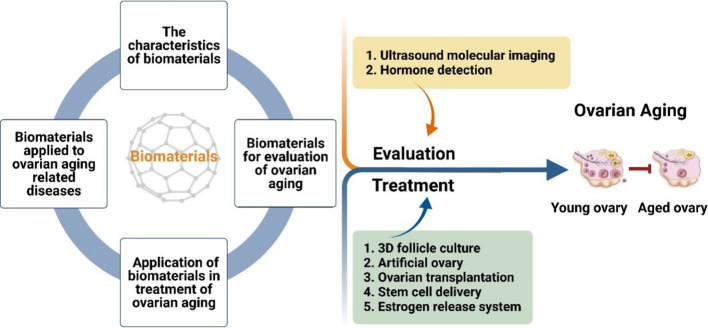
Abstract
Ovarian aging is characterized by a progressive decline in ovarian function. With the increase in life expectancy worldwide, ovarian aging has gradually become a key health problem among women. Over the years, various strategies have been developed to preserve fertility in women, while there are currently no clinical treatments to delay ovarian aging. Recently, advances in biomaterials and technologies, such as three-dimensional (3D) printing and microfluidics for the encapsulation of follicles and nanoparticles as delivery systems for drugs, have shown potential to be translational strategies for ovarian aging. This review introduces the research progress on the mechanisms underlying ovarian aging, and summarizes the current state of biomaterials in the evaluation and treatment of ovarian aging, including safety, potential applications, future directions and difficulties in translation.
Graphical Abstract
Keywords: Ovarian aging, Biomaterials, Evaluation, Treatment
Introduction
Ovarian aging is characterized by a progressive decline of ovarian function, manifested by a decrease in the quantity and quality of oocytes with advancing age. The ovary is one of the first organ systems to show hallmarks of aging, in comparison to other organs. Most countries show an increasing number of women’s first pregnancies at what is considered an advanced reproductive age (≥ 35 years). With advancing age, difficulty in conceiving and infertility increased. Similarly, oocytes derived from women of advanced age have higher chance of resulting in miscarriage, and/or aneuploid offspring [1]. The end point of ovarian aging is menopause, most women experience menopause around the age of 50 years [2]. The average life expectancy of women has increased to more than 78 years that means nearly a third of a woman's life will be spent after menopause, accompanied by hot flashes, night sweats, irritability, depression, and other menopausal syndrome. Importantly, ovarian aging drives the aging of multiple organs, which is considered as the pacemaker of female body aging [3]. Ovarian aging can lead to obesity, diabetes, Alzheimer's disease, urogenital atrophy, osteoporosis and fracture, cardiovascular disease, and an increased all-cause mortality, which seriously decrease the life quality of aged female [4, 5]. Therefore, the treatment strategies that can delay ovarian aging would improve fertility and health in females.
Over the last two decades, some therapeutic strategies to improve, reverse or slow ovarian aging have emerged. Hormone replacement therapy (HRT) is a universal treatment for ovarian aging, which could allow women to free themselves from the malediction of menopause and conserve their fertility [6]. However the use of HRT has been vigorously debated [7], previous studies revealed that HRT was associated with an increased risk of venous thromboembolism [8], cancer risk [9], and ischemic stroke [10]. In recent years, interest has rapidly grown in studies exploring the therapeutic potential of stem cells in ovarian aging. Different types of stem cells, including embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), stem cells from extraembryonic tissues, induced pluripotent stem cells (iPSCs) and ovarian stem cells [11], have therapeutic effects on ovarian damage. However, transplantation rejection, tumorigenicity, genetic instability and ethical issues with stem cells limited their use [12–14]. Furthermore, some other methods, such as mitochondrial therapy, antioxidants, epigenetic regulators, telomerase activators and traditional Chinese medicine, have been used to prevent ovarian aging, while clinical trials have not yet been conducted on most of these therapies. Therefore, advanced therapeutic strategies to delay, or partially reverse symptoms of ovarian aging are urgently needed.
Biomaterials have the advantages of promoting cell interactions, good passive and active targeting, good stability and biodegradability, high drug loading content and controlled drug release [15–20]. For decades, a large number of studies have focused on evaluating the potential of biomaterials for various applications including regenerative medicine and anti-aging. For example, in age-related macular degeneration (AMD), Suri et al. for the first time delivered chitosan modified poly (lactic-co-glycolic acid) (PLGA) nanoparticles containing sirolimus to the posterior segment of the eye via the subconjunctival route for the treatment of AMD in rat models, achieving slow degradation and the necessary long-term sustained drug release while minimizing systemic exposure [21]. In addition, in age-related brain diseases, Chang et al. constructed electrically magnetized gold nanoparticles (AuNPs) to improve cognitive function and memory consolidation by promoting adult hippocampal neurogenesis [22]. Based on the advantages and significant effects of biomaterials in the field of antiaging, the application potential and value of biomaterials for the management of ovarian aging have been gradually recognized by researchers.
In this review, we focus on the research progress on the potential mechanisms of ovarian aging and summarize the current state of biomaterials in the diagnosis and treatment of ovarian aging, including safety, potential applications, future directions and the difficulties in translation, which could help to provide support and guidance for future scientific research and clinical applications.
Ovarian aging
Factors of ovarian aging
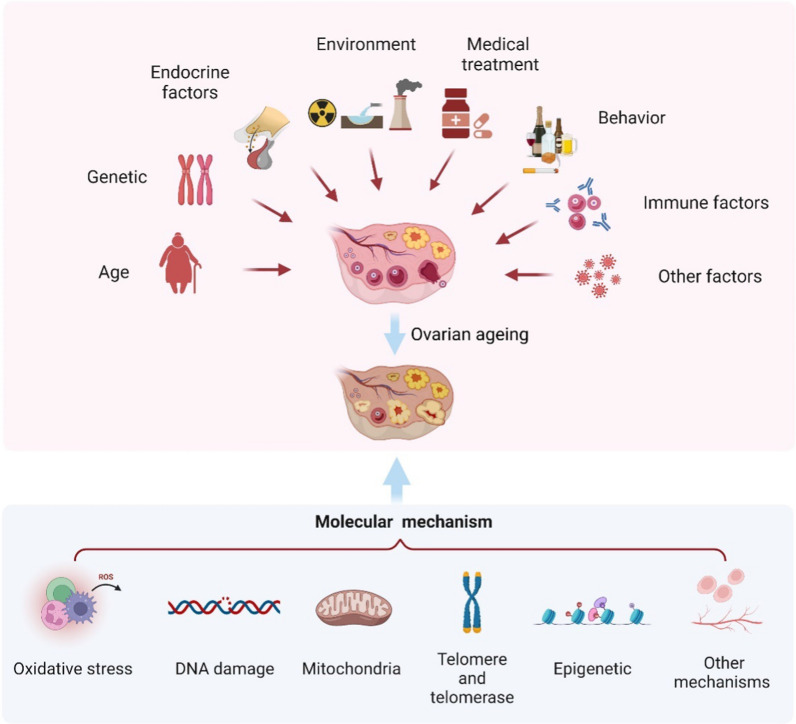
Ovarian aging is a complex process of multifactor and multilink interactions, and the etiology of ovarian aging has not yet been fully elucidated. The main factors of ovarian aging include age, genetics, the hypothalamus and pituitary glands, environment, medical treatments, behaviors, infection, immunity, the endocrine system, and social psychology (Fig. 1).
Fig. 1.
The factors and the molecular mechanisms of ovarian aging
Age
The number of follicles decreases with the increasing age. After 38 years of age, the number of follicles is rapidly consumed, and they number fewer than 1000 in the ovary at the time of menopause [23]. The same is observed for the quality of follicles. From the age of 38, the follicle quality declines rapidly, leading to greatly reduced pregnancy and live birth rates. Therefore, the number and quality of follicles are closely related to age, and age is one of the most important factors in ovarian aging.
Genetics
Genetic causes account for approximately 20% to 25% of patients with premature ovarian failure (POF). POF shows a high degree of heterogeneity in genetic variation, including abnormalities in chromosome number and structure, chromosome fragment abnormalities, and single-gene perturbations [24]. The mutated genes associated with ovarian aging are mainly related to the processes of oocyte meiosis, follicle development, hormone synthesis and secretion, DNA damage and repair, and mitochondrial function [25, 26]. However, the related genes known currently can only explain 15% of the genetic causes of ovarian aging [27]. Therefore, the application of clinical orientations for genetic testing is needed for the evaluation of ovarian aging.
Environment
A large number of epidemiological investigations have shown that environmental factors can adversely affect primordial follicle establishment, oocyte meiosis, follicle formation, steroid hormone synthesis and fertility, which are associated with decreased ovarian reserve [28]. For example, high concentrations of PM2.5 in the air, polycyclic aromatic hydrocarbons (PAHs) in cigarette smoke and automobile exhaust, heavy metals (lead, mercury, cadmium) in polluted water sources, pesticides remaining in fruits and vegetables, plastic components in packaging bags and other possible environmental factors can affect the reproductive health of female mammals, suggesting that such exposures can lead to premature ovarian aging (POA) in women [29]. However, more experimental investigations in humans are needed to identify their direct and indirect effects on the ovary function, and to characterize their mechanisms of action.
Medical treatment
In the process of clinical treatment, many medically related factors such as chemotherapy drugs, radiotherapy and surgical injury, can damage ovarian function. The adverse effects of chemotherapy, radiotherapy and surgery on ovarian function have long been recognized, and there have been increasingly detailed data documenting the effects on short-term markers of ovarian function, longer-term fertility and risk of early menopause [30–32]. The last decade has seen the development of a number of potential methods for protecting the ovaries against damage from chemotherapy or radiotherapy. However, most of that work has been performed using animal models, and it is worth exploring how to minimize the risk of ovarian damage with inevitable medical injury.
Behaviors
Poor living habits and behaviors also have adverse effects on ovarian function. A meta-analysis suggested that smoking is associated with a decreased age of menopause of 0.90 years (95% CI 1.58–0.21) [33]. Evidence on the impact of alcohol consumption on female fertility has been quite inconsistent, although the majority of studies have suggested that drinking alcohol damages to ovarian function [34]; nevertheless, moderate alcohol consumption might be unrelated to female fertility [35]. Moreover, both smoking and alcohol consumption might lead to epigenetic changes and DNA damage in germ cells, potentially resulting in inherited imprinting and genetic defects [36].
Endocrine factors
The endocrine system maintains and regulates various complicated vital life activities by secreting hormones. The ovary, together with two major neuroendocrine organs, the hypothalamus and the pituitary gland, constitutes the hypothalamic pituitary ovarian (HPO) axis, which is considered to be a classical circuit regulating the female reproductive endocrine system. Abnormal function and endocrine organ diseases, such as thyroid disease and diabetes, affect ovarian function via direct and indirect interactions with the HPO axis. Pooling the results of several studies that have investigated the prevalence of autoimmune thyroid disease (AITD) in women with infertility demonstrated a significantly increased incidence of AITD compared to controls, with an overall estimated relative risk of 2.1 (P < 0.0001) [37]. Hypothyroidism can impair pulsatile secretion of gonadotropin-releasing hormone (GnRH), resulting in ovulatory dysfunction and insufficient corpus luteum development [38]. In patients with diabetes mellitus, a hyperglycemic environment promotes neuronal apoptosis, leading to disordered HPO axis secretion [39]. Additionally, diabetes can also directly cause follicle dysfunction [40]. Although there have been many studies of the correlation between the endocrine system and ovarian aging, the underlying mechanism has yet to be fully elucidated.
Immune factors
It is well known that immune factors play a crucial role in ovarian aging. Studies have shown that autoimmune abnormalities account for 10% to 30% of premature ovarian insufficiency (POI), including anti-ovarian autoantibodies, immune oophoritis, thyroiditis and rheumatoid arthritis [41]. The most abundantly present types of innate immune cells in the ovaries are macrophages. Zhang et al. revealed a significantly M2 polarized and increasingly monocyte-derived macrophage population in the old ovary compared to that in the young ovary [42]. Intriguingly, M2 macrophages are known to deposit collagens in the extracellular matrix (ECM), in turn contributing to the development of fibrosis in the ovary during aging [43]. Furthermore, cytokines are the key substances that mediate immune biological processes. Mechanistically, there is evidence that cytokines can influence oocyte quality, ovarian reserve, ovarian steroid production, and the follicular microenvironment, thereby further contributing to ovarian aging [44–47]. For example, elevated levels of the proinflammatory cytokines interleukin-1 alpha (IL-1α), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) have been found in the serum of patients with POI [48]. Therefore, anti-immune inflammation aging therapies will become an important strategy in the prevention and treatment of ovarian aging.
Other factors
Infection is also one of the influencing factors of ovarian aging. Studies have demonstrated that bacterial or viral infection can lead to abnormal menstruation, decreased reproductive function, and even amenorrhea or POA [34].
The molecular mechanisms of ovarian aging
Ovarian aging is essentially a process of gradual depletion of the primordial follicle pool, influenced by the complex regulatory network inside and outside the body, such as DNA damage, epigenetic changes, free radical balance disorders, and abnormal mitochondrial function and so on (Fig. 1).
Oxidative stress
Free radicals play an indispensable role in the physiological changes in the ovaries, such as angiogenesis, sex hormone synthesis, ovulation, and formation and dissolution of the luteum [49]. Oxidative stress, caused by the imbalance between the production and destruction of reactive oxygen species (ROS), directly damages the intraovarian environment and many other cells. Excessive ROS induce apoptosis of granulosa cells (GC) and/or oocytes, leading to follicular atresia, directly or indirectly activating primordial follicles, and accelerating the decline of ovarian reserve function [50]. Some studies have shown an increase in ROS and a decrease in antioxidant levels in the oocytes, cumulus cells and follicular fluid of older women [51, 52]. Oxidative damage to the ovaries is generally caused by the propagation of lipid peroxidation cascades, which seriously influencing folliculogenesis, meiosis, and ovulation and eventually leading to ovarian aging.
DNA damage
Recent evidence has suggested that the DNA damage accumulates with age, possibly due to reduced DNA repair capacity with age in the oocytes of humans and mice [53]. Katherine et al. revealed that the genes related to the DNA damage response (DDR) process regulate ovarian reserve and its depletion rate and determine the age of natural menopause [54]. With the development of sequencing technology, an increasing number of candidate genes related to DNA damage and repair in ovarian aging have been found, including MCM8, MCM9, MEIOB, MND1, PSMC3IP, HFM1, and MSH5, which affect oogenesis mainly by regulating the process of homologous recombination in meiosis [55–57].
Mitochondria
As an important energy-supplying organelle, the mitochondria play a key role in the regulation of calcium homeostasis, oxidative phosphorylation, the cell cycle, senescence and apoptosis. As age-related alterations have been documented in mitochondrial function, the mitochondrial DNA mutation load and mitochondrial DNA copy numbers in mammalian oocytes have been investigated as potential biomarkers of oocyte quality [58]. Women undergoing in vitro fertilization (IVF) who are carriers of mitochondrial DNA mutations demonstrate decreased ovarian reserve based on lower anti-Müllerian hormone (AMH), lower antral follicle count (AFC), and a smaller number of oocytes retrieved than healthy volunteers [59]. Similarly, the mitochondrial DNA copy number is also lower in the unfertilized oocytes from women with infertility problems [60]. Therefore, mitochondria play a key role in ovarian aging.
Telomeres and telomerase
Telomeres and telomerase are closely related to aging and apoptosis. In recent years, studies have revealed that changes in telomere length and telomerase activity might be among the important mechanisms of ovarian aging [61]. It has been shown that oocytes in women with advanced age have shorter telomeres than young women, and this difference leads to a higher percentage of miscarriages or aneuploid embryos [62]. Similarly, women with a low pregnancy rate or POI showed shorter telomeres than healthy controls [62, 63]. Uysal et al. suggested that decreased telomerase reverse transcriptase (TERT) and telomere-binding protein expression might underlie the telomere shortening of ovaries with age, which could be associated with female fertility loss [64]. These data together argue that the telomere pathway is critical in ovarian aging.
Epigenetics
Epigenetics is considered to be an important cause of ovarian aging. It has been reported that common epigenetic modifications such as DNA methylation, ribonucleic acid (RNA) methylation, histone acetylation, phosphorylation and ubiquitin, could be involved in the occurrence and development of ovarian aging [65]. Kristina et al. reported differential methylation variability between diminished ovarian reserve (DOR) and normal ovarian function, indicating that the unstable methylome in granulosa cells can cause epigenetic dysfunction, resulting in poor ovarian reserve [66]. A study of histone modification showed that phosphorylation of histone H3 regulates the initiation of granulosa cell differentiation [67]. Currently, there are still few studies of epigenetic modification in ovarian aging, and some of the conclusions have been controversial. Therefore, future in-depth studies of epigenetics could have profound implications for ovarian aging.
Other mechanisms
The ovarian microenvironment is involved in follicular formation, development, maturation and ovulation [68], imbalances in which will lead to abnormal ovarian function. The accumulation of extracellular matrix, abnormalities in the vascular system, and the accumulation of senescent cells will lead to ovarian microenvironment disorder [69]. In addition, recent studies have demonstrated that there are oogonial stem cells in the ovaries [70], and the loss of stem cell function or instability of stem cell nests leads to ovarian imbalance, resulting in ovarian aging.
The characteristics of biomaterials
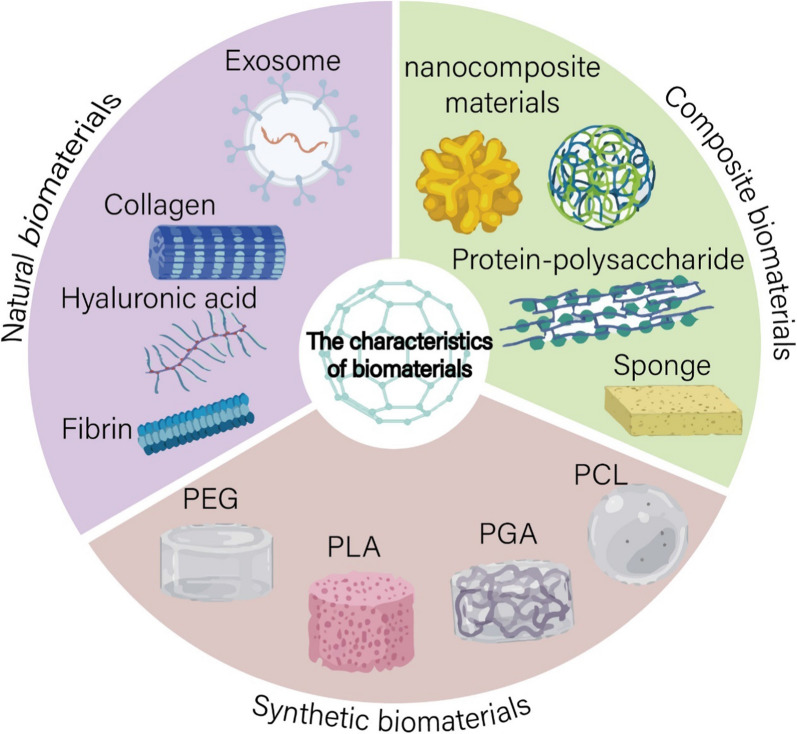
Biomaterials can be classified into three basic categories: natural biomaterials (including extracellular vesicle, collagen, hyaluronic acid, fibrin, etc.), synthetic biomaterials (including polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), polyethylene glycol (PEG), etc.) and composite biomaterials (including protein-polysaccharide composite biomaterials, nanocomposite biomaterials, sponges, etc.) (Fig. 2). They have been designed as theranostics showing unparalleled advantages, such as promoting favorable cellular interaction, relatively high drug loading content, controllable drug release, excellent passive and active targeting, good stability, biodegradability, biocompatibility and low toxicity [15, 16, 71, 72]. Some biomaterials have been utilized for reproductive tissue engineering and regenerative medicine [73, 74]. The characteristics of biomaterials are discussed below, with a focus on those that have been investigated for the tissue engineering.
Fig. 2.
The characteristics of biomaterials
Natural biomaterials
Natural biomaterials are well positioned to play a significant role in the development of the next generation of biomaterials for recovery of ovarian function. They are derived from naturally occurring substances and are easily available, have excellent biocompatibility and biodegradability and are well tolerated in vivo. Furthermore, natural polymers contain biomolecules that are natural to the cells, which can support and guide the cells to proliferate and differentiate at particular time interval and consequently can enhance the biological interaction with them [75]. Natural biomaterials can be divided into protein biomaterials (including fibrin, collagen), polysaccharide biomaterials (including hydrogel, alginate, and hyaluronic acid), and natural nanomaterials (extracellular vesicles, EVs). Protein-based biomaterials are flexible in structure and have good biocompatibility [76]. In addition, they have natural cell adhesion sites, making them ideal materials for biomaterials engineering [77]. Polysaccharide biomaterials that can be readily available from plants, animals, bacteria, etc. [78], are therefore inexpensive and non-toxic [79]. In addition, chitosan, sodium alginate and starch are natural sources of many non-conjugated luminescent polymers (NLP), which show great potential for future application in the design and development of luminescent drug carriers [80]. As a natural nanomaterial, EVs are a heterogeneous group of cell-derived membranous structures comprising exosomes and macrovesicles, which originate from the endosomal system or which are shed from the plasma membrane, respectively [81]. EVs perform an important role in cell-to-cell communication and are involved in multiple physiological and pathological processes, so they have good biocompatibility with immune system and low toxicity.
Synthetic biomaterials
With the further development of science and technology and economy, the application range of synthetic polymer materials is gradually expanding. Synthetic polyesters, including PLA, PGA, PCL and PEG approved by Food and Drug Administration (FDA) are the most widely studied biodegradable polymers in the reproductive tissue engineering and regenerative medicine field. The synthetic materials have the special properties of being able to cross biological barriers or passively target tissues [82] and avoid some drug delivery problems that could not be effectively solved in the past, including overcoming multidrug resistance and penetrating cell barriers that restrict drugs from reaching their intended targets [83]. Compared with natural biomaterials, the physical, chemical, mechanical and biological properties synthetic biomaterials can be modified to suit the needs of material design [84, 85]. In addition, their materials are more plentiful and therefore cheaper, making them cheaper to manufacture. For example, Obireddy et al. have used inexpensive 2-hydroxyethyl starch synthetic biomaterials to produce co-released particles for use in a variety of drugs [86]. However, although the biocompatibility and biodegradability of synthetic biomaterials are well established in almost all cases, have reached similar safety levels to natural compounds, they carry the risk of toxicity and immunogenicity to the host due to their significant difference to native tissue [84].
Composite biomaterials
The composite biomaterials definition is basically used to refer to new types of materials that are created by using two or more natural or synthetic polymers together. Nanocomposite materials can be categorized due to its polymer including such as polymer based and non-polymer based (inorganic) [87]. A variety of bioactive composites have been investigated over the last three decades as substitute materials for diseased or damaged tissues in the human body [88]. Proteins (including fibrin, collagen, and elastin) and polysaccharides (including chitosan, cellulose, and alginate) are widely used in composite biomaterials [89]. Proteins can provide better biocompatibility, and polysaccharide can provide further thermal stability and antibacterial properties. The combination of the two can be used as better biomaterials in the field of regenerative medicine [90].
Biomaterials for the evaluation of ovarian aging
Timely diagnosis of ovarian aging has become an urgent need to improve the quality of life of contemporary women. Bioinformatics involves the research, development, or application of computational tools and methods to obtain, store, visualize, and interpret medical or biological data [91, 92]. In addition, machine learning has become an indispensable tool influencing the fields of bioinformatics and medicine. Machine learning automatically learns complex patterns or rules from big data, mainly for data representation and prediction problems [93]. For example, He et al. developed a Python tool, MRMD2.0, to achieve dimensionality reduction during machine learning [94]. Using bioinformatics to construct biomaterials to detect ovarian function markers for dynamic monitoring of ovarian aging is a noninvasive, effective and convenient technology with high sensitivity and specificity. Next, we summarize innovative strategies for the evaluation of ovarian aging with different biomaterials.
Biomaterials for anti-Müllerian hormone detection
AMH is a dimer glycoprotein that is a member of the transforming growth factor β (TGF-β) family of growth and differentiation factors [95]. AMH is synthesized by granulosa cells of follicles and released into follicular fluid. It enters the blood circulation through the perifollicular vascular network, so it can be measured in peripheral blood [96]. AMH has been implicated as the most valuable marker of ovarian reserve function because it is consistent throughout the menstrual cycle, with no significant variability between menstrual cycle and not affected by short term use of oral contraceptives [97].
Early detection of AMH is an immune cell chemical detection and immunoradiometric analysis technology, focusing on AMH positioning and functional studies in animal tissues, which have been unable to meet the needs of clinical detection diagnosis [98–100]. The first generation of AMH enzyme-linked immunosorbent assay (ELISA) detection technology uses a pair of paired monoclonal antibodies to AMH, and it has begun to meet the needs of clinical detection, but there is no unified detection standard [101], and its results are susceptible to sample storage and repeated freeze–thaw cycles [102]. In addition, there was a large difference in test results between commercial kits [103]. Beckman Coulter established the second-generation AMH ELISA detection technology and unified the detection standard for AMH [104], but the problem of unstable AMH detection results persisted [102]. In addition, the above detection methods indirectly evaluate ovarian function through AMH in the blood, showing limitations in assessing the real ovarian reserve.
Molecular probes are constructed by modifying antibodies or ligands of disease-specific molecules on the surface of biomaterials, and they constitute a noninvasive, effective and convenient detection technology that can achieve early detection and real-time monitoring of diseases at the molecular level [105]. Zhang et al. developed AMH-targeted nanobubbles (NBAMH) by integrating AMH antibodies into the surface of nanobubbles (NBs) [105]. NBAMH showed high affinity for ovarian granulosa cells in vitro, and the ultrasound signal of transplanted ovaries was significantly enhanced compared to that of untargeted NBs. By designing NBAMH as an ovarian tissue-specific molecular probe, Zhang et al. provided a promising noninvasive tool for the study of dynamic monitoring of early ovarian function after ovarian transplantation. Moreover, Mu’s study also devised a nanoscale AMH targeted contrast agent, which could improve the targeted development ability of rat transplanted ovaries and effectively solve the existing problems of micron grade contrast agents, such as their only being for blood pool imaging and their lack of tissue specificity; in contrast, they could facilitate noninvasive evaluation of transplantation of ovarian function to realize the in vivo transplantation of ovarian development and functional evaluation. Furthermore, Liu et al. introduced time-resolved immunochromatographic technology into the detection of AMH using nanoenhanced time-resolved fluorescence microspheres and prepared a quantitative AMH detection strip combined with a time-resolved fluorescence immunoassay, which effectively improved the sensitivity of the platform and the detection effect of the low-value fluorescence signal. The invention realized the simple, rapid and low-cost detection of AMH, with high sensitivity and small differences between batches, and it provided a method for the realization of bedside detection.
Biomaterials in estrogen detection
Estrogen can be divided into estrone (E1), estradiol (E2) and estriol (E3), among which E2, produced by granulosa cells of the ovarian follicles, is crucial to maintaining female secondary sexual characteristics and reproductive function [106]. E2 levels are commonly assessed during the early follicular phase of the menstrual cycle, it is a simple, inexpensive, and effective screening tool [107]. Basal levels of E2 have been shown to related with ovarian aging, it falls with age throughout a woman's life [108].
At present, radioimmunoassay is the main method to detect estradiol [109]. Although radioimmunoassay has high sensitivity and specificity, it has the disadvantages of short shelf life and radioactive hazard [109]. In recent years, methods for quantitative detection of serum estrogen have emerged. The main products were enzyme-linked immunosorbent assay [110], fluorescence immunoassay [111], chemiluminescence immunoassay [112] and electrochemiluminescence immunoassay [113]. Conventional immunoassay techniques have been under scrutiny for some time with their selectivity, accuracy and precision coming into question [114]. Chromatographic analysis [115] and ultraviolet (UV) detection [116] have also been used for estrogen detection. However, these methods are expensive, complicated, time-consuming and have different levels of assay sensitivity (0.014–0.04 ng/mL) [110].
Recently, the increasing availability of nanoparticles has attracted widespread attention in the determination of estrogen analysis, because of their high surface areas, high activity, and high selectivity. Li et al. developed a method in which gold nanoparticles enhanced chemiluminescence methods for the measurement of estrogens [117]. Based on the advantages of electrochemical techniques, Jin et al. developed choline derivative-modified electrodes for the assay of estrogens [118]. Subsequently, the same team prepared a Pt nanoclusters/multiwalled carbon nanotube electrochemical biosensor, which had high sensitivity and good reproducibility and stability and could be used as a current-type biosensor for routine analysis of total estrogen in serum [119]. In addition, Huang et al. used AuNP-coupled adaptors to enhance the quantitative detection of 17β-estradiol (17β-E2) specificity by ELISA [120]. Additionally, for the purpose of low-cost and sensitive electrochemical detection of 17β-E2, another study used a multiwall carbon nanotube-Nafion modified electrode [121]. Ovarian 17β-E2 is normally converted into E3, which acts preventively against the occurrence of diseases in women, such as cardiovascular complications and osteoporosis [122, 123]. One study by Gomes et al. created a voltametric sensor based on a cobalt-poly(methionine)-modified glassy carbon electrode that did not require sophisticated instruments or any separation steps, allowing for E3 quantification without laborious and time-consuming procedures [124].
Biomaterials for follicle stimulating hormone detection
Follicle stimulating hormone (FSH) is a heterodimer expressed by the anterior pituitary gonadotropin, composed of two different subunits, α and β [125], mainly plays a role in the regulation of ovarian follicular generation and steroid generation [125], and is an important indicator of clinical detection of ovarian function [126]. Therefore, timely detection of FSH dynamic changes in women is conducive to the evaluation of ovarian function.
In 1953, Steelman and Pohley first proposed an in vivo specific quantitative determination of FSH, namely the rat ovarian weight gain method [127]. However, this method is cumbersome and not suitable for routine clinical studies, and its sensitivity is too low to detect serum FSH level [128]. With the development of technology, immunoassay methods such as ELISA [129], electrochemiluminescence [130] and chemiluminescence [131] have been widely used in clinical detection of FSH. However, these methods have the disadvantages of requiring many samples, long test time, high cost, low sensitivity and large measurement uncertainty [132]. Therefore, it is urgent to develop a fast, economical and simplified FSH detection and analysis method.
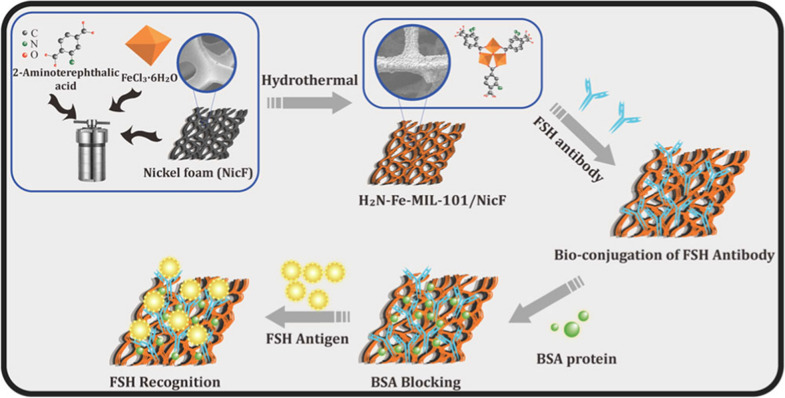
The development of nanotechnology provides new conditions for the development of biomolecular electrical detection systems, but it also provides a new direction for FSH detection [133, 134]. Luo et al. developed a label-free electrochemical immunosensor for the rapid detection of FSH using graphene nanocomposite materials [132]. The method had high sensitivity, fast response and substantial clinical application value. In addition, Lee et al. used a metal-oxide semiconductor silicon nanowire field effect transistor (SINW-FET) device to achieve accurate and rapid detection of FSH [135]. This sensitive, inexpensive, and miniaturized SINW-FET device could serve as an effective sensing method for rapid screening of FSH and menopausal diagnosis. Moreover, Palanisamy et al. synthesized an iron-containing metal–organic framework (H2N –Fe-MIL-101 MOFs) on a porous nickel foam (NicF) substrate by in situ hydrothermal methods, as depicted in Fig. 3. The H2N–Fe-MIL-101/NicF electrode labeled with FSH antibody (Ab-FSH) was applied for specific recognition of an FSH glycoprotein [136]. The material showed fast and excellent sensitivity to FSH. Furthermore, Pareek et al. constructed a novel nanomaterial (NiCo2O4/rGO)-modified indium tin oxide (ITO) electrode for the detection of FSH [137]. The biosensor could help to overcome the disadvantages of current FSH detection methods, such as high cost, long time consumption and low sensitivity, while also providing a dynamic detection range (0.1 pM–1 µM) and a low detection limit (0.1 pM).
Fig. 3.
Illustration of the synthesis of iron containing 3D H2N–Fe-MIL-101 nanosheets MOFs on porous NicF substrate by in situ hydrothermal methods derived from FeCl3·6H2O salt and H2Bdc-NH2 ligand precursors and NicF solid support producing uniformly decorated H2N–Fe-MIL-101/NicF electrodes followed by bioconjugation of FSH antibody for FSH detection. (the figure is reproduced from Palanisamy et al. [136] with required copyright permission)
Biomaterials in ovary ultrasound molecular imaging
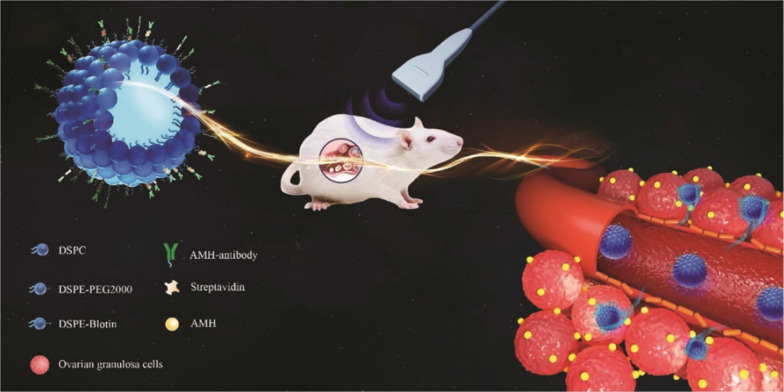
Ultrasonic molecular imaging is a molecular imaging technique based on traditional ultrasound imaging to monitor the level of disease-specific molecular expression [138–140]. With the characteristics of realizing early, noninvasive detection and real-time monitoring of disease at the molecular level [138–140], ultrasonic molecular imaging has great application potential in evaluating follicle survival after early ovarian transplantation. As shown in Fig. 4, Zhang et al. successfully prepared AMH-targeted nanobubbles, which exhibited a high affinity for ovarian granulosa cells in vitro and enhanced ultrasound signals in the ovaries [105]. This new method could be used for early follicle survival detection after ovarian transplantation.
Fig. 4.
Schematic of AMH-targeted nanobubbles (NBAMH) and their targeting ability to rat ovarian granulosa cells expressing AMH. (the figure is reproduced from Zhang et al. [105] with required copyright permission)
Biomaterials for ovarian aging therapy
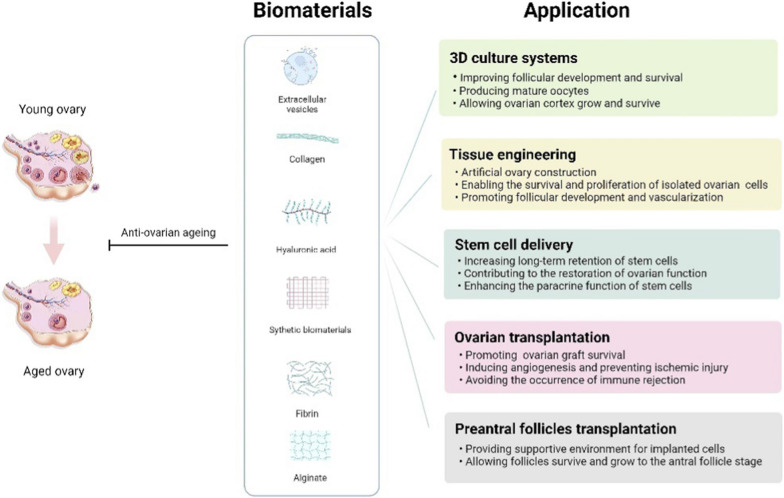
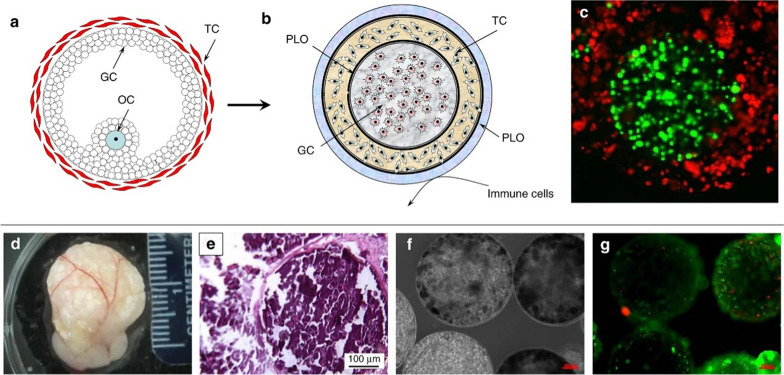
Over the last decade, biomaterial technologies have shown great promise as potential treatments for ovarian aging. The ideal biomaterials for ovarian aging therapy should be nontoxic, biocompatible, biodegradable, and bioresorbable. Furthermore, biomaterials should be able to support the regeneration of new cells and tissue without producing an inflammatory reaction [141]. Biomaterials techniques used to treat ovarian aging include the construction of artificial ovaries, systems for the development of follicles, biomaterial encapsulation of cells or drugs, and delivery of natural extracellular vesicles (Fig. 5). Although not yet in the clinical stage, there have been significant developments in this area, including assessments of the effects, safety and feasibility of anti-ovarian aging using biomaterials in animals. The use of these biomaterials and their success in treating ovarian aging (Table 1) and diseases related to ovarian aging are discussed in detail in the following sections.
Fig. 5.
Application of biomaterials in treatment of ovarian aging
Table 1.
List of biomaterials used for the treatment of ovarian aging
| Categories | Author | Year | Materials | Model | Major finding | |
|---|---|---|---|---|---|---|
| Extracellular vesicles | Bo Sun [145] | 2019 | BMSC-derived exosomes | Cisplatin-induced POF mouse model | Inhibited the apoptosis of granulosa cell. | |
| Meiling Yang [147] | 2020 | BMSC-derived exosomes | Cyclophosphamide-induced POF mouse model | Prevented follicular atresia and GCs apoptosis. | ||
| Zhongkang Li [148] | 2021 | hUCMSC-derived exosomes | Cyclophosphamide-induced POI mouse model | Reduced cell apoptosis and enhanced proliferation. | ||
| Conghui Liu [149] | 2020 | hUCMSC-derived exosomes | Busulfan and cyclophosphamide-induced POI mouse model | Improved the fertility of POI mice without adverse effects on the cognitive behavior of their offspring. | ||
| Ziling Yang [150] | 2019 | hUCMSC-derived exosomes | Busulfan and cyclophosphamide-induced POI mouse model | Restored ovarian function by promoting angiogenesis. | ||
| Chenyue Ding [151] | 2020 | hUCMSC-derived exosomes | Cyclophosphamide-induced POI mouse model | Reduced ROS levels in the damaged ovary and suppressed SIRT7 expression. | ||
| Jin Zhang [152] | 2020 | hUCMSC-derived exosomes | Cisplatin-damaged granulosa cells | Promoted resistance to cisplatin-induced granulosa cells apoptosis and restored synthesis and secretion of steroid hormone in granulosa cells. | ||
| Liping Sun [153] | 2017 | hUCMSC-derived exosomes | Cisplatin-damaged granulosa cells | Ameliorated cisplatin-induced granulosa cells stress and apoptosis in vitro. | ||
| Boxian Huang [155] | 2018 | hADMSC-derived exosomes | Cyclophosphamide-induced POI mouse model | Inhibited expression of the apoptosis genes in human granulosa cells and improved ovarian function. | ||
| Chenyue Ding [157] | 2020 | hAMSCs-derived exosomes | Cyclophosphamide-induced POI mouse model | Improved proliferation, inhibited apoptosis, reduced ROS level and decreased the expression of SIRT4 and relative genes in POI hGCs and ovaries. | ||
| Qiuwan Zhang [14] | 2019 | hAECs-derived exosomes | Busulfan and cyclophosphamide-induced POI mouse model | Increased follicles,inhibited GCs apoptosis and protected the ovarian vasculature from damage in POF mice. | ||
| Guan-Yu Xiao [163] | 2016 | AFMSCs-derived exosome | Busulfan and cyclophosphamide-induced POF mouse model | Inhibited apoptosis in damaged GCs and prevented ovarian follicles from atresia. | ||
| Eman Thabet [164] | 2020 | AFMSCs-derived extracellular vesicles | Cyclophosphamide-induced premature ovarian dysfunction rats model | Restored total follicular counts, AMH levels,regular estrous cycles and fruitful conception. | ||
| Siwen Zhang [166] | 2021 | MenSCs-derived exosomes | 4-Vinylcyclohexene diepoxide-induced POI mouse model | Promoted follicular development, restored fertility and improved live birth. | ||
| Chenfeng Yuan [172] | 2021 | Follicular fluid exosomes | Porcine granulosa cells | Increased the proliferation and progesterone synthesis of porcine ovarian granulosa cells. | ||
| Samuel Gebremedhn [173] | 2020 | Follicular fluid exosomes | Bovine granulosa cells | Protected against heat stress by reducing the amount of ROS accumulation. | ||
| Thais A Rodrigues [174] | 2019 | Follicular fluid exosomes | Cultured cumulus—oocyte complex | Increased the resistance of the oocyte to heat shock and improved the cleavage and blastocyst rates. | ||
| Extracellular matrix | Monica M Laronda [180] | 2015 | SDS | Ovariectomized mice | It could significantly change ECM, and had a strong destructive effect on the ultrastructure of natural tissues. | |
| S E Pors [186] | 2019 | 0.1% SDS and DNA enzymes | Immunodeficient mice | Adequately decellularized both human ovarian medullary and cortical tissue by eliminating all cells and leaving the ECM intact. | ||
| Wen-Yue Liu [187] | 2017 | Triton X-100 solution and DNA enzyme | Rats | Had no cytotoxicity to rat ovarian cells in vitro and only caused minimal immunogenic response in vivo. | ||
| Maryam Nezhad Sistani [188] | 2021 | 1%Triton X-100 and 0.5%SDS | The endometrial mesenchymal cells | It could effectively decellularize human ovarian tissue and highly preserve ECM content and non-cytotoxic properties. | ||
| Farideh Eivazkhani [184] | 2019 | NaOH used as a satisfactory decellularization agent | Ovariectomized mice | It supported follicular reconstruction better than SDS. | ||
| Ashraf Hassanpour [179] | 2018 | SLES as an ionic detergent | Ovariectomized rats | Preserved the structure and composition of ovarian ECM, and promoted in vitro and in vivo biocompatibility and neovascularization of biological ovarian scaffides. | ||
| Hossein Nikniaz [190] | 2021 | Human and bovine acellular ovarian scaffold | Mouse preantral follicles | Sodium alginate containing acellular ovarian scaffold could maintain follicular viability in vitro. | ||
| Sanaz Alaee[191] | 2021 | Decellularized rat ovarian scaffold | Preantral follicles from prepubertal mice | The preantral follicles transformed into antral follicles, and produced mature meiosis oocytes. | ||
| Wen-Yue Liu[187] | 2017 | Porcine acellular scaffold | Rat ovarian tissue | Supported the adhesion, migration, and proliferation of immature female rat granulose cells and showed estradiol secretion. | ||
| S E Pors [186] | 2019 | Acellular human ovarian tissue | Human preantral follicles | Supported the survival of human follicles. | ||
| Eun Jung Kim [192] | 2020 | ECM-derived hydrogel | Mouse ovarian follicles | Supported follicular morphology and growth, and promoted oocyte maturation. | ||
|
Ashraf Hassanpour [179] |
2018 | Acellular scaffold of human ovarian tissue | Ovariectomized mice | Increased vaginal opening and estrogen levels after implantation and confirmed the onset of puberty. | ||
| Monica M Laronda [180] | 2015 | Acellular bovine ovarian scaffold | Ovariectomized mice | Supported the growth of isolated mouse follicles, and produced estrogen and reconstructed menstrual cycles. | ||
| Georgia Pennarossa [193] | 2021 | Porcine ovarian 3D biological scaffold | Female germ line stem cells | Represented a powerful tool for in vitro recreation of a bioengineered ovary that might constitute a promising solution for hormone and fertility function restoring. | ||
| Kutluk Oktay[194] | 2016 | Human extracellular tissue matrix scaffold | Human | Pregnancies had been reported following minimally invasive transplantation of previously cryopreserved ovarian tissue. | ||
| Collagen | Sunyoung Joo[198] | 2016 | Collagen-rich, biomimetic 3D shells | Rodent ovarian follicles | Collagen hydrogel properties were important for follicular phenotype and function maintenance. | |
| C Torrance [199] | 1989 | A collagen gel matrix | Mouse preantral follicles | Allowed mouse follicles to separate and grow in vitro for at least 2 weeks. | ||
| G Taru Sharma [200] | 2009 | A 3D collagen gel culture system | Buffalo preantral follicles | Maintained follicle viability and growth by providing surface interaction and increasing attachment of follicles. | ||
| Kossowska-Tomaszczuk [205] | 2010 | A three-dimensional culture system containing type I collagen | Immunodeficient mice | Allowed granulosa cell subpopulations isolated from mature follicles to survive and grow, and supported their proliferation into steroid-producing spherical structures. | ||
| Saori Itami [206] | 2011 | A three-dimensional collagen gel | Mouse preantral follicles | The follicle could maintain its three-dimensional shape, and increase its size in response to FSH stimulation. | ||
| R Abir [201] | 1999 | collagen gel | Monolayer follicles from human ovarian tissue | Reported an increase in the GC layer and oocyte diameter of human follicles. | ||
| Catherine M H Combelles [202] | 2005 | 3D collagen gel matrix | Cumulus cells | Established for the first time an effective in vitro fertilization combined culture system of human denuded oocytes and cumulus cells. | ||
| L Vanhoutte [213] | 2009 | Collagen (type I) gel | Dermished foamed oocytes | The fertilization rate of 3D pre-cultured oocytes was significantly higher than that of conventional IVM oocytes. | ||
| Yanjun Yang [214] | 2019 | The collagen scaffold loaded with hUCMSCs | Cyclophosphamide-induced POF mouse model | Increased the levels of E2 and AMH, ovarian volume and the number of antral follicles. | ||
| Jing Su [154] | 2016 | The collagen scaffold with ADSCs | Tripterygium Glycosides -induced POF rat model | Increased long-term retention of ADSCs in the ovary and contributed to the restoration of ovarian function. | ||
| Lijun Ding [215] | 2018 | The collagen scaffold with umbilical cord mesenchymal stem cells | Infertile POF patients | Saved overall ovarian function and leaded to a successful clinical pregnancy. | ||
| Hyaluronic acid | Nina Desai [219] | 2012 | A tyramine-based HA hydrogel | Mouse preantral follicles | Promoted the secretion of estradiol and increased the survival rate, GV rupture rate and MII formation rate of cultured follicles. | |
| I R Brito [220] | 2016 | A novel hyaluronic acid hydrogel based on tyramine-substituted sodium hyaluronate dihydroxyphenyl bond | Goat preantral follicles | Failed to maintain survival and improve antral formation. | ||
| Parisa Jamalzaei [222] | 2020 | A HAA composed of HA and ALG | Mouse preantral follicles | Promoted the development of preantral follicles and oocyte maturation in mice and enhanced estrogen secretion. | ||
| L M G Paim [224] | 2015 | A vitrification solution with 1% hyaluronic acid | The cumulus oocyte complex | Improved the meiotic recovery rate and nuclear maturation rate of norvegicus oocytes. | ||
| Somayeh Tavana [225] | 2016 | The HABH | Ovariectomized rats | Prevented or reduced early ischemia-induced follicular loss, promoted follicular survival and angiogenesis. | ||
| Maryam Akhavan Taheri [226] | 2016 | HA hydrogel | Ovariectomized rats | Had no negative effect on estrus cycle recovery and ovarian preservation,and improved the outcome of autologous transplantation. | ||
| Or Friedman [227] | 2012 | HA—rich biogel | Immunodeficient mice | Improved ovarian graft survival. | ||
| Wenlin Jiao [228] | 2022 | A combination of UCMSCs and HA gel | 4-Vinylcyclohexene diepoxide -induced POI mouse model | Improved follicular survival. | ||
| Eun-Young Shin [229] | 2021 | HA gel scaffolder | Cisplatin-induced POI mouse model | Restored the ovarian structure and function and improved the quality of oocyte and embryo as well as the regularity of estrus cycle. | ||
| Guangfeng Zhao [230] | 2015 | HA | Immunosuppressive drug-induced POI-like rat model | Prevented chemotherapy-induced ovarian damage. | ||
| Fibrin | Seyedeh Zeynab Sadr [235] | 2018 | Fibrinalginate scaffold | Mouse preluminal follicles | Improved follicular development and survival, and produced mature oocytes. | |
| Shi Ying Jin [240] | 2010 | A fibrinalginate hydrogel matrix | Mouse secondary follicles | Supported the growth of secondary follicles to the antral follicles stage and produced mature oocytes. | ||
| Ariella Shikanov [239, 241] |
2011 2009 |
The FA-IPN | Mouse secondary follicles | Contributed to increased meiosis maturation rates of oocytes. | ||
| I R Brito [220] | 2016 | Fibrinalginate | Goat preluminal follicles | Restored oocyte meiosis and promoted oocyte maturation to produce parthenotes. | ||
| J Xu [242] | 2011 | A fibrin alginate matrix | Rhesus monkey secondary follicles | Supported the growth of secondary follicles to antral follicles stage, and promoted the maturation of oocytes to MII stage. | ||
| J Xu [243] | 2013 | Fibrinin-sodium alginate 3D capsule | Primate rhesus monkey primary follicles | Primate oocytes derived from primary follicles developed in vitro had the ability to restart meiosis for fertilization. | ||
| Alireza Rajabzadeh [245] | 2020 | A fibrin hydrogel scaffold supplemented with platelet lysates | Mouse preantral follicles | Improved the local vascularization of follicles, and the survival rate of follicles, and promoted the growth of follicles to the stage of antral follicles. | ||
| Valérie Luyckx [246] | 2014 | A fibrin matrix containing low concentrations of fibrinogen and thrombin | Mouse preantral follicles and ovarian cells | All follicles were found to be alive or only slightly damaged and to grow to the antral follicular stage. | ||
| M C Chiti [247] | 2016 | Fibrinogen and thrombin (F12.5/T1) substrates | SCID mice | Isolated secondary follicles survived and grew to the antral follicle stage. | ||
| Rachel M Smith [248] | 2014 | Fibrin hydrogel | Infertile mouse model | Restored ovarian endocrine function. | ||
| Fernanda Paulini [249] | 2016 | A fibrin matrix containing fibrinogen and thrombin | Nude mice | Isolated human follicles were viable after encapsulation in fibrin clots and short-term xenotransplantation. | ||
| Ariella Shikanov [244] | 2011 | Heparin modified fibrin | Infertile mouse model | Reduced ischemia and improved vascular remodeling. | ||
| Jiang-Man Gao[250] | 2013 | Fibrin hydrogels mixed | Adult female mice | Increased follicular survival and improved revascularization. | ||
| Chungmo Yang [251] | 2021 | Fibrin hydrogel containing NO-NPs | Ovariectomized mice | Improved the total number and quality of follicles, induced angiogenesis, and prevented ischemic injury. | ||
| Elham Shojafar [252] | 2019 | Platelette-rich fibrin biofolders | Ovariectomized mice | Reduced oxidative stress, promoted revascularization, and protected follicular cisterns from ischemia–reperfusion injury. | ||
| Maria Costanza Chiti [237] | 2018 | A novel fibrin matrix | Human ovarian follicles | Fibrin matrix composed of F50/T50 most closely resembled human ovarian cortex. | ||
| Valérie Luyckx [238] | 2013 | A artificial ovary composed of fibrinogen and thrombin | Human ovarian cells | Enabled the survival and proliferation of isolated human ovarian stromal cells. | ||
| Alginate | Hudson H V Correia [259] | 2020 | A sodium alginate 3D culture system | Goat primordia follicles | Showed appropriate survival rate, high follicular activation rate and continued to grow throughout culture. | |
| Samaneh Sadeghnia [261] | 2016 | A sodium alginate three-dimensional culture system | Sheep primordial/primary follicles | 2% sodium alginate supported follicle growth better than 1% sodium alginate. | ||
| Min Xu [262] | 2006 | An alginate hydrogel matrix | Pseudopregnant female mice | Produced healthy and fertile progenies. | ||
| Jing Xu [263] | 2010 | Alginate | Rhesus monkey secondary follicles | Grew to the antral follicle stage, produced steroids and growth factors, and produced healthy oocytes within 40 days. | ||
| Min Xu [264] | 2009 | Alginate | Rhesus monkey secondary follicles | The follicles survived and continued to grow. | ||
| Alon Kedem [266] | 2011 | Macropores sodium alginate scaffold | Human ovarian cortex slices | There was an increase in developing follicle culture and a decrease in atretic follicles. | ||
| Monica M Laronda [267] | 2014 | Sodium alginate hydrogel | Human ovarian cortex containing primordial follicles | The ovarian cortex grew, survived, and supported follicular development for up to 6 weeks. | ||
| Christiani A Amorim [268] | 2009 | alginate matrix | Small human preantral follicles | Survived in vitro culture in alginate matrix for 7 days. | ||
| Antonella Mastrorocco[269] | 2021 | Alginate microspheres | Lamb cumulus oocyte complexes | Increased the nuclear maturation rate of preadolescent oocytes and reduced the incidence of chromosome abnormality. | ||
| Parisa Jamalzaei [270] | 2020 | ALG hydrogel | Mouse preantral follicles | Survival rate of 0.5%ALG cultured follicles was significantly higher than 0.75% and 1%ALG cultured follicles. | ||
| Cyrus Jalili [271] | 2020 | Sodium alginate | Mouse preantral follicles | 0.5% alginate was the most favorable concentration. | ||
| Erin R West[258] | 2007 | Alginate gel | Mouse secondary oocytes | Reducing alginate matrix hardness could maintain intercellular tension homeostasis, promote cell process, create local paracrine environment and improve oocyte quality. | ||
| Julie Vanacker [273] | 2014 | Alginate saline gel | Immunodeficient mice | Promoted follicular development and vascularization. | ||
| Sivanandane Sittadjody [274] | 2017 | Sr++ cross-linked alginate | Ovariectorized rats | Achieved stable hormone secretion and improved the adverse effects of hormone deficiency. | ||
| Shani Felder [275] | 2019 | Macrofenate scaffold | Ovariectorized mice | Showed high serum hormone levels and the appearance of the vaginal area. | ||
| Sythetic biomaterials | Jiwon Kim [276] | 2016 | A synthetic hydrogel, PEG-VS | Ovariectorized mice | It was found to wrap immature follicles successfully functioned as an artificial ovarian tissue in vivo for 60 days. | |
| Uziel Mendez [277] | 2018 | A three-dimensional PEG-based culture system | Mice follicles | Improved the survival and maturation rates of small follicles. | ||
| Zhonghua Shi [278] | 2021 | A supramolecular hydrogel | Aged mice | Delayed ovarian aging in aged mice,stimulated ovaries to secrete estrogen and progesterone, and developed more antral follicles for reproduction. | ||
| Anu David [279] | 2017 | A TheraCyte device | Ovariectomized mice | Restored follicular development and ovarian endocrine function and reduced FSH levels. |
Application of biomaterials in the treatment of ovarian aging
Extracellular vesicles
EVs, known as nano-sized, are a heterogeneous group of cell-derived membranous structures comprising exosomes (~ 50–150 nm) and microvesicles (~ 100–1000 nm), which carry bioactive material such as mRNAs, microRNAs (miRNAs), and protein in different body fluids and deliver their contents to recipient cells [142]. In recent years, EVs has developed into an effective nanocarrier for advanced drug delivery due to its multiple advantages [143]. EVs, as a natural vector produced by endogenous cells, have good biocompatibility with the immune system and low toxicity. In addition, EVs avoid phagocytosis by macrophages and penetrates blood vessels into the extracellular matrix. Furthermore, EVs can cross biological barriers to treat refractory diseases, such as the blood–brain barrier [144]. With the exploration of EVs, stem cell-derived EVs have attracted much scientific attention due to its broad prospects for treatment of various diseases. Regarding the function of EVs depends on their parent cells, accumulating studies have shown that stem cell-derived EVs can treat ovarian aging by transferring functional miRNAs and proteins. The following sections will mainly elucidate the therapeutic effects of stem cell-derived EVs in the treatment of ovarian aging (Fig. 6).
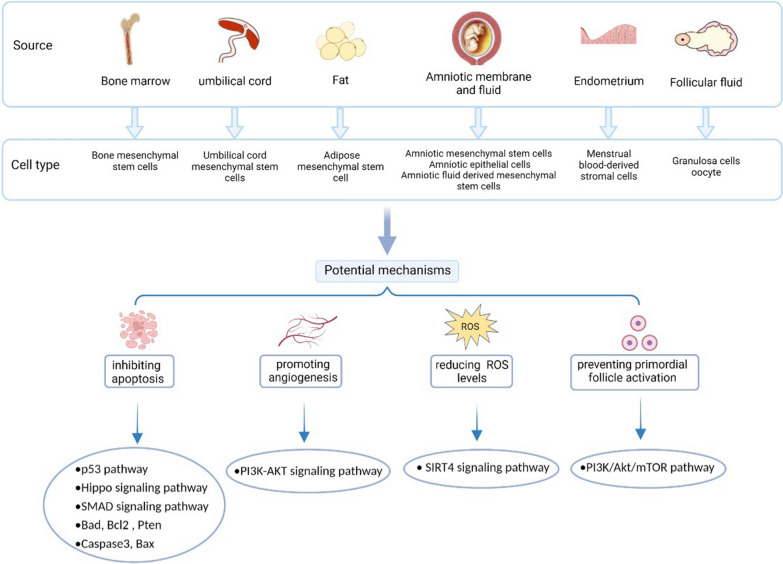
Fig. 6.
The therapeutic effects of stem cell-derived EVs in the treatment of ovarian aging
Bone marrow mesenchymal stem cell (BMSC)-derived exosomes
Bone marrow mesenchymal stem cells (BMSCs) are a cell subpopulation with multiple differentiation potential, and they constitute a popular research topic in the field of stem cell therapy [145]. Studies have shown that exosomes derived from BMSC (BMSC-exos) can also participate in tissue repair, and they are expected to replace stem cells as a new therapeutic tool for tissue repair [146]. It has been reported that miR-644-5p carried by BMSC-derived exosomes improved follicle injury and ovarian function by inhibiting the apoptosis of ovarian granulosa cells induced by cisplatin through targeting of the p53 pathway [145]. Their results suggested that inhibition of the apoptosis pathway in granulosa cells might occur via horizontal transfer of mRNAs by BMSC-exos. In another chemotherapy-induced POF rat model, the prominent role of the contents of BMSC-exos was studied. BMSC-exos exerted anti-apoptotic effects on tubular granulosa cells via the delivery of miR-144-5p, which regulated proliferative/anti-apoptotic pathways, leading to restoration of ovarian function [147].
Human umbilical cord mesenchymal stem cell (hUCMSC)-derived exosomes
The human umbilical cord is a promising source of MSCs, and hUCMSCs have a painless collection procedure and faster self-renewal properties. hUCMSC-derived exosomes (hUCMSC-exos) help to maintain tissue homeostasis and enable the recovery of critical cellular functions by initiating the process of repair and regeneration. hUCMSC-exos have also proven to be effective in recovering ovarian function and improving fertility in ovarian aging. Li et al. investigated the effect of hUCMSC-exos on POF induced by cyclophosphamide (CTX) in a mouse model [148]. The results showed that hUCMSC-exos could reduce cell apoptosis and enhance proliferation through the Hippo signaling pathway, leading to ovarian cells recovery and overall improvement of ovarian function. In a recent animal study, Liu et al. studied the effect of hUCMSC-exos on POI induced by chemotherapy in mice [149]. They demonstrated that hUCMSC-exos improved the fertility of POI mice by inhibiting the apoptosis of ovarian cells mediated by mRNAs and miRNAs transferred by the hUCMSC-exos. In another study, Yang et al. explored the proangiogenesis effect of hUCMSC-exos in a mouse model of POI [150]. They demonstrated that hUCMSC-exos transplantation could restore ovarian function by promoting angiogenesis through activation of the PI3K-AKT signaling pathway. hUCMSC-exos also exerted ovary protection via their anti-inflammatory effects. In a CTX-induced POI mouse model, injection of hUCMSC-exos reduced ROS levels by suppressing SIRT7 expression in the damaged ovary [151]. Furthermore, an in vitro study by Zhang et al. showed that hUCMSC-exos increased the proportion of Bcl-2/Bax and decreased the expression of the proapoptotic gene Caspase-3, therefore playing important roles in promoting resistance to cisplatin-induced granulosa cell apoptosis and restoring the synthesis and secretion of steroid hormones in granulosa cells [152]. In a similar study, hUCMSC-exos treatment ameliorated cisplatin-induced granulosa cell stress and apoptosis in vitro [153].
Human adipose mesenchymal stem cell (hADMSC)-derived exosomes
hADMSCs are derived from human adipose tissue and are superior biomaterials that can be suitable for allotransplantation and regenerative medicine [154]. Hung et al. established a mouse POI model by CTX administration to study the therapeutic effect of hADMSC-derived exosomes (hADMSC-exos) in chemotherapy-induced ovarian aging. The results showed that hADMSC-exos inhibited the expression of apoptosis-related genes in granulosa cells and improved ovarian function via regulation of the SMAD signaling pathway [155].
Human amniotic mesenchymal stem cell (hAMSC)-derived exosomes
hAMSCs are derived from the human amniotic membrane, are easy to obtain, are less invasive and are ethical, and they can be used in allotransplantation and regenerative medicine [156]. hAMSCs have been shown to be effective in recovering ovarian function in a mouse model of POF [156]. However, little is still known about the underlying molecular mechanism of hAMSC treatment in ovarian damage, and much remains to be further clarified. In another study, Ding et al. first reported that hAMSC derived exosomes (hAMSC-exos) reversed apoptosis in a chemotherapy-induced POF mouse model. This study indicated that miR-320 in hAMSC-exos reduced ROS levels via SIRT4 signaling to exert protective effects on ovarian function [157].
Human amniotic epithelial cell (hAEC)-derived exosomes
hAEC-based therapy mediates tissue regeneration in a variety of diseases, and increasing evidence has suggested that the therapeutic efficacy of hAECs mainly depends on paracrine action [158–160]. The effects of hAEC-derived exosomes (hAEC-exos) were investigated in POF induced by busulfan and cyclophosphamide in mice. hAEC-exos significantly improved ovarian function by ameliorating the granulosa cell apoptosis and preventing the ovarian vasculature damage. An in vitro study showed that hAEC-exos increased the expression of anti-apoptotic genes, such as Bad, Bcl2, and PTEN, and decreased the expression of pro-apoptosis genes, such as Caspase-3 and Bax, by transferring functional miRNAs, such as miR-1246 [14]. In addition, the results showed that hAEC-exos prevented primordial follicle activation in chemotherapy-treated mice through the PI3K/AKT/mTOR pathway.
Amniotic fluid-derived mesenchymal stem cell (AFMSC)-derived exosomes
AFMSCs are adult, fibroblast-like, self-regenerating pluripotent stem cells [161]. Accordingly, AFMSCs serve as a rich source of MSCs in terms of the number of potential donors and the simplicity of the harvesting procedure [162]. Based on their enormous differentiation capacity and immunomodulatory characteristics, the therapeutic potential of AFMSCs has been extensively explored in animal models of degenerative diseases. In a mouse model of POF induced by CTX, Xiao et al. showed that injection of AFMSC-derived exosomes (AFMSC-exos) protected mice from ovarian damage by reducing apoptosis of granulosa cells [163]. They revealed that AFMSC-exos contained two miRNAs, miR-146a and miR-10a, which inhibited apoptosis in damaged granulosa cells and prevented the atresia of ovarian follicles induced by CTX [163]. Thabet et al. showed that AFMSC-exos were able to repair CTX-induced POF in rats [164]. They found that AFMSC-exos containing miRNA-21 could inhibit the expression of target genes, such as PTEN and Caspase-3 in ovarian cells. These target genes are involved in the apoptosis and physiology of follicles [164].
Menstrual blood-derived stromal cell (MenSC)-derived exosomes
Mesenchymal stromal cells isolated from menstrual blood (MenSCs), exhibiting a potent proangiogenic and immunomodulatory capacity, have become an important source of stromal cells for cell therapy [165]. Their therapeutic effect is mediated by paracrine mediators released by their secretomes. Previous studies have shown that MenSCs play a paracrine role in ovarian therapy, such as promoting the number of follicles and ovarian angiogenesis and reducing the apoptosis of granulosa cells [142, 145]. Recently, Zhang et al. found that menstrual blood-derived stromal cell-derived exosomes (MenSC-exos) transplantation could effectively promote follicular development, restore fertility and improve live birth rates in a chemotherapy-induced POI rat model [166]. These protective effects might mainly be due to improvement of the ovarian extracellular matrix and the proliferation of granulosa cells.
Follicular fluid-derived exosomes
Follicular fluid has been recognized as a source of biochemical factors that can be predictive of oocyte quality, and it contains a variety of important secretory factors, such as proteins, amino acids, nucleotides, hormones and so on [167]. The microenvironment provided by follicular fluid plays an important role in follicular growth and maturation [168]. Follicular fluid exosomes are new molecules in follicular fluid, and they have been successfully isolated from human, bovine, and pig ovaries [169–171]. Juliano et al. proved that microvesicles isolated from follicular fluid could be taken up by surrounding granulosa cells [168]. Recently, Yuan et al. found that follicular fluid exosomes increased the proliferation and progesterone synthesis of porcine ovarian granulosa cells, in which the MAPK/ERK and WNT/B-CATENIN pathways were involved [172]. Another study investigated the role of the antioxidative properties of follicular fluid exosomes in bovine granulosa cells [173]. The results showed that follicular fluid exosomes had protective effects against heat stress by reducing the amount of ROS accumulation. In a similar study, to improve cumulus cell expansion and oocyte competence for fertilization, treatment with follicular fluid exosomes increased the resistance of oocytes to heat shock and improved the cleavage and blastocyst rates [174].
Together, EVs, especially exosomes, have attracted significant interest with regard to their use in the treatment of ovarian aging. EVs can be readily isolated from stem cells of various origins and carry biologically active molecules that can be transferred to target cells to exert their therapeutic effects. EVs prevent ovarian aging by promoting angiogenesis, modulating the immune system, suppressing cell apoptosis, and exerting many other beneficial effects. However, the functional mechanisms of EVs must be determined to take full advantage of EVs in ovarian aging therapy.
Extracellular matrix
Tissues and organs contain a mixture of cellular and noncellular components that form well-organized networks called ECM. The ECM not only provides a physical scaffold for cell embedding, but also regulates many cellular processes such as cell growth, migration, differentiation, survival, homeostasis and morphogenesis [175, 176]. The major constituents of ECMs are fibrous forming proteins, such as collagens, elastin, fibronectin (FN), laminins, glycoproteins, proteoglycans (PGs) and glycosaminoglycans (GAGs), which are highly acidic and hydrated molecules [177] and synthetic ECM is a promising material for tissue engineering [178]. The ovarian tissue engineering concept presents a 3D system for folliculogenesis resumption, supporting follicle survival and growth, providing a new strategy for the treatment of ovarian aging [73].
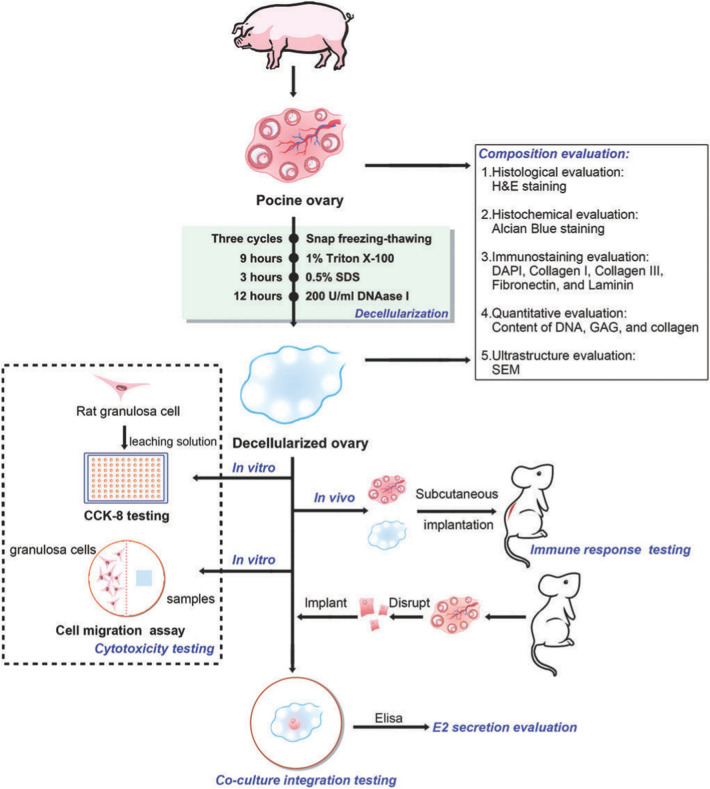
Detergents for the decellularization of whole organs or tissues are key factors in the preparation of acellular scaffolds with ECM structural integrity [179]. Originally, human and bovine ovaries were decellularized using sodium dodecyl sulfate (SDS) as an ionic detergent [180]. However, the long-term application of SDS can significantly change the ECM and has a strong, destructive effect on the ultrastructure of natural tissues, including the reduction of polysaccharides and cytokines, cytotoxicity, poor adhesion, and induced inflammation and thrombosis after transplantation [181–183]. Since then, the ovarian decellularization protocol has been improved. Studies have shown that the harmful effects of SDS are related to its concentration and exposure time [184, 185]. Pors et al. treated human ovarian tissue with 0.1% SDS as a cell detergent for 18–24 h, and they added DNA enzymes to decellularized human ovarian tissue and maintained ECM integrity [186]. Similarly, Liu et al. improved porcine ovarian decellularization strategies by adding Triton X-100 solution and shortening the SDS culture time, and further shortening the chemical treatment time by adding DNA enzyme digestion and freezing and thawing steps, and they developed a novel xenogenic ovarian regeneration decellularization protocol [187] as depicted in Fig. 7. Sistani et al. felt that the simplified procedure might better preserve the biochemical properties of the scaffold, so they applied three freezing/thawing cycles using a combined regimen of 1% Triton X-100 for 15 h and 0.5%SDS for 72 h without any enzyme treatment. The regiment could effectively decellularize human ovarian tissue and highly preserve ECM content and noncytotoxic properties [188]. Eivazkhania et al. demonstrated that sodium hydroxide (NaOH) could be used as a satisfactory decellularization agent for ovarian decellularization and regeneration of follicle-like structures [184]. Hassanpour et al. investigated a novel decellularization protocol based on sodium dodecyl sulfate (SLES) treatment, which avoided the disadvantages of SDS treatment, preserved the structure and composition of ovarian ECM, and promoted in vitro and in vivo biocompatibility and neovascularization of biological ovarian scaffolds [179].
Fig. 7.
Flow diagram of study design. Color images available online at www.liebertpub.com/tec (the figure is reproduced from Liu et al. [187] with required copyright permission)
Acellular tissue can improve follicular activity and growth by providing natural ECM components, growth factors and porous structures [189], making it an ideal scaffold for in vitro follicular culture. Nikniaz et al. cultured isolated mouse preantral follicles into an acellular ovarian scaffold and tested the survival rate of follicles for the first time, demonstrating that sodium alginate-containing acellular ovarian scaffolds could maintain follicular viability in vitro for 6 days [190]. Similarly, Alaee et al. cultured preantral follicles from mice in a decellularized rat ovarian scaffold for 12 days [191]. In the acellular ovarian scaffold, the preantral follicles transformed into antral follicles, and the mature follicles secreted E2 and progesterone (P4), and they could grow and develop normally and produce mature meiosis oocytes. Liu et al. implanted rat ovarian tissue into a porcine acellular scaffold, as depicted in Fig. 7. The acellular ovaries supported the adhesion, migration, and proliferation of immature female rat granulosa cells and showed estradiol secretion in vitro [187]. Pors et al. cultured human preantral follicles in vitro from acellular human ovarian tissue and demonstrated that the scaffold could support the survival of human follicles, while further research is needed to improve the recovery and survival of retransplanted follicles [186]. Kim et al. for the first time used ECM-derived hydrogels to perform 3D follicular culture in vitro and showed that this culture system could effectively improve the results of in vitro follicular culture, support follicular morphology and growth, and promote oocyte maturation [192].
Reconstruction of bioengineered ovaries could pave the way for possible in vitro reconstruction of ovarian tissue, which could in turn lead to overall improvements in reproductive technology and possibly future applications in organ transplantation to restore hormonal and reproductive function. Hassanpour et al. created artificial ovaries by implanting rat primary ovarian cells into an acellular scaffold of human ovarian tissue and implanted them into ovariectomized mice. Increased vaginal opening and estrogen levels after implantation confirmed the recovery of puberty [179]. Similarly, Laronda et al. showed that acellular bovine ovarian scaffolds supported the growth of isolated mouse follicles, produced estrogen and reconstructed menstrual cycles in ovariectomized mice [180]. Recently, Pennarossa et al. created a complete porcine ovarian 3D biological scaffold and refilled the ECM scaffold with female germline stem cells (FGSCs) to form a bioengineered ovary [193]. Notably, pregnancies have been reported following minimally invasive transplantation of previously cryopreserved ovarian tissue using human extracellular tissue matrix scaffolds assisted by robotic surgery [194].
Collagen
Collagen, which belongs to the fibrin family, is the most abundant extracellular matrix protein in the animal kingdom. It transmits loads in tissues and provides a highly biocompatible environment for cells [195]. This good biocompatibility, biodegradability, low inflammatory response, and low antigenicity make collagen a perfect biomaterial for regenerative medicine and tissue engineering [196].
There are 28 different members of the collagen family. Collagen type I, III and IV are the most abundant of the various collagen types in ovaries of vertebrates. The presence of normal collagen retains primordial follicle dormancy, follicles development, ovulation and steroidogenesis [197]. Therefore, collagen is a promising hydrogel for encapsulation of ovarian follicles. Joo et al. created collagen-rich, biomimetic 3D shells to culture rodent ovarian follicles [198]. They found that differences in cell survival, follicular growth and development, sex hormone production, and oocyte maturation were associated with changes in the density and elasticity of collagen hydrogel, suggesting that collagen hydrogel properties were important for follicular phenotype and function maintenance in 3D culture systems. In addition, follicles from several species have been successfully implanted into 3D collagen gel for culture. Torrance et al. developed a technique for isolating and growing intact mouse preantral follicles in a collagen gel matrix, and it allowed mouse follicles to separate and grow in vitro for at least 2 weeks [199]. Sharma et al. developed for the first time a 3D collagen gel culture system for the in vitro growth and survival of buffalo preantral follicles [200]. In addition, human [201, 202], pig [203] and bovine [204] follicles could be cultured in collagen gel, and it has been proven that the collagen gel culture system could provide maximal support for the growth of follicles, and maintaining their three-dimensional structure. Furthermore, a 3D matrix culture system consisting of type I collagen was constructed, which, together with leukemia inhibitors, allowed granulosa cell subpopulations isolated from mature follicles to survive and grow and supported their proliferation into steroid-producing spherical structures [205]. To study how the cell layer of the follicular membrane is formed, Itami et al. constructed a three-dimensional follicular culture system. Using this culture system, the follicles could maintain their three-dimensional shape by embedding in collagen gel, increasing their size in response to FSH stimulation, and replicating the formation of the cell layer of the follicle membrane when cultured with mesenchymal cells [206].
In vitro maturation (IVM) of human oocytes has the potential to provide some patients with the opportunity to receive fertility therapy; however, the conditions of human oocyte IVM remain to be improved [207]. Abir et al. embedded monolayer follicles from human ovarian tissue in collagen gel and cultured them for 24 h in vitro, establishing the first step of successful IVM of human small follicular oocytes. They reported for the first time an increase in the granulosa cell layer and oocyte diameter of human follicles isolated and cultured in collagen gel [201]. It is now clear that bidirectional communication between oocytes and their surrounding cumulus cells plays an important role in obtaining oocyte development capacity and subsequent embryogenesis [208–210]. Combelles et al. embedded cumulus cells into a 3D collagen gel matrix, adding a single oocyte to each gel, and they established an effective in vitro fertilization combined culture system of human denuded oocytes and cumulus cells [202]. In addition, it was found that the developmental potential of oocytes could be increased by temporarily inhibiting spontaneous meiosis maturation [211, 212]. Vanhoutte et al. precultured germinal foamed (GV) oocytes from the controlled ovarian overstimulation (COH) cycle in a collagen (type I) gel containing free cumulus cells and a specific phosphodiesterase 3 inhibitor (PDE3-I, inhibiting meiosis) for 24 h [213]. The results showed that the fertilization rate of 3D precultured oocytes was significantly higher than that of conventional IVM oocytes.
Indeed, the use of collagen as a scaffold for stem cell-based ovarian aging therapy is well documented. Yang et al. transplanted a collagen scaffold loaded with hUCMSCs into the ovaries of POF mice for the first time [214]. They demonstrated that the collagen scaffold increased the levels of E2 and AMH, the ovarian volume and the number of antral follicles. However, the mechanism of interaction between collagen scaffolds and stem cells remains unclear and requires further study. The collagen scaffold with hUCMSCs transplantation could represent an ideal and promising treatment for POF. In another study, Su et al. explored the transplantation of collagen scaffolds with adipose-derived stem cells (ADSCs) in a rat model of POF [154]. They observed that collagen scaffolds increased the long-term retention of ADSCs in the ovary and contributed to the restoration of ovarian function, including a regular estrus cycle, elevated E2 levels and improved fertility. These protective effects might be due to the growth factors secreted from ADSCs in the collagen scaffold, contributing to granulosa cell proliferation and angiogenesis within follicles. Although collagen scaffolds promoted the long-term retention of adipose stem cells in the ovary, the retention of these cells did not exceed 1 month and must be further optimized. Ding et al. showed that umbilical cord mesenchymal stem cells on collagen scaffolds (collagen/UC-MSCs) could activate primordial follicles in vitro by phosphorylation of FOXO3a and FOXO1 and activate follicles to grow to the preovulation stage in vivo [215]. In addition, they transplanted collagen/UC-MSCs into the ovaries of patients with POF, preserving overall ovarian function and a successful clinical pregnancy.
Hyaluronic acid
Hyaluronic acid (HA) is a biopolymer composed of disaccharide repeat units, including d-glucuronic acid molecules and N-acetylglucosamine molecules linked by B-(1–4) and B-(1–3) glycosides. It is present in all vertebrates and is an important component of the ECM in most mature tissues [216]. Hyaluronic acid has been widely used for its excellent physicochemical properties such as biodegradability, biocompatibility, non-toxicity, non-immunogenicity and as an excellent tool in biomedical applications such as osteoarthritis surgery, eye surgery, plastic surgery, tissue engineering and drug delivery [217].
In the ovary, HA is found not only in the cumulus matrix but also in the theca cells and follicular fluid, and they play a significant role in establishing a microenvironment conducive to the development of follicles [218]. Desai et al. described for the first time a novel tyramine-based HA hydrogel that supported the in vitro culture of mouse preantral follicles [219]. It was proven that oocytes from HA-encapsulated follicles could resume meiosis and produce mature MII oocytes. Brito et al. also used a novel HA hydrogel based on a tyramine-substituted sodium hyaluronate dihydroxyphenyl bond for the in vitro culture of caprine preantral follicles [220]. However, it was found that the follicles enclosed in HA failed to survive, possibly due to differences in experimental conditions (number of cultured follicles, culture medium and species). Compared with alginate (ALG), HA hydrogel lost the ability to increase follicular survival and the antral formation rate [220]. This outcome might be due to poor mechanical properties or a lack of pores in the HA microstructure, which are necessary for follicular nutrition and growth [221]. Jamalzaei et al. created a composite hydrogel (HAA) composed of HA and ALG to optimize the poor mechanical properties of HA and to form porous microstructures [222]. They found that the application of HAA hydrogel produced good results in follicular culture. Vitrification of embryos has been successful, while cryopreservation of oocytes has still failed to achieve the expected results [223]. Paim et al. used a vitrification solution with 1% hyaluronic acid to freeze the cumulus oocyte complex (COC) for 7 days and then heated and matured it in vitro for 30 h [224]. The results showed that adding 1% hyaluronic acid to vitrified frozen solution could improve the meiotic recovery rate and nuclear maturation rate of Rattus norvegicus oocytes in vitro.
Cryopreservation and transplantation of ovarian tissue is an effective method for the treatment of iatrogenic ovarian aging. Tavana et al. used a hyaluronic acid-based hydrogel (HABH) as a scaffold to improve ovarian tissue transplantation [225]. They found that ovarian encapsulation with HABH could prevent or reduce early ischemia-induced follicular loss and promote follicular survival and angiogenesis. However, the underlying mechanisms and clinical translational applications of HABH require further investigation. The same group used HA hydrogel as a scaffold to wrap vitrified ovarian tissue in autologous intramuscular transplantation, and they showed that it increased angiogenesis and reduced the follicular apoptosis rate in transplanted ovaries [226]. The results showed that the use of HA in combination with growth factors seemed to improve the outcome of autologous transplantation. Friedman et al. found that the coincubation of human ovarian grafts with HA-rich biogels in combination with vascular endothelial growth factor A (VEGF-A) and vitamin E resulted in improved ovarian graft survival [227].
Self-linked HA is a good cell scaffold to improve the transplantation of stem cells in the treatment of ovarian aging. Jiao et al. explored a combination of hUCMSCs and HA gel to rescue ovarian reserve and fecundity in a POI mouse model [228]. The HA gel not only increased the local retention of stem cells in the ovary, but also enhanced the paracrine function of hUCMSCs. The authors demonstrated that transplantation of hUCMSCs combined with HA gel could improve follicular survival by activating the PI3K-AKT pathway. Shin et al. transplanted embryonic stem cell-derived mesenchymal progenitor cells (ESC-MPCs) into cisplatin-induced POI mouse models by using HA gel scaffolds [229]. This method could effectively restore the ovarian structure and function of POI mice and improve the quality of oocytes and embryos, as well as the regularity of the estrus cycle. Interestingly, Zhao et al. demonstrated that HA supplementation prevented the occurrence of POI induced by treatment with the immunosuppressive agent tripterygium glycosides (TGs) [230]. This study indicated that HA promoted granulosa cell proliferation by upregulating PGRMC1 expression.
Fibrin
Fibrin (FIB), composed of fibrinogen and thrombin, is a natural scaffold formed after tissue injury, which can cause hemostasis and provide a useful initial matrix for cell adhesion, migration, proliferation and differentiation [231]. Fibrin has attracted the attention of tissue engineers because of its excellent biocompatibility, controllability and biodegradability as well as its ability to transfer cells and biomolecules. Fibrin is widely used in the development of cell-induced scaffolders [232], stem cell delivery [233] and induction of angiogenesis [234].
Fibrin by itself does not support follicular development in vitro because the encapsulated follicles secrete matrix-degrading proteolytic enzymes that cause follicle extrusion [235]. However, a combined culture system of fibrin alginate and fibrin thrombin can be successfully applied for in vitro follicular culture and artificial ovary construction [236–239]. Sadr et al. encapsulated mouse follicles in fibrin-alginate scaffolds and cultured them for 12 days. This culture system could improve follicular development and survival and produced mature oocytes [235]. Jin et al. isolated mouse secondary follicles and cultured them in a fibrin-alginate (FA) hydrogel matrix for 12 days [240]. The 3D culture system supported the growth of secondary follicles to the antral follicle stage and produced mature oocytes suitable for fertilization. Shikanov et al. developed a culture system based on the fibrin-sodium alginate interpenetration network (FA-IPN), which was subsequently used to grow mouse secondary follicles. This combination provided a dynamic mechanical environment that mimics the natural ovarian environment and contributed to increased meiosis maturation rates of oocytes [239, 241]. Brito used a culture system of FA to support the development of caprine preantral follicles, restore oocyte meiosis and promote oocyte maturation to produce parthenotes [220]. Notably, Xu et al. cultured isolated rhesus monkey secondary follicles in a fibrin alginate matrix for 40 days. The results showed that this culture system supported the growth of secondary follicles to the antral follicle stage in nonhuman primates and promoted the maturation of oocytes to the MII stage for the first time [242]. Subsequently, Xu et al. reported for the first time that fibrin-alginate 3D capsules could promote the development of primary follicles in primate rhesus monkeys and increase the production of follicle E2 and AMH in vitro [243]. In addition, they demonstrated that primate oocytes derived from primary follicles cultured in fibrin-alginate 3D capsules had the ability to restart meiosis for fertilization.
Cryo-thawed follicles have a better survival rate due to faster vascularization compared to the rate of ovarian tissue transplantation. The fibrin scaffold can control the release of growth factors and create a continuous path of cell infiltration between the host and graft [244], making it an ideal scaffold for follicular transplantation. Rajabzadeh et al. transplanted preantral follicles encapsulated in a fibrin hydrogel scaffold supplemented with platelet lysate [245]. The results showed that the culture system could significantly improve the local vascularization, survival rate, and growth of follicles. Luyckx et al. transplanted mouse preantral follicles and ovarian cells by wrapping them in a fibrin matrix containing low concentrations of fibrinogen and thrombin [246]. Almost all follicles were alive and grew to the antral follicular stage. The fibrin matrix also allowed for the proliferation of transplanted endothelial cells and capillary formation. Chiti et al. transplanted mouse primordial-primary and secondary follicles into severe combined immunodeficiency (SCID) mice by coating them with fibrinogen and thrombin (F12.5/T1) substrates. The results showed that isolated secondary follicles in the fibrin matrix could survive and grow to the antral follicle stage after short-term transplantation [247]. Smith et al. coated primordial follicles in fibrin hydrogel and transplanted them into an infertile mouse model [248]. The transplanted follicles could survive in infertile mice, develop into antral follicles and restore ovarian endocrine function. The surviving follicles were surrounded by the host interstitial tissue and produced luteum after ovulation. Paulini et al. xenografted human preantral follicles coated with a fibrin matrix containing fibrinogen and thrombin into the peritoneal sacs of nude mice [249]. The results showed that isolated human follicles were viable after encapsulation in fibrin clots.
Revascularization has always been an obstacle to the development of ovarian transplantation [244]. Shikanov et al. hypothesized that fibrin scaffolds provided a physical bridge between graft and host tissue and had the potential to enhance angiogenesis [244]. They transplanted vitrified/thawed ovarian tissue from mice coated with heparin binding peptide (HBP) and heparin-modified fibrin and loaded with vascular endothelial growth factor (VEGF) into infertile mouse models. The results showed that fibrin gel grafts could reduce ischemia and improve vascular remodeling after transplantation. The protocol also restored endocrine function and fertility in transplanted mice and allowed for natural conception. In addition, Gao et al. coated mouse ovarian tissues in fibrin hydrogels mixed with different concentrations of basic fibroblast growth factor (bFGF) and transplanted them under the skin of adult female mice for 1 week [250]. They demonstrated that bFGF and fibrin hydrogels could increase follicular survival and improve revascularization after ovarian transplantation. In addition, the high concentration of bFGF promoted the revascularization of transplanted ovarian tissue. Yang et al. transplanted mouse ovaries by wrapping them in fibrin hydrogels containing nitric oxide-releasing nanoparticles (NO-NPs) [251]. The results showed that the NO-NP/fibrin hydrogel improved the total number and quality of follicles after transplantation, induced angiogenesis, and prevented ischemic injury in the early stage of ovarian transplantation in mice. Shojafar et al. demonstrated that platelet-rich fibrin bioscaffolds indirectly reduced oxidative stress, promoted revascularization, and protected follicular cisterns from ischemia–reperfusion injury, thereby improving endocrine function and follicular formation in transplanted ovaries [252].
Fibrin-based scaffolders are also an alternative for the construction of artificial ovarian prototypes [253]. Chiti et al. compared the fiber thickness of four different fibrin formulations with the human ovarian cortex to optimize the composition of fibrin matrix and mimic the structure of human ovarian tissue, creating artificial ovaries that could grow human follicles [237]. Luyckx et al. reported that artificial ovaries formed by two optimal combinations of fibrinogen and thrombin (F12.5/T1 and F25/T4) enabled the survival and proliferation of isolated human ovarian stromal cells [238].
Alginate
Alginate is a group of non-branched polysaccharides composed of 1, 4-bound B-d-mannuronic acid (M) and A-l-guluronic acid (G) produced by brown algae and some bacteria [254]. Since alginate is non-toxic, rich in resources and easy to obtain, it is used as scaffold material for two-dimensional (2D) and 3D culture of mammalian cells [255]. In addition, alginate is able to form a soft hydrogel under physiological conditions, with pores large enough to allow nutrients and growth factors to pass through freely, while cells are trapped in the polymer network [256]. Their beneficial properties also include biocompatibility and biodegradability in human [257]. Based on the above advantages, alginate has become a very important biological material in pharmaceutical and biomedical fields.
Alginate hydrogels have been widely investigated in the culture of follicles from numerous animal species. The ultimate goal of an alginate system is to promote follicle development to obtain healthy oocytes that can be further matured and fertilized to produce embryos for fertility restoration [258]. Preantral follicles are the largest follicle population and represent an important source of potentially competent oocytes for further use in assisted reproductive technology [259]. There is evidence that the preantral follicle requires a hard tissue similar to the ovarian cortex to initiate in vitro development [260]. Alginate is an ideal scaffold for follicular culture in vitro and has been successfully applied in preantral follicle culture in many species. When Correia et al. coated goat primordial follicles in a sodium alginate 3D culture system, they showed an appropriate survival rate and high follicular activation rate [259]. Sadeghnia et al. evaluated sheep primordial/primary follicles in a sodium alginate three-dimensional culture system and found that the system supported the structural integrity of the follicles, with 2% sodium alginate supporting follicle growth better than 1% sodium alginate and increasing the diameter of the follicle [261]. The sodium alginate hydrogel matrix designed by Xu et al. promoted the follicular development of mature oocytes in vitro, and embryos extracted from cultured oocytes fertilized in vitro were transplanted into pseudopregnant female mice to produce healthy and fertile progeny [262]. In addition, studies have shown that, when coated with alginate and cultured, rhesus monkey secondary follicles could grow to the antral follicle stage and produce healthy oocytes for a long time [263, 264]. It is noteworthy that sodium alginate hydrogels can also be used for human ovary or follicle culture [265]. Kedem’s group tested the feasibility of culturing human ovarian cortex slices on macroporous sodium alginate scaffolds [266]. This study showed that there was an increase in the developing of follicle culture and a decrease in atretic follicles on sodium alginate scaffolds. Similarly, Laronda et al. coated human ovarian cortex-containing primordial follicles in sodium alginate hydrogel and found that the ovarian cortex grew, survived, and supported follicular development for up to 6 weeks in vitro [267]. Amorim et al. demonstrated that small human preantral follicles from frozen and thawed ovarian tissue could survive in vitro culture in alginate matrix for 7 days [268]. In addition, alginate culture systems can be used for IVM. Recently, Mastrorocco et al. developed a 3D IVM protocol for lamb COC encapsulated in the core of alginate microspheres [269]. This technique could increase the nuclear maturation rate of preadolescent oocytes and reduce the incidence of chromosome abnormalities, thus improving the in vitro performance of preadolescent lamb oocytes.
Jamalzaei et al. found that both the hardness and concentration of alginate ALG hydrogel affected follicle survival and found that the survival rate of 0.5%ALG cultured follicles was significantly higher than that of 0.75% and 1% ALG cultured follicles [270]. In addition, Jalili et al. compared the development of mouse preantral follicles in 3D media containing 0.25%, 0.5% and 1% sodium alginate [271]. They found that appropriate concentrations of sodium alginate hydrogels promoted follicular growth, maturation, and steroid hormones, with 0.5% alginate being the most favorable concentration. West’s group formed alginate brine gels of different hardnesses by changing the alginate solid concentration or by radiating or chemically oxidizing the polymer [258]. Secondary oocytes were coated with alginate gel with different hardnesses and growth, morphology and hormone secretion were observed. The results showed that reducing alginate matrix hardness could maintain intercellular tension homeostasis, promote cell processes, create a local paracrine environment and improve oocyte quality.
Alginate not only can provide a supportive environment for implanted cells, allowing for full diffusion of nutrients and oxygen, but it also can act as a barrier between host and graft to prevent rejection caused by infiltration of host immune cells, making it suitable for ovarian transplantation [272]. For the first time, Vanacker et al. successfully constructed an artificial ovary with alginate saline gel encapsulated in mouse preantral follicles and transplanted it into immunodeficient mice [273]. The results showed that the artificial ovary promoted follicular development and vascularization. Sittadjody et al. constructed a 3D bioengineered ovary with Sr-cross-linked alginate and coated it with granulosa and theca cells by a cell encapsulation technique, as depicted in Fig. 8. After implantation in ovariectomized rats, the artificial ovary achieved stable hormone secretion for 90 days and improved the adverse effects of hormone deficiency, including osteoporosis, uterine hypertrophy and obesity [274]. In addition, Felder et al. constructed alginate scaffolds with affinity-bound bone morphogenetic protein-4 (BMP-4) to mimic the ovary microenvironment, and it supported the culture and growth of primordial follicles. The study showed the restoration of ovarian function in ovariectomized SCID mice after transplantation of this scaffold coated with porcine primordial follicles [275].
Fig. 8.
Schematic diagram of a native ovarian follicle (a) compared to the bioengineered ovarian construct (b). 3D-confocal images of bioengineered ovarian construct (c) demonstrating compartmentalization of different cells within the constructs as determined through the use of CellTracker green-labeled cells (granulosa) in the inner layer and CellTracker orange-labeled cells (theca) in the outer layer. Images of bioengineered ovarian construct retrieved 90 days after transplantation into ovariectomized rats including the presence of the vascularized omentum pouch enclosing the constructs following explantation (d). Explanted constructs showed minimal fibrous encapsulation as indicated by H&E staining (e). Phase-contrast images of the microcapsules after retrieval show that the constructs remain intact throughout the 90-day period tested in vivo (f). Live/dead imaging of the retrieved capsules (g), where green indicates live and red indicates dead cells, which shows that most cells in the constructs remained viable during the 90-day implantation period. Scale bars are 100 μm for e–g (the figure is reproduced from Sittadjody et al. [274] with required copyright permission)
Synthetic biomaterials
Synthetic biomaterials can be tailored according to the physicochemical and mechanical properties of biological tissues, which have attracted great attention in the field of tissue engineering and regenerative medicine. At present, some studies also show that synthetic biomaterials play an important role in the recovery of ovarian function.
PEG is a commonly used biocompatible polymer that has been used to increase solubility, reduce accumulation, and prolong the blood half-life of various nanoparticles. Kim et al. engineered artificial ovarian tissue using a synthetic hydrogel, poly(ethylene glycol) vinyl sulfone (PEG-VS) as a supportive matrix [276]. The PEG-VS synthetic hydrogel was found to wrap immature follicles successfully and functioned as an artificial ovarian tissue in vivo for 60 days, demonstrating that PEG hydrogels provided a good microenvironment for follicles. Furthermore, Mendez et al. developed a three-dimensional PEG-based in vitro follicular culture system that could improve the survival and maturation rates of small follicles [277].
Supramolecular hydrogels, as a new type of soft biomaterial, have attracted extensive exploration because of their good biocompatibility and biodegradability. As shown in Fig. 9, Shi et al. designed a supramolecular hydrogel (Nap-Phe-Phe-Asp-Arg-Leu-Tyr-OH, Y)-coated receptor tyrosine kinase (RTK) inhibitor, called Gel Y + Inh (inhibitor of RTK), and developed an RTK responsive hydrogel to release Inh [278]. The results showed that the moderate release of Inh effectively delayed ovarian aging in aged mice by downregulation mTOR activity.
Fig. 9.
a Schematic illustration of RTK-instructed disassembly of hydrogel Gel Y + Inh for RTKs/PI3K signaling pathway inhibition. b RTK-instructed disassembly of Gel Y + Inh and the chemical structures of hydrogelator Y, its corresponding phosphate Yp, and a RTK inhibitor Inh. Photographs: Gel Y + Inh (left frame) and Gel Y + Inh incubated with SCFR (one type of RTKs) at 37 °C for 3 h (right frame). c Illustration of RTK-insusceptible hydrolgel Gel F + Inh and the chemical structures of hydrogelator F and Inh. Photographs: Gel F + Inh (left frame) and Gel F + Inh incubated with SCFR at 37 °C for 3 h (right frame) (the figure is reproduced from Shi et al. [278] with required copyright permission)
TheraCyte is an FDA-approved device made of a polytetrafluoroethylene (PTFE) membrane, which is impermeable to cells but allows for diffusion of soluble molecules through its 0.4-µm pores. David et al. transplanted a TheraCyte device coated with ovarian tissue into ovariectomized mice [279]. The TheraCyte device effectively isolated the graft from immune recognition and avoided the occurrence of immune rejection. The ovarian grafts encapsulated in TheraCyte devices could restore follicular development and ovarian endocrine function in ovariectomized mice.
Alhough synthetic biomaterials have been used in the treatment of ovarian aging, the low degradation rate, high hydrophobicity and low electrical conductivity of some synthetic polymer biomaterials remain major challenges in clinical application [280]. Conversely, the use of polymer biomaterials is often challenged by concerns about the lack of biological activity and foreign body reactions.
Biomaterials applied to ovarian aging-related diseases
Ovarian aging can lead to endometrial dysfunction, cardiovascular disease, osteoporosis, central nervous system-related disease, hyperlipidemia, and stress urinary incontinence, seriously decreasing the quality of life of aged women. Based on the advantages and significant effects of biomaterials in the field of antiaging, different biomaterials have good effects on the diseases associated with ovarian aging. Next, we summarize the current application of biomaterials for the treatment of diseases related to ovarian aging (Fig. 10).
Fig. 10.
Biomaterials applied to ovarian aging related diseases
Endometrial dysfunction
Steroid hormones are the basis of normal endometrial function, and the initiation and regulation of endometrial shedding and repair are tightly controlled by these ovarian steroids [281]. When ovarian function is defective, insufficient secretion of steroid hormones will lead to endometrial dysfunction, such as a thin endometrium and infertility [282]. Yoon et al. used a rat ovariectomy model to harvest autologous ovarian cells to construct ovarian spherical vascularized hydrogels (VHOS) for hormone autocrination, and they implanted them into the hind limbs of ovariectomized rats [283]. Such VHOS could significantly promote the recovery of endocrine function and release of hormones, thus achieving complete regeneration of the endometrium.
Cardiovascular disease
Cardiovascular disease is the leading cause of death among women globally, and cardiovascular risk increases substantially following menopause. Premature menopause is associated with cardiovascular disease, with higher cardiovascular risks observed at progressively earlier menopausal ages [284]. Studies have shown that ovarian hormones, mainly estrogen, may play key roles in reducing the risk of heart disease [285]. Han et al. implanted 17β-E2 eluting stents into the abdominal aortae of rabbits fed a high-fat diet. They observed that the implantation of 17β-E2-coated stents inhibited ERK activation and reduced the formation of new intima after angiotensin conversion, thereby preventing restenosis [286]. Similarly, New et al. assessed the effect of 17β-E2-eluting stents on neovascularization in a pig model and showed that 17β-E2-eluting stents had potential benefits in the prevention and treatment of in-stent restenosis [287].
Osteoporosis (OP)
Osteoporosis is a common disease in postmenopausal women characterized by reduced bone mass, deterioration of microstructures, and brittle fractures, affecting nearly one-third of women after the age of 50 [288]. Postmenopausal osteoporosis is largely the result of quantitative and qualitative bone changes caused by estrogen deficiency. Guo et al. constructed a drug delivery system based on PLGA nanoparticles (NPs), containing 17β-E2 and ferric oxide (Fe3O4) and modified it with alendronate sodium to achieve bone targeting and magnetic remote drug delivery [289]. In an osteoporosis model in ovariectomized (OVX) rats, the NPs showed a high encapsulation ability of E2 and were enriched in bone tissue. The three-month study showed that the NPs improved OVX-induced bone loss, increased bone strength and induced new bone formation with fewer adverse effects on other tissues. In another study, Takeuchi et al. explored an alternative administration route in a rat model of ovariectomized osteoporosis [290]. They prepared a transdermal delivery system of E2-loaded PLGA NPs for the treatment of osteoporosis. The results showed that E2-loaded PLGA NPs could effectively restore the bone mineral density of cancellous bone and prolong the interval between E2 administrations. In addition, Wang et al. developed a localized E2 delivery system incorporating β-cyclodextrin (CD-MBGNPs) and silk fibroin to sustain the constant release of E2 [291], as Fig. 11. The system could be utilized as a bone void filler for localized E2 delivery and bone regeneration in osteoporotic patients.
Fig. 11.
The schematic diagram of the design and preparation of beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers. (the figure is reproduced from Wang et al. [291]with required copyright permission)
The central nervous system
Decreased estrogen levels before, during and after menopause can affect memory and cognitive function [292]. Alzheimer's disease (AD) is the most common neurodegenerative disorder, and female sex is a key risk factor for AD, especially in postmenopausal women [293]. In addition, there has been considerable evidence that estrogen has important neuroregulatory and neuroprotective effects [294]. Therefore, estrogen could be a potential treatment for ameliorating postmenopausal degenerative diseases of the central nervous system [295]. One study by Kreuter showed that PLGA micro/nanocarriers could enhance the therapeutic activity of E2 in the central nervous system (CNS) [296]. Prakapenka et al. subcutaneously injected E2-loaded PLGA NPs into rats undergoing menopause induced by OVX surgery and evaluated spatial learning and memory [297]. They demonstrated that delivery of E2 from PLGA NPs could enhance the beneficial cognitive effects of E2 relative to free E2 or non-hormone loaded nanoparticle controls. As shown in Fig. 12, D'Amato et al. designed a biomaterial scaffold composed of electrospun fibers and films completely composed of poly (pro-E2), which released E2 locally during in vitro for a long period of 1–10 years [298]. These scaffolds demonstrated the ability to promote and guide neurite extension and protect neurons from oxidative stress damage.
Fig. 12.
a Step-growth polymerization is used to synthesize P1 from pro-E2 and PEG dithiol monomers to create a copolymer that can be processed into thin films and electrospun fibers that deliver E2 as they degrade via hydrolysis. b Three different neuron culture models are used to demonstrate that electrospun P1 fibers provide contact guidance for extending neurites, and P1 films are neurotrophic and neuroprotective against oxidative stress (the figure is reproduced from D'Amato et al. [298] with required copyright permission)
Hyperlipidemia
Menopause is associated with potentially adverse changes in serum lipids and lipoproteins, likely partially explaining the increase in cardiovascular disease (CVD) risk following menopause [299]. Estrogen deficiency due to ovarian function loss during menopause is a major cause of abnormal lipid metabolism. Improving estrogen levels is a promising approach for treating menopausal hyperlipidemia. Mittal et al. designed 17β-E2 capsule NPs to evaluate their efficacy in treating postmenopausal dyslipidemia in a rat model of hyperlipidemia induced by ovariectomy with a high-fat diet [300]. The results showed that these NPs improved the bioavailability of E2 and its effect on hyperlipidemia and alleviated the adverse effects of traditional hormone replacement therapy.
Stress urinary incontinence
Stress urinary incontinence (SUI) refers to the involuntary loss of urine in the absence of any prior sensation or need to empty; it occurs during physical activity and affects more than 50% of postmenopausal women [301]. The increased incidence of SUI in postmenopausal women is thought to be associated with decreased levels of 17β-E2 [301]. ADSCs are considered a promising method for the treatment of intrinsic sphincter deficiency due to urethral sphincter weakness and subsequent sphincter reconstruction [302, 303]. 17β-E2 has been shown to regulate the multidifferentiation ability of stem cells in bone, muscle, cartilage, and adipose tissue [304–306]. Based on this information, Feng's group developed a poly(l-lactide)/poly(e-caprolactone) electrospun nanoscaffold to combine ADSCs and E2 [307]. They found that the biocompatible cell/nanoscaffold with E2 could enhance the proliferation and myogenic differentiation of ADSCs and might be a feasible new option for SUI treatment.
Practical challenges with biomaterials for the clinical evaluation and treatment of ovarian aging
It is evident that a number of preclinical studies have described the potential clinical value of biomaterials in treating ovarian aging. However, despite the theoretical benefits of biomaterials in treating ovarian aging and improving ovarian function, practical challenges remain in translating them into clinical practice.
First, safety is a major concern. As an organ to exercise fertility, the ovary not only affects women's own health but also plays an important role in the safety of offspring. Therefore, the materials used in the evaluation and treatment of ovarian aging must ensure their safety. Compared to natural biomaterials, it is challenging to obtain regulatory approval for synthetic biomaterials, and they might need to demonstrate additional safety and efficacy. The different physicochemical properties of NPs can also have adverse effects on humans, animal cells and invertebrate models, ultimately leading to toxicological consequences [308, 309]. For example, Sirotkin et al. compared the effects of different morphologies of copper nanoparticles (CuNPs) (spherical, triangular, and hexagonal) on the function of porcine ovarian granulosa cells [310]. The results showed that the cell viability of granulosa cells decreased after treatment with hexagonal CuNPs but increased after treatment with other CuNPs. Stelzer et al. demonstrated by coincubating rat ovarian granulosa cells that AuNPs could enter mammalian ovarian granulosa cells and affect steroid production. In addition, the ovaries contain a rich and highly porous network of blood vessels [311] so that biomaterials can easily accumulate in the ovaries through blood circulation [312]. Through in vivo experiments, Schadlich et al. detected the accumulation of nanoparticles, nanocapsules and nanolipid emulsions at specific locations in mouse ovaries [313]. Moreover, evidence accumulated from in vivo and in vitro models has suggested that the accumulation of nanoparticles in the ovary could an impair ovarian function by affecting sex hormone synthesis [314, 315], follicular development [31, 316] and oocyte quality [317, 318]. Therefore, the ovarian toxicity of NPs should be considered when selecting materials for the diagnosis and treatment of ovarian aging and a better examination of the ovarian toxicity of NPs will help to improve NPs regulation and design safer NPs.
Second, the effectiveness of biomaterials in the evaluation and treatment of ovarian aging must be clarified, and there is a lack of effective preclinical models to accurately predict the efficacy outcomes of these biomaterials. The effect of biomaterials on improving ovarian function is mostly based on the results of animal experiments. However, animal models differ from humans under study in biology, immunology and genetics, leading to the failure of biomaterials to successfully transition from animal to human therapy. For example, mouse follicles require only 350 μm in diameter to mature, while human follicles must be at least 5 mm. The alginate 3D culture system allows for follicle growth and maintenance in mice, but its nondegradability limits follicle growth in humans [277]. Moreover, the properties of biomaterials themselves also affect their effectiveness. Hyaluronic acid hydrogels cannot provide nutritional support for follicular growth due to their poor mechanical properties or the lack of pores in their microstructure [221]. Fibrin itself is easily degraded by proteolytic enzymes secreted by enveloping follicles and therefore cannot support follicular development in vitro [235]. Furthermore, the long-term effectiveness of the biomaterials in the treatment of ovarian aging must be confirmed. Nikniaz et al. cultured isolated mouse antral follicles in vitro for only 7 days [190], which was too short to evaluate follicular development. Pors et al. reseeded human antral follicles on decellularized scaffolds and grafted them subcutaneously into immunodeficient mice for 3 weeks [186]. However, the three-week transplant period was not sufficient for prolonged follicular development in humans. In addition, immune rejection affects the effectiveness of biomaterials. Biomaterials are usually classified as materials placed in the body, not only as materials in contact with the outside of the body [319]. As exogenous substances, biomaterials could activate the immune system to attack them and cause immune rejection. Hassanpou et al. established a human decellularized ovarian scaffold and found that residual cellular substances and cytotoxic detergents in the ECM of the recellularized scaffold might promote some immune cell infiltration within the graft [179]. Therefore, the efficacy of some materials in the treatment of ovarian aging must be improved.
Other potential challenges of biomaterials for the clinical evaluation and treatment of ovarian aging cannot be ignored. For example, moving biomaterials from the laboratory and into clinical use requires demonstrating biocompatibility with human tissue, which in turn requires additional years of preclinical studies. In addition, the therapeutic mechanisms of many biomaterials in ovarian aging are unclear, which could limit their clinical translation. Another challenge to clinical translation is the FDA approval process, because nanomedicines are the most heavily regulated consumer products throughout the premarket and postmarket phases.
Therefore, the clinical transformation of biological materials used for the evaluation and treatment of ovarian aging requires a long period of scientific research to achieve. With the progress of future science and technology, it could be possible to innovate and transform safe and novel biological materials to meet clinical needs.
Conclusion and perspective
Timely diagnosis and treatment of ovarian aging and its related diseases have become urgent needs to improve the quality of life of women. This review highlights that the use of biomaterials could provide new directions for the diagnosis and treatment of ovarian aging. We have summarized innovative strategies for the evaluation and treatment of ovarian aging with different biomaterials. First, biological materials were constructed to detect hormone levels and were used for ultrasonic molecular imaging technology to achieve dynamic monitoring of ovarian function with high sensitivity and specificity. Furthermore, scientists have utilized the excellent properties of biomaterials to treat ovarian aging, such as improving the maturation and fertilization rates of oocytes, enhancing the treatment efficacy of stem cells, and developing artificial ovaries. Finally, different biomaterials used for the delivery of estrogen have good effects on the diseases associated with ovarian aging. All of these different options represent promising, albeit still experimental, strategies for improving ovarian aging. However, problems such as safety and effectiveness issues, lack of effective preclinical models, and unclear therapeutic mechanisms bring challenges to the clinical transformation of biomaterials. With advances in the regulatory mechanisms of biomaterials and enhanced safety assessments, biomaterials in the management of ovarian aging could be part of an exciting new strategy.
Acknowledgements
Not applicable.
Abbreviations
- HRT
Hormone replacement therapy
- ESCs
Embryonic stem cells
- MSCs
Mesenchymal stem cells
- iPSCs
Induced pluripotent stem cells
- AMD
Age-related macular degeneration
- PLGA
Poly lactic-co-glycolic acid
- AuNPs
Gold nanoparticles
- POF
Premature ovarian failure
- DNA
Deoxyribonucleic acid
- PM
Particulate matter
- PAHs
Polycyclic aromatic hydrocarbons
- POA
Premature ovarian aging
- HPO
Hypothalamic pituitary ovarian
- AITD
Autoimmune thyroid disease
- GnRH
Gonadotropin-releasing hormone
- IL-1α
Interleukin-1 alpha
- IFN-γ
Interferon gamma
- TNF-α
Tumor necrosis factor alpha
- POI
Premature ovarian insufficiency
- ROS
Reactive oxygen species
- DDR
DNA damage response
- MCM8
Minichromosome maintenance deficient 8
- MCM9
Minichromosome maintenance proteins 9
- MEIOB
Meiosis specific with OB domains
- MND1
Meiotic nuclear divisions 1
- PSMC3IP
Proteasome 26S ATPase subunit 3-interacting protein
- HFM1
ATP-dependent DNA helicase homolog
- MSH5
MutS protein homolog 5
- IVF
In vitro fertilization
- AMH
Anti-Müllerian hormone
- AFC
Antral follicle count
- TERT
Telomerase reverse transcriptase
- RNA
Ribonucleic acid
- H3
Histone H3
- DOR
Diminished ovarian reserve
- PLA
Polylactic acid
- PGA
Polyglycolic acid
- PCL
Polycaprolactone
- PEG
Polyethylene glycol
- EVs
Extracellular vesicles
- NLP
Non-conjugated luminescent polymers
- FDA
Food and Drug Administration
- MRMD2.0
A Python tool for machine learning
- TGF-β
Transforming growth factor β
- ELISA
Enzyme linked immunosorbent assay
- NBAMH
AMH-targeted nanobubbles
- NBs
Nanobubbles
- E1
Estrone
- E2
Estradiol
- E3
Estriol
- UV
Ultraviolet
- 17β-E2
17β-Estradiol
- FSH
Follicle stimulating hormone
- SINW-FET
Silicon nanowire field effect transistor
- H2N–Fe-MIL-101 MOFs
Amine-functionalized iron-containing metal–organic framework
- NicF
Porous nickel foam
- Ab-FSH
FSH antibody
- NiCo2O4/rGO
A novel nanomaterial
- ITO
Indium tin oxide
- mRNA
Messenger RNA
- miRNA
Micro RNA
- BMSCs
Bone marrow mesenchymal stem cells
- BMSC-exos
Exosomes derived from bone marrow mesenchymal stem cell
- p53
Tumor protein p53
- MSC
Mesenchymal stem cell
- hUCMSCs
Human umbilical cord mesenchymal stem cells
- hUCMSC-exos
Human umbilical cord mesenchymal stem cell derived exosomes
- CTX
Cyclophosphamide
- PI3K
Phosphatidylinositol 3-kinase
- AKT
Protein kinase B
- SIRT7
Sirtuin-7
- Bcl2
B-cell lymphoma-2
- Bax
Apoptosis regulator BAX
- Caspase
Cysteinyl aspartate specific proteinase
- hADMSCs
Human adipose mesenchymal stem cells
- hADMSC-exos
Human adipose mesenchymal stem cell derived exosomes
- SMAD
Recombinant mothers against decapentaplegic homolog
- hAMSCs
Human amniotic mesenchymal stem cells
- hAMSC-exos
Human amniotic mesenchymal stem cell derived exosomes
- SIRT4
Sirtuin-4
- hAECs
Human amniotic epithelial cells
- hAEC-exos
Human amniotic epithelial cell derived exosomes
- Bad
Bcl-xL/Bcl-2 associated death promoter
- PTEN
Gene of phosphate and tension homology deleted on chromosome ten
- mTOR
Molecular target of rapamycin
- AFMSCs
Amniotic fluid-derived mesenchymal stem cells
- AFMSC-exos
Amniotic fluid-derived mesenchymal stem cell derived exosomes
- MenSCs
Menstrual blood-derived stromal cells
- MenSC-exos
Menstrual blood-derived stromal cell-derived exosomes
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular regulated protein kinases
- WNT/B-CATENIN
Canonical Wnt/β-catenin pathway
- ECM
Extracellular matrix
- FN
Fibronectin
- PGs
Proteoglycans
- GAGs
Glycosaminoglycans
- SDS
Sodium dodecyl sulfate
- NAOH
Sodium hydroxide
- SLES
Sodium dodecyl sulfate
- P4
Progesterone
- FGSCs
Female germ line stem cells
- 3D
Three-dimensional
- IVM
In vitro maturation
- GC
Granulosa cell
- GV
Germinal foamed
- COH
Controlled ovarian overstimulation
- PDE3-I
Phosphodiesterase 3 inhibitor
- ADSCs
Adipose-derived stem cells
- collagen/UC-MSCs
Umbilical cord mesenchymal stem cells on collagen scaffolded
- FOXO3a
Forkhead box O3
- FOXO1
Forkhead box protein O1
- HA
Hyaluronic acid
- ALG
Alginate
- HAA
A composite hydrogel composed of HA and ALG
- COC
Cumulus oocyte complex
- HABH
Hyaluronic acid-based hydrogel
- VEGF
Vascular endothelial growth factor
- ESC-MPCs
Embryonic stem cells-derived mesenchymal progenitor cells
- TG
Tripterygium glycosides
- PGRMC1
Progesterone receptor membrane component 1
- FIB
Fibrin
- FA
Fibrin-alginate
- FA-IPN
Fibrin-sodium alginate interpenetration network
- SCID
Severe combined immunodeficiency
- F/T
Fibrinogen/thrombin
- HBP
Heparin binding peptide
- bFGF
Basic fibroblast growth factor
- NO-NPs
Nitric oxide releasing nanoparticles
- M
1, 4-Bound B-d-mannuronic acid
- G
A-l-guluronic acid
- 2D
Two-dimensional
- BMP-4
Bone morphogenetic protein-4
- PEG-VS
Poly (ethylene glycol) vinyl sulfone
- RTK
Receptor tyrosine kinase
- PTFE
Polytetrafluoroethylene
- VHOS
Ovarian spherical vascularized hydrogels
- OP
Osteoporosis
- NPs
Nanoparticles
- Fe3O4
Ferric oxide
- OVX
Ovariectomized
- CD-MBGNPs
A localized E2 delivery system with incorporation of β-cyclodextrin
- AD
Alzheimer's disease
- CNS
Central nervous system
- pro-E2
A biomaterial scaffold composed of electrospinning fibers and films completely composed of poly
- CVD
Cardiovascular disease
- SUI
Stress urinary incontinence
- CuNPs
Copper nanoparticles
Author contributions
SW, JD and WW guided the writing. MW and YG defined the focus of the review. MW and YG were major contributors in writing and drawing the manuscript. YG and SW were mainly responsible for drawing the manuscript. LX, WT, DC, JX, YH, FF, CW, YC, SZ, JZ, and YL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (nos. 81873824, 82001514, 82001498, 82101703, 22104040, 81701420, 81902669) and the Fundamental Research Funds for the Central Universities (HSUT: 2021yjsCXCY087).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng Wu and Yican Guo contributed equally to this work
Contributor Information
Wenwen Wang, Email: petrawang@163.com.
Shixuan Wang, Email: sxwang@tjh.tjmu.edu.cn.
References
- 1.Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur J Public Health. 2007;17:221–225. doi: 10.1093/eurpub/ckl103. [DOI] [PubMed] [Google Scholar]
- 2.Smith PE. Menopause assessment, treatment, and patient education. Nurse Pract. 2005;30:32–43. doi: 10.1097/00006205-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Laisk T, Tsuiko O, Jatsenko T, Horak P, Otala M, Lahdenpera M, Lummaa V, Tuuri T, Salumets A, Tapanainen JS. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update. 2019;25:34–50. doi: 10.1093/humupd/dmy031. [DOI] [PubMed] [Google Scholar]
- 4.Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, Lumsden MA, Mack WJ, Shapiro S, Baber RJ. Prevention of diseases after menopause. Climacteric. 2014;17:540–556. doi: 10.3109/13697137.2014.933411. [DOI] [PubMed] [Google Scholar]
- 5.Koothirezhi R, Ranganathan S. Postmenopausal Syndrome. Treasure Island: StatPearls; 2022. [PubMed] [Google Scholar]
- 6.Paciuc J. Hormone therapy in menopause. Adv Exp Med Biol. 2020;1242:89–120. doi: 10.1007/978-3-030-38474-6_6. [DOI] [PubMed] [Google Scholar]
- 7.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13:220–231. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 8.Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral contraceptives and HRT risk of thrombosis. Clin Appl Thromb Hemost. 2018;24:217–225. doi: 10.1177/1076029616683802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 11.Volarevic V, Bojic S, Nurkovic J, Volarevic A, Ljujic B, Arsenijevic N, Lako M, Stojkovic M. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. Biomed Res Int. 2014;2014:507234. doi: 10.1155/2014/507234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filip S, Mokry J, Horacek J, English D. Stem cells and the phenomena of plasticity and diversity: a limiting property of carcinogenesis. Stem Cells Dev. 2008;17:1031–1038. doi: 10.1089/scd.2007.0234. [DOI] [PubMed] [Google Scholar]
- 13.Zarzeczny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research & and the current role of the moral status of the embryo. Stem Cell Rev Rep. 2009;5:96–101. doi: 10.1007/s12015-009-9062-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Sun J, Huang Y, Bu S, Guo Y, Gu T, Li B, Wang C, Lai D. Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring microRNAs against apoptosis. Mol Ther Nucleic Acids. 2019;16:407–418. doi: 10.1016/j.omtn.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: a review. Bioact Mater. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Hu S, Wang S, Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6:141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brokesh AM, Gaharwar AK. Inorganic biomaterials for regenerative medicine. ACS Appl Mater Interfaces. 2020;12:5319–5344. doi: 10.1021/acsami.9b17801. [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Wu M, Wang Q, Ding S, Dong X, Xue L, Zhu Q, Zhou J, Xia F, Wang S, Hong Y. Red blood cell membrane-camouflaged nanoparticles loaded with AIEgen and Poly(I : C) for enhanced tumoral photodynamic-immunotherapy. Natl Sci Rev. 2021;8:nwab039. doi: 10.1093/nsr/nwab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He G, Yan X, Miao Z, Qian H, Ma Y, Xu Y, Gao L, Lu Y, Zha Z. Anti-inflammatory catecholic chitosan hydrogel for rapid surgical trauma healing and subsequent prevention of tumor recurrence. Chin Chem Lett. 2020;31:1807–1811. [Google Scholar]
- 20.Liu G, Bao Z, Wu J. Injectable baicalin/F127 hydrogel with antioxidant activity for enhanced wound healing. Chin Chem Lett. 2020;31:1817–1821. [Google Scholar]
- 21.Suri R, Neupane YR, Mehra N, Nematullah M, Khan F, Alam O, Iqubal A, Jain GK, Kohli K. Sirolimus loaded chitosan functionalized poly (lactic-co-glycolic acid) (PLGA) nanoparticles for potential treatment of age-related macular degeneration. Int J Biol Macromol. 2021;191:548–559. doi: 10.1016/j.ijbiomac.2021.09.069. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Cho B, Lee E, Kim J, Yoo J, Sung JS, Kwon Y, Kim J. Electromagnetized gold nanoparticles improve neurogenesis and cognition in the aged brain. Biomaterials. 2021;278:121157. doi: 10.1016/j.biomaterials.2021.121157. [DOI] [PubMed] [Google Scholar]
- 23.Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71:132–143. doi: 10.1016/j.ando.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Laven JS. Genetics of early and normal menopause. Semin Reprod Med. 2015;33:377–383. doi: 10.1055/s-0035-1567825. [DOI] [PubMed] [Google Scholar]
- 25.Huhtaniemi I, Hovatta O, La Marca A, Livera G, Monniaux D, Persani L, Heddar A, Jarzabek K, Laisk-Podar T, Salumets A, et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol Metab. 2018;29:400–419. doi: 10.1016/j.tem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Tesarik J, Galan-Lazaro M, Mendoza-Tesarik R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci. 2021;22:1371. doi: 10.3390/ijms22031371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry JR, Hsu YH, Chasman DI, Johnson AD, Elks C, Albrecht E, Andrulis IL, Beesley J, Berenson GS, Bergmann S, et al. DNA mismatch repair gene MSH6 implicated in determining age at natural menopause. Hum Mol Genet. 2014;23:2490–2497. doi: 10.1093/hmg/ddt620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haruty B, Friedman J, Hopp S, Daniels R, Pregler J. Reproductive health and the environment: counseling patients about risks. Cleve Clin J Med. 2016;83:367–372. doi: 10.3949/ccjm.83a.14070. [DOI] [PubMed] [Google Scholar]
- 29.Hipwell AE, Kahn LG, Factor-Litvak P, Porucznik CA, Siegel EL, Fichorova RN, Hamman RF, Klein-Fedyshin M, Harley KG. program collaborators for Environmental influences on Child Health O: exposure to non-persistent chemicals in consumer products and fecundability: a systematic review. Hum Reprod Update. 2019;25:51–71. doi: 10.1093/humupd/dmy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21:5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao G, Ze Y, Li B, Zhao X, Zhang T, Sheng L, Hu R, Gui S, Sang X, Sun Q, et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 32.Ates U, Ata B, Ortakuz S, Seyhan A, Urman B. Prevention of adhesion formation following ovarian surgery in a standardized animal model: comparative study of Interceed and double layer Surgicell. J Obstet Gynaecol Res. 2008;34:12–17. doi: 10.1111/j.1447-0756.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 33.Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, Dvornyk V. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19:126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- 34.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142:2554–2563. doi: 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Angelis C, Nardone A, Garifalos F, Pivonello C, Sansone A, Conforti A, Di Dato C, Sirico F, Alviggi C, Isidori A, et al. Smoke, alcohol and drug addiction and female fertility. Reprod Biol Endocrinol. 2020;18:21. doi: 10.1186/s12958-020-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mussa A, Russo S, De Crescenzo A, Freschi A, Calzari L, Maitz S, Macchiaiolo M, Molinatto C, Baldassarre G, Mariani M, et al. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2016;24:183–190. doi: 10.1038/ejhg.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol. 2007;66:309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 38.Poppe K. Management of endocrine disease: thyroid and female infertility: more questions than answers?! Eur J Endocrinol. 2021;184:R123–R135. doi: 10.1530/EJE-20-1284. [DOI] [PubMed] [Google Scholar]
- 39.Arrais RF, Dib SA. The hypothalamus-pituitary-ovary axis and type 1 diabetes mellitus: a mini review. Hum Reprod. 2006;21:327–337. doi: 10.1093/humrep/dei353. [DOI] [PubMed] [Google Scholar]
- 40.Inhasz Kiss AC, Woodside B, Sinzato YK, Bernardi MM, De Grava KW, Anselmo-Franci JA, Damasceno DC. Neonatally induced mild diabetes: influence on development, behavior and reproductive function of female Wistar rats. Diabetol Metab Syndr. 2013;5:61. doi: 10.1186/1758-5996-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dragojevic-Dikic S, Marisavljevic D, Mitrovic A, Dikic S, Jovanovic T, Jankovic-Raznatovic S. An immunological insight into premature ovarian failure (POF) Autoimmun Rev. 2010;9:771–774. doi: 10.1016/j.autrev.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Schlamp F, Huang L, Clark H, Brayboy L. Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction. 2020;159:325–337. doi: 10.1530/REP-19-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, Duncan FE. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152:245–260. doi: 10.1530/REP-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2016;23:1–18. doi: 10.1093/humupd/dmw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 46.Chang HM, Cheng JC, Klausen C, Taylor EL, Leung PC. Effects of recombinant activins on steroidogenesis in human granulosa-lutein cells. J Clin Endocrinol Metab. 2014;99:E1922–1932. doi: 10.1210/jc.2014-1223. [DOI] [PubMed] [Google Scholar]
- 47.Chang HM, Cheng JC, Klausen C, Leung PC. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol. 2013;27:2093–2104. doi: 10.1210/me.2013-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiao X, Zhang X, Li N, Zhang D, Zhao S, Dang Y, Zanvit P, Jin W, Chen ZJ, Chen W, Qin Y. Treg deficiency-mediated TH 1 response causes human premature ovarian insufficiency through apoptosis and steroidogenesis dysfunction of granulosa cells. Clin Transl Med. 2021;11:e448. doi: 10.1002/ctm2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12:655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- 52.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update. 2020;26:43–57. doi: 10.1093/humupd/dmz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruth KS, Day FR, Hussain J, Martinez-Marchal A, Aiken CE, Azad A, Thompson DJ, Knoblochova L, Abe H, Tarry-Adkins JL, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–397. doi: 10.1038/s41586-021-03779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heddar A, Dessen P, Flatters D, Misrahi M. Novel STAG3 mutations in a Caucasian family with primary ovarian insufficiency. Mol Genet Genomics. 2019;294:1527–1534. doi: 10.1007/s00438-019-01594-4. [DOI] [PubMed] [Google Scholar]
- 56.Luo M, Yang F, Leu NA, Landaiche J, Handel MA, Benavente R, La Salle S, Wang PJ. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nat Commun. 2013;4:2788. doi: 10.1038/ncomms3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jolly A, Bayram Y, Turan S, Aycan Z, Tos T, Abali ZY, Hacihamdioglu B, Coban Akdemir ZH, Hijazi H, Bas S, et al. Exome sequencing of a primary ovarian insufficiency cohort reveals common molecular etiologies for a spectrum of disease. J Clin Endocrinol Metab. 2019;104:3049–3067. doi: 10.1210/jc.2019-00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasapoglu I, Seli E. Mitochondrial dysfunction and ovarian aging. Endocrinology. 2020;161:bqaa001. doi: 10.1210/endocr/bqaa001. [DOI] [PubMed] [Google Scholar]
- 59.Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, Platero-Luengo A, Martinez-Redondo P, Ma H, Lee Y, et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- 60.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 61.Polonio AM, Chico-Sordo L, Cordova-Oriz I, Medrano M, Garcia-Velasco JA, Varela E. Impact of ovarian aging in reproduction: from telomeres and mice models to ovarian rejuvenation. Yale J Biol Med. 2020;93:561–569. [PMC free article] [PubMed] [Google Scholar]
- 62.Rocca MS, Foresta C, Ferlin A. Telomere length: lights and shadows on their role in human reproduction. Biol Reprod. 2019;100:305–317. doi: 10.1093/biolre/ioy208. [DOI] [PubMed] [Google Scholar]
- 63.Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20:15–30. doi: 10.1093/molehr/gat055. [DOI] [PubMed] [Google Scholar]
- 64.Uysal F, Kosebent EG, Toru HS, Ozturk S. Decreased expression of TERT and telomeric proteins as human ovaries age may cause telomere shortening. J Assist Reprod Genet. 2021;38:429–441. doi: 10.1007/s10815-020-01932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng J, Huang J, Yuan S, Zhou S, Yan W, Shen W, Chen Y, Xia X, Luo A, Zhu D, Wang S. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS ONE. 2017;12:e0177888. doi: 10.1371/journal.pone.0177888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsen KW, Castillo-Fernandez J, Chan AC, la Cour FN, Zedeler A, Bungum M, Cardona A, Perry JRB, Skouby SO, Hoffmann ER, et al. Identification of a unique epigenetic profile in women with diminished ovarian reserve. Fertil Steril. 2021;115:732–741. doi: 10.1016/j.fertnstert.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Salvador LM, Park Y, Cottom J, Maizels ET, Jones JC, Schillace RV, Carr DW, Cheung P, Allis CD, Jameson JL, Hunzicker-Dunn M. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem. 2001;276:40146–40155. doi: 10.1074/jbc.M106710200. [DOI] [PubMed] [Google Scholar]
- 68.Fortune JE, Rivera GM, Yang MY. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci. 2004;82–83:109–126. doi: 10.1016/j.anireprosci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Geng X, Zheng W, Tang J, Xu B, Shi Q. Current understanding of ovarian aging. Sci China Life Sci. 2012;55:659–669. doi: 10.1007/s11427-012-4352-5. [DOI] [PubMed] [Google Scholar]
- 70.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Zhang S, Wang J. Photo-crosslinkable hydrogel and its biological applications. Chin Chem Lett. 2021;32:1603–1614. [Google Scholar]
- 72.Liu H, Xiang X, Huang J, Zhu B, Wang L, Tang Y, Du F, Li L, Yan F, Ma L, Qiu L. Ultrasound augmenting injectable chemotaxis hydrogel for articular cartilage repair in osteoarthritis. Chin Chem Lett. 2021;32:1759–1764. [Google Scholar]
- 73.Dadashzadeh A, Moghassemi S, Shavandi A, Amorim CA. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021;135:48–63. doi: 10.1016/j.actbio.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 74.Yh A, Xy A, Lh A, Xin LA, Sw B, Zq C, Ls A. 3D porous acellular cartilage matrix scaffold with surface mediated sustainable release of TGF- β 3 for cartilage engineering. Chin Chem Lett. 2020;31:1797–1800. [Google Scholar]
- 75.Asti A, Gioglio L. Natural and synthetic biodegradable polymers: different scaffolds for cell expansion and tissue formation. Int J Artif Organs. 2014;37:187–205. doi: 10.530/ijao.5000307. [DOI] [PubMed] [Google Scholar]
- 76.Gagner JE, Kim W, Chaikof EL. Designing protein-based biomaterials for medical applications. Acta Biomater. 2014;10:1542–1557. doi: 10.1016/j.actbio.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. ScientificWorldJournal. 2015;2015:685690. doi: 10.1155/2015/685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fehlner-Peach H, Magnabosco C, Raghavan V, Scher JU, Tett A, Cox LM, Gottsegen C, Watters A, Wiltshire-Gordon JD, Segata N, et al. Distinct polysaccharide utilization profiles of human intestinal Prevotella copri isolates. Cell Host Microbe. 2019;26(680–690):e685. doi: 10.1016/j.chom.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Q, Qi C, Wang H, Xiao X, Zhuang Y, Gu S, Zhou Y, Wang L, Yang H, Xu W. Biocompatible and degradable Bletilla striata polysaccharide hemostasis sponges constructed from natural medicinal herb Bletilla striata. Carbohydr Polym. 2019;226:115304. doi: 10.1016/j.carbpol.2019.115304. [DOI] [PubMed] [Google Scholar]
- 80.Lai W-F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J Drug Deliv Sci Technol. 2020;59:101916. [Google Scholar]
- 81.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 82.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 83.Srikanth M, Kessler JA. Nanotechnology-novel therapeutics for CNS disorders. Nat Rev Neurol. 2012;8:307–318. doi: 10.1038/nrneurol.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pascual-Gil S, Garbayo E, Diaz-Herraez P, Prosper F, Blanco-Prieto MJ. Heart regeneration after myocardial infarction using synthetic biomaterials. J Control Release. 2015;203:23–38. doi: 10.1016/j.jconrel.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 85.Afnan MAM, Saxena AK. Tissue repair in neonatal and paediatric surgery: analysis of infection in surgical implantation of synthetic resorbable biomaterials. Biomed Mater Eng. 2018;29:799–808. doi: 10.3233/BME-181024. [DOI] [PubMed] [Google Scholar]
- 86.Obireddy SR, Lai W-F. Preparation and characterization of 2-hydroxyethyl starch microparticles for co-delivery of multiple bioactive agents. Drug Delivery. 2021;28:1562–1568. doi: 10.1080/10717544.2021.1955043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akgol S, Ulucan-Karnak F, Kuru CI, Kusat K. The usage of composite nanomaterials in biomedical engineering applications. Biotechnol Bioeng. 2021;118:2906–2922. doi: 10.1002/bit.27843. [DOI] [PubMed] [Google Scholar]
- 88.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133–2151. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 89.Bealer EJ, Kavetsky K, Dutko S, Lofland S, Hu X. Protein and polysaccharide-based magnetic composite materials for medical applications. Int J Mol Sci. 2019;21:186. doi: 10.3390/ijms21010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 1974;2016:17. doi: 10.3390/ijms17121974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akalin PK. Introduction to bioinformatics. Mol Nutr Food Res. 2006;50:610–619. doi: 10.1002/mnfr.200500273. [DOI] [PubMed] [Google Scholar]
- 92.Sargazi S, Ahmadi Z, Barani M, Rahdar A, Amani S, Desimone MF, Pandey S, Kyzas GZ. Can nanomaterials support the diagnosis and treatment of human infertility? A preliminary review. Life Sci. 2022;299:120539. doi: 10.1016/j.lfs.2022.120539. [DOI] [PubMed] [Google Scholar]
- 93.Cho YR, Kang M. Interpretable machine learning in bioinformatics. Methods. 2020;179:1–2. doi: 10.1016/j.ymeth.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 94.He SD, Guo F, Zou Q, Ding H. MRMD2.0: a Python tool for machine learning with feature ranking and reduction. Curr Bioinform. 2020;15:1213–1221. [Google Scholar]
- 95.Shrikhande L, Shrikhande B, Shrikhande A. AMH and its clinical implications. J Obstet Gynaecol India. 2020;70:337–341. doi: 10.1007/s13224-020-01362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kruszynska A, Slowinska-Srzednicka J. Anti-Mullerian hormone (AMH) as a good predictor of time of menopause. Prz Menopauzalny. 2017;16:47–50. doi: 10.5114/pm.2017.68591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 98.Tran D, Josso N. Localization of anti-Mullerian hormone in the rough endoplasmic reticulum of the developing bovine sertoli cell using immunocytochemistry with a monoclonal antibody. Endocrinology. 1982;111:1562–1567. doi: 10.1210/endo-111-5-1562. [DOI] [PubMed] [Google Scholar]
- 99.Bezard J, Vigier B, Tran D, Mauleon P, Josso N. Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J Reprod Fertil. 1987;80:509–516. doi: 10.1530/jrf.0.0800509. [DOI] [PubMed] [Google Scholar]
- 100.Vigier B, Picard JY, Campargue J, Forest MG, Heyman Y, Josso N. Secretion of anti-Mullerian hormone by immature bovine Sertoli cells in primary culture, studied by a competition-type radioimmunoassay: lack of modulation by either FSH or testosterone. Mol Cell Endocrinol. 1985;43:141–150. doi: 10.1016/0303-7207(85)90077-2. [DOI] [PubMed] [Google Scholar]
- 101.Ferguson JM, Pepin D, Duru C, Matejtschuk P, Donahoe PK, Burns CJ. Towards international standardization of immunoassays for Mullerian inhibiting substance/anti-Mullerian hormone. Reprod Biomed Online. 2018;37:631–640. doi: 10.1016/j.rbmo.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, Nardo LG, Pemberton PW. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27:3085–3091. doi: 10.1093/humrep/des260. [DOI] [PubMed] [Google Scholar]
- 103.Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375:162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 104.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Zhang S, Di N, Tayier B, Guan L, Wang G, Lu H, Yan F, Mu Y. Early evaluation of survival of the transplanted ovaries through ultrasound molecular imaging via targeted nanobubbles. Biomater Sci. 2020;8:5402–5414. doi: 10.1039/d0bm01125h. [DOI] [PubMed] [Google Scholar]
- 106.Shimizu Y. Estrogen: estrone (E1), estradiol (E2), estriol (E3) and estetrol (E4) Nihon Rinsho. 2005;63(Suppl 8):425–438. [PubMed] [Google Scholar]
- 107.Domingues TS, Rocha AM, Serafini PC. Tests for ovarian reserve: reliability and utility. Curr Opin Obstet Gynecol. 2010;22:271–276. doi: 10.1097/GCO.0b013e32833b4f5c. [DOI] [PubMed] [Google Scholar]
- 108.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 109.Skenandore CS, Pineda A, Bahr JM, Newell-Fugate AE, Cardoso FC. Evaluation of a commercially available radioimmunoassay and enzyme immunoassay for the analysis of progesterone and estradiol and the comparison of two extraction efficiency methods. Domest Anim Endocrinol. 2017;60:61–66. doi: 10.1016/j.domaniend.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 110.Caron E, Sheedy C, Farenhorst A. Development of competitive ELISAs for 17beta-estradiol and 17beta-estradiol +estrone+estriol using rabbit polyclonal antibodies. J Environ Sci Health B. 2010;45:145–151. doi: 10.1080/03601230903472090. [DOI] [PubMed] [Google Scholar]
- 111.Leivo J, Kivimaki L, Juntunen E, Pettersson K, Lamminmaki U. Development of anti-immunocomplex specific antibodies and non-competitive time-resolved fluorescence immunoassay for the detection of estradiol. Anal Bioanal Chem. 2019;411:5633–5639. doi: 10.1007/s00216-019-01952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taieb J, Benattar C, Diop R, Birr AS, Lindenbaum A. Use of the Architect-i2000 estradiol immunoassay during in vitro fertilization. Clin Chem. 2003;49:183–186. doi: 10.1373/49.1.183. [DOI] [PubMed] [Google Scholar]
- 113.Xue J, Zhao Q, Yang L, Ma H, Wu D, Liu L, Ren X, Ju H, Wei Q. Dual-mode sensing platform guided by intramolecular electrochemiluminescence of a ruthenium complex and cationic N, N-Bis(2-(trimethylammonium iodide)propylene) Perylene-3,4,9,10-tetracarboxydiimide for estradiol assay. Anal Chem. 2021;93:6088–6093. doi: 10.1021/acs.analchem.0c04563. [DOI] [PubMed] [Google Scholar]
- 114.Denver N, Khan S, Homer NZM, MacLean MR, Andrew R. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mol Biol. 2019;192:105373. doi: 10.1016/j.jsbmb.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stella A, Dey S. A sensitive and specific ESI-Positive LC-MS/MS method for the quantitation of estrogens in human serum in under three minutes. J Chromatogr Sci. 2021;59:280–288. doi: 10.1093/chromsci/bmaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siren H, Seppanen-Laakso T, Oresic M. Capillary electrophoresis with UV detection and mass spectrometry in method development for profiling metabolites of steroid hormone metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:375–382. doi: 10.1016/j.jchromb.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Yang P, Wang P, Wang L. Development of a novel luminol chemiluminescent method catalyzed by gold nanoparticles for determination of estrogens. Anal Bioanal Chem. 2007;387:585–592. doi: 10.1007/s00216-006-0925-0. [DOI] [PubMed] [Google Scholar]
- 118.Jin GP, Lin XQ. Voltammetric behavior and determination of estrogens at carbamylcholine modified paraffin-impregnated graphite electrode. Electrochim Acta. 2005;50:3556–3562. [Google Scholar]
- 119.Lin X, Li Y. A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosens Bioelectron. 2006;22:253–259. doi: 10.1016/j.bios.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y, Zhang L, Li Z, Gopinath SCB, Chen Y, Xiao Y. Aptamer-17beta-estradiol-antibody sandwich ELISA for determination of gynecological endocrine function. Biotechnol Appl Biochem. 2021;68:881–888. doi: 10.1002/bab.2008. [DOI] [PubMed] [Google Scholar]
- 121.Terui N, Fugetsu B, Tanaka S. Voltammetric behavior and determination of 17 beta-estradiol at multi-wall carbon nanotube-Nafion modified glassy carbon electrode. Anal Sci. 2006;22:895–898. doi: 10.2116/analsci.22.895. [DOI] [PubMed] [Google Scholar]
- 122.Naftolin F, Friedenthal J, Nachtigall R, Nachtigall L. Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment. F1000Res. 2019 doi: 10.12688/f1000research.15548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Management of osteoporosis in postmenopausal women the 2021 position statement of The North American Menopause Society. Menopause. 2021;28:973–997. doi: 10.1097/GME.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 124.Gomes ES, Leite FRF, Ferraz BRL, Mourao H, Malagutti AR. Voltammetric sensor based on cobalt-poly(methionine)-modified glassy carbon electrode for determination of estriol hormone in pharmaceuticals and urine. J Pharm Anal. 2019;9:347–357. doi: 10.1016/j.jpha.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol. 2018;60:R131–R155. doi: 10.1530/JME-17-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.La Marca A, Papaleo E, Grisendi V, Argento C, Giulini S, Volpe A. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle-stimulating hormone starting dose in in vitro fertilisation cycles. BJOG. 2012;119:1171–1179. doi: 10.1111/j.1471-0528.2012.03412.x. [DOI] [PubMed] [Google Scholar]
- 127.Steelman SL, Pohley FM. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology. 1953;53:604–616. doi: 10.1210/endo-53-6-604. [DOI] [PubMed] [Google Scholar]
- 128.Wang C. Bioassays of follicle stimulating hormone. Endocr Rev. 1988;9:374–377. doi: 10.1210/edrv-9-3-374. [DOI] [PubMed] [Google Scholar]
- 129.Ongaro L, Alonso CAI, Zhou X, Brule E, Li Y, Schang G, Parlow AF, Steyn F, Bernard DJ. Development of a highly sensitive ELISA for measurement of FSH in serum, plasma, and whole blood in mice. Endocrinology. 2021;162:bqab014. doi: 10.1210/endocr/bqab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y, Zheng H. Noncompetitive immunoassay for follicle-stimulating hormone in human serum using capillary electrophoresis with chemiluminescence detection. J Immunoassay Immunochem. 2010;31:193–204. doi: 10.1080/10739149.2010.488610. [DOI] [PubMed] [Google Scholar]
- 131.Tan X, David A, Day J, Tang H, Dixon ER, Zhu H, Chen YC, Khaing Oo MK, Shikanov A, Fan X. Rapid mouse follicle stimulating hormone quantification and estrus cycle analysis using an automated microfluidic chemiluminescent ELISA system. ACS Sens. 2018;3:2327–2334. doi: 10.1021/acssensors.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo J, Kong Z, Wang Y, Xie J, Liu J, Jin H, Cai X. Label-free Paper-based Immunosensor with Graphene Nanocomposites for Electrochemical Detection of Follicle-stimulating Hormone. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:2901–2904. doi: 10.1109/EMBC.2018.8512923. [DOI] [PubMed] [Google Scholar]
- 133.Patolsky F, Zheng G, Lieber CM. Nanowire-based biosensors. Anal Chem. 2006;78:4260–4269. doi: 10.1021/ac069419j. [DOI] [PubMed] [Google Scholar]
- 134.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 135.Lee M, Palanisamy S, Zhou BH, Wang LY, Chen CY, Lee CY, Yuan SF, Wang YM. Ultrasensitive electrical detection of follicle-stimulating hormone using a functionalized silicon nanowire transistor chemosensor. ACS Appl Mater Interfaces. 2018;10:36120–36127. doi: 10.1021/acsami.8b11882. [DOI] [PubMed] [Google Scholar]
- 136.Palanisamy S, Wu HM, Lee LY, Yuan SF, Wang YM. Fabrication of 3D amino-functionalized metal-organic framework on porous nickel foam skeleton to combinate follicle stimulating hormone antibody for specific recognition of follicle-stimulating hormone. JACS Au. 2021;1:2249–2260. doi: 10.1021/jacsau.1c00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pareek S, Jain U, Balayan S, Chauhan N. Ultra-sensitive nano- molecular imprinting polymer-based electrochemical sensor for Follicle-Stimulating Hormone (FSH) detection. Biochem Eng J. 2022;180:108329. [Google Scholar]
- 138.Zlitni A, Gambhir SS. Molecular imaging agents for ultrasound. Curr Opin Chem Biol. 2018;45:113–120. doi: 10.1016/j.cbpa.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol. 2010;65:567–581. doi: 10.1016/j.crad.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: moving toward clinical translation. Eur J Radiol. 2015;84:1685–1693. doi: 10.1016/j.ejrad.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, Hu XB, Xiang DX. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19:242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. doi: 10.1016/j.biomaterials.2020.120467. [DOI] [PubMed] [Google Scholar]
- 145.Sun B, Ma Y, Wang F, Hu L, Sun Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10:360. doi: 10.1186/s13287-019-1442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang M, Lin L, Sha C, Li T, Zhao D, Wei H, Chen Q, Liu Y, Chen X, Xu W, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest. 2020;100:342–352. doi: 10.1038/s41374-019-0321-y. [DOI] [PubMed] [Google Scholar]
- 148.Li Z, Zhang M, Zheng J, Tian Y, Zhang H, Tan Y, Li Q, Zhang J, Huang X. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the hippo signaling pathway. Front Endocrinol. 2021;12:711902. doi: 10.3389/fendo.2021.711902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu C, Yin H, Jiang H, Du X, Wang C, Liu Y, Li Y, Yang Z. Extracellular vesicles derived from mesenchymal stem cells recover fertility of premature ovarian insufficiency mice and the effects on their offspring. Cell Transpl. 2020;29:963689720923575. doi: 10.1177/0963689720923575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y, Jiang H. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther. 2019;10:250. doi: 10.1186/s13287-019-1327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, Li H, Huang B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 2020;38:1137–1148. doi: 10.1002/stem.3204. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J, Yin H, Jiang H, Du X, Yang Z. The protective effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on cisplatin-damaged granulosa cells. Taiwan J Obstet Gynecol. 2020;59:527–533. doi: 10.1016/j.tjog.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 153.Sun L, Li D, Song K, Wei J, Yao S, Li Z, Su X, Ju X, Chao L, Deng X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7:2552. doi: 10.1038/s41598-017-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su J, Ding L, Cheng J, Yang J, Li X, Yan G, Sun H, Dai J, Hu Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31:1075–1086. doi: 10.1093/humrep/dew041. [DOI] [PubMed] [Google Scholar]
- 155.Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9:216. doi: 10.1186/s13287-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ding C, Li H, Wang Y, Wang F, Wu H, Chen R, Lv J, Wang W, Huang B. Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Res Ther. 2017;8:173. doi: 10.1186/s13287-017-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding C, Qian C, Hou S, Lu J, Zou Q, Li H, Huang B. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to Prevent reactive oxygen species generation in POI. Mol Ther Nucleic Acids. 2020;21:37–50. doi: 10.1016/j.omtn.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4:124. doi: 10.1186/scrt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152. doi: 10.1186/s13287-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leaw B, Zhu D, Tan J, Muljadi R, Saad MI, Mockler JC, Wallace EM, Lim R, Tolcos M. Human amnion epithelial cells rescue cell death via immunomodulation of microglia in a mouse model of perinatal brain injury. Stem Cell Res Ther. 2017;8:46. doi: 10.1186/s13287-017-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harrell CR, Gazdic M, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Therapeutic Potential of amniotic fluid derived mesenchymal stem cells based on their differentiation capacity and immunomodulatory properties. Curr Stem Cell Res Ther. 2019;14:327–336. doi: 10.2174/1574888X14666190222201749. [DOI] [PubMed] [Google Scholar]
- 162.Spitzhorn LS, Rahman MS, Schwindt L, Ho HT, Wruck W, Bohndorf M, Wehrmeyer S, Ncube A, Beyer I, Hagenbeck C, et al. Isolation and molecular characterization of amniotic fluid-derived mesenchymal stem cells obtained from caesarean sections. Stem Cells Int. 2017;2017:5932706. doi: 10.1155/2017/5932706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thabet E, Yusuf A, Abdelmonsif DA, Nabil I, Mourad G, Mehanna RA. Extracellular vesicles miRNA-21: a potential therapeutic tool in premature ovarian dysfunction. Mol Hum Reprod. 2020;26:906–919. doi: 10.1093/molehr/gaaa068. [DOI] [PubMed] [Google Scholar]
- 165.Sanchez-Mata A, Gonzalez-Munoz E. Understanding menstrual blood-derived stromal/stem cells: definition and properties. Are we rushing into their therapeutic applications? iScience. 2021;24:103501. doi: 10.1016/j.isci.2021.103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang S, Huang B, Su P, Chang Q, Li P, Song A, Zhao X, Yuan Z, Tan J. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther. 2021;12:178. doi: 10.1186/s13287-021-02255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86:71. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- 169.Sohel MM, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, Rings F, Uddin MJ, Spencer TE, Schellander K, Tesfaye D. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS ONE. 2013;8:e78505. doi: 10.1371/journal.pone.0078505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzi P, Rizzari S, Maugeri M, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102(1751–1761):e1751. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 171.Matsuno Y, Onuma A, Fujioka YA, Yasuhara K, Fujii W, Naito K, Sugiura K. Effects of exosome-like vesicles on cumulus expansion in pigs in vitro. J Reprod Dev. 2017;63:51–58. doi: 10.1262/jrd.2016-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yuan C, Li Z, Zhao Y, Wang X, Chen L, Zhao Z, Cao M, Chen T, Iqbal T, Zhang B, et al. Follicular fluid exosomes: Important modulator in proliferation and steroid synthesis of porcine granulosa cells. FASEB J. 2021;35:e21610. doi: 10.1096/fj.202100030RR. [DOI] [PubMed] [Google Scholar]
- 173.Gebremedhn S, Gad A, Aglan HS, Laurincik J, Prochazka R, Salilew-Wondim D, Hoelker M, Schellander K, Tesfaye D. Extracellular vesicles shuttle protective messages against heat stress in bovine granulosa cells. Sci Rep. 2020;10:15824. doi: 10.1038/s41598-020-72706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rodrigues TA, Tuna KM, Alli AA, Tribulo P, Hansen PJ, Koh J, Paula-Lopes FF. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod Fertil Dev. 2019;31:888–897. doi: 10.1071/RD18450. [DOI] [PubMed] [Google Scholar]
- 175.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 178.Ford AJ, Rajagopalan P. Extracellular matrix remodeling in 3D: implications in tissue homeostasis and disease progression. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10:e1503. doi: 10.1002/wnan.1503. [DOI] [PubMed] [Google Scholar]
- 179.Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther. 2018;9:252. doi: 10.1186/s13287-018-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–29. doi: 10.1016/j.biomaterials.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kawecki M, Labus W, Klama-Baryla A, Kitala D, Kraut M, Glik J, Misiuga M, Nowak M, Bielecki T, Kasperczyk A. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix' scaffolds and their role in regenerative medicine. J Biomed Mater Res B Appl Biomater. 2018;106:909–923. doi: 10.1002/jbm.b.33865. [DOI] [PubMed] [Google Scholar]
- 182.Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng. 2014;42:1517–1527. doi: 10.1007/s10439-013-0963-7. [DOI] [PubMed] [Google Scholar]
- 184.Eivazkhani F, Abtahi NS, Tavana S, Mirzaeian L, Abedi F, Ebrahimi B, Montazeri L, Valojerdi MR, Fathi R. Evaluating two ovarian decellularization methods in three species. Mater Sci Eng C Mater Biol Appl. 2019;102:670–682. doi: 10.1016/j.msec.2019.04.092. [DOI] [PubMed] [Google Scholar]
- 185.Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brannstrom M, Hellstrom M. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res. 2019;12:58. doi: 10.1186/s13048-019-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pors SE, Ramlose M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, Kristensen SG. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34:1523–1535. doi: 10.1093/humrep/dez077. [DOI] [PubMed] [Google Scholar]
- 187.Liu WY, Lin SG, Zhuo RY, Xie YY, Pan W, Lin XF, Shen FX. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng Part C Methods. 2017;23:61–71. doi: 10.1089/ten.tec.2016.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sistani MN, Zavareh S, Valujerdi MR, Salehnia M. Characteristics of a decellularized human ovarian tissue created by combined protocols and its interaction with human endometrial mesenchymal cells. Prog Biomater. 2021;10:195–206. doi: 10.1007/s40204-021-00163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24:262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nikniaz H, Zandieh Z, Nouri M, Daei-Farshbaf N, Aflatoonian R, Gholipourmalekabadi M, Jameie SB. Comparing various protocols of human and bovine ovarian tissue decellularization to prepare extracellular matrix-alginate scaffold for better follicle development in vitro. BMC Biotechnol. 2021;21:8. doi: 10.1186/s12896-020-00658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alaee S, Asadollahpour R, Hosseinzadeh Colagar A, Talaei-Khozani T. The decellularized ovary as a potential scaffold for maturation of preantral ovarian follicles of prepubertal mice. Syst Biol Reprod Med. 2021;67:413–427. doi: 10.1080/19396368.2021.1968542. [DOI] [PubMed] [Google Scholar]
- 192.Kim EJ, Yang C, Lee J, Youm HW, Lee JR, Suh CS, Kim SH. The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology. 2020;144:33–40. doi: 10.1016/j.theriogenology.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 193.Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TAL. Creation of a bioengineered ovary: isolation of female germline stem cells for the repopulation of a decellularized ovarian bioscaffold. Methods Mol Biol. 2021;2273:139–149. doi: 10.1007/978-1-0716-1246-0_9. [DOI] [PubMed] [Google Scholar]
- 194.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(94):e91–99. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online. 2019;18:24. doi: 10.1186/s12938-019-0647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ding L, Li X, Sun H, Su J, Lin N, Peault B, Song T, Yang J, Dai J, Hu Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35:4888–4900. doi: 10.1016/j.biomaterials.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 197.Lind AK, Weijdegard B, Dahm-Kahler P, Molne J, Sundfeldt K, Brannstrom M. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet Gynecol Scand. 2006;85:1476–1484. doi: 10.1080/00016340601033741. [DOI] [PubMed] [Google Scholar]
- 198.Joo S, Oh SH, Sittadjody S, Opara EC, Jackson JD, Lee SJ, Yoo JJ, Atala A. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed Mater. 2016;11:065009. doi: 10.1088/1748-6041/11/6/065009. [DOI] [PubMed] [Google Scholar]
- 199.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87:367–374. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 200.Sharma GT, Dubey PK, Meur SK. Survival and developmental competence of buffalo preantral follicles using three-dimensional collagen gel culture system. Anim Reprod Sci. 2009;114:115–124. doi: 10.1016/j.anireprosci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 201.Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14:1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- 202.Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20:1349–1358. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- 203.Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil. 1994;100:333–339. doi: 10.1530/jrf.0.1000333. [DOI] [PubMed] [Google Scholar]
- 204.Alm H, Katska-Ksiazkiewicz L, Rynska B, Tuchscherer A. Survival and meiotic competence of bovine oocytes originating from early antral ovarian follicles. Theriogenology. 2006;65:1422–1434. doi: 10.1016/j.theriogenology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 205.Kossowska-Tomaszczuk K, Pelczar P, Guven S, Kowalski J, Volpi E, De Geyter C, Scherberich A. A novel three-dimensional culture system allows prolonged culture of functional human granulosa cells and mimics the ovarian environment. Tissue Eng Part A. 2010;16:2063–2073. doi: 10.1089/ten.TEA.2009.0684. [DOI] [PubMed] [Google Scholar]
- 206.Itami S, Yasuda K, Yoshida Y, Matsui C, Hashiura S, Sakai A, Tamotsu S. Co-culturing of follicles with interstitial cells in collagen gel reproduce follicular development accompanied with theca cell layer formation. Reprod Biol Endocrinol. 2011;9:159. doi: 10.1186/1477-7827-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol. 2004;16:211–219. doi: 10.1097/00001703-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 208.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 209.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 210.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 211.Anderiesz C, Fong CY, Bongso A, Trounson AO. Regulation of human and mouse oocyte maturation in vitro with 6-dimethylaminopurine. Hum Reprod. 2000;15:379–388. doi: 10.1093/humrep/15.2.379. [DOI] [PubMed] [Google Scholar]
- 212.Herrick JR. Reversible meiotic arrest in feline oocytes. Reprod Fertil Dev. 2014;26:258–267. doi: 10.1071/RD12341. [DOI] [PubMed] [Google Scholar]
- 213.Vanhoutte L, Nogueira D, De Sutter P. Prematuration of human denuded oocytes in a three-dimensional co-culture system: effects on meiosis progression and developmental competence. Hum Reprod. 2009;24:658–669. doi: 10.1093/humrep/den420. [DOI] [PubMed] [Google Scholar]
- 214.Yang Y, Lei L, Wang S, Sheng X, Yan G, Xu L, Liu J, Liu M, Zhen X, Ding L, Sun H. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell Dev Biol Anim. 2019;55:302–311. doi: 10.1007/s11626-019-00337-4. [DOI] [PubMed] [Google Scholar]
- 215.Ding L, Yan G, Wang B, Xu L, Gu Y, Ru T, Cui X, Lei L, Liu J, Sheng X, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci. 2018;61:1554–1565. doi: 10.1007/s11427-017-9272-2. [DOI] [PubMed] [Google Scholar]
- 216.Salwowska NM, Bebenek KA, Zadlo DA, Wcislo-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016;15:520–526. doi: 10.1111/jocd.12237. [DOI] [PubMed] [Google Scholar]
- 217.Sudha PN, Rose MH. Beneficial effects of hyaluronic acid. Adv Food Nutr Res. 2014;72:137–176. doi: 10.1016/B978-0-12-800269-8.00009-9. [DOI] [PubMed] [Google Scholar]
- 218.Marei WF, Ghafari F, Fouladi-Nashta AA. Role of hyaluronic acid in maturation and further early embryo development of bovine oocytes. Theriogenology. 2012;78:670–677. doi: 10.1016/j.theriogenology.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 219.Desai N, Abdelhafez F, Calabro A, Falcone T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod Biol Endocrinol. 2012;10:29. doi: 10.1186/1477-7827-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brito IR, Silva GM, Sales AD, Lobo CH, Rodrigues GQ, Sousa RF, Moura A, Calderon C, Bertolini M, Campello CC, et al. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod Domest Anim. 2016;51:997–1009. doi: 10.1111/rda.12779. [DOI] [PubMed] [Google Scholar]
- 221.Bhattacharjee P, Kundu B, Naskar D, Kim HW, Maiti TK, Bhattacharya D, Kundu SC. Silk scaffolds in bone tissue engineering: an overview. Acta Biomater. 2017;63:1–17. doi: 10.1016/j.actbio.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 222.Jamalzaei P, Rezazadeh Valojerdi M, Montazeri L, Baharvand H. Applicability of hyaluronic acid-alginate hydrogel and ovarian cells for in vitro development of mouse preantral follicles. Cell J. 2020;22:49–60. doi: 10.22074/cellj.2020.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shahedi A, Hosseini A, Khalili MA, Norouzian M, Salehi M, Piriaei A, Nottola SA. The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol. 2013;167:69–75. doi: 10.1016/j.ejogrb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 224.Paim LM, Gal LL, Lopes RF, Oliveira AT. Vitrification of Rattus norvegicus immature cumulus-oocyte complexes using hyaluronic acid. In Vitro Cell Dev Biol Anim. 2015;51:995–1002. doi: 10.1007/s11626-015-9940-9. [DOI] [PubMed] [Google Scholar]
- 225.Tavana S, Azarnia M, Valojerdi MR, Shahverdi A. Hyaluronic acid-based hydrogel scaffold without angiogenic growth factors enhances ovarian tissue function after autotransplantation in rats. Biomed Mater. 2016;11:055006. doi: 10.1088/1748-6041/11/5/055006. [DOI] [PubMed] [Google Scholar]
- 226.Taheri MA, Valojerdi MR, Ebrahimi B. Intramuscular autotransplantation of vitrified rat ovary encapsulated with hyaluronic acid hydrogel. Biopreserv Biobank. 2016;14:114–121. doi: 10.1089/bio.2015.0021. [DOI] [PubMed] [Google Scholar]
- 227.Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, Abir R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–482. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 228.Jiao W, Mi X, Yang Y, Liu R, Liu Q, Yan T, Chen ZJ, Qin Y, Zhao S. Mesenchymal stem cells combined with autocrosslinked hyaluronic acid improve mouse ovarian function by activating the PI3K-AKT pathway in a paracrine manner. Stem Cell Res Ther. 2022;13:49. doi: 10.1186/s13287-022-02724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Shin EY, Kim DS, Lee MJ, Lee AR, Shim SH, Baek SW, Han DK, Lee DR. Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells. Stem Cell Res Ther. 2021;12:431. doi: 10.1186/s13287-021-02479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhao G, Yan G, Cheng J, Zhou X, Fang T, Sun H, Hou Y, Hu Y. Hyaluronic acid prevents immunosuppressive drug-induced ovarian damage via up-regulating PGRMC1 expression. Sci Rep. 2015;5:7647. doi: 10.1038/srep07647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomed. 2017;12:4937–4961. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 234.Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med. 2009;4:527–538. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sadr SZ, Fatehi R, Maroufizadeh S, Amorim CA, Ebrahimi B. Utilizing fibrin-alginate and matrigel-alginate for mouse follicle development in three-dimensional culture systems. Biopreserv Biobank. 2018;16:120–127. doi: 10.1089/bio.2017.0087. [DOI] [PubMed] [Google Scholar]
- 236.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, Van Ruymbeke E, Champagne SD, Donnez J, Amorim CA. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35:41–48. doi: 10.1007/s10815-017-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Luyckx V, Dolmans MM, Vanacker J, Scalercio SR, Donnez J, Amorim CA. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J Ovarian Res. 2013;6:83. doi: 10.1186/1757-2215-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shikanov A, Xu M, Woodruff TK, Shea LD. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp. 2011 doi: 10.3791/2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–2200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–3104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rajabzadeh A, Jahanpeyma F, Talebi A, Moradi F, Hamidieh AA, Eimani H. Fibrin scaffold incorporating platelet lysate enhance follicle survival and angiogenesis in cryopreserved preantral follicle transplantation. Galen Med J. 2020;9:e1558. doi: 10.31661/gmj.v9i0.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Luyckx V, Dolmans MM, Vanacker J, Legat C, Fortuno Moya C, Donnez J, Amorim CA. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril. 2014;101:1149–1156. doi: 10.1016/j.fertnstert.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 247.Chiti MC, Dolmans MM, Orellana R, Soares M, Paulini F, Donnez J, Amorim CA. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum Reprod. 2016;31:427–435. doi: 10.1093/humrep/dev299. [DOI] [PubMed] [Google Scholar]
- 248.Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A. 2014;20:3021–3030. doi: 10.1089/ten.tea.2013.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Paulini F, Vilela JM, Chiti MC, Donnez J, Jadoul P, Dolmans MM, Amorim CA. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod Biomed Online. 2016;33:425–432. doi: 10.1016/j.rbmo.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 250.Gao JM, Yan J, Li R, Li M, Yan LY, Wang TR, Zhao HC, Zhao Y, Yu Y, Qiao J. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum Reprod. 2013;28:2784–2793. doi: 10.1093/humrep/det296. [DOI] [PubMed] [Google Scholar]
- 251.Yang C, Chung N, Song C, Youm HW, Lee K, Lee JR. Promotion of angiogenesis toward transplanted ovaries using nitric oxide releasing nanoparticles in fibrin hydrogel. Biofabrication. 2021;14:011001. doi: 10.1088/1758-5090/ac3f28. [DOI] [PubMed] [Google Scholar]
- 252.Shojafar E, Mehranjani MS, Shariatzadeh SM. Utilizing platelet-rich fibrin bioscaffold at the graft site improves the structure and function of mice ovarian grafts. Regen Med. 2019;14:409–422. doi: 10.2217/rme-2018-0050. [DOI] [PubMed] [Google Scholar]
- 253.Chiti MC, Dolmans MM, Donnez J, Amorim CA. Fibrin in reproductive tissue engineering: a review on its application as a biomaterial for fertility preservation. Ann Biomed Eng. 2017;45:1650–1663. doi: 10.1007/s10439-017-1817-5. [DOI] [PubMed] [Google Scholar]
- 254.Dalheim MO, Vanacker J, Najmi MA, Aachmann FL, Strand BL, Christensen BE. Efficient functionalization of alginate biomaterials. Biomaterials. 2016;80:146–156. doi: 10.1016/j.biomaterials.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 255.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rokstad AM, Brekke OL, Steinkjer B, Ryan L, Kollarikova G, Strand BL, Skjak-Braek G, Lacik I, Espevik T, Mollnes TE. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011;7:2566–2578. doi: 10.1016/j.actbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 257.Uyen NTT, Hamid ZAA, Tram NXT, Ahmad N. Fabrication of alginate microspheres for drug delivery: a review. Int J Biol Macromol. 2020;153:1035–1046. doi: 10.1016/j.ijbiomac.2019.10.233. [DOI] [PubMed] [Google Scholar]
- 258.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Correia HHV, Lima LF, Sousa FGC, Ferreira ACA, Cadenas J, Paes VM, Alves BG, Shikanov A, Figueiredo JR. Activation of goat primordial follicles in vitro: influence of alginate and ovarian tissue. Reprod Domest Anim. 2020;55:105–109. doi: 10.1111/rda.13582. [DOI] [PubMed] [Google Scholar]
- 260.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sadeghnia S, Akhondi MM, Hossein G, Mobini S, Hosseini L, Naderi MM, Boroujeni SB, Sarvari A, Behzadi B, Shirazi A. Development of sheep primordial follicles encapsulated in alginate or in ovarian tissue in fresh and vitrified samples. Cryobiology. 2016;72:100–105. doi: 10.1016/j.cryobiol.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 262.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67:64–69. doi: 10.1016/j.cryobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 266.Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, Dor J, Freud E, Felz C, Abir R. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28:761–769. doi: 10.1007/s10815-011-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 269.Mastrorocco A, Cacopardo L, Lamanna D, Temerario L, Brunetti G, Carluccio A, Robbe D, Dell'Aquila ME. Bioengineering approaches to improve in vitro performance of prepubertal lamb oocytes. Cells. 2021;10:1458. doi: 10.3390/cells10061458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jamalzaei P, Valojerdi MR, Montazeri L, Baharvand H. Effects of alginate concentration and ovarian cells on in vitro development of mouse preantral follicles: a factorial study. Int J Fertil Steril. 2020;13:330–338. doi: 10.22074/ijfs.2020.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jalili C, Khani Hemmatabadi F, Mansouri K, Bakhtiyari M. Effects of sodium alginate capsules as 3D scaffolds on hormones and genes expression in preantral follicles of mice compared to 2D medium: an experimental study. Int J Reprod Biomed. 2020;18:517–530. doi: 10.18502/ijrm.v13i7.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vanacker J, Dolmans MM, Luyckx V, Donnez J, Amorim CA. First transplantation of isolated murine follicles in alginate. Regen Med. 2014;9:609–619. doi: 10.2217/rme.14.33. [DOI] [PubMed] [Google Scholar]
- 274.Sittadjody S, Saul JM, McQuilling JP, Joo S, Register TC, Yoo JJ, Atala A, Opara EC. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun. 1858;2017:8. doi: 10.1038/s41467-017-01851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Felder S, Masasa H, Orenbuch A, Levaot N, Shachar Goldenberg M, Cohen S. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials. 2019;205:11–22. doi: 10.1016/j.biomaterials.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 276.Kim J, Perez AS, Claflin J, David A, Zhou H, Shikanov A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. NPJ Regen Med. 2016;1:16010. doi: 10.1038/npjregenmed.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mendez U, Zhou H, Shikanov A. Synthetic PEG hydrogel for engineering the environment of ovarian follicles. Methods Mol Biol. 2018;1758:115–128. doi: 10.1007/978-1-4939-7741-3_9. [DOI] [PubMed] [Google Scholar]
- 278.Shi Z, Li X, Wei M, Chen P, Zhang T, Ling X, Zhang J, Zhao C, Wang F, Liang G. Receptor tyrosine kinases-instructed release of its inhibitor from hydrogel to delay ovarian aging. Biomaterials. 2021;269:120536. doi: 10.1016/j.biomaterials.2020.120536. [DOI] [PubMed] [Google Scholar]
- 279.David A, Day JR, Cichon AL, Lefferts A, Cascalho M, Shikanov A. Restoring ovarian endocrine function with encapsulated ovarian allograft in immune competent mice. Ann Biomed Eng. 2017;45:1685–1696. doi: 10.1007/s10439-016-1780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Amani H, Kazerooni H, Hassanpoor H, Akbarzadeh A, Pazoki-Toroudi H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: a review. Artif Cells Nanomed Biotechnol. 2019;47:3524–3539. doi: 10.1080/21691401.2019.1639723. [DOI] [PubMed] [Google Scholar]
- 281.Maybin JA, Critchley HO. Steroid regulation of menstrual bleeding and endometrial repair. Rev Endocr Metab Disord. 2012;13:253–263. doi: 10.1007/s11154-012-9228-2. [DOI] [PubMed] [Google Scholar]
- 282.Bachmann GA, Leiblum SR. The impact of hormones on menopausal sexuality: a literature review. Menopause. 2004;11:120–130. doi: 10.1097/01.GME.0000075502.60230.28. [DOI] [PubMed] [Google Scholar]
- 283.Yoon HJ, Lee YJ, Baek S, Chung YS, Kim DH, Lee JH, Shin YC, Shin YM, Ryu C, Kim HS, et al. Hormone autocrination by vascularized hydrogel delivery of ovary spheroids to rescue ovarian dysfunctions. Sci Adv. 2021;7:eabe8873. doi: 10.1126/sciadv.abe8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, Nakao T, Whitsel EA, Farland LV, Laurie C, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143:410–423. doi: 10.1161/CIRCULATIONAHA.120.051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Fortini F, Vieceli Dalla Sega F, Caliceti C, Lambertini E, Pannuti A, Peiffer DS, Balla C, Rizzo P. Estrogen-mediated protection against coronary heart disease: the role of the Notch pathway. J Steroid Biochem Mol Biol. 2019;189:87–100. doi: 10.1016/j.jsbmb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 286.Han Y, Liang M, Kang J, Qi Y, Deng J, Xu K, Yan C. Estrogen-eluting stent implantation inhibits neointimal formation and extracellular signal-regulated kinase activation. Catheter Cardiovasc Interv. 2007;70:647–653. doi: 10.1002/ccd.21156. [DOI] [PubMed] [Google Scholar]
- 287.New G, Moses JW, Roubin GS, Leon MB, Colombo A, Iyer SS, Tio FO, Mehran R, Kipshidze N. Estrogen-eluting, phosphorylcholine-coated stent implantation is associated with reduced neointimal formation but no delay in vascular repair in a porcine coronary model. Catheter Cardiovasc Interv. 2002;57:266–271. doi: 10.1002/ccd.10339. [DOI] [PubMed] [Google Scholar]
- 288.Gosset A, Pouilles JM, Tremollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2021;35:101551. doi: 10.1016/j.beem.2021.101551. [DOI] [PubMed] [Google Scholar]
- 289.Guo Y, Liu Y, Shi C, Wu T, Cui Y, Wang S, Liu P, Feng X, He Y, Fu D. Remote-controllable bone-targeted delivery of estradiol for the treatment of ovariectomy-induced osteoporosis in rats. J Nanobiotechnol. 2021;19:248. doi: 10.1186/s12951-021-00976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Takeuchi I, Kobayashi S, Hida Y, Makino K. Estradiol-loaded PLGA nanoparticles for improving low bone mineral density of cancellous bone caused by osteoporosis: application of enhanced charged nanoparticles with iontophoresis. Colloids Surf B Biointerfaces. 2017;155:35–40. doi: 10.1016/j.colsurfb.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 291.Wang D, Steffi C, Wang Z, Kong CH, Lim PN, Shi Z, Thian ES, Wang W. Beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers as an implantable estradiol delivery system for the potential treatment of osteoporosis. Nanoscale. 2018;10:18341–18353. doi: 10.1039/c8nr05268a. [DOI] [PubMed] [Google Scholar]
- 292.Boyle CP, Raji CA, Erickson KI, Lopez OL, Becker JT, Gach HM, Kuller LH, Longstreth W, Jr, Carmichael OT, Riedel BC, Thompson PM. Estrogen, brain structure, and cognition in postmenopausal women. Hum Brain Mapp. 2021;42:24–35. doi: 10.1002/hbm.25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD. Increased Alzheimer's risk during the menopause transition: a 3-year longitudinal brain imaging study. PLoS ONE. 2018;13:e0207885. doi: 10.1371/journal.pone.0207885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. J Steroid Biochem Mol Biol. 2003;85:473–482. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- 295.Prakapenka AV, Bimonte-Nelson HA, Sirianni RW. Engineering poly(lactic-co-glycolic acid) (PLGA) micro- and nano-carriers for controlled delivery of 17beta-Estradiol. Ann Biomed Eng. 2017;45:1697–1709. doi: 10.1007/s10439-017-1859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 297.Prakapenka AV, Quihuis AM, Carson CG, Patel S, Bimonte-Nelson HA, Sirianni RW. Poly(lactic-co-glycolic acid) nanoparticle encapsulated 17beta-estradiol improves spatial memory and increases uterine stimulation in middle-aged ovariectomized rats. Front Behav Neurosci. 2020;14:597690. doi: 10.3389/fnbeh.2020.597690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.D'Amato AR, Puhl DL, Ellman SAT, Balouch B, Gilbert RJ, Palermo EF. Vastly extended drug release from poly(pro-17beta-estradiol) materials facilitates in vitro neurotrophism and neuroprotection. Nat Commun. 2019;10:4830. doi: 10.1038/s41467-019-12835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Reiner Z. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14:401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 300.Mittal G, Chandraiah G, Ramarao P, Ravi Kumar MN. Pharmacodynamic evaluation of oral estradiol nanoparticles in estrogen deficient (ovariectomized) high-fat diet induced hyperlipidemic rat model. Pharm Res. 2009;26:218–223. doi: 10.1007/s11095-008-9725-x. [DOI] [PubMed] [Google Scholar]
- 301.Augoulea A, Sioutis D, Rizos D, Panoulis C, Triantafyllou N, Armeni E, Deligeoroglou E, Chrelias C, Creatsa M, Liapis A, Lambrinoudaki I. Stress urinary incontinence and endogenous sex steroids in postmenopausal women. Neurourol Urodyn. 2017;36:121–125. doi: 10.1002/nau.22885. [DOI] [PubMed] [Google Scholar]
- 302.Yamamoto T, Gotoh M, Hattori R, Toriyama K, Kamei Y, Iwaguro H, Matsukawa Y, Funahashi Y. Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: report of two initial cases. Int J Urol. 2010;17:75–82. doi: 10.1111/j.1442-2042.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 303.Cui L, Meng Q, Wen J, Gao Z, Yan Z, Tian Y, Xu P, He X, Yu H, Lian P. A functional comparison of treatment of intrinsic sphincter deficiency with muscle-derived and adipose tissue-derived stem cells. IUBMB Life. 2018;70:976–984. doi: 10.1002/iub.1896. [DOI] [PubMed] [Google Scholar]
- 304.Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, Hunziker W, Weber P, Martin I, Bendik I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology. 2004;145:848–859. doi: 10.1210/en.2003-1014. [DOI] [PubMed] [Google Scholar]
- 305.Dang Z, Lowik CW. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J Bone Miner Res. 2004;19:853–861. doi: 10.1359/JBMR.040120. [DOI] [PubMed] [Google Scholar]
- 306.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229:1127–1135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 307.Feng C, Hu J, Liu C, Liu S, Liao G, Song L, Zeng X. Association of 17-beta estradiol with adipose-derived stem cells: new strategy to produce functional myogenic differentiated cells with a nano-scaffold for tissue engineering. PLoS ONE. 2016;11:e0164918. doi: 10.1371/journal.pone.0164918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4:347–363. doi: 10.3109/17435390.2010.509519. [DOI] [PubMed] [Google Scholar]
- 309.Dwivedi S, Saquib Q, Ahmad B, Ansari SM, Azam A, Musarrat J. Toxicogenomics: a new paradigm for nanotoxicity evaluation. Adv Exp Med Biol. 2018;1048:143–161. doi: 10.1007/978-3-319-72041-8_9. [DOI] [PubMed] [Google Scholar]
- 310.Sirotkin AV, Radosova M, Tarko A, Martin-Garcia I, Alonso F. Effect of morphology and support of copper nanoparticles on basic ovarian granulosa cell functions. Nanotoxicology. 2020;14:683–695. doi: 10.1080/17435390.2020.1736680. [DOI] [PubMed] [Google Scholar]
- 311.Brown HM, Russell DL. Blood and lymphatic vasculature in the ovary: development, function and disease. Hum Reprod Update. 2014;20:29–39. doi: 10.1093/humupd/dmt049. [DOI] [PubMed] [Google Scholar]
- 312.Stelzer R, Hutz RJ. Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in-vitro estrogen accumulation. J Reprod Dev. 2009;55:685–690. doi: 10.1262/jrd.20241. [DOI] [PubMed] [Google Scholar]
- 313.Schadlich A, Hoffmann S, Mueller T, Caysa H, Rose C, Gopferich A, Li J, Kuntsche J, Mader K. Accumulation of nanocarriers in the ovary: a neglected toxicity risk? J Control Release. 2012;160:105–112. doi: 10.1016/j.jconrel.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 314.Qu Y, Yang B, Jiang X, Ma X, Lu C, Chen C. Multiwalled carbon nanotubes inhibit steroidogenesis by disrupting steroidogenic acute regulatory protein expression and redox status. J Nanosci Nanotechnol. 2017;17:914–925. doi: 10.1166/jnn.2017.12647. [DOI] [PubMed] [Google Scholar]
- 315.Zhao X, Ze Y, Gao G, Sang X, Li B, Gui S, Sheng L, Sun Q, Cheng J, Cheng Z, et al. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS ONE. 2013;8:e59378. doi: 10.1371/journal.pone.0059378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 316.Santacruz-Marquez R, Solorio-Rodriguez A, Gonzalez-Posos S, Garcia-Zepeda SP, Santoyo-Salazar J, De Vizcaya-Ruiz A, Hernandez-Ochoa I. Comparative effects of TiO2 and ZnO nanoparticles on growth and ultrastructure of ovarian antral follicles. Reprod Toxicol. 2020;96:399–412. doi: 10.1016/j.reprotox.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 317.Courbiere B, Auffan M, Rollais R, Tassistro V, Bonnefoy A, Botta A, Rose J, Orsiere T, Perrin J. Ultrastructural interactions and genotoxicity assay of cerium dioxide nanoparticles on mouse oocytes. Int J Mol Sci. 2013;14:21613–21628. doi: 10.3390/ijms141121613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhai QY, Ge W, Wang JJ, Sun XF, Ma JM, Liu JC, Zhao Y, Feng YZ, Dyce PW, De Felici M, Shen W. Exposure to Zinc oxide nanoparticles during pregnancy induces oocyte DNA damage and affects ovarian reserve of mouse offspring. Aging. 2018;10:2170–2189. doi: 10.18632/aging.101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kashi A, Saha S. Ethics in biomaterials research. J Long Term Eff Med Implants. 2009;19:19–30. doi: 10.1615/jlongtermeffmedimplants.v19.i1.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.