Abstract
Background
To estimate the prevalence of malocclusion in individuals with autism spectrum disorders (ASD) and to assess the relationship between ASD and malocclusion.
Methods
We searched electronic databases including PubMed, Scopus, Web of Science, Cochrane, Embase, SciELO LILACS, Proquest, OpenGrey and Google Scholar. There were no language or publication dates restrictions. Two researchers independently performed selection, data extraction and quality assessment. Quality assessment and risk of bias were evaluated through the Newcastle–Ottawa scale and ROBINS-E tool. Meta-analyses using random effect models were used to estimate pooled measures of prevalence of malocclusion characteristics in individuals with ASD and pooled odds ratio (OR) on the relationship between ASD and malocclusion characteristics. Subgroup meta-analyses were conducted according to children and adolescents, history of orthodontic treatment, and occurrence of other syndromes and medical conditions.
Results
Searching identified 5549 papers with 238 were selected for full assessment. Eighteen cross-sectional studies were included according to inclusion criteria. Of them, eleven studies were considered of moderate quality. A judgement of critical risk of bias occurred for thirteen studies. The most prevalent malocclusion characteristics in individuals with ASD were crowding (33%; 95% CI 22 to 44%) and increased maxillary overjet (39%; 95% CI 23 to 54%). Individuals with ASD had higher odds of Angle’s Class II (OR 1.92; 95% CI 1.36 to 2.72), Angle’s Class III (OR 2.33; 95% CI 1.29 to 4.23), open bite (OR 1.96; 95% CI 1.21 to 3.16), and increased maxillary overjet (OR 1.53; 95% CI 1.06 to 2.21) than individuals without ASD.
Conclusions
Angle’s Class II, Angle’s Class III, anterior open bite and increased maxillary overjet were more prevalent in individuals with ASD than those without ASD. Further high-quality studies are needed.
Keywords: Autistic disorder, Malocclusion, Angle Class II, Malocclusion, Angle Class III, Open bite, Systematic review, Meta-analysis
Background
Autism spectrum disorder (ASD) is a lifelong and complex developmental condition linked to the atypical neurodevelopment usually diagnosed between the ages of one to six years depending on access to healthcare services [1]. It is estimated that 1 in 270 people have ASD, with abilities and needs varying between individuals from those living independently with minimum support to those requiring lifelong care [2]. Environmental and genetic factors have previously been linked to the occurrence of ASD, although the aetiologic mechanisms remain unknown [3]. Individuals with ASD may experience persistent challenges in social interaction and communication [4]. Intellectual disability is often a coexisting condition in approximately 50% of individuals with ASD and frustration with communication challenges, coupled with an unsupportive environment may often lead to behavioural outbursts [5, 6]. The dimensions of social interaction and communication as well as restrictive and repetitive behaviour are part of the assessment procedure for ASD in The Diagnostic and Statistical Manual of Mental Disorders (DSM-5), although diagnosis is not always straightforward [7, 8].
Recent evidence suggests that a diagnosis of ASD may be accompanied by the occurrence of dental problems and health impairing behaviours, such as poor oral hygiene, which predisposes individuals with ASD to gingivitis and poorer periodontal health [9, 10]. Individuals with ASD experience increased rates of immunological and gastrointestinal problems, sleeping disorders, mental health problems, convulsion, obesity, hypertension, and diabetes [11]. A previous systematic review including ten primary studies indicates a lack of consensus whether the incidence of dental caries is higher among people with ASD [9]. Furthermore, children with ASD present with greater prevalence of halitosis, oral lesions, and dental pain and many individuals with ASD have at least one dental problem creating negative impacts on their quality of life [12]. Pharmacological interventions for people with ASD and coexisting conditions often control behaviour [13]. Side effects of some of the drugs are gingival bleeding, gingival overgrowth, hyperplasia, aphthous ulcers, delayed healing, and xerostomia [14]. The associated challenges may lead to poorer oral health, often compounded by the lack of effective health promotion for individuals with ASD and their carers compared to individuals without ASD, resulting in an increased demand and use of health services [15, 16].
For individuals without ASD, malocclusion is a craniofacial developmental disorder affecting teeth, bones, and facial muscles. The multifactorial aetiology of malocclusion includes genetic and environmental factors as well as persistent harmful oral habits [17, 18]. A previous systematic review revealed the global prevalence of Angle’s Class I, Class II and Class III as 74.7%, 19.6% and 5.9%, respectively. In addition, an increased maxillary overjet and deep overbite were estimated as 20.1% and 22.0%. The prevalence of open bite and posterior cross bite were 4.9% and 9.4% [18]. The negative impact of malocclusion on quality of life has been extensively reported in children. Children without ASD and diagnosed with malocclusion perceive more functional problems, including speaking, chewing, and sleeping, as well as impacts affecting social interaction, self-esteem, and oral health satisfaction [19].
ASD is a diverse condition and there appear to be morphological facial differences arising from genetic mechanisms for some individuals [20]. For example, fragile X syndrome is associated with ASD, with studies indicating a higher occurrence of malocclusion among individuals with the syndrome [21]. However, testing for fragile X remains a subject for debate because there is no available treatment and it may be unknown whether an individual with ASD also has fragile X [22]. Another overlapping syndrome with ASD is Rett syndrome, often misdiagnosed as ASD and can occur as a syndrome without ASD [23]. A systematic review on oral health and Rett syndrome suggested a higher prevalence of anterior open-bite and mouth breathing in affected individuals, but the study did not identify whether there was an interplay with ASD [24]. Another syndrome associated with ASD is Phelan-McDermid with a high frequency of malocclusion [25]. The genetic landscape of ASD and its association with other syndromes appears inconclusive and complex.
Harmful oral habits, including para-functional habits, are more common in individuals with ASD than those without [26]. Compared to controls, individuals with ASD reported greater prevalence of bruxism, mouth breathing, biting objects, lips or tongue, nail biting and finger sucking [12, 26]. The influence of harmful oral habits on malocclusion and the greater prevalence of para-functional oral habits in individuals with ASD raises the question as to whether ASD predisposes distinct types of malocclusions. Therefore, the aims of this study were to systematically review the existing literature on the prevalence of the different malocclusion characteristics in individuals with ASD and to examine the association between ASD and malocclusion.
Methods
Protocol registration
The protocol for the present systematic review was registered on the National Institute of Health Research Database (registration number CRD42019151794; http://www.crd.york.ac.uk/PROSPERO).
Eligibility criteria
The studies included in this systematic review met the following selection criteria. (1) Participants: Individuals of any age group who had or not had undergone previous orthodontic treatment. (2) Exposure: Individuals with ASD diagnosis. (3) Comparator: Studies had to report at least one malocclusion characteristic of individuals diagnosed with ASD. They could include one or more comparison groups such as individuals without ASD or individuals with other syndromes or intellectual disabilities. (4) Outcome measures: Malocclusion characteristics on clinical examination was the main outcome. The condition must have been assessed through clinical visual inspection using malocclusion indices such as the Dental Aesthetic Index (DAI), clinical classifications, such as Angle’s Class, or through the presence of horizontal or vertical malocclusions. (5) Study design: Prospective or retrospective cohort studies, case–control and cross-sectional studies were retrieved for inclusion. Ineligible papers included interventional studies and previous review papers.
Literature search strategy and selection of papers
Databases searched included PubMed, Scopus, Web of Science, Cochrane, Embase, SciELO and LILACS, up to November 2021. Grey literature was examined through Proquest, OpenGrey and Google Scholar. There were no language restrictions. The electronic searches were carried using a combination of search terms linked through Boolean operators (Table 1). Manual searching took place of the reference lists of included articles and those from previously identified systematic and narrative reviews.
Table 1.
Study search strategy
| Search groups | (1) | (2) |
|---|---|---|
| Key-words |
(a) Malocclusion Malocclusion, angle class Malocclusion, angle class II malocclusion, angle class III (b) Orthodontics Orthodontic, corrective Index of orthodontic treatment needs Dental aesthetic index Stomatognathic System Abnormalities Stomatognathic diseases Tooth Abnormalities Dental Care for disabled Dental care, disability |
Handicapped Mentally handicapped Learning disability* Intellectual disability* (c) Asperger’s Neurodiversity Child development disorders, pervasive (d) Autism Autism spectrum disorders Autistic disorder Neurodevelopmental disorders |
| Database | Search strategy | |
| PubMed | (1 AND 2) | |
| Scopus | (1 AND 2) | |
| Web of Science | (1 AND 2) | |
| Cochrane | (1 AND 2) | |
| Embase | (1 AND 2) | |
| Scielo | (1 AND 2) | |
| Lilacs | (1 AND 2) | |
| Proquest | (a OR b AND c OR d) | |
| OpenGrey | (a OR b AND c OR d) | |
| Google Scholar* | (a OR b AND c OR d) | |
*On Google Scholar database search, only the first hundred hits were considered
Total: 5549
Titles and abstracts of all retrieved papers were independently screened and selected for inclusion by two authors (T.P.M. and S.A.T.). A third author (M.V.V.) who did not participate in the original screening and selection of papers was involved in the discussion to resolve any disagreements.
Data extraction
Relevant data of included papers were independently extracted in duplicate by two authors (T.P.M. and M.V.V.). The following information was recorded: (1) author and year of publication; (2) study design; (3) country; (4) study setting; (5) participants: sample size, gender rate, participant’s age; (6) malocclusion measures, including examiners’ background, clinical calibration, and examination conditions; (7) eligibility criteria; and (8) comparison group.
Quality assessment
The quality assessment was carried out independently by two authors (T.P.M. and M.V.V.) using the Newcastle–Ottawa scale (NOS) [27]. Any disagreements were resolved by consensus. The NOS evaluates the methodological quality of individual studies following a star system based on 8 domains grouped into 3 main domains: patient selection, comparability of study groups, and outcome assessment. Cohort and case–control studies may receive up to 9 stars and cross-sectional studies may receive up to 10 stars. Studies were categorized as high-quality, moderate quality and low quality if they reached 7–9 (cohort and case–control studies) or 7–10 (cross-sectional studies) stars, 4–6 stars and 0–3 stars, respectively.
Risk of bias of individual studies
The risk of bias of individual studies was carried out independently by two authors (T.P.M. and S.T.D.) using the Risk of Bias in Nonrandomized Studies of Exposures (ROBINS-E) tool [28]. Any disagreements were resolved by consensus with input from a third reviewer (M.V.V.). The application of the Risk of bias (RoB) instrument followed the three steps. In the first step, reviewers revised the review question and specific aspects of sources of bias, such as confounders, and exposure and outcome measurements. The second step involved the description of a hypothetical ideal study and specific confounders. Finally, each study was compared to the ideal study considering the RoB criteria across the seven items: (1) bias due to confounding, (2) bias in selection of participants into the study, (3) bias in classification of exposures, (4) bias due to departures from intended exposures, (5) bias due to missing data, (6) bias in measurement of outcomes, (7) bias in selection of the reported result. Initially, the examiners answer the ROBINS-E questions using the options “yes,” “Probably yes,” “Probably no,” or “No.” Then, each RoB item was assessed as ‘low,’ ‘moderate,’ ‘serious,’ or ‘critical,’ to judge RoB at study-level and at item-level.
Quantitative synthesis and statistical analysis
Meta-analyses were conducted to obtain summary measures (prevalence) and pooled effect sizes (odds ratios) and 95% confidence interval (CI) using random effects models to account for the heterogeneity between primary estimates. Both pooled prevalence measures and pooled odds ratios were estimated using the inverse variance method. Producing forest plots related to the different malocclusion classifications (e.g., Angle’s classification, Dental Aesthetic Index (DAI)) and malocclusion characteristics. Meta-analyses were conducted for all studies that provided data. Sub-group analyses were conducted for (i) studies including only children and adolescents, (ii) studies excluding individuals with history of orthodontic treatment, and (iii) studies that excluding or providing information about other syndromes and medical conditions.
To obtain pooled prevalence, the original estimates were submitted to logit transformations to account for the distribution asymmetry. Then, the transformed estimates were weighted by the logit. The pooled prevalence estimates were generated thereafter. The following definitions of malocclusion characteristics were used in the meta-analyses of prevalence and in those comparing malocclusion characteristics between individuals with ASD and without ASD: increased maxillary overjet ≥ 3 mm, anterior cross-bite ≥ 0 mm, and open bite ≥ 0 mm [29].
Studies comparing malocclusion measures between individuals with ASD and those without any deficiency were included to obtain pooled effect sizes. Studies reporting an odds ratio and 95%CI were reported or could be obtained through numerical transformation using continuous measures (e.g., mean differences, correlations) were included [30]. I2 statistics assessed the proportion of the variance due to statistical heterogeneity among studies comparing malocclusion measures between individuals with and without ASD [31]. Meta-analyses reporting I2 as equal or less than 50% acknowledged heterogeneity [32]. Assessing heterogeneity in the studies reporting the prevalence of malocclusion among individuals with ASD occurred through prediction intervals (PI) [33]. The decision not to conduct a publication bias assessment resulted from power issues, because only one meta-analysis included more than 10 studies [34]. All statistical analyses were performed using Stata software (version 16.0) using the commands ‘metaprop’ and ‘metan’ to obtain pooled prevalence estimates and pooled effect sizes.
Results
Study selection
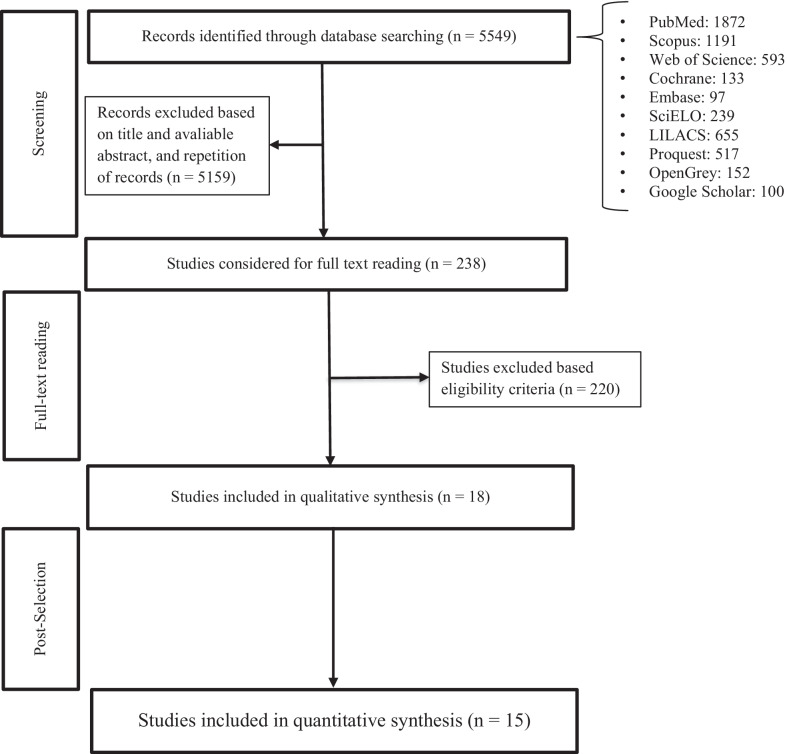
A PRISMA flow chart reports the number of outputs retrieved, screened, and selected (Fig. 1). The initial electronic search yielded 5549 articles after removing duplicates. The search of references did not retrieve any further relevant publications. After the initial screening of titles and abstracts 238 articles were selected for full assessment. After the full-text analysis, 18 articles assessing 2194 individuals with ASD and 10,846 without ASD were included in the systematic review [35–51]. The Kappa coefficient regarding the agreement between authors involved in selection of papers was 0.70.
Fig. 1.
Flow chart of studies identification and selection
Characteristics of included studies
The characteristics of the included studies are summarized in Table 2. Eighteen cross-sectional studies were identified. Of these, two studies, originally classified as case–control studies [40, 44] selected participants with a diagnosis of ASD (exposure of interest in this study) and reclassified as cross-sectional studies. Three studies included individuals solely with ASD [41, 50, 51]. Included studies from 13 countries selected participants from rehabilitation centres, healthcare services, schools for disabled children, university dental hospitals and mainstream schools. Sample size for the studies ranged from 54 to 844. Of the 18 studies, 13 assessed children and adolescents up to 18 years of age. Only five studies excluded individuals with a history of orthodontic treatment [38, 43, 45, 46, 52]. The occurrence of other syndromes and medical conditions were considered in eight studies [35, 36, 39, 42, 43, 45, 46, 52]. Different malocclusion measures and malocclusion indices used included Angle’s classification, DAI, crowding, posterior crossbite, increased maxillary overjet, anterior crossbite, open bite and deep bite.
Table 2.
Characteristics of selected studies (N = 18)
| References | Study design | Country | Setting | Participants | ASD diagnosis | Malocclusion measures | Eligibility criteria* | Group of comparison |
|---|---|---|---|---|---|---|---|---|
| Vittek et al. [35] | Cross-sectional | United States | Healthcare services |
N total = 458; N ASD = 26 boys (63.8%), girls (36.2%) Age group: 6–87 years-old |
Medical records |
Angle's classification, crowding, crossbite, open bite, overbite and overjet Examiner: N.I Clinical calibration: N.I Examination conditions: dental chair using mouth mirroe and probe |
Exclusion criteria Edentulous patients and those without molar relationship |
Yes Children without intellectual and mental disabilities (N = 8841), organic brain (N = 238), seizure disorder (N = 90), cerebral palsy (N = 47), down (N = 57) |
| Manzano et al. [36] | Cross-sectional | Venezuela | Special education institutes |
N total = 133; N ASD = 23 boys (55.6%), girls (44.4%) Age group: 3–4 years-old |
Medical diagnosis |
Angle's classification (só relata no resultado) Examiner: N.I Clinical calibration: N.I Local of examination: N.I |
Inclusion criteria Age between 3 and 4 years |
Yes Down syndrome (N = 65), deaf and speech impaired (N = 26), sight impaired (N = 7), cerebral palsy (N = 12) |
| DeMattei et al. [37] | Cross-sectional | United States | Three schools for disabled children |
N total = 55; N ASD = 39 boys (72.7%) girls (27.3%) Age group: 2.6–21.0 years-old |
Medical records and school files |
Angle´s classification, crowding, crossbite Examiner: dental hygienists Clinical calibration: N.I Examination conditions: portable dental chairs |
None |
Yes Children with other developmental disorders (N = 16) |
| Luppanapornlarp et al. [38] | Cross-sectional | Thailand | Division of Dentistry of University |
N total = 80; N ASD = 32 boys (78.1%), girls (21.8%) Age group: 8–12 years-old Mean age = 9.8 ± 1.1 |
Not reported |
Dental Aesthetic Index Examiner: N.I Clinical calibration: Intrarater Correlation Coefficient = 0.98 Examination conditions: N.I |
Inclusion criteria Age between 8 and 12 years, history of orthodontic treatment Exclusion criteria Inability to cooperate in the oral examination |
Yes Children without ASD (N = 48) |
| Soni et al. [39] | Cross-sectional | India | Special schools |
N total = 78, N ASD = 10 boys (66.7%) and girls (33.3) Age group:12–15 years |
Not reported |
IOTN Examiner: N.I Clinical calibration: Kappa > 0.82 Examination conditions: use of natural daylight |
Exclusion criteria Age between 12 and 15 years, Inability to cooperate in the oral examination |
Yes Down syndrome (N = 4), hearing impaired (N = 11), learning disability (N = 2), mental retardation (N = 43), orthopedic disability (N = 2), spastic paraplegia (N = 1) and visually impaired (N = 5) |
| Orellana et al. [40] | Cross-sectional | Spain | Two day-centres for people with autism |
N total = 60; N ASD = 30 boys (90.0%), girls (10.0%) Age group: 20–41 years-old Mean age = 27.8 ± 5.8 |
Not reported |
Dental crowding, open bite Examiner: dentists Clinical calibration: Kappa > 0.81 Examination conditions: portable dental chair and lamp |
None |
Yes Individuals without ASD (N = 30) |
| Rekha et al. [41] | Cross-sectional | India | Twelve special education schools, three autistic child centres and three therapy centres |
N ASD = 483 boys (75.1%), girls (24.9%) Age group: 4–16 years-old |
School files |
Proclination, crowding, anterior open bite, rotation Examiner: pediatric dentistry Clinical calibration: N.I Local of examination: mouth mirror in broad daylight |
None | No |
| Muppa et al. [42] | Cross-sectional | India | Eleven special schools |
N total = 844; N ASD = 40 boys (80.0%), girls (20.0%) Age group: 6–30 years-old |
Not reported |
Class I, Class II, Class III, anterior crowding, anterior spacing, deep bite, open bite, and anterior cross bite Examiner: N.I Clinical calibration: N.I Examination conditions: under natural light, with the child in the knee to knee position |
None |
Yes Mild Intellectual Disability (MID) (N = 308), moderate ID (N = 201), severe ID (N = 83, Hearing and speech (N = 172), cerebral palsy (N = 40) |
| Vellappally et al. [43] | Cross-sectional | India | Fourteen special schools for disabled |
N total = 243; N ASD = 14 boys (60.1%), girls (39.9%) Age group: 12–18 years-old Mean age 14.1 ± 2.0 |
Not reported |
Dental Aesthetic Index Examiner: N.I Clinical calibration: reproducibility = 90% Examination conditions: N.I |
Inclusion criteria Ages ≥ 12 and < 19 years, intelligence Quotient (IQ) ≤ 85 Exclusion criteria Inability to cooperate in the oral examination, history of orthodontic treatment |
Yes Intellectual disability (ID) alone (N = 108), ID and cerebral palsy (N = 55), Down’s syndrome (N = 36), ID and a learning disability (N = 18) and ID and a speech-hearing impairment (N = 12) |
| Du et al. [44] | Cross-sectional | China | Nineteen special child care centres |
N total = 514; N ASD = 257 boys (84.4%), girls (15.6%) Age group: 2.7–6.4 years-old Mean age = 4.9 ± 0.8 |
Not reported |
Deep overbite, anterior open bite, increased overjet, anterior crossbite and posterior crossbite Examiner: N.I Clinical calibration: Kappa > 0.70 Examination conditions: chair using an intra-oral mirror with a LED light source |
None |
Yes Children without ASD (N = 257) |
| Alkhadra [45] | Cross-sectional | Kingdom of Saudi Arabia | Five rehabilitation centres for disabled children |
N total = 72; N ASD = 55 boys (72.7%) girls (27.3%) Age group: 6–14 years-old |
Medical records |
Angle’s classification, overjet, overbite, incisor open bite, cross bite in the right and left side on both anterior and posterior Examiner: N.I Clinical calibration: N.I Examination conditions: portable chair under natural light using a disposable mouth mirror and tongue blade |
Exclusion criteria History of ongoing medical treatment, extraction, and orthodontic treatment |
Yes Children with Down syndrome (N = 100) |
| Fontaine-Sylvestre et al. [46] | Cross-sectional | Canada | Division of dentistry, children's hospital |
N total = 200; N ASD = 99 boys (78.8%), girls (21.2%) Age group: 5–18 years-old Mean age = 11.0 ± 3.7 |
Medical diagnosis |
Angle's classification, midline deviation, crossbite, open bite, overbite, crowding, Examiner: N.I Clinical calibration: N.I Examination conditions: dental chair |
Inclusion criteria Age between 5 to 18 years Mixed or permanent dentition Exclusion criteria Another disorder or syndrome than ASD, history of orthodontic treatment, Incomplete files with respect to the child's diagnosis of ASD |
Yes Children without ASD (N = 101) |
| Alkhabuli et al.[47] | Cross-sectional | United Arab Emirates | Rehabilitation centres for disabled children |
N total = 54; N ASD = 9 boys (70.4%) girls (29.6%) Age group: 3–17 years-old |
Medical records |
Angle’s classification, crowding; spacing, anterior open bite, IOTN Examiner: N.I Clinical calibration: Kappa = 0.83 Examination conditions: adjustable chair using torchlight, dental explorer, and mouth mirror |
Inclusion criteria Age ≤ 17 years |
No |
| Kuter and Guler [48] | Cross-sectional | Turkey | Regular schools |
N total = 407; N ASD = 285 boys (80%), girls (20%) Age group: 5–16 years-old |
Not reported |
crowding, open bite, deep-palate Examiner: N.I Clinical calibration: N.I Examination conditions: chair using dental mirror and explorer |
None |
Yes Children without ASD (N = 122) |
| Leiva-Garcia et al. [49] | Cross-sectional | Spain | Rehabilitation centre for disabled children |
N total = 146; N ASD = 55 boys (74%), girls (26%) Age group: 6–18 years-old Mean age = 10.7 ± 3.0 |
Medical diagnosis |
Angle's classification, open bite, crossbite Examiner: one pediatric dentist Clinical calibration: Kappa = 0.80 Examination conditions: chair under natural light and using a sterile exploration kit comprising an oral probe and mirror, and cotton tips |
Exclusion criteria Special diets, food allergies or medications capable of modifying dietary intake and oral health |
Yes Children with typical development (N = 91) |
| Orellana et al. [50] | Cross-sectional | Chile | Institutions for people with ASD |
N ASD = 123 boys (82.9%), girls (17.1%) Age group: 4–23 years-old Mean age = 9.4 ± 4.3 |
Not reported |
Deep/ogival palate, anterior open bite, anterior and posterior crossbite Examiner: dentists Clinical calibration: Kappa > 0.81 Examination conditions: portable dental chair and lamp |
Inclusion criteria Understanding very simple instructions |
No |
| Mangione et al. [51] | Cross-sectional | France | special dental care department Division of Dentistry of University |
N ASD = 118 boys (75.4%), girls (24.6%) Age group: 4–53 years-old Mean age = 23.3 |
Medical records |
mild to severe dental and/or alveolar malocclusions Examiner: N.I Clinical calibration: N.I Examination conditions: dental chairs |
None | No |
| Bagattoni et al. [52] | Cross-sectional | Italy | Special Needs Dentistry Unit of University |
N total = 128, N ASD = 64 boys (66%), girls (34%) Age group: 9.0 ± 2.9 years |
Medical diagnosis |
Angle’s classification, posterior crossbite, anterior open bite and deep bite Examiner: dentists Clinical calibration: N.I Examination conditions: dental chair using a dental mirror and a WHO periodontal probe |
Exclusion criteria: Medical condition associated with oral diseases, inability to cooperate in the oral examination, dental prophylaxis in the previous 6 months, history of orthodontic treatment |
Children without ASD (N = 64) |
*Diagnostic methods of ASD and non-ASDs were not considered as eligibility criteria
N.I.: not informed
Quality assessment
The modified Newcastle–Ottawa scale for cross-sectional studies was used to score the methodologic quality (Table 3). Five studies achieved a maximum of 3 stars or less and were assigned as having low quality [39, 40, 42, 44, 50]. Eleven studies were considered as having moderate quality [35–37, 41, 43, 45, 47–49, 51, 52], and two studies were assessed as high quality [38, 46]. Fifteen studies achieved 2 or less stars for selection of the study groups. Only one study selected a representative sample. Two studies reached 2 stars for comparability and all studies achieved two or more stars for outcome.
Table 3.
Quality assessment according to Newcastle–Ottawa of the included studies (n = 18)
| References | Selection | Comparability | Outcome | Stars | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure | Control for confounders | Assessment of the outcome | Statistical test | ||
| Vitek et al. [35] | b | b | c | a** | c | b** | b | 4* |
| Manzano et al. [36] | c | a* | c | a** | c | b** | c | 5* |
| DeMattei et al. [37] | c | b | c | a** | c | b** | c | 4* |
| Luppanapornlarp et al. [38] | c | b | c | a** | ab** | b** | a* | 7* |
| Soni et al. [39] | c | b | c | c | c | a** | c | 2* |
| Orellana et al. [40] | c | b | c | c | b* | b** | b | 3* |
| Rekha et al. [41] | c | b | c | a** | c | b** | c | 4* |
| Muppa et al. [42] | c | b | c | c | c | b** | c | 2* |
| Vellappally et al. [43] | c | b | c | a** | c | a** | c | 4* |
| Du et al. [44] | c | b | c | c | b* | b** | b | 3* |
| Alkhadra [45] | c | b | c | a** | c | b** | c | 4* |
| Fontaine-Sylvestre et al. [46] | c | b | c | a** | ab** | b** | a* | 7* |
| Alkhabuli et al. [47] | c | b | c | a** | c | b** | c | 4* |
| Kuter and Guler [48] | b* | b | c | c | b* | b** | b | 4* |
| Leiva-Garcia et al. [49] | c | b | c | a** | b* | b** | b | 5* |
| Orellana et al. [50] | c | b | c | c | c | a** | c | 2* |
| Mangione et al. [51] | c | b | c | a** | c | b** | c | 4* |
| Bagattoni et al. [52] | c | b | c | a** | c | b** | c | 4* |
Risk of bias of individual studies
The ROBINS-E tool was used to assess RoB (Table 4). Nine were judged as critical risk of bias and four at serious risk. All studies were at low risk of selection bias. Nine studies were at critical risk of bias due to the measurement of exposure and nine at moderate risk. The departure from exposure domain was not relevant for all studies. One study was at moderate risk of bias due to missing data, 12 at serious risk, and five at critical risk. Thirteen studies were at critical risk of bias due to measurement of outcomes and five studies at serious risk. Twelve studies were at moderate risk of bias due to the reported results, and six studies were at critical risk. Of the 18 studies, none was judged as of low risk of bias, one [52] was assessed as having a moderate risk of bias, four studies [35, 38, 47, 51] were assessed as having a serious risk of bias, and 13 at critical risk of bias.
Table 4.
ROBINS-E risk of bias assessment
| References | Confounding | Selection | Measurement of exposure | Departure from exposure | Missing data | Measurement of outcomes | Reported Results | Study-level RoB judgment |
|---|---|---|---|---|---|---|---|---|
| Vitek et al. [35] | C | L | M | NR | S | C | M | S |
| Manzano et al. [36] | C | L | C | NR | C | C | M | C |
| DeMattei et al. [37] | C | L | M | NR | C | C | M | C |
| Luppanapornlarp et al. [38] | S | L | C | NR | S | S | M | S |
| Soni et al. [39] | C | L | C | NR | S | S | C | C |
| Orellana et al. [40] | C | L | C | NR | S | C | M | C |
| Rekha et al. [41] | NR | L | C | NR | C | C | M | C |
| Muppa et al. [42] | C | L | C | NR | S | C | C | C |
| Du et al. [43] | C | L | M | NR | C | S | M | C |
| Vellappally et al. [44] | S | L | C | NR | S | S | M | C |
| Alkhadra [45] | S | L | M | NR | S | C | C | C |
| Fontaine-Sylvestre et al. [46] | S | L | M | NR | S | C | C | C |
| Alkhabuli et al. [47] | NR | L | M | NR | M | C | M | S |
| Kuter & Guler [48] | C | L | C | NR | C | C | C | C |
| Leiva-Garcia et al. [49] | C | L | M | NR | S | C | C | C |
| Orellana et al. [50] | NR | L | C | NR | S | C | M | C |
| Mangione et al. [51] | NR | L | M | NR | S | C | M | S |
| Bagattoni et al. [52] | M | L | M | NR | S | S | M | M |
L low, M moderate, S Serious, C Critical, NR not relevant
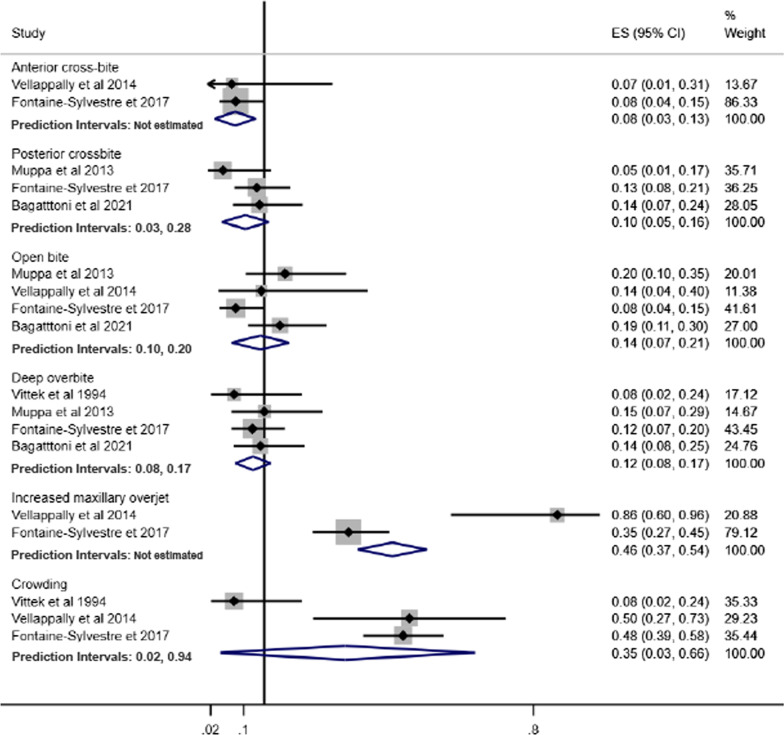
Prevalence of malocclusion in individuals with ASD
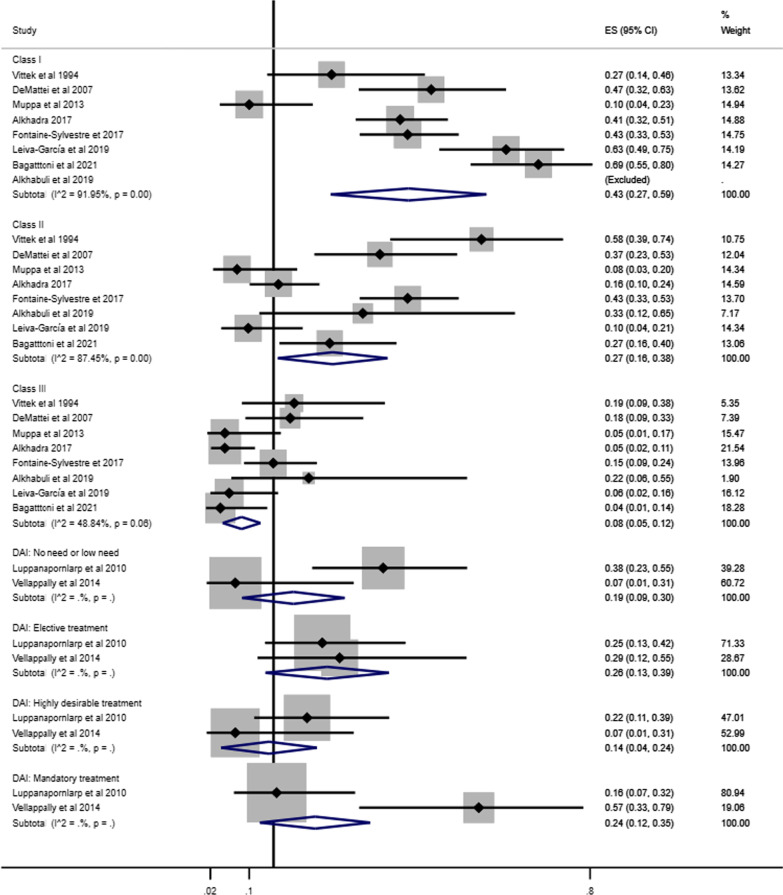
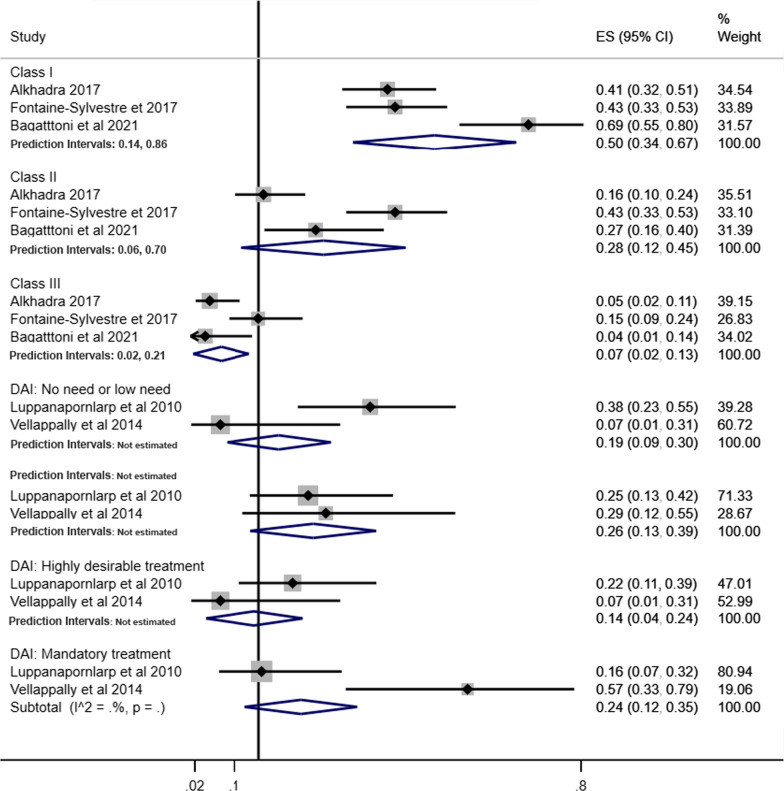
The forest plot combining the prevalence of malocclusion classifications (Angle’s Class and DAI) and characteristics of malocclusion in individuals with ASD derived from 15 studies involving 1458 individuals are shown in Figs. 2 and 3 [35, 37, 38, 40–50, 52]. The pooled prevalence of Angle’s Class I, Class II and Class III in individuals with ASD were 43% (95% CI 27%–59%), 27% (95% CI 16%–38%) and 8% (95% CI 5%–12%), respectively. The pooled measures of highly desirable treatment and mandatory treatment according to DAI were 14% (95% CI 4%–24%) and 24% (95% CI 12%–35%), respectively (Fig. 2).
Fig. 2.
Forest-plot for prevalence of malocclusion according to Angle´s Class and DAI among individuals with ASD
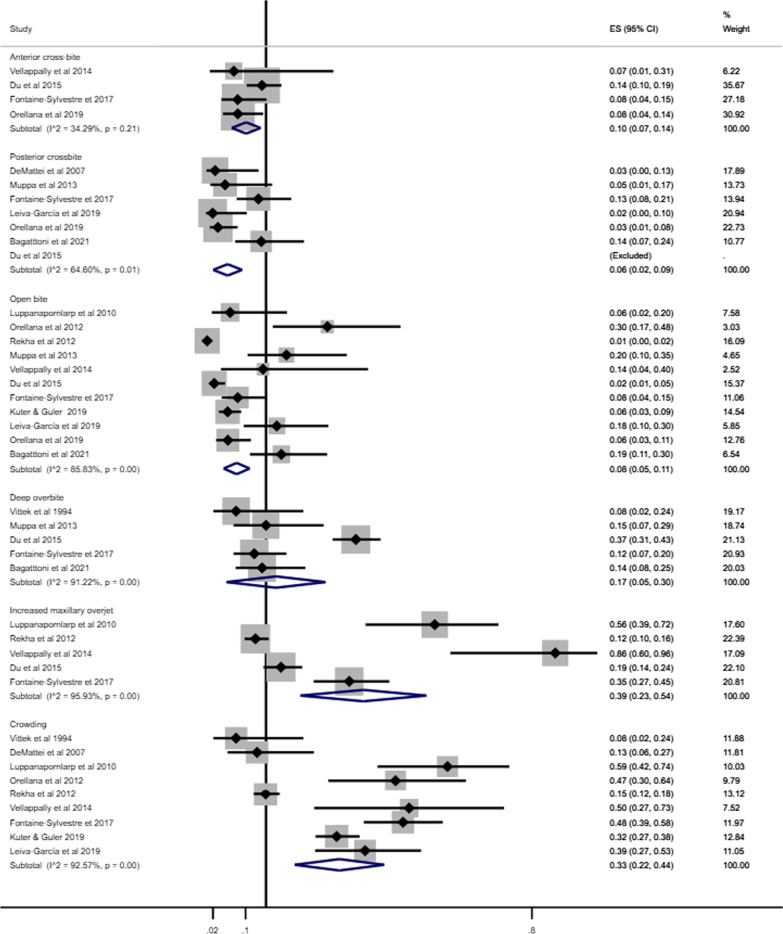
Fig. 3.
Forest-plot for prevalence of malocclusion characteristics among individuals with ASD
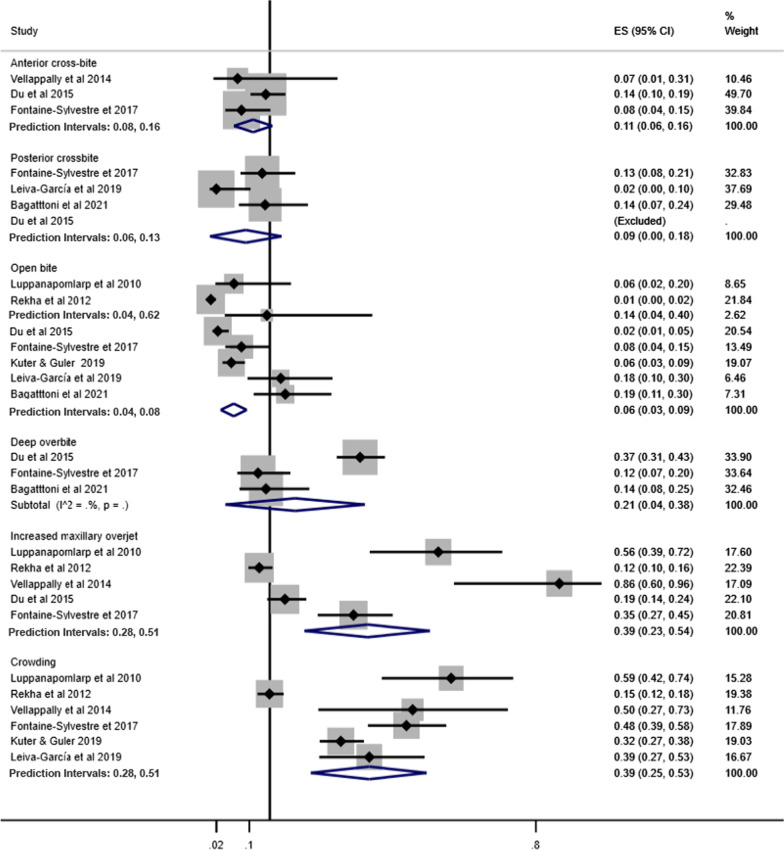
Increased maxillary overjet (39%, 95% CI 23%–54%) and crowding (33%, 95% CI 23%–44%) were the most prevalent malocclusion characteristics in individuals with ASD. The least common malocclusion conditions were posterior crossbite (6%, 95% CI 2%–9%) and open bite (8%, 95% CI 6%–11%) (Fig. 3).
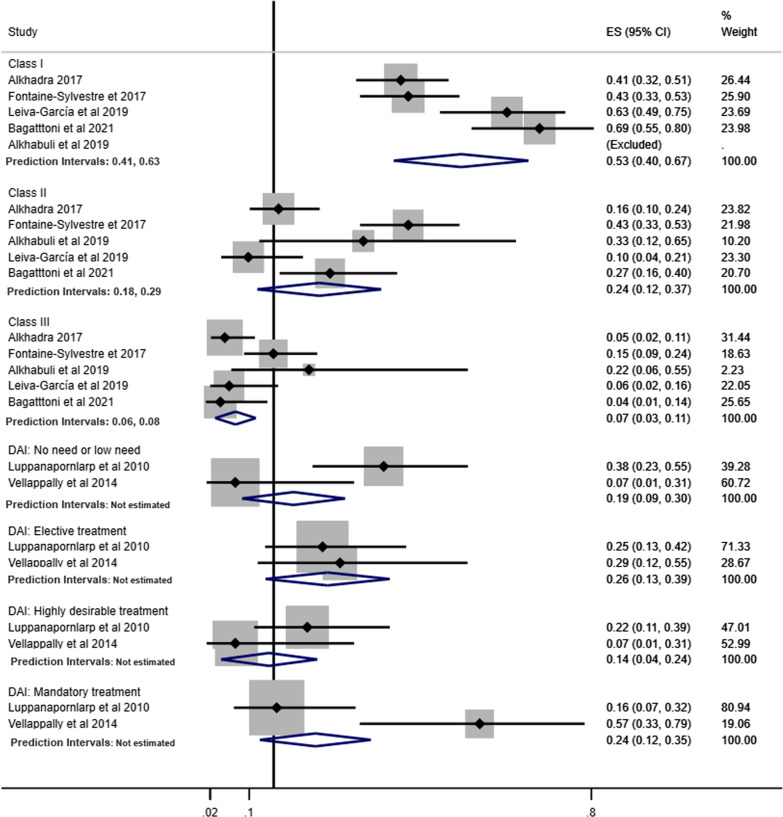
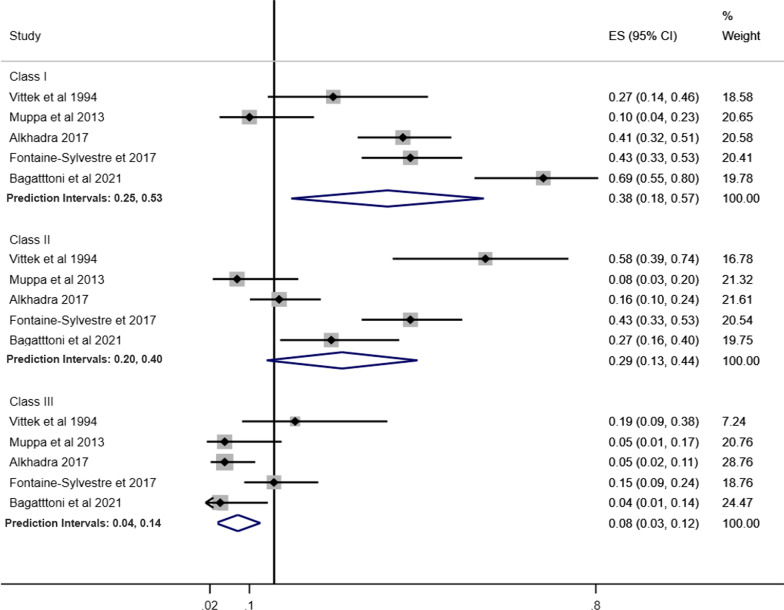
Pooled prevalence of Angle’s Class was estimated according to children and adolescents (Fig. 4), history of orthodontic treatment (Fig. 5) and studies that excluded or provided information about other syndromes and medical conditions (Fig. 6). The pooled estimates of DAI categories were obtained from two studies including only children and adolescents and participants without previous orthodontic treatment.
Fig. 4.
Prevalence of Angle’s Class and DAI in children and adolescents with ASD
Fig. 5.
Prevalence of Angle’s Class and DAI in individuals with ASD without history of orthodontic treatment
Fig. 6.
Prevalence of Angle’s Class and DAI in individuals with ASD involving studies that excluded or provided information about other syndromes and medical conditions
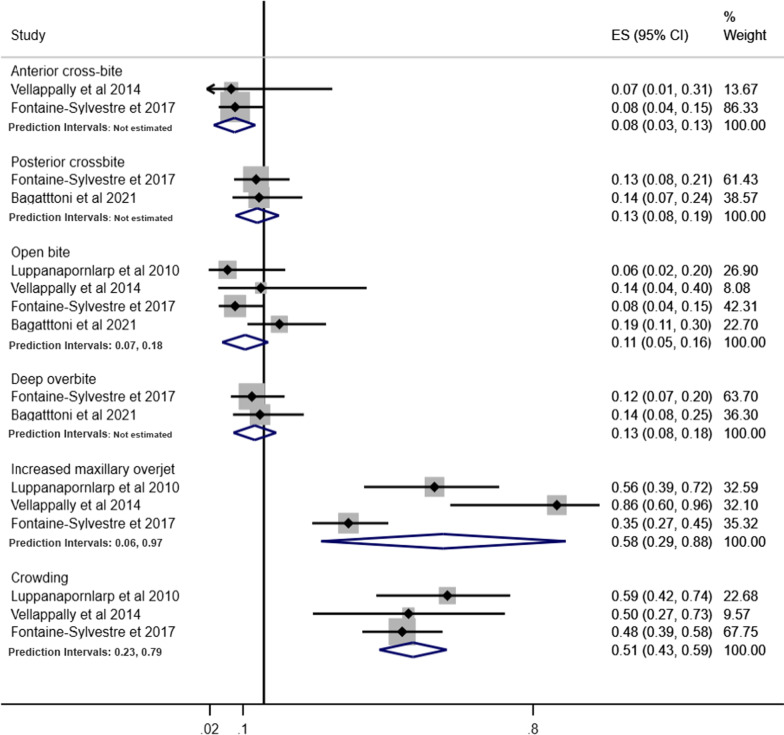
Pooled prevalence of malocclusion characteristics according to children and adolescents, history of orthodontic treatment and studies that excluded or provided information about other syndromes and medical conditions are presented in Figs. 7, 8 and 9, respectively.
Fig. 7.
Prevalence of malocclusion characteristics in children and adolescents with ASD
Fig. 8.
Prevalence of malocclusion characteristics in individuals with ASD without history of orthodontic treatment
Fig. 9.
Prevalence of malocclusion characteristics in individuals with ASD involving studies that excluded or provided information about other syndromes and medical conditions
The pooled prevalence of malocclusion characteristics including all studies tended to be lower than the subgroup analyses. For instance, the pooled prevalence of posterior cross-bite including all studies was 6%, while in the subgroup analyses, prevalence estimates were children and adolescents (9%), history of orthodontic treatment (13%) and studies that excluded or provided information about other syndromes and medical conditions (10%). Similarly, the pooled prevalence of increased maxillary overjet for all studies, and the subgroup analyses of studies that excluded previous orthodontic treatment and studies that excluded or provided information about other syndromes and medical conditions were 39%, 58% and 46%, respectively.
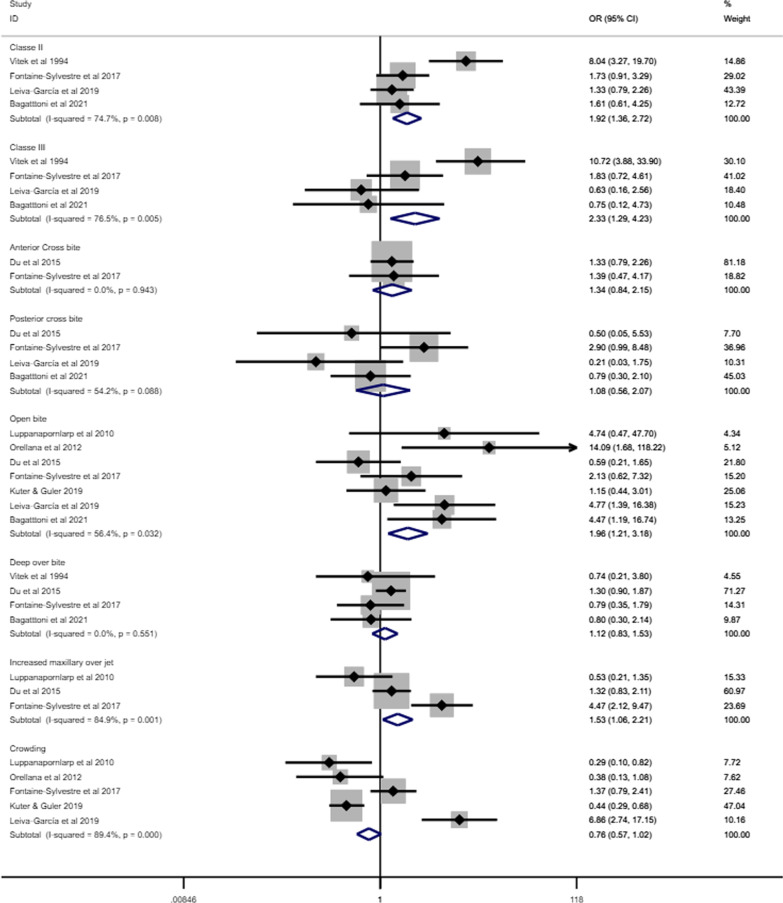
Association between ASD and malocclusion
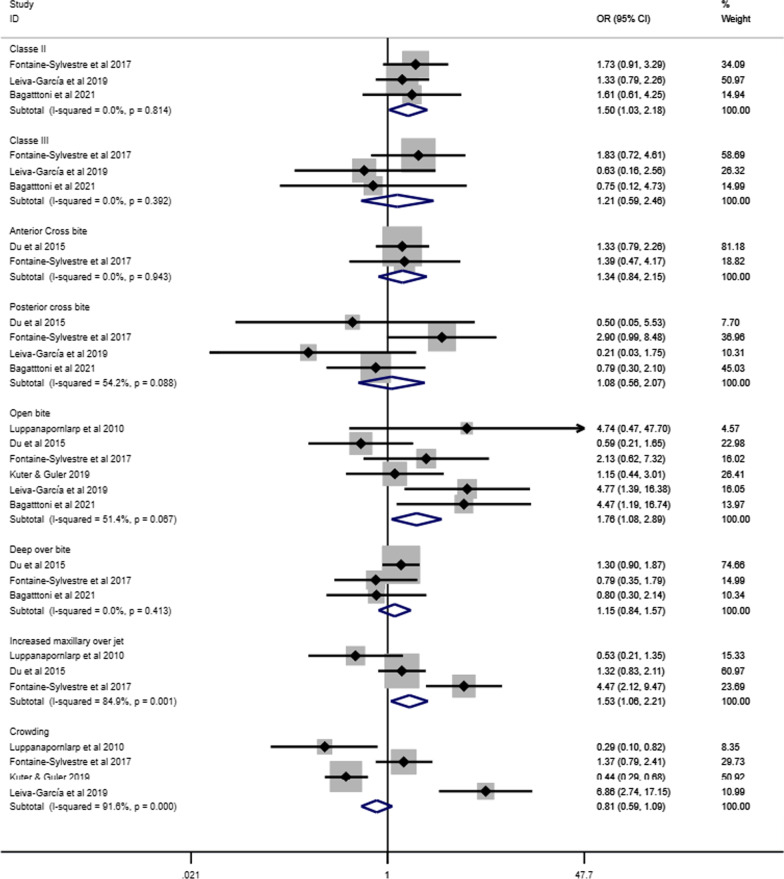
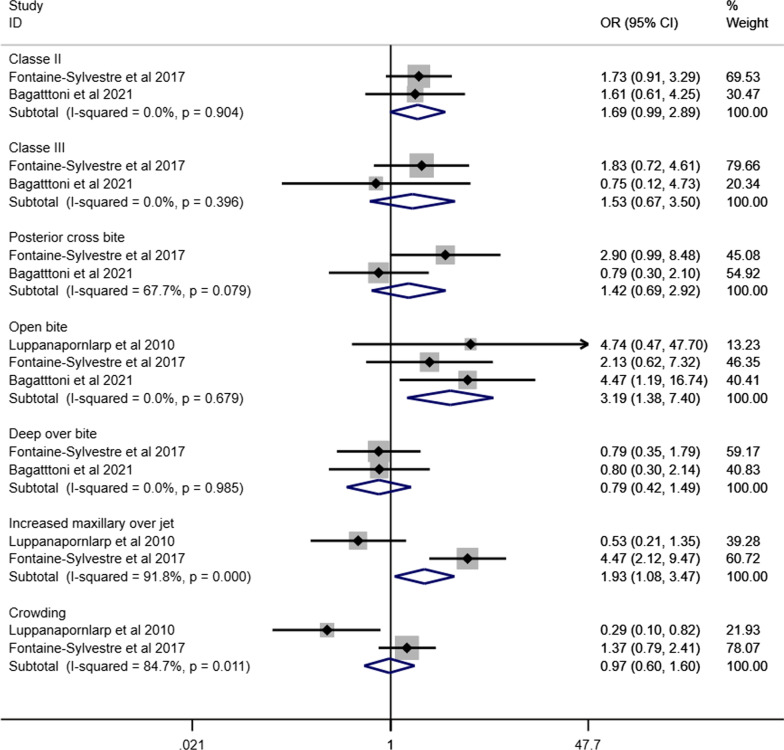
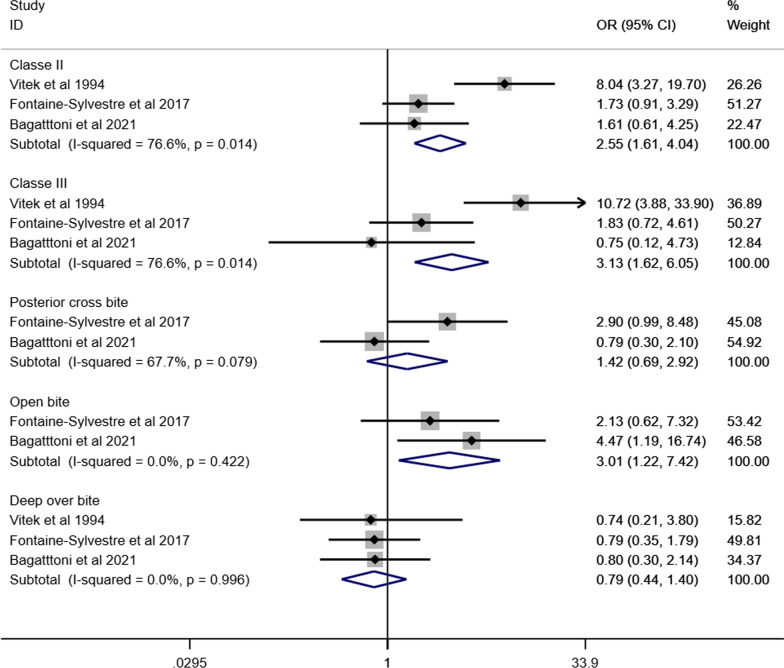
Figure 10 presents the forest plot of the meta-analyses assessing the association between different malocclusion characteristics and ASD based on data extracted from eight articles involving 848 individuals with ASD and 9554 individuals without ASD [35, 38, 40, 44, 46, 48, 49, 52]. Individuals with ASD had significantly higher odds of Angle’s class II (OR 1.92, 95% CI 1.36–2.72), Angle’s Class III (OR 2.33, 95% CI 1.29–4.23) and open bite (OR 1.96, 95% CI 1.21–3.18) than those without ASD. The odds of having increased maxillary overjet were 53% higher for individuals with ASD than those without ASD (OR 1.53, 95% IC: 1.06–2.21). Heterogeneity was observed in Angle’s Class II (I2 = 75%), Angle’s Class III (I2 = 77%), open bite (I2 = 56%), increased maxillary overjet (I2 = 85%), and crowding (I2 = 89%). The association between ASD and malocclusion characteristics in subgroup analyses is presented according to children and adolescents (Fig. 11), history of orthodontic treatment (Fig. 12) and studies that excluded or provided information about other syndromes and medical conditions (Fig. 13). The association of ASD with Angle’s Class II and Angle’s Class III was not significant when pooling data from studies excluding participants with previous orthodontic treatment.
Fig. 10.
Forest-plot for association between ASD and malocclusion
Fig. 11.
Association between ASD and malocclusion in children and adolescents with ASD
Fig. 12.
Association between ASD and malocclusion in individuals without history of orthodontic treatment
Fig. 13.
Association between ASD and malocclusion involving studies that excluded or provided information about other syndromes and medical conditions
Discussion
The present systematic review and meta-analysis was conducted to examine the following research questions: (i) what are the malocclusion characteristics and the most common occlusal disorders of individuals with ASD? and (ii) do individuals with ASD have a greater likelihood of malocclusion than those without ASD? It was hypothesised that individuals with ASD had more severe occlusal deviations than those without ASD. Overall, 18 primary studies addressing these research questions were identified. These studies used six different clinical occlusal measures and two malocclusion classification systems. According to the first research question, our findings demonstrated that occlusal deviation in individuals with ASD was represented by horizontal occlusal disorders and reduced spacing, including increased maxillary overjet and crowding. Vertical and transversal occlusal problems, represented by a posterior crossbite and open bite, were less commonly found in these individuals, although the likelihood of an open bite among individuals with ASD was significantly higher than among individuals without ASD. The occurrence of Angle’s Class II was more than three times higher than Angle’s Class III in individuals with ASD. In addition, 38% of individuals with ASD were classified as highly desirable and in need of treatment for malocclusion according to DAI [53]. The second research question and the study’s hypothesis were confirmed into some extent. Individuals with ASD had higher odds of Angle’s Class II, Angle’s Class III, open bite, and increased maxillary overjet than individuals without ASD. The remaining four malocclusion characteristics investigated were not associated with ASD.
There is a dearth of systematic reviews aiming to characterize the characteristics and prevalence of malocclusion in individuals with ASD as well as investigating the relationship between malocclusion and ASD. Most of the previous review papers on oral health status and ASD have assessed dental caries and periodontal disease. The only previous review on this topic indicated a prevalence of malocclusion in children with and without ASD of 60% and 40%, respectively. However, these figures did not differ statistically [9].
According to our findings, individuals with ASD are at higher risk of malocclusion. It could be argued that the influence of ASD on malocclusion might be explained by behavioural factors [26, 54]. For example, children diagnosed with ASD had lower breastfeeding rates, were weaned earlier, had a preference for liquid foods and transitioned later to solid foods [54]. The lack of adequate dietary masticatory stimulation during development directly influences human craniofacial growth and consequently may predispose the occurrence of occlusal deviations [55]. Moreover, individuals with ASD had higher rates of persistent parafunctional habits, including mouth breathing and biting objects than those without ASD [26]. Mouth breathing, for instance, is closely associated with an open bite. Mouth breathers may also exhibit vestibular inclination of the upper incisors and clockwise rotation of the mandible, contributing, in part, to the increased maxillary overjet [56, 57] and may also exhibit deformity of the dental arches, which may lead to tooth-size/arch-length discrepancy and space problems [58, 59]. Finally, the challenges involved in the management of individuals with ASD in the dental setting, may lead to the late diagnosis of malocclusions and preclude the early treatment of any occlusal alteration [60].
A relevant aspect of the present meta-analysis worth mentioning is the use of different malocclusion measures, which considered distinct transversal, horizontal and vertical occlusal deviations. Thus, data were combined according to type of malocclusion, enabling identification of the prevalence of different occlusal problems as well as malocclusions associated with ASD. The use of a random effects model in meta-analyses of observational studies is considered a valid strategy to account for some of the between-study variation. Heterogeneity was observed in some of the meta-analyses in terms of prevalence of malocclusion and on the association between ASD and malocclusion notwithstanding. This might be considered an expected finding since all studies included in this review were cross-sectional designs, with frequent methodological discrepancies.
The meta-analyses on the link between ASD and malocclusion characteristics only included eight studies. The paucity of primary studies assessing the influence of ASD on malocclusion characteristics may have affected the statistical power of the quantitative synthesis, particularly in the subgroup analyses of studies including only children and adolescents, studies excluding individuals with history of orthodontic treatment, and studies that excluded or provided information about other syndromes and medical conditions. The need for further studies is paramount to ascertain the role of ASD on malocclusion. However, the use of robust methodology in future research is essential to reach valid conclusions. One suggestion is that future studies evaluating the possible influence of ASD on malocclusion should use representative samples of individuals with ASD, select an adequate group for comparison, and assess potential confounding factors, including previous orthodontic treatment, other syndromes, parafunctional habits, and history of feeding habits.
The monitoring of preventive and risk factors for malocclusion as well as orthodontic treatment should be carried out by multidisciplinary health teams, including orthodontists, paediatric dentists, paediatricians, occupational therapists, speech and language therapists and psychologists [60]. Multidisciplinary approaches could also enhance oral health related quality of life along with the functional aspects of oral health. The difficulties and barriers to accessing specialized dental care, including orthodontic care, among individuals with ASD in most countries reinforces the importance of early diagnosis of malocclusion for children diagnosed with ASD. Moreover, the benefits of orthodontic treatment on masticatory and speech function, orofacial musculature as well as quality of life supports the development of orthodontic therapies for individuals with ASD [32].
There are methodological limitations of this systematic review. First, all included studies are cross-sectional which imposes important constraints because they do not infer cause and effect and are only a snapshot in time. Although this might not be considered a meaningful problem since ASD (the exposure) is an innate exposure and malocclusion can only be observed after the first years of life, most research on this topic adopted an exploratory approach. Therefore, testing the association between ASD and malocclusion was limited in most studies due to lack of appropriate comparison groups and an insufficient analytical approach.
Second, only five primary studies included in this systematic review and meta-analysis investigated whether individuals with and without ASD were already treated for occlusal deviations. The remaining studies did not inform whether or not individuals received orthodontic treatment, if the malocclusion was corrected among those who were treated for malocclusion. Eight studies recorded the occurrence of other syndromes and medical conditions, but failed to discuss any relationship. Moreover, age group was a selection criterion in only six studies. This may suggest the confounding effect of previous orthodontic treatment and other factors in studies reporting the association between ASD and malocclusion. No manuscripts conducted power calculations to estimate the sample size, leaving the studies subject to type I and type II errors. This means that the magnitude of any significant difference and precision and variance within the samples is unclear.
Third, there was no information and discussion around syndromes that may be associated with ASD, the level of commitment of the individual’s autistic spectrum, dietary patterns and tooth loss of individuals with ASD, as well as the facial profile and malocclusion of their parents and genetic influences. Most studies included in this review addressed ASD as a homogenous condition, failing to report the interplay of associated syndromes, the level of commitment of the individual’s autistic spectrum, behavioural mechanisms (eg. use of bottle feeding) and parental factors which may exert an effect on facial and skeletal morphology and increase the prevalence of malocclusion. A more nuanced approach, distinguishing between essential autism and complex (syndromic) autism, across different degrees of the ASD spectrum for individuals, could be a potential starting point for future research. Furthermore, behavioural factors and parental characteristics related to malocclusion should be collected in the forthcoming studies.
Fourth, according to the eligibility criteria for the present study there were no restrictions regarding participant age limits. This approach was adopted to identify and include all relevant publications on this topic. Although published studies involved mostly children and adolescents, at least five studies included adults. Conducting a sub-group analysis for adults was not possible due to the limited number of included studies.
Finally, 13 of the 18 studies included were classified as having critical risk or serious risk of bias due to the limitations, along with other methodological flaws. Additionally, primary studies did not consider the ASD spectrum when reporting malocclusion characteristics in individuals with ASD. Thus, future studies should acknowledge and overcome the methodological limitations highlighted in this systematic review and meta-analysis consider the wide spectrum of ASD.
Our study has demonstrated the prevalence of different malocclusion characteristics in individuals with ASD varied meaningfully according to different malocclusion measures. Angle’s Class II, DAI elective treatment need, DAI mandatory need treatment, increased maxillary overjet and crowding were the most common occlusal deviations. The present findings also provide evidence to support specific occlusal deviations, including Angle’s Class II, Angle’s Class III, open bite, and increased maxillary overjet were more prevalent among individuals with ASD than those without ASD. Early diagnosis of malocclusion may assist in prompt intervention and improvement of the oral health of people with ASD across the life course.
Acknowledgements
Not applicable.
Author contributions
T.P.M, J.O., F.V.F. and M.V.V. conceptualized the study; T.P.M, J.O., L.G.A., S.A.T.D., F.V.F. and M.V.V. elaborated the methods of the study; T.P.M., L.G.A., S.A.T.D. and M.V.V. extracted and analysed the data; T.P.M, J.O., L.G.A., S.A.T.D., F.V.F. and M.V.V. wrote the first draft of the manuscript; T.P.M, J.O., L.G.A., S.A.T.D., F.V.F. and M.V.V. revised and prepared the final version for submission. All authors have read and agreed to the published version of the manuscript.
Funding
There is no funding related to the present study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thiago Peixoto da Motta, Email: thiagopmotta@hotmail.com.
Janine Owens, Email: janine.owens@manchester.ac.uk.
Lucas Guimarães Abreu, Email: lucasgabreu01@gmail.com.
Suélen Alves Teixeira Debossan, Email: su.alvesteixeira@gmail.com.
Fabiana Vargas-Ferreira, Email: fabivfer@gmail.com.
Mario Vianna Vettore, Email: mario.vettore@uia.no.
References
- 1.Valicenti-Mcdermott M, Hottinger K, Seijo R, Shulman L. Age at diagnosis of autism spectrum disorders. J Pediatr. 2012;161:554–556. doi: 10.1016/j.jpeds.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Dutra SR, Pretti H, Martins MT, Bendo CB, Vale MP. Impact of malocclusion on the quality of life of children aged 8 to 10 years. Dental Press J Orthod. 2018;23:46–53. doi: 10.1590/2177-6709.23.2.046-053.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76:1275–1297. doi: 10.1007/s00018-018-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahvik-Planefeld C, Herrström P. Dental care of autistic children within the non-specialized Public Dental Service. Swed Dent J. 2001;25:113–118. [PubMed] [Google Scholar]
- 5.Osgood T. Supporting positive behaviour in intellectual disabilities and autism: practical strategies for addressing challenging behaviour. London: Jessica Kingsley Publications; 2019. [Google Scholar]
- 6.Nakao S, Scott JAM, Masterson EE, Chi DL. Non-traumatic dental condition-related emergency department visits and associated costs for children and adults with autism spectrum disorders. J Autism Dev Disord. 2015;45:1396–1407. doi: 10.1007/s10803-014-2298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasari C, Brady N, Lord C, Tager-Flusberg H. Assessing the minimally verbal school-aged child with autism spectrum disorder. Autism Res. 2013;6:479–493. doi: 10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5ª ed. Arlington: American Psychiatric Association; 2013.
- 9.Bartolomé-Villar B, Rosa Mourelle-Martínez M, Diéguez-Pérez M, de Nova-García M-J. Incidence of oral health in paediatric patients with disabilities: sensory disorders and autism spectrum disorder. Systematic review II. J Clin Exp Dent. 2016;8:344–51. [DOI] [PMC free article] [PubMed]
- 10.Benevides TW, Shore SM, Andresen ML, Caplan R, Cook B, Gassner DL, et al. Interventions to address health outcomes among autistic adults: a systematic review. Autism. 2020;24:1345–1359. doi: 10.1177/1362361320913664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, et al. The health status of adults on the autism spectrum. Autism. 2015;19:814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- 12.Qiao Y, Shi H, Wang H, Wang M, Chen F. Oral health status of Chinese children with autism spectrum disorders. Front Psychiatry. 2020;11:398. doi: 10.3389/fpsyt.2020.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimland B, Baker SM. Brief report: Alternative approaches to the development of effective treatments for autism. J Autism Dev Disord. 1996;26:237–241. doi: 10.1007/BF02172019. [DOI] [PubMed] [Google Scholar]
- 14.Klein U. Characteristics of patients with Autistic Disorder (AD) presenting for dental treatment: a survey and chart review. Spec Care Dent. 1999;19:200–207. doi: 10.1111/j.1754-4505.1999.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 15.Naidoo M, Singh S. The Oral health status of children with autism Spectrum disorder in KwaZulu-Nata. South Africa BMC Oral Health. 2018;18:1–9. doi: 10.1186/s12903-017-0444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerbo O, Qian Y, Ray T, Sidney S, Rich S, Massolo M, et al. Health care service utilization and cost among adults with autism spectrum disorders in a U.S. integrated health care system. Autism Adulthood. 2019;1:27–36. doi: 10.1089/aut.2018.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montaldo L, Montaldo P, Cuccaro P, Caramico N, Minervini G. Effects of feeding on non-nutritive sucking habits and implications on occlusion in mixed dentition. Int J Paediatr Dent. 2011;21:68–73. doi: 10.1111/j.1365-263X.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 18.Alhammadi MS, Halboub E, Fayed MS, Labib A, El-Saaidi C. Global distribution of malocclusion traits: a systematic review. Dental Press J Orthod. 2018;23:e1–10. doi: 10.1590/2177-6709.23.6.40.e1-10.onl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kragt L, Dhamo B, Wolvius EB, Ongkosuwito EM. The impact of malocclusions on oral health-related quality of life in children—a systematic review and meta-analysis. Clin Oral Investig. 2016;20:1881–1894. doi: 10.1007/s00784-015-1681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozgen H, Hellemann GS, Stellato RK, Lahuis B, van Daalen E, Staal WG, et al. Morphological features in children with autism spectrum disorders: a matched case-control study. J Autism Dev Disord. 2011;41:23–31. doi: 10.1007/s10803-010-1018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shellart WC, Casamassimo PS, Hagerman RJ, Belanger GK. Oral findings in fragile X syndrome. Am J Med Genet. 1986;23:179–187. doi: 10.1002/ajmg.1320230112. [DOI] [PubMed] [Google Scholar]
- 22.Rosti RO, Sadek AA, Vaux KK, Gleeson JG. The genetic Landscape of autism spectrum disorders. Dev Med Child Neurol. 2014;56:12–18. doi: 10.1111/dmcn.12278. [DOI] [PubMed] [Google Scholar]
- 23.Young DJ, Bebbington A, Anderson A, Ravine D, Ellaway C, Kulkami A, et al. The diagnosis of autism in a female: could it be Rett syndrome? Eur J Pediatr. 2008;167:661–669. doi: 10.1007/s00431-007-0569-x. [DOI] [PubMed] [Google Scholar]
- 24.Mahdi SS, Jafri HA, Allana R, Amenta F, Khawaja M, Qasim SSB. Oral manifestations of Rett syndrome—a systematic review. Int J Environ Res Public Health. 2021;18:1162. doi: 10.3390/ijerph18031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolevzon A, Angarita B, Bush L, Wang AT, Frank Y, Yang A, et al. Phelan-McDermid syndrome: a review of the literature and practice parameters for medical assessment and monitoring. J Neurodevelop Disord. 2014;6:39. doi: 10.1186/1866-1955-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Sehaibany FS. Occurrence of oral habits among preschool children with autism spectrum disorder. Pakistan J Med Sci. 2017;33:1156–1160. doi: 10.12669/pjms.335.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O’Connell DA, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 14, 2021.
- 28.Morgan RL, Thayer KA, Santesso N, Holloway AC, Blain R, Eftim SE, et al. A risk of bias instrument for non-randomized studies of exposures: a users’ guide to its application in the context of GRADE. Environ Int. 2019;122:168–184. doi: 10.1016/j.envint.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proffit W, Fields HW, Sarver DM. Contemporary orthodontics. 5. St. Louis: Mosby; 2012. [Google Scholar]
- 30.Lipsey M, Wilson D. Practical meta-analysis—IDoStatistics. Thousand Oaks: US Sage Publications; 2001. [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javidi H, Vettore M, Benson PE. Does orthodontic treatment before the age of 18 years improve oral health-related quality of life? A systematic review and meta-analysis. Am J Orthod Dentofac Orthop. 2017;151:644–655. doi: 10.1016/j.ajodo.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. R Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BM J. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 35.Vittek J, Winik S, Winik A, Sioris C, Tarangelo AM, Chou M. Analysis of orthodontic anomalies in mentally retarded developmentally disabled (MRDD) persons. Spec Care Dentist. 1994;14:198–202. doi: 10.1111/j.1754-4505.1994.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 36.Manzano AP, Salazar CR, Manzano MA. Patología Bucal prevalente en niños excepcionales. Acta Odontol Venez. 1999;37:193–198. [Google Scholar]
- 37.DeMattei R, Cuvo A, Maurizio S. Oral assessment of children with an autism spectrum disorder. J Dent Hyg. 2007;81:65. [PubMed] [Google Scholar]
- 38.Luppanapornlarp S, Leelataweewud P, Putongkam P, Ketanont S. Periodontal status and orthodontic treatment need of autistic children. World J Orthod. 2010;11:256–261. [PubMed] [Google Scholar]
- 39.Soni S, Aggarwal P, Dua V. The use of Index of Orthodontic Treatment Needs (IOTN) in children with special need. Int J Contemp Dent. 2011;2:72–79. [Google Scholar]
- 40.Orellana L-M, Silvestre F-J, Martínez-Sanchis S, Martínez-Mihi V, Bautista D. Oral manifestations in a group of adults with autism spectrum disorder. Med Oral Patol Oral Cir Bucal. 2012;17:415–424. doi: 10.4317/medoral.17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rekha CV, Aranganna P, Shahed H. Oral health status of children with autistic disorder in Chennai. Eur Arch Paediatr Dent. 2012;13:126–131. doi: 10.1007/BF03262858. [DOI] [PubMed] [Google Scholar]
- 42.Muppa R, Bhupathiraju P, Duddu MK, Dandempally A, Karre DL. Prevalence and determinant factors of malocclusion in population with special needs in South India. J Indian Soc Pedod Prev Dent. 2013;31:87–90. doi: 10.4103/0970-4388.115701. [DOI] [PubMed] [Google Scholar]
- 43.Vellappally S, Gardens SJ, Al Kheraif AAA, Krishna M, Babu S, Hashem M, et al. The prevalence of malocclusion and its association with dental caries among 12–18-year-old disabled adolescents. BMC Oral Health. 2014;14:123. doi: 10.1186/1472-6831-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du RY, Yiu CKY, King NM, Wong VCN, McGrath CPJ. Oral health among preschool children with autism spectrum disorders: A case-control study. Autism. 2015;19(6):746–751. doi: 10.1177/1362361314553439. [DOI] [PubMed] [Google Scholar]
- 45.Alkhadra T. Characteristic of malocclusion among Saudi special need group children. J Contemp Dent Pract. 2017;18:959–963. doi: 10.5005/jp-journals-10024-2156. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine-Sylvestre C, Roy A, Rizkallah J, Dabbagh B, Ferraz dos Santos B. Prevalence of malocclusion in Canadian children with autism spectrum disorder. Am J Orthod Dentofac Orthop. 2017;152:38–41. doi: 10.1016/j.ajodo.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Alkhabuli JOS, Essa EZ, Al-Zuhair AM, Jaber AA. Oral health status and treatment needs for children with special needs: a cross-sectional study. Pesqui Bras Odontopediatria Clin Integr. 2019;19:e4877. [Google Scholar]
- 48.Kuter B, Guler N. Caries experience, oral disorders, oral hygiene practices and sociodemographic characteristics of autistic children. Eur J Paediatr Dent. 2019;20(3):237–241. doi: 10.23804/ejpd.2019.20.03.13. [DOI] [PubMed] [Google Scholar]
- 49.Leiva-García B, Planells E, Planells del Pozo P, Molina-López J. Association Between Feeding Problems and Oral Health Status in Children with Autism Spectrum Disorder. J Autism Dev Disord. 2019;49:4997–5008. [DOI] [PubMed]
- 50.Orellana LM, Cantero-Fuentealba C, Schmidlin-Espinoza L, Luengo L. Oral health, hygiene practices and oral habits of people with autism spectrum disorder. Rev Cubana Estomatol. 2019;56:1–13. doi: 10.4317/medoral.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mangione F, Bdeoui F, Monnier-Da Costa A, Dursun E. Autistic patients: a retrospective study on their dental needs and the behavioural approach. Clin Oral Investig. 2020;24:1677–1685. doi: 10.1007/s00784-019-03023-7. [DOI] [PubMed] [Google Scholar]
- 52.Bagattoni S, Lardani L, D’Alessandro G, Piana G. Oral health status of Italian children with Autism Spectrum Disorder. Eur J Paediatr Dent. 2021;22:243–247. doi: 10.23804/ejpd.2021.22.03.12. [DOI] [PubMed] [Google Scholar]
- 53.Cons NC, Jenny J, Kohout FJ. DAI: the dental aesthetic index. Iowa City: University of Iowa College of Dentistry; 1986. [Google Scholar]
- 54.Lemcke S, Parner ET, Bjerrum M, Thomsen PERH, Lauritsen MB. Early regulation in children who are later diagnosed with autism spectrum disorder: a longitudinal study within the danish national birth cohort. Infant Ment Health J. 2018;39:170–182. doi: 10.1002/imhj.21701. [DOI] [PubMed] [Google Scholar]
- 55.Ankum AMV. Changes in dietary consistency and the epidemiological occlusal transition. Compass. 2018;2:1–17. doi: 10.29173/comp51. [DOI] [Google Scholar]
- 56.Farronato M, Lanteri V, Fama A, Maspero C. Correlation between malocclusion and allergic rhinitis in pediatric patients: a systematic review. Children (Basel) 2020;7:260. doi: 10.3390/children7120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Z, Zheng L, Huang X, Li C, Liu J, Hu Y. Effects of mouth breathing on facial skeletal development in children: a systematic review and meta-analysis. BMC Oral Health. 2021;21:108. doi: 10.1186/s12903-021-01458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNamara JA. Influence of respiratory pattern on craniofacial growth. Angle Orthod. 1981;51:269–300. doi: 10.1043/0003-3219(1981)051<0269:IORPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Tervahauta E, Nokela J, Vuollo V, Pirttiniemi P, Silvola AS. Associations of sagittal malocclusions with dental arch characteristics and crowding in Northern Finland Birth Cohort 1966. Orthod Craniofac Res. 2021. [DOI] [PubMed]
- 60.Koskela A, Neittaanmäki A, Rönnberg K, Palotie A, Ripatti S, Palotie T. The relation of severe malocclusion to patients’ mental and behavioral disorders, growth, and speech problems. Eur J Orthod. 2021;43:159–164. doi: 10.1093/ejo/cjaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.