Graphical abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a serious threat to individuals with underlying chronic kidney disease (CKD). People with CKD are immunocompromised and therefore result in poorer outcomes including increased risk of hospitalization and mortality after COVID-19.1 Despite the availability of COVID-19 vaccines, current data on the vaccine efficacy in individuals with CKD are limited to surrogate endpoints such as antibody titers. As a result, a dedicated study is required to evaluate the effectiveness and safety of COVID-19 vaccines for the CKD population.
In Hong Kong Special Administrative Region, China, a territory-wide vaccination program with BNT162b2 (Comirnaty; BioNTech/Pfizer/Fosun) and CoronaVac (CoronaVac Life Sciences) commenced on March 6, 2021, and February 23, 2021, respectively. BNT162b2 vaccine was the first SARS-CoV-2 mRNA vaccine approved by the US Food and Drug Administration,2 whereas CoronaVac is an inactivated whole-virion SARS-CoV-2 vaccine using adjuvant aluminum hydroxide.3 Using territory-wide electronic medical records and vaccination records, we conducted this population-based, retrospective study to evaluate the effectiveness and safety of COVID-19 vaccines in the CKD population.
Results
A total of 144,591 individuals with CKD before the matched first vaccination date were included, and 220 subjects aged below 18 years and 3130 subjects who received different brands of vaccines were excluded (Supplementary Figure S1).
Vaccine effectiveness using a retrospective cohort
After excluding individuals without matched second vaccination date and who died or who contracted COVID-19 before the matched second vaccination date, 28,374 nonvaccinated, 27,129 two-dose BNT162b2 vaccine recipients, and 47,640 two-dose CoronaVac vaccine recipients were included in the retrospective cohort study. After inverse probability of treatment weighting with 1% extreme values trimming, we obtained a well-balanced cohort with all standardized mean difference <0.1. The baseline characteristics are shown in Supplementary Table S1.
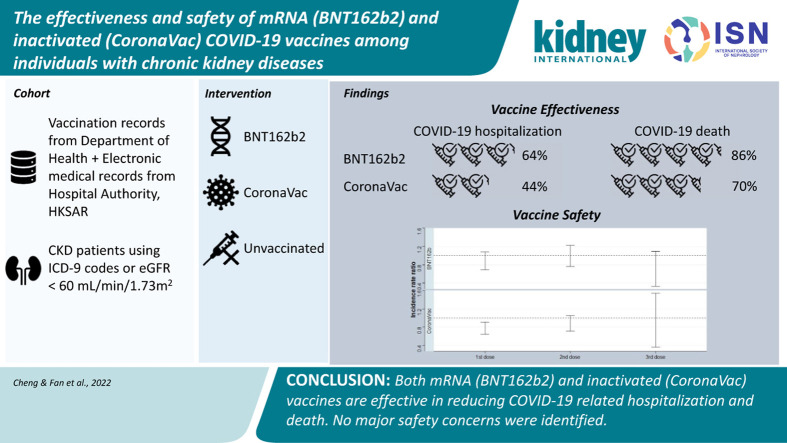
The incidence rates of the COVID infection in the BNT162b2 (25.58 [95% confidence interval {CI}: 24.63–26.56] per 100 person-years) and CoronaVac group (38.07 [95% CI: 37.02–39.13] per 100 person-years) were lower than that in the nonvaccinated group (45.55 [95% CI: 44.28–46.84] per 100 person-years). The vaccine effectiveness was estimated to be 38% (95% CI: 34%–41%) for BNT162b2 and 4% (95% CI: 0%–8%) for CoronaVac when compared with the nonvaccinated group.
Contrary to the COVID-19 infection, both vaccines demonstrated a reduction in COVID-19–related hospitalization and death. For BNT162b2 recipients, the vaccine effectiveness was 64% (95% CI: 57%–69%) for COVID-19–related hospitalization and 86% (95% CI: 80%–90%) for COVID-19–related death. For CoronaVac, the vaccine effectiveness was 44% (95% CI: 37%–49%) and 70% (95% CI: 64%–75%) for COVID-19–related hospitalization and death, respectively. Table 1 summarizes the results of the vaccine effectiveness for both vaccines.
Table 1.
Vaccine effectiveness of BNT162b2 and CoronaVac against COVID-19 infection, COVID-19–related hospitalization, and COVID-19–related death using a retrospective cohort design
| Exposure | Events | Time to events, d, median (IQR) | Follow-up time, person-years | Incidence, 100 person-years, (95% CI) | Adjusted IRRa | P value |
|---|---|---|---|---|---|---|
|
COVID-19 infection | ||||||
| Nonvaccinated | 4933 | 157 (34–220) | 10,830.67 | 45.55 (44.28–46.84) | Ref | — |
| BNT162b2 | 2714 | 156.5 (51–201) | 10,608.71 | 25.58 (24.63–26.56) | 0.62 (0.59–0.66) | <0.001 |
| CoronaVac |
5048 |
120.5 (26–186) |
13,260.88 |
38.07 (37.02–39.13) |
0.96 (0.92–1.00) |
0.05 |
|
COVID-19 hospitalization | ||||||
| Nonvaccinated | 1488 | 154 (34–217.25) | 11,028.46 | 13.49 (12.82–14.2) | Ref | — |
| BNT162b2 | 293 | 84 (26–175) | 10,767.85 | 2.72 (2.42–3.05) | 0.36 (0.31–0.43) | <0.001 |
| CoronaVac |
620 |
61 (19.75–160) |
13,536.92 |
4.58 (4.23–4.96) |
0.56 (0.51–0.63) |
<0.001 |
|
COVID-19 death | ||||||
| Nonvaccinated | 792 | 144 (35–211.25) | 11,101.85 | 7.13 (6.65–7.65) | Ref | — |
| BNT162b2 | 49 | 94 (34–196) | 10,785.68 | 0.45 (0.34–0.6) | 0.14 (0.10–0.20) | <0.001 |
| CoronaVac | 177 | 80 (29–166) | 13,569.68 | 1.3 (1.12–1.51) | 0.30 (0.25–0.36) | <0.001 |
CI, confidence interval; COVID-19: coronavirus disease 2019; IQR, interquartile range; IRR, incidence rate ratio.
Vaccine effectiveness = (1 – IRR) × 100%.
These findings were generally consistent among all subgroups, except for those aged below 65 years, on dialysis or transplant. The vaccine effectiveness against the COVID-19 infection was smaller among CoronaVac recipients in these subgroups. There was also an increased risk of COVID-19–related hospitalization in dialysis patients who received CoronaVac (Supplementary Figure S2, Supplementary Tables S2–S4).
Vaccine safety using modified self-controlled case series
After excluding individuals who contracted the COVID-19 infection, a total of 20,344 incident adverse event of special interest cases were included in the modified self-controlled case series (SCCS) analysis. Among them, 11,574 individuals were nonvaccinated, 2589 received BNT162b2, and 6181 received CoronaVac.
The incidence rate of any adverse events of special interest after the first dose of vaccine is 34.28 (95% CI: 29.81–39.23) and 38.39 (95% CI: 34.81–42.23) per 10,000 doses of BNT162b2 and CoronaVac, respectively. Compared with the baseline period, no increased risk of overall adverse event of special interest was observed in any risk period for both BNT162b2 (incidence rate ratio [95% CI]: first dose: 0.86 [0.69–1.08]; second dose: 0.96 [0.76–1.22]; third dose: 0.60 [0.33–1.10]) and CoronaVac (incidence rate ratio [95% CI]: first dose: 0.76 [0.64–0.91]; second dose: 0.86 [0.71–1.05]; third dose: 0.74 [0.36–1.54]) (Table 2 , Supplementary Table S5).
Table 2.
Adjusted incidence rate ratio of any AESI after BNT162b2 and CoronaVac vaccines using modified SCCS
| Period | Events |
Follow-up time, person-years |
IRR (95% CI) |
P value |
Events |
Follow-up time, person-years |
IRR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|---|
| BNT162b2 | CoronaVac | |||||||
| Baseline | 13,948 | 15,262.14 | — | — | 17,331 | 18,842.38 | — | — |
| Day 0 after first dose | 2 | 7.09 | — | — | 0 | 16.92 | — | — |
| Days 1–13 after first dose | 44 | 89.42 | 0.65 (0.46–0.90) | 0.010 | 124 | 213.45 | 0.67 (0.54–0.84) | 0.001 |
| Days 14–27 after first dose | 56 | 58.95 | 1.20 (0.90–1.59) | 0.211 | 131 | 204.12 | 0.82 (0.66–1.02) | 0.074 |
| Days 1–27 after first dose | 100 | 148.37 | 0.86 (0.69–1.08) | 0.207 | 255 | 417.57 | 0.76 (0.64–0.91) | 0.002 |
| Day 0 after second dose | 2 | 5.60 | — | — | 2 | 10.85 | — | — |
| Days 1–13 after second dose | 41 | 68.26 | 0.84 (0.61–1.16) | 0.290 | 84 | 122.85 | 0.84 (0.64–1.10) | 0.205 |
| Days 14–27 after second dose | 54 | 62.72 | 1.15 (0.86–1.53) | 0.344 | 70 | 96.68 | 0.83 (0.63–1.07) | 0.154 |
| Days 1–27 after second dose | 95 | 130.98 | 0.96 (0.76–1.22) | 0.757 | 154 | 219.52 | 0.86 (0.71–1.05) | 0.141 |
| Day 0 after third dose | 0 | 1.56 | — | — | 0 | 1.59 | — | — |
| Days 1–13 after third dose | 8 | 17.91 | 0.58 (0.26–1.29) | 0.181 | 7 | 18.36 | 0.73 (0.29–1.82) | 0.498 |
| Days 14–27 after third dose | 8 | 14.37 | 0.70 (0.32–1.53) | 0.370 | 6 | 14.23 | 0.66 (0.27–1.62) | 0.362 |
| Days 1–27 after third dose | 16 | 32.29 | 0.60 (0.33–1.10) | 0.101 | 13 | 32.59 | 0.74 (0.36–1.54) | 0.424 |
AESI, adverse event of special interest; CI, confidence interval; IRR, incidence rate ratio; SCCS, self-controlled case series.
Discussion
Our study demonstrated that both BNT162b2 and CoronaVac were effective against severe outcomes after the COVID-19 infection by reducing hospitalization and mortality and safe with no increased risk of adverse events of special interest observed during the study period. These results are consistent with the findings among the general population in Hong Kong.4
For both BNT162b2 and CoronaVac, the vaccine effectiveness against the infection is substantially lower than the one for more severe outcomes as this is more likely to be affected by time since vaccination. A recent study from Qatar on waning vaccine protection found that the effectiveness against the COVID-19 infection peaked in the first month after the second dose and dropped drastically after 6 months (from 77.5% to 17.3%).5 However, the effectiveness against severe outcomes was maintained at 88.9% 6 months after the second dose.
Our subgroup analyses indicated that the vaccines appeared to be less effective to prevent the COVID-19 infection in dialysis, transplant, and younger individuals. This could be attributed to several reasons. Younger subjects are generally more active and may have a higher rate of social contact. Patients on hemodialysis require frequent hospital or hemodialysis center visits, while those younger individuals may still be employed. Notably, these results were also consistent with the current data on COVID-19 vaccination efficacy in individuals with CKD using the immunogenicity data. In a recent meta-analysis, Ma et al. 6 reported a significant reduction in antibody response in patients with renal replacement therapy, leading to reduced protection against the COVID-19 infection.
In addition, we also observed an increased risk of COVID-19 hospitalization among individuals on dialysis. For patients on hemodialysis with the COVID-19 infection, admission may be mandatory to keep the dialysis schedule, irrespective of the COVID-19 severity. In fact, there was no increased risk of mortality despite the higher rate of infection and hospitalization in these patients, indicating that the vaccines remained effective against mortality despite the increased hospitalization rate.
Our study has several strengths. We used a retrospective cohort study to evaluate vaccine effectiveness, whereas modified SCCS to assess vaccine safety. A cohort design enabled us to examine multiple outcomes for a given exposure and calculate the incidence rate over time.7 The use of territory-wide electronic records reduced the risk of selection, recall, and misclassification biases in the cohort study. At the same time, with the increasing vaccine coverage, SCCS remains the most appropriate method to evaluate the safety by eliminating confounding by all time-independent variables. This study applied the modified SCCS method to avoid the violation of the assumption on the event-dependent exposure as well as event-dependent censoring of observation of SCCS. Our findings provide more information about vaccine effectiveness and safety, which are the core elements affecting vaccine uptake in Hong Kong.8
This study is subject to several limitations. First, we defined the COVID-19 infection as SARS-CoV-2 polymerase chain reaction positive, whereas a positive rapid antigen test was regarded as the COVID-19 infection in Hong Kong after March 7, 2022. As a result, we may have underestimated the overall incidence of the COVID-19 infection that affects the estimation of the vaccine effectiveness. However, our results may have underestimated the vaccine effectiveness instead, because vaccinated individuals presumably have a higher rate of social contact and lower adherence to safety measures.9 Furthermore, polymerase chain reaction tests remain to be the gold standard to confirm the diagnosis, and our results are consistent in COVID-19–related hospitalization and death. Secondly, our study did not consider the waning of vaccine protection over time, which may underestimate the vaccine effectiveness as well. Our subgroup analysis with subjects receiving the booster dose demonstrated that the vaccine is more effective against the COVID-19 infection and severe outcomes than in the main analysis. Lastly, our dataset does not contain information with regard to the SARS-CoV-2 variants. Vaccine effectiveness toward different variants could not be evaluated in this study.
In conclusion, we found that both mRNA (BNT162b2) and inactivated (CoronaVac) vaccines were effective against the severe outcomes of the COVID-19 infection, namely, hospitalization and mortality. Both vaccines were less effective in protecting individuals from the COVID-19 infection. No safety concerns were identified. With these findings, vaccination is strongly encouraged for individuals with CKD.
Disclosure
CKHW reports the receipt of General Research Fund, Research Grants Council (RGC), Government of Hong Kong Special Administrative Region (HKSAR) and EuroQol Research Foundation, all outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong RGC, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; and personal fees from Primevigilance Ltd., outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong RGC and has received research grants from Food and Health Bureau of the Government of the HKSAR, outside the submitted work. XL received research grants from Research Fund Secretariat of the Food and Health Bureau (Health and Medical Research Fund [HMRF], HKSAR), RGC/Early Career Scheme (RGC/ECS, HKSAR), Janssen, and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme, unrelated to this work. EYFW has received research grants from the Food and Health Bureau of the Government of the HKSAR and the Hong Kong RGC, outside the submitted work. EWYC reports grants from RGC (Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of HKSAR; and honorarium from Hospital Authority, outside the submitted work. SCWT reports research funding outside the submitted work from Sanofi, RGC of Hong Kong, HMRF of Hong Kong, and National Natural Science Foundation of China; and also received speaker fees from AstraZeneca, Novartis, and Bayer in the previous 3 years. He is also on the Executive Committee of Kidney Disease: Improving Global Outcomes (KDIGO) and the Steering Committee of Eledon Pharmaceuticals, and provides scientific advice to Travere Therapeutics. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong HMRF, National Institute for Health Research in England, European Commission, and National Health and Medical Research Council in Australia; and also received speaker fees from Janssen and Medicine in the previous 3 years. He is also an independent nonexecutive director of Jacobson Medical in Hong Kong. All the other authors declared no competing interests.
Data Statement
Data will not be available for others as the data custodians have not given permission.
Acknowledgments
The authors thank the Hospital Authority and the Department of Health for the generous provision of data for this study and Vincent Yan for technical support. Research grant has been received from The Food and Health Bureau, the Government of Hong Kong Special Administrative Region, China. The funders did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. FTTL and ICKW’s post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK, administered by the Innovation and Technology Commission.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Flowchart for inclusion and exclusion in the retrospective cohort and self-controlled case series (SCCS) design.
Figure S2. Vaccine effectiveness of BNT162b2 and CoronaVac against coronavirus disease 2019 (COVID-19) infection, COVID-19–related hospitalization, and COVID-19–related death across subgroups.
Figure S3. Observation timeline of individuals in the modified self-controlled case series.
Table S1. Baseline characteristics in retrospective cohort design.
Table S2. Incidence rate ratio of BNT162b2 and CoronaVac against coronavirus disease 2019 (COVID-19) infection in subgroups using a retrospective cohort design.
Table S3. Vaccine effectiveness of BNT162b2 and CoronaVac against coronavirus disease 2019 (COVID-19)–related hospitalization in subgroups using a retrospective cohort design.
Table S4. Vaccine effectiveness of BNT162b2 and CoronaVac against coronavirus disease 2019 (COVID-19)–related death in subgroups using a retrospective cohort design.
Table S5. Adjusted incidence rate ratio (IRR) of adverse events of special interest (AESIs) after BNT162b2 and CoronaVac using modified self-controlled case series (SCCS).
Table S6. Definitions of chronic kidney disease (CKD) using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Table S7. Definitions of adverse events of special interest (AESIs).
Supplementary Material
References
- 1.Jdiaa S.S., Mansour R., El Alayli A., et al. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022;35:69–85. doi: 10.1007/s40620-021-01206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
- 3.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. Preprint. Posted online March 24, 2022 doi: 10.1101/2022.03.22.22272769. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly H., Tang P., Hasan M.R., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma B.M., Tam A.R., Chan K.W., et al. Immunogenicity and safety of COVID-19 vaccines in patients receiving renal replacement therapy: a systematic review and meta-analysis. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.827859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann C.J. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20:54–60. doi: 10.1136/emj.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang S.J. Predicting COVID-19 vaccine hesitancy in Hong Kong: vaccine knowledge, risks from coronavirus, and risks and benefits of vaccination. Vaccine X. 2022;11 doi: 10.1016/j.jvacx.2022.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usherwood T., LaJoie Z., Srivastava V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.