To the Editor,
We recently came accross an interesting article published in this journal by Usman Ayub Awan et al.,1 which highlighted that the monkeypox virus had become another global emergency threatening human health under the COVID-19 pandemic. An outbreak of the Monkeypox virus, which was first reported in the United Kingdom in May of 2022 and has since spread to more than 72 territories causing up to 14,533 cases according to the World Health Organization (WHO), as of the 20th of July 2022 (www.who.int/). In response to rising concerns over monkeypox, researchers are focused on acquiring structures of essential monkeypox proteins and have succeeded in producing crystal structures of the A42R Profilin-like protein (PDB: 4QWO), as well as simulating the structure of envelope protein F13, also known as C19L.2 The structures of most of the monkeypox virus proteins remain unknown, knowledge of which would significantly enhance our understanding of the molecular mechanisms underlying critical viral processes such as viral entry and replication. AlphaFold2 is a powerful open-source computational approach developed to help predict protein structures,3 which has been used successfully in acquiring accurate protein structures of the human and SARS- CoV-2 proteomes.4, 5, 6
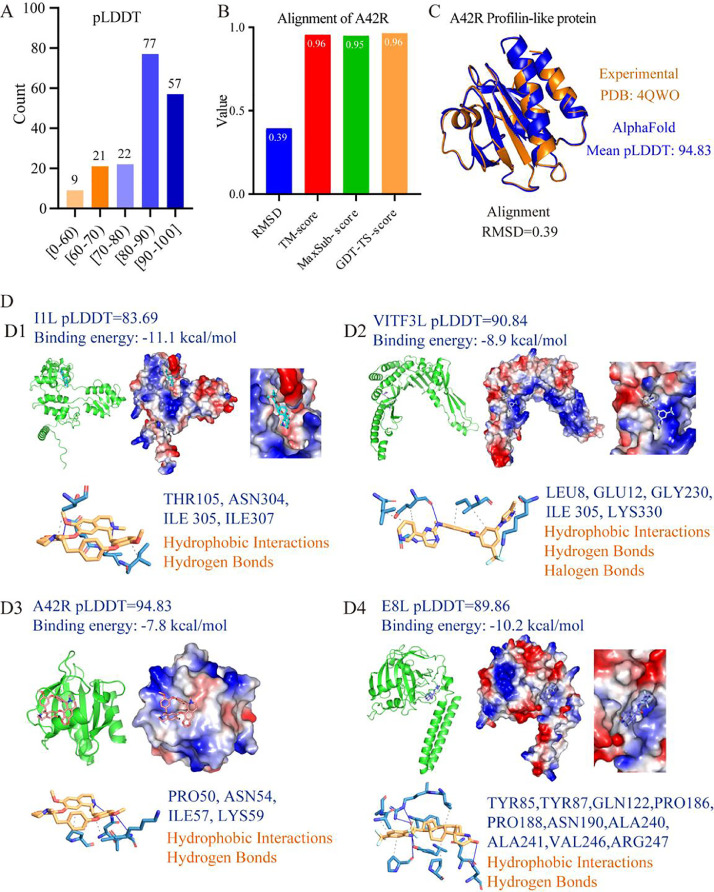
Here, we used AlphaFold2 to predict the protein structures of the reference monkeypox virus proteome (Uniprot ID: UP1012697), yielding a total of 186 highly accurate protein structures (Supplemental Table S1). The mean predicted Local Distance Difference Test (pLDDT) values of 156 of the 186 proteins are above the threshold of 70, which suggests that the predicted structures for these proteins are highly accurate (Fig. 1 A). Furthermore, among these proteins, 77 protein structures had mean pLDDT values of between 80 and 90, while 57 protein structures showed mean pLDDT values above 90. To further evaluate the predicted protein structures, we compared the AlphaFold-predicted structure with the experimental crystal structure (PDB: 4QWO) of A42R Profilin-like protein. The resulting Template Modelling (TM), Global Distance Test (GDT)-TS, and the maximal subset (MaxSub) scores between two protein structures are above 0.95 (Fig. 1B). The aligned structure between two proteins has a root means square deviation (RMSD) value of 0.39 (Figs. 1B and 1C), suggesting that the two protein structures are highly similar (Figs. 1B and 1C). Taken together the above suggests that the predicted structures can be considered to be highly accurate.
Fig. 1.
Protein structural analysis and drug screening of the monkeypox virus. (A) The pLDDT values of all 186 monkeypox virus proteins. (B) Comparison between experimental structure (PDB: 4QWO) and AlphaFold2-predicted structure of A42R profiling-like protein. (C) The alignment of experimental structure and AlphaFold2-predicted structure of A42R profiling-like protein. (D) The interactions between Cepharanthine and four monkeypox virus proteins, including I1L (D1), VITF3L (D2), A42R (D3), and E8L (D4). The molecular structures of cepharanthine in different protein complexes are shown in different colors. The green "cartoon" model and the electrostatic surface present the protein structures. Red and blue indicate negative and positive charges on the electrostatic surface. The detailed interactions between amino acids (light blue) and cepharanthine (yellow) are under the four protein structures. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Previously approved drugs for smallpox, tecovirimat and brincidofovir have been shown to be effective against the monkeypox virus, both in vitro and in animals8; however, given the global emergency, it is necessary to identify alternative drugs that could be used to fight the outbreak. Thus, we endeavored to uncover potential drugs to treat monkeypox based on accurate prediction of the monkeypox protein structures and 5903 approved drugs from the Zinc database9 (Supplemental Table S2). We first selected ten target proteins based on their high pLDDT values, essential functions, and potential pharmacophores. The selected proteins were B4R, A42R, PRO132, VITF3L, E8L, I1L, D10L, P28, and G9R. We subsequently calculated the binding energies of all ten proteins with the 5903 drugs (Supplemental Table S3). The top best docking drugs targeting the ten proteins showed low binding energies (Table 1 , S3, and FigureS1-S10), suggesting they might have strong interactions.
Table 1.
Virtual screening of potential drugs targeting the ten proteins. Related to Table S3.
| Proteins | Drugs | Values | Proteins | Drugs | Values |
|---|---|---|---|---|---|
| B4R | ZINC164528615 | −11.3 | G9R | Trypan Blue | −9.4 |
| Trypan Blue | −11.2 | ZINC3934128 | −8.7 | ||
| ZINC208774715 | −11.1 | ZINC40164432 | −8.7 | ||
| Ixabepilone | −10.9 | ZINC14879959 | −8.6 | ||
| ZINC40165257 | −10.8 | Irinotecan | −8.5 | ||
| Differin | −10.7 | ZINC8220175 | −8.5 | ||
| ZINC43195321 | −10.6 | ZINC14879961 | −8.4 | ||
| Vumon | −10.5 | Indacaterol-8-O-Glucuronide | −8.4 | ||
| P28 | Yaz | −9.7 | D10L | Lumacaftor | −9.3 |
| Ergotamine | −9.2 | ZINC3934128 | −9.1 | ||
| Xaliproden | −9 | Dihydroergotamine | −9 | ||
| Indocyanine Green | −9 | Trypan Blue | −9 | ||
| Trypan Blue | −9 | Dihydroergotoxine | −8.9 | ||
| Bisoctrizole | −8.9 | Gliquidone | −8.8 | ||
| Orobronze | −8.9 | Lestaurtinib | −8.8 | ||
| ZINC1530886 | −8.8 | ZINC253632968 | −8.7 | ||
| I1L | Cepharanthine | −11.1 | E4R | Glycyrrhizinate Dipotassium | −9.7 |
| Trypan Blue | −11.1 | Trypan Blue | −9.7 | ||
| ZINC3934128 | −10.2 | Nilotinib | −9.6 | ||
| ZINC14880001 | −10.2 | Naldemedine | −9.6 | ||
| Avodart | −10.1 | Lifitegrast | −9.5 | ||
| ZINC3917540 | −9.9 | ZINC936069565 | −9.5 | ||
| Lumacaftor | −9.9 | ZINC14880001 | −9.4 | ||
| Nilotinib | −9.7 | ZINC43195321 | −9.4 | ||
| PRO132 | Antrafenine | −11.4 | VITF3L | Trypan Blue | −9.6 |
| Dihydroergotamine | −11 | ZINC164528615 | −9.1 | ||
| ZINC43195321 | −11 | Midostaurin | −9 | ||
| Cabozantinib | −11 | Nilotinib | −8.9 | ||
| Trypan Blue | −11 | Cepharanthine | −8.9 | ||
| Lorazepam Glucuronide | −10.9 | ZINC253633751 | −8.9 | ||
| ZINC8234383 | −10.8 | Avodart | −8.8 | ||
| Lomitapide | −10.8 | ZINC14880001 | −8.8 | ||
| E8L | Trypan Blue | −11.4 | A42R | Tipranavir | −7.9 |
| Dihydroergotoxine | −10.9 | Trypan Blue | −7.9 | ||
| Naldemedine | −10.9 | ZINC936069565 | −7.9 | ||
| ZINC14880001 | −10.6 | Cepharanthine | −7.8 | ||
| Irinotecan | −10.3 | Daclatasvir | −7.8 | ||
| Cepharanthine | −10.2 | Dihydroergotamine | −7.7 | ||
| Fluspirilene | −10.1 | Penfluridol | −7.7 | ||
| Dihydroergotamine | −10.1 | Dihydroergotoxine | −7.7 |
Trypan Blue and Cepharanthine display significant binding affinities to all ten target proteins (Supplemental Table S3). Trypan Blue interacts with the proteins by forming hydrogen bonds, salt bridges and hydrophobic and pi-cation interactions (supplemental materials). Cepharanthine shows high binding affinities to I1L (Fig. 1D1), VITF3L (Fig. 1 D2), A42R (Fig. 1 D3), and E8L (Fig. 1D4) through hydrophobic interactions and hydrogen bond formation. We have generated an extensive drug dataset consisting of 43,800 protein-drug paired data for the monkeypox virus, which could contribute to discovering the potential drugs for fighting monkeypox. Combatting the outbreak would require rapid research in the following: 1. Development of suitable pseudovirus and animal models of the current subtype of monkeypox virus; 2. Clarification of the mechanism of infection, reproduction, and transmission of monkeypox virus; 3. Identify and validate potential drugs that can be used to treat the viral infection.
In conclusion, here we acquired 186 highly accurate protein structures of the monkeypox virus reference proteome using AlphaFold2, which provided the most comprehensive database of monkeypox virus protein structures worldwide. Moreover, we have screened the potential drugs for binding the ten crucial proteins of the monkeypox virus, including B4R, A42R, PRO132, VITF3L, E8L, I1L, D10L, P28, and G9R, and generated a drug dataset containing total 43,800 protein-drug paired data, which could be helpful for drug discovery to the monkeypox virus.
Authors' contributions
Q.Y. and S.Y. conceived and designed the study. Q.Y. and X.S. analyzed and interpreted the data. Q.Y., A.A.S.S, and S.Y. wrote the first draft. Z.W. provided the softwares.
Data availability
The detailed methods are described in supplemental Methods. The protein structural files predicted by AlphaFold2 and the interaction analysis results between drugs and proteins were available on Github (https://github.com/lg10is1/monkeypox-virus-protein-structures). The RStudio code used in this study to perform statistical analysis and visualize data are available upon request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), Natural Science Foundation of China (32070679, U1804284, 81871055), the National Key R&D Program of China (2019YFA0905400, 2017YFC0908105, 2021YFC2702103), Taishan Scholar Program of Shandong Province (tsqn201812153) and Natural Science Foundation of Shandong Province (ZR2019YQ14). The computations in this paper were run on the Siyuan cluster supported by the Center for High-Performance Computing at Shanghai Jiao Tong University.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.08.006.
Appendix. Supplementary materials
References
- 1.Awan U.A., et al. Monkeypox: a new threat at our doorstep! J Infect. 2022;85:e47–e48. doi: 10.1016/j.jinf.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D., Liu Y., Li K., Zhang L. Targeting F13 from monkeypox virus and variola virus by tecovirimat: molecular simulation analysis. J Infect. 2022 doi: 10.1016/j.jinf.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Jumper J., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tunyasuvunakool K., et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590–596. doi: 10.1038/s41586-021-03828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q., et al. Structural comparison and drug screening of spike proteins of ten SARS-CoV-2 variants. Research. 2022;2022 doi: 10.34133/2022/9781758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q., Syed A.A.S., Fahira A., Shi Y. Structural analysis of the SARS-CoV-2 omicron variant proteins. Research. 2021;2021 doi: 10.34133/2021/9769586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shchelkunov S.N., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/s0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaune D., Iseni F. Drug development against smallpox: present and future. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/aac.01683-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterling T., Irwin J.J. ZINC 15–ligand discovery for everyone. J Chem Inf Model. 2015;55:2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The detailed methods are described in supplemental Methods. The protein structural files predicted by AlphaFold2 and the interaction analysis results between drugs and proteins were available on Github (https://github.com/lg10is1/monkeypox-virus-protein-structures). The RStudio code used in this study to perform statistical analysis and visualize data are available upon request.