosomal entry site [74]. miR-802 showed the same results on viral replication in hepatitis B [75]. Viral RNAs of heterogeneous lengths have also been observed circulating in blood as capsid-antibody complexes in hepatitis B [76].
Role of extracellular RNA in lung fibrosis
Similar to liver fibrosis, exRNAs also have a versatile role in lung fibrosis (LF). The extracellular matrix (ECM) contains many subcellular entities that accumulate during myofibroblastic transition, resulting in several exRNA entities being released out of the cell. Exosomal miR-1343 plays a role as a potent inhibitor of TGF-β signaling, and it has shown high expression in HL-69 human leukemia cells. This high expression was then transferred to the A549 lung epithelium, where it inhibited TGF-β receptors 1 and 2 [65]. A research study by Yao et al. [77] showed that miR-328 was overexpressed in M2 macrophages and contributed to pulmonary fibroblast proliferation and the development of LF by regulating FAM13A in rats. Another study also showed the impact of let-7d-5p downregulation, which indicates its involvement in pulmonary inflammation by regulating several pathophysiological processes, such as the release of ECM for the development of idiopathic pulmonary fibrosis (IPF). A positive correlation between let-7d-5p and the diffusing capacity of the lungs for carbon monoxide/alveolar volume showed its association with IPF severity [78].
Role of extracellular RNAs in diabetes and obesity
As discussed earlier, an exRNA can envelop itself into lipids and plasma lipoproteins, which are important distributary agents that can be converted to a different form of lipid, resulting in many lipid-related illnesses [79]. Obesity may also lead to insulin resistance (IR), which causes the body to be inactive in producing insulin. Various diagnostic practices ranging from glucose testing to oral testing are performed to diagnose an individual with obesity [80]. In a recent review, certain exRNAs were shown to function as potential therapeutics for IR [81]. Recently, a study revealed a relationship between the etiology of diabetes and exRNAs. Furthermore, the findings of exRNAs in serum and plasma offer possible targets for the development of novel drugs [82]. In a cohort comparative study, it was found that individuals with diabetes showed higher levels of circulating EVs than control patients. The same study also concluded that patients who developed the disease in 5 years (or more) also had higher levels of EVs [83]. Moreover, studies have aimed to form a library of exRNA in human aging after the detection of short and long RNAs in a single sequencing step [84].
Role of extracellular RNAs in aging
Aging is an irreversible process that is influenced by numerous factors. In a recent study, the levels of various kinds of exRNAs increased with age [85]. The results offered timely and pertinent information regarding the serum exRNA repertoire and how it changes as people age. The potential diagnostic and therapeutic use of EVs in age-related diseases has sparked renewed interest in these nanosized vesicles [86]. Extracellular vesicles have some unique biological features and play several important physiological roles. miRNAs and their mRNA targets are essential regulators of cellular senescence, and their expression changes with age in circulating peripheral blood mononuclear cells (PBMCs) [87]. Long noncoding RNAs (lncRNAs) are noncoding RNAs (ncRNAs) with transcript lengths greater than 200 nucleotides that play vital roles in the regulation of gene expression. Therefore, unveiling the molecular mechanisms of lncRNAs that underlie senescence may make it easier to diagnose and treat disorders associated with aging [88]. Many future studies will aim to elucidate the complexities of age and its relation to exRNAs. Another study evaluated exRNAs in PBMCs of healthy subjects, and the results showed biological inactivity due to aging. The study also identified key alterations in many aging-associated biological processes through differential expression analysis [89]. Likewise, another study indicated that exRNA and age-related expressions coincided with certain previously known genes, again indicating the potential of exRNAs as biomarkers for aging [90].
Clinical trials
The use of exRNAs and their potential was first properly taken into account by the NIH Common Fund-supported Extracellular RNA Communication Consortium (ERCC1) in 2013. ERCC1 documented numerous manuscripts and publications. Moreover, they allocated various sources and their correlating applications [91]. Since exRNAs may function as EVs, various studies have shown a positive impact on cancer therapeutics and the use of exRNAs, specifically in lung cancer [92]. Doxorubicin, a drug used in for cancer treatment, has been optimized to be more effective in codelivery with exRNAs [93]. Other diseases, such as heart failure, have also shown an association with exRNA expression. Whether it be the subdual, production, or removal of EVs, these strategies are all notable approaches [94]. Many clinical trials have tried to modify exRNAs to target a specific cell or cell type [95]. Another investigation discussed how EVs support numerous immune responses, such as immunosuppression, and aid in cancer countermeasures [96]. EVs have also shown many similarities to enveloped viruses, which can be used as prototypes to better understand the potential for these vesicles to operate as vectors for RNA delivery [97]. One study concluded that battling cancer with combination therapy has proven to be more effective than conventional monotherapy [98]. When cancer cells were subjected to 8 Gy radiation, CDCP1 was shown to be elevated as an ideal tumor-associated antigen (TAA) in lung cancer cells. These overexpressed TAAs are subsequently transported to dendritic cells by EVs, accelerating CD4+ and CD8+ T-cell aggregation, infiltration, and tumor killing [99]. Conversely, during the priming of the premetastatic niche, tumor-derived exosomes have been shown to carry molecular signals important in angiogenesis and stromal remodeling for tumor cell adhesion and proliferation [100]. Exosomes hold potential in cancer therapeutics, as evident from various studies; however, further preclinical trials are necessary to consider them a viable approach [101, 102].
exRNAs as therapeutics
Since exRNAs are thought to be associated with many local biomolecules, including exosomes, exRNAs have a multitude of biological functions and therefore show correlation to diseases [103]. For clinically meaningful treatment routines, strategies for effectively delivering RNA are frequently needed. The use of viral vectors as RNA carriers with a high transfection efficiency has been investigated. Such use of viral vectors, including adeno-associated viruses, lentiviruses, and retroviruses, is limited owing to the risk of insertional mutagenesis and immunogenicity. In a recent study, fetal bovine serum (FBS) and its influence were tested on cell cultures, revealing that current analysis and protocols lack any notable changes [104]. For more reliable and scalable cancer treatment methods, modification of miRNAs that span both tumor cell proliferation and T-cell-mediated antitumor immunity will lead to crucial developments in the near future [105].
In regard to the translational uses of exRNAs, researchers have begun to think outside the box. Stem cell modification of exosomes has suggested the possibility of facilitating the multiplication of cells at a critical site, such as acute kidney injury [106]. Another study concluded that implementing exRNAs as epigenetic modifications could lead to advancements in transplantation and genome-wide studies practices; however, few studies have focused on this topic [107]. Furthermore, a number of studies have documented that miRNA absorption into recipient cells via EVs alters gene expression and physiological activities and that miRNA profiles are altered in patients with various disease types and/or statuses [1].
Directed distribution of exRNA-loaded EVs for gene therapy and treatments targeting exRNAs have been implicated in kidney disease development, which is a potential therapeutic strategy [106]. Recently, a study investigated the effectiveness of cell-free DNA (cfDNA) [108] and exRNAs in cancer cases by identifying viable biomarkers [109].
Conclusions
After exploring the nature of the circulating marker exRNA, we conclude that its use as a biomarker has opened an entirely new realm of diagnostics in various diseases, ranging from cancer to obesity. Although there is increasing interest in exRNA and EV biology, there is still a critical need for rigorous, hypothesis-driven investigations to develop model systems that allow for a molecular understanding of exRNA. In developing such models, we will come closer to unveiling the full potential of exRNA in medical fields. In the future, alternative unknown carriers for transporting exRNA as well as the importance of molecular function in cell biology can be investigated. Hence, the significance of circulating biomarkers as diagnostic and prognostic indicators of disease will be increased when exRNAs are progressively considered in various studies and cohorts.
Acknowledgements
The authors are thankful to the Vice-chancellor of the University of Narowal, Narowal, Pakistan, for providing support for the accomplishment of this study.
Abbreviations
- ALT
Alanine aminotransferase
- CCN2
Cellular communication network factor 2
- CCN2 3′-UTR
Untranslated regions from 3′ of CCN2
- circRNAs
Circulating RNAs
- CKD
Chronic kidney disease
- ECM
Extracellular matrix
- ERCC1
Extracellular RNA communication consortium
- exRNAs
Extracellular RNAs
- EVs
Extracellular vehicles
- FBS
Fetal bovine serum
- GC
Gastric cancer
- GGT
γ-Glutamyltransferase
- HDL
High-density lipoproteins
- HSCs
Hepatic stellate cells
- IPF
Idiopathic pulmonary fibrosis
- IR
Insulin resistance
- LDL
Low density lipoproteins
- LF
Lung fibrosis
- LN
Lupus nephritis
- lncRNAs
Long noncoding RNAs
- miRNA
MicroRNA
- MMP2
Matrix metalloproteinase 2
- mRNA
Messenger RNA
- MS
Multiple sclerosis
- MVBs
Multivesicular bodies
- NASH
Nonalcoholic steatohepatitis
- ncRNAs
Noncoding RNAs
- PBMCs
Peripheral blood mononuclear cells
- piRNAs
PIWI-interacting RNAs
- RNPs
Ribonucleoproteins
- sncRNAs
Small noncoding RNAs
- TAA
Tumor-associated antigen
- TGF-β1
Transforming growth factor-β1
- tRNA
Transfer RNA
Author contributions
AMS, AA, MBK and SS wrote the manuscript. AH and AA prepared the table and figure. MBK proposed the idea and revised and supervised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Abdullah Muhammad Sohail, Email: sohailabdullah7028@gmail.com.
Muhammad Babar Khawar, Email: babar.khawar@uon.edu.pk, Email: babarkhawar@yahoo.com.
Ali Afzal, Email: aliafzal2615@gmail.com.
Ali Hassan, Email: alihassanvirk4512@gmail.com.
Sara Shahzaman, Email: sarashahzaman9@gmail.com.
Ahmed Ali, Email: ahmadxha7@gmail.com.
References
- 1.Kim S, Jeon OH, Jeon YJ. Extracellular RNA: emerging roles in cancer cell communication and biomarkers. Cancer Lett. 2020;495:33–40. doi: 10.1016/j.canlet.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Tosar JP, Witwer K, Cayota A. Revisiting extracellular RNA release, processing, and function. Trends Biochem Sci. 2021;46(6):438–445. doi: 10.1016/j.tibs.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosar JP. Die hard: resilient RNAs in the blood. Nat Rev Mol Cell Biol. 2021;22(6):373. doi: 10.1038/s41580-021-00355-9. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20(9):509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 7.Tamura T, Yoshioka Y, Sakamoto S, Ichikawa T, Ochiya T. Extracellular vesicles as a promising biomarker resource in liquid biopsy for cancer. Extracell Vesicles Circ Nucleic Acids. 2021;2(2):148–174. [Google Scholar]
- 8.Nik Mohamed Kamal NNSB, Shahidan WNS. Non-exosomal and exosomal circulatory micrornas: Which are more valid as biomarkers? Front Pharmacol. 2020;10:1500. doi: 10.3389/fphar.2019.01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galagali H, Kim JK. The multifaceted roles of microRNAs in differentiation. Curr Opin Cell Biol. 2020;67:118–140. doi: 10.1016/j.ceb.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 11.Musilová K, Mráz M. MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia. 2015;29(5):1004–1017. doi: 10.1038/leu.2014.351. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18(1):74. doi: 10.1186/s12943-019-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahra S, Singh A, Poddar N, Kumar S. Transfer RNA-derived non-coding RNAs (tncRNAs): hidden regulation of plants' transcriptional regulatory circuits. Comput Struct Biotechnol J. 2021;19:5278–5291. doi: 10.1016/j.csbj.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23(22):2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman JE, Gerstein M, Mick E, Rozowsky J, Levy D, Kitchen R, et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun. 2016;7:11106. doi: 10.1038/ncomms11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatosyan KA, Ustyantsev IG, Kramerov DA. RNA degradation in eukaryotic cells. Mol Biol (Mosk) 2020;54(4):542–561. doi: 10.1134/S0026893320040159. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski MP, Krude T. Functional roles of non-coding Y RNAs. Int J Biochem Cell Biol. 2015;66:20–29. doi: 10.1016/j.biocel.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Taylor DW, Fowler CC, Galan JE, Wang HW, Wolin SL. An RNA degradation machine sculpted by Ro autoantigen and noncoding RNA. Cell. 2013;153(1):166–177. doi: 10.1016/j.cell.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Sun X, Li L. Biogenesis and function of extracellular miRNAs. ExRNA. 2019;1:38. doi: 10.1186/s41544-019-0039-4. [DOI] [Google Scholar]
- 22.Vickers KC, Michell DL. HDL-small RNA export, transport, and functional delivery in atherosclerosis. Curr Atheroscler Rep. 2021;23(7):38. doi: 10.1007/s11883-021-00930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of micrornas in living cells. J Biol Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand S, Samuel M, Kumar S, Mathivanan S. Ticket to a bubble ride: cargo sorting into exosomes and extracellular vesicles. Biochim Biophys Acta Proteins Proteom. 2019;1867(12):140203. doi: 10.1016/j.bbapap.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Mcclelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, et al. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci. 2015;129(12):1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 26.Rennert RC, Hochberg FH, Carter BS. ExRNA in biofluids as biomarkers for brain tumors. Cell Mol Neurobiol. 2016;36(3):353–360. doi: 10.1007/s10571-015-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migita K, Komori A, Kozuru H, Jiuchi Y, Nakamura M, Yasunami M, et al. Circulating microRNA profiles in patients with type-1 autoimmune hepatitis. PLoS ONE. 2015;10(11):e0136908. doi: 10.1371/journal.pone.0136908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen L, Hansen T, Venø M, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–565. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ömerhoca S, Akkaş SY, İçen NK. Multiple sclerosis: diagnosis and differential diagnosis. Noro Psikiyatr Ars. 2018;55(Suppl 1):S1–9. doi: 10.29399/npa.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Happel C, Ganguly A, Tagle DA. Extracellular RNAs as potential biomarkers for cancer. J Cancer Metastasis Treat. 2020;6(9):20–37. doi: 10.20517/2394-4722.2020.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebrahimkhani S, Vafaee F, Young PE, Hur SS, Hawke S, Devenney E, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7(1):14293. doi: 10.1038/s41598-017-14301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Yoshizawa JM, Kim KM, Kanjanapangka J, Grogan TR, Wang X, et al. Discovery and validation of salivary extracellular RNA biomarkers for noninvasive detection of gastric cancer. Clin Chem. 2018;64(10):1513–1521. doi: 10.1373/clinchem.2018.290569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochberg FH, Atai NA, Gonda D, Hughes MS, Mawejje B, Balaj L, et al. Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn. 2014;14(4):439–452. doi: 10.1586/14737159.2014.905202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters LJ, Floege J, Biessen EA, Jankowski J, Van Der Vorst EP. MicroRNAs in chronic kidney disease: four candidates for clinical application. Int J Mol Sci. 2020;21(18):6547. doi: 10.3390/ijms21186547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29(7):749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 37.Huleihel L, Scarritt ME, Badylak SF. The influence of extracellular RNA on cell behavior in health, disease and regeneration. Curr Pathobiol Rep. 2017;5(1):13–22. doi: 10.1007/s40139-017-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roderburg C, Urban G-W, Bettermann K, Vucur M, Zimmermann H, Schmidt S, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 39.Bala S, Petrasek J, Mundkur S, Catalano D, Ward J, Levin I, et al. Serum microRNA-122 and miR-155 as biomarkers of liver injury and inflammation in models of acute and chronic liver disease. Hoagland Pincus Conference Center; 2012.
- 40.Guess MG, Barthel KK, Harrison BC, Leinwand LA. miR-30 family microRNAs regulate myogenic differentiation and provide negative feedback on the microRNA pathway. PLoS ONE. 2015;10(2):e0118229. doi: 10.1371/journal.pone.0118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schueller F, Roy S, Loosen SH, Alder J, Koppe C, Schneider AT, et al. miR-223 represents a biomarker in acute and chronic liver injury. Clin Sci. 2017;131(15):1971–1987. doi: 10.1042/CS20170218. [DOI] [PubMed] [Google Scholar]
- 42.Hallal S, Ebrahimkhani S, Shivalingam B, Graeber MB, Kaufman KL, Buckland ME. The emerging clinical potential of circulating extracellular vesicles for non-invasive glioma diagnosis and disease monitoring. Brain Tumor Pathol. 2019;36(2):29–39. doi: 10.1007/s10014-019-00335-0. [DOI] [PubMed] [Google Scholar]
- 43.Fukumoto I, Hanazawa T, Kinoshita T, Kikkawa N, Koshizuka K, Goto Y, et al. MicroRNA expression signature of oral squamous cell carcinoma: functional role of microRNA-26a/b in the modulation of novel cancer pathways. Br J Cancer. 2015;112(5):891–900. doi: 10.1038/bjc.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3(10):985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machida T, Tomofuji T, Ekuni D, Maruyama T, Yoneda T, Kawabata Y, et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int J Mol Sci. 2015;16(9):21294–21309. doi: 10.3390/ijms160921294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng L, Huang Y, Li L, Chen H, Su J. Serum miR-29a/b expression in gestational diabetes mellitus and its influence on prognosis evaluation. J Int Med Res. 2020;48(9):0300060520954763. doi: 10.1177/0300060520954763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SLT, Wong MT, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(12):E2271–E2276. doi: 10.1210/jc.2012-1996. [DOI] [PubMed] [Google Scholar]
- 48.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48(1):61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 49.Sebastiani G, Spagnuolo I, Patti A, Grieco FA, Cataldo D, Ferretti E, et al. MicroRNA expression fingerprint in serum of type 1 diabetic patients. Diabetologia. 2012;55:S48. [Google Scholar]
- 50.Yu Y, Naoyuki T, Shingo T, Shinsaku T, Tatsuki F, Kazuto T, et al. Serum microRNA profiles in patients with chronic hepatitis B, chronic hepatitis C, primary biliary cirrhosis, autoimmune hepatitis, nonalcoholic steatohepatitis, or drug-induced liver injury. Clin Biochem. 2017;50(18):1034–1039. doi: 10.1016/j.clinbiochem.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Kacperska MJ, Jastrzebski K, Tomasik B, Walenczak J, Konarska-Krol M, Glabinski A. Selected extracellular microRNA as potential biomarkers of multiple sclerosis activity—preliminary study. J Mol Neurosci. 2015;56(1):154–163. doi: 10.1007/s12031-014-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eissa S, Matboli M, Aboushahba R, Bekhet MM, Soliman Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications. 2016;30(8):1585–1592. doi: 10.1016/j.jdiacomp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Zang J, Maxwell AP, Simpson DA, Mckay GJ. Differential expression of urinary exosomal microRNAs miR-21-5p and miR-30b-5p in individuals with diabetic kidney disease. Sci Rep. 2019;9(1):10900. doi: 10.1038/s41598-019-47504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiabotto G, Gai C, Deregibus MC, Camussi G. Salivary extracellular vesicle-associated exRNA as cancer biomarker. Cancers (Basel) 2019;11(7):891. doi: 10.3390/cancers11070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Xia D, Wang RX, Zhang YT, Zhang SY, Yang C, et al. Identification of potential biomarkers for digestive system cancers from serum-derived extracellular vesicle RNA. Clin Chim Acta. 2022;531:36–47. doi: 10.1016/j.cca.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4(7–8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao L, Ning Q, Zheng G, Luo J, Dong D. exRNAdisease: an extracellular RNA transcriptome atlas in human diseases. Gene. 2022;836:146662. doi: 10.1016/j.gene.2022.146662. [DOI] [PubMed] [Google Scholar]
- 58.Larson MH, Pan W, Kim HJ, Mauntz RE, Stuart SM, Pimentel M, et al. A comprehensive characterization of the cell-free transcriptome reveals tissue-and subtype-specific biomarkers for cancer detection. Nat Commun. 2021;12(1):2357. doi: 10.1038/s41467-021-22444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dogra N, Ahsen ME, Kozlova EEG, Chen TY, Allette K, Olsen R, et al. exRNA signatures in extracellular vesicles and their tumor-lineage from prostate cancer. medRxiv. 2020 doi: 10.1101/2020.09.28.20190009:2020.09.28.20190009. [DOI] [Google Scholar]
- 60.Tamehri Zadeh SS, Taheri D, Shivarani S, Khatami F, Kazemi R. Liquid biopsy in prostate cancer diagnosis and prognosis: a narrative review. Transl Res Urol. 2020;2(4):139–146. [Google Scholar]
- 61.Ji J, Chen R, Zhao L, Xu Y, Cao Z, Xu H, et al. Circulating exosomal mRNA profiling identifies novel signatures for the detection of prostate cancer. Mol Cancer. 2021;20(1):58. doi: 10.1186/s12943-021-01349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, et al. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61(1):221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ingenito F, Roscigno G, Affinito A, Nuzzo S, Scognamiglio I, Quintavalle C, et al. The role of Exo-miRNAs in cancer: a focus on therapeutic and diagnostic applications. Int J Mol Sci. 2019;20(19):4687. doi: 10.3390/ijms20194687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Wang J, Jia E, Liu Z, Ge Q, Zhao X. Plasma RNA sequencing of extracellular RNAs reveals potential biomarkers for non-small cell lung cancer. Clin Biochem. 2020;83:65–73. doi: 10.1016/j.clinbiochem.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Qin XJ, Zhang JX, Wang RL. Exosomes as mediators and biomarkers in fibrosis. Biomark Med. 2020;14(8):697–712. doi: 10.2217/bmm-2019-0368. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Chen R, Velazquez VM, Brigstock DR. Fibrogenic signaling is suppressed in hepatic stellate cells through targeting of connective tissue growth factor (CCN2) by cellular or exosomal microRNA-199a-5p. Am J Pathol. 2016;186(11):2921–2933. doi: 10.1016/j.ajpath.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uzzaman A, Zhang X, Qiao Z, Zhan H, Sohail A, Wahid A, et al. Discovery of small extracellular vesicle proteins from human serum for liver cirrhosis and liver cancer. Biochimie. 2020;177:132–141. doi: 10.1016/j.biochi.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Weis A. Investigating the potential of circulating microRNAs as non-invasive biomarkers in cirrhosis and hepatocellular carcinoma. Australia: The University of Queensland; 2019. [Google Scholar]
- 69.Iravani F, Hosseini N, Mojarrad M. Role of micrornas in pathophysiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Middle East J Dig Dis. 2018;10(4):213–219. doi: 10.15171/mejdd.2018.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48(6):1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gruner HN, Mcmanus MT. Examining the evidence for extracellular RNA function in mammals. Nat Rev Genet. 2021;22(7):448–458. doi: 10.1038/s41576-021-00346-8. [DOI] [PubMed] [Google Scholar]
- 72.Kim TH, Lee Y, Lee YS, Gim JA, Ko E, Yim SY, et al. Circulating miRNA is a useful diagnostic biomarker for nonalcoholic steatohepatitis in nonalcoholic fatty liver disease. Sci Rep. 2021;11(1):14639. doi: 10.1038/s41598-021-94115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang P, Tu B, Liao HJ, Huang FZ, Li ZZ, Zhu KY, et al. Elevation of plasma tRNA fragments as a promising biomarker for liver fibrosis in nonalcoholic fatty liver disease. Sci Rep. 2021;11(1):5886. doi: 10.1038/s41598-021-85421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schult P, Roth H, Adams RL, Mas C, Imbert L, Orlik C, et al. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9(1):2613. doi: 10.1038/s41467-018-05053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Cao J, Zhang S, Sun L, Nan Y, Yao H, et al. MicroRNA-802 induces hepatitis B virus replication and replication through regulating SMARCE1 expression in hepatocellular carcinoma. Cell Death Dis. 2019;10(10):783. doi: 10.1038/s41419-019-1999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai L, Zhang X, Kozlowski M, Li W, Wu M, Liu J, et al. Extracellular hepatitis B virus RNAs are heterogeneous in length and circulate as capsid-antibody complexes in addition to virions in chronic hepatitis B patients. J Virol. 2018;92(24):e00798–e818. doi: 10.1128/JVI.00798-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao MY, Zhang WH, Ma WT, Liu QH, Xing LH, Zhao GF. microRNA-328 in exosomes derived from M2 macrophages exerts a promotive effect on the progression of pulmonary fibrosis via FAM13A in a rat model. Exp Mol Med. 2019;51(6):1–16. doi: 10.1038/s12276-019-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax. 2019;74(3):309–312. doi: 10.1136/thoraxjnl-2018-211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sadik N, Cruz L, Gurtner A, Rodosthenous RS, Dusoswa SA, Ziegler O, et al. Extracellular RNAs: a new awareness of old perspectives. Methods Mol Biol. 2018;1740:1–15. doi: 10.1007/978-1-4939-7652-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and sarcopenic obesity: pathogenesis, diagnosis, and treatments. Front Endocrinol (Lausanne) 2020;11:568. doi: 10.3389/fendo.2020.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y, Yang J. Implications of microRNA in kidney metabolic disorders. ExRNA. 2020;2:4. doi: 10.1186/s41544-019-0042-9. [DOI] [Google Scholar]
- 82.Deng J, Guo F. MicroRNAs and type 2 diabetes. ExRNA. 2019;1:36. doi: 10.1186/s41544-019-0038-5. [DOI] [Google Scholar]
- 83.Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67(11):2377–2388. doi: 10.2337/db17-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noren HN. Extracellular vesicles and extracellular RNA in aging and age-related disease. Transl Med Aging. 2020;4:96–98. doi: 10.1016/j.tma.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dluzen DF, Noren Hooten N, De S, Wood Iii WH, Zhang Y, Becker KG, et al. Extracellular RNA profiles with human age. Aging Cell. 2018;17(4):e12785. doi: 10.1111/acel.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, et al. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. GeroScience. 2020;42(1):1–17. doi: 10.1007/s11357-019-00115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alibhai FJ, Lim F, Yeganeh A, Distefano PV, Binesh-Marvasti T, Belfiore A, et al. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell. 2020;19(3):e13103. doi: 10.1111/acel.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He J, Tu C, Liu Y. Role of lncRNAs in aging and age-related diseases. Aging Med. 2018;1(2):158–175. doi: 10.1002/agm2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nevalainen T, Autio A, Puhka M, Jylhä M, Hurme M. Composition of the whole transcriptome in the human plasma: cellular source and modification by aging. Exp Gerontol. 2021;143:111119. doi: 10.1016/j.exger.2020.111119. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Z, Wu Q, Yan Z, Zheng H, Chen CJ, Liu Y, et al. Extracellular RNA in a single droplet of human serum reflects physiologic and disease states. Proc Natl Acad Sci USA. 2019;116(38):19200–19208. doi: 10.1073/pnas.1908252116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das S, Abdel-Mageed AB, Adamidi C, Adelson PD, Akat KM, Alsop E, et al. The extracellular RNA communication consortium: establishing foundational knowledge and technologies for extracellular RNA research. Cell. 2019;177(2):231–242. doi: 10.1016/j.cell.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarasov VV, Svistunov AA, Chubarev VN, Dostdar SA, Sokolov AV, Brzecka A, et al. Extracellular vesicles in cancer nanomedicine. Semin Cancer Biol. 2021;69:212–225. doi: 10.1016/j.semcancer.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 93.Ouyang Y, Liu Y, Wang ZM, Liu Z, Wu M. FLIM as a promising tool for cancer diagnosis and treatment monitoring. Nanomicro Lett. 2021;13(1):133. doi: 10.1007/s40820-021-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sundararajan V, Sarkar FH, Ramasamy TS. The versatile role of exosomes in cancer progression: diagnostic and therapeutic implications. Cell Oncol. 2018;41(3):223–252. doi: 10.1007/s13402-018-0378-4. [DOI] [PubMed] [Google Scholar]
- 95.Wiklander Oscar PB, Brennan M, Lötvall J, Breakefield Xandra O, Samir ELA. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheehan C, D'souza-Schorey C. Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer. J Cell Sci. 2019;132(20):jcs235085. doi: 10.1242/jcs.235085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diamond JM, Vanpouille-Box C, Spada S, Rudqvist N-P, Chapman JR, Ueberheide BM, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. 2018;6(8):910–920. doi: 10.1158/2326-6066.CIR-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin W, Xu Y, Chen X, Liu J, Weng Y, Zhuang Q, et al. Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics. 2020;10(11):4871–4884. doi: 10.7150/thno.43539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soe ZY, Park EJ, Shimaoka M. Integrin regulation in immunological and cancerous cells and exosomes. Int J Mol Sci. 2021;22(4):2193. doi: 10.3390/ijms22042193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.You B, Xu W, Zhang B. Engineering exosomes: a new direction for anticancer treatment. Am J Cancer Res. 2018;8(8):1332. [PMC free article] [PubMed] [Google Scholar]
- 102.Xu L, Faruqu FN, Liam-Or R, Abu Abed O, Li D, Venner K, et al. Design of experiment (DoE)-driven in vitro and in vivo uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. J Extracell Vesicles. 2020;9(1):1779458. doi: 10.1080/20013078.2020.1779458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mithraprabhu S, Morley R, Chen M, Ramachandran M, Choi K, Kalff A, et al. Extracellular RNA: an emerging biomarker for therapeutic monitoring in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S68–S69. doi: 10.1016/S2152-2650(21)02189-3. [DOI] [Google Scholar]
- 104.Lehrich BM, Liang Y, Fiandaca MS. Foetal bovine serum influence on in vitro extracellular vesicle analyses. J Extracell Vesicles. 2021;10(3):e12061. doi: 10.1002/jev2.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He W, Xu J, Huang Z, Zhang J, Dong L. MiRNAs in cancer therapy: focusing on their bi-directional roles. ExRNA. 2019;1:7. doi: 10.1186/s41544-019-0005-1. [DOI] [Google Scholar]
- 106.Zhou Y, Yang J. Extracellular RNA in renal diseases. ExRNA. 2019;1:5. doi: 10.1186/s41544-018-0001-x. [DOI] [Google Scholar]
- 107.Kuscu C, Eason JD, Kuscu C. Technical advancements in epigenomics and applications in transplantation. Curr Opin Organ Transplant. 2021;26(1):23–29. doi: 10.1097/MOT.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 108.Arshad S, Khawar MB, Hassan A, Afzal A, Muhammad Sohail A, Mukhtar M, et al. Cell free DNA; diagnostic and prognostic approaches to oncology. Adv Cancer Biol Metastasis. 2022;5:100052. doi: 10.1016/j.adcanc.2022.100052. [DOI] [Google Scholar]
- 109.Mithraprabhu S, Morley R, Khong T, Kalff A, Bergin K, Hocking J, et al. Monitoring tumour burden and therapeutic response through analysis of circulating tumour DNA and extracellular RNA in multiple myeloma patients. Leukemia. 2019;33(8):2022–2033. doi: 10.1038/s41375-019-0469-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


